Abstract
Enzyme engineering plays a central role in the development of biocatalysts for biotechnology, chemical and pharmaceutical manufacturing, and environmental remediation. Rational design of proteins has historically relied on targeting active site residues to confer a protein with desirable catalytic properties. However, additional “hotspots” are also known to exist beyond the active site. Structural elements such as subunit–subunit interactions, entrance tunnels, and flexible loops influence enzyme catalysis and serve as potential “hotspots” for engineering. For the Rieske oxygenases, which use a Rieske cluster and mononuclear iron center to catalyze a challenging set of reactions, these outside of the active site regions are increasingly being shown to drive catalytic outcomes. Therefore, here, we highlight recent work on structurally characterized Rieske oxygenases that implicates architectural pieces inside and outside of the active site as key dictators of catalysis, and we suggest that these features may warrant attention in efforts aimed at Rieske oxygenase engineering.
Introduction
Enzymes are the original catalysts and Nature’s most creative chemists. For thousands of years, the ability of enzymes to catalyze a broad range of chemical transformations and operate under mild, biologically relevant temperatures and pH values, has been harnessed and exploited to create valuable compounds [1–3]. In fact, engineered enzymes, produced using structure-guided rational mutagenesis or directed evolution techniques, have been used in a myriad of applications, including regenerative medicine, food production, waste degradation, biosensing, and chemical and pharmaceutical production [1–3]. Traditionally, enzyme engineering efforts by rational design have heavily focused on modifying an enzyme active site to exploit an identified promiscuous activity or to evolve a new function or reaction selectivity [1–3]. This active site region is often predicted based on knowledge of the enzyme mechanism and the presence of characteristic sequence motifs. However, the active site generally accounts for only a small percentage of the enzyme architecture, and solely introducing mutations into this region often limits the achievable chemistry, substrate scope, or desired selectivity for a reaction of interest [4]. Changes made to an enzyme active site can also result in lower or complete loss of activity, making engineering campaigns that focus on identifying residues, protein features, and dynamic elements outside of the active site region critical to enzyme engineering [4–6]. However, in many cases, the rational identification of atypical hotspots found outside of the active site is complicated by a lack of structure–function information for an enzyme or enzyme class of interest.
Importantly, however, and notably in some heme-based and other metalloenzyme families, residues found outside of the active site that influence activity have been identified by making random mutations using directed evolution techniques [7–9]. Combined with extensive computational and experimental work on multiple protein families, several emerging architectural features for engineering outside the active site have been revealed to exist across multiple enzyme classes [4,10–14]. Additional protein subunits or domains, substrate tunnels, and flexible connector loops have each been shown to impact reaction outcome [4,10–14].
For instance, the presence of an extra subunit or partner protein is known to influence the structural stability, reaction trajectory, and substrate scope of many types of enzymes [13]. Likewise, by playing an integral role in the shuttling of a substrate from the cellular milieu into an enzyme active site, tunnels have been shown to influence enzyme stability, and the temporal progress, selectivity, and substrate scope of a reaction [4,10,12,15]. Of equal importance, the flexible loops that join secondary structural elements and protein domains, as well as mediate interactions with partner proteins are known to play critical roles in the stability and allosteric behavior of enzymes [13,14]. Loops have also been implicated in dictating the substrate scope or selectivity of an enzyme-catalyzed reaction [5,11]. These observations highlight the potential of tunnels, loops, and subunit modulation as valuable pieces of the enzyme engineering toolbox.
Members of the Rieske non-heme iron oxygenase enzyme class have been rapidly gaining attention for their biocatalytic promise. These metalloenzymes, commonly referred to as Rieske oxygenases, contain a [2Fe–2S] Rieske cluster and mononuclear iron center (Figure 1a). The Rieske cluster shuttles electrons from a dedicated reductase protein to the mononuclear iron center where oxygen is activated to form a reactive intermediate. This species is subsequently used to facilitate monooxygenation, dioxygenation, sequential monooxygenation, or other disparate reactions (Figure 1b) [16–20]. Rieske oxygenases accept substrates with chemical structures of varying complexity, synthesize natural products with medical, environmental, and industrial relevance, and degrade environmental contaminants (Figure 1b) [16–20]. However, despite their known substrate specificities and widespread reactivities, members of the Rieske oxygenase enzyme class share the same catalytic machinery (Figure 1) [19]. As a growing amount of literature suggests that the specificity and catalytic functions of a Rieske oxygenase can be attributed to auxiliary protein components, tunnels, and loops, here, we provide an up-to-date discussion on the structurally characterized Rieske oxygenases and the discoveries that are informing rational protein engineering efforts (Figure 1).
Figure 1.
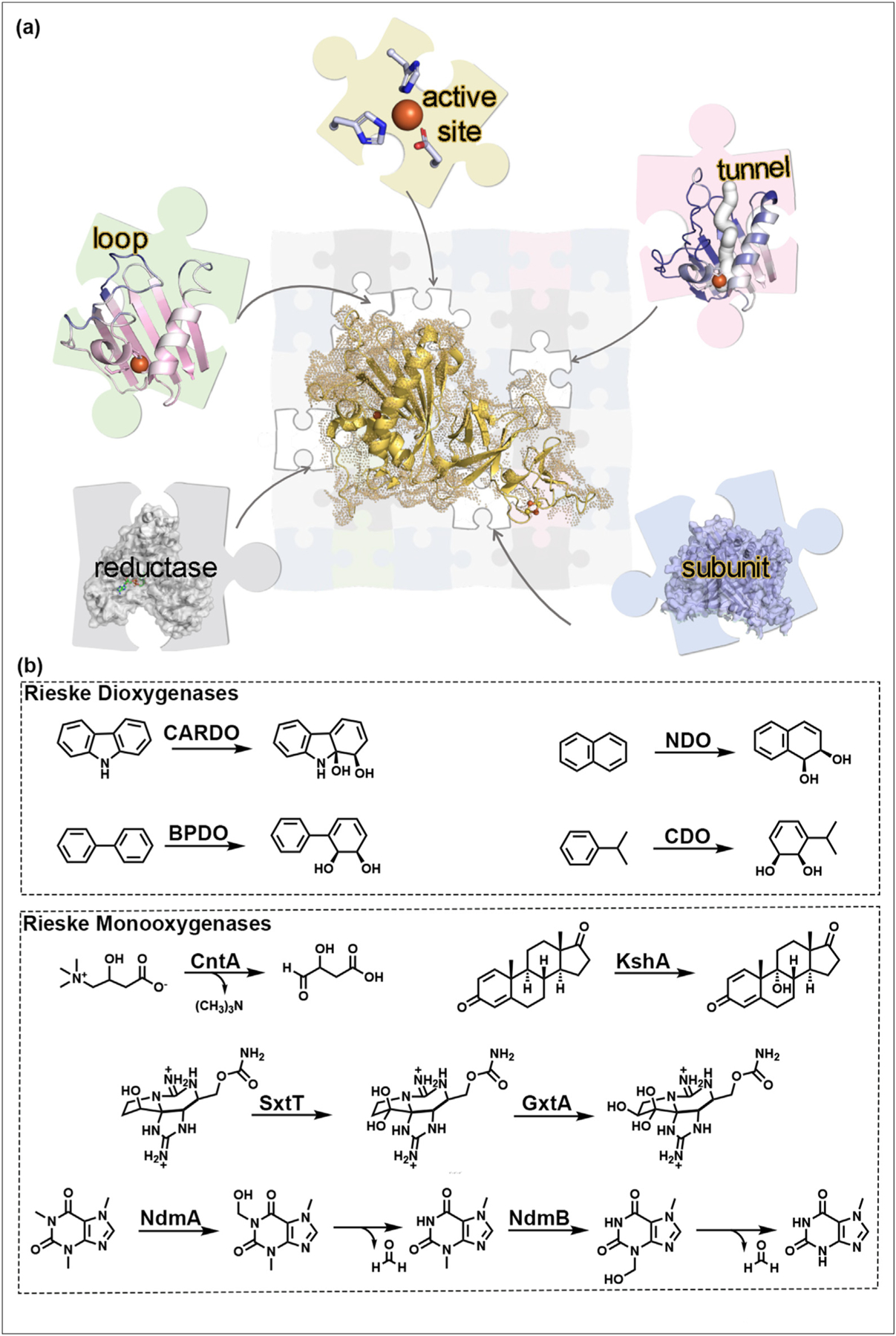
Rieske oxygenase activity is controlled using regions both inside and outside of the active site. (a) Rieske oxygenases use electrons, molecular oxygen, and a common catalytic metallocluster-containing scaffold (the α subunit) to catalyze a myriad of transformations. A growing amount of literature suggests that the selectivity of a reaction catalyzed by a Rieske oxygenase can be customized by the addition of an extra subunit, the microenvironment of a substrate entrance tunnel, or the presence of flexible loops near the active site. (b) Rieske oxygenases are traditionally recognized for their ability to catalyze dioxygenation and monooxygenation reactions. The reactions shown here are catalyzed by carbazole dioxygenase (CARDO), biphenyl 2,3-dioxygenase (BPDO), naphthalene dioxygenase (NDO), cumene dioxygenase (CDO), carnitine monooxygenase (CntA), 3-ketosteroid 9α-hydroxylase (KshA), the saxitoxin biosynthetic enzymes (SxtT and GxtA), and the caffeine demethylases (NdmA and NdmB). Of note, each transformation shown in this figure also requires O2 and electrons.
The quaternary architecture of a Rieske oxygenase
Based upon approximately 20 unique crystal structures, it has been revealed that Rieske oxygenases assemble into heterohexameric (α3β3) [21–30], homohexameric (α3α3) [31,32], and homotrimeric (α3) [33–40] architectures (Figure 2a–c). Some reports have also suggested that the Rieske oxygenases chlorophyll(ide) a oxygenase and phthalate dioxygenase (PDO) from Micromonas pusilla and Pseudomonas cepacia, respectively, adopt alternative dimeric and tetrameric quaternary structures [32,41,42]. However, more recent structural and biochemical data suggest that these systems instead fold into one of the above-noted traditional assemblies [32,41,42]. In the α3β3, α3α3, and α3 structures, three α subunits, which each contain both metallocenters, arrange into a trimeric architecture that places Rieske cluster and iron site within electron transfer distance of one another across a subunit–subunit interface (Figure 2a–c). In the α3 architecture, the subunit–subunit interfaces are also the site where the partner reductase proteins bind [43] (Figure 2d).
Figure 2.
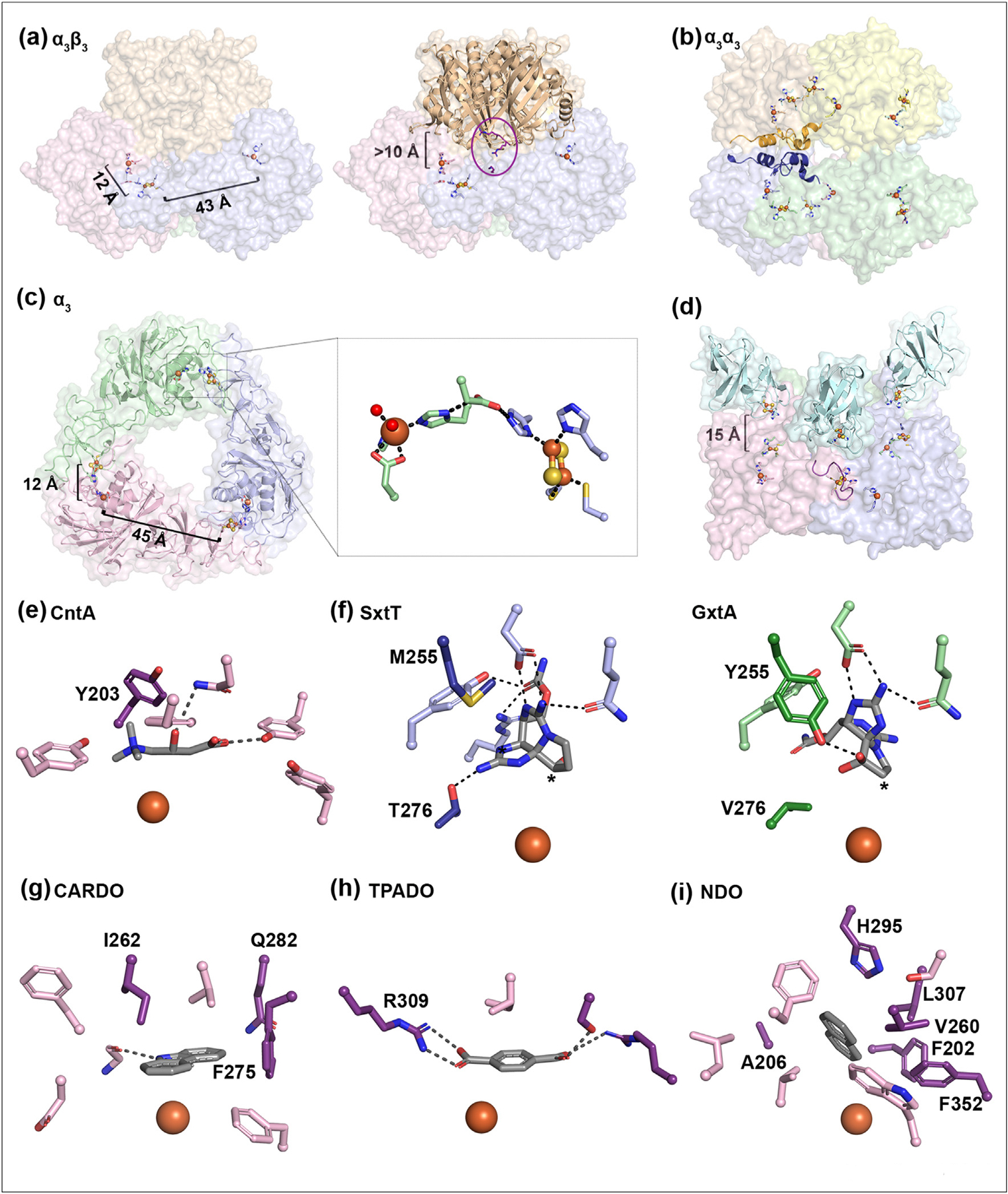
Rieske oxygenases are found in different architectures, each of which contains a trimer of α-subunits to coordinate the metallocenters and anchor the substrate near the mononuclear iron center. (a) In the α3β3 Rieske oxygenases, the metallocenters in a single α protomer are too far apart for electron transfer. However, assembly of the α subunits into a trimeric architecture moves the metallocenters from adjacent subunits close together (PDB:1NDO) [30]. The β subunits of the α3β3 enzymes sit more than 10 Å away from the active site. Residues implicated in binding the reductase are shown in an oval and highlighted in purple [49]. (b) The α3α3 architecture of PDO reveals that the trimers are staggered and likely stabilized by additional secondary structures found in each α subunit (dark blue and orange). (c) The α3 Rieske oxygenase architecture, as seen in the α3β3 and α3α3 architectures, arranges its Rieske cluster and mononuclear iron center across a subunit–subunit interface bridged by a conserved Asp residue (inset). (d) For the enzyme CARDO, three reductase proteins sit on top of the trimer such that the Fe–S clusters are aligned with the Rieske clusters of the oxygenase [43]. The loop that is suggested to stabilize the trimeric CARDO structure (and negate the need for a β subunit) is shown in purple for the light pink protomer. (e) The active site of CntA has a Tyr residue (Tyr203) that is important for substrate binding (PDB: 6Y9D). (f) Two active site residues in SxtT (left, PDB: 7SZH) and GxtA (right, PDB: 7SZE) (Met/Tyr255 and Thr/Val276) are important for positioning the native substrates for hydroxylation at the C12 and C11 positions, respectively (starred) [37,38]. Comparison between the panels reveals that the tricyclic substrate scaffold undergoes an approximate 120° rotation in the active site. (g) Residues Ile262, Gln282, and Phe275 are important to the selectivity of the CARDO-catalyzed reaction (PDB: 1WW9). (h) Arg309 in terephthalate dioxygenase (TPADO) is important for substrate binding and is conserved in additional Rieske oxygenases that perform chemistry on structurally similar molecules (PDB:7Q05). (i) Several residues (Phe202, Ala206, Val260, His295, Leu307, and Phe352) have been implicated in the selectivity of NDO-catalyzed reaction. In panels e, g, h, and i, specific residues involved in dictating reaction selectivity are shown in dark purple.
For the α3α3 and α3β3 architectures, a second trimer of α or β subunits stacks on top of the α3 trimer more than 10 Å away from the active site (Figure 2a–b) [21–32] Chemically speaking, many of the α3β3 Rieske oxygenases have been demonstrated to function as dioxygenases. However, it does not appear as though the reaction catalyzed by a Rieske oxygenase is enough to assign it a specific architecture. Carbazole dioxygenase (CARDO), for example, has been shown to exist in an α3 architecture [44] and PDO adopts an α3α3 arrangement (Figure 2b) [32]. Similarly, some oxidative demethylase enzymes adopt an α3 structure, whereas the caffeine demethylases NdmA and NdmB assemble into an α3α’3 heterohexameric complex [31–33,40]. As an alternative to dictating the type of reaction catalyzed, the extra α and β subunits of the α3α3 and α3β3 proteins, have been proposed to serve a structural role [21,30,45]. In agreement with this proposal, CARDO and PDO have been noted to contain loop regions, in lieu of a β subunit, that form interactions with the adjacent subunits to provide additional stability (Figure 2b, d) [32,44]. Other reports suggest that the β subunits could be involved in dictating the substrate scopes and preferences of different Rieske oxygenases [46–48]. Most recently, residues from both the α and β subunits were reported to mediate interactions with the ferredoxin component of a dedicated partner reductase system [49]. Mapping of these residues onto naphthalene dioxygenase (NDO) reveals that the reductase, as observed in the α3 enzymes, also sits at the subunit–subunit interfaces (Figure 2a, d). The multitude of different roles proposed for the β subunit suggests that it is a critical architectural element that can be used to customize the specificity and catalytic functions of individual Rieske oxygenases.
The microenvironment of the active site contributes to catalysis
In each of the described architectures, residues found within the active site of the α subunit not only coordinate the metallocenters, but also play key roles in dictating the site-, stereo-, and chemoselectivity of a Rieske oxygenase catalyzed reaction. For example, the presence of an aromatic residue in the active site of carnitine monooxygenase (CntA) is indispensable for positioning the carnitine substrate for catalysis via a π–cation interaction in the active site (Figure 2e) [39]. Based on sequence alignments, this residue appears to be a hallmark of quaternary-ammonium-substrate-oxidizing Rieske oxygenases [39]. Along these lines, the α3 Rieske oxygenases SxtT and GxtA, which catalyze monooxygenation reactions at the C12 and C11 positions of a tricyclic saxitoxin scaffold, respectively, use two active site residues to hold the substrate such that the correct carbon-atom is positioned for hydroxylation (Figure 2f) [37,38,50]. Two important structural studies on CARDO have revealed that mutations in the active site can change the openness of the substrate-binding pocket, the orientation in which the substrate binds, and the outcome of the hydroxylation event (Figure 2g) [36,51]. Similarly, in the structure of the α3β3 enzyme terephthalate dioxygenase, an active site Arg residue is critical for correctly positioning the terephthalate substate near the iron center for dioxygenation (Figure 2h) [29]. This Arg residue is conserved in several other Rieske oxygenases that also catalyze reactions on carboxylate-containing substrates [29]. Changes in the active site of the α3β3 enzyme NDO have also been shown to affect reaction outcome. Some active site NDO variants produce a mixture of native naphthalene dihydrodiol product enantiomers, others form isomeric distributions when provided with biphenyl or phenanthrene substrates, and some create monooxygenated, rather than dioxygenated products using non-native substrates (Figure 2i) [52–54]. In accordance with these NDO studies, active site residues in toluene dioxygenase can be targeted to increase the yield, or change the enantio-, chemo-, or regioselectivity of a reaction when provided with a non-natural substrate [55,56].
However, in each of these noted examples, these focused active-site changes alone do not typically result in a full transformation of the native reactivity. Rather, some of the active site variants of CARDO and NDO described above form mixtures of products and other variants showcase altered substrate specificities [36]. For SxtT and GxtA, the active site variants have added functionality and hydroxylate at both the native and non-native positions but remain biased toward the native selectivity of the enzymes [38]. Collectively, these studies suggest that the outcome of a Rieske oxygenase catalyzed reaction depends on additional protein motifs that are located outside of the active site.
The influence of the substrate entrance tunnel
Aside from the quaternary architecture of a Rieske oxygenase, several protein engineering efforts have provided insight into regions outside of the active site involved in the selectivity of a catalyzed reaction. Rieske oxygenases, like the majority of other metalloproteins, bury their active sites far beneath the protein surface [4,15] (Figure 2, 3a). This arrangement serves to protect the metallocenters and reactive intermediates from solvent and molecular oxygen, and at the same time, necessitates the presence of a tunnel for shuttling substrate into the active site.
Figure 3.
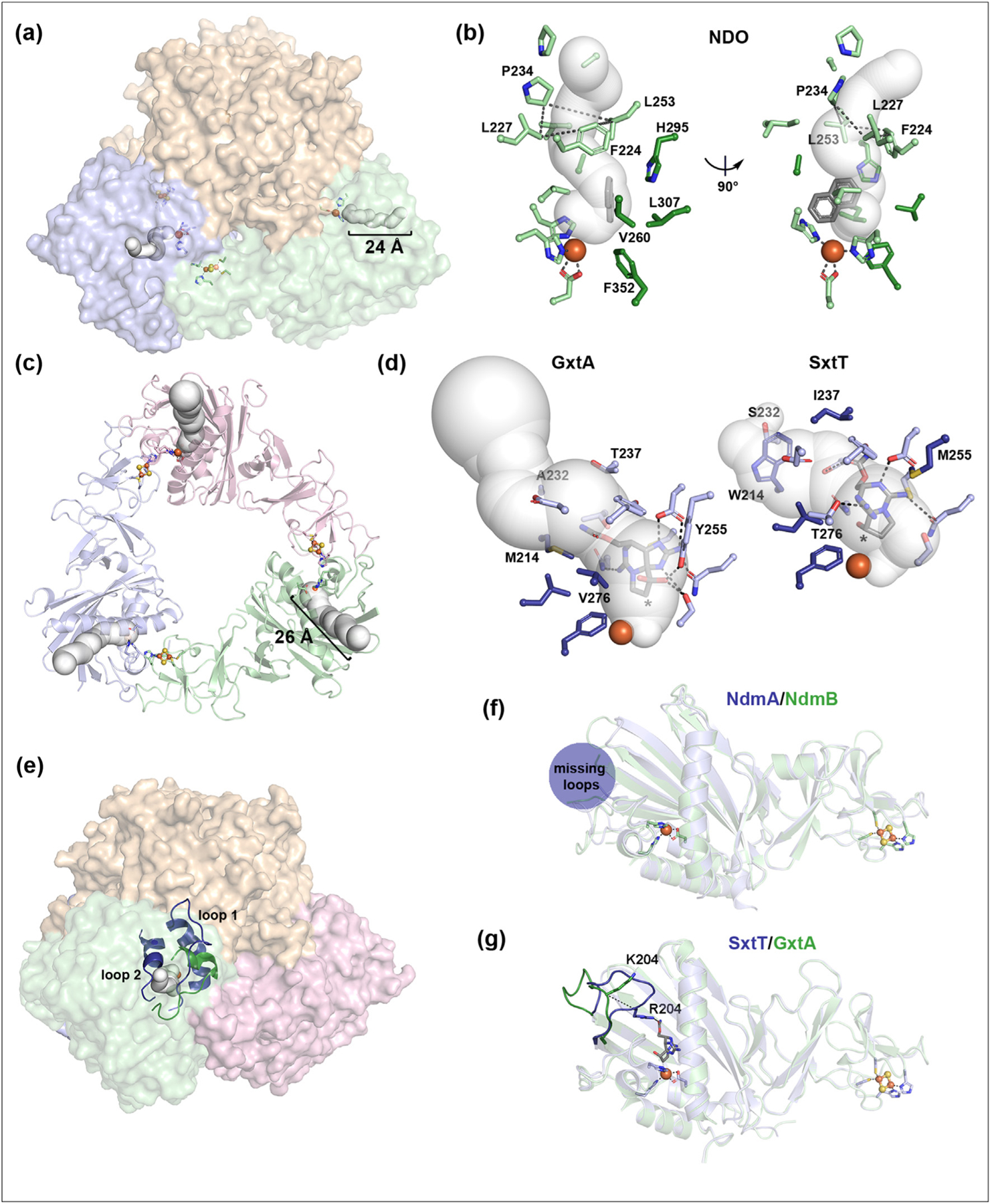
A substrate entrance tunnel and flexible loop are important architectural elements involved in Rieske oxygenase chemistry. (a) The α3β3 architecture of a Rieske oxygenase is comprised of stacked α3 and β3 trimers (PDB:1NDO, 1OG7) [30]. An approximate 24 Å long substrate entrance tunnel can be calculated in each α subunit using the MOLEonline server [63]. (b) The bottleneck of the NDO tunnel is formed by four residues, Phe224, Leu227, Pro234, and Leu253 [15]. In this panel, residues that line the tunnel are shown in light green, residues experimentally revealed to impact the selectivity of the NDO-catalyzed reaction are shown in dark green, and naphthalene is shown in gray (PDB:1NDO, 1O7G) [54,64]. (c) The α3 Rieske oxygenase GxtA has a 33 Å long substrate entrance tunnel that was identified using Xe-pressurization experiments (PDB: 7SZG) [37]. (d) A comparison of the microenvironments of the GxtA (left, PDB: 7SZE) and SxtT (right, PDB: 7SZH) tunnels reveals key differences that are important for positioning the native saxitoxin and βsaxitoxinol substrates (gray) of these enzymes in the active site for hydroxylation (* indicates where the substrate is oxygenated). Key differences in these proteins in the tunnel (214, 232, and 237) and active site (255 and 276) are important for the site-selectivity of the reactions [37]. (e) A loop (forest green) in NDO is important for the positioning of substrate in the active site. NDO is shown in this panel rotated approximately 60° from the orientation in panel a. Similar studies on cumene dioxygenase, which is overlaid with the structure of NDO, revealed that the analogous loop (loop 1) and one other loop (loop 2) both impact the outcome of the reaction (these loops are shown in dark blue). (f) The β13-to-β14 connecting loop of NdmA (dark blue, PDB:6ICK) and NdmB (green, PDB:6ICL) is important to the selectivity and substrate scopes of the enzyme-catalyzed reactions but was too disordered to be built into the structures [31]. (g) The β13-to-β14 connecting loop of SxtT (dark blue) and GxtA (green) are found in remarkably different orientations that differ in position by 5.5 Å (dashed line). Loop residue Arg204 interacts with the β-saxitoxinol substrate of SxtT and along with active site residues, Met255 and Thr276, contribues to the site-selectivity of the catalyzed reaction [37].
The design of the substrate entrance tunnel in these proteins has been suggested to resemble a funnel: the narrowest region sits at the entrance of the active site and gates the entry of non-substrate molecules [57]. Consistently, a comparison of the NDO and biphenyl 2,3-dioxygenase (BPDO) crystal structures, which both perform dioxygenation chemistry on a biphenyl substrate, reveals that the size of the active site entrance in BPDO is larger than that of NDO [57]. This architectural difference is credited with conferring BPDO the ability to also oxygenate large four- and five-ring-containing aromatic compounds [57]. More recently, molecular dynamics simulations were used to predict the identity of substrate molecules that can be oxidized by NDO. By accounting for the energetics of substrate migration into the active site through a computationally predicted tunnel, this study showed an approximate 90-percent success rate for predicting known substrates of NDO [15]. Four residues found at the bottleneck of the 24 Å long entrance tunnel provide the largest energetic barrier to substrate entry, suggesting that the chemical properties of an entrance tunnel are integral to determining the substrate scope of a Rieske oxygenase (Figure 3a–b) [15]. Interestingly, several of the previously identified NDO variants that show changes in reaction specificity and in the stereo- and site-selectivity of the catalyzed reaction are found within the active site and along the computationally identified tunnel (Figure 2i and 3b) [54].
Related to these computational studies, Xepressurization X-ray crystallographic studies allowed for identification of a substrate-entrance tunnel in the α3 Rieske oxygenase GxtA (Figure 3c) [37,38]. Using the identified tunnel and mapping its position to SxtT allowed for the discovery of three residues that can be mutated to endow SxtT with a broader substrate scope, which closely resembles that of GxtA, and the ability to preferentially hydroxylate the non-native C11 position of its substrate [37]. These results suggest that, as described for NDO, the tunnel is a key determinant in dictating the nature of substrates that enter the active site. These results also suggest that the tunnel is involved in the selectivity of the hydroxylation event [37]. Key differences in the size and hydrophobicity of the SxtT and GxtA tunnel-lining residues affect the geometry by which the substrate enters the active site and thus influence the positioning of the substrate relative to the activated oxygen intermediate (Figure 3d) [37].
The roles of flexible connecting loops
Experimental, computational, and structural studies have revealed that the loops of Rieske oxygenases and several other protein classes are not simple connectors but key players in dictating enzyme activity [5,11,14,58]. Through a comparative structural study of the α3β3 Rieske oxygenases BPDO and NDO, it was shown that loop residues impact the positioning of substrates in the active site and influence the size and shape of accepted substrates [57]. Recently, both the equivalent loop and an auxiliary loop that also folds over the opening to the active site in cumene dioxygenase (CDO) were subjected to an engineering campaign (Figure 3e). Here, libraries of loop variants that were lengthened, truncated, or subjected to mutagenesis were created. In this work, CDO was shown to be an adaptable biocatalyst: variants in these two loops showcased more robust activity, shifted product distributions, and altered selectivity [59].
The site-selectivity of NdmA and NdmB is known to be dependent on a flexible loop that connects the β13 and β14 strands of the protein [31] (Figure 3f). An NdmA variant that mimics NdmB in the active site and loop regions intriguingly exhibits the selectivity and substrate preference of NdmB [31]. Further studies on the analogous β13-to-β14 connecting loop of the α3 Rieske oxygenases SxtT and GxtA revealed the importance of a single residue for conferring rigorous selectivity to SxtT [37] (Figure 3g). Mutation of the Arg204 loop residue, which interacts with the bound substrate, into the corresponding Lys residue of GxtA results in creation of a variant that hydroxylates at both the native and non-native positions [37]. The non-native selectivity of R204K-SxtT is amplified, but not fully inverted, by mutation of two active site residues (Thr276 and Met255) that also interact with the substrate and contribute to its positioning (Figure 2f and 3g) [37,38]. This triple variant of SxtTalso has a substrate scope that more closely resembles that of GxtA [37]. A similar phenomenon has also been observed in another Rieske oxygenase 3-ketosteroid 9α-hydroxylase (KshA): changing the loop sequence to mirror that of its homologs results in an altered substrate profile [60].
The cooperative impact of three architectural regions: completing the puzzle
Whereas engineering of the active site, substrate entrance tunnel, and flexible loop have been individually shown to play essential roles in tuning the activity of a Rieske oxygenase, it was recently demonstrated that mutation of six residues distributed among these regions in SxtT results in creation of a SxtT variant that wholly displays the site-selectivity and substrate specificities of GxtA (Figure 4a) [37]. Active site and loop residues (Arg204, Thr276, and Met255) interact with the substrate of SxtT and hold it in place for hydroxylation. Changing the identity of these residues to match those found in GxtA allows for some amount of movement in the active site, formation of new interactions, and some level of non-native selectivity. On the other hand, tunnel residues are credited with geometrically positioning the substrate and delivering it to the active site in the correct orientation for catalysis. A SxtT variant that contains the GxtA tunnel residues is heavily biased to hydroxylate at the non-native position of its substrate [37]. Thus, the collective use of these three regions to hold, position, and direct the substrate toward the active site for site-selective catalysis means that they must be cooperatively targeted to observe the altered activity. More broadly, this work showed that in many of the structurally characterized Rieske oxygenases, a flexible β13-to-β14 connecting loop and analogous tunnel that traverses a similar secondary structure also exists (Figure 4b) [37]. As active site-centered site-saturation mutagenesis experiments have successfully influenced reaction outcome in different systems [61,62], it is possible that making random or rational mutations in the active site, flexible connector loop, and tunnel may be a broadly applicable strategy in Rieske oxygenase engineering.
Figure 4.
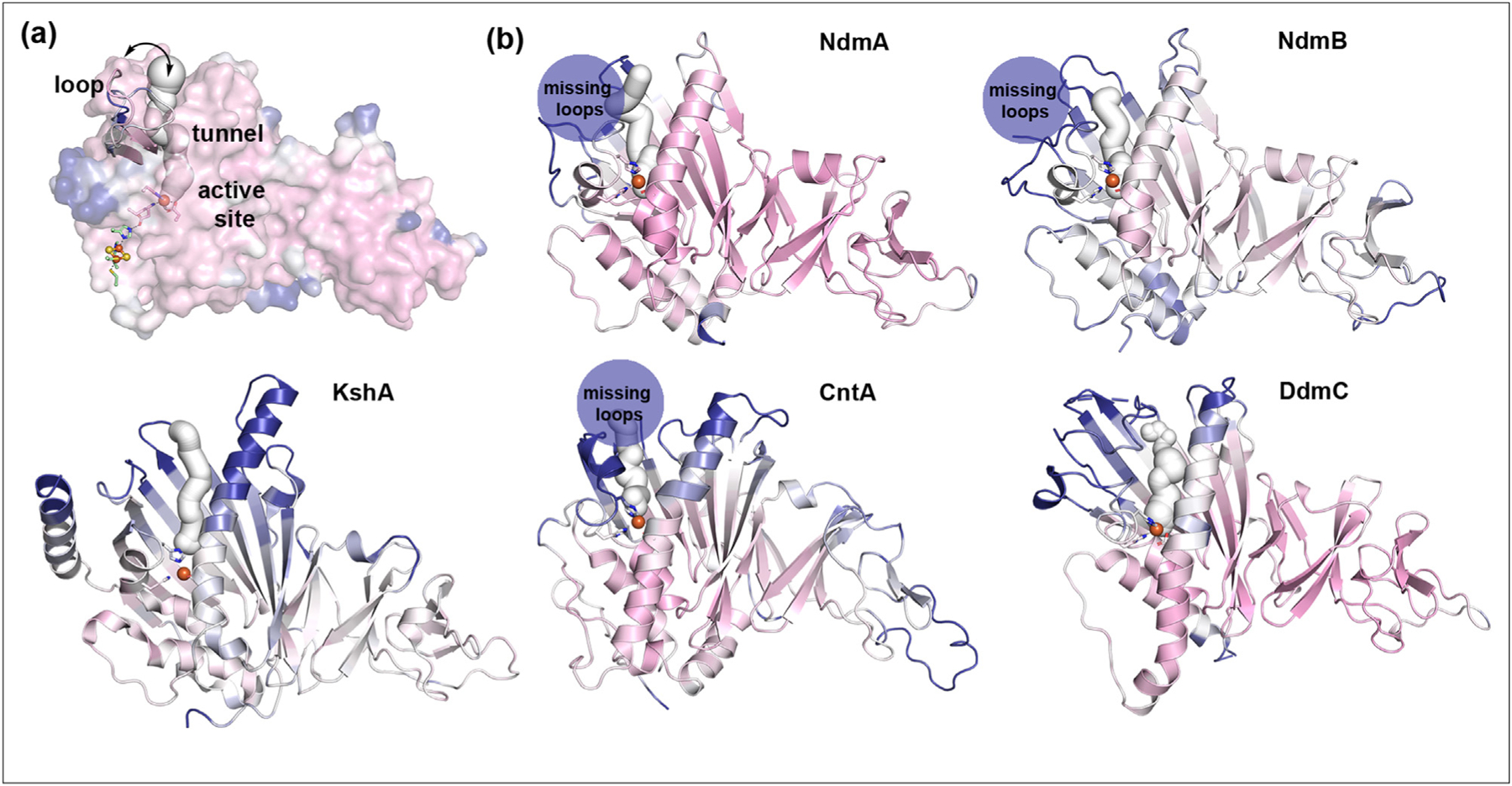
Three architectural regions important to the site-selectivity of the catalyzed reactions of SxtT and GxtA are also found in other Rieske oxygenases. (a) In SxtT and GxtA, residues found in the active site, on the flexible loop that connects β13-to-β14, and lining the tunnel contribute to the site-selectivity of the catalyzed reactions [37,38]. The different loop orientations of SxtA and GxtA are both overlaid with the surface and can also be seen in Figure 3g. (b) In other structurally characterized α3 Rieske oxygenases, a flexible loop, as shown by a plot of the B factors (plotted on a scale of 20–60 Å2 and colored from pink-white-blue), can also be observed in the same region as SxtT and GxtA. In these proteins, tunnels can also be computationally identified [37]. The Rieske oxygenases shown here, in order, are NdmA and NdmB (PDB: 6ICK and 6ICL) [31], KshA (PDB: 4QCK) [34], CntA (PDB: 6Y9D) [39], and DdmC (PDB: 3GKE) [40]. In several cases, the mobility of the loop in the structure resulted in it not being modeled and is indicated by a dark blue circle that says “missing loop”.
Final thoughts
Our understanding of how Rieske oxygenases participate in biochemical pathways and achieve their impressive chemical feats is ever-expanding. Here, we provide a structurally informed starting point to identify architectural elements, both inside and outside the active site of a Rieske oxygenase, that promote changes in reaction selectivity, broaden substrate scope, and/or amplify a useful promiscuous activity. Importantly, through merging structural and biochemical experiments, the studies described here not only pinpoint the so-called “hotspots” for Rieske oxygenase engineering, but also, in many cases, explain why these different architectural regions impact catalytic outcome. Through these experiments, several trends regarding the importance of thus far underappreciated protein regions of interest have emerged. The additional α and β subunits, as described for thermophilic proteins, likely provide an increased number of weak interactions to stabilize protein structure or create a better interaction with the needed electron donor to facilitate catalysis [13,32,44,49]. The entrance tunnels of Rieske oxygenases interact with substrate, geometrically position substrate for catalysis, and are designed to gate access of non-substrate molecules into the active site [4,15,37]. The flexible loops interact with substrate, gate entry to the tunnel, or direct substrate toward the active site also have profound effects on catalysis [14,31,37,57,59]. Thus, from these studies, it appears that the reactivity and selectivity of a Rieske oxygenase, like a puzzle, is assembled through careful selection of a quaternary architecture, and through tinkering with the chemical composition of the active site, substrate entrance tunnel, and flexible protein loops. Consequently, the importance of Rieske oxygenases in diverse industrial, pharmaceutical, and chemical applications necessitates further efforts to capitalize on our growing molecular understanding of these remarkable catalysts.
Acknowledgements
The work in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35 GM138271 (J.B.R.). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Jennifer Bridwell-Rabb is a co-editor for the Biocatalysis and Biotransformation issue.
Data availability
No data was used for the research described in the article.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
* * of outstanding interest
- 1.Bornscheuer UT, Buchholz K: Highlights in biocatalysis - historical landmarks and current trends. Eng Life Sci 2005, 5:309–323. [Google Scholar]
- 2.Sheldon RA, Pereira PC: Biocatalysis engineering: the big picture. Chem Soc Rev 2017, 46:2678–2691. [DOI] [PubMed] [Google Scholar]
- 3.Bornscheuer UT, Hauer B, Jaeger KE, Schwaneberg U: Directed evolution empowered redesign of natural proteins for the sustainable production of chemicals and pharmaceuticals. Angew Chem Int Ed Engl 2019, 58:36–40. [DOI] [PubMed] [Google Scholar]
- 4.*.Kokkonen P, Bednar D, Pinto G, Prokop Z, Damborsky J: Engineering enzyme access tunnels. Biotechnol Adv 2019, 37, 107386. [DOI] [PubMed] [Google Scholar]; This article spotlights the significance of regions that are distant to the active site on catalysis. It highlights the keyhole-lock-key model for enzyme catalysis, which emphasizes the impact of the substrate entrance channel, or the keyhole, on enzymatic reactions. Along with this paradigm-shifting view of enzyme chemistry, this article details the computational tools that can be used to identify enzyme tunnels and analyze ligand passage through these tunnels.
- 5.Kress N, Halder JM, Rapp LR, Hauer B: Unlocked potential of dynamic elements in protein structures: channels and loops. Curr Opin Chem Biol 2018, 47:109–116. [DOI] [PubMed] [Google Scholar]
- 6.Campbell E, Kaltenbach M, Correy GJ, Carr PD, Porebski BT, Livingstone EK, Afriat-Jurnou L, Buckle AM, Weik M, Hollfelder F, et al. : The role of protein dynamics in the evolution of new enzyme function. Nat Chem Biol 2016, 12:944–950. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Jiang Y, Guengerich FP, Ma L, Li S, Zhang W: Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J Biol Chem 2020, 295:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang RK, Huang X, Arnold FH: Selective CH bond functionalization with engineered heme proteins: new tools to generate complexity. Curr Opin Chem Biol 2019, 49:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y, Yeung N, Sieracki N, Marshall NM: Design of functional metalloproteins. Nature 2009, 460:855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingsley LJ, Lill MA: Substrate tunnels in enzymes: structure-function relationships and computational methodology. Proteins 2015, 83:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nestl BM, Hauer B: Engineering of flexible loops in enzymes. ACS Catal 2014, 4:3201–3211. [Google Scholar]
- 12.**.Banerjee R, Lipscomb JD: Small-molecule tunnels in metalloenzymes viewed as extensions of the active site. Acc Chem Res 2021, 54:2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review article summarizes the characteristics of tunnels in metalloenzymes that transport dissolved gases and methane. An intriguing implication of this article is that since tunnels allow for exquisite substrate selectivity and positioning, which can not be provided from active site residues alone, tunnels should be considered as extensions of the active site.
- 13.Littlechild JA, Guy J, Connelly S, Mallett L, Waddell S, Rye CA, Line K, Isupov M: Natural methods of protein stabilization: thermostable biocatalysts. Biochem Soc Trans 2007, 35: 1558–1563. [DOI] [PubMed] [Google Scholar]
- 14.Papaleo E, Saladino G, Lambrughi M, Lindorff-Larsen K, Gervasio FL, Nussinov R: The role of protein loops and linkers in conformational dynamics and allostery. Chem Rev 2016, 116:6391–6423. [DOI] [PubMed] [Google Scholar]
- 15.**.Escalante DE, Aukema KG, Wackett LP, Aksan A: Simulation of the bottleneck controlling access into a rieske active site: predicting substrates of naphthalene 1,2-dioxygenase. J Chem Inf Model 2017, 57:550–561. [DOI] [PubMed] [Google Scholar]; To complement the existing experimental studies on naphthalene dioxygenase (NDO), the authors computationally investigate entry of substrates into the active site. Based on the simulated entrance tunnel and free energies, an algorithm was created to predict whether a substrate would be oxidized by NDO. This study is the first to accurately identify substrate molecules that can be oxidized by a Rieske oxygenase, suggesting its utility for protein engineering.
- 16.Kovaleva EG, Lipscomb JD: Versatility of biological non-heme Fe(II) centers in oxygen activation reactions. Nat Chem Biol 2008, 4:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry SM, Challis GL: Mechanism and catalytic diversity of Rieske non-heme iron-dependent oxygenases. ACS Catal 2013, 3:2362–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knapp M, Mendoza J, Bridwell-Rabb J: An aerobic route for C-H bond functionalization: the rieske non-heme iron oxygenases. In Encyclopedia of biological chemistry III. Edited by Jez J 3rd ed., Elsevier; 2021:413–424. [Google Scholar]
- 19.Ferraro DJ, Gakhar L, Ramaswamy S: Rieske business: structure-function of Rieske non-heme oxygenases. Biochem Biophys Res Commun 2005, 338:175–190. [DOI] [PubMed] [Google Scholar]
- 20.Perry C, de Los Santos ELC, Alkhalaf LM, Challis GL: Rieske non-heme iron-dependent oxygenases catalyse diverse reactions in natural product biosynthesis. Nat Prod Rep 2018, 35:622–632. [DOI] [PubMed] [Google Scholar]
- 21.Friemann R, Ivkovic-Jensen MM, Lessner DJ, Yu CL, Gibson DT, Parales RE, Eklund H, Ramaswamy S: Structural insight into the dioxygenation of nitroarene compounds: the crystal structure of nitrobenzene dioxygenase. J Mol Biol 2005, 348: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 22.Kumari A, Singh D, Ramaswamy S, Ramanathan G: Structural and functional studies of ferredoxin and oxygenase components of 3-nitrotoluene dioxygenase from Diaphorobacter sp. strain DS2. PLoS One 2017, 12, e0176398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friemann R, Lee K, Brown EN, Gibson DT, Eklund H, Ramaswamy S: Structures of the multicomponent Rieske non-heme iron toluene 2,3-dioxygenase enzyme system. Acta Crystallogr D Biol Crystallogr 2009, 65:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong X, Fushinobu S, Fukuda E, Terada T, Nakamura S, Shimizu K, Nojiri H, Omori T, Shoun H, Wakagi T: Crystal structure of the terminal oxygenase component of cumene dioxygenase from Pseudomonas fluorescens IP01. J Bacteriol 2005, 187:2483–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furusawa Y, Nagarajan V, Tanokura M, Masai E, Fukuda M, Senda T: Crystal structure of the terminal oxygenase component of biphenyl dioxygenase derived from Rhodococcus sp. strain RHA1. J Mol Biol 2004, 342: 1041–1052. [DOI] [PubMed] [Google Scholar]
- 26.Jakoncic J, Jouanneau Y, Meyer C, Stojanoff V: The catalytic pocket of the ring-hydroxylating dioxygenase from Sphingomonas CHY-1. Biochem Biophys Res Commun 2007, 352: 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou YJ, Guo Y, Li DF, Zhou NY: Structural and biochemical analysis reveals a distinct catalytic site of salicylate 5-monooxygenase NagGH from rieske dioxygenases. Appl Environ Microbiol 2021:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahto JK, Neetu N, Sharma M, Dubey M, Vellanki BP, Kumar P: Structural insights into dihydroxylation of terephthalate, a product of polyethylene terephthalate degradation. J Bacteriol 2022, 204, e0054321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.*.Kincannon WM, Zahn M, Clare R, Lusty Beech J, Romberg A, Larson J, Bothner B, Beckham GT, McGeehan JE, DuBois JL: Biochemical and structural characterization of an aromatic ring-hydroxylating dioxygenase for terephthalic acid catabolism. Proc Natl Acad Sci U S A 2022, 119, e2121426119. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reveals the structure of terephthalate dioxygenase which is involved in microbial degradation of poly(ethylene terephthalate), a synthetic and widely used industrial molecule. Through biochemical characterization of this enzyme, the authors reveal key protein and substrate features that are important for catalysis. More broadly, the authors highlight an important protein residue that appears widely conserved in other Rieske oxygenases that also bind a carboxylate-containing substrate.
- 30.Kauppi B, Lee K, Carredano E, Parales RE, Gibson DT, Eklund H, Ramaswamy S: Structure of an aromatic-ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure 1998, 6: 571–586. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Kim BH, Brooks S, Kang SY, Summers RM, Song HK: Structural and mechanistic insights into caffeine degradation by the bacterial N-demethylase complex. J Mol Biol 2019, 431: 3647–3661. [DOI] [PubMed] [Google Scholar]
- 32.Mahto JK, Neetu N, Waghmode B, Kuatsjah E, Sharma M, Sircar D, Sharma AK, Tomar S, Eltis LD, Kumar P: Molecular insights into substrate recognition and catalysis by phthalate dioxygenase from Comamonas testosteroni. J Biol Chem 2021, 297, 101416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daughtry KD, Xiao Y, Stoner-Ma D, Cho E, Orville AM, Liu P, Allen KN: Quaternary ammonium oxidative demethylation: X-ray crystallographic, resonance Raman, and UV-visible spectroscopic analysis of a Rieske-type demethylase. J Am Chem Soc 2012, 134:2823–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capyk JK, D’Angelo I, Strynadka NC, Eltis LD: Characterization of 3-ketosteroid 9a-hydroxylase, a Rieske oxygenase in the cholesterol degradation pathway of Mycobacterium tuberculosis. J Biol Chem 2009, 284:9937–9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martins BM, Svetlitchnaia T, Dobbek H: 2-Oxoquinoline 8-monooxygenase oxygenase component: active site modulation by Rieske-[2Fe-2S] center oxidation/reduction. Structure 2005, 13:817–824. [DOI] [PubMed] [Google Scholar]
- 36.Inoue K, Usami Y, Ashikawa Y, Noguchi H, Umeda T, Yamagami-Ashikawa A, Horisaki T, Uchimura H, Terada T, Nakamura S, et al. : Structural basis of the divergent oxygenation reactions catalyzed by the rieske nonheme iron oxygenase carbazole 1,9a-dioxygenase. Appl Environ Microbiol 2014, 80:2821–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.**.Liu J, Tian J, Perry C, Lukowski AL, Doukov TI, Narayan ARH, Bridwell-Rabb J: Design principles for site-selective hydroxylation by a Rieske oxygenase. Nat Commun 2022, 13: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]; By engineering and juxtaposing key residues in the Rieske oxygenases SxtT and GxtA, in this work, the authors spotlight the importance of six residues—two from the active site, three from the substrate entrance tunnel, and one from a flexible loop—to the native site-selectivity and substrate scope of these enzymes. Importantly, the identified tunnel and loop, appear to be common architectural features found in other structurally characterized Rieske oxygenases, providing a guide for engineering these enzymes.
- 38.Lukowski AL, Liu J, Bridwell-Rabb J, Narayan ARH: Structural basis for divergent C-H hydroxylation selectivity in two Rieske oxygenases. Nat Commun 2020, 11:2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.*.Quareshy M, Shanmugam M, Townsend E, Jameson E, Bugg TDH, Cameron AD, Chen Y: Structural basis of carnitine monooxygenase CntA substrate specificity, inhibition, and intersubunit electron transfer. J Biol Chem 2021, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this work, the authors structurally characterize CntA, an α3 Rieske oxygenase that is involved in the conversion of carnitine to trimethylamine in microbes. Importantly, as oxidized trimethylamine is linked to heart disease, characterization of enzymes involved in its biosynthesis is key to understanding its formation in the gut microbiome. Through identification of a Tyr residue that is essential for substrate binding, the authors uncover a pivotal interaction for binding an inhibitor.
- 40.Dumitru R, Jiang WZ, Weeks DP, Wilson MA: Crystal structure of dicamba monooxygenase: a Rieske nonheme oxygenase that catalyzes oxidative demethylation. J Mol Biol 2009, 392: 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Knapp M, Jo M, Dill Z, Bridwell-Rabb J: Rieske oxygenase catalyzed C–H bond functionalization reactions in chlorophyll b biosynthesis. ACS Cent Sci 2022, 8:1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunugi M, Takabayashi A, Tanaka A: Evolutionary changes in chlorophyllide a oxygenase (CAO) structure contribute to the acquisition of a new light-harvesting complex in micro-monas. J Biol Chem 2013, 288:19330–19341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashikawa Y, Fujimoto Z, Noguchi H, Habe H, Omori T, Yamane H, Nojiri H: Electron transfer complex formation between oxygenase and ferredoxin components in Rieske nonheme iron oxygenase system. Structure 2006, 14: 1779–1789. [DOI] [PubMed] [Google Scholar]
- 44.Nojiri H, Ashikawa Y, Noguchi H, Nam JW, Urata M, Fujimoto Z, Uchimura H, Terada T, Nakamura S, Shimizu K, et al. : Structure of the terminal oxygenase component of angular dioxygenase, carbazole 1,9a-dioxygenase. J Mol Biol 2005, 351: 355–370. [DOI] [PubMed] [Google Scholar]
- 45.Jiang H, Parales RE, Gibson DT: The alpha subunit of toluene dioxygenase from Pseudomonas putida F1 can accept electrons from reduced FerredoxinTOL but is catalytically inactive in the absence of the beta subunit. Appl Environ Microbiol 1999, 65:315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirose J, Suyama A, Hayashida S, Furukawa K: Construction of hybrid biphenyl (bph) and toluene (tod) genes for functional analysis of aromatic ring dioxygenases. Gene 1994, 138: 27–33. [DOI] [PubMed] [Google Scholar]
- 47.Hurtubise Y, Barriault D, Sylvestre M: Involvement of the terminal oxygenase beta subunit in the biphenyl dioxygenase reactivity pattern toward chlorobiphenyls. J Bacteriol 1998, 180:5828–5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chebrou H, Hurtubise Y, Barriault D, Sylvestre M: Heterologous expression and characterization of the purified oxygenase component of Rhodococcus globerulus P6 biphenyl dioxygenase and of chimeras derived from it. J Bacteriol 1999, 181: 4805–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.**.Tsai PC, Chakraborty J, Suzuki-Minakuchi C, Terada T, Kotake T, Matsuzawa J, Okada K, Nojiri H: The alpha- and beta-subunit boundary at the stem of the mushroom-like alpha3beta3-type oxygenase component of rieske non-heme iron oxygenases is the rieske-type ferredoxin-binding site. Appl Environ Micro-biol 2022, 88, e0083522. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work used computational and experimental methods to identify positively charged residues distributed between the subunits of the α 3β3 Rieske oxygenase cumene dioxygenase that are critical for interaction with the Rieske-type electron-donating ferredoxin protein. Most importantly, these residues were shown to be conserved among other α3β3 Rieske oxygenases that use a similar reductase system and uncovered a previously unknown binding site.
- 50.Lukowski AL, Ellinwood DC, Hinze ME, DeLuca RJ, Du Bois J, Hall S, Narayan ARH: C-H hydroxylation in paralytic shellfish toxin biosynthesis. J Am Chem Soc 2018, 140:11863–11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashikawa Y, Uchimura H, Fujimoto Z, Inoue K, Noguchi H, Yamane H, Nojiri H: Crystallization and preliminary X-ray diffraction studies of the ferredoxin reductase component in the Rieske nonhaem iron oxygenase system carbazole 1,9a-dioxygenase. Acta Crystallogr, Sect F: Struct Biol Cryst Commun 2007, 63:499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parales RE, Lee K, Resnick SM, Jiang H, Lessner DJ, Gibson DT: Substrate specificity of naphthalene dioxygenase: effect of specific amino acids at the active site of the enzyme. J Bacteriol 2000, 182:1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu CL, Parales RE, Gibson DT: Multiple mutations at the active site of naphthalene dioxygenase affect regioselectivity and enantioselectivity. J Ind Microbiol Biotechnol 2001, 27: 94–103. [DOI] [PubMed] [Google Scholar]
- 54.Halder JM, Nestl BM, Hauer B: Semirational engineering of the naphthalene dioxygenase from Pseudomonas sp. NCIB 9816–4 towards selective asymmetric dihydroxylation. Chem-CatChem 2018, 10:178–182. [Google Scholar]
- 55.Wissner JL, Schelle JT, Escobedo-Hinojosa W, Vogel A, Hauer B: Semi-rational engineering of toluene dioxygenase from Pseudomonas putida F1 towards oxyfunctionalization of bicyclic aromatics. Adv Synth Catal 2021, 363:4905–4914. [Google Scholar]
- 56.Wissner JL, Escobedo-Hinojosa W, Vogel A, Hauer B: An engineered toluene dioxygenase for a single step biocatalytical production of (−)-(1S,2R)-cis-1,2-dihydro-1,2-naphthalenediol. J Biotechnol 2021, 326:37–39. [DOI] [PubMed] [Google Scholar]
- 57.Ferraro DJ, Brown EN, Yu CL, Parales RE, Gibson DT, Ramaswamy S: Structural investigations of the ferredoxin and terminal oxygenase components of the biphenyl 2,3-dioxygenase from Sphingobium yanoikuyae B1. BMC Struct Biol 2007, 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu S, Acevedo JP, Reetz MT: Induced allostery in the directed evolution of an enantioselective Baeyer-Villiger monooxygenase. Proc Natl Acad Sci U S A 2010, 107:2775–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.**.Heinemann PM, Armbruster D, Hauer B: Active-site loop variations adjust activity and selectivity of the cumene dioxygenase. Nat Commun 2021, 12:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here, the authors focus on two active site loop regions in cumene dioxygenase. Through making loop modifications—mutations, insertions, and deletions—the authors demonstrate increased product yields and shifted reaction selectivity with non-native substrates. The generation of thorough libraries of CDO with loop variants based on different techniques represents an exemplary model for future loop engineering.
- 60.Petrusma M, Dijkhuizen L, van der Geize R: Structural features in the KshA terminal oxygenase protein that determine substrate preference of 3-ketosteroid 9alpha-hydroxylase enzymes. J Bacteriol 2012, 194:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ang EL, Obbard JP, Zhao H: Probing the molecular determinants of aniline dioxygenase substrate specificity by saturation mutagenesis. FEBS J 2007, 274:928–939. [DOI] [PubMed] [Google Scholar]
- 62.Lee JK, Ang EL, Zhao H: Probing the substrate specificity of aminopyrrolnitrin oxygenase (PrnD) by mutational analysis. J Bacteriol 2006, 188:6179–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berka K, Hanak O, Sehnal D, Banas P, Navratilova V, Jaiswal D, Ionescu CM, Svobodova Varekova R, Koca J, Otyepka M: MOLEonline 2.0: interactive web-based analysis of biomacromolecular channels. Nucleic Acids Res 2012, 40: W222–W227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karlsson A, Parales JV, Parales RE, Gibson DT, Eklund H, Ramaswamy S: Crystal structure of naphthalene dioxygenase: side-on binding of dioxygen to iron. Science 2003, 299: 1039–1042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


