Abstract
Objectives
To investigate temporal trends in incidence and severity of COVID-19 among patients with systemic autoimmune rheumatic diseases (SARDs) from the first wave through the initial Omicron wave.
Methods
We conducted a retrospective cohort study investigating COVID-19 outcomes among patients with SARD systematically identified to have confirmed COVID-19 from 1 March 2020 to 31 January 2022 at Mass General Brigham. We tabulated COVID-19 counts of total and severe cases (hospitalisations or deaths) and compared the proportion with severe COVID-19 by calendar period and by vaccination status. We used logistic regression to estimate the ORs for severe COVID-19 for each period compared with the early COVID-19 period (reference group).
Results
We identified 1449 patients with SARD with COVID-19 (mean age 58.4 years, 75.2% female, 33.9% rheumatoid arthritis). There were 399 (28%) cases of severe COVID-19. The proportion of severe COVID-19 outcomes declined over calendar time (p for trend <0.001); 46% of cases were severe in the early COVID-19 period (1 March 2020–30 June 2020) vs 15% in the initial Omicron wave (17 December 2021–31 January 2022; adjusted OR 0.29, 95% CI 0.19 to 0.43). A higher proportion of those unvaccinated were severe compared with not severe cases (78% vs 60%).
Conclusions
The proportion of patients with SARD with severe COVID-19 has diminished since early in the pandemic, particularly during the most recent time periods, including the initial Omicron wave. Advances in prevention, diagnosis and treatment of COVID-19 may have improved outcomes among patients with SARD.
INTRODUCTION
In the 2 years since COVID-19 was recognised as a global pandemic, significant strides have been made in the testing, prevention and treatment of COVID-19. The initial wave of infections caused by the ‘Omicron” variant has been reported to cause less severe outcomes in the general population compared with prior waves.1–4 Multiple factors including vaccinations, prior infection, increased testing and effective treatments as well as intrinsic features of the variant likely contribute to these observations. However, whether such improved outcomes have also been observed in people with systemic autoimmune rheumatic diseases (SARDs) remain unclear. Some people with SARDs have been found to have an increased risk of severe outcomes from COVID-19, such as hospitalisation and death. This has been attributed to altered underlying immunity, immunosuppression contributing to blunted responses to both natural infection and vaccination, and end-organ damage from the SARD.5 6 Advances in vaccination, testing, and outpatient and inpatient treatments during the initial Omicron wave in the USA may have also contributed to improved temporal COVID-19 outcomes among people with SARDs.
Previous studies have investigated temporal trends in COVID-19 outcomes among patients with SARD prior to the initial Omicron wave. A Swedish study compared patients with inflammatory joint diseases to matched comparators and found worse outcomes, particularly early in the pandemic.7 A small cohort study in Ireland found no improvement in hospitalisation or mortality rates in the first three waves of the pandemic.8 Two other studies performed about 6 months into the pandemic showed that excess risk of severe COVID-19 among patients with SARD was similar to the general population.9 10 It remains unclear whether outcomes have improved in recent time periods for patients with SARDs.
We aimed to investigate temporal trends in incidence and severity of COVID-19 among patients with SARD. We hypothesised that the proportion of patients with SARD experiencing severe COVID-19 has improved since early in the pandemic due to several factors including vaccinations, testing availability, as well as outpatient and inpatient treatments.
METHODS
Study design and population
We performed a retrospective cohort study investigating temporal trends of COVID-19 outcomes among patients with SARD throughout the pandemic (from 1 March 2020 to 31 January 2022) at the Mass General Brigham (MGB) HealthCare system in the greater Boston, Massachusetts area. MGB is composed of 14 hospitals including Massachusetts General Hospital and Brigham and Women’s Hospital and affiliated community health centres.
Identification of COVID-19 cases and patients with SARD
As previously described in more detail we systematically identified all patients with SARD with PCR-confirmed SARS-CoV-2 infection using electronic query.6 10–14 Our EHR also flags patients who had positive testing either at home (eg, patients who notified their rheumatologist about a positive rapid antigen test through the secure patient portal) or outside of the MGB system (eg, admitted patient with COVID-19 transferred from an outside hospital). These lists were filtered by the presence of at least one International Classification of Diseases 9th revision (ICD-9) or ICD-10 code for SARD as a sensitive screen. This was further supplemented by direct referrals to our study team from rheumatologists who learnt of patients’ positive tests during a clinical encounter. Among these lists, the presence of prevalent SARD and SARS-CoV-2 infection was confirmed by medical record review. As in our previous studies, we excluded participants being treated for osteoarthritis, fibromyalgia, mechanical back pain, Raynaud’s phenomenon, gout or pseudogout alone since these conditions are not typically treated with systemic immunomodulators and are often managed by non-rheumatologists.6 10–14 Thus, all patients included had a confirmed SARD diagnosis with verified SARS-CoV-2 infection between 1 March 2020 and 31 January 2022 (See online supplemental figure 1 for flow diagram of analyzed sample).
Exposure variable: time periods throughout the pandemic
The date of COVID-19 onset was determined by the first date of SARS-CoV-2 test positivity for those with PCR tests in the MGB system, date of first COVID-19 flag or date of positive test from medical record review or referral. We a priori divided calendar time into periods based on changes in viral epidemiology and care advances. The ‘Early COVID-19 period’ was 1 March 2020 to 30 June 2020, corresponding to when Massachusetts experienced the first wave of COVID-19. The ‘Early treatment period’ was 1 July 2020 to 31 January 2021, corresponding to seminal advances in treatment of hospitalised patients with dexamethasone and remdesivir as well as a wave of cases in the fall/winter.15 16 The ‘Early vaccination’ period was 1 February 2021 to 30 June 2021, corresponding to the initial roll-out of COVID-19 vaccines to high-risk populations starting on 1 February 2021 in Massachusetts, USA.17–19 The ‘Additional vaccination and Delta wave’ period was 1 July 2022 to 16 December 2022, corresponding to recommendations for additional doses of vaccines to immunocompromised patients as well as a fall surge of cases due to the Delta variant.20 The ‘Omicron wave’ period was 17 December 2021 to 31 January 2022, corresponding to the initial large local surge of cases due to the Omicron variant.
Outcome variables: severe COVID-19
As in previous studies, we defined severe COVID-19 as a composite of hospitalisation or death within 30 days from COVID-19 date.5 Medical record review was performed to determine these outcomes and to identify patients who received mechanical ventilation. This was supplemented by electronic query and information from the referring rheumatologist for those who were hospitalised outside the MGB system. We also examined the individual outcomes of hospitalisation, mechanical ventilation and death from COVID-19.
Other characteristics
We collected information on demographics, comorbidities, vaccination status, and rheumatic disease characteristics and medications. Age, sex, race (white, black, Asian/Hawaiian/Pacific Islander or other/unknown) and Hispanic ethnicity were identified from electronic query. Comorbidities were collected from medical record review and included hypertension, diabetes mellitus, obesity, cardiovascular disease, obstructive lung disease (including asthma, chronic obstructive pulmonary disease, and obstructive sleep apnoea), and interstitial lung disease. We classified vaccination status as follows: (1) unvaccinated or prevaccine, (2) partially vaccinated (occurring between day 0 and day 13 of 2-dose mRNA vaccines or between day 0 and day 13 of the one-dose Johnson & Johnson-Janssen (J&J) vaccine), (3) two-dose mRNA or one dose J&J (14 days after completion of the initial series) and (4) additional doses (day 14 or later after third vaccine dose for those who initially received two-dose mRNA vaccines or day 14 or later after second vaccine dose for those who initially received one-dose J&J vaccine per US Centers for Disease Control and Prevention (CDC) recommendations for an additional dose)21 (See online supplemental figure 2 for diagram illustrating vaccine status definitions).
SARD characteristics were obtained from medical record review. These included specific type of SARD, glucocorticoid use/dose (none, low dose (1–10 mg/day of prednisone-equivalent), moderate/high dose (>10 mg) and unknown dose), disease-modifying antirheumatic drug (DMARD) use (categorised by specific drug for conventional synthetic DMARDs or mechanism of action for biologic DMARDs) at time of COVID-19 diagnosis, and preceding SARD activity per review of notes from the medical record (categorised as remission/low, moderate/severe or unknown).
Treatment of COVID-19 was also collected by medical record review and supplemented by electronic query: neutralising monoclonal antibodies (typically given as outpatient for COVID-19 treatment rather than inpatient or as postexposure prophylaxis during our study period), remdesivir (only used as inpatient during our study period), convalescent plasma, dexamethasone (or other high-dose glucocorticoids used inpatient to treat COVID-19), tocilizumab (to treat inpatient COVID-19 rather than underlying SARD), baricitinib (used inpatient to treat COVID-19 rather than underlying SARD).22 No patients with SARD were documented to have received nirmatrelvir/ritonavir (Paxlovid) or molnupiravir for COVID-19 treatment during the study period.23
Statistical analysis
Calendar time was the exposure of interest for our study. We graphed weekly counts of total COVID-19 cases and severe outcomes among patients with SARD throughout the entire study period. We reported frequencies and proportions of demographics, comorbidities, vaccination status, type of COVID-19 test and SARD characteristics overall and stratified by the five calendar periods. Since severe COVID-19 during the initial Omicron wave period has been reported to be relatively uncommon in the general population,1 we also reported on characteristics of patients with SARD with severe versus not severe COVID-19 during the period. We also reported a case series of the patients with SARD with COVID-19 who died during the Omicron wave period.
We reported the proportion of severe COVID-19 outcomes that occurred during each calendar period and calculated a p for trend across the five calendar periods. We also stratified cases by severe versus not severe and calculated the proportion in each category by vaccination status. We performed logistic regression to estimate the ORs and 95% CIs) for severe COVID-19 for each period compared with the early pandemic period as the reference. The base model was unadjusted. The multivariable model adjusted for age, sex and race.
We performed several sensitivity analyses to assess the robustness of our findings with different definitions of time periods and alternative reference groups. Some of the COVID-19 advances were iterative, rather than discrete (eg, inpatient treatments for severe COVID-19), and may have had slow uptake (eg, monoclonal antibody and vaccine uptake). First, we divided the original period ‘Early treatment’ into two separate time periods, before and after 9 November 2020. This date was chosen as the date that monoclonal antibodies first received emergency use authorisation from the US Food and Drug Administration and coincided with the beginning of the second wave of COVID-19. Second, we considered the entire ‘prevaccine era’ (1 March 2020 to 31 January 2021) as a single reference group. Third, we changed the reference group to the ‘Early treatment’ time period (1 July 2020 to 31 January 2021) in case the original choice of the reference group influenced results.
Since monoclonal antibodies were the only effective outpatient treatment available during our study period, we performed two analyses to investigate how their availability may have impacted results. First, we removed all patients who received monoclonal antibodies from the primary analysis to investigate whether there was still improved outcomes over calendar time. Second, we investigated the association of monoclonal antibody use versus no use with severe COVID-19 in an exploratory analysis using logistic regression. For that analysis, we limited the sample to those diagnosed with COVID-19 on 9 November 2020 or later since monoclonal antibodies were not available through routine clinical care prior to then.
We considered a two-sided p<0.05 as statistically significant. SAS V.9.4 was used for all analyses.
RESULTS
COVID-19 cases among patients with SARD
From 1 March 2020 to 31 January 2022, we identified 1449 patients with SARD with confirmed COVID-19 (see flow diagram for the analysed sample in online supplemental figure 1). Figure 1 shows the weekly counts of total and severe COVID-19 cases. The tallest peak of cases occurred during the initial Omicron wave period. There were 261, 492, 123, 172 and 401 total cases in the early COVID-19, early treatment, early vaccination, additional vaccination and Delta wave, and initial Omicron wave periods, respectively.
Figure 1.
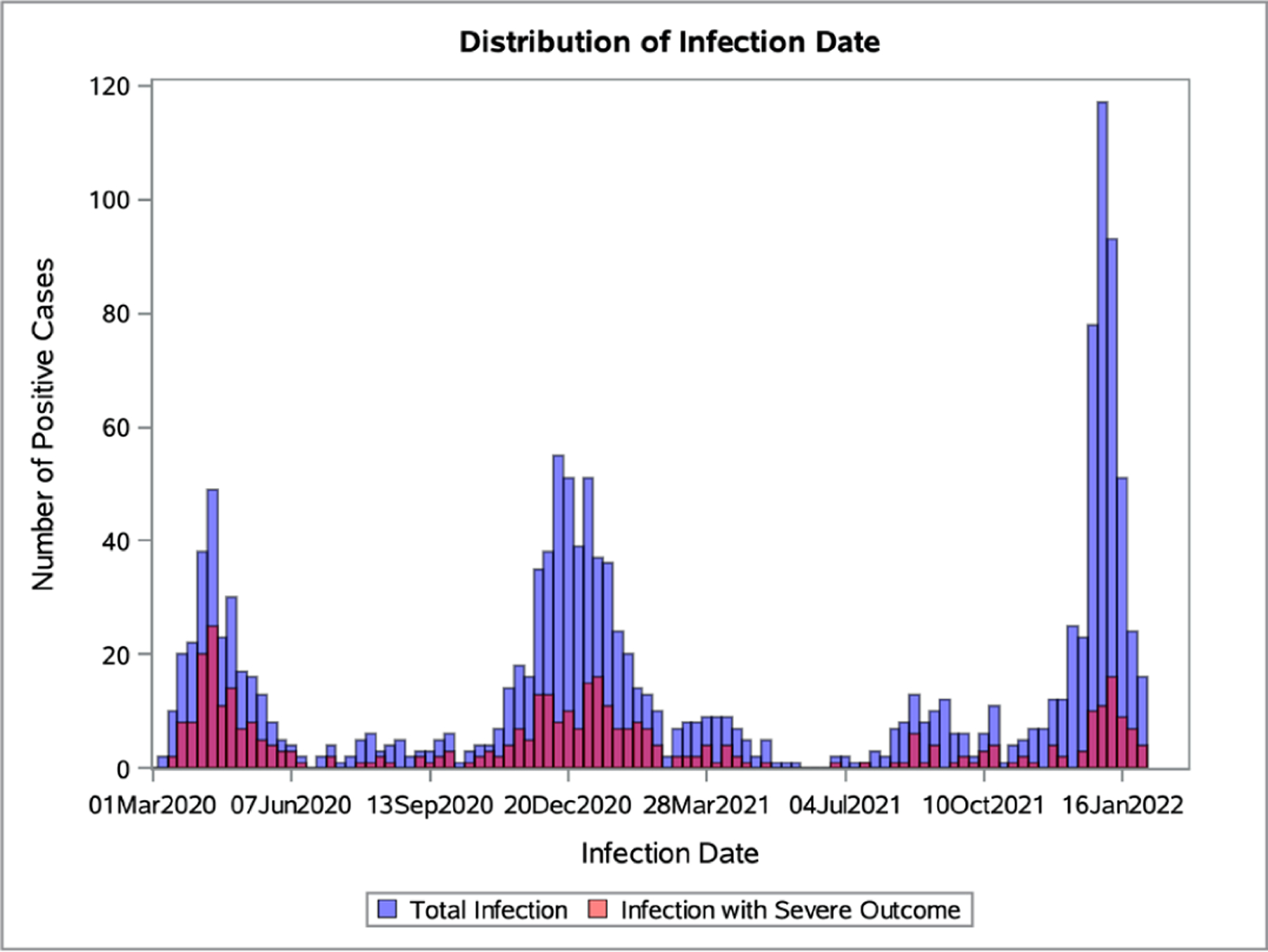
Total and severe COVID-19 case counts over time. Case counts of SARS-CoV-2 infections over calendar time with total infections shown in blue and infections with severe outcomes in red.
Characteristics of patients with SARD with COVID-19
Table 1 shows the demographics, comorbidities, SARS-CoV-2 testing type and COVID-19 vaccination status. Mean age of the entire study sample was 58.4 years (SD 17.5). Cases in the early COVID-19 period tended to be older compared with cases in the initial Omicron wave period (mean age 63.1 years vs 54.2). The overall sample was 71.4% white, 11.2% black and 3.6% Asian/Hawaiian/Pacific Islander. The most common comorbidity was hypertension (43.8%). The vaccination status for the entire sample at the time of infection was 64.7% prevaccination/unvaccinated, 3.5% partially vaccinated, 15.7% two-dose mRNA or one-dose J&J and 16.1% additional doses. Breakthrough infections were particularly common in the initial Omicron wave: 136 cases (33.8% of cases in the period) occurred among those who received 2-dose mRNA or 1-dose J&J and 205 cases (51.1% of cases in this period) occurred among those with additional vaccine doses. Most cases were diagnosed with PCR testing. The initial Omicron wave was the only period where a substantial portion of cases were diagnosed with home rapid antigen testing (122/401 (30.4%) during this period).
Table 1.
Demographics and clinical characteristics of rheumatic disease patients with COVID-19 over calendar time.
| Time period | ||||||
|---|---|---|---|---|---|---|
| Overall (n=1449) | Early COVID-19 | Early treatment | Early vaccination | Additional vaccination and Delta wave | Omicron wave | |
| 1 March 2020–30 June 30 (n=261) | 1 July 2020–31 January 2021 (n=492) | 1 February 2021–30 June 2021 (n=123) | 1 July 2021–16 December 2021 (n=172) | 17 December 2021–31 January 2022 (n=401) | ||
| Age, years (mean±SD) | 58.4 (17.5) | 63.1 (16.6) | 59.3 (17.0) | 55.5 (16.9) | 56.8 (17.7) | 54.2 (17.6) |
| Female sex, n (%) | 1090 (75.2) | 196 (75.1) | 370 (75.2) | 90 (73.2) | 111 (64.5) | 323 (80.5) |
| Race, n (%) | ||||||
| White | 1035 (71.4) | 154 (59.0) | 353 (71.7) | 93 (75.6) | 136 (79.1) | 299 (74.6) |
| Black | 162 (11.2) | 52 (19.9) | 50 (10.2) | 15 (12.2) | 11 (6.4) | 34 (8.5) |
| Asian, Hawaiian or Pacific Islander | 52 (3.6) | 9 (3.4) | 19 (3.9) | 6 (4.9) | 7 (4.1) | 11 (2.7) |
| Other or unknown | 200 (13.8) | 46 (17.6) | 70 (14.2) | 9 (7.3) | 18 (10.5) | 57 (14.2) |
| Hispanic ethnicity, n (%) | 67 (4.6) | 13 (5.0) | 31 (6.3) | 5 (4.1) | 4 (2.3) | 14 (3.5) |
| Comorbidities, n (%) | ||||||
| Hypertension | 635 (43.8) | 154 (59.0) | 220 (44.7) | 49 (39.8) | 63 (36.6) | 149 (37.2) |
| Diabetes mellitus | 234 (16.1) | 61 (23.4) | 92 (18.7) | 20 (16.3) | 20 (11.6) | 41 (10.2) |
| Obesity | 427 (29.5) | 92 (35.2) | 156 (31.7) | 49 (39.8) | 35 (20.3) | 95 (23.7) |
| Cardiovascular disease | 210 (14.5) | 62 (23.8) | 72 (14.6) | 15 (12.2) | 20 (11.6) | 41 (10.2) |
| Obstructive lung disease | 310 (21.4) | 73 (28.0) | 109 (22.2) | 25 (20.3) | 31 (18.0) | 72 (18.0) |
| Interstitial lung disease | 81 (6.0) | 17 (6.5) | 25 (5.1) | 8 (6.5) | 9 (5.2) | 22 (5.5) |
| Vaccination status, n (%) | ||||||
| Unvaccinated or prevaccine | 938 (64.7) | 261 (100) | 492 (100) | 102 (82.9) | 40 (23.3) | 43 (10.7) |
| Partially vaccinated | 50 (3.5) | 0 (0) | 0 (0) | 19 (15.4) | 14 (8.1) | 17 (4.2) |
| Two doses mRNA or one dose J&J | 228 (15.7) | 0 (0) | 0 (0) | 2 (1.6) | 90 (52.3) | 136 (33.9) |
| Additional doses | 233 (16.1) | 0 (0) | 0 (0) | 0 (0) | 28 (16.3) | 205 (51.1) |
| SARS-CoV2 diagnosis method, n (%) | ||||||
| PCR | 1126 (77.7) | 246 (94.3) | 426 (86.6) | 100 (81.3) | 129 (75.0) | 225 (56.1) |
| Antigen/rapid test | 123 (8.5) | 0 (0) | 0 (0) | 0 (0) | 1 (0.6) | 122 (30.4) |
| Other or unknown | 200 (13.8) | 15 (5.7) | 66 (13.4) | 23 (18.7) | 42 (24.4) | 54 (13.5) |
J&J, Johnson and Johnson/Janssen
Table 2 shows the SARD characteristics for the COVID-19 cases, stratified by the five calendar periods. The most common type of SARD was rheumatoid arthritis (33.9%), followed by psoriatic arthritis and spondyloarthritis (14.7%) and systemic lupus erythematosus (13.1%). Most patients (74.1%) were in remission or had low disease activity at time of COVID-19 onset. Baseline glucocorticoids were used in 26.0% of cases. The most commonly used DMARD was methotrexate (22.0%), followed by antimalarials (21.7%) and TNF inhibitors (20.2%). Rituximab was used in 9.2% of cases.
Table 2.
Rheumatic disease characteristics of patients with SARD with COVID-19 over calendar time
| Time period | ||||||
|---|---|---|---|---|---|---|
| Overall (n=1449) | Early COVID-19 | Early treatment | Early vaccination | Additional vaccination and Delta wave | Omicron wave | |
| 1 March 2020–30 June 2020 (n=261) | 1 July 2020–31 January 2021 (n=492) | 1 February 2021–30 June 2021 (n=123) | 1 July 2021–16 December 2021 (n=172) | 17 December 2021–31 January 2022 (n=401) | ||
| Rheumatic disease diagnosis, n () | ||||||
| Rheumatoid arthritis | 491 (33.9) | 90 (34.5) | 174 (35.4) | 48 (39.0) | 45 (26.2) | 134 (33.4) |
| Psoriatic arthritis and spondyloarthritis | 213 (14.7) | 31 (11.9) | 75 (15.2) | 17 (13.8) | 32 (18.6) | 58 (14.5) |
| Systemic lupus erythematosus | 190 (13.1) | 39 (14.9) | 64 (13.0) | 16 (13.0) | 21 (12.2) | 50 (12.5) |
| Other inflammatory arthritis* | 106 (7.3) | 19 (7.3) | 38 (7.7) | 4 (3.3) | 13 (7.6) | 32 (8.0) |
| PMR and/or GCA | 101 (7.0) | 23 (8.8) | 31 (6.3) | 7 (5.7) | 14 (8.1) | 26 (6.5) |
| ANCA-associated vasculitis | 68 (4.7) | 11 (4.2) | 21 (4.3) | 7 (5.7) | 12 (7.0) | 17 (4.2) |
| Other vasculitis† | 33 (2.3) | 8 (3.1) | 7 (1.4) | 4 (3.3) | 2 (1.2) | 12 (3.0) |
| Sjogren’s syndrome | 36 (2.5) | 2 (0.8) | 13 (2.6) | 3 (2.4) | 5 (2.9) | 13 (3.2) |
| Systemic sclerosis | 35 (2.4) | 6 (2.3) | 11 (2.2) | 5 (4.1) | 3 (1.7) | 10 (2.5) |
| Inflammatory myopathy | 33 (2.3) | 6 (2.3) | 13 (2.6) | 1 (0.8) | 6 (3.5) | 7 (1.8) |
| Other connective tissue diseases‡ | 37 (2.6) | 9 (3.4) | 8 (1.6) | 3 (2.4) | 3 (1.7) | 14 (3.5) |
| Sarcoidosis | 43 (3.0) | 9 (3.4) | 22 (4.5) | 1 (0.8) | 4 (2.3) | 7 (1.8) |
| Multiple rheumatic diagnoses | 35 (2.4) | 6 (2.3) | 9 (1.8) | 4 (3.3) | 4 (2.3) | 12 (3.0) |
| Other diagnoses§ | 28 (1.9) | 2 (0.8) | 6 (1.2) | 3 (2.4) | 8 (4.7) | 9 (2.2) |
| Disease activity, n (%) | ||||||
| Remission or low activity | 1074 (74.1) | 181 (69.3) | 365 (74.2) | 91 (74.0) | 131 (76.2) | 306 (76.3) |
| Moderate or severe activity | 259 (17.9) | 50 (19.2) | 100 (20.3) | 23 (18.7) | 27 (15.7) | 59 (14.7) |
| Unknown | 116 (8.0) | 30 (11.5) | 27 (5.5) | 9 (7.3) | 14 (8.1) | 36 (9.0) |
| Rheumatic disease medications at time of infection, n (%) | ||||||
| Glucocorticoids | 377 (26.0) | 80 (30.7) | 125 (25.4) | 29 (23.6) | 42 (24.4) | 101 (25.2) |
| None | 1072 (74.0) | 181 (69.3) | 367 (74.6) | 94 (76.4) | 130 (75.6) | 300 (74.8) |
| Low dose (1–10 mg) | 317 (21.9) | 70 (26.8) | 106 (21.5) | 26 (21.1) | 36 (20.9) | 79 (19.7) |
| Moderate/high dose (>10 mg) | 53 (3.7) | 10 (3.8) | 18 (3.7) | 3 (2.4) | 4 (2.3) | 18 (4.5) |
| Unknown dose | 7 (0.5) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 2 (1.2) | 4 (1.0) |
| Conventional synthetic DMARDs and immunosuppressants | ||||||
| Methotrexate | 319 (22.0) | 49 (18.8) | 108 (22.0) | 21 (17.1) | 38 (22.1) | 103 (25.7) |
| Antimalarials | 314 (21.7) | 58 (22.2) | 102 (20.7) | 20 (16.3) | 36 (20.9) | 98 (24.4) |
| Sulfasalazine | 31 (2.1) | 5 (1.9) | 8 (1.6) | 2 (1.6) | 3 (1.7) | 13 (3.2) |
| Leflunomide | 54 (3.7) | 12 (4.6) | 23 (4.7) | 1 (0.8) | 5 (2.9) | 13 (3.2) |
| Mycophenolate mofetil | 101 (7.0) | 13 (5.0) | 27 (5.5) | 13 (10.6) | 17 (9.9) | 31 (7.7) |
| Azathioprine | 27 (1.9) | 9 (3.4) | 6 (1.2) | 4 (3.3) | 2 (1.2) | 6 (1.5) |
| Calcineurin inhibitor | 19 (1.3) | 4 (1.5) | 5 (1.0) | 5 (4.1) | 3 (1.7) | 2 (0.5) |
| Cyclophosphamide | 4 (0.3) | 3 (1.2) | 0 (0) | 0 (0) | 1 (0.6) | 0 (0) |
| Biologic DMARDs | ||||||
| TNF inhibitors | 292 (20.2) | 32 (12.3) | 90 (18.3) | 26 (21.1) | 40 (23.3) | 104 (25.9) |
| Rituximab | 133 (9.2) | 17 (6.5) | 33 (6.7) | 18 (14.6) | 25 (14.5) | 40 (10.0) |
| Belimumab | 20 (1.4) | 4 (1.5) | 1 (0.2) | 1 (0.8) | 3 (1.7) | 11 (2.7) |
| Abatacept | 31 (2.1) | 4 (1.5) | 6 (1.2) | 3 (2.4) | 3 (1.7) | 15 (3.7) |
| IL-6 inhibitors | 45 (3.1) | 3 (1.2) | 18 (3.7) | 3 (2.4) | 5 (2.9) | 16 (4.0) |
| IL-17, IL-12/23, and IL-23 inhibitors | 36 (2.5) | 8 (3.1) | 16 (3.3) | 2 (1.6) | 3 (1.7) | 7 (1.7) |
| IL-1 inhibitors | 6 (0.4) | 0 (0) | 0 (0) | 0 (0%) | 3 (1.7) | 3 (0.8) |
| Targeted synthetic DMARDs | ||||||
| JAK inhibitors | 55 (3.8) | 8 (3.1) | 21 (4.3) | 4 (3.3) | 7 (4.1) | 15 (3.7) |
| Apremilast | 5 (0.4) | 0 (0) | 2 (0.4) | 0 (0) | 0 (0) | 3 (0.8) |
| IVIG | 19 (1.3) | 2 (0.8) | 7 (1.4) | 2 (1.6) | 4 (2.3) | 4 (1.0) |
Includes juvenile idiopathic arthritis, other unspecified inflammatory arthritis.
Includes Takayasu’s arteritis, Kawasaki disease, Behcet’s disease, polyarteritis nodosa, other vasculitis.
Includes undifferentiated connective tissue disease, mixed connective tissue disease, antiphospholipid syndrome (without concurrent systemic lupus erythematosus).
Includes relapsing polychondritis, IgG4-related disease, sclerosing mediastinitis, periodic fever syndromes, adult-onset Still’s disease.
ANCA, antineutrophil cytoplasmic antibody; DMARD, disease-modifying antirheumatic drug; GCA, giant cell arteritis; IL, interleukin; IVIG, intravenous immune globulin; JAK, Janus kinase; PMR, polymyalgia rheumatica; SARD, systemic autoimmune rheumatic disease; TNF, tumour necrosis factor.
Treatment and severe COVID-19 outcomes
The most common treatments for COVID-19 were remdesivir (14.2%), dexamethasone (13.2%) and neutralising monoclonal antibodies (12.6%). Few patients with SARD received tocilizumab (1.0%), baricitinib (0.2%) or convalescent plasma (0.5%) to treat COVID-19.
There were 399 (27.5%) who had the composite outcome of severe COVID-19 of hospitalisation or death (table 3). There were 391 (27.0%) hospitalisations and 60 (4.1%) deaths. The proportion of patients with SARD experiencing severe COVID-19 decreased over the calendar periods. The total and proportion of severe COVID-19 in each calendar period was 119 (45.6%), 144 (29.3%), 41 (33.3%), 36 (20.9%) and 59 (14.7%) in the early COVID-19, early treatment, early vaccination, additional vaccination and Delta wave, and Omicron wave periods, respectively (figure 2, p for trend <0.001). Compared with the reference of the early COVID-19 period, the multivariable ORs and 95%CIs for severe COVID-19 were 0.58 (0.41 to 0.81) in the early treatment period, 0.89 (0.54 to 1.46) in the early vaccination period, 0.39 (0.24 to 0.62) in the additional vaccination and Delta wave period, and 0.29 (0.19 to 0.43) in the Omicron wave period, adjusted for age, sex and race.
Table 3.
Outcomes and treatments of patients with SARD over time
| Time period | ||||||
|---|---|---|---|---|---|---|
| Overall (n=1449) | Early COVID-19 | Early treatment | Early vaccination | Additional vaccination and Delta wave | Omicron wave | |
| 1 March 2020–30 June 2020 (n=261) | 1 July 2020–31 January 2021 (n=492) | 1 February 2021–30 June 2021 (n=123) | 1 July 2021–16 December 2021 (n=172) | 17 December 2021–31 January 2022 (n=401) | ||
| Hospitalisation, n (%) | 391 (27.0) | 115 (44.1) | 142 (28.9) | 40 (32.5) | 36 (20.9) | 58 (14.5) |
| Mechanical ventilation, n (%) | 57 (3.9) | 29 (11.1) | 12 (2.4) | 6 (4.9) | 3 (1.7) | 7 (1.7) |
| Death, n (%) | 60 (4.1) | 23 (8.8) | 12 (2.4) | 9 (7.3) | 8 (4.7) | 8 (2.0) |
| Severe COVID-19* | 399 (27.5) | 119 (45.6) | 144 (29.3) | 41 (33.3) | 36 (20.9) | 59 (14.7) |
| OR | Reference | 0.49 (0.36, 0.67) | 0.60 (0.38, 0.93) | 0.32 (0.20, 0.49) | 0.21 (0.14,0.30) | |
| Adjusted OR† | Reference | 0.58 (0.41, 0.81) | 0.89 (0.54, 1.46) | 0.39 (0.24, 0.62) | 0.29 (0.19, 0.43) | |
| Treatments Received, n (%) | ||||||
| Monoclonal antibodies‡ | 183 (12.6) | 1 (0.4) | 17 (3.5) | 18 (14.6) | 86 (50.0) | 61 (15.2) |
| Remdesivir | 206 (14.2) | 18 (6.9) | 90 (18.3) | 29 (23.6) | 21 (12.2) | 48 (12.0) |
| Convalescent plasma | 7 (0.5) | 1 (0.4) | 1 (0.2) | 5 (4.1) | 0 (0.0) | 0 (0.0) |
| Dexamethasone | 191 (13.2) | 11 (4.2) | 92 (18.7) | 29 (23.6) | 20 (11.6) | 39 (9.7) |
| Tocilizumab | 15 (1.0) | 11 (4.2) | 2 (0.4) | 2 (0.4) | 0 (0.0) | 0 (0.0) |
| Baricitinib | 3 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 2 (0.5) |
Bolding indicates p<0.05.
Composite outcome of hospitalisation, mechanical ventilation or death.
Adjusted for age, sex and race.
Includes bamlanivimab/etesevimab, casirivimab/imdevimab and sotrovimab. One patient received monoclonal antibodies in the Early treatment period through compassionate use prior to routine clinical availability.
SARD, systemic autoimmune rheumatic disease.
Figure 2.

Proportion of cases with severe and non-severe COVID-19 in each time period. P value is for the trend across categories.
Figure 3 displays the vaccination status stratified by severe or not severe COVID-19. Among the 399 severe cases, 78.4% were unvaccinated. Among the 1050 cases that were not severe, 59.5% were unvaccinated.
Figure 3.
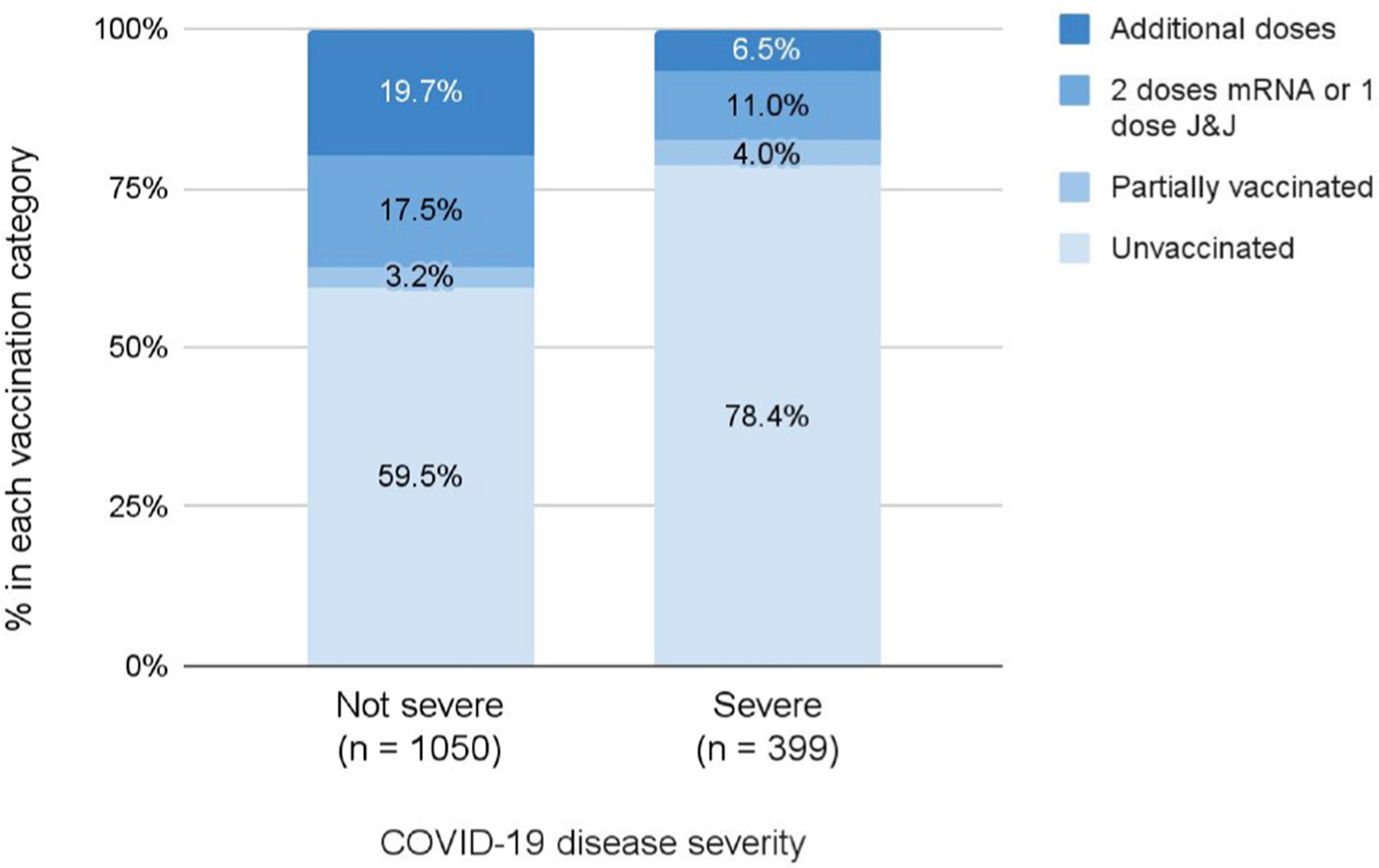
Vaccination status stratified by severe or not severe COVID-19. J&J, Johnson & Johnson-Janssen.
Severe COVID-19 outcomes during the Omicron wave period
Online supplemental table 1 shows characteristics of the 401 patients with SARD in the initial Omicron wave, stratified by COVID-19 severity. Mean age of those with severe COVID-19 was 66.9 years (SD 19.1), 76.3% were female, 10.2% had interstitial lung disease, 37.3% had rheumatoid arthritis, 47.5% were on glucocorticoids, 18.6% were on methotrexate and 15.3% were on rituximab. Online supplemental table 2 shows the case series of the eight patients with SARD who died during the initial Omicron wave.
Sensitivity analyses
The results of the sensitivity analyses with alternative calendar time periods and reference groups are shown in online supplemental tables 3–5. These showed significantly lower odds for severe COVID-19 in the initial Omicron wave compared with the reference group, similar to the main analysis. Results were also similar when removing patients who received monoclonal antibodies (online supplemental table 6). Monoclonal antibody use had an adjusted OR of 0.42 (95% CI 0.25 to 0.68) for severe COVID-19 compared with no use (online supplemental table 7).
DISCUSSION
In this large cohort study, we found that outcomes of COVID-19 in patients with SARDs have improved since the beginning of the pandemic. In particular, SARS-CoV-2 infections in the initial Omicron wave were associated with a 71% reduction in the risk of hospitalisation or death compared with the earliest time period. Despite these improvements, the absolute number of cases of severe COVID-19 was similar to that observed in other waves, suggesting that despite a reduced risk of severe disease, the initial Omicron wave had a substantial impact on patients with SARDs and the healthcare systems caring for them. The temporal improvement in outcomes is likely multifactorial, including differences in availability of testing, vaccination and other preventative strategies, hospital capacity, availability of effective treatments, depletion of susceptible individuals and virulence of SARS-CoV2 variants.
Previous studies conducted earlier in the pandemic found improvement in hospitalisation, mechanical ventilation, death and other outcomes for patients with SARDs over time, even during the first 6 months of the pandemic.9 10 However, no other study to date has examined the temporal trends in COVID-19 outcomes among SARDs extending up to the initial Omicron wave. These findings are particularly relevant given the blunted vaccine response and associated higher risk of breakthrough infections that have been observed in some patients with SARDs.24
The introduction of effective vaccines represented a key turning point in the pandemic and despite waning efficacy with time and against novel variants, they continue to provide important protection against severe disease.25 In our study of patients with SARDs, the majority of whom were on immunosuppressive treatments previously associated with blunted vaccine responses, we found that vaccination was associated with less severe COVID-19. These findings suggest that while some patients with SARD on immunosuppressives may be at higher risk for breakthrough infection, vaccination provided important benefits for many of these patients if infected with SARS-CoV-2. Additional studies are needed to further evaluate the efficacy of COVID-19 vaccinations among patients with SARDs during the most recent Omicron wave, a time characterised by substantial waning efficacy against breakthrough infection in the general population.26 27
Despite temporal improvements in the risk of severe COVID-19, some patients with SARDs during the initial Omicron wave experienced hospitalisation or death. Ours is one of the first to describe deaths from COVID-19 among SARDs that occurred during the initial Omicron wave. Several of these patients had serious comorbidities other than SARDs but were also on treatments associated with substantially blunted immune response to vaccine and infection (eg, B cell depletion),12 28–30 so it is difficult to ascribe the contribution of underlying SARD and immunosuppression to these individual cases. These findings highlight the need for ongoing risk mitigating strategies for many patients with SARD on such treatments as well as those with other comorbidities that may be related to their SARD (eg, interstitial lung disease) or its treatment (eg, cardiovascular comorbidities). In addition to shielding practices such as masking, social distancing, and avoiding indoor congregation, the recent introduction of pre-exposure prophylaxis with tixagevimab/cilgavimab, a monoclonal antibody against SARS-CoV-2, represents an important strategy for protecting our highest risk patients. However, tixagevimab/cilgavimab was studied in high risk, unvaccinated patients, the vast majority of whom did not have SARDs or similar conditions.31 Real-world effectiveness studies of tixagevimab/cilgavimab are now being reported and will be informative for guiding ongoing risk mitigating strategies for patients with SARDs.31
In addition to temporal improvements in the outcomes of COVID-19 among patients with SARDs during the ongoing pandemic, we also found other notable trends. First, there were shifts in the demographics of patients with COVID-19 during the course of the pandemic. For instance, there was a decrease in the proportion of patients with SARD with COVID-19 who identify as black or Hispanic and a decrease in the age of patients during the study period. These shifts are likely multifactorial, reflecting depletion of susceptible patients, changes in access to diagnostics and treatments, rates of vaccination and other factors. Second, a large portion of infections are now diagnosed at home using rapid antigen tests. This shift in diagnostics will make it increasingly difficult to capture more mild infections for the purpose of epidemiological studies like this one in both the general population and among SARDs. Leveraging electronic health record data, as we did here, will be an important way to capture and include patients like these in future studies.
Strengths of our study include the systematic identification of patients with SARDs in a large healthcare system that includes both tertiary care hospitals as well as community hospitals and their affiliated outpatient clinics. In contrast to studies relying only on administrative claims data, we were also able to capture COVID-19 diagnoses made using home rapid antigen testing because of the way these are captured in our electronic health record when reported to providers in our healthcare system. Additionally, we were able to confirm the SARD diagnosis and treatment, vaccination status at the time of infection, and details surrounding deaths and other outcomes with manual chart review.
Despite these strengths, our study has certain limitations. First, although we found temporal improvements in the outcomes of COVID-19 among patients with SARDs and an association between vaccination status and less severe disease, we cannot establish the causal effects of vaccinations, other risk mitigating strategies and COVID-19 treatments on these improvements. Second, this study was conducted in a single healthcare system in Massachusetts, USA. Our findings may not be generalisable to other areas of the USA or world because of differences in demographics as well as access to testing and treatment. Third, although we systematically identified cases in our healthcare system, patients who received COVID-19 diagnoses outside of our system may not have been captured, severe events occurring after diagnosis may have been missed, and patients with asymptomatic disease or mild courses may have been less likely to have testing or report their positive test. Thus, we were limited in our ability to calculate incidence and the true proportion of patients experiencing severe outcomes may be lower than we report with a larger denominator. However, those with SARDs, especially those on immunosuppression, are likely to contact their clinicians in the context of a COVID-19 infection to receive guidance regarding the management of their SARD treatments and to obtain treatments.
In conclusion, there have been substantial improvements in the outcomes associated with COVID-19 among patients with SARDs since the pandemic began in March 2020. In particular, the initial Omicron wave was associated with the largest number of cases but the lowest risk of severe disease. Despite these findings, some patients with SARDs continue to experience severe disease, especially those on immunosuppressives known to blunt the response to vaccine and infection, as well as those with other serious comorbidities. Additional studies are needed to refine risk mitigating strategies for patients at highest risk for severe outcomes.
Supplementary Material
WHAT IS ALREADY KNOWN ABOUT THIS TOPIC.
Patients with systemic autoimmune rheumatic diseases (SARDs) may be at increased risk for severe COVID-19, defined as hospitalisation or death.
Previous studies of patients with SARD suggested improving COVID-19 outcomes over calendar time, but most were performed prior to the wide availability of COVID-19 vaccines or the initial Omicron wave that was characterised by high infectivity.
WHAT THIS STUDY ADDS.
The proportion of patients with SARD with severe COVID-19 outcomes was lower over calendar time.
The adjusted OR of severe COVID-19 in the initial Omicron wave was 0.29 (95% CI 0.19 to 0.43) compared with early COVID-19 period.
The absolute number of severe COVID-19 cases during the peak of the Omicron variant wave was similar to the peaks of other waves.
Patients with SARD and severe COVID-19 were more likely to be unvaccinated than patients with SARD whose COVID-19 was not severe.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY.
These findings suggest that advances in COVID-19 prevention, diagnosis and treatment have contributed to improved outcomes among patients with SARD over calendar time.
Future studies should extend findings into future viral variants and consider the roles of waning immunity after vaccination or natural infection among patients with SARD who may still be vulnerable to severe COVID-19.
Funding
YK and TY-TH are supported by the National Institutes of Health Ruth L. Kirschstein Institutional National Research Service Award (T32 AR007530). NJP is supported by the Rheumatology Research Foundation Scientist Development Award. ZSW is funded by NIH/NIAMS (K23 AR073334 and R03 AR078938). JAS is funded by NIH/NIAMS (grant numbers R01 AR077607, P30 AR070253, and P30 AR072577), the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care.
Competing interests
NJP reports consulting fees from FVC Health unrelated to this work. MEW reports research support from Bristol Meyers Squibb, Sanofi, Eli Lilly, Amgen, and consulting fees from Abbvie, Aclaris, Amgen Bristol Myers Squibb, Corevitas, EQRx, Genosco, GlaxoSmithKline, Gilead, Horizon, Johnson & Johnson, Eli Lilly, Pfizer, Roche, Sanofi, Scipher, Set Point, and Tremeau, and stock options in Can-Fite, Inmediz, and Scipher unrelated to this work. ZSW reports research support from Bristol-Myers Squibb and Principia/Sanofi and consulting fees from Viela Bio, Zenas BioPharma, and MedPace. JAS reports research support from Bristol Myers Squibb and consultancy fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum and Pfizer unrelated to this work. All other authors report no competing interests.
Footnotes
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval This study was approved by the Mass General Brigham Institutional Review Board (2020P000833).
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Additional supplemental material is published online only. To view, please visit the journal online (http://dx.doi.org/10.1136/ard-2022-222954).
Data availability statement
Data are available on reasonable request. This study includes patient data from Mass General Brigham. The data that support the findings of this study may be made available upon reasonable request by contacting the corresponding author, JAS.
REFERENCES
- 1.Madhi SA, Kwatra G, Myers JE, et al. Population immunity and COVID-19 severity with omicron variant in South Africa. N Engl J Med 2022;386:1314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui DSC, Zumla A. Advances in the epidemiology, clinical features, diagnosis, clinical management and prevention of coronavirus disease 2019. Curr Opin Pulm Med 2022;28:166–73. [DOI] [PubMed] [Google Scholar]
- 3.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022;399:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022;399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway R, Grimshaw AA, Konig MF, et al. SARS-CoV-2 infection and COVID-19 outcomes in rheumatic diseases: a systematic literature review and meta-analysis. Arthritis Rheumatol 2022;74:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis 2020;79:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bower H, Frisell T, Di Giuseppe D, et al. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis 2021;80:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway R, Nikiphorou E, Demetriou CA, et al. Temporal trends in COVID-19 outcomes in people with rheumatic diseases in Ireland: data from the COVID-19 global rheumatology alliance registry. Rheumatology 2022;61:SI151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorge A, D’Silva KM, Cohen A, et al. Temporal trends in severe COVID-19 outcomes in patients with rheumatic disease: a cohort study. Lancet Rheumatol 2021;3:e131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serling-Boyd N, D’Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis 2021;80:660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from Hospital: a cohort study. Lancet 2021;397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook C, Patel NJ, D’Silva KM, et al. Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis 2022;81:289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu TY-T, D’Silva KM, Patel NJ, et al. Laboratory trends, hyperinflammation, and clinical outcomes for patients with a systemic rheumatic disease admitted to hospital for COVID-19: a retrospective, comparative cohort study. Lancet Rheumatol 2021;3:e638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Silva KM, Serling-Boyd N, Hsu TY. SARS-CoV-2 antibody response after COVID-19 in patients with rheumatic disease. Ann Rheum Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of COVID-19 - Final Report. N Engl J Med 2020;383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med 2021;384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanni KM, Patel NJ, DiIorio M, et al. Breakthrough infection after three doses of COVID-19 mRNA vaccine in systemic autoimmune rheumatic diseases: two cases in patients on TNF inhibitor monotherapy. RMD Open 2022;8:e002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Giuseppe D, Alfredsson L, Bottai M, et al. Long term alcohol intake and risk of rheumatoid arthritis in women: a population based cohort study. BMJ 2012;345:e4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 2021;326:499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with COVID-19. N Engl J Med 2021;384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik JJ, Sparks JA, Kim AHJ. Immunogenicity, breakthrough infection, and underlying disease flare after SARS-CoV-2 vaccination among individuals with systemic autoimmune rheumatic diseases. Curr Opin Pharmacol 2022;65:102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenforde MW, Self WH, Gaglani M, et al. Effectiveness of mRNA Vaccination in Preventing COVID-19-Associated Invasive Mechanical Ventilation and Death - United States, March 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tartof SY, Slezak JM, Puzniak L, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir Med 2022;10:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deepak P, Kim W, Paley MA, et al. Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2 : A Prospective Cohort Study. Ann Intern Med 2021;174:1572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2022;4:e177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of COVID-19. N Engl J Med 2022;386:2188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. This study includes patient data from Mass General Brigham. The data that support the findings of this study may be made available upon reasonable request by contacting the corresponding author, JAS.


