Abstract
Background
Diabetes is associated with long‐term damage, dysfunction and failure of various organs, especially the eyes, kidneys, nerves, heart and blood vessels. The risk of developing type 2 diabetes increases with age, obesity and lack of physical activity. Insulin resistance is a fundamental aspect of the aetiology of type 2 diabetes. Insulin resistance has been shown to be associated with atherosclerosis, dyslipidaemia, glucose intolerance, hyperuricaemia, hypertension and polycystic ovary syndrome. The mineral zinc plays a key role in the synthesis and action of insulin, both physiologically and in diabetes mellitus. Zinc seems to stimulate insulin action and insulin receptor tyrosine kinase activity.
Objectives
To assess the effects of zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance.
Search methods
This review is an update of a previous Cochrane systematic review published in 2007. We searched the Cochrane Library (2015, Issue 3), MEDLINE, EMBASE, LILACS and the ICTRP trial register (from inception to March 2015). There were no language restrictions. We conducted citation searches and screened reference lists of included studies.
Selection criteria
We included studies if they had a randomised or quasi‐randomised design and if they investigated zinc supplementation compared with placebo or no intervention in adults with insulin resistance living in the community.
Data collection and analysis
Two review authors selected relevant trials, assessed risk of bias and extracted data.
Main results
We included three trials with a total of 128 participants in this review. The duration of zinc supplementation ranged between four and 12 weeks. Risk of bias was unclear for most studies regarding selection bias (random sequence generation, allocation concealment) and detection bias (blinding of outcome assessment). No study reported on our key outcome measures (incidence of type 2 diabetes mellitus, adverse events, health‐related quality of life, all‐cause mortality, diabetic complications, socioeconomic effects). Evaluation of insulin resistance as measured by the Homeostasis Model Assessment of Insulin Resistance (HOMA‐IR) showed neutral effects when comparing zinc supplementation with control (two trials; 114 participants). There were neutral effects for trials comparing zinc supplementation with placebo for total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol and triglycerides (2 studies, 70 participants). The one trial comparing zinc supplementation with exercise also showed neutral effects for total cholesterol, HDL and LDL cholesterol, and a mean difference in triglycerides of ‐30 mg/dL (95% confidence interval (CI) ‐49 to ‐10) in favour of zinc supplementation (53 participants). Various surrogate laboratory parameters were also analysed in the included trials.
Authors' conclusions
There is currently no evidence on which to base the use of zinc supplementation for the prevention of type 2 diabetes mellitus. Future trials should investigate patient‐important outcome measures such as incidence of type 2 diabetes mellitus, health‐related quality of life, diabetic complications, all‐cause mortality and socioeconomic effects.
Plain language summary
Zinc supplementation for the prevention of type 2 diabetes mellitus
Review question
What are the effects of zinc supplementation compared with placebo or no treatment for the prevention of type 2 diabetes in adults with insulin resistance?
Background
Some studies have shown that zinc improves glucose levels (glycaemic control) in people with diabetes. As a consequence of diabetes long‐term complications may develop, such as kidney, nerve and eye disease. Also, the risk of cardiovascular complications like heart attacks and strokes is raised. Type 1 diabetes is a form of diabetes where the body cannot produce insulin any more. The risk of developing type 2 diabetes increases with age, obesity and lack of physical activity and is characterised by an increasing inability of the body to make good use of insulin (insulin resistance). The mineral zinc plays a key role in the action of insulin and theoretically zinc supplementation used by people with insulin resistance could prevent the onset of diabetes.
Study characteristics
We included three randomised controlled studies with a total of 128 participants in this review. The duration of zinc supplementation ranged between four and 12 weeks.
Key results
No study reported on our patient‐important key outcomes (new onset of type 2 diabetes mellitus, side effects, health‐related quality of life, all‐cause mortality, diabetic complications, socioeconomic effects). The effects of zinc supplementation are uncertain regarding insulin resistance and lipid levels in the blood (mainly cholesterol and triglycerides).
Quality of evidence
The overall quality of the included studies was unclear because study authors did not provide important information for us to judge how the studies were performed (unclear risk of bias in most cases). In addition, the number of studies and participants is low and the study authors did not investigate important outcomes such as new onset of type 2 diabetes mellitus or side effects of zinc supplementation.
Currentness of evidence
This evidence is up to date as of March 2015.
Summary of findings
for the main comparison.
| Zinc supplementation for the prevention of type 2 diabetes mellitus | ||||||
|
Population: non‐diabetic adults with insulin resistance Settings: not specified/outpatients Intervention: zinc supplementation Comparison: placebo or exercise | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or exercise | Zinc supplementation | |||||
| Incidence of type 2 diabetes mellitus | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Diabetic complications | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Adverse events | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
This is an update of a Cochrane Review first published in the Cochrane Library in 2007 (Beletate 2007), which included one study that did not provide evidence on patient‐important outcome measures such as incidence of new‐onset type 2 diabetes (Marreiro 2006).
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (that is elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased (ADA 1999). There are two major forms of diabetes: type 1 diabetes (insulin‐dependent diabetes mellitus (IDDM)) and type 2 diabetes (non‐insulin‐dependent diabetes mellitus (NIDDM) (ADA 1999). Type 2 diabetes mellitus, the most prevalent form of the disease, is often asymptomatic and may remain undiagnosed for many years (ADA 1998). Type 2 diabetes may be seen in children and young adults. In type 2 diabetes the pancreatic islet cells are capable of compensating insulin resistance (see below) and producing larger quantities of insulin, at least at the beginning of the disease (Chausmer 1998).
Prevalence and costs
The worldwide prevalence of diabetes has been estimated to be around 347 million people (Danaei 2011). In 2004, an estimated 3.4 million people died from consequences of high blood sugar (WHO 2009). The World Health Organization (WHO) projects that diabetes will be the 7th leading cause of death in 2030 (WHO 2011). Diabetes accounts for over EUR 77 billion (USD 98) in healthcare costs (Tracey 2003).
The risk of developing type 2 diabetes and insulin resistance
The risk of developing type 2 diabetes increases with age, obesity and lack of physical activity. Insulin resistance is a fundamental aspect of the aetiology of type 2 diabetes (Kahn 2000). The majority of people who develop type 2 diabetes are insulin resistant, a condition in which the pancreas produces insulin but the body's cells become increasingly resistant to the regular effects of insulin, which results in hyperglycaemia (Tracey 2003). Insulin resistance has been shown to be associated with atherosclerosis (Howard 1996), dyslipidaemia (Moro 2003), glucose intolerance, hyperuricaemia, hypertension (Bonora 1998), and polycystic ovary syndrome (Kahn 2000).
Insulin resistance can be measured by using the glucose clamp technique (DeFronzo 1979), which is considered the gold standard in the assessment of insulin sensitivity (Karelis 2004). However, this method is laborious, expensive and inadequate for large‐scale or epidemiological studies (Bonora 2000). Matthews et al developed the Homeostasis Model Assessment (HOMA) method, which derives an estimate of insulin sensitivity by taking fasting plasma glucose and insulin concentrations into account. HOMA is supposed to evaluate insulin resistance and the function of ß‐cells. Insulin resistance is calculated with the following formula: (fasting serum insulin (µU/ml) x fasting plasma glucose (mmol/L)/22.5 (Matthews 1985).
Impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) are associated with insulin resistance. IFG and IGT refer to an intermediate metabolic stage between normal glucose homeostasis and diabetes. This stage includes individuals with fasting glucose levels equal to or greater than 110 mg/dL but lower than 126 mg/dL or after an orale glucose tolerance test with two‐hour values equal to or greater than 140 mg/dL but lower than 200 mg/dL.
Description of the intervention
Every single cell in the body needs zinc for structural and energy‐producing functions. Zinc is an essential trigger for many biochemical reactions and also for protein production. Zinc plays an important role in cell division, growth and repair. It helps with wound healing and maintaining a normal sense of taste and smell. Zinc works as an immune booster and can be instrumental in fighting colds, flu and other infections. Zinc is a component of more than 200 enzymes, most of them involved in protein and DNA synthesis. Zinc has beneficial effects on sex and thyroid hormones. The human body does not produce zinc on its own, so it must be obtained from outside sources. The mineral zinc can be found in both animal and plant food sources, but the richest source of zinc comes from animal food.
The daily recommended dose of zinc is 12 mg for women and 15 mg for men. Studies suggest oral supplementation of zinc sulphate from 30 mg to 200 mg per day. The effect of supplementation with zinc was assessed in 10 patients with liver cirrhosis (Marchesini 1998). The patients presented with impaired glucose tolerance and zinc deficiency. Supplementation was approximately 136 mg zinc per day. The study showed that zinc supplementation produced a significant improvement in glucose disposal. The action of zinc seemed to be related to the increased activities of insulin independent glucose transporters (Marchesini 1998).
Adverse effects of the intervention
Amounts of zinc of two or more grams per day can cause gastrointestinal irritation and vomiting (Marchesini 1998; Marreiro 2004).
How the intervention might work
Zinc ions have an insulin‐like effect. A particularly sensitive target of zinc ions is protein tyrosine phosphatase 1B, a key regulator of the phosphorylation state of the insulin receptor (Haase 2005). Several studies have investigated the role of zinc status in insulin secretion and metabolism. In vitro studies show that insulin may form a complex with zinc, improving the solubility of this hormone in the pancreatic β‐cells (Rossetti 1990). Moreover, the binding ability of insulin to its receptor may be increased (Rossetti 1990). Alterations in zinc concentration and distribution in tissues, as well as improvements in insulin sensitivity after supplementation with this element have been demonstrated (Marchesini 1998).
Why it is important to do this review
Given the worldwide public health relevance of type 2 diabetes mellitus any intervention thought to be associated with only minor or no adverse effects should be investigated by means of a systematic review of the available evidence.
Objectives
To assess the effects of zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised controlled clinical trials with a minimum duration of four weeks.
Types of participants
Non‐diabetic adults (18 years or older) living in the community with insulin resistance.
Diagnostic criteria
Insulin resistance had to be measured by the Homeostasis Model Assessment of Insulin Resistance (HOMA‐IR) or the glucose clamp technique.
To be consistent with changes in the classification of and diagnostic criteria for diabetes mellitus over the years, the diagnosis should have been established using the standard criteria valid at the time of the trial commencing (for example ADA 1999; WHO 1999). Ideally, the diagnostic criteria should have been described. If necessary, we would have used the study authors' definition of diabetes mellitus. We planned to subject diagnostic criteria to a sensitivity analysis.
Types of interventions
We planned to investigate the following comparisons of intervention versus control/comparator.
Intervention
Zinc
Comparator
Placebo.
No intervention.
Another dose of zinc.
Concomitant interventions had to be the same in the intervention and comparator groups to establish fair comparisons.
Types of outcome measures
Primary outcomes
Incidence of type 2 diabetes mellitus.
Adverse events.
Secondary outcomes
Insulin resistance.
Health‐related quality of life.
All‐cause mortality.
Diabetic complications.
Socioeconomic effects.
Lipid levels.
Method of outcome measurement
Incidence of type 2 diabetes mellitus: new onset of type 2 diabetes mellitus as defined by standard diagnostic criteria (such as ADA 1997; ADA 1999; ADA 2003; WHO 1980; WHO 1985).
Adverse events: for example, diarrhoea, nausea, fatigue, gastrointestinal discomfort, anaemia.
Insulin resistance: estimated by the HOMA‐IR or the glucose clamp technique.
Health‐related quality of life: measured with a validated instrument such as the Quality of Well‐Being Scale (QWB), Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36), EuroQol (EQ‐5D) and specific instruments such as the Diabetes Care Profile (DCP) and the Diabetes Quality of Life Measure (DQOL).
All‐cause mortality: defined as death from any cause.
Diabetic complications: defined as hypoglycaemia, diabetic ketoacidosis, hyperosmolar hyperglycaemic state, retinopathy, coronary heart disease, nephropathy, neuropathy, stroke and amputation.
Socioeconomic effects: such as consequences on income, wealth, education, occupation.
Lipid levels: as measured by total cholesterol, LDL and HDL cholesterol, triglycerides and leptin concentration.
Timing of outcome measurement
We planned to collect data for all primary and secondary outcomes for any time of outcome measurement.
'Summary of findings' table
We present a 'Summary of findings' table reporting the following outcomes listed according to priority.
Incidence of type 2 diabetes mellitus.
Diabetic complications.
Adverse events.
Health‐related quality of life.
All‐cause mortality.
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception to the specified date without language restriction.
-
Cochrane Library (2015, Issue 3)
Cochrane Database of Systematic Reviews (CDSR)
Cochrane Central Register of Controlled Trials (CENTRAL)
Database of Abstracts of Reviews of Effects (DARE)
Health Technology Assessment (HTA) reports
MEDLINE (until March 2015)
EMBASE (until March 2015)
LILACS (until March 2015)
-
International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/):
Australian New Zealand Clinical Trials Registry (2 March 2015)
ClinicalTrials.gov (2 March 2015)
EU Clinical Trials Register (2 March 2015)
International Standard Randomised Controlled Trial Number (ISRCTN) (2 March 2015)
Brazilian Clinical Trials Registry (2 March 2015)
Chinese Clinical Trial Registry (2 March 2015)
Clinical Trials Registry ‐ India (2 March 2015)
Clinical Research Information Service ‐ Republic of Korea (2 March 2015)
Cuban Public Registry of Clinical Trials (2 March 2015)
German Clinical Trials Register (2 March 2015)
Iranian Registry of Clinical Trials (2 March 2015)
Japan Primary Registries Network (2 March 2015)
Pan African Clinical Trial Registry (2 March 2015)
Sri Lanka Clinical Trials Registry (2 March 2015)
The Netherlands National Trial Register (2 March 2015)
Thai Clinical Trials Register (2 March 2015)
For detailed search strategies see Appendix 1. We continuously applied a MEDLINE (via Ovid SP) email alert service to identify newly published studies (Appendix 1). If we detected new studies for inclusion we would have evaluated these and incorporated findings in our review before submission of the final review draft (Beller 2013).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses and health technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors (LFOG, MSPO) independently scanned the title or abstract of every record retrieved. We retrieved full articles for further assessment if the information given suggested that the study: 1) included patients with insulin resistance, 2) compared a zinc intervention with placebo. Where differences in opinion existed, they were resolved by a third party (RED). If resolving disagreement was not possible, we planned to add the article to those 'awaiting classification' and contact the study authors for clarification. We present an adapted Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram to show the process of study selection (Liberati 2009).
Data extraction and management
For studies that fulfilled the inclusion criteria, two review authors (LFOG, MSPO) independently abstracted relevant population and intervention characteristics, using standard data extraction templates as supplied by the Cochrane Metabolic and Endocrine Disorders (CMED) Group, with any disagreements to be resolved by discussion, or, if required, by a third review author (RED) (for details see Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6;Appendix 7; Appendix 8; Appendix 9; Appendix 10).
1. Overview of study populations.
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible [N] | Randomised [N] | ITT [N] | Analysed [N] | Finishing study [N] | Randomised finishing study [%] | Follow‐up timeb | |
| (1) Gómez‐García 2006 | I: 100 mg/day zinc sulfate | 12 (6 participants per group; 80% power) | 14 | 7 | ‐ | 7 | 7 | 100 | 1 month |
| C: placebo | 7 | ‐ | 7 | 7 | 100 | ||||
| total: | 14 | ‐ | 14 | 14 | 100 | ||||
| (2) Marreiro 2006 | I: 30 mg/day zinc amino chelate | ‐ | ‐ | 28 | ‐ | 28 | 28 | 100 | 1 month |
| C: placebo | 28 | ‐ | 28 | 28 | 100 | ||||
| total: | 56 | ‐ | 56 | 56 | 100 | ||||
| (3) Soheilykhah 2012 | I: 50 mg/day zinc sulfate | ‐ | ‐ | ‐ | ‐ | 28 | 28 | 100 | 12 weeks |
| C: regular exercise and weight control | ‐ | ‐ | 25 | 25 | 100 | ||||
| total: | 58 | ‐ | 53 | 53 | 91.4 | ||||
| Grand total | All interventions | 63 | 63 | ||||||
| All comparators | 60 | 60 | |||||||
| All interventions and comparators | 128 | 123 | |||||||
aAccording to power calculation in study publication or report bDuration of intervention and/or follow‐up under randomised conditions until end of study
"‐" denotes not reported
C: comparator; I: intervention; ITT: intention‐to‐treat.
We planned to provide information including trial identifier about potentially relevant ongoing studies in the 'Characteristics of ongoing studies' table and in a joint appendix. We attempted to find the protocol for each included study and had planned to report primary, secondary and other outcomes in comparison with data in publications in a joint appendix 'Matrix of study endpoint (publications and trial documents)'.
We sent an email request to the authors of included studies to enquire whether they were willing to answer questions regarding their trials. Appendix 11 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the study authors of the article, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximised the yield of information by collating all available data. In case of doubt the publication reporting the longest follow‐up associated with our primary or secondary outcomes had priority.
Assessment of risk of bias in included studies
Two review authors (LFOG, MSPO) assessed each trial independently. We resolved possible disagreement by consensus, or with consultation of a third review author in case of disagreement (RED). In cases of disagreement, we consulted the rest of the group and made a judgement based on consensus.
We used the 'Risk of bias' assessment toll of the Cochrane Collaboration (Higgins 2011a; Higgins 2011b) and evaluated the following bias criteria:
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other potential sources of bias
We judged the above 'Risk of bias' criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We have presented a 'Risk of bias' graph and a 'Risk of bias' summary. We assessed the impact of individual bias domains on study results at endpoint and study levels.
We planned to assess outcome reporting bias by integrating the results of the appendix 'Matrix of study endpoints (publications and trial documents)' and the appendix 'Examination of outcome reporting bias' (Kirkham 2010). This analysis would have formed the basis of the judgement of selective reporting (reporting bias).
For blinding of participants and personnel (performance bias), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data) we intended to evaluate risk of bias separately for subjective and objective outcomes (Hróbjartsson 2013). We considered the implications of missing outcome data from individual participants.
We defined the following endpoints as subjective outcomes.
Adverse events
Health‐related quality of life
We defined the following endpoints as objective outcomes.
Incidence of type 2 diabetes mellitus
Diabetic complications
All‐cause mortality
Lipid levels
Socioeconomic effects
Measures of treatment effect
We planned to express dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We planned to express continuous data as mean differences (MDs) with 95% CIs.
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
We planned to obtain relevant missing data from authors, if feasible, and we evaluated important numerical data such as screened, eligible and randomised participants, as well as intention‐to‐treat (ITT), as‐treated and per‐protocol populations. We investigated attrition rates, for example drop‐outs, losses to follow‐up and withdrawals, and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Where standard deviations for outcomes were not reported, we planned to impute these values by assuming the standard deviation of the missing outcome to be the average of the standard deviations from those studies where this information was reported. We planned to investigate the impact of imputation on meta‐analyses by means of sensitivity analysis.
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, we planned not to report study results as the pooled effect estimate in a meta‐analysis.
We wanted to identify heterogeneity by visual inspection of the forest plots and by using a standard Chi² test with a significance level of α = 0.1. In view of the low power of this test, we also planned to examine heterogeneity using the I² statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I² statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011a).
Had we found heterogeneity, we would have attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We planned to use funnel plots to assess small study effects, if there were at least 10 studies for a particular outcome. There can be several explanations for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We planned therefore to interpret the results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across studies we planned primarily to summarise low risk of bias data by means of a random‐effects model (Wood 2008). We planned to interpret random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual study (Riley 2011). In addition, we planned to perform statistical analyses according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We wanted to present the overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity such as directness of results. Two review authors (LFOG, MSPO) would have independently rated the quality of evidence for each outcome. We planned to present a summary of the evidence in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies, numbers of participants, and studies addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented results for the outcomes as described in Types of outcome measures. If meta‐analysis was not possible, we planned to present the results in a narrative 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
There were insufficient data to allow for any subgroup analyses. Should sufficient data in the future permit, we plan to carry out the following subgroup analyses and plan to investigate interaction.
Gender (female/male)
Age (depending on data but especially older versus younger participants)
Participants with or without comorbidities (for example, heart attack, stroke, peripheral vascular disease)
Duration of intervention
Different doses of zinc
Sensitivity analysis
There were insufficient data to allow for any sensitivity analyses to explore the influence of the following factors on effect size by restricting the analysis to:
Published studies.
Taking into account risk of bias, as specified in the 'Assessment of risk of bias in included studies' section.
Very long or large studies to establish how much they dominate the results.
Studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), or country.
We also planned to test the robustness of the results by repeating the analysis using different measures of effect size (RRs, ORs etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of studies, see the tables Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
In the first version of the review (2007) we evaluated 192 records. Following the assessment of the full articles, we considered only two publications for inclusion. We excluded one study (Marchesini 1998), and included one study that met our inclusion criteria (Marreiro 2006). For this first update, our comprehensive literature searches identified 132 records; from these, we identified 14 full‐text publications for further examination. We excluded the other studies on the basis of their titles or abstracts, because they did not meet the inclusion criteria or were not relevant to the question under study (Figure 1). After screening the full text of the selected publications, two new studies (Gómez‐García 2006; Soheilykhah 2012), and a further unpublished manuscript of Marreiro 2006 met the inclusion criteria for this update. We excluded nine studies (Czernichow 2006; Czernichow 2009; Islam 2013; Kelishadi 2010; Kim 2012; Marreiro 2002; Marchesini 1998; Ramos 2007; Stewart 2011). One further study is an ongoing trial (Ranasinghe 2013).
1.

Study flow diagram.
Included studies
In this first update we included two published studies (Gómez‐García 2006; Soheilykhah 2012), and a further manuscript (Marreiro 2006), in addition to the original included study (Marreiro 2002), with a total of 128 randomised participants.
Overview of study populations
The Gómez‐García 2006 study enrolled 14 obese men. The Marreiro 2006 study included 56 normal glucose‐tolerant obese women, while the Soheilykhah 2012 study assessed 58 overweight or obese participants with a normal oral glucose tolerance test.
Study design
All included studies claimed to be randomised controlled trials.
Settings
Two of the included studies did not report the setting where the trials were conducted (Gómez‐García 2006; Soheilykhah 2012). One study involved outpatients visiting hospital clinics (Marreiro 2006).
Participants
Gómez‐García 2006 evaluated 14 men between 21 and 30 years old, while Marreiro 2006 assessed 56 women aged 25 to 45 years. The mean age of participants in Soheilykhah 2012 was 38 years.
Gómez‐García 2006 recruited study participants with a prediabetic state and stable weight for at least three months before the beginning of the study. Marreiro 2006 recruited glucose‐tolerant obese women. Soheilykhah 2012 recruited overweight or obese participants. The mean body mass index (BMI) at baseline ranged from 31 kg/m² in Gómez‐García 2006 to 36 kg/m² in Marreiro 2006. Soheilykhah 2012 evaluated 58 first degree relatives of diabetic people with a normal oral glucose tolerance test and a BMI > 25 kg/m²; the mean BMI was 29 kg/m².
Interventions
In the Gómez‐García 2006 study participants received 100 mg zinc per day for four weeks (n = 7) or a matching placebo (n = 7). In the Marreiro 2006 study treatment with zinc consisted of 30 mg per day for four weeks (n = 28) or a matching placebo (n = 28). In the Soheilykhah 2012 study 28 participants received 50 mg zinc per day for 12 weeks and for 25 participants regular exercise and weight control were recommended.
Outcomes
None of the included studies investigated the incidence of type 2 diabetes mellitus, adverse events, health‐related quality of life, all‐cause mortality, diabetic complications or socioeconomic effects.
In the Gómez‐García 2006 study the authors evaluated insulin sensitivity, leptin levels, biochemical profile and androgens. Outcome measures in the Marreiro 2006 study were insulin resistance, anthropometric parameters, diet parameters, leptin levels, insulin levels, zinc levels, lipid metabolism and fasting plasma glucose. In the Soheilykhah 2012 study adiponectin, fasting blood glucose, insulin, insulin resistance and lipid profile were assessed.
Excluded studies
Nine studies are described in the Characteristics of excluded studies (Czernichow 2006; Czernichow 2009; Islam 2013; Kelishadi 2010; Kim 2012; Marreiro 2002; Marchesini 1998; Ramos 2007; Stewart 2011). The main reason for exclusion was that the study was not a randomised controlled trial (RCT) or it was a RCT evaluating a combination of another oxidant and vitamins or included only children.
The Ranasinghe 2013 study is described in detail under Characteristics of ongoing studies. This is a RCT performed in Sri Lanka that evaluated participants in a prediabetic stage. The anticipated end of study was the end of June 2012. Participants received 20 mg zinc per day or a matching placebo for a period of 12 months. The objectives of this study were as follows: "The study aims to evaluate the effects of zinc supplementation on the progression of disease in patients with pre‐diabetes from Sri Lanka and determine the metabolic effects of zinc supplementation on glycemic control. Furthermore, we aim to evaluate the effects of zinc supplementation on appetite and body weight in patients with pre‐diabetes" (Ranasinghe 2013). None of our review's primary or secondary outcome measures were planned to be analysed.
Risk of bias in included studies
For details on the risk of bias of the included studies see Characteristics of included studies. For an overview of review authors' judgements about each risk of bias item for individual studies and across all studies see Figure 2 and Figure 3.
2.
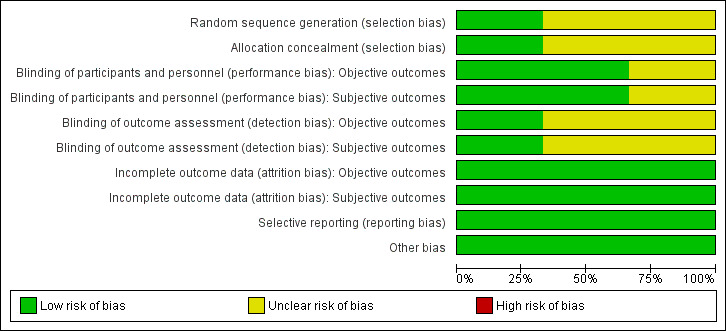
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies had an unclear risk of selection bias with the exception of Gómez‐García 2006 that used opaque and sealed envelopes in a simple randomisation process.
Blinding
Two studies were double‐blinded for both investigators and participants resulting in a low risk of performance bias (Gómez‐García 2006; Marreiro 2006). Two of the included studies did not report on blinding of outcome assessors (Marreiro 2006; Soheilykhah 2012) and were ranked as unclear risk of bias for this domain. However, blinding of outcome assessors in Gómez‐García 2006 was done for both objective and subjective outcomes resulting in a low risk of detection bias.
Incomplete outcome data
All participants in the Gómez‐García 2006 and Marreiro 2006 studies completed the trial. In the Soheilykhah 2012 study 53 of 58 participants completed the study. Altogether we ranked attrition bias as low risk for all studies.
Selective reporting
We noted no evidence of selective reporting in any of the included studies.
Other potential sources of bias
We noted no evidence of other potential sources of bias in any of the included studies.
Effects of interventions
See: Table 1
Zinc supplementation versus placebo or no intervention
Primary outcomes
Incidence of type 2 diabetes mellitus
No study reported this outcome.
Adverse events
No study reported this outcome.
Secondary outcomes
Insulin resistance
Marreiro 2006 reported neutral effects for the Homeostasis Model Assessment of Insulin Resistance (HOMA‐IR), showing a mean difference (MD) of ‐0.10 (95% confidence interval (CI) ‐1.28 to 1.08), P = 0.87; 56 participants; Analysis 1.1 when comparing zinc supplementation with placebo. Soheilykhah 2012 reported a MD in the HOMA‐IR of ‐0.27 (95% CI ‐1.02 to 0.48), P = 0.48; 58 participants; Analysis 2.2 for zinc supplementation versus exercise.
1.1. Analysis.
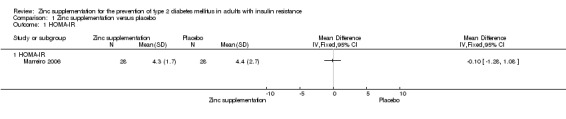
Comparison 1 Zinc supplementation versus placebo, Outcome 1 HOMA‐IR.
2.2. Analysis.

Comparison 2 Zinc supplementation versus exercise, Outcome 2 Lipids.
Health‐related quality of life
No study reported this outcome.
All‐cause mortality
No study reported this outcome.
Diabetic complications
No study reported this outcome.
Socioeconomic effects
No study reported this outcome.
Lipid levels
All included studies reported on this outcome (Gómez‐García 2006; Marreiro 2006; Soheilykhah 2012). There were neutral effects for studies comparing zinc supplementation with placebo for total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol and triglycerides (Gómez‐García 2006; Marreiro 2006) (Analysis 1.2). The one study comparing zinc supplementation with exercise, Soheilykhah 2012, also showed neutral effects for total, HDL andLDL cholesterol, and a MD in triglycerides of ‐30 mg/dL (95% CI ‐49 to ‐10) in favour of zinc supplementation (Analysis 2.2).
1.2. Analysis.
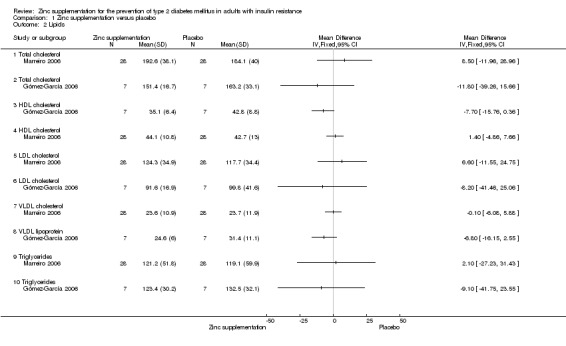
Comparison 1 Zinc supplementation versus placebo, Outcome 2 Lipids.
Additional parameters evaluated in the included studies
One study reported on body mass index, body perimeter, body constitution, skinfold thickness below the scapula, skinfold thickness above the hip bone, skinfold thickness of the upper arm, body mass index, waist, waist‐hip ratio, energy and nutrient intake, and percentage of fat as obtained by bioimpedance (Marreiro 2006). Marreiro 2006 also measured zinc concentrations in plasma, erythrocytes and urine.
One study reported on insulin, glucose, creatinine, uric acid, leptin, testosterone and sex hormone binding globulin (Gómez‐García 2006).
One study measured adiponectin, insulin and fasting blood sugar (FBS) (Soheilykhah 2012).
Discussion
Summary of main results
This systematic review offers up‐to‐date but very limited evidence supported by only three small randomised controlled trials regarding the effects of zinc supplementation for the prevention of type 2 diabetes mellitus. The relationship between obesity and insulin resistance is seen across all ethnic groups and is evident across the full range of body weights. Insulin resistance is thought to be a major feature of type 2 diabetes, particularly since high basal plasma insulin concentrations are often found in obese type 2 diabetic people. Unfortunately, none of our patient‐important outcome measures were reported in the included studies. Only parameters of insulin resistance and lipid levels were reported, showing neutral effects.
Overall completeness and applicability of evidence
None of the included studies investigated our predefined outcome measure, therefore the body of evidence is very limited. Marreiro 2006 suggested future trials with higher doses of zinc and longer follow‐up intervals, since four weeks and a dose of 30 mg of zinc are not sufficient to assess a long‐term process like the development of glucose intolerance and diabetes.
Quality of the evidence
The methodological quality of the included studies could not be judged according to the GRADE approach because none of our key endpoints were evaluated in the included studies.
Potential biases in the review process
Despite our thorough search in various databases we might have overlooked trials, especially with regard to the grey literature. However, we contacted the authors of the included studies to ask whether they had done another trial dealing with zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance.
Agreements and disagreements with other studies or reviews
The results of our review reflect those of the previous version of this review (Beletate 2007). Another systematic review of randomised controlled trials evaluated the effects of zinc supplementation on biomarkers of glycaemic control included type 1 and type 2 diabetic participants, participants with metabolic syndrome, participants with obesity and healthy persons (Capdor 2013). When comparing participants with metabolic conditions to healthy volunteers the study authors showed that zinc supplementation produced a greater reduction in glucose concentrations.
Authors' conclusions
Implications for practice.
There is currently no evidence on which to base the use of zinc supplementation for the prevention of type 2 diabetes mellitus.
Implications for research.
There are only three included studies evaluating the effects of zinc supplementation for the prevention of type 2 diabetes mellitus. Future randomised controlled clinical trials should have standardised outcome measures, such as incidence of type 2 diabetes mellitus, insulin resistance, diabetic complications, health‐related quality of life, all‐cause mortality and socioeconomic outcome measures.
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2015 | New search has been performed | This review is an update of the previous Cochrane systematic review (Beletate 2007). |
| 8 May 2014 | New citation required but conclusions have not changed | The two new included studies, Gómez‐García 2006 and Soheilykhah 2012, did not change our conclusions. |
Acknowledgements
The authors would like to thank Regis Bruni Andriolo for his useful advice in the development of the original review. We also would like to thank Karla Bergerhoff and Maria‐Inti Metzendorf for the searches done for this review update. We also are thankful for Dr. Esperanza Martínez‐Abundis and Dr. Gómez‐García who provided us with further information and details of their publications.
Appendices
Appendix 1. Search strategies
| The Cochrane Library |
| #1 [mh Zinc/AD,AE,ST,TU,TH] #2 (zinc near/4 supplement*):ti,ab,kw #3 #1 or #2 #4 [mh "Glucose tolerance test"] #5 [mh "Glucose intolerance"] #6 [mh "Diabetes mellitus"/PC] #7 [mh "Insulin resistance"] #8 [mh "Metabolic syndrome X"] #9 [mh "Prediabetic state"] #10 (glucose near/4 (intolerance or tolerance test*)):ti,ab,kw #11 (impaired and (fasting near/4 (glucose or glycemia* or glycaemia*))):ti,ab,kw #12 (impaired and (glucose near/4 (toleran* or stat* or respons* or control* or regul* or metabol* or homeost*))):ti,ab,kw #13 (reduced and (glucose near/4 (metab* or toleran*))):ti,ab,kw #14 (praediabet* or prae diabet* or prediabet* or pre diabet*):ti,ab,kw #15 (metabolic syndrom* or syndrome X):ti,ab,kw #16 ((borderline or mild) near/4 diabet*):ti,ab,kw #17 (insulin* near/4 resistan*):ti,ab,kw #18 (((impaired or reduced) near/4 insulin) near/4 secret*):ti,ab,kw #19 {or #4‐#18} #20 #3 and #19 |
| MEDLINE (via Ovid SP) |
| 1 (zinc adj3 supplement*).tw,ot.
2 exp Zinc/ad, ae, st, tu, th [Administration & Dosage, Adverse Effects, Standards, Therapeutic Use, Therapy]
3 1 or 2
4 exp Glucose Tolerance Test/ or exp Glucose Intolerance/
5 exp Diabetes Mellitus, Type 2/pc [Prevention & Control]
6 exp Insulin Resistance/
7 exp Metabolic Syndrome X/
8 exp Prediabetic State/
9 (glucose adj3 (intolerance or tolerance test*)).tw,ot.
10 (impaired fasting adj3 (glucose or glyc?emia*)).tw,ot.
11 (impaired glucose adj3 (toleran* or stat* or respons* or control* or regul* or metab* or homeost*)).tw,ot.
12 (reduced glucose adj3 (metab* or toleran*)).tw,ot.
13 (pr?ediabet* or pr?e diabet*).tw,ot.
14 (metabolic syndrom* or syndrome X).tw,ot.
15 ((borderline or mild) adj3 diabet*).tw,ot.
16 insulin resistan*.tw,ot.
17 ((impaired or reduced) adj3 insulin secret*).tw,ot.
18 or/4‐17
19 3 and 18 [20‐30:Lefebvre 2011RCT filter ‐ max. sensitivity version] 20 randomized controlled trial.pt. 21 controlled clinical trial.pt. 22 randomized.ab. 23 placebo.ab. 24 randomly.ab. 25 drug therapy.fs. 26 trial.ab. 27 groups.ab. 28 or/20‐27 29 exp animals/ not humans/ 30 28 not 29 31 19 and 30 |
| EMBASE (via Ovid SP) |
| 1 exp zinc/ae, do, dt, th [Adverse Drug Reaction, Drug Dose, Drug Therapy, Therapy]
2 (zinc adj3 supplement*).tw,ot.
3 1 or 2
4 exp glucose tolerance test/
5 exp glucose intolerance/
6 exp non insulin dependent diabetes mellitus/pc [Prevention]
7 exp insulin resistance/
8 exp metabolic syndrome X/
9 exp impaired glucose tolerance/
10 (glucose adj3 (intolerance or tolerance test*)).tw,ot.
11 (impaired fasting adj3 (glucose or glyc?emia*)).tw,ot.
12 (impaired glucose adj3 (toleran* or stat* or respons* or control* or regul* or metab* or homeost*)).tw,ot.
13 (reduced glucose adj3 (metab* or toleran*)).tw,ot.
14 (pr?e diabet* or pr?ediabet*).tw,ot.
15 (metabolic syndrom* or syndrom* X).tw,ot.
16 ((borderline or mild) adj3 diabet*).tw,ot.
17 insulin resistan*.tw,ot.
18 ((impaired or reduced) adj3 insulin secret*).tw,ot.
19 or/4‐18
20 3 and 19 [21:Wong 2006"treatment studies" filter ‐ SDSSGS version] 21 random*.tw. or clinical trial*.mp. or exp treatment outcome/ 22 20 and 21 |
| LILACS (via IAHx) |
| zinc AND diabet* |
| ICTRP Search Platform (Standard search) |
| [searched as one string] zinc* AND glucose OR zinc* AND diabet* OR zinc* AND insulin OR zinc* AND metabol* OR zinc* AND prediabet* OR zinc* AND glycemia OR zinc* AND glycaemia |
Appendix 2. Description of interventions
| Intervention(s) [route, frequency, total dose/day] |
Adequatea intervention [Yes/No] |
Comparator(s) [route, frequency, total dose/day] |
Adequatea comparator [Yes/No] |
|
| Gómez‐García 2006 | 100 mg/day zinc sulfate orally | Yes | Placebo | Yes |
| Marreiro 2006 | 30 mg/day zinc amino chelate | Yes | Placebo | Yes |
| Soheilykhah 2012 | 50 mg/day zinc sulfate orally | Yes | Regular exercise and weight control | Yes |
| aThe term 'adequate' refers to sufficient use of the intervention/comparator with regard to dose, dose escalation, dosing scheme, provision for contraindications and other features necessary to establish a fair contrast between intervention and comparator | ||||
Appendix 3. Baseline characteristics (I)
| Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) | Description of participants | Study period [year to year] | Country | Setting | Ethnic groups [%] | Duration of condition [mean years (SD)] | |
| Gómez‐García 2006 | I: zinc | 1 month (1 month) |
Obese men | ‐ | Mexico | Medical research unit | ‐ | ‐ |
| C: placebo | ||||||||
| Marreiro 2006 | I: zinc | 1 month (1 month) |
Obese women | ‐ | Brazil | Outpatients (hospital clinics) | ‐ | ‐ |
| C: placebo | ||||||||
| Soheilykhah 2012 | I: zinc | 12 weeks (12 weeks) |
Overweight or obese participants | 2009 to 2010 | Iran | Yazd Diabetes Research Center | ‐ | ‐ |
| C: regular exercise and weight control | ||||||||
| "‐" denotes not reported C: comparator; I: intervention; SD: standard deviation | ||||||||
Appendix 4. Baseline characteristics (II)
| Intervention(s) and comparator(s) | Sex [female %] | Age [mean/range years (SD), or as reported] | HbA1c [%] | BMI [mean kg/m² (SD)] | Co‐medications/ Co‐interventions | Comorbidities | |
| Gómez‐García 2006 | I: zinc | 0 | 21.8 (2.8) | ‐ | 30.7 (2.6) | No co‐medications/co‐interventions | Obesity |
| C: placebo | 0 | 25.1 (4.5) | ‐ | 30.5 (3.9) | |||
| Marreiro 2006 | I: zinc | 100 | 35.5 (6.5) | ‐ | 35.8 (2.2) | No co‐medications/co‐interventions | Obesity |
| C: placebo | 100 | 33.9 (5.4) | ‐ | 36.5 (2.5) | |||
| Soheilykhah 2012 | I: zinc | 57a | 37.6 (7.4) | ‐ | 28.8 (3.5) | No co‐medications/co‐interventions | BMI more than 25 kg/m² with normal 75 g OGTT |
| C: regular exercise and weight control | |||||||
| "‐" denotes not reported aRefers to 53/58 participants completing the study BMI: body mass index; C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; OGTT: oral glucose tolerance test; SD: standard deviation | |||||||
Appendix 5. Matrix of study endpoints (publications and trial documents)
| Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c | |
| Gómez‐García 2006 | Source: N/T |
Primary outcome measure(s): ‐ |
Primary outcome measure(s): ‐ |
|
Secondary outcome measure(s): ‐ |
Secondary outcome measure(s): ‐ |
||
|
Other outcome measure(s): Insulin sensitivity, leptin levels, biochemical profile and androgens |
Other outcome measure(s): Insulin sensitivity, leptin levels, biochemical profile and androgens |
||
| Marreiro 2006 | Source: N/T |
Primary outcome measure(s): ‐ |
Primary outcome measure(s): ‐ |
|
Secondary outcome measure(s): ‐ |
Secondary outcome measure(s): ‐ |
||
|
Other outcome measure(s): BMI, fasting glucose and zinc concentration in plasma, insulin, HOMA‐IR, leptin, waist circumference, waist‐to‐hip ratio, percentage of fat |
Other outcome measure(s): BMI, fasting glucose, zinc concentration in plasma, urine and erythrocytes, insulin, HOMA‐IR, leptin, glucose |
||
| Soheilykhah 2012 | Source: N/T |
Primary outcome measure(s): ‐ |
Primary outcome measure(s): ‐ |
|
Secondary outcome measure(s): ‐ |
Secondary outcome measure(s): ‐ |
||
|
Other outcome measure(s): Adiponectin, fasting blood glucose, insulin and insulin resistance and lipid profile |
Other outcome measure(s): Adiponectin, fasting blood glucose, insulin and insulin resistance and lipid profile |
||
| ‐ denotes not reported aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers). bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary study). cOther outcome measures refer to all outcomes not specified as primary or secondary outcome measures. BMI: body mass index; EMA: European Medicines Agency; FDA: Food and Drug Administration (US); HOMA‐IR: Homeostasis Model Assessment of Insulin Resistance; N/A: not applicable; N/T: no trial document available | |||
Appendix 6. Examination of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d | |
| Gómez‐García 2006 | N/A | N/A | N/A | N/A | N/A |
| Marreiro 2006 | N/A | N/A | N/A | N/A | N/A |
| Soheilykhah 2012 | N/A | N/A | N/A | N/A | N/A |
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but only reports that result was not significant (Classification 'A', table 2, Kirkham 2010).
bClear that outcome was measured and analysed; trial report states that outcome was analysed but no results reported (Classification 'D', table 2, Kirkham 2010).
cClear that outcome was measured; clear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results (Classification 'E', table 2, Kirkham 2010).
dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results (Classification 'G', table 2, Kirkham 2010). N/A: not applicable | |||||
Appendix 7. Definition of endpoint measurement
| Incidence of type 2 diabetes mellitus | Insulin resistance | Diabetic complications | All‐cause mortality | Health‐related quality of life | Socioeconomic effects | Lipid levels | Severe/serious adverse events | |
| Gómez‐García 2006 | N/I | Insulin sensitivity measured by the glucose clamp technique | N/I | N/I | N/I | N/I | Total cholesterol, HDL cholesterol, triglycerides measured by enzymatic methods; LDL cholesterol was estimated by the Friedewald formula and the very low density lipoproteins were calculated by triglycerides/5 | N/I |
| Marreiro 2006 | N/I | HOMA‐IR | N/I | N/I | N/I | N/I | N/I | N/I |
| Soheilykhah 2012 | N/I | HOMA‐IR | N/I | N/I | N/I | N/I | Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides | N/I |
| HOMA‐IR: Homeostasis Model Assessment of Insulin Resistance; N/I: not investigated | ||||||||
Appendix 8. Adverse events (I)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Deaths [N] | Deaths [%] | All adverse events [N] | All adverse events [%] | Severe/serious adverse events [N] | Severe/serious adverse events [%] | |
| Gómez‐García 2006 | I: zinc | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Marreiro 2006 | I: zinc | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Soheilykhah 2012 | I: zinc | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| "‐" denotes not reported C: comparator; I: intervention | ||||||||
Appendix 9. Adverse events (II)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Discontinued study due to adverse events [N] | Discontinued study due to adverse events [%] | Hospitalisation [N] | Hospitalisation [%] | Outpatient treatment [N] | Outpatient treatment [%] | |
| Gómez‐García 2006 | I: zinc | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Marreiro 2006 | I: zinc | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Soheilykhah 2012 | I: zinc | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| "‐" denotes not reported C: comparator; I: intervention | ||||||||
Appendix 10. Adverse events (III)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Specific adverse events [description] | Specific adverse events [N participants] | Specific adverse events [% participants] | |
| Gómez‐García 2006 | I: zinc | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | |
| Marreiro 2006 | I: zinc | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | |
| Soheilykhah 2012 | I: zinc | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | |
| "‐" denotes not reported C: comparator; I: intervention | |||||
Appendix 11. Survey of authors providing information on included trials
| Study author contacted | Study author replied | Study author asked for additional information [short summary] | Study author provided data [short summary] | |
| Gómez‐García 2006 | 16 April 2015 | 18 April 2015 |
|
|
| Marreiro 2006 | 16 April 2015 | We are awaiting a reply | N/A | N/A |
| Soheilykhah 2012 | 16 April 2015 | We are awaiting a reply | N/A | N/A |
| N/A: not applicable; RCT: randomised controlled trial | ||||
Data and analyses
Comparison 1. Zinc supplementation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HOMA‐IR | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 HOMA‐IR | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Lipids | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Total cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 HDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 HDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 LDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 LDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 VLDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 VLDL lipoprotein | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Triglycerides | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Triglycerides | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Zinc supplementation versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HOMA‐IR | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Lipids | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 HDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 LDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Triglycerides | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
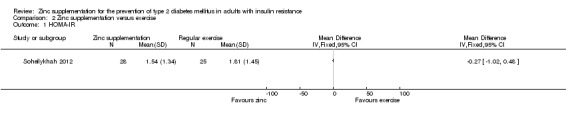
Comparison 2 Zinc supplementation versus exercise, Outcome 1 HOMA‐IR.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gómez‐García 2006.
| Methods |
Design: single‐centre, randomised, triple‐blind, placebo‐controlled clinical trial (information provided by contact with author) Setting: Medical Research Unit in Clinical Epidemiology, Hospital of Specialties, West National Medical Center of the Mexican Institute of Social Security, Guadalajara, Mexico (information provided by contact with author) Sample size: the sample size was calculated with a formula for clinical trials with a confidence level of 95% power to test a standard deviation of 80% of the euglycaemic hyperinsulinaemic clamp 0.8 mg/Kg/min and an expected difference of at least twice the standard deviation, so that a score of 6 participants per group was obtained; due to the possibility of loss during the follow‐up, 7 participants were included per group Intention‐to‐treat analysis: "We contemplated an intention‐to‐treat analysis, however, no subject had to be excluded from the study" [information retrieved by contact with the study authors] Follow‐up: 1 month |
|
| Participants |
N = 14 Inclusion criteria: participants included 14 obese men aged between 21 to 30 years old (body mass index (BMI) 27 kg/m²). Also, these volunteers had first degree relatives with diabetes mellitus type 2 and sustained a stable body weight for at least 3 months before the beginning of the study Exclusion criteria: Quote from contact with the study author by email: "The exclusion criterion was the intake of any medication with effect on the metabolism of carbohydrates, lipids, zinc, or insulin during the intervention. However, no subject had to be excluded from the study" Diagnostic criteria: insulin sensitivity measured by the glucose clamp method |
|
| Interventions |
Number of study centres: 1 Treatment before study: Quote from contact with the study author by email: "The volunteers did not ingest any treatment at least in the month preceding the study" Intervention and control groups: 100 mg/day of zinc sulfate orally (n = 7) versus placebo (n = 7) |
|
| Outcomes | Outcomes reported in abstract of publication: insulin sensitivity, leptin levels, biochemical profile and androgens | |
| Study details |
Run‐in period: 1 month Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: Spanish Commercial funding/non‐commercial funding/other funding: Fondo de Fomento a la Investigacion del Insituto Mexicano del Seguro Social ‐ Fund of Scientific Support of The Mexican Institute of Social Security Publication status: journal article |
|
| Stated aim for study | Quote from publication: "To assess the effects of zinc sulfate on insulin sensitivity, leptin and androgens in obese individuals" | |
| Notes | We contacted the author on 16 April 2015 to request information about risk of bias. The author kindly replied with all the information requested on 18 April 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "La aleatorización de los participantes para recibir sulfato de zinc o placebo se realizó mediante el azar simple" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "...con la selección de un sobre cerrado que contenía la opción A o B." Quote from contact with the study author by email: "We used opaque and sealed envelopes" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote from publication: "un ensayo clínico al azar, doble ciego, controlado con placebo" Comment: the study drugs were double‐blinded to both participants and investigators |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote from publication: "un ensayo clínico al azar, doble ciego, controlado con placebo" Comment: the study drugs were double‐blinded to both participants and investigators |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from contact with author by email: "The study was triple blind, the patients received one capsule of 100 mg/day of zinc sulfate orally daily or placebo capsule approved with identical presentation. Neither the researchers, nor the patients nor the person who performed the statistical analyses knew which group participants were allocated." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote from contact with author by email: "The study was triple blind, the patients received one capsule of 100 mg/day of zinc sulfate orally daily or placebo capsule approved with identical presentation. Neither the researchers, nor the patients nor the person who performed the statistical analyses knew which group participants were allocated." Quote from contact with the study author by email: "The blinding of the outcome assessors was for both objective and subjective outcomes" |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: all participants completed the study |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: all participants completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Quote from contact with author by email: "There was no conflict of interest." |
Marreiro 2006.
| Methods |
Design: randomised, double‐blind, placebo‐controlled study Setting: outpatient (hospital clinics) Intention‐to‐treat analysis: not reported Follow‐up: 4 weeks |
|
| Participants |
N = 56 Inclusion criteria: age ranging between 25 and 45 years, obesity class I and II, absence of vitamin and mineral supplementation and/or use other medication that could interfere with zinc and insulin action, nonsmokers, absence of hormone replacement therapy and/or use of oral contraceptives, and without diseases that could interfere with the nutritional status regarding zinc and insulin resistance, such as type 2 diabetes, polycystic ovary syndrome, hypertension or chronic kidney failure Exclusion criteria: not reported Diagnostic criteria: determination of insulin resistance was performed using the Homeostasis Model Assessment (HOMA) model as proposed by Matthews and co‐workers |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Intervention and control groups: participants received oral zinc consisted 30 mg per day for 4 weeks (n = 28) or placebo (n = 28) |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, fasting glucose, zinc concentration in plasma, insulin, HOMA‐IR, and leptin | |
| Study details |
Run‐in period: 4 weeks Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Commercial funding/non‐commercial funding/other funding: not reported |
|
| Stated aim for study | Quote from publication: "This study was conducted with the purpose to evaluating the effect of zinc supplementation on leptin levels in obese women" | |
| Notes | There is a further unpublished manuscript reporting complementary outcomes We contacted the author on 16 April 2015 to request information about risk of bias. We are awaiting a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote from publication: "Both of the groups received pills identified by color...However, neither the researchers nor the patients knew which pill contained zinc." Comment: the study drugs were double‐blinded to both participants and investigators |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote from publication: "Both of the groups received pills identified by color...However, neither the researchers nor the patients knew which pill contained zinc." Comment: the study drugs were double‐blinded to both participants and investigators |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not reported |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: all participants completed the study |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: all participants completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
Soheilykhah 2012.
| Methods |
Design: randomised clinical trial Setting: not reported Intention‐to‐treat analysis: not reported Follow‐up: 12 weeks |
|
| Participants |
N = 53 Inclusion criteria: more than 25 years and BMI more than 25 kg/m² with normal 75 g oral glucose tolerance test Exclusion criteria: first degree relatives of participants who consumed vitamin D or magnesium supplementation in the past 6 months Diagnostic criteria: prediabetic participants |
|
| Interventions |
Number of study centres: 1 Treatment before study: none reported Intervention and control groups: 50 mg/day of oral zinc sulfate (n = 28) versus control group with regular exercise and weight control (n = 25) |
|
| Outcomes |
Outcomes reported in abstract of publication Adiponectin, fasting blood glucose, insulin and insulin resistance, and lipid profile |
|
| Study details |
Run‐in period: 4 weeks Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Commercial funding/non‐commercial funding/other funding: Yazd Diabetes Research Center |
|
| Stated aim for study | Quote from publication: "This study was conducted to assess the effect of zinc supplementation on serum adiponectin and insulin resistance in first degree relatives of diabetic patients." | |
| Notes | We contacted the author on 16 April 2015 to request information about risk of bias. We are awaiting a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not reported |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote from publication: "In this randomized clinical control study 58 subjects from first degree relatives of diabetic patients...of these, 53 subjects." Comment: low attrition rate with probably only minor influence on outcome measures |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote from publication: "In this randomized clinical control study 58 subjects from first degree relatives of diabetic patients...of these, 53 subjects." Comment: low attrition rate with probably only minor influence on outcome measures |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
BMI: body mass index HOMA‐IR: Homeostasis Model Assessment of Insulin Resistance
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Czernichow 2006 | RCT, but evaluated vitamin C, vitamin E, ß‐carotene, selenium, and zinc or placebo |
| Czernichow 2009 | RCT, but evaluated a combination of antioxidants (vitamins C and E, ß‐carotene, zinc and selenium) at nutritional doses or a placebo in metabolic syndrome |
| Islam 2013 | Cross‐sectional study |
| Kelishadi 2010 | RCT, but only evaluated children |
| Kim 2012 | Controlled clinical trial |
| Marchesini 1998 | Case series study |
| Marreiro 2002 | Controlled clinical trial and only evaluated children and adolescents |
| Ramos 2007 | Controlled clinical trial |
| Stewart 2011 | RCT, but evaluated vitamin B12 |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Ranasinghe 2013.
| Trial name or title | Zinc supplementation in pre‐diabetes: study protocol for a randomised controlled trial |
| Methods |
Design: single‐centre, randomised, double‐blind, placebo‐controlled clinical trial Setting: Faculty of Medicine, University of Colombo, Sri Lanka Sample size: the number of participants required for determination of a 20% reduction of fasting plasma glucose in the treatment arm in comparison with the placebo arm at 90% power and 95% confidence interval with a dropout rate of 30% is 100 patients per arm. Hence, a total of 200 participants with prediabetes will be recruited for the study Intention‐to‐treat analysis: not reported Follow‐up: 12 months |
| Participants |
Inclusion criteria: both genders between the ages of 18 and 60 years, eligible for the study through a screening test confirming the presence of prediabetes. Prediabetes is defined as the presence of fasting plasma glucose levels between 110 and 125 mg/dL or 2‐hour post‐oral glucose plasma glucose levels between 140 and 199 mg/dL, or both Exclusion criteria: on any other vitamin or mineral supplementation or the current use of a weight loss medicine or dietary modification; history of diabetes mellitus or any metabolic disease; alcohol consumption > 20 g/day; presently having acute diseases or other untreated illness requiring treatment; impaired hepatic or renal functions; lactation, pregnancy or unwillingness to use an effective form of birth control for women of child‐bearing years; history or presence of any condition that would endanger the individual's safety or affect the study result |
| Interventions |
Number of study centres: 1 Treatment before study: none Titration period: none The treatment drug is a capsule containing elemental zinc 20 mg as the active ingredient; the placebo had a similar appearance, shape, weight, taste and colour as the zinc 20 mg capsule; the participants receive either 1 capsule of 20 mg zinc or an identical placebo daily, taken 1 hour before breakfast for a period of 12 months |
| Outcomes |
Primary outcomes: the following biochemical assessments will be done at baseline, at stated intervals and at completion: fasting plasma glucose, oral glucose tolerance test, serum insulin, HbA1c, total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, and serum zinc. Furthermore, appetite will be evaluated using a visual analogue scale (VAS) Secondary outcomes: measurement of systolic (SBP) and diastolic blood pressure (DBP), anthropometric assessment such as body weight, height, body mass index (BMI), waist circumference (WC), hip circumference (HC) and waist:hip ratio (WHR), dietary assessment using a validated food frequency questionnaire (FFQ) |
| Starting date | Not reported |
| Contact information | Dr Priyanga Ranasinghe: priyanga.ranasinghe@gmail.com |
| Notes | Trial registration: Sri Lanka Clinical Trial Registry: SLCTR/2012/010 |
HDL: high‐density lipoprotein LDL: low‐density lipoprotein
Differences between protocol and review
This review is an update of the previous Cochrane systematic review, which included one RCT (Beletate 2007).
The previous author Álvaro Atallah decided not to participate in the update of the review. For this update the following new authors contributed: Luis Felipe Orsi Gameiro, Matheus Senna Pereira Ogata, Norma Sueli Pinheiro Modolo, Leandro Gobbo Braz, Eliane C Jorge and Paulo do Nascimento Junior.
We updated the Methods section (added the 'Summary of findings' table and the new 'Risk of bias' assessment). We changed adverse events from a secondary to a primary outcome measure.
Contributions of authors
Regina El Dib (RED): co‐ordinating the review, data management, interpretation of data, writing the review, securing funding for the review, guarantor for the review, responsible for reading and checking the review before submission.
Luis Felipe Orsi Gameiro (LFOG): undertaking manual searches, screening search results, organising retrieval of publications, screening retrieved publications against inclusion criteria, appraising quality of publications, abstracting data from publications, obtaining and screening data on unpublished studies, data management for the review, entering data into Review Manager, double entry of data.
Matheus SP Ogata (MSPO): providing additional data about publications, abstracting data from publications, obtaining and screening data on unpublished studies, entering data into Review Manager, double entry of data.
Norma SP Módolo (NSPM): responsible for reading and checking the review before submission.
Leandro G Braz (LGB): responsible for reading and checking the review before submission.
Eliane C Jorge: responsible for reading and checking the review before submission.
Paulo do Nascimento Junior (PNJ): responsible for reading and checking the review before submission.
Vânia Beletate (VB): conceiving the review.
Sources of support
Internal sources
Brazilian Cochrane Center, Brazil.
External sources
No sources of support supplied
Declarations of interest
RED: none known.
LFOG: none known.
MSPO: none known.
NSPM: none known.
LGB: none known.
ECJ: none known.
PNJ: none known.
VB: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Gómez‐García 2006 {published data only}
- Gómez‐García A, Hernández‐Salazar E, González‐Ortiz M, Martínez‐Abundis E. Effect of oral zinc administration on insulin sensitivity, leptin and androgens in obese males. Revista Medica de Chile 2006;134:279‐84. [DOI] [PubMed] [Google Scholar]
Marreiro 2006 {published data only}
- Marreiro DN. The effect of zinc supplementation in insulin resistance in obesity women. [Efeito da suplementação com zinco na resistência à insulina em mulheres obesas]. Tese de doutorado ‐ Faculdade de Ciências Farmacêuticas ‐ USP, São Paulo, Brazil. São Paulo, 2002:109p..
- Marreiro DN, Geloneze B, Tambascia M, Lerário A, Halpern A, Cozzolino SM. Effect of zinc supplementation on serum leptin levels and insulin resistance of obese women. Biological Trace Element Research 2006;112:109‐18. [DOI] [PubMed] [Google Scholar]
Soheilykhah 2012 {published data only}
- Soheilykhah S, Dehestani M, Mohammadi S, Afkhami‐Ardeni M, Eghbali S, Dehghan F. The effect of zinc supplementation on serum adiponectin concentration and insulin resistance in first degree relatives of diabetic patients. Iranian Journal of Diabetes and Obesity 2012;4(2):57‐62. [Google Scholar]
References to studies excluded from this review
Czernichow 2006 {published data only}
- Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P, et al. Antioxidant supplementation does not affect fasting plasma glucose in the supplementation with antioxidant vitamins and minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. American Journal of Clinical Nutrition 2006;84:395‐9. [DOI] [PubMed] [Google Scholar]
Czernichow 2009 {published data only}
- Czernichow S, Vergnaud AC, Galan P, Arnaud J, Favier A, Faure H, et al. Effects of long‐term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Journal of Clinical Nutrition 2009;90:329‐35. [DOI] [PubMed] [Google Scholar]
Islam 2013 {published data only}
- Islam R, Arslan I, Attia J, McEvoy M, McElduff P, Basher A, et al. Is serum zinc level associated with prediabetes and diabetes?: A cross‐sectional study from Bangladesh. PloS One 2013;8(4):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelishadi 2010 {published data only}
- Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian‐Attar A, Shapouri J, et al. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metabolic Syndrome and Related Disorders 2010;8(6):1‐6. [DOI: 10.1089/met.2010.0020] [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim J, Lee S. Effect of zinc supplementation on insulin resistance and metabolic risk factors in obese Korean women. Nutrition Research and Practice 2012;6(3):221‐5. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchesini 1998 {published data only}
- Marchesini G, Bugianesi E, Ronchi M, Flamia R, Thomaseth K, Pacini G. Zinc supplementation improves glucose disposal in patients with cirrhosis. Metabolism Baltimore 1998;47:792‐8. [DOI] [PubMed] [Google Scholar]
Marreiro 2002 {published data only}
- Marreiro DN, Fisberg M, Cozzolino SM. Zinc nutritional status in obese children and adolescents. Biological Trace Element Research 2002;86(2):107‐22. [DOI] [PubMed] [Google Scholar]
Ramos 2007 {published data only}
- Ramos RI, Atalah E, Urteaga C, Castañeda R, Orozco M, Avila L, et al. Effect of the consumption of a food supplement on plasma zinc concentrations of free‐living Chilean elderly adults. Revista Medica de Chile 2007;135:1015‐24. [DOI] [PubMed] [Google Scholar]
Stewart 2011 {published data only}
- Stewart C, Christian P, Schulze K, Arguello M, LeClerq S, Khatry S, et al. Low maternal vitamin B‐12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. Journal of Nutrition 2011;141:1912‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Ranasinghe 2013 {published data only}
- Ranansinghe P, Jayawardena R, Pigera A, Katulanda P, Constantine G, Galappaththy P. Zinc supplementation in pre‐diabetes: study protocol for a randomized controlled trial. Trials 2013;14(52):2‐6. [DOI: 10.1186/1745-6215-14-52] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ADA 1997
- American Diabetes Association. Report on the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20(Suppl 1):S5–20. [DOI] [PubMed] [Google Scholar]
ADA 1998
- American Diabetes Association. Screening for type 2 diabetes. Diabetes Care 1998;22(Suppl 1):S20‐3. [Google Scholar]
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22(Suppl 1):S5‐19. [DOI] [PubMed] [Google Scholar]
ADA 2003
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5–20. [DOI] [PubMed] [Google Scholar]
Beller 2013
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2(1):36. [2046‐4053: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonora 1998
- Bonora E, Kiechi E, Willeit J, Oberhollenzer F, Egger G, Targher G, et al. Prevalence of insulin resistance in metabolic disorders. Diabetes 1998;47:1643‐9. [DOI] [PubMed] [Google Scholar]
Bonora 2000
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 2000;23(1):57‐63. [DOI] [PubMed] [Google Scholar]
Capdor 2013
- Capdor J, Foster M, Petocz P, Samman S. Zinc and glycemic control: a meta‐analysis of randomised placebo controlled supplementation trials in humans. Journal of Trace Elements in Medicine and Biology 2013;27:137‐42. [DOI] [PubMed] [Google Scholar]
Chausmer 1998
- Chausmer AB. Zinc, insulin and diabetes. Journal of the American College of Nutrition 1998;17(2):109‐15. [DOI] [PubMed] [Google Scholar]
Danaei 2011
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2.7 million participants. Lancet 2011;378:31‐40. [DOI] [PubMed] [Google Scholar]
DeFronzo 1979
- DeFronzo RA, Tobin JD, Andres R. Glucose Clamp technique: a method for quantifying insulin secretion and resistance. American Journal of Physiology 1979;237:E214‐23. [DOI] [PubMed] [Google Scholar]
Haase 2005
- Haase H, Maret W. Protein tyrosine phosphatases as targets of the combined insulinomimetic effects of zinc and oxidants. Biometals 2005;18(4):333‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:555‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 1996
- Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, et al. Insulin sensitivity and atherosclerosis: the Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation 1996;93:1809‐17. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahn 2000
- Kahn BB, Flier JS. Obesity and insulin resistance. Journal of Clinical Investigation 2000;106(4):473‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karelis 2004
- Karelis AD, Henry JF, Malita F, St‐Pierre DH, Vigneault I, Poehlman ET, et al. Comparison of insulin sensitivity values using the hyperinsulinemic euglycemic clamp: 2 vs 3 hours. Diabetes & Metabolism 2004;30:413‐4. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marreiro 2004
- Marreiro DN, Geloneze B, Tambascia MA, Lerário AC, Halpern A, Cozzolino SMF. Participation of zinc in insulin resistance [Participaçäo do zinco na resistência à insulina]. Arquivos brasileiros de endocrinologia e metabologia 2004;48(2):234‐9. [DOI] [PubMed] [Google Scholar]
Matthews 1985
- Matthews DR, Hosker JP, Rudenski BA, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412‐9. [DOI] [PubMed] [Google Scholar]
Moro 2003
- Moro E, Gallina P, Pais M, Cazzolato G, Alessandrini P, Bittolo‐Bon G. Hypertriglyceridemia is associated with increased insulin resistance in subjects assessed with the 1999 World Health Organization criteria for the classification of diabetes. Metabolism: Clinical and Experimental 2003;52(5):616‐9. [DOI] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Rossetti 1990
- Rossetti L, Giaccari A, Klein‐Robbenhaar E, Vogel LR. Insulinomimetic properties of trace elements and characterization of their in vivo mode of action. Diabetes 1990;39:1243‐50. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Tracey 2003
- Tracey L, Mclaughlin MD, Gerald M, Reaven MD. Beyond type 2 diabetes: The need for a clinically useful way to identify insulin resistance. American Journal of Medicine 2003;114:501‐2. [DOI] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second Report. Technical Report Series 646. Geneva: World Health Organization 1980. [PubMed]
WHO 1985
- WHO Expert Committee on Diabetes Mellitus. Technical Report Series 727. Geneva: World Health Organization 1985. [PubMed]
WHO 1999
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization 1999.
WHO 2009
- World Health Organization. Global health risks. Mortality and burden of disease attributable to selected major risks. World Health Organization 2009.
WHO 2011
- World Health Organization. Global status report on noncommunicable diseases 2010. World Health Organization 2011.
Wong 2006
- Wong SSL, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Beletate 2007
- Beletate V, Dib RP, Atallah AN. Zinc supplementation for the prevention of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005525.pub2] [DOI] [PubMed] [Google Scholar]


