Abstract
Schwann cells in the peripheral nervous system (PNS) are essential for the support and myelination of axons, ensuring fast and accurate communication between the central nervous system and the periphery. Schwann cells and related glia accompany innervating axons in virtually all tissues in the body, where they exhibit remarkable plasticity and the ability to modulate pathology in extraordinary, and sometimes surprising, ways. Here, we provide a brief overview of the various glial cell types in the PNS and describe the cornerstone cellular and molecular processes that enable Schwann cells to perform their canonical functions. We then dive into discussing exciting noncanonical functions of Schwann cells and related PNS glia, which include their role in organizing the PNS, in regulating synaptic activity and pain, in modulating immunity, in providing a pool of stem cells for different organs, and, finally, in influencing cancer.
Keywords: Schwann cells, satellite cells, boundary cap cells, terminal Schwann cells
1. DEVELOPMENT AND DIVERSITY OF SCHWANN CELLS
The peripheral nervous system (PNS) conveys bidirectional information between the central nervous system (CNS) and the periphery, thus connecting organisms to the external world. The information is transmitted through axons derived from central and peripheral neurons in cooperation with other cells, including barrier- and structure-forming endoneurial, perineurial, and epineurial cells and vascular, immune, and glial cells, which associate intimately with axons and perikarya (Gerber et al. 2021).
The main glial cells in the PNS are axon-associated Schwann cells (SCs) in nerves, synapse-associated SCs, and perikaryon-associated satellite cells in ganglia. Together, they cover most of the functions that oligodendrocytes, microglia, and astrocytes perform in the CNS. SCs are more plastic that CNS glia and maintain the ability to de-/transdifferentiate, even when terminally differentiated. This unique ability provides greater adaptability to stress, disease, and injury, endowing the PNS with great resilience and the capacity for regeneration and repair.
All SCs derive from the neural crest and accompany migrating axons, surrounding their growth cones (Wanner et al. 2006). One of the earliest subpopulations of SCs are boundary cap cells (BCCs), which transiently congregate at the boundary between the CNS and PNS (see below). Later, BCCs migrate along motor and sensory roots and give rise to neurons in sensory ganglia and to most of the SCs in spinal roots. BCCs and neural crest cells also differentiate into satellite cells that surround the somas of sensory and autonomic neurons.
Trunk neural crest cells that migrate ventrally give rise to SCs that surround motor and sensory axons in peripheral nerves (Le Douarin & Teillet 1974) by first generating Schwann cell precursors (SCPs), which differentiate into immature SCs (Figure 1). Immature SCs deposit a basal lamina and begin to separate axons into progressively smaller groups; they first select and sort single large axons (e.g., motor and fusimotor fibers) that become myelinated by myelin-forming SCs, while smaller axons (e.g., afferent fibers to skin, pain fibers, and postganglionic autonomic axons) remain associated with SCs, forming a so-called Remak bundle (reviewed by Jessen et al. 2015).
Figure 1.
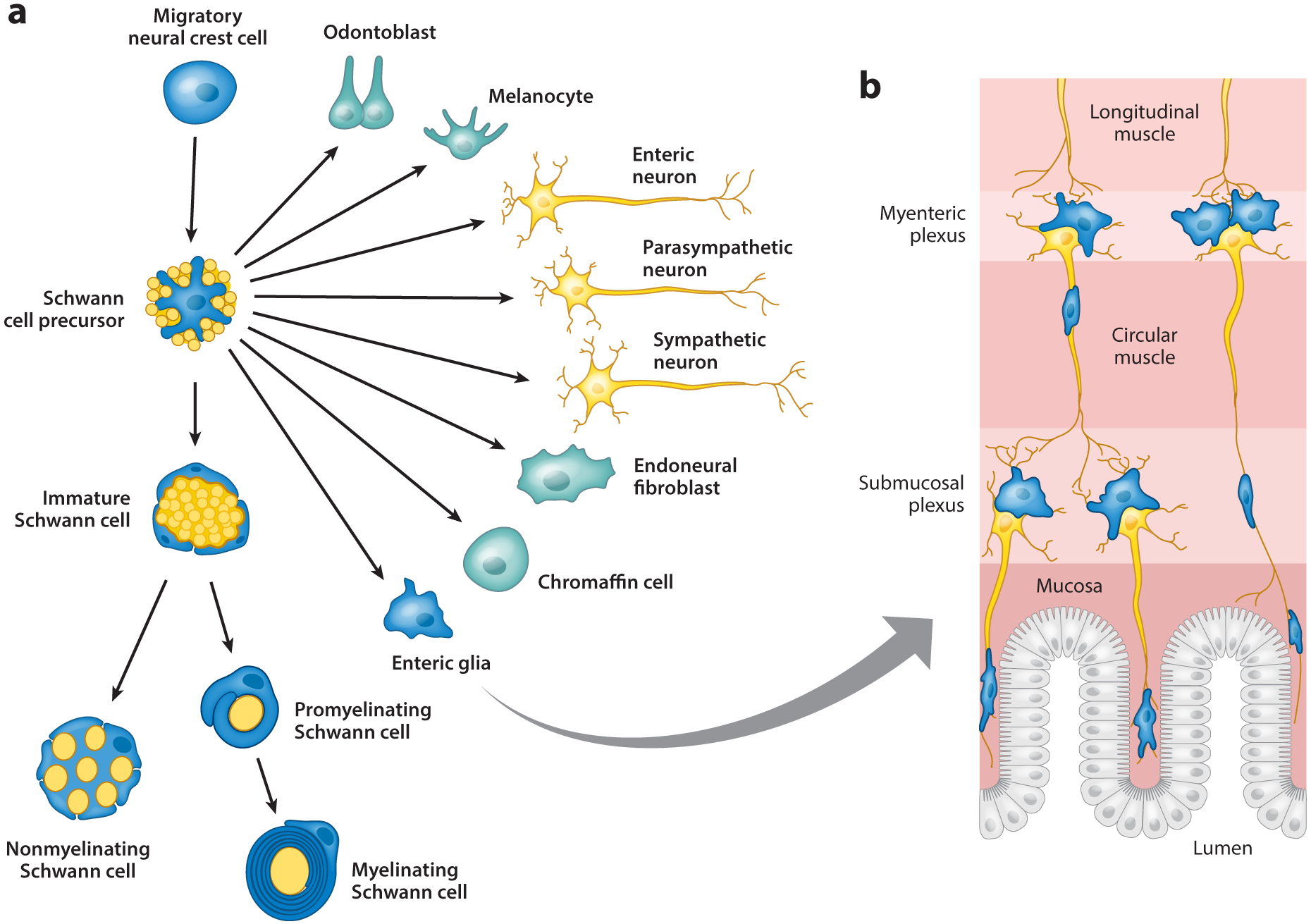
Schwann cells and Schwann cell precursors are endowed with significant plasticity. (a) Schwann cell precursors derive from the neural crest and maintain some of the neural crest’s multipotency. They can act as multipotent progenitors for several glia and nonglial cell types such as odontoblasts, melanocytes, autonomic neurons, chondrocytes, endoneurial fibroblasts, chromaffin cells, and enteric glia. Enteric glia are shown in detail in panel b. Schwann cell precursors’ main function is to develop into immature and then myelinating or nonmyelinating Schwann cells, which fulfill the canonical functions of axonal ensheathment and myelination. In a process known as axonal sorting, large axons that are destined to be myelinated, such as motor axons or sensory axons that transmit positional information, are segregated by immature Schwann cells from axon bundles into a 1:1 Schwann cell relationship (promyelinating Schwann cells) and finally wrapped by multiple layers of membrane to form the myelin sheath. Nonmyelinating Schwann cells form Remak bundles by ensheathing the small-caliber axons (e.g., sensory axons that transmit pain and temperature information) that remain after the large axons have been sorted out.
Neural crest cells that migrate with olfactory nerve fibers give rise to a specialized glial population known as olfactory ensheathing cells (reviewed in Barnett & Chang 2004), which share some molecular characteristics with SCs but do not deposit a basal lamina and do not make myelin. However, olfactory ensheathing cells are extremely plastic and regenerative, as they can myelinate CNS axons after transplantation and contribute to the unique renewal capacity of the olfactory nervous system. Neural crest cells also generate neurons of the enteric ganglia and their axons, which organize into enteric plexi. These plexi are ensheathed by enteric glia, which share many characteristics with Remak SCs (Figure 1) (reviewed in Veiga-Fernandes & Pachnis 2017).
At synapses, SCs specialize to perform different functions, such as the terminal or perisynaptic SCs at the neuromuscular junction (NMJ) (see below). SCs of the skin also originate from the neural crest and are associated with subepidermal nerve plexi and specialized nerve endings.
Three recent articles have illuminated our understanding of peripheral glial diversity and nerve cellular composition, and confirmed the developmental origins and lineage paths of glia (Gerber et al. 2021, Tasdemir-Yilmaz et al. 2021, Yim et al. 2022). These studies indicate that satellite glial cells from dorsal root ganglia (DRG) and auditory ganglia are regionally heterogenous and that there are at least three subtypes of trunk SCs. For a more detailed description of peripheral glial diversity, please refer to Reed et al. (2021).
2. CANONICAL FUNCTIONS OF SCHWANN CELLS
2.1. Axonal Ensheathment and Support
One of the most important functions of SCs is to support axons. Axons can extend to more than 1 meter in length. This remarkable distance from their cell bodies makes them vulnerable and dependent on axonal transport to survive. Indeed, axon degeneration is an early event of many neurological diseases. SCs are evenly distributed along the entire length of the axon and are therefore perfectly poised to provide local structural and metabolic support to axons, similar to oligodendrocytes in the CNS. This support function is evolutionarily conserved (Nave & Werner 2021), is independent from myelination, and relies on the intimate interaction between SCs and axons. Presumably, SCs in close contact with axons sense their metabolic status. Although the molecular mechanisms that mediate this sensing and energy delivery are still largely unknown, they are the subject of intense investigation, and some aspects of this crucial function are starting to emerge. Earlier investigations indicated that proteins such as myelin-associated protein (MAG), proteolipid protein, and CNPase on the inner SC membrane engage axonal receptors to mediate some of these exchanges (Edgar et al. 2009, Griffiths et al. 1998, Yin et al. 1998). However, the corresponding receptor(s) on the axonal side has been difficult to identify, but there are reports implicating gangliosides such as GD1a and GT1b and Nogo as MAG receptors (Schnaar & Lopez 2009). In the CNS, these molecules may create cytoplasmic channels equipped with gap junctions, which enable the transfer of metabolites to the axon (Rosenbluth et al. 2006, Snaidero et al. 2017). Some of these molecules also form cytoplasmic channels in peripheral myelin called Schmidt-Lanterman incisures, which probably fulfill the same purpose (Balice-Gordon et al. 1998). Ensheathing glia in Caenorhabditis elegans and Drosophila similarly support axons and form axoglial septate junctions that are similar in morphology and molecular composition to analogous structures that flank the nodes of Ranvier in vertebrate myelinated fibers (reviewed by Nave & Werner 2021). In these regions, close contact between the axolemma and the glial membrane is mediated by several adhesion molecules (see below). Loss of function of these molecules due to autoantibodies or mutations results in demyelinating neuropathies and axonal degeneration in patients, supporting the idea that axo-glia communications at these sites are important for axonal support (reviewed in Fehmi et al. 2018). The nature of the energy that is transferred is also under investigation, and many studies suggest that SCs are metabolically coupled to axons and provide them with energy-rich substrates such as lactose (Boucanova et al. 2021, Funfschilling et al. 2012, Jha et al. 2020), glucose (Saab et al. 2016), glycogen (Brown et al. 2012), and the bioenergetic cofactor NAD+ (Coleman & Hoke 2020). SCs can also sense axonal injury and respond via mammalian target of rapamycin complex 1 (mTORC1) by upregulating glycolytic enzymes, effectively shifting SC metabolism to support axons (Babetto et al. 2020).
Axonal ensheathment by SCs also serves to recognize and isolate larger axons destined to be myelinated. This process, named radial axonal sorting (Peters & Muir 1959), is essential for the differentiation of small- and large-caliber axons and of SCs. During radial sorting, immature SCs interdigitate lamellipodium-like processes among axons that expose the tyrosine kinase Erb2/3 receptors, which, in turn, sense the amount of axon-bound neuregulin-1 type III (Nrg1-III) (Taveggia et al. 2005), a key signal for SC terminal differentiation and myelination (see below). This process is modulated by laminins, which are deposited by SCs in the basal lamina and serve different purposes. One purpose is to inhibit PKA-mediated Nrg1-III signaling to prevent myelination until radial sorting is completed (Ghidinelli et al. 2017). The second is to activate the cotranscriptional activators yes-associated protein 1 (YAP1) and WW domain–containing transcription regulator protein 1 (WWTR1 or TAZ), which induce proliferation and the expression of laminin receptors such as dystroglycan and integrin α6β1 and of transcription factors such as Egr2/Krox20 (Poitelon et al. 2016). In turn, laminin receptors activate downstream signaling (e.g., ILK, FAK, Rac1, Cdc42, and PKA) to promote the formation of lamellipodium-like processes, which interdigitate and contact axons (Benninger et al. 2007, Grove & Brophy 2014, Nodari et al. 2007, Pellegatta et al. 2013, Pereira et al. 2009). Experimental deletion of any of the above-mentioned components in SCs or mutations in the human gene coding for laminin 211 prevent radial sorting of axons and peripheral nerve differentiation. For a detailed review on this process, see Feltri et al. (2016).
2.2. Myelination
Among molecules controlling PNS myelination, Nrg1-III represents the best example of a do-it-all signal. Indeed, several studies have demonstrated that the binding of NRG1 to ErbB2/3 receptors is necessary for many steps that lead to PNS myelination (reviewed in Birchmeier & Nave 2008). The relevance of this signaling mechanism (Michailov et al. 2004, Taveggia et al. 2005) is underscored by the fact that its role has been maintained throughout evolution (Lyons et al. 2005). The functionality of NRG1-ErbB receptors in the PNS is strictly regulated, in part by processing mediated by secretases that either activate [the β-secretase BACE1 (Hu et al. 2006, Willem et al. 2006)] or inhibit [the α-secretase ADAM17 (La Marca et al. 2011)] NRG1 activity. The engagement of ErbB receptors on the plasma membrane of SCs activates downstream effectors. Two main pathways are critical for the formation of PNS myelin: the PI3K/AKT/mTOR pathway (Goebbels et al. 2010, Sherman et al. 2012) and the ERK pathway (Ishii et al. 2021, Newbern et al. 2011). A role for a third pathway, calcineurin/NFAT (Kao et al. 2009), has not been confirmed (Reed et al. 2020).
Other pathways are also critical for peripheral myelination. Among them, the G protein–coupled receptor Gpr126 cell autonomously increases the levels of cAMP and activates PKA signaling in myelinating SCs (Monk et al. 2009). Gpr126 activity requires interaction with molecules in the SC extracellular matrix (Paavola et al. 2014, Petersen et al. 2015). In addition, Gpr44, a receptor of prostaglandin D2, regulates myelination following cleavage of Nrg1-III by the γ-secretase complex (Trimarco et al. 2014). Other molecules important for myelination include those in the Jagged/Delta/Notch signaling pathway (Woodhoo et al. 2009) and members of the Lgi4/ADAM22 complex (Bermingham et al. 2006, Ozkaynak et al. 2010).
The above-mentioned pathways convey signals to the nucleus that control transcription factors, which orchestrate the expression of myelin proteins or enzymes implicated in lipid synthesis. Among them are Yin Yang 1 (He et al. 2010), Zeb2 (Wu et al. 2016), the sterol regulatory element–binding protein (SREBP) (Verheijen et al. 2009), and early growth response gene 20 (Egr2), also known as Krox-20. Krox-20 is considered the master transcription factor for PNS myelination. Krox-20 is induced in promyelinating SCs, and without its activity, SCs cannot form myelin (Topilko et al. 1994). The timely activation of Krox-20 is under the control of another transcription factor, Pou3f1/Oct6/SCIP (Jaegle et al. 1996), which is expressed in premyelinating SCs; downregulation of Oct6 induces Krox-20 expression. Other important transcription factors belong to the SRY-related HMG box family, including Sox-10, whose expression is maintained in SCs regardless of their stage or phenotype (Woodhoo 2018) and is essential for SCs throughout life (Finzsch et al. 2010). Sox-10 synergizes with Krox-20 to induce myelination (Jagalur et al. 2011, Srinivasan et al. 2012; for comprehensive reviews, see Salzer 2015, Wegner 2000). Future studies should aim to disentangle the complex interconnection occurring between these pathways. It will be similarly important to clarify which of these molecules maintain myelin in adulthood, a critical aspect for the homeostasis of the nervous system.
2.3. Differentiation of Axonal Domains
The textbook function of myelination is to enable fast and reliable conduction of action potentials along axons, which is critical for nervous system function and represents the evolutionary foundation for the complexity of the vertebrate nervous system (Zalc & Colman 2000). The high capacitance of the myelin segments (called internodes) favors regeneration of the action potential exclusively at nodes of Ranvier (Huxley & Stampfli 1949), where there is a high density of voltage-gated sodium channels (Vabnick et al. 1996); voltage-gated potassium channels, which mediate membrane repolarization, are positioned at juxtaparanodes (Arroyo et al. 1999, Einheber et al. 1997). These voltage-gated channel clusters, as well as paranodal junctions, are formed and stabilized by heterotypic adhesion mechanisms, which occur between glial, axonal, and extracellular matrix proteins. In this way, SCs play an active role in the formation of the specific domains (Ching et al. 1999) (Figure 2), where gliomedin and perlecan interact with axonal NrCam and neurofascin 186 at nodes (Colombelli et al. 2015, Eshed et al. 2005, Tait et al. 2000), neurofascin 155 apposes axonal contactin and Caspr at paranodes (Bhat et al. 2001), and Caspr2 interacts with contactin 2–TAG1 at juxtaparanodes (Traka et al. 2003). These complexes in turn are linked to axonal cytoskeletal proteins such as ankyrins and protein 1.4 (Dzhashiashvili et al. 2007; for recent reviews of the process of axonal domain differentiation, see Faivre-Sarrailh 2020, Rasband & Peles 2021).
Figure 2.
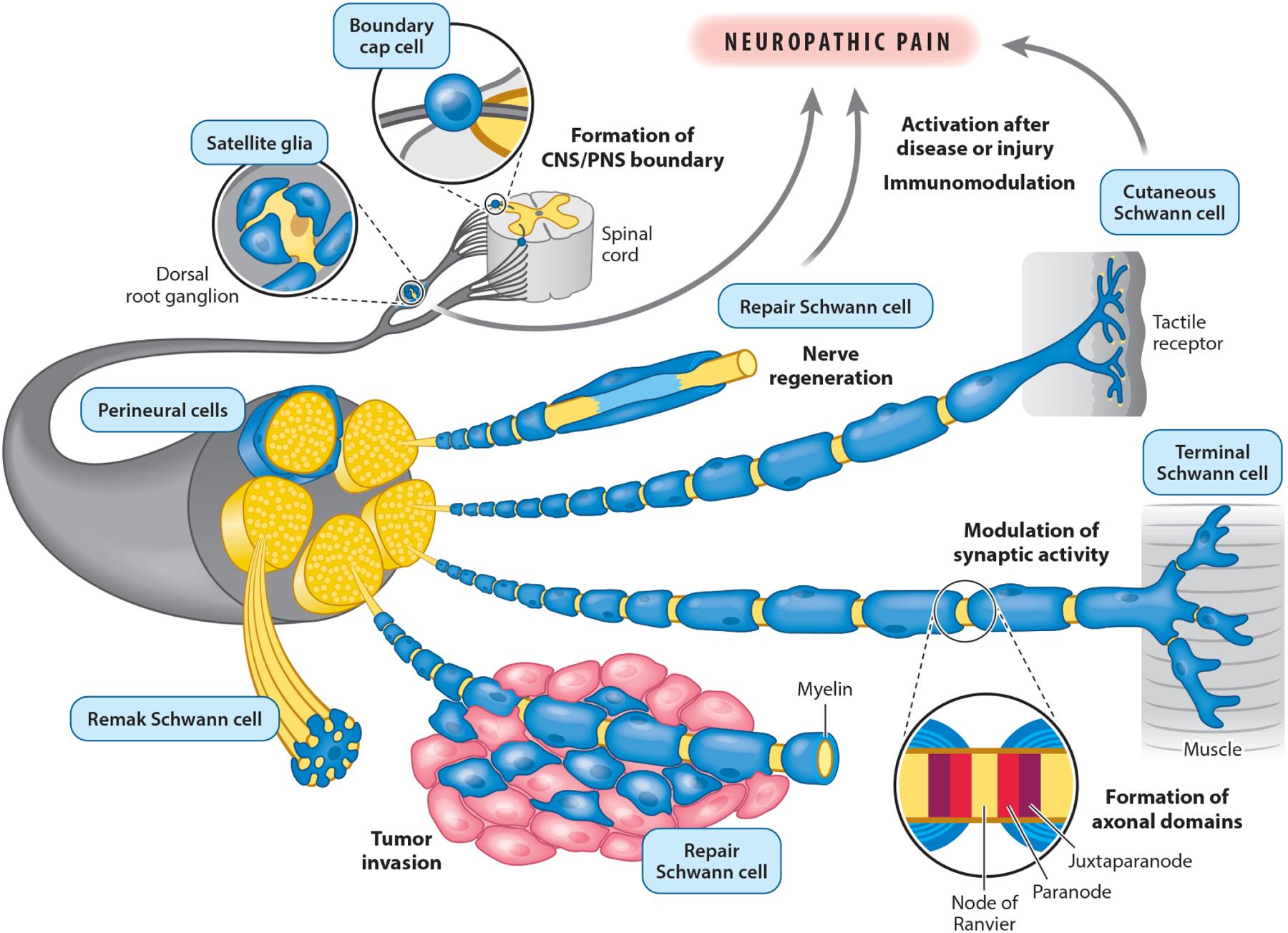
Schwann cell functions in physiological and pathological conditions. In addition to canonical functions such as wrapping and myelinating axons and instructing the formation of the node of Ranvier, Schwann cells exert other functions that are required for the physiological function of several organs. For example, a subset of Schwann cells called boundary cap cells actively contribute to the formation of the boundary between the central and peripheral nervous systems (CNS and PNS). Another subset called satellite glia surround neuronal cell bodies in ganglia, such as dorsal root sensory ganglia, where they modulate neuronal activity. Terminal Schwann cells surround the pre- and postsynaptic portion of the neuromuscular junction and actively regulate the activity of this synapse. Other noncanonical Schwann cell functions include the modulation of the innate and adaptive immune response, and the formation and regulation of tactile receptors in the epidermis. Due to their remarkable plasticity, fully differentiated myelinating, nonmyelinating (Remak), and terminal Schwann cells are able to transdifferentiate after injury into repair Schwann cells, which actively contribute to the process of nerve regeneration by clearing myelin and cellular debris and creating basal lamina tracks used by axons to regrow. In pathological conditions, the plasticity, immunomodulatory, and neuromodulatory activities of Schwann cells contribute to neuropathic pain and to the invasion and spreading of cancer cells, the latter likely by dedifferentiating to a phenotype resembling that of repair Schwann cells. Figure adapted with permission from Reed et al. (2021).
2.4. Nerve Regeneration and Repair
A characteristic of the PNS that is not shared with the CNS is its capacity to regenerate damaged nerves. If two nerve stumps remain close, a tissue bridge forms through which SCs and axons can regrow (Morris et al. 1972). In the nerve stump distal to the cell body, axons degenerate and trigger a cascade of signals resulting in the transdifferentiation of terminally differentiated SCs into an entirely different phenotype (repair phenotype) (Figure 2), characterized by a high rate of proliferation and the expression of a distinct set of genes (Arthur-Farraj et al. 2017, Stierli et al. 2019). The master regulator of this transition is the transcription factor c-Jun (Arthur-Farraj et al. 2012, Parkinson et al. 2008), but other key factors include STAT3 activation (Benito et al. 2017) and methylation of lysine 27 on histone 3 (Arthur-Farraj et al. 2017, Ma et al. 2016). Collectively, these changes affect the expression of an entire repertoire of molecules that are necessary to activate the repair process and are accompanied by the downregulation of genes encoding myelin proteins (reviewed by Jessen & Arthur-Farraj 2019).
Immediately after damage, repair SCs actively proliferate and initiate to phagocyte myelin (Gomez-Sanchez et al. 2015, Jang et al. 2016). Though the majority of myelin is eventually removed by macrophages that are recruited from the periphery, this initial process is essential, as myelin is inhibitory for axonal regrowth (Filbin 2003). Repair SCs and fibroblasts actively recruit macrophages from the bloodstream by secreting cytokines such as MCP1/CCL2 (Martini et al. 2008). Next, transdifferentiating SCs align to regrowing axons to form the bands of Büngner, regenerative paths that originate along the SC basal lamina and are necessary to support axon regrowth (Weinberg & Spencer 1978). After injury, the recruitment of macrophages is also facilitated by prostaglandin D2, which is also responsible for blood-nerve barrier (BNB) integrity (Forese et al. 2020).
Repair SCs engage mechanisms similar but complementary to those occurring in development, further underscoring their uniqueness (Jessen et al. 2015). For example, although axonal Nrg1-III is key for myelination, its role is replaced in part by SC-derived Nrg1-I for remyelination (Stassart et al. 2013). Correspondingly, the β-secretase BACE1 promotes nerve remyelination most likely by acting on glial Nrg1-I (reviewed by Pellegatta & Taveggia 2019), which activates glial ErbB receptors and stimulates the ERK1/2 pathway, a response required for the repair process (Napoli et al. 2012).
SCs within the tissue bridge have a higher proliferative capacity (Clements et al. 2017) and acquire a migratory behavior triggered by interactions between Ephrin B2 on fibroblasts and glial EphB2. These interactions relocalize N-cadherin to switch SCs from a repulsive to an attractive phenotype (Parrinello et al. 2010). SCs within the tissue bridge may also acquire mesenchymal characteristics (Arthur-Farraj et al. 2017, Clements et al. 2017).
3. NONCANONICAL FUNCTIONS OF SCHWANN CELLS
3.1. Schwann Cell, Boundary Cap Cell, and Peripheral Nervous System Organization
SCs interact with cells other than neurons to orchestrate nerve architecture, including formation of the BNB, the perineurium, and the boundary between the PNS and the CNS. This boundary comprises BCCs (Figure 2) at regions where axons projecting from CNS-located motor neurons exit the ventral spinal cord [ventral root transitional zone, or motor exit points (MEPs)] and regions where sensory neuron axons enter the dorsal spinal cord (dorsal root transitional zone, or sensory entry points). The BCCs that comprise and maintain this boundary are in part molecularly distinct from SCs and display early and transient expression of Krox-20. Depletion of these cells via Krox-20-Cre enables motor neuron somas to exit the spinal cord (reviewed by Radomska & Topilko 2017). BCCs also share characteristics with MEP cells, which has been shown in the zebrafish (Smith et al. 2014).
Peripheral nerves are surrounded by connective tissue layers, including the perineurium, that form a barrier and a protective layer (Figure 2). In developing mammals, the perineurium is formed by mesenchymal cells that are recruited to embryonic nerves. These cells undergo a mesenchymal-to-epithelial transformation to form tight junctions, deposit a basal lamina and collagen fibers, and form a multilayered tube that is a component of the BNB (Kristensson & Olsson 1971). Recent experiments suggest that at least a subset of perineurial cells originate not from the mesoderm but in the CNS (Clark et al. 2014). Nevertheless, the formation of the perineurium depends on the release of desert hedgehog (Dhh) by developing SCs, which acts on patched receptors on perineurial cells (Parmantier et al. 1999). Pericytes and macrophages that line endothelial blood vessels also contribute to the development of the BNB (Malong et al. 2019). Finally, recent data indicate that Gli1 in endoneurial fibroblasts is also required for development of a normal endoneurium architecture (Zotter et al. 2022). Thus, a series of complex multicellular interactions are necessary for normal nerve formation.
3.2. Schwann Cells and Immunomodulation
SCs are activated in pathological settings in which they express immune-related molecules (Lisak et al. 1997) and act as immune modulators, but they are essentially quiescent under physiological conditions (DeFrancesco-Lisowitz et al. 2015, Stierli et al. 2019). SCs act as immune surveillance cells in Charcot-Marie-Tooth hereditary neuropathies, Guillain-Barré syndrome, and chronic inflammatory demyelinating polyneuropathy (reviewed by Martini & Willison 2016).
SCs affect both innate and adaptive immune systems, as they express a plethora of molecules directly regulating these systems, including Toll-like receptors, receptors for advanced glycation end products (RAGEs), the mannose receptor, C-type leptin receptor, and the low-density-lipoprotein receptor-related protein-1 (LRP1) (reviewed by Tzekova et al. 2014). These molecules are expressed after injury concomitantly with proinflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF), and IL-6 and chemokines such as leukemia inhibitory factor and monocyte chemoattractant protein (reviewed by Tzekova et al. 2014). SCs also induce the anti-inflammatory cytokine IL-10 to modulate the innate immune response (Siqueira Mietto et al. 2015).
SCs trigger the adaptive immune response by expressing major histocompatibility complex (MHC)-I and MCH-II as well as costimulatory molecules such as CD80, CD86, and CD58 (Murata & Dalakas 2000, Spierings et al. 2001, Van Rhijn et al. 2000). Under pathological conditions, these molecules can reactivate T lymphocytes in peripheral nerves and establish functional immunological synapses on SC plasma membranes (Meyer zu Horste et al. 2010).
The inflammatory response is critical in several nerve disorders. For example, studies with animal models of Charcot-Marie-Tooth neuropathies due to genetic defects in SCs revealed that inflammation amplifies the pathological features (Groh et al. 2015), and the ablation of T and B lymphocytes ameliorates the disease course. Similarly, the inflammatory response of enteric glia is critical in gastrointestinal disorders (Seguella & Gulbransen 2021, Veiga-Fernandes & Pachnis 2017). For instance, an IFN-γ, CXCL10 axis in enteric glia is essential to limit inflammation after infection (Progatzky et al. 2021). Furthermore, the migration of hematopoietic stem cells and leukocytes is in part regulated by sympathetic nerves (Chen et al. 2021). Of note, sensory nerves were also shown to influence the primed immune response via their contribution to lymph node organization (Huang et al. 2021). Results from these studies raise a number of compelling questions; in particular, does the immune system reciprocally influence and shape nerve activity? For a detailed description of the pathogenetic mechanisms at the basis of inflammation in peripheral neuropathies, we refer readers to Martini & Willison (2016) and Ydens et al. (2013).
3.3. Schwann Cell Precursors as Multipotent Progenitors
Recent studies have highlighted new functions for SCPs as a class of multipotent progenitors. A combination of elegant fate mapping studies and single-cell RNA sequencing revealed that SCPs can generate melanocytes (Adameyko et al. 2009, Colombo et al. 2022), endoneurial fibroblasts (Joseph et al. 2004), parasympathetic neurons (Dyachuk et al. 2014, Espinosa-Medina et al. 2014), enteric neurons, dental pulp mesenchymal stromal cells (Kaukua et al. 2014), and adrenal chromaffin cells (Furlan et al. 2017) (Figure 1). For a comprehensive review, we refer the reader to Furlan & Adameyko (2018). SCPs also contribute to the patterning and differentiation of arterial branching, as they can instruct remodeling of the nascent vasculature and guide primitive vessels into arterioles. The relationship between nerve and vasculature is also fundamental to the organization of nascent axons through the bridge after nerve transection (Cattin et al. 2015). The molecular mechanisms regulating the above-described phenomena, which include secreted factors and contact-dependent signals, have been described in reviews (Aquino & Sierra 2018, Furlan & Adameyko 2018, Parfejevs et al. 2018). For the purposes of this review, the multipotent features of SCPs translate as phenotypic characteristics of some PNS tumors such as neurofibromatosis type 1 due to mutation in the neurofibromin 1 gene (NF1) (Cram et al. 2022). Likely due to the fact that SCPs can generate melanocytes, almost all neurofibromatosis type 1 patients present pigmented patches on the skin, substantial numbers of melanocytes, and a melanocyte molecular signature in melanotic schwannoma and desmoplastic melanomas (Van Raamsdonk & Deo 2013).
3.4. Modulation of Synaptic and Neuronal Activities by Terminal Schwann Cells and Satellite Cells
One of the best understood synapses in the PNS is the NMJ, formed between motor nerve terminals and the postsynaptic regions of muscle cells. The presynaptic nerve terminal and the postsynaptic muscle are capped by nonmyelin-forming SCs called perisynaptic or terminal SCs (Figure 2). Transcriptomic studies indicate that terminal SCs have unique patterns of gene expression, which include genes known to be important for NMJ and synapse formation (Castro et al. 2020). Terminal SCs are required for the maturation of the NMJ and aid in the regeneration of the nerve terminal after injury (Love & Thompson 1999). Terminal SCs modulate synaptic activity at NMJs and participate in the formation of a bona fide tripartite synapse (Araque et al. 1999). In the frog, ablation of terminal SCs halves synaptic transmission (Reddy et al. 2003). Terminal SCs sense (Jahromi et al. 1992, Reist & Smith 1992) and decode (Todd et al. 2010) synaptic activity via G protein–coupled receptors and regulate the release of calcium via muscarinic and purinergic receptors, which in turn influences the amount of transmitter released into the synapse (Robitaille 1995). For a comprehensive review, please see Darabid et al. (2014).
Like terminal SCs, satellite cells are also capable of modulating neuronal activities as a result of their contact with the neuronal soma (George et al. 2018) (Figure 2). They also share some functional characteristics with astrocytes (Avraham et al. 2020, Hanani & Spray 2020). For example, satellite cells respond to and modulate sensory neuron stimulation, and experimental manipulation of this bidirectional communication influences neuronal activity (Suadicani et al. 2010, Zhang et al. 2007). For a comprehensive review, we refer readers to Hanani & Spray (2020). Transcriptomic analyses further support the overlapping functions of astrocytes and satellite cells (Avraham et al. 2020, Tasdemir-Yilmaz et al. 2021).
4. PATHOLOGY
4.1. Pathology Due to Defects in Canonical Functions
Defects in the canonical function of SCs cause a plethora of diseases, ranging from inherited Charcot-Marie-Tooth neuropathies to common acquired neuropathies such as diabetic, toxic, and aging-associated neuropathies. We refer the reader to excellent reviews on these topics (Cavaletti & Marmiroli 2010, Laura et al. 2019, Mizukami & Osonoi 2020), as the focus of this review is on normal SC functions and physiopathological noncanonical functions of SCs.
4.2. Pathology Due to Defects in Noncanonical Functions
The noncanonical functions of SCs and related PNS glia described above are required for normal homeostasis of the PNS and other organs. Here, we review some instances in which dysregulation of noncanonical SC functions contributes to disease.
4.2.1. Schwann cells and nerve-cancer interaction.
The plasticity and immunomodulatory function of SCs can influence cancer. Several lines of evidence indicate that nerves play an important role in cancer pathogenesis and dissemination, which is particularly relevant in solid tumors affecting highly innervated organs (Martyn et al. 2019). Seminal studies have demonstrated that nerves stimulate the progression of prostate (Magnon et al. 2013) and gastric (Zhao et al. 2014) cancers. Indeed, denervation experiments in animal models for prostate, gastric, pancreatic, and skin cancer all resulted in reductions of tumor growth. However, denervation could also cause cancer progression (Bunimovich et al. 2017, Dubeykovskaya et al. 2016, Pawlowski & Weddell 1967); thus, the mechanisms regulating the nerve-tumor cross talk are likely context dependent and regulated by the tumor microenvironment, as well as by the neurotransmitters released by the innervating fibers (Gysler & Drapkin 2021, Hayakawa et al. 2017, Renz et al. 2018).
Nerves can directly influence the angiogenic process by enhancing endothelial cell metabolism (Zahalka et al. 2017). Immune cells and fibroblasts, which are part of the tumor microenvironment, can influence both nerves and cancer, as they release factors that activate cancer cells and remodel the extracellular matrix, facilitating the invasion of the tumor by nerves (reviewed by Gysler & Drapkin 2021). Recent studies have shown that tumor-associated SCs can also modify the ECM upon metabolite-driven epigenetic modifications, ultimately potentiating metastasis formation (Pascual et al. 2021).
Given the centrality of nerves in tumor growth and metastasis, several studies have investigated whether SCs participate in nerve-cancer interactions. Indeed, SCs have a central role in the progression of cancers characterized by perineural invasion, which is defined as the presence of cancer cells along or inside the nerves. Perineural invasion is present in pancreatic adenocarcinoma and in cancers of the prostate and head and neck and is often associated with increased tumor aggressiveness and a poor prognosis (Crippa et al. 2020, Gasparini et al. 2019).
SCs colonize tumors before the onset of cancer invasion (Demir et al. 2014) and actively degrade the extracellular matrix to instruct cancer cell invasion by direct contact (Deborde et al. 2016). Detailed analyses of the molecules expressed by SCs associated with cancer cells have revealed a strong similarity with those expressed in repair SCs (reviewed by Boilly et al. 2017) (Figure 2). Furthermore, nerves growing in the tumor microenvironment undergo sprouting similar to that of regenerating nerves. As during regeneration, SCs may also recruit immune cells to the tumor at the site of nerve invasion. Thus, the contribution of SCs to the nerve-tumor interaction is multifaceted: They can directly affect cancer cell migration and invasion and indirectly modulate the tumor microenvironment by acting on angiogenic processes and the inflammatory milieu.
Despite the recent advances in this field, many issues remain open. It will be important to discover the signal(s) that triggers SC dedifferentiation in early cancer development. Furthermore, whether the molecules that govern the interaction between all the cells implicated in nerve regeneration serve as a platform for perineural invasion and nerve-cancer interaction is poorly understood. Finally, and most important for patients, the essential checkpoints in nerve-cancer interaction and perineurial invasion that could be targeted to develop more-effective treatments need to be deciphered.
4.2.2. Neuropathic pain.
Satellite cells in both the DRG and SCs in peripheral nerves affect neuropathic pain as a result of their influences on neural activity and immune modulation. As mentioned above, satellite cells can modulate the activity of DRG neurons. Experimental evidence and the similarities to CNS astrocytes indicate that SCs are activated after injury or pathology and contribute to the increased neuronal activity that can lead to chronic pain (Figure 2). Experimental blockade of some of these changes is sufficient to reduce the activation of DRG neurons and may provide a target to treat chronic neuropathic pain (De Logu et al. 2022, Grace et al. 2014, Ohara et al. 2008; for an in-depth review of satellite cells in neuropathic pain, see Hanani & Spray 2020).
SCs release factors under pathological conditions that can increase or decrease pain sensitivity. Pain-inducing factors include TNF-α and nitric oxide synthase, whereas protective analgesic factors include SC basal lamina components, erythropoietin, and LRP1. Of note, genetic or pharmacological manipulation of these SC-released factors can induce (Wagner & Myers 1996) or reduce pain (Keswani et al. 2004, Orita et al. 2013) independently from demyelination and axonal degeneration. Deletion of structural genes in SCs elicits neuropathic behavior in mice (Gillespie et al. 2000, Saito et al. 2003) by unknown mechanisms. Remak SCs modulate neuropathic pain as a result of their association with small pain C fibers (Murinson et al. 2005b) and their exquisite responsiveness to peripheral nerve injuries (Murinson et al. 2005a, Wu et al. 2002; for a more comprehensive review of the role of SCs in neuropathic pain, see Campana 2007).
Specialized SCs in skin can respond directly to mechanical stimuli and trigger neuropathic pain. The skin derma is innervated by a plexus of myelinated and unmyelinated fibers, some of which project to the epidermis. Some SCs associated with these fibers form an extensive network of processes in the subepidermal region and can be activated directly by optogenetic stimulation, which results in a pain response and increased nociceptor firing rates. Neurophysiological studies determined that these skin SCs respond directly to mechanical stimuli and are necessary and sufficient for propagating action potentials in associated axons, thus initiating mechanical pain sensation (Abdo et al. 2019) (Figure 2). Finally, SCs associated with skin mechanoreceptors, but not their afferent DRG neurons, express the protein usherin, the gene for which is mutated in a genetic syndrome that includes impaired touch and pallesthesic (vibratory) sensation (Schwaller et al. 2021). Thus, the participation of PNS glial cells in the transduction of sensory inputs may be substantially greater than previously appreciated.
5. CONCLUDING REMARKS
Our increasing understanding of the multifaceted role of SCs and nerves raises many questions that trigger new opportunities for research. For instance, as nerves reach all organs, does SC plasticity also influence other body systems? Is it possible, for example, that SCs provide immunological surveillance in the skin, as enteric glia do in the gut? If so, is this system affected in dermatological disorders? Do SCs and nerves play additional roles in the gastrointestinal tract; for example, do they influence the action of the local microbiota? Can glial cells influence the specialization of different neuronal subtypes in ganglia similarly to their actions in the auditory system? Given the remarkable plasticity of SCPs and their role as a stem cell reservoir, can we exploit SCPs to develop new treatments? Answering these questions will further reveal the centrality of peripheral nerves in shaping and maintaining multiple biological systems.
ACKNOWLEDGMENTS
We thank all previous and current members of our laboratories for their amazing work and dedication and Karen Dietz for her critical reading of the manuscript. We apologize to colleagues whose relevant work we were unable to cite due to space limitations. Work in the laboratory of C.T. is supported by grants from the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) (R01NS099102) and AIRC (IG 2020-24774). Work in the laboratory of M.L.F. is supported by grants from NIH NINDS (R01 NS100464, R01 NS045630, R01 NS111715), US Army Medical Research Acquisition Activity (MS170085), the Charcot-Marie-Tooth Association, and the Legacy of Angels Foundation.
Footnotes
DISCLOSURE STATEMENT
M.L.F. is the president-elect and a board member of the Peripheral Nerve Society, a Scientific Board member of the Charcot-Marie-Tooth Association, and a Scientific Board member of the Charcot-Marie-Tooth 4B3 Foundation.
LITERATURE CITED
- Abdo H, Calvo-Enrique L, Lopez JM, Song J, Zhang MD, et al. 2019. Specialized cutaneous Schwann cells initiate pain sensation. Science 365:695–99 [DOI] [PubMed] [Google Scholar]
- Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, et al. 2009. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 139:366–79 [DOI] [PubMed] [Google Scholar]
- Aquino JB, Sierra R. 2018. Schwann cell precursors in health and disease. Glia 66:465–76 [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22:208–15 [DOI] [PubMed] [Google Scholar]
- Arroyo EJ, Xu YT, Zhou L, Messing A, Peles E, et al. 1999. Myelinating Schwann cells determine the inter-nodal localization of Kv1.1, Kv1.2, Kvβ2, and Caspr. J. Neurocytol 28:333–47 [DOI] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, et al. 2012. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, Morgan CC, Adamowicz M, Gomez-Sanchez JA, Fazal SV, et al. 2017. Changes in the coding and non-coding transcriptome and DNA methylome that define the Schwann cell repair phenotype after nerve injury. Cell Rep. 20:2719–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham O, Deng PY, Jones S, Kuruvilla R, Semenkovich CF, et al. 2020. Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun 11:4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babetto E, Wong KM, Beirowski B. 2020. A glycolytic shift in Schwann cells supports injured axons. Nat. Neurosci 23:1215–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Bone LJ, Scherer SS. 1998. Functional gap junctions in the Schwann cell myelin sheath. J. Cell Biol 142:1095–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SC, Chang L. 2004. Olfactory ensheathing cells and CNS repair: going solo or in need of a friend? Trends Neurosci. 27:54–60 [DOI] [PubMed] [Google Scholar]
- Benito C, Davis CM, Gomez-Sanchez JA, Turmaine M, Meijer D, et al. 2017. STAT3 controls the long-term survival and phenotype of repair Schwann cells during nerve regeneration. J. Neurosci 37:4255–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, et al. 2007. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J. Cell Biol 177:1051–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham JR Jr., Shearin H, Pennington J, O’Moore J, Jaegle M, et al. 2006. The claw paw mutation reveals a role for Lgi4 in peripheral nerve development. Nat. Neurosci 9:76–84 [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, et al. 2001. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 30:369–83 [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Nave KA. 2008. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56:1491–97 [DOI] [PubMed] [Google Scholar]
- Boilly B, Faulkner S, Jobling P, Hondermarck H. 2017. Nerve dependence: from regeneration to cancer. Cancer Cell 31:342–54 [DOI] [PubMed] [Google Scholar]
- Boucanova F, Pollmeier G, Sandor K, Morado Urbina C, Nijssen J, et al. 2021. Disrupted function of lac-tate transporter MCT1, but not MCT4, in Schwann cells affects the maintenance of motor end-plate innervation. Glia 69:124–36 [DOI] [PubMed] [Google Scholar]
- Brown AM, Evans RD, Black J, Ransom BR. 2012. Schwann cell glycogen selectively supports myelinated axon function. Ann. Neurol 72:406–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunimovich YL, Keskinov AA, Shurin GV, Shurin MR. 2017. Schwann cells: a new player in the tumor microenvironment. Cancer Immunol. Immunother 66:959–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana WM. 2007. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav. Immun 21:522–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R, Taetzsch T, Vaughan SK, Godbe K, Chappell J, et al. 2020. Specific labeling of synaptic Schwann cells reveals unique cellular and molecular features. eLife 9:e56935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, et al. 2015. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 162:1127–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaletti G, Marmiroli P. 2010. Chemotherapy-induced peripheral neurotoxicity. Nat. Rev. Neurol 6:657–66 [DOI] [PubMed] [Google Scholar]
- Chen CS, Weber J, Holtkamp SJ, Ince LM, de Juan A, et al. 2021. Loss of direct adrenergic innervation after peripheral nerve injury causes lymph node expansion through IFN-γ. J. Exp. Med 218:e20202377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Zanazzi G, Levinson SR, Salzer JL. 1999. Clustering of neuronal sodium channels requires contact with myelinating Schwann cells. J. Neurocytol 28:295–301 [DOI] [PubMed] [Google Scholar]
- Clark JK, O’Keefe A, Mastracci TL, Sussel L, Matise MP, Kucenas S. 2014. Mammalian Nkx2.2+ perineurial glia are essential for motor nerve development. Dev. Dyn 243:1116–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MP, Byrne E, Camarillo Guerrero LF, Cattin AL, Zakka L, et al. 2017. The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron 96:98–114.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Hoke A. 2020. Programmed axon degeneration: from mouse to mechanism to medicine. Nat. Rev. Neurosci 21:183–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombelli C, Palmisano M, Eshed-Eisenbach Y, Zambroni D, Pavoni E, et al. 2015. Perlecan is recruited by dystroglycan to nodes of Ranvier and binds the clustering molecule gliomedin. J. Cell Biol 208:313–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo S, Petit V, Wagner RY, Champeval D Yajima I, et al. 2022. Stabilization of β-catenin promotes melanocyte specification at the expense of the Schwann cell lineage. Development 149:dev194407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram JP, Wu J, Cover RA, Rizvi TA, Chaney KE, et al. 2022. P2RY14 cAMP signaling regulates Schwann cell precursor self-renewal, proliferation, and nerve tumor initiation in a mouse model of neurofibromatosis. eLife 11:e73511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa S, Pergolini I, Javed AA, Honselmann KC, Weiss MJ, et al. 2020. Implications of perineural invasion on disease recurrence and survival after pancreatectomy for pancreatic head ductal adenocarcinoma. Ann. Surg In press [DOI] [PubMed] [Google Scholar]
- Darabid H, Perez-Gonzalez AP, Robitaille R. 2014. Neuromuscular synaptogenesis: coordinating partners with multiple functions. Nat. Rev. Neurosci 15:703–18 [PubMed] [Google Scholar]
- De Logu F, Nassini R, Hegron A, Landini L, Jensen DD, et al. 2022. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat. Commun 13:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborde S, Omelchenko T, Lyubchik A, Zhou Y, He S, et al. 2016. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig 126:1538–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE. 2015. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience 302:174–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir IE, Boldis A, Pfitzinger PL, Teller S, Brunner E, et al. 2014. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J. Natl. Cancer Inst 106:dju184. [DOI] [PubMed] [Google Scholar]
- Dubeykovskaya Z, Si Y, Chen X, Worthley DL, Renz BW, et al. 2016. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat. Commun 7:10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyachuk V, Furlan A, Shahidi MK, Giovenco M, Kaukua N, et al. 2014. Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science 345:82–87 [DOI] [PubMed] [Google Scholar]
- Dzhashiashvili Y, Zhang Y, Galinska J, Lam I, Grumet M, Salzer JL. 2007. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J. Cell Biol 177:857–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Werner HB, McCulloch MC, Barrie JA, et al. 2009. Early ultrastructural defects of axons and axon-glia junctions in mice lacking expression of Cnp1. Glia 57:1815–24 [DOI] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, et al. 1997. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J. Cell Biol 139:1495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, et al. 2005. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron 47:215–29 [DOI] [PubMed] [Google Scholar]
- Espinosa-Medina I, Outin E, Picard CA, Chettouh Z, Dymecki S, et al. 2014. Parasympathetic ganglia derive from Schwann cell precursors. Science 345:87–90 [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C 2020. Molecular organization and function of vertebrate septate-like junctions. Biochim. Biophys. Acta Biomembr 1862:183211. [DOI] [PubMed] [Google Scholar]
- Fehmi J, Scherer SS, Willison HJ, Rinaldi S. 2018. Nodes, paranodes and neuropathies. J. Neurol. Neurosurg. Psychiatry 89:61–71 [DOI] [PubMed] [Google Scholar]
- Feltri ML, Poitelon Y, Previtali SC. 2016. How Schwann cells sort axons: new concepts. Neuroscientist 22:252–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. 2003. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci 4:703–13 [DOI] [PubMed] [Google Scholar]
- Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, et al. 2010. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J. Cell Biol 189:701–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forese MG, Pellegatta M, Canevazzi P, Gullotta GS, Podini P, et al. 2020. Prostaglandin D2 synthase modulates macrophage activity and accumulation in injured peripheral nerves. Glia 68:95–110 [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, et al. 2012. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan A, Adameyko I. 2018. Schwann cell precursor: a neural crest cell in disguise? Dev. Biol 444(Suppl. 1):S25–35 [DOI] [PubMed] [Google Scholar]
- Furlan A, Dyachuk V, Kastriti ME, Calvo-Enrique L, Abdo H, et al. 2017. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 357:eaal3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini G, Pellegatta M, Crippa S, Lena MS, Belfiori G, et al. 2019. Nerves and pancreatic cancer: new insights into a dangerous relationship. Cancers 11:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D, Ahrens P, Lambert S. 2018. Satellite glial cells represent a population of developmentally arrested Schwann cells. Glia 66:1496–506 [DOI] [PubMed] [Google Scholar]
- Gerber D, Pereira JA, Gerber J, Tan G, Dimitrieva S, et al. 2021. Transcriptional profiling of mouse peripheral nerves to the single-cell level to build a sciatic nerve ATlas (SNAT). eLife 10:e58591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidinelli M, Poitelon Y, Shin YK, Ameroso D, Williamson C, et al. 2017. Laminin 211 inhibits protein kinase A in Schwann cells to modulate neuregulin 1 type III-driven myelination. PLOS Biol. 15:e2001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CS, Sherman DL, Fleetwood-Walker SM, Cottrell DF, Tait S, et al. 2000. Peripheral demyelination and neuropathic pain behavior in periaxin-deficient mice. Neuron 26:523–31 [DOI] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, et al. 2010. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci 30:8953–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, et al. 2015. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol 210:153–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. 2014. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol 14:217–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, et al. 1998. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280:1610–13 [DOI] [PubMed] [Google Scholar]
- Groh J, Klein I, Hollmann C, Wettmarshausen J, Klein D, Martini R. 2015. CSF-1-activated macrophages are target-directed and essential mediators of Schwann cell dedifferentiation and dysfunction in Cx32-deficient mice. Glia 63:977–86 [DOI] [PubMed] [Google Scholar]
- Grove M, Brophy PJ. 2014. FAK is required for Schwann cell spreading on immature basal lamina to coordinate the radial sorting of peripheral axons with myelination. J. Neurosci 34:13422–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysler SM, Drapkin R. 2021. Tumor innervation: peripheral nerves take control of the tumor microenvironment. J. Clin. Investig 131:e147276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanani M, Spray DC. 2020. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci 21:485–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, et al. 2017. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Kim JY, Dupree J, Tewari A, Melendez-Vasquez C, et al. 2010. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat. Neurosci 13:1472–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, et al. 2006. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci 9:1520–25 [DOI] [PubMed] [Google Scholar]
- Huang S, Ziegler CGK, Austin J, Mannoun N, Vukovic M, et al. 2021. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 184:441–59.e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF, Stampfli R. 1949. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J. Physiol 108:315–39 [PubMed] [Google Scholar]
- Ishii A, Furusho M, Bansal R. 2021. Mek/ERK1/2-MAPK and PI3K/Akt/mTOR signaling plays both independent and cooperative roles in Schwann cell differentiation, myelination and dysmyelination. Glia 69:2429–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaegle M, Mandemakers W, Broos L, Zwart R, Karis A, et al. 1996. The POU factor Oct-6 and Schwann cell differentiation. Science 273:507–10 [DOI] [PubMed] [Google Scholar]
- Jagalur NB, Ghazvini M, Mandemakers W, Driegen S, Maas A, et al. 2011. Functional dissection of the Oct6 Schwann cell enhancer reveals an essential role for dimeric Sox10 binding. J. Neurosci 31:8585–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi BS, Robitaille R, Charlton MP. 1992. Transmitter release increases intracellular calcium in perisynaptic Schwann cells in situ. Neuron 8:1069–77 [DOI] [PubMed] [Google Scholar]
- Jang SY, Shin YK, Park SY, Park JY, Lee HJ, et al. 2016. Autophagic myelin destruction by Schwann cells during Wallerian degeneration and segmental demyelination. Glia 64:730–42 [DOI] [PubMed] [Google Scholar]
- Jessen KR, Arthur-Farraj P. 2019. Repair Schwann cell update: adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 67:421–37 [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R, Lloyd AC. 2015. Schwann cells: development and role in nerve repair. Cold Spring Harb. Perspect. Biol 7:a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Lee Y, Russell KA, Yang F, Dastgheyb RM, et al. 2020. Monocarboxylate transporter 1 in Schwann cells contributes to maintenance of sensory nerve myelination during aging. Glia 68:161–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, et al. 2004. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 131:5599–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SC, Wu H, Xie J, Chang CP, Ranish JA, et al. 2009. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science 323:651–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, et al. 2014. Glial origin of mesenchymal stem cells in a tooth model system. Nature 513:551–54 [DOI] [PubMed] [Google Scholar]
- Keswani SC, Leitz GJ, Hoke A. 2004. Erythropoietin is neuroprotective in models of HIV sensory neuropathy. Neurosci. Lett 371:102–5 [DOI] [PubMed] [Google Scholar]
- Kristensson K, Olsson Y. 1971. The perineurium as a diffusion barrier to protein tracers. Differences between mature and immature animals. Acta Neuropathol. 17:127–38 [DOI] [PubMed] [Google Scholar]
- La Marca R, Cerri F, Horiuchi K, Bachi A, Feltri ML, et al. 2011. TACE (ADAM17) inhibits Schwann cell myelination. Nat. Neurosci 14:857–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laura M, Pipis M, Rossor AM, Reilly MM. 2019. Charcot-Marie-Tooth disease and related disorders: an evolving landscape. Curr. Opin. Neurol 32:641–50 [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. 1974. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol 41:162–84 [DOI] [PubMed] [Google Scholar]
- Lisak RP, Skundric D, Bealmear B, Ragheb S. 1997. The role of cytokines in Schwann cell damage, protection, and repair. J. Infect. Dis 176(Suppl. 2):S173–79 [DOI] [PubMed] [Google Scholar]
- Love FM, Thompson WJ. 1999. Glial cells promote muscle reinnervation by responding to activity-dependent postsynaptic signals. J. Neurosci 19:10390–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DA, Pogoda HM, Voas MG, Woods IG, Diamond B, et al. 2005. erbb3 and erbb2 are essential for Schwann cell migration and myelination in zebrafish. Curr. Biol 15:513–24 [DOI] [PubMed] [Google Scholar]
- Ma KH, Hung HA, Svaren J. 2016. Epigenomic regulation of Schwann cell reprogramming in peripheral nerve injury. J. Neurosci 36:9135–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, et al. 2013. Autonomic nerve development contributes to prostate cancer progression. Science 341:1236361. [DOI] [PubMed] [Google Scholar]
- Malong L, Napoli I, White IJ, Stierli S, Bossio A, Lloyd AC. 2019. Macrophages enforce the blood nerve barrier. bioRxiv 493494. 10.1101/493494 [DOI] [Google Scholar]
- Martini R, Fischer S, Lopez-Vales R, David S. 2008. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia 56:1566–77 [DOI] [PubMed] [Google Scholar]
- Martini R, Willison H. 2016. Neuroinflammation in the peripheral nerve: cause, modulator, or bystander in peripheral neuropathies? Glia 64:475–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn GV, Shurin GV, Keskinov AA, Bunimovich YL, Shurin MR. 2019. Schwann cells shape the neuroimmune environs and control cancer progression. Cancer Immunol. Immunother 68:1819–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Horste G, Heidenreich H, Mausberg AK, Lehmann HC, ten Asbroek AL, et al. 2010. Mouse Schwann cells activate MHC class I and II restricted T-cell responses, but require external peptide processing for MHC class II presentation. Neurobiol. Dis 37:483–90 [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, et al. 2004. Axonal neuregulin-1 regulates myelin sheath thickness. Science 304:700–3 [DOI] [PubMed] [Google Scholar]
- Mizukami H, Osonoi S. 2020. Pathogenesis and molecular treatment strategies of diabetic neuropathy collateral glucose-utilizing pathways in diabetic polyneuropathy. Int. J. Mol. Sci 22:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, et al. 2009. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 325:1402–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JH, Hudson AR, Weddell G. 1972. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. II. The development of the “regenerating unit.” Z. Zellforsch. Mikrosk. Anat 124:103–30 [PubMed] [Google Scholar]
- Murata K, Dalakas MC. 2000. Expression of the co-stimulatory molecule BB-1, the ligands CTLA-4 and CD28 and their mRNAs in chronic inflammatory demyelinating polyneuropathy. Brain 123(Pt. 8):1660–66 [DOI] [PubMed] [Google Scholar]
- Murinson BB, Archer DR, Li Y, Griffin JW. 2005a. Degeneration of myelinated efferent fibers prompts mitosis in Remak Schwann cells of uninjured C-fiber afferents. J. Neurosci 25:1179–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murinson BB, Hoffman PN, Banihashemi MR, Meyer RA, Griffin JW. 2005b. C-fiber (Remak) bundles contain both isolectin B4-binding and calcitonin gene-related peptide-positive axons. J. Comp. Neurol 484:392–402 [DOI] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, et al. 2012. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 73:729–42 [DOI] [PubMed] [Google Scholar]
- Nave KA, Werner HB. 2021. Ensheathment and myelination of axons: evolution of glial functions. Annu. Rev. Neurosci 44:197–219 [DOI] [PubMed] [Google Scholar]
- Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, et al. 2011. Specific functions for ERK/MAPK signaling during PNS development. Neuron 69:91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A, Zambroni D, Quattrini A, Court FA, D’Urso A, et al. 2007. β1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J. Cell Biol 177:1063–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara PT, Vit JP, Bhargava A, Jasmin L. 2008. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J. Neurophysiol 100:3064–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita S, Henry K, Mantuano E, Yamauchi K, De Corato A, et al. 2013. Schwann cell LRP1 regulates Remak bundle ultrastructure and axonal interactions to prevent neuropathic pain. J. Neurosci 33:5590–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E, Abello G, Jaegle M, van Berge L, Hamer D, et al. 2010. Adam22 is a major neuronal receptor for Lgi4-mediated Schwann cell signaling. J. Neurosci 30:3857–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola KJ, Sidik H, Zuchero JB, Eckart M, Talbot WS. 2014. Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci. Signal 7:ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfejevs V, Antunes AT, Sommer L. 2018. Injury and stress responses of adult neural crest-derived cells. Dev. Biol 444(Suppl. 1):S356–65 [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, et al. 2008. c-Jun is a negative regulator of myelination. J. Cell Biol 181:625–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmantier E, Lynn B, Lawson D, Turmaine M, Namini SS, et al. 1999. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 23:713–24 [DOI] [PubMed] [Google Scholar]
- Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, et al. 2010. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 143:145–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G, Dominguez D, Elosua-Bayes M, Beckedorff F, Laudanna C, et al. 2021. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 599:485–90 [DOI] [PubMed] [Google Scholar]
- Pawlowski A, Weddell G. 1967. Induction of tumours in denervated skin. Nature 213:1234–37 [Google Scholar]
- Pellegatta M, De Arcangelis A, D’Urso A, Nodari A, Zambroni D, et al. 2013. α6β1 and α7β1 integrins are required in Schwann cells to sort axons. J. Neurosci 33:17995–8007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegatta M, Taveggia C. 2019. The complex work of proteases and secretases in Wallerian degeneration: beyond neuregulin-1. Front. Cell Neurosci 13:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Benninger Y, Baumann R, Goncalves AF, Ozcelik M, et al. 2009. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J. Cell Biol 185:147–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Muir AR. 1959. The relationship between axons and Schwann cells during development of peripheral nerves in the rat. Q. J. Exp. Physiol. Cogn. Med. Sci 44:117–30 [DOI] [PubMed] [Google Scholar]
- Petersen SC, Luo R, Liebscher I, Giera S, Jeong SJ, et al. 2015. The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron 85:755–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitelon Y, Lopez-Anido C, Catignas K, Berti C, Palmisano M, et al. 2016. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat. Neurosci 19:879–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progatzky F, Shapiro M, Hui Chng S, Garcia-Cassani B, Classon CH, et al. 2021. Regulation of intestinal immunity and tissue repair by enteric glia. Nature 599:125–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomska KJ, Topilko P. 2017. Boundary cap cells in development and disease. Curr. Opin. Neurobiol 47:209–15 [DOI] [PubMed] [Google Scholar]
- Rasband MN, Peles E. 2021. Mechanisms of node of Ranvier assembly. Nat. Rev. Neurosci 22:7–20 [DOI] [PubMed] [Google Scholar]
- Reddy LV, Koirala S, Sugiura Y, Herrera AA, Ko CP. 2003. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron 40:563–80 [DOI] [PubMed] [Google Scholar]
- Reed CB, Feltri ML, Wilson ER. 2021. Peripheral glia diversity. J. Anat In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CB, Frick LR, Weaver A, Sidoli M, Schlant E, et al. 2020. Deletion of calcineurin in Schwann cells does not affect developmental myelination, but reduces autophagy and delays myelin clearance after peripheral nerve injury. J. Neurosci 40:6165–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist NE, Smith SJ. 1992. Neurally evoked calcium transients in terminal Schwann cells at the neuromuscular junction. PNAS 89:7625–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, et al. 2018. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 8:1458–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R 1995. Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. J. Neurosci 15:7121–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J, Nave KA, Mierzwa A, Schiff R. 2006. Subtle myelin defects in PLP-null mice. Glia 54:172–82 [DOI] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, et al. 2016. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91:119–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, et al. 2003. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron 38:747–58 [DOI] [PubMed] [Google Scholar]
- Salzer JL. 2015. Schwann cell myelination. Cold Spring Harb. Perspect. Biol 7:a020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL, Lopez PH. 2009. Myelin-associated glycoprotein and its axonal receptors. J. Neurosci. Res 87:3267–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller F, Begay V, Garcia-Garcia G, Taberner FJ, Moshourab R, et al. 2021. USH2A is a Meissner’s corpuscle protein necessary for normal vibration sensing in mice and humans. Nat. Neurosci 24:74–81 [DOI] [PubMed] [Google Scholar]
- Seguella L, Gulbransen BD. 2021. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol 18:571–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Krols M, Wu LM, Grove M, Nave KA, et al. 2012. Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. J. Neurosci 32:1817–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira Mietto B, Kroner A, Girolami EI, Santos-Nogueira E, Zhang J, David S. 2015. Role of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injury. J. Neurosci 35:16431–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Morris AD, Welsh TG, Kucenas S. 2014. Contact-mediated inhibition between oligodendrocyte progenitor cells and motor exit point glia establishes the spinal cord transition zone. PLOS Biol. 12:e1001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaidero N, Velte C, Myllykoski M, Raasakka A, Ignatev A, et al. 2017. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 18:314–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierings E, de Boer T, Wieles B, Adams LB, Marani E, Ottenhoff TH. 2001. Mycobacterium leprae-specific, HLA class II-restricted killing of human Schwann cells by CD4+ Th1 cells: a novel immunopathogenic mechanism of nerve damage in leprosy. J. Immunol 166:5883–88 [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Sun G, Keles S, Jones EA, Jang SW, et al. 2012. Genome-wide analysis of EGR2/SOX10 binding in myelinating peripheral nerve. Nucleic Acids Res. 40:6449–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, et al. 2013. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat. Neurosci 16:48–54 [DOI] [PubMed] [Google Scholar]
- Stierli S, Imperatore V, Lloyd AC. 2019. Schwann cell plasticity-roles in tissue homeostasis, regeneration, and disease. Glia 67:2203–15 [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Cherkas PS, Zuckerman J, Smith DN, Spray DC, Hanani M. 2010. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol. 6:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S, Gunn-Moore F, Collinson JM, Huang J, Lubetzki C, et al. 2000. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. J. Cell Biol 150:657–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir-Yilmaz OE, Druckenbrod NR, Olukoya OO, Dong W, Yung AR, et al. 2021. Diversity of developing peripheral glia revealed by single-cell RNA sequencing. Dev. Cell 56:2516–35.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, et al. 2005. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47:681–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd KJ, Darabid H, Robitaille R. 2010. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J. Neurosci 30:11870–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, et al. 1994. Krox-20 controls myelination in the peripheral nervous system. Nature 371:796–99 [DOI] [PubMed] [Google Scholar]
- Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, et al. 2003. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J. Cell Biol 162:1161–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarco A, Forese MG, Alfieri V, Lucente A, Brambilla P, et al. 2014. Prostaglandin D2 synthase/GPR44: a signaling axis in PNS myelination. Nat. Neurosci 17:1682–92 [DOI] [PubMed] [Google Scholar]
- Tzekova N, Heinen A, Kury P. 2014. Molecules involved in the crosstalk between immune- and peripheral nerve Schwann cells. J. Clin. Immunol 34(Suppl. 1):S86–104 [DOI] [PubMed] [Google Scholar]
- Vabnick I, Novakovic SD, Levinson SR, Schachner M, Shrager P. 1996. The clustering of axonal sodium channels during development of the peripheral nervous system. J. Neurosci 16:4914–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Deo M. 2013. Links between Schwann cells and melanocytes in development and disease. Pigment Cell Melanoma Res. 26:634–45 [DOI] [PubMed] [Google Scholar]
- Van Rhijn I, Van den Berg LH, Bosboom WM, Otten HG, Logtenberg T. 2000. Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain 123(Pt. 10):2020–29 [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Pachnis V. 2017. Neuroimmune regulation during intestinal development and homeostasis. Nat. Immunol 18:116–22 [DOI] [PubMed] [Google Scholar]
- Verheijen MH, Camargo N, Verdier V, Nadra K, de Preux Charles AS, et al. 2009. SCAP is required for timely and proper myelin membrane synthesis. PNAS 106:21383–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Myers RR. 1996. Endoneurial injection of TNF-α produces neuropathic pain behaviors. Neuroreport 7:2897–901 [DOI] [PubMed] [Google Scholar]
- Wanner IB, Mahoney J, Jessen KR, Wood PM, Bates M, Bunge MB. 2006. Invariant mantling of growth cones by Schwann cell precursors characterize growing peripheral nerve fronts. Glia 54:424–38 [DOI] [PubMed] [Google Scholar]
- Wegner M 2000. Transcriptional control in myelinating glia: the basic recipe. Glia 29:118–23 [PubMed] [Google Scholar]
- Weinberg HJ, Spencer PS. 1978. The fate of Schwann cells isolated from axonal contact. J. Neurocytol 7:555–69 [DOI] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, et al. 2006. Control of peripheral nerve myelination by the β-secretase BACE1. Science 314:664–66 [DOI] [PubMed] [Google Scholar]
- Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D’Antonio M, et al. 2009. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat. Neurosci 12:839–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhoo ASL. 2018. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia 56:1481–90 [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, et al. 2002. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J. Neurosci 22:7746–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LM, Wang J, Conidi A, Zhao C, Wang H, et al. 2016. Zeb2 recruits HDAC-NuRD to inhibit Notch and controls Schwann cell differentiation and remyelination. Nat. Neurosci 19:1060–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ydens E, Lornet G, Smits V, Goethals S, Timmerman V, Janssens S. 2013. The neuroinflammatory role of Schwann cells in disease. Neurobiol. Dis 55:95–103 [DOI] [PubMed] [Google Scholar]
- Yim AKY, Wang PL, Bermingham JR Jr., Hackett A, Strickland A, et al. 2022. Disentangling glial diversity in peripheral nerves at single-nuclei resolution. Nat. Neurosci 25:238–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Crawford TO, Griffin JW, Tu P, Lee VM, et al. 1998. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J. Neurosci 18:1953–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahalka AH, Arnal-Estape A, Maryanovich M, Nakahara F, Cruz CD, et al. 2017. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358:321–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalc B, Colman DR. 2000. Origins of vertebrate success. Science 288:271–72 [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Wang C, Huang LY. 2007. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. PNAS 104:9864–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, et al. 2014. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med 6:250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotter B, Dagan O, Brady J, Baloui H, Samanta J, Salzer JL. 2022. Gli1 regulates the postnatal acquisition of peripheral nerve architecture. J. Neurosci 42:183–201 [DOI] [PMC free article] [PubMed] [Google Scholar]


