Abstract
Background
The role of various serum tumor markers (TMs) has been reported in non‐small cell lung cancer (NSCLC). However, the prognosis of patients with multiple TM‐negative NSCLC remain unclear.
Aims
This study aimed to describe the characteristics and outcomes of patients with NSCLC undergoing surgery and to investigate their prognostic association with preoperative serum TM‐negative cases.
Methods and results
We retrospectively evaluated 442 patients who underwent complete resection of stage I NSCLC between January 2004 and December 2019. These 442 patients were classified into a group whose preoperative serum levels of carcinoembryonic antigen (CEA), cytokeratin‐19 fragment (CYFRA21‐1), carbohydrate antigen 19‐9 (CA19‐9), and squamous cell carcinoma antigen (SCC Ag) were all negative (TM‐negative group; n = 249, 56%) and a group with at least one positive marker (TM‐positive group; n = 193, 44%). Among all patients, the TM‐negative group showed higher 5‐year recurrence‐free survival (RFS) (92.6% vs. 79.1%; p < .01), and overall survival (OS) rates (86.3% vs. 68.6%; p < .01). After propensity score matching, patients in the TM‐negative group still exhibited good 5‐year RFS (92.1% vs. 81.4%; p = .01) and OS rates (87.6% vs. 72.6%; p < .01).
Conclusion
Our study suggests that NSCLC patients who are preoperatively negative for all serum TMs, such as CEA, CYFRA21‐1, CA19‐9, and SCC Ag, represent a subgroup with a particularly good prognosis.
Keywords: biology, lung cancer, prognosis, surgery, tumor markers
1. INTRODUCTION
Lung cancer is the leading cause of cancer‐related deaths. 1 In 2021, the American Cancer Society estimated that the prevalence of lung cancer is the second highest among all cancer types, and almost one‐quarter of all cancer‐related deaths are due to lung cancer. 2 Early diagnosis of recurrence after surgery for lung cancer contributes to an improved prognosis. The role of various serum tumor markers (TMs) has been reported in non‐small cell lung cancer (NSCLC), but their efficiency in early diagnosis is limited. High preoperative serum levels of several TMs are associated with a poor prognosis in patients with NSCLC. In particular, the preoperative value of carcinoembryonic antigen (CEA) and cytokeratin‐19 fragment (CYFRA21‐1) may provide prognostic and predictive information for both recurrence and mortality risk in NSCLC. 3 , 4 , 5 , 6 , 7 , 8 Carbohydrate antigen 19‐9 (CA19‐9) is widely used to predict prognosis in patients with colorectal or pancreatic cancer. Accumulated evidence suggests a high positivity rate in lung adenocarcinoma as well. 9 In addition, squamous cell carcinoma antigen (SCC Ag) is the most used prognostic marker for lung SCC. 10 , 11 The combined use of multiple TMs with relevant clinical factors, such as age or sex, may increase their prognostic accuracy; however, the results obtained using this approach have been inconsistent. 12 , 13 , 14 The association between elevated preoperative serum TM levels and prognosis is well known. However, the prognosis of patients with multiple TM‐negative NSCLC remains unclear. It is important to investigate these patients as they are easy to follow‐up and may possibly represent a subgroup with a particularly good prognosis.
This study aimed to describe the characteristics and outcomes of patients with NSCLC undergoing surgery and to examine the prognosis in preoperatively TM‐negative patients.
2. METHODS
2.1. Patients and study design
We conducted a retrospective study data of patients who underwent surgery for NSCLC between January 2004 and December 2019. We reviewed the medical records of patients to examine their socio‐demographic profiles (age, sex, and smoking history), clinical status (comorbid chronic obstructive pulmonary disease or interstitial pneumonia), surgical treatment, tumor characteristics (pathological tumor size, histological subclassification, lymphovascular invasion, visceral pleural invasion, pathological stage, mutation status of epidermal growth factor receptor [EGFR]), adjuvant therapy (chemotherapy and/or radiation), and preoperative serum CEA, CYFRA21‐1, CA19‐9, and SCC Ag levels. Pathological stage was determined according to the eighth edition of the International Tumor Node Metastasis (TNM) staging system. 15 Thus, pathological tumor size was defined as the largest dimension of the invasive portion.
We included patients with completely resected stage I NSCLC and with complete data of preoperative serum TM levels (CEA, CYFRA21‐1, CA19‐9, and SCC Ag) (n = 446). Patients who had received prior induction or definitive treatment were excluded from the study (n = 4). Ultimately, 442 patients were classified into a group whose preoperative serum levels of CEA, CYFRA21‐1, CA19‐9, and SCC Ag were all negative (TM‐negative group; n = 249, 56%) or a group with at least one positive marker (TM‐positive group; n = 193, 44%) (Figure 1).
FIGURE 1.
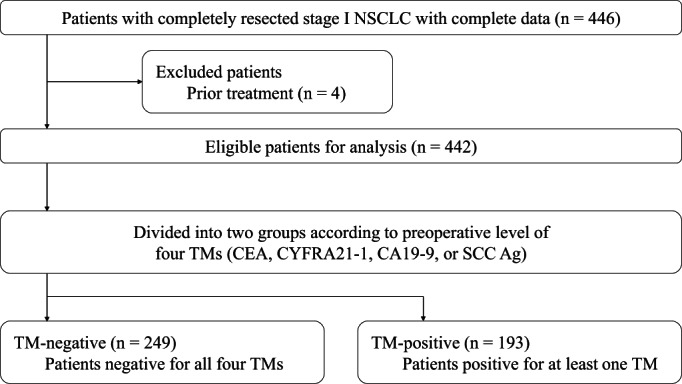
Study cohort flowchart. CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CYFRA21‐1, cytokeratin‐19 fragment; NSCLC, non‐small cell lung cancer; SCC Ag, squamous cell carcinoma antigen; TM, tumor marker
Patients were generally followed up every 3 months for the first 2 years after surgery and every 6–12 months thereafter. Computed tomography (CT) was performed every 6 months for the first 2 years after surgery and every 12 months after that. Additional examinations were also performed when the related symptoms occurred. Recurrent disease in the study was examined based on combined pathological examination and imaging evidence of CT or positron emission tomography (PET)‐CT and was confirmed by a radiologist and thoracic surgeon. This study was approved by the Institutional Review Board of the Yamanashi University Hospital (approval No. 2506). Informed consent was obtained in the form of opt‐out on the website (https://www.med.yamanashi.ac.jp/rinri/ippan.html) by the decision of the Institutional Review Board.
2.2. TM assays
We assessed four TMs, that is, CEA, CYFRA21‐1, CA19‐9, SCC Ag. Blood samples for TM measurements were obtained at least 1 month before surgery. To analyze the correlation between the preoperative TM levels and recurrence, TM levels were measured using an electrochemiluminescence immunoassay on the Cobas8000/e801® module (Roche Diagnostics, Pleasanton, CA, United States of America K.K., Tokyo, Japan). According to manufacturer's instructions, the cut‐off values were as follows: CEA, 5 ng/ml; CYFRA21‐1, 2.8 ng/ml; CA19‐9, 37 U/ml; and SCC Ag, 2.5 ng/ml. Any individual TM levels below the cut‐off value were defined as negative. We considered patients who were negative for all four TMs as “TM‐negative.”
2.3. Endpoints
The primary study endpoint was recurrence‐free survival (RFS), and the secondary study endpoint was overall survival (OS) after surgery in both the TM‐negative and TM‐positive groups.
2.4. Statistical analyses
Categorical and continuous variables were compared between the groups using Fisher's exact test or Pearson's chi‐square test and Student's t test, respectively. We estimated the survival rate using the Kaplan–Meier method and examined differences between groups using the log‐rank test. Furthermore, propensity score matching analysis was used to balance the characteristics of each group. The groups were adjusted through 1:1 matching, which was performed based on a logistic regression model that included clinicopathological factors, such as age, sex, smoking history, preoperative comorbidities, surgical treatment, histological subclassification, lymphovascular invasion, pathological stage, and adjuvant therapy. The identified caliper value was set at 0.2. All p values were two‐sided tested, and a p value less than .05 was considered statistically significant. All statistical analyses were performed using EZR version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R version 4.03 (The R Foundation for Statistical Computing, Vienna, Austria). 16
3. RESULTS
Table 1 shows the preoperative positive rate of each TM. CEA and CA19‐19 positivity gradually increased with increasing cancer stage, even in stages IA1 to IB. The baseline characteristics of patients in the TM‐negative and TM positive groups are summarized in Table 2. The average follow‐up period was 50.4 and 43.8 months in the TM‐negative and TM positive group, respectively. Compared with the TM‐positive group, the TM‐negative group comprised patients who were younger (p < .001), had fewer preoperative comorbidities (chronic obstructive pulmonary disease [p = .025] and interstitial pneumonia [p = .002]), and had smaller tumors (p < .001), as well as fewer smokers (p = .001). In addition, adenocarcinoma (p < .001) and early pathological stages (p = .001) were more common; while lymphovascular invasion (p < .001), visceral pleural invasion (p = .001), and EGFR expression (p < .001) were less common. Postoperative recurrence was more common in the TM‐positive group (15 patients [6.0%] vs. 34 patients [17.6%]).
TABLE 1.
Preoperative positive rates of tumor markers
| Variables | Pathological stage (n = 442) | ||||
|---|---|---|---|---|---|
| IA1 (n = 154) | IA2 (n = 124) | IA3 (n = 45) | IB (n = 119) | All (n = 442) | |
| CEA | 17 (11.0) | 22 (17.7) | 13 (28.9) | 38 (31.9) | 90 (20.4) |
| CYFRA21‐1 | 33 (21.4) | 33 (26.6) | 12 (26.7) | 45 (37.8) | 123 (27.8) |
| CA19‐9 | 12 (7.8) | 5 (4.0) | 6 (13.3) | 10 (8.4) | 33 (7.5) |
| SCC Ag | 12 (7.8) | 3 (2.4) | 2 (4.4) | 13 (10.9) | 30 (6.8) |
Note: Values are presented as n (%).
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CYFRA21‐1, cytokeratin‐19 fragment; SCC Ag, squamous cell carcinoma antigen.
TABLE 2.
Baseline characteristics of the overall cohort
| Variable | TM‐negative (n = 249) | TM‐positive (n = 193) | p Value |
|---|---|---|---|
| Sex | .203 | ||
| Male | 143 (57.4) | 123 (63.7) | |
| Female | 106 (42.6) | 70 (36.3) | |
| Age (years) | 68.6 (±8.6) | 71.2 (±7.5) | <.001 |
| Follow‐up period (months) | 50.4 (±31.4) | 43.8 (±31.7) | .031 |
| Smoking history | .001 | ||
| Yes | 140 (56.2) | 138 (71.5) | |
| No | 109 (43.8) | 55 (28.5) | |
| Preoperative comorbidity | |||
| COPD | 70 (28.1) | 74 (38.3) | .025 |
| IP | 18 (7.2) | 33 (17.1) | .002 |
| Extent of resection | .261 | ||
| Pneumonectomy | 1 (0.4) | 2 (1.0) | |
| Lobectomy | 183 (73.5) | 128 (66.3) | |
| Segmentectomy | 39 (15.7) | 43 (22.3) | |
| Wedge resection | 26 (10.4) | 20 (10.4) | |
| Pathological tumor size (cm) | 1.5 (±0.9) | 1.8 (±1.1) | <.001 |
| Tumor histology | <.001 | ||
| Adenocarcinoma | 206 (82.7) | 127 (65.8) | |
| Squamous cell carcinoma | 34 (13.7) | 48 (24.9) | |
| Others | 9 (3.6) | 18 (9.3) | |
| Lymphovascular invasion | <.001 | ||
| Yes | 50 (20.1) | 70 (36.3) | |
| No | 194 (77.9) | 119 (61.7) | |
| Not available | 5 (2.0) | 4 (2.1) | |
| Visceral pleural invasion | .001 | ||
| Yes | 38 (15.3) | 54 (28.0) | |
| No | 211 (84.7) | 139 (72.0) | |
| Pathological stage | .005 | ||
| IA1 | 98 (39.4) | 56 (29.0) | |
| IA2 | 75 (30.1) | 49 (25.4) | |
| IA3 | 25 (10.0) | 20 (10.4) | |
| IB | 51 (20.5) | 68 (35.2) | |
| EGFR mutation | <.001 | ||
| Yes | 108 (43.4) | 51 (26.4) | |
| No | 123 (49.4) | 125 (64.8) | |
| Not available | 18 (7.2) | 17 (8.8) | |
| Adjuvant therapy | .102 | ||
| Yes | 19 (7.6) | 7 (3.6) | |
| No | 230 (92.4) | 186 (96.4) | |
| Postoperative recurrence | 15 (6.0) | 34 (17.6) | <.001 |
| Preoperative TM levels | |||
| CEA (ng/ml) | 2.4 (±1.0) | 7.4 (±19.5) | <.001 |
| CYFRA21‐1 (U/ml) | 1.7 (±0.5) | 3.6 (±2.2) | <.001 |
| CA19‐9 (ng/ml) | 11.3 (±7.3) | 34.2 (±35.0) | .008 |
| SCC Ag (ng/ml) | 1.0 (±0.4) | 1.8 (±4.4) | .003 |
Note: Values are presented as n (%) or means (±SDs).
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; COPD, chronic obstructive pulmonary disease; CYFRA21‐1, cytokeratin‐19 fragment; EGFR, epidermal growth factor receptor; IP, interstitial pneumonia; SCC Ag, squamous cell carcinoma antigen; TM, tumor marker.
Next, we performed a 1:1 propensity score matching analysis. The baseline characteristics of the matched stage I NSCLC patients are listed in Table 3. There were no statistically significant differences in clinicopathological factors, except preoperative recurrences and TM levels, between the two groups. On the other hand, preoperative TM levels of CEA, CYFRA21‐1, CA19‐9, and SCC Ag remained significantly different.
TABLE 3.
Baseline characteristics of propensity score matched pairs
| Variable | Propensity score matched pairs | ||
|---|---|---|---|
| TM‐negative (n = 150) | TM‐positive (n = 150) | p Value | |
| Sex | ˃.999 | ||
| Male | 92 (61.3) | 93 (62.0) | |
| Female | 58 (38.7) | 57 (38.0) | |
| Age (years) | 70.4 (±8.1) | 70.3 (±7.5) | .900 |
| Follow‐up period (months) | 46.4 (±27.5) | 47.3 (±33.2) | .803 |
| Smoking history | .904 | ||
| Yes | 96 (64.0) | 98 (65.3) | |
| No | 54 (36.0) | 52 (34.7) | |
| Preoperative comorbidity | |||
| COPD | 53 (35.3) | 47 (31.3) | .540 |
| IP | 13 (8.7) | 14 (9.3) | ˃.999 |
| Extent of resection | .951 | ||
| Pneumonectomy | 1 (0.7) | 1 (0.7) | |
| Lobectomy | 102 (68.0) | 106 (70.7) | |
| Segmentectomy | 29 (19.3) | 26 (17.3) | |
| Wedge resection | 18 (12.0) | 17 (11.3) | |
| Pathological tumor size (cm) | 1.5 (±1.0) | 1.7 (±1.0) | .214 |
| Tumor histology | .891 | ||
| Adenocarcinoma | 115 (76.7) | 115 (76.7) | |
| Squamous cell carcinoma | 27 (18.0) | 25 (16.7) | |
| Others | 8 (5.3) | 10 (6.7) | |
| Lymphovascular invasion | .629 | ||
| Yes | 38 (25.3) | 42 (28.0) | |
| No | 112 (74.7) | 108 (72.0) | |
| Not available | 5 (2.0) | 4 (2.1) | |
| Visceral pleural invasion | ˃.999 | ||
| Yes | 29 (19.3) | 30 (20.0) | |
| No | 121 (80.7) | 120 (80.0) | |
| Pathological stage | .867 | ||
| IA1 | 58 (38.7) | 52 (34.7) | |
| IA2 | 40 (26.7) | 43 (28.7) | |
| IA3 | 13 (8.7) | 16 (10.7) | |
| IB | 39 (26.0) | 39 (26.0) | |
| EGFR mutation | .533 | ||
| Yes | 54 (36.0) | 46 (30.7) | |
| No | 89 (59.3) | 90 (60.0) | |
| Not available | 7 (4.7) | 14 (9.3) | |
| Adjuvant therapy | ˃.999 | ||
| Yes | 6 (4.0) | 7 (4.7) | |
| No | 144 (96.0) | 186 (95.3) | |
| Postoperative recurrence | 10 (6.7) | 24 (16.0) | .017 |
| Preoperative TM levels | |||
| CEA (ng/ml) | 2.6 (±1.1) | 7.9 (±22.0) | .003 |
| CYFRA21‐1 (U/ml) | 1.8 (±0.5) | 3.3 (±1.8) | <.001 |
| CA19‐9 (ng/ml) | 11.6 (±0.4) | 37.2 (±152.2) | .041 |
| SCC Ag (ng/ml) | 1.0 (±0.4) | 1.8 (±4.9) | .045 |
Note: Values are presented as n (%) or means (±SDs).
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; COPD, chronic obstructive pulmonary disease; CYFRA21‐1, cytokeratin‐19 fragment; EGFR, epidermal growth factor receptor; IP, interstitial pneumonia; SCC Ag, squamous cell carcinoma antigen; TM, tumor marker.
3.1. Survival analyses
In the unmatched cohort, the 5‐year RFS (92.6% vs. 79.1%; p < .01) and OS (86.3% vs. 68.6%; p < .01) rates showed significant differences between the two groups (Figure 2A,B). After propensity score matching, the patients in the TM‐negative group still exhibited improved 5‐year RFS (92.1% vs. 81.4%; p = .01) and OS (87.6% vs. 72.6%; p < .01) rates (Figure 2C,D). These data suggested that combined negativity of preoperative serum TMs, such as CEA, CYFRA21‐1, CA19‐9, and SCC Ag, is an independent prognostic factor.
FIGURE 2.
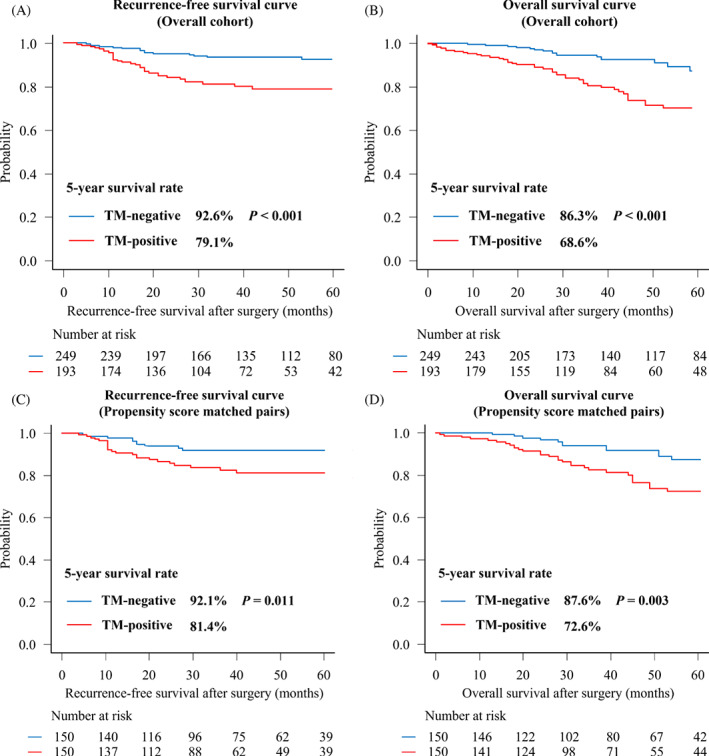
Kaplan–Meier curves for stage I non‐small cell lung cancer according to preoperative tumor marker (TM) levels (TM‐negative; blue, TM‐positive; red). (A) Recurrence‐free survival (RFS) and (B) overall survival curves for overall patients, (C) RFS, and (D) OS curves after propensity score matching
4. DISCUSSION
Although many biomarkers for lung cancer have been identified, 17 , 18 several serum TMs are used more widely, and the use of such assays is minimally invasive, convenient, and relatively inexpensive in clinical practice. However, the clinical significance of serum TM measurement remains controversial. 19 , 20 The overall TM positivity rates were lower in our study than in previous studies (Table 1). For example, studies that also included patients with stage I–III completely resected NSCLC 19 , 21 reported preoperative serum CEA levels of 33%–38%, compared with the 20.4% reported in our study focused on stage I patients. This finding could be explained by the high rate of surgery for early‐stage lung cancer. In general, serum TM levels gradually increase as lung cancer progresses. 3 , 22 , 23 , 24 In contrast, the frequency of surgery for early‐stage lung cancer is increasing, especially in Japan. 25 , 26 In 2017, stage I lung cancer accounted for 70.9% of invasive lung cancer surgeries, according to a report by the Japanese Association for Thoracic Surgery. 25 The most recently reported preoperative TM levels are possibly lower than the levels reported previously. 27 , 28 In patients with elevated preoperative TM levels, re‐elevation of postoperative TM levels could help rule out recurrence. However, in patients with TM‐negative lung cancer, it can be considered as a predictor of prognosis.
Identifying EGFR mutations that may be the target of molecular therapy is crucial for NSCLC treatment. In fact, the use of tyrosine kinase inhibitors dramatically improves RFS and OS, especially in patients with advanced NSCLC. The previous studies reported an association between the levels of TMs, such as CEA, CYFRA21‐1, CA19‐9, and SCC Ag, and the rate of EGFR mutations. 28 , 29 More specifically, the rate of EGFR mutations increases in proportion to CEA levels. Demographic analyses have shown that a high prevalence of EGFR mutation is observed in women, nonsmokers, East Asian populations, and patients with adenocarcinoma, 30 , 31 which is consistent with the findings of our study. Our TM‐negative group had greater rates of EGFR mutations (43.4% vs. 26.4%) probably because of the predominance of nonsmokers and adenocarcinoma, as well as early‐stage lung cancer. However, after propensity score matching, there was no difference in the characteristics of patients with EGFR mutation, and EGFR status did not affect prognosis.
Regarding follow‐up methods after lung cancer surgery, the European Society for Medical Oncology guidelines recommend that medical history be monitored and physical examination performed every 6 months for 2 years after surgery when a period of relatively high recurrence rate is observed, with regular annual consultation thereafter. 32 In addition, it is recommended that contrast‐enhanced CT be performed at least 12 and 24 months after surgery. The American Society of Clinical Oncology also recommends surveillance via a clinical examination (including CT) every 6 months for 2 years after surgery but does not recommend TM measurement for surveillance. 33 In our study, pathological tumor size, visceral pleural invasion, and lymphovascular invasion, which are known prognostic factors in lung cancer, were significantly greater in the TM‐positive group. Although it would be complicated to try and to predict the prognosis by combining these factors, it may be possible to simplify postoperative follow‐up by classifying stage I NSCLC patients into TM‐negative and TM‐positive groups.
This study has some limitations. First, our study was not based on multicenter cohort data, which complicates the generalizability of our findings. Second, we limited ourselves to the examination of only four TMs, which are routinely measured at our institution. Further clinical studies are needed to assess the prognostic importance of other markers in TM‐negative cases.
In conclusion, our study suggests that patients with NSCLC who are preoperatively negative for all serum TMs, such as CEA, CYFRA21‐1, CA19‐9, and SCC Ag, represent a subgroup with a particularly good prognosis.
AUTHOR CONTRIBUTIONS
Yuichiro Onuki: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Hirochika Matsubara: Conceptualization (equal); methodology (equal); supervision (equal); validation (equal); writing – review and editing (equal). Ryunosuke Koizumi: Data curation (equal); resources (equal). Mamoru Muto: Data curation (equal); resources (equal). Harunobu Sasanuma: Data curation (equal); resources (equal). Daisuke Sato: Data curation (equal); validation (equal). Aya Sugimura: Data curation (equal); validation (equal). Tsuyoshi Uchida: Data curation (equal); validation (equal). Hiroyasu Matsuoka: Data curation (equal); software (equal). Hiroyuki Nakajima: Project administration (equal); supervision (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ETHICS STATEMENT
This study was approved by the Institutional Review Board of the Yamanashi University Hospital (Approval No. 2506).
PATIENT CONSENT STATEMENT
Informed consent was obtained in the form of opt‐out on the website (https://www.med.yamanashi.ac.jp/rinri/ippan.html) by the decision of the Institutional Review Board.
ACKNOWLEDGMENTS
The authors wish to thank Editage (www.editage.com) for English language editing.
Onuki Y, Matsubara H, Koizumi R, et al. Prognostic evaluation of preoperative serum tumor marker‐negative cases in non‐small cell lung cancer: A retrospective study. Cancer Reports. 2023;6(2):e1696. doi: 10.1002/cnr2.1696
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941‐1953. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. [DOI] [PubMed] [Google Scholar]
- 3. Icard P, Regnard JF, Essomba A, Panebianco V, Magdeleinat P, Levasseur P. Preoperative carcinoembryonic antigen level as a prognostic indicator in resected primary lung cancer. Ann Thorac Surg. 1994;58(3):811‐814. [DOI] [PubMed] [Google Scholar]
- 4. Hsu WH, Huang CS, Hsu HS, et al. Preoperative serum carcinoembryonic antigen level is a prognostic factor in women with early non‐small‐cell lung cancer. Ann Thorac Surg. 2007;83(2):419‐424. [DOI] [PubMed] [Google Scholar]
- 5. Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76(2):138‐143. [DOI] [PubMed] [Google Scholar]
- 6. Nasralla A, Lee J, Dang J, Turner S. Elevated preoperative CEA is associated with subclinical nodal involvement and worse survival in stage I non‐small cell lung cancer: a systematic review and meta‐analysis. J Cardiothorac Surg. 2020;15(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cedrés S, Nuñez I, Longo M, et al. Serum tumor markers CEA, CYFRA21‐1, and CA‐125 are associated with worse prognosis in advanced non‐small‐cell lung cancer (NSCLC). Clin Lung Cancer. 2011;12(3):172‐179. [DOI] [PubMed] [Google Scholar]
- 8. Reinmuth N, Brandt B, Semik M, et al. Prognostic impact of Cyfra21‐1 and other serum markers in completely resected non‐small cell lung cancer. Lung Cancer. 2002;36(3):265‐270. [DOI] [PubMed] [Google Scholar]
- 9. Lee T, Teng TZJ, Shelat VG. Carbohydrate antigen 19–9—tumor marker: past, present, and future. World J Gastrointest Surg. 2020;12(12):468‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holdenrieder S, Molina R, Qiu L, et al. Technical and clinical performance of a new assay to detect squamous cell carcinoma antigen levels for the differential diagnosis of cervical, lung, and head and neck cancer. Tumour Biol. 2018;40(4):1010428318772202. [DOI] [PubMed] [Google Scholar]
- 11. Sánchez De Cos J, Masa F, de la Cruz JL, Disdier C, Vergara C. Squamous cell carcinoma antigen (SCC Ag) in the diagnosis and prognosis of lung cancer. Chest. 1994;105(3):773‐776. [DOI] [PubMed] [Google Scholar]
- 12. Wang J, Chu Y, Li J, et al. Development of a prediction model with serum tumor markers to assess tumor metastasis in lung cancer. Cancer Med. 2020;9(15):5436‐5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molina R, Marrades RM, Augé JM, et al. Assessment of a combined panel of six serum tumor markers for lung cancer. Am J Respir Crit Care Med. 2016;193(4):427‐437. [DOI] [PubMed] [Google Scholar]
- 14. Mazzone PJ, Wang XF, Han X, et al. Evaluation of a serum lung cancer biomarker panel. Biomark Insights. 2018;13:1177271917751608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39‐51. [DOI] [PubMed] [Google Scholar]
- 16. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D'Amico TA, Brooks KR, Joshi MB, et al. Serum protein expression predicts recurrence in patients with early‐stage lung cancer after resection. Ann Thorac Surg. 2006;81(6):1982‐1987. discussion 1987. [DOI] [PubMed] [Google Scholar]
- 18. Seijo LM, Peled N, Ajona D, et al. Biomarkers in lung cancer screening: achievements, promises, and challenges. J Thorac Oncol. 2019;14(3):343‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeda R, Yoshida J, Hishida T, et al. Late recurrence of non‐small cell lung cancer more than 5 years after complete resection: incidence and clinical implications in patient follow‐up. Chest. 2010;138(1):145‐150. [DOI] [PubMed] [Google Scholar]
- 20. Matsuoka K, Sumitomo S, Nakashima N, Nakajima D, Misaki N. Prognostic value of carcinoembryonic antigen and CYFRA21‐1 in patients with pathological stage I non‐small cell lung cancer. Eur J Cardiothorac Surg. 2007;32(3):435‐439. [DOI] [PubMed] [Google Scholar]
- 21. Díez M, Torres A, Maestro ML, et al. Prediction of survival and recurrence by serum and cytosolic levels of CEA, CA125 and SCC antigens in resectable non‐small‐cell lung cancer. Br J Cancer. 1996;73(10):1248‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Q, Jia Z, Gao J, et al. Auxiliary diagnosis of lung cancer on the basis of a serum protein biomarker panel. J Cancer. 2021;12(10):2835‐2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Gaast A, Schoenmakers CH, Kok TC, Blijenberg BG, Cornillie F, Splinter TA. Evaluation of a new tumour marker in patients with non‐small‐cell lung cancer: Cyfra 21.1. Br J Cancer. 1994;69(3):525‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moro D, Villemain D, Vuillez JP, Delord CA, Brambilla C. CEA, CYFRA21–1 and SCC in non‐small cell lung cancer. Lung Cancer. 1995;13(2):169‐176. [DOI] [PubMed] [Google Scholar]
- 25. Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery , Shimizu H, Okada M, et al. Thoracic and cardiovascular surgeries in Japan during 2017: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2020;68(4):414‐449. [DOI] [PubMed] [Google Scholar]
- 26. Sawabata N, Miyaoka E, Asamura H, et al. Japanese Joint Committee for Lung Cancer Registration. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6(7):1229‐1235. [DOI] [PubMed] [Google Scholar]
- 27. Zhang H, He M, Wan R, Zhu L, Chu X. Establishment and evaluation of EGFR mutation prediction model based on tumor markers and CT features in NSCLC. J Healthc Eng. 2022;2022:8089750‐8089756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okada M, Nishio W, Sakamoto T, et al. Effect of histologic type and smoking status on interpretation of serum carcinoembryonic antigen value in non‐small cell lung carcinoma. Ann Thorac Surg. 2004;78(3):1004‐1009. discussion 1009. [DOI] [PubMed] [Google Scholar]
- 29. Jiang R, Wang X, Li K. Predictive and prognostic value of preoperative serum tumor markers is EGFR mutation‐specific in resectable non‐small‐cell lung cancer. Oncotarget. 2016;7(18):26823‐26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339‐346. [DOI] [PubMed] [Google Scholar]
- 31. Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98(12):1817‐1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non‐small‐cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28(suppl_4):iv1‐iv21. [DOI] [PubMed] [Google Scholar]
- 33. Colt HG, Murgu SD, Korst RJ, Slatore CG, Unger M, Quadrelli S. Follow‐up and surveillance of the patient with lung cancer after curative‐intent therapy: diagnosis and management of lung cancer, 3rd edn: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143:437‐454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


