Abstract
BACKGROUND:
Malignant hyperthermia (MH), a pharmacogenetic disorder of skeletal muscle, presents with a potentially lethal hypermetabolic reaction to certain anesthetics. However, some MH-susceptible patients experience muscle weakness, fatigue, and exercise intolerance in the absence of anesthetic triggers. The objective of this exploratory study was to elucidate the pathophysiology of exercise intolerance in patients tested positive for MH with the caffeine-halothane contracture test. To this end, we used phosphorus magnetic resonance spectroscopy, blood oxygen level–dependent functional magnetic resonance imaging (MRI), and traditional exercise testing to compare skeletal muscle metabolism in MH-positive patients and healthy controls.
METHODS:
Skeletal muscle metabolism was assessed using phosphorus magnetic resonance spectroscopy and blood oxygen level–dependent functional MRI in 29 MH-positive patients and 20 healthy controls. Traditional measures of physical capacity were employed to measure aerobic capacity, anaerobic capacity, and muscle strength.
RESULTS:
During 30- and 60-second exercise, MH-positive patients had significantly lower ATP production via the oxidative pathway compared to healthy controls. MH-positive patients also had a longer recovery time with blood oxygen level–dependent functional MRI compared to healthy controls. Exercise testing revealed lower aerobic and anaerobic capacity in MH-positive patients compared to healthy controls.
CONCLUSIONS:
Results of this exploratory study suggest that MH-positive patients have impaired aerobic metabolism compared to healthy individuals. This could explain the exercise intolerance exhibited in MH-susceptible patient population.
Malignant hyperthermia (MH), associated with mutations in RYR1, CACNA1S, or STAC3 genes,1–3 is a hypermetabolic reaction to anesthesia caused by an uncontrollable release of Ca2+ from the sarcoplasmic reticulum. There is an increasing number of reports of MH-susceptible (MHS) patients, as per a positive caffeine-halothane contracture test (CHCT)/in vitro contracture test, suffering from heat stroke,4 exercise-induced rhabdomyolysis,5 postexertional muscle cramping,6 and idiopathic elevated creatine kinase (CK).7 Some of MHS patients experience muscle pain, weakness, and cramping, which tend to worsen over time.8–10 These reports, collectively, suggest that MHS individuals may have skeletal muscle metabolism abnormalities even in the absence of triggering anesthetics. Studies on animal models of MH11,12 have indeed indicated a bioenergetic compromise in these animals in the absence of anesthetic triggers.
These reports and studies contradict the conventional perception of MH susceptibility as a subclinical anesthetic-induced disorder. To understand the exercise intolerance in these patients and to realize all aspects of MH susceptibility as the only “specific disease to anesthesiologists,” we conducted the first human study to examine the metabolic dysfunction in MHS patients in the absence of anesthetic triggers.
The aim of our study was to assess skeletal muscle metabolism in MHS patients and to explore mechanisms of functional impairment, especially exercise intolerance. We hypothesized that MHS patients have impaired skeletal muscle metabolism with reduced oxidative phosphorylation (OXPHOS) ATP production, which can affect their functional performance and exercise capacity.
To test our hypothesis, we utilized phosphorus magnetic resonance spectroscopy (31P-MRS), an in vivo tool to assess skeletal muscle metabolism,13 which provides a noninvasive way to measure cellular high-energy phosphates (ATP), phosphocreatine (PCr), inorganic phosphate (Pi), and intracellular pH. 31P-MRS allows probing of the 3 bioenergetic systems: the PCr system (ATP-CP), anaerobic glycolysis, and OXPHOS that are active during exercise. We also used blood oxygen level–dependent functional magnetic resonance imaging (BOLD fMRI), another in vivo technique, for the first time in MHS patients to assess changes that reflect exercise-induced alteration in the deoxyhemoglobin-to-oxyhemoglobin ratio that serves as a surrogate marker of altered muscle metabolism, blood volume, and blood flow.14 Previous use of 31P-MRS in MHS patients showed equivocal results.15–17
METHODS
Participants
This article adheres to the applicable Equator guidelines. Following research ethics board approval, for this cohort study from December 2012 to September 2015, consecutive patients who tested positive with the CHCT, performed according to the North American criteria,18 were approached by the Malignant Hyperthermia Investigation Unit at Toronto General Hospital. Thirty-five patients were recruited; however, 2 patients dropped out of the study due to scheduling constraints, and 4 patients failed to attend the magnetic resonance imaging (MRI) session, leaving 29 in the CHCT-positive group. Of these, 19 patients were tested due to either a previous MH reaction or family history of MH, and 10 patients were tested due to frequent exercise or heat-induced rhabdomyolysis, determined by neurologists’ reports. Participants were informed of the risks and benefits associated with this study and signed a written informed consent. Results on full screening of RYR1 and CACNA1S genes, resting CK and histopathology (as per previous published methods)19 on the CHCT-positive patients were also included. Twenty healthy age- and sex-matched controls (HCs) were recruited from University of Toronto campus and surrounding community. HCs and CHCT-positive patients were included if they were able to tolerate moderate to hard exercise. Because participants only completed the physical activity questionnaire on the day of testing, no formal matching for physical activity level was administered. However, participants were matched as best as possible based on information gathered from informal interviews over the phone. Participants were excluded if they were deemed unable to perform strenuous physical activity, had any neurological or muscular disease besides MH, or had any metal implants or devices that were not MR compatible.
Anthropometric Measures and Physical Activity Questionnaire
Height and weight were measured with a wall-mounted stadiometer (Scale-Tronix; Global Medical Products, Burlington, ON, Canada) and a low-profile stand-on scale (Scale-Tronix, 5122), respectively. The Habitual Activity Estimation Scale was administered to assess physical activity level. This questionnaire is used to estimate hours spent “inactive” (time spent lying down), “somewhat inactive” (time spent sitting), “somewhat active” (time spent standing or walking), and “very active” (activity in which someone is sweating or breathing hard) during a typical weekday and weekend.20 The questionnaire accuracy has been validated in adults with chronic illnesses.21
Magnetic Resonance Imaging and Spectroscopy
All MRI and spectroscopy data were collected using a Siemens Magnetom Tim Trio 3 Tesla MRI (Siemens AG; Medical Solutions, Erlangen, Germany) at The Hospital for Sick Children. A dual-tuned 1H/31P transmit/receive surface coil was used for imaging and spectroscopy acquisition. Patients were placed supine and head first inside the magnet. First, T1-weighted anatomical images were acquired axially from the mid-quadriceps region. Following anatomical imaging, with a participant remaining in the scanner, 31P-MRS and BOLD MRI scanning were performed with the same coil.
Ten resting 31P-MRS spectra were acquired and then averaged to determine baseline high-energy phosphate content. Subsequently, participants performed a leg extension exercise using an up-down ergometer with power meter (Lode BV Medical Technology, Groningen, the Netherlands) while inside the MRI. They performed 30 seconds of maximal exercise (very high intensity exercise), 60 seconds at 85% of maximum (high intensity exercise), and 5 bouts of 30-second exercise at 65% of maximum, separated by 15 seconds of rest (moderate intensity exercise). Spectra were obtained immediately following each exercise bout and in between each bout of the 5 × 30-second exercise. ATP production rates in each of the bioenergetics systems were calculated according to the equations developed by Newcomer and Boska.22 This protocol has been described previously by our group.23 ATP production is the fundamental energy currency for all cellular function in the human body, but we chose to measure this in skeletal muscle because the bioenergetic systems that create ATP can be stressed voluntarily and safely. This allows us to probe physiology noninvasively. The technique allows for the measurement of ATP production with a resolution of 0.01 mM/s.
Following 31P-MRS data acquisition, participants remained in the MRI in the same position for the BOLD signal acquisition. Measures were taken before and after three 1-minute bouts of exercise separated by 2 minutes of rest, using the same up-down ergometer as used for the 31P-MRS protocol. T2*-weighted BOLD images were obtained using a gradient echo planar imaging sequence. More details regarding the parameters for MR data acquisition are provided in Supplemental Digital Content, Appendix, http://links.lww.com/AA/B811.
MR Data Analysis
Spectral analysis was performed using Java-based magnetic resonance user interface (v. 4.0).24 The areas under the curve for Pi, PCr, and the 3 peaks of ATP (γ, α, and β) were calculated and then normalized to 41.3 mmol, the total sum of muscle phosphate,25 to determine the concentration of each peak. Changes in intracellular magnesium (Mg2+) and pH during exercise were calculated from the chemical shift of β-ATP with respect to PCr and Pi with respect to PCr, respectively.26
Two authors, who were blinded to the group allocations, performed the 31P-MRS and BOLD fMRI analysis. Both authors have extensive experience in this type of analysis, and there was concordance between the 2 assessors in 100% of the cases. See Supplemental Digital Content, Appendix, http://links.lww.com/AA/B811 for more details regarding MR data analysis.
Functional Exercise Performance Measurements
Aerobic Capacity.
To estimate maximal oxygen uptake (Vo2max), the Young Men′s Christian Association cycle ergometer submaximal test27 was performed using a cycle ergometer (Upright Corival, Lode, the Netherlands). This cycling protocol involves 3-minute bouts of incremental workloads until approximately 85% of age-predicted maximum heart rate (220 – age) is achieved. This test is used as a measure of aerobic fitness according to the CSEP Canadian Physical Activity, Fitness and Lifestyle Approach (2010).28
Skeletal Muscle Strength.
To assess lower body strength and power, the vertical jump test was performed using a vertical measuring device (Vertec, Sports Imports, Hilliard, OH). Participants performed a countermovement jump by squatting and then jumping in 1 fluid motion. They repeated the vertical jump test until they were unable to improve their jump height, and their vertical jump height was recorded (in centimeters). Lower body power was then calculated to correct for body mass.29 To assess upper body strength, participants used a handgrip dynamometer (Lafayette Instrument Company model 78010; Lafayette, IN). The highest score after 3 trials was recorded (in kilograms).
Anaerobic Capacity.
Anaerobic capacity was assessed using the Wingate Anaerobic Test30 on a cycle ergometer (Ergomedic 849E, Monark, Sweden). Following a warm-up, consisting of 3 minutes of easy pedaling and three 10-second sprints, participants were instructed to cycle as fast as possible with zero resistance on the bike. As soon as the participant reached maximum pedaling speed, predetermined during warm-up, resistance was automatically applied and the participant biked at maximal effort for 30 seconds. Load was set at 8% of body weight. This is an estimate of optimal load for a recreationally active adult population based on recommendations by Bar-Or and coworkers.31
Statistical Analysis and Sample Size Calculation
Independent samples t tests and Fisher exact tests were performed to compare numerical and categorical parameters, respectively. For habitual physical activity levels, nonparametric tests were performed (Mann-Whitney U and Kruskal-Wallis independent samples tests) due to high variability in the data. The Pearson r coefficient was performed to determine the relationship between measures of functional exercise capacity and in vivo measures of metabolism. All statistical analyses were performed with SPSS (version 22.0, released 2013; IBM Corporation, Armonk, NY). Measures were reported as mean ± standard deviation. Statistical significance was set at P < .05; however, correction for multiple comparisons was done using Benjamini-Hochberg procedure, with false discovery rate set at 0.2.
The primary outcome for this study was ATP production rate as measured by 31P-MRS. There was no previous study on measurement of ATP production in MHS patients; however, a priori power was calculated based on a previous study by our group on Turner Syndrome.23 Minimal detectable differences in ATP production were calculated to be 0.3 ± 0.3 mM/s (mean ± standard deviation). To achieve a power of 0.8, 34 total participants (CHCT and HC) were required.
RESULTS
Anthropometric Measures and Habitual Physical Activity Levels
Participants’ descriptive characteristics, including the results on resting CK values, genetic testing, and histopathology for CHCT-positive patients, are shown in Table 1. There were no significant differences in age, height, weight, or body mass index between the CHCT+ and HC groups.
Table 1.
Descriptive Characteristics of CHCT-Positive Patients and HCs
| Characteristic | HC (n = 20) | CHCT+ (n = 29) | P Valuea |
|---|---|---|---|
| Sex (F/M) | 11 F/9 M | 14 F/15 M | |
| Age (y), mean ± SD | 39.7 ± 14.6 | 39.32 ± 14.1 | .92 |
| Height (cm), mean ± SD | 171.3 ± 8.7 | 172.0 ± 7.6 | .75 |
| Weight (kg), mean ± SD | 75.2 ± 14.7 | 76.2 ± 20.3 | .85 |
| BMI (kg/m2), mean ± SD | 25.5 ± 4.0 | 25.7 ± 6.5 | .90 |
| Genetic testing | |||
| RYR1 | N/A | Causative (n = 3)b R2454H; G2434R |
|
| Polymorphism (n = 3) G2060C; E3583Q; Q3756E |
|||
| VUS (n = 11) C64R; R401C, P978L; I1571V; R3366H; Y3540F; Y3933C; A3421V; R4737W; V4849I |
|||
| Negative (n = 12) | N/A | ||
| Resting CK range (IU/L) | N/A | 43–729 | N/A |
| Abnormal histopathologyc | N/A | 7/29 Type II atrophy Fiber size variation |
N/A |
Abbreviations: BMI, body mass index; CHCT, caffeine-halothane contracture test; CK, creatine kinase; F, female; HC, healthy age- and sex-matched controls; M, male; N/A, not applicable; SD, standard deviation; VUS, variant of unknown significance.
Independent samples t test was used to compare means between HC and CHCT+ groups.
Two patients carried the same mutation.
Histopathological examinations were performed by a neuromuscular pathologist on the samples excised for CHCT.
There were significant differences between CHCT-positive patients and HC regarding the hours spent “very active” on weekdays (CHCT+: median, 0.0 [interquartile range {IQR}, 0.0–0.98] hours versus HC: median, 0.92 [IQR, 0.8–1.28] hours, P = .004) and the hours spent “very active” on weekends (CHCT+: median, 0.0 [IQR, 0.0–0.0] hours versus HC: median, 0.88 [IQR, 0.0–1.6] hours, P = .002).
Magnetic Resonance Imaging and Spectroscopy
A summary of ATP production rate during exercise as measured by 31P-MRS is shown in Table 2. During 30- and 60-second exercise tests, CHCT-positive patients had significantly lower ATP production via OXPHOS than HC (Figure 1). Recovery of PCr, postexercise Pi, ratio of Pi to PCr, and Mg2+ were calculated as secondary outcome measures. Resting levels of Pi, PCr, ATP, pH, Mg2+, or Pi:PCr, as well as postexercise Pi, Pi:PCr, or Mg2+, were not significantly different between CHCT-positive patients and HC.
Table 2.
ATP Production Rate as Measured by 31P-MRS for CHCT-Positive Patients and HCs During Exercise
| 31P-MRS Measure | Exercise Bouta | HC (n = 19) | CHCT+ (n = 27)b | P Valuec |
|---|---|---|---|---|
| ATP production rate (mM/s), ATP-CP System (mean ± SD) | 30 s | 0.32 ± 0.11 | 0.30 ± 0.11 | .38 |
| MH: 0.29 ± 0.11d | ||||
| EHI: 0.31 ± 0.12d | ||||
| 60 s | 0.20 ± 0.06 | 0.20 ± 0.06 | .84 | |
| MH: 0.20 ± 0.06 | ||||
| EHI: 0.19 ± 0.06 | ||||
| 5 × 30 s | 0.33 ± 0.11 | 0.36 ± 0.11 | .39 | |
| MH: 0.37 ± 0.11 | ||||
| EHI: 0.34 ± 0.13 | ||||
| ATP production rate (mM/s), a naerobic glycolysis (mean ± SD) | 30 s | 0.62 ± 0.31 | 0.53 ± 0.30 | .18 |
| MH: 0.48 ± 0.25 | ||||
| EHI: 0.56 ± 0.32 | ||||
| 60 s | 0.49 ± 0.28 | 0.49 ± 0.24 | .91 | |
| MH: 0.51 ± 0.24 | ||||
| EHI: 0.41 ± 0.22 | ||||
| 5 × 30 s | 0.74 ± 0.53 | 0.89 ± 0.53 | .40 | |
| MH: 0.94 ± 0.52 | ||||
| EHI: 0.72 ± 0.54 | ||||
| ATP production rate (mM/s), oxidative phosphorylation (mean ± SD) | 30 s | 0.33 ± 0.13 | 0.25 ± 0.15 | .05e |
| MH: 0.25 ± 0.15 | ||||
| EHI: 0.23 ± 0.13 | ||||
| 60 s | 0.34 ± 0.11 | 0.27 ± 0.11 | .03e | |
| MH: 0.26 ± 0.11 | ||||
| EHI: 0.27 ± 0.09 | ||||
| 5 × 30 s | 0.25 ± 0.08 | 0.24 ± 0.09 | .71 | |
| MH: 0.25 ± 0.09 | ||||
| EHI: 0.20 ± 0.07 |
Abbreviations: ATP-CP system, phosphocreatine system; CHCT, caffeine-halothane contracture test; EHI, exertional heat illness; HC, healthy age- and sex-matched controls; MH, malignant hyperthermia; 31P-MRS, phosphorus magnetic resonance spectroscopy; SD, standard deviation.
Measures were calculated for each of the exercise bouts: 30-s exercise (30 s), 60-s exercise (60 s), and 5 × 30-s exercise (5 × 30 s).
Missing data for 2 CHCT patients due to claustrophobia and poor signal quality.
Independent samples t test was used to compare means between HC and CHCT groups.
The CHCT+ group was divided into those with a history or family history of MH (MH group) and those with exertional heat illness (EHI group); however, because of small sample sizes, no statistical analyses were performed on these subgroups.
Significant, based on Benjamini-Hochberg correction.
Figure 1.
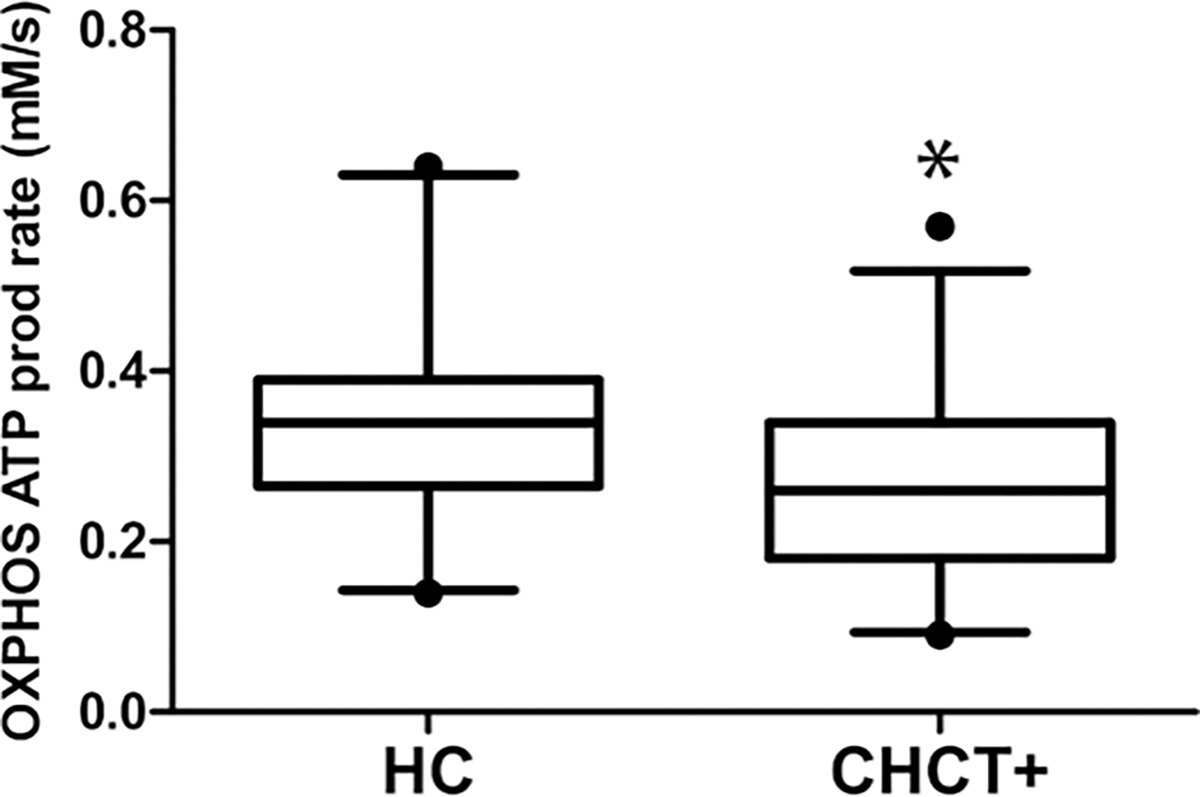
Box and whiskers plot of ATP production rate as measured by phosphorus magnetic resonance spectroscopy (31P-MRS). CHCT-positive patients had significantly lower OXPHOS ATP production rates than HCs (HC: 0.34 ± 0.11 mM/s versus CHCT+: 0.27 ± 0.11 mM/s, P = .03, r = 0.30). Whiskers display data from minimum to maximum with 5%–95% percentile; box shows interquartile range from first to third quartile, and median value is shown as a horizontal line within the box, according to Tukey post hoc test. CHCT indicates caffeine-halothane contracture test; HC, healthy age- and sex-matched control; OXPHOS, oxidative phosphorylation.
A summary of the BOLD fMRI results is presented in Table 3. A significant difference was detected between CHCT-positive patients and HC for the response time of the BOLD fMRI signal recovery curve following exercise (CHCT+: 12.86 ± 3.07 seconds versus HC: 9.54 ± 3.73 seconds, P = .01, r = .44). Baseline BOLD fMRI signal intensity, change in signal intensity, and the half-time recovery were not significantly different between CHCT-positive patients and HC.
Table 3.
BOLD fMRI Measures CHCT-Positive Patients and HC Patients
| BOLD fMRI Measure | HC (n = 15) | CHCT+ (n = 20)a | P Valueb |
|---|---|---|---|
| S0 (arbitrary units), mean ± SD | 0.92 ± 0.04 | 0.92 ± 0.04 | 1.00 |
| MH: 0.92 ± 0.04c | |||
| EHI: 0.91 ± 0.04c | |||
| κ (arbitrary units), mean ± SD | 0.10 ± 0.03 | 0.09 ± 0.03 | .54 |
| MH: 0.10 ± 0.03 | |||
| EHI: 0.09 ± 0.04 | |||
| β (s), mean ± SD | 25.94 ± 6.81 | 25.65 ± 10.55 | .93 |
| MH: 27.15 ± 11.63 | |||
| EHI: 21.13 ± 4.61 | |||
| α (s), mean ± SD | 9.54 ± 3.73 | 12.86 ± 3.07 | .01d |
| MH: 12.47 ± 2.98 | |||
| EHI: 14.02 ± 3.41 |
Abbreviations: BOLD fMRI, blood oxygen level-dependent functional magnetic resonance imaging; CHCT, caffeine-halothane contracture test; EHI, exertional heat illness; HC, healthy age- and sex-matched controls; MH, malignant hyperthermia; S0, baseline signal intensity; SD, standard deviation; α, response of the sigmoid function; β, recovery half-time; κ, change in baseline BOLD signal intensity.
Missing data for 5 HC and 9 CHCT patients due to motion artifacts.
Independent samples t test were used to compare means between HC and CHCT+ groups.
The CHCT+ group was divided into those with a history or family history of MH (MH group) and those with exertional heat illness (EHI group); however, because of small sample sizes, no statistical analyses were performed on these subgroups.
Significant, based on Benjamini-Hochberg correction.
Exercise Performance
Aerobic and Anaerobic Capacity.
CHCT-positive patients had significantly lower predicted Vo2max than HC (CHCT+: 33.2 ± 7.1 mL/kg/min versus HC: 38.2 ± 6.7 mL/kg/min, P = .02; Figure 2A).
Figure 2.
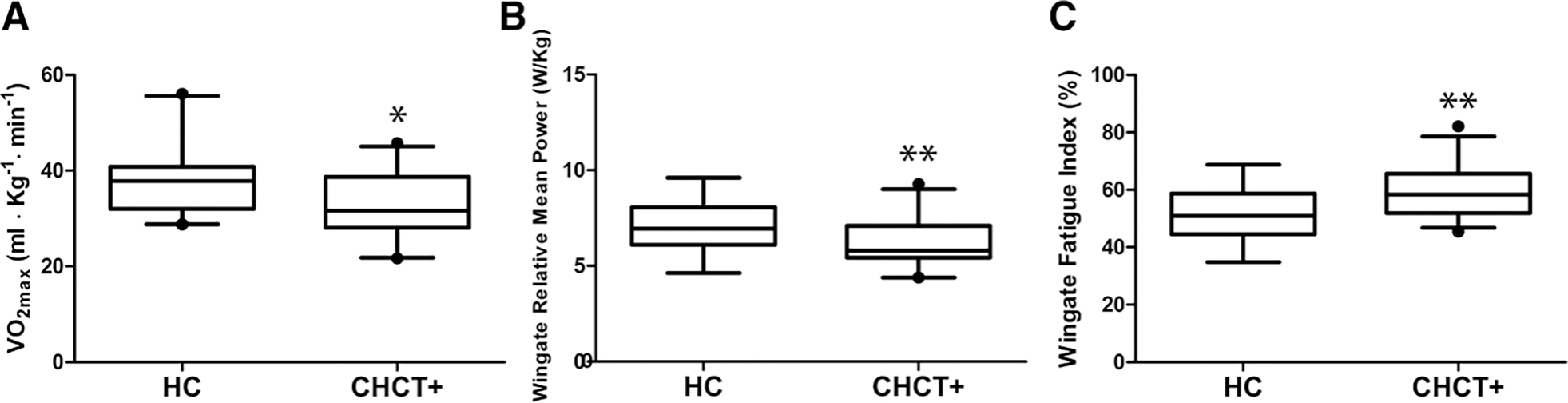
Exercise performance of CHCT-positive patients compared to HCs. A, CHCT-positive patients had significantly lower predicted Vo2max than HC (CHCT+: 33.2 ± 7.1 mL/kg/min versus HC: 38.2 ± 6.7 mL/kg/min, P = .02). B, Significantly lower relative mean power W/kg (watts per kilogram) in CHCT-positive patients compared to HC (HC: 7.2 ±1.3 W/kg versus CHCT+: 6.2 ± 1.2 watts), P = .01. C, Significantly higher fatigue index (% drop in power) in CHCT-positive patients compared to HC (HC: 51.7% ± 8.4% versus CHCT+: 59.5% ± 8.6 %), P = .004. CHCT indicates caffeine-halothane contracture test; HC, healthy age- and sex-matched controls.
CHCT-positive patients had significantly lower absolute mean power (CHCT+: 446.1 ± 119.7 W versus HC: 544.7 ± 144.6 W, P = .02) and relative mean power (CHCT+: 6.2 ± 1.2 W/kg versus HC: 7.2 ± 1.3 W/kg, P = .01) during the 30-second Wingate Anaerobic Test than HC (Figure 2B). CHCT-positive patients had a greater fatigue index or the percentage drop from peak power to minimum power (CHCT+: 59.6% ± 8.8% versus HC: 51.7% ± 8.4% P = .004) compared to HC (Figure 2C).
Upper and Lower Body Muscle Strength.
Results from the participants’ upper and lower body muscle strength tests are shown in Table 4. There were no significant differences in handgrip strength, vertical jump height, or lower body power between the groups.
Table 4.
Results of the Upper and Lower Body Strength Tests for CHCT-Positive Patients and HCs
| Strength Measure | HC (n = 20) | CHCT+ (n = 29) | P Valuea |
|---|---|---|---|
| Handgrip maximum (kg), mean ± SD | 45.0 ± 13.6 | 39.2 ± 9.7 | .09 |
| MH: 39.1 ± 9.2b | |||
| EHI: 39.4 ± 10.9b | |||
| Vertical jump (cm), mean ± SD | 41.5 ± 12.6 | 38.8 ± 13.1 | .48 |
| MH: 41.7 ± 13.8 | |||
| EHI: 32.9 ± 9.41 | |||
| Sayers peak power (W), mean ± SD | 3867.2 ± 1199.9 | 3506.9 ± 952.0 | .26 |
| MH: 3593.4 ± 1003.1 | |||
| EHI: 3333.7 ± 869.8 |
Abbreviations: CHCT, caffeine-halothane contracture test; EHI, exertional heat illness; HC, healthy age- and sex-matched controls; MH, malignant hyperthermia; SD, standard deviation.
Independent samples t test was used to compare means between HC and CHCT+ groups.
The CHCT+ group was divided into those with a history or family history of MH (MH group) and those with exertional heat illness (EHI group); however, because of small sample sizes, no statistical analyses were performed on these subgroups.
DISCUSSION
We demonstrated that patients tested positive for MH based on CHCT, when compared to HC, showed a significant decrease in ATP production via OXPHOS following 30- and 60-second high-intensity exercise bouts, lower maximal oxygen uptake (Vo2max) during the endurance cycle ergometer test, impaired anaerobic capacity, as measured by decreased mean power, and greater fatigue during the Wingate Anaerobic Test, as well as a significantly longer response time of the sigmoidal function in BOLD fMRI. None of the CHCT-positive patients demonstrated any overt myopathic features nor did they have any significant structural changes, such as cores or minicores in their histopathology. Due to their association with mutations in the ryanodine receptor gene, RYR1, core myopathies (central core and multiminicore disorders) carry a high risk of MH susceptibility.32
Previous studies of in vivo metabolic changes in MHS patients have shown equivocal results.15–17 The observed longer PCr recovery in CHCT-positive patients was consistent with Monsieurs et al15 who detected longer recovery of PCr following exercise as an indication of impaired aerobic metabolism. Our approach enabled us to gain insights into each of the 3 primary energy systems that provide energy for metabolism in muscle (ATP-CP, anaerobic glycolytic and aerobic oxidative), and for the first time, by measuring ATP production, we were able to identify impairment specifically in the OXPHOS pathway, which is a primary source of energy during exercise.33 The reduced ATP production after 30 or 60 seconds of intense exercise demonstrates that the exercise intolerance is observable only following elevated metabolic stress. From a clinical relevance perspective, it suggests that MHS patients may not experience physiological problems during activities of daily living (walking, cleaning, and gardening) but that they would likely experience limitations with more intense activities (walking up flights of stairs, running for a bus, or carrying groceries).
Because ATP is the fundamental energy currency for many metabolic processes and pathways in the human body, lower ATP production rate can have wide-ranging consequences metabolically and clinically. More specifically, the observed difference in ATP production rate of approximately 0.1 mM/s in MHS patients represents a 21% slower rate of ATP production when compared to HCs in the postexercise recovery period. Since ATP production in the postexercise recovery phase is primarily driven by mitochondrial function, this helps to focus the pathophysiology of exercise intolerance to mitochondrial dysfunction. The slower postexercise recovery of 21% also manifests itself in a patient having to rest 21% longer or experiencing more postexercise discomfort (muscle discomfort, heavy breathing, and fatigue) after an exercise bout than a healthy individual. Individuals who experience exercise intolerance may be less inclined to participate in physical activity, which can lead to a negative cycle of deconditioning. Therefore, decreased ATP production via this critical energy pathway can be the cause of muscle weakness, cramping, and exercise intolerance both in MHS patients with a family history of anesthetic reaction and in patients with exertional heat illness who tested positive for MH susceptibility with CHCT.
Decreased aerobic capacity, as demonstrated in the endurance cycle ergometer test, suggests that the decreased ATP production observed at the molecular level is associated with impaired functional capacity. Specifically, MHS patients have an impaired ability to sustain moderate level activity. The impaired anaerobic capacity observed during the Wingate Anaerobic Test demonstrates the inability to sustain very high intensity physical activity. This can be possibly explained by greater reliance on anaerobic metabolism to compensate for impaired aerobic function,16 which leads to decreased power and faster fatigue with the accumulation of glycolytic metabolites and impediment of contractile function.33 Indeed, a negative correlation was observed between fatigue index during the Wingate Anaerobic Test and OXPHOS ATP production rate during the 31P-MRS 30-second exercise bout.
Our study is the first to use BOLD fMRI to assess skeletal muscle hemodynamics following exercise in this group of patients. Within our experimental framework, changes in BOLD signal are dominated, albeit indirectly, through changes in tissue perfusion.12 The accumulation of metabolites, such as carbon dioxide, H+, and Pi, during exercise elicits a local increase in muscle blood flow due to vasodilation of microvessels,34 impairing bodily ability to eliminate these waste products from the muscle.23 Indeed, we observed a significantly longer response time of the sigmoidal function in CHCT-positive patients compared to HC. This longer response time might be indicative of a longer washout of deoxyhemoglobin,35 suggesting that CHCT-positive patients have impaired tissue perfusion following exercise. The BOLD signal is representative of the ratio of oxygenated hemoglobin to deoxygenated hemoglobin. After exercise, deoxygenated hemoglobin was observed to be predominant in the measured tissue, and there was a gradual return to higher levels of oxygenated hemoglobin. The rate of recovery was slower in CHCT-positive patients, which may be indicative of impaired aerobic oxidation in muscle or impaired perfusion. Further research is required to validate the sigmoidal function and to determine if the difference in BOLD signal is due to impaired oxygen utilization (aerobic oxidation) or oxygen delivery (impaired perfusion).
We acknowledge that there is only estimated and indirect evidence for the resultant sigmoidal slope (α), representing the washout time of deoxyhemoglobin postexercise. However, there is good evidence for α representing the washout of deoxyhemoglobin following a hyperemic or exercise stimulus.36,37 In addition, given that extravascular effects on the BOLD response are essentially negligible at 3.0 Tesla MRI scanners, we believe that α is a reasonable estimate of the rate of deoxyhemoglobin washout postexercise.
The impairment shown on BOLD fMRI might explain the progression of symptoms during an exercise-induced hypermetabolic reaction, because skeletal muscle requires increased blood supply to offset the oxygen deficiency during exercise. Thus, this study demonstrates the value of BOLD fMRI in assessing metabolic dysfunction.
Impairment of OXPHOS may be caused by mitochondrial damage in skeletal muscle of MHS patients. It has been suggested, based on experiments on the murine model of MH, that even in the absence of an MH episode, there is an increase in cytosolic Ca2+, due to “leaky” RyR1,38 and, therefore, mitochondrial Ca2+ might also be persistently elevated. Ca2+ overload causes metabolic insufficiency of the mitochondria,39 resulting in the production of reactive oxygen species and reactive nitrogen species and subsequent decrease in mitochondrial mass and OXPHOS. Durham et al,40 studying a mouse model, proposed that reactive nitrogen species cause remodeling of RyR1 through protein nitrosylation, leading to an even “leakier” channel that is more susceptible to future triggers. This feed-forward mechanism of accumulated Ca2+ increased reactive oxygen species production, and worsening mitochondrial damage is one hypothesis to explain the wide range of phenotypic severities in the MHS population. Continuous mitochondrial damage and subsequent RyR1 remodeling can lead to increased sensitivity to both anesthetic and heat- and exercise-induced triggers and a worsening phenotype over time. Due to the small sample size, no statistical analyses were performed to compare subgroups of CHCT-positive patients with and without musculoskeletal symptoms. However, both metabolic and functional impairments were observed in symptomatic CHCT-positive patients, particularly in those with severe symptoms.
There are several limitations to this study. Most notably, there were potentially confounding variables that we were unable to correct for. One particular limitation is that, with many CHCT-positive patients in our study group leading an inactive lifestyle (as demonstrated by the physical activity questionnaire), it remains uncertain if the metabolic and functional impairments are due to the disease itself or if they are secondary to physical inactivity. Because consistent exercise brings about numerous physiological adaptations, such as enhanced ability to transport oxygen, increased enzyme activity, and greater size and number of mitochondria,33 it might be possible that the impaired OXPHOS and aerobic capacity were due to the deconditioned nature of these patients. However, our patients did not have changes in such physiological measures as ATP-CP and anaerobic glycolysis (as measured by 31P-MRS) nor did they exhibit decrease in skeletal muscle strength compared to healthy participants. Therefore, it is unlikely that their metabolic and functional impairments were caused solely by physical inactivity. In addition, although measurement of physical activity by means of a questionnaire, as done in this study, is an accurate marker of habitual physical activity,21 future studies should consider the use of accelerometry to obtain a quantitative measurement of physical activity.
Another limitation is a relatively small sample size due to patient dropout. In addition, some data were excluded due to poor signal acquisition/motion artifacts (as was the case during MRS and BOLD fMRI testing) or noncompliance during the exercise testing, decreasing the power of the study. In addition, we have combined the patients with a personal or family history of MH reaction with those who tested positive with CHCT following exertional rhabdomyolysis. Arguably, these 2 groups are different for their reasons for referral, but due to the small sample size and since they had been tested positive for CHCT, we treated them as the same group.
Further, we have assumed that the change in BOLD signal is predominantly through altered metabolism. To more directly confirm this, a study using a spiral or echo planar imaging-based arterial spin labeling approach to directly measure microvascular perfusion would be helpful. However, such a sequence is not widely available on MRI scanners; hence, we decided to use BOLD.
No mitochondrial or inflammatory markers were measured directly from the muscle tissue. Future studies are warranted to analyze mitochondrial function in patients tested positive for MH at the cellular level to confirm our in vivo findings, as we have done previously in patients with cystic fibrosis.41
In summary, these findings interweave to provide insights into the pathophysiology of exercise intolerance in MHS patients. There were 3 primary observations: (1) impaired ATP production rate after intense exercise, which is suggestive of secondary mitochondrial impairment; (2) lower mean power and greater fatigue during the Wingate cycling test, which is suggestive of impaired energy production during intense exercise and impaired fatigue resistance during exercise; and (3) a longer response time for the BOLD signal, which demonstrates that the muscles of MHS patients take longer to return to homeostasis following exercise than HCs. These findings, taken in aggregate, clearly demonstrate that MHS patients have exercise intolerance, especially when doing intense exercise (such as running up a flight of stairs, lifting heavy objects, or sprinting for a bus), that they experience heightened fatigue during and after such activities, and that they take longer to recover from activity when compared to HCs. These findings can be used as a foundation for evidence-based physical activity and exercise recommendations that could help to prevent or alleviate these challenges. In addition, a better understanding of the pathophysiology of MH can help anesthesiologists and other medical professionals help patients better manage their clinical symptoms.
Supplementary Material
Funding:
This work was supported by the University of Toronto, Department of Anesthesia Translational Research Award. S.J.T. was supported by the Master’s Canadian Graduate Scholarship (Canadian Institutes of Health Research). S.R. is supported by the Department of Anesthesia Merit Award.
Footnotes
The authors declare no conflicts of interest.
DISCLOSURES
Name: Sara J. Thompson, MSc.
Contribution: This author designed the study, analyzed the data, and wrote and revised the manuscript.
Name: Sheila Riazi, MD.
Contribution: This author designed the study and wrote and revised the manuscript.
Name: Natalia Kraeva, PhD.
Contribution: This author designed the study and wrote and revised the manuscript.
Name: Michael D. Noseworthy, PhD.
Contribution: This author designed BOLD MRI, analyzed the data, and wrote and revised the manuscript.
Name: Tammy E. Rayner, RMRT, RTR.
Contribution: This author performed the tests and analyzed the data.
Name: Jane E. Schneiderman, PhD.
Contribution: This author helped in design and wrote and revised the manuscript.
Name: Barbara Cifra, MD.
Contribution: This author helped in design and wrote and revised the manuscript.
Name: Greg D. Wells, PhD.
Contribution: This author designed the study, analyzed the data, and wrote and revised the manuscript.
This manuscript was handled by: Ken B. Johnson, MD.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
Part of this project was presented at European MH Group Meeting, Lille, France, June 11–13, 2015.
REFERENCES
- 1.Eltit JM, Bannister RA, Moua O, et al. Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc Natl Acad Sci USA. 2012;109:7923–7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horstick EJ, Linsley JW, Dowling JJ, et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun. 2013;4:1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacLennan DH, Duff C, Zorzato F, et al. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990;343:559–561. [DOI] [PubMed] [Google Scholar]
- 4.Tobin JR, Jason DR, Challa VR, Nelson TE, Sambuughin N. Malignant hyperthermia and apparent heat stroke. JAMA. 2001;286:168–169. [DOI] [PubMed] [Google Scholar]
- 5.Wappler F, Fiege M, Steinfath M, et al. Evidence for susceptibility to malignant hyperthermia in patients with exercise-induced rhabdomyolysis. Anesthesiology. 2001;94:95–100. [DOI] [PubMed] [Google Scholar]
- 6.Ogletree JW, Antognini JF, Gronert GA. Postexercise muscle cramping associated with positive malignant hyperthermia contracture testing. Am J Sports Med. 1996;24:49–51. [DOI] [PubMed] [Google Scholar]
- 7.Capacchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065–1069. [DOI] [PubMed] [Google Scholar]
- 8.Timmins MA, Rosenberg H, Larach MG, Sterling C, Kraeva N, Riazi S. Malignant hyperthermia testing in probands without adverse anesthetic reaction. Anesthesiology. 2015;123:548–556. [DOI] [PubMed] [Google Scholar]
- 9.Riazi S, Larach MG, Hu C, Wijeysundera D, Massey C, Kraeva N. Malignant hyperthermia in Canada: characteristics of index anesthetics in 129 malignant hyperthermia susceptible probands. Anesth Analg. 2014;118:381–387. [DOI] [PubMed] [Google Scholar]
- 10.Wappler F, Fiege M, Antz M, Schulte am Esch J. Hemodynamic and metabolic alterations in response to graded exercise in a patient susceptible to malignant hyperthermia. Anesthesiology. 2000;92:268–272. [DOI] [PubMed] [Google Scholar]
- 11.Corona BT, Rouviere C, Hamilton SL, Ingalls CP. Eccentric contractions do not induce rhabdomyolysis in malignant hyperthermia susceptible mice. J Appl Physiol (1985). 2008;105:1542–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Allaire S, DeRoth L. Physiological responses to treadmill exercise and ambient temperature in normal and malignant hyperthermia susceptible pigs. Can J Vet Res. 1986;50:78–83. [PMC free article] [PubMed] [Google Scholar]
- 13.Zanconato S, Buchthal S, Barstow TJ, Cooper DM. 31P-magnetic resonance spectroscopy of leg muscle metabolism during exercise in children and adults. J Appl Physiol (1985). 1993;74:2214–2218. [DOI] [PubMed] [Google Scholar]
- 14.Noseworthy MD, Bulte DP, Alfonsi J . BOLD magnetic resonance imaging of skeletal muscle. Semin Musculoskelet Radiol. 2003;7:307–315. [DOI] [PubMed] [Google Scholar]
- 15.Monsieurs K, Heytens L, Kloeck C, Martin JJ, Wuyts F, Bossaert L. Slower recovery of muscle phosphocreatine in malignant hyperthermia-susceptible individuals assessed by 31P-MR spectroscopy. J Neurol. 1997;244:651–656. [DOI] [PubMed] [Google Scholar]
- 16.Bendahan D, Kozak-Ribbens G, Confort-Gouny S, et al. A noninvasive investigation of muscle energetics supports similarities between exertional heat stroke and malignant hyperthermia. Anesth Analg. 2001;93:683–689. [DOI] [PubMed] [Google Scholar]
- 17.Webster DW, Thompson RT, Gravelle DR, Laschuk MJ, Driedger AA. Metabolic response to exercise in malignant hyperthermia-sensitive patients measured by 31P magnetic resonance spectroscopy. Magn Reson Med. 1990;15:81–89. [DOI] [PubMed] [Google Scholar]
- 18.Larach MG. Standardization of the caffeine halothane muscle contracture test. North American Malignant Hyperthermia Group. Anesth Analg. 1989;69:511–515. [PubMed] [Google Scholar]
- 19.Orlov D, Keith J, Rosen D, Croul S, Kraeva N, Riazi S. Analysis of histomorphology in malignant hyperthermia-susceptible patients. Can J Anaesth. 2013;60:982–989. [DOI] [PubMed] [Google Scholar]
- 20.Hay J, Cairney J. Development of the habitual activity estimation scale for research: a systematic approach. Pediatr Exerc Sci. 2006;18:193–202. [Google Scholar]
- 21.Ruf KC, Fehn S, Bachmann M, et al. Validation of activity questionnaires in patients with cystic fibrosis by accelerometry and cycle ergometry. BMC Med Res Methodol. 2012;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newcomer BR, Boska MD. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve. 1997;20:336–346. [DOI] [PubMed] [Google Scholar]
- 23.Wells GD, O’Gorman CS, Rayner T, et al. Skeletal muscle abnormalities in girls and adolescents with Turner syndrome. J Clin Endocrinol Metab. 2013;98:2521–2527. [DOI] [PubMed] [Google Scholar]
- 24.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–286. [DOI] [PubMed] [Google Scholar]
- 25.Boska M ATP production rates as a function of force level in the human gastrocnemius/soleus using 31P MRS. Magn Reson Med. 1994;32:1–10. [DOI] [PubMed] [Google Scholar]
- 26.Iotti S, Frassineti C, Alderighi L, Sabatini A, Vacca A, Barbiroli B. In vivo (31)P-MRS assessment of cytosolic [Mg(2+)] in the human skeletal muscle in different metabolic conditions. Magn Reson Imaging. 2000;18:607–614. [DOI] [PubMed] [Google Scholar]
- 27.Golding LA, Myers CR, Sinning WE. Y’s Way to Physical Fitness: The Complete Guide to Fitness Testing and Instruction. 3rd ed. Champaign: Human Kinetics Publishers; 1989. [Google Scholar]
- 28.The Canadian Physical Activity, Fitness & Lifestyle Approach (CPAFLA). 3rd ed. Canadian Society for Exercise Physiology: Ottawa, ON, Canada; 2010. [Google Scholar]
- 29.Sayers SP, Harackiewicz DV, Harman EA, Frykman PN, Rosenstein MT. Cross-validation of three jump power equations. Med Sci Sports Exerc. 1999;31:572–577. [DOI] [PubMed] [Google Scholar]
- 30.Inbar O, Bar-Or O, Skinner JS. The Wingate Anaerobic Test. Champaign: Human Kinetics Publishers; 1996. [Google Scholar]
- 31.Bar-Or O The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med. 1987;4:381–394. [DOI] [PubMed] [Google Scholar]
- 32.Brislin RP, Theroux MC. Core myopathies and malignant hyperthermia susceptibility: a review. Paediatr Anaesth. 2013;23:834–841. [DOI] [PubMed] [Google Scholar]
- 33.Wells GD, Selvadurai H, Tein I. Bioenergetic provision of energy for muscular activity. Paediatr Respir Rev. 2009;10:83–90. [DOI] [PubMed] [Google Scholar]
- 34.Partovi S, Karimi S, Jacobi B, et al. Clinical implications of skeletal muscle blood-oxygenation-level-dependent (BOLD) MRI. MAGMA. 2012;25:251–261. [DOI] [PubMed] [Google Scholar]
- 35.McGrath DM, Naish JH, O’Connor JP, et al. Oxygen-induced changes in longitudinal relaxation times in skeletal muscle. Magn Reson Imaging. 2008;26:221–227. [DOI] [PubMed] [Google Scholar]
- 36.Schewzow K, Andreas M, Moser E, Wolzt M, Schmid AI. Automatic model-based analysis of skeletal muscle BOLD-MRI in reactive hyperemia. J Magn Reson Imaging. 2013;38:963–969. [DOI] [PubMed] [Google Scholar]
- 37.Towse TF, Slade JM, Ambrose JA, DeLano MC, Meyer RA. Quantitative analysis of the postcontractile blood-oxygenation-level-dependent (BOLD) effect in skeletal muscle. J Appl Physiol (1985). 2011;111:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giulivi C, Ross-Inta C, Omanska-Klusek A, et al. Basal bioenergetic abnormalities in skeletal muscle from ryanodine receptor malignant hyperthermia-susceptible R163C knock-in mice. J Biol Chem. 2011;286:99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adam-Vizi V, Starkov AA. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J Alzheimers Dis. 2010;20(suppl 2):S413–S426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durham WJ, Aracena-Parks P, Long C, et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I. Cystic fibrosis transmembrane conductance regulator in human muscle: dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol. 2010;67:802–808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


