Abstract
Introduction:
This histological study analyzed silk sericin as a potential direct pulp capping biomaterial in contact with pulp and comparing its response to calcium hydroxide.
Methods and Materials:
Twenty maxillary first molars from Wistar male rats were used, with 60 days of age, between 200 and 300 gr, which were divided in 4 groups (n=5): G1 and G3, controls, capped with calcium hydroxide in 7 and 30 days, respectively; G2 and G4, capped with silk sericin in 7 and 30 days, respectively. Circular cavities were prepared for pulp exposure, where capping materials were applied, being posteriorly restored with glass ionomer cement. After completion of each observation period, the animals were sacrificed and molars were histologically processed for analysis in light microscopy to evaluate presence of necrosis in pulp tissue, inflammatory cells infiltration and tertiary dentin formation. Data analysis was carried out using Kruskal-Wallis and Dunn’s post hoc tests.
Results:
After 7 days, there was less necrosis and inflammatory cells infiltration in G1 when compared to G2 (P=0.007 and P=0.008, respectively). After 30 days, a sample of G3 induced tertiary dentin formation and G4 showed decrease in inflammation (P=0.041) compared to G2.
Conclusion:
Among the determined experiment conditions, it was concluded that contact between silk sericin and pulp tissue showed improved inflammatory response throughout treatment and new cells proliferation. However, silk sericin adhibition in pure form did not show capability for induction of tertiary dentin formation.
Key Words: Biocompatible Materials, Calcium Hydroxide, Dental Pulp Capping, Sericin
Introduction
Complete dental pulp vitality is of utmost importance for teeth, not only for providing nutrition, but also as biological sensors to detect external stimuli. Preservation of pulp vitality, when possible, should always be the essential objective of the restoring treatment. Direct pulp capping is a viable and valid method for isolating external stimulation and can deliver an excellent result [1, 2].
Originally, healing of the exposed pulp occurs through formation of a new dentin barrier under the exposure, being calcium hydroxide capable of accomplishing this in about three to four weeks. On the other hand, it is known that the initial effect of calcium hydroxide on the pulp is destructive, producing chemical damage due to its alkaline pH (high concentration of hydroxyl ions), leading to necrosis of the pulp [3, 4]. Therefore, the search for biocompatible substances which can minimize, or even cause no additional tissue aggression during pulp capping would represent a scientific achievement on the field [5].
Recent studies show that natural substances, such as silk sericin, obtained from silkworm cocoons, have been utilized as biomaterials in healing tissue process, in their pure form as well as modified, and even in combination with other materials, resulting in a great variety of application means [6-10].
Sericin is a highly hydrophilic protein with molecular weight ranging from 20 to 400 kDa, and is composed of 18 amino acids. The polar groups (carboxyl, hydroxyl and amine groups) of lateral chains of amino acids and its organic composition,
solubility and structural organization allow crossed bonds, copolymerizations and combinations with other polymers, that together add antioxidant, moisturizing, healing, antibacterial and antimicrobial, anti-ultraviolet radiation and antitumor properties to silk sericin [11-13].
According to Lamboni et al. [7], silk sericin incorporation on skin healing and healing materials of wounds leads to greater adhesion, migration and fibroblast and keratinocyte growth; it also increases collagen production and skin wound reepithelialization. Regarding its effect on connective tissue, such as dental pulp, Xie et al. [8] reported that the use of silk sericin in sciatic nerve of mice allowed functional recovery similar to autologous nerve graft, displaying function and nerve morphology drastically improved.
Observing the potential application of silk sericin in regenerative and curative medicine and tissue engineering [7, 8, 14-17], this silk protein holds great interest in odontology, mostly in procedures such as direct pulp capping, since there is a direct contact between material and connective tissue, which may offer an effective and valuable alternative to this technique.
Regarding calcium hydroxide, its effect on pulp capping is seen as the result of chemical damage caused by hydroxyl ions liberation. Initially, calcium hydroxide application to exposed pulp causes a development of superficial necrosis and mild inflammation due to its alkaline pH, stimulating pulp defense and tissue healing to form a restorative dentin bridge through cell differentiation, extracellular matrix secretion and subsequent mineralization [18].
Based on these considerations, it is opportune to analyze silk sericin as a potential biomaterial in contact with dental pulp, histologically comparing its response to direct pulp capping in mice with calcium hydroxide, so that it is possible to evaluate the presence of necrosis on pulp tissue, infiltration of inflammatory cells and tertiary dentin formation during two periods of time: 7 and 30 days.
Materials and Methods
The experimental procedures of this paper are in accordance with the Ethical Principles on Animal Experimentation, employed by the Brazilian College of Animal Experimentation (COBEA) and were analyzed and approved by the Ethics Committee on Animal Use (CEUA) of Universidade Estadual do Oeste do Paraná under protocol # 60/17.
This study was performed on 20 maxillary first molars of 60-day male mice (Rattus norvegicus albinus, Wistar), weighing about 200 to 300 gr, held in a controlled ambient, bright-dark cycle of 12 h, room temperature of 21±1°C, water and pasty ration ad libitum. These molar teeth were histologically evaluated under two periods of time: 7 and 30 days distributed equally and randomly in 4 groups (n=5), being group 1 (control): calcium hydroxide in 7 days; group 2: silk sericin in 7 days; group 3 (control): calcium hydroxide in 30 days; group 4: silk sericin in 30 days.
Silk sericin preparation
Silk sericin protein was extracted from silkworm cocoons (Bombyx mori), obtained from the sericulture company (BRATAC Silk do Brasil®, Londrina, Paraná, Brazil). Cocoons were cut into small pieces (about 1 cm²) and the equivalent of 6 gr of cocoons were submerged in 100 mL of distilled water, in a 500 mL Erlenmeyer. The solution was autoclaved, (autoclave CS 30–Prismatec) at 120°C and pressure of 1 kgf/cm2, for 60 min. The raw extract was then filtered with an 18 mesh sieve, to separate/remove fibroin, from which a silk sericin hydrolyzed was obtained. This protocol was based on studies by Gimenes et al. [19], who utilized water, without any chemical additive, for silk sericin extraction. The hydrolyzed silk sericin was then frozen and freeze-dried (Liofilizador LT 1000, Terroni Equipamentos Ltda®, São Carlos, SP, Brazil), for approximately 54 h, obtaining silk sericin powder, stored in room temperature until its utilization (Figure 1).
Figure 1.
The sericin powder on a glass plate and Angelus MTA applicator of 0.6 mm in diameter can be seen
Direct pulp capping
Animals were weighted previously and anesthetized with a combination of ketamine 10% 0.1 mL/100 gr of weight (Dopalen injetável®, Ceva Saúde Animal, Paulínia, SP, Brazil) and 2% xylazine 0.05 mL/100 gr of weight (Anasedan injetável®, Ceva Saúde Animal, Paulínia, SP, Brazil), through an intraperitoneal injection, and were positioned on appropriate operating table [20], which allows wide mouth opening, enabling access to teeth in maxillary region (Figure 2).
Figure 2.
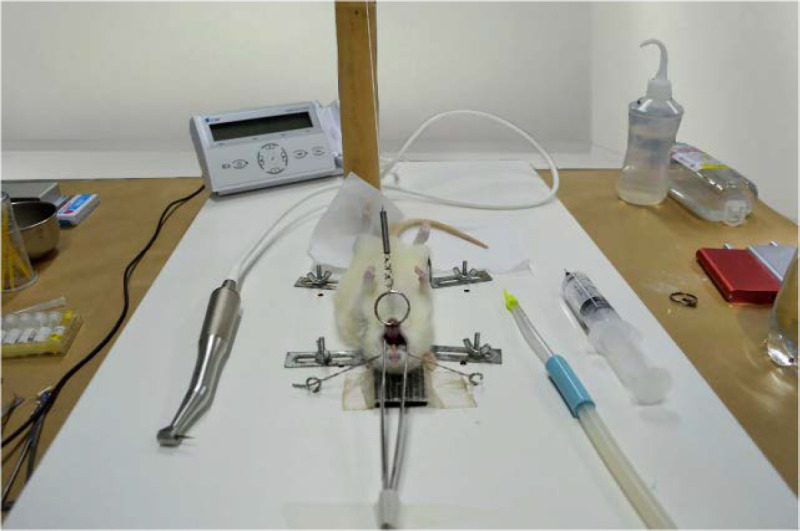
The operating table for immobilization and conservation of the animal’s mouth opening
Circular cavities were prepared on the maxillary first molars, at the occlusal surface above mesial central surface of the teeth, aided by an endodontic motor VDW Silver (VDW®, Munich, Germany) at 400 rpm, with a ¼ round diamond bur (KG Sorensen Ind & Com®, Alphaville, São Paulo, Brazil), which was used under microscopic magnification and saline solution watering until bleeding was obtained. The diameter of exposed pulp area ranged from 0.5 to 0.7 mm. Pulp hemorrhage is controlled and cavity is dried with sterilized paper cones, adapted from Shinkai et al. [21]. Materials were applied using Angelus MTA applicator of 0.6 mm in diameter metallic device (Angelus®, Londrina, PR, Brazil) and cavities were sealed with Ketac Molar Easymix glass ionomer cement (3M ESPE®, São Paulo-SP, Brazil) (Figure 3).
Figure 3.
These images show procedure sequences: A) Cavity preparation of maxillary first molar; B) Bleeding control with paper cone; C) Material insertion with Angelus MTA applicator; D) Sealing with Ketac molar Easymix
Samples obtainment
After completion of each observation period (7 and 30 days after pulp capping), the animals were sacrificed, using intraperitoneal injections with an overdose of the same anesthetics used in operating procedure (four times greater). Maxilla was then removed and properly labeled, stored in glass container and underwent fixation in 10% formaldehyde solution for 48 h, and later in 70% alcohol for 15 days.
Histological processing
Hemimaxilla was decalcified with 5% trichloroacetic acid (TCA), in room temperature for 20 days. Pieces were analyzed to evaluate expected decalcification degree, with renewal of the TCA solution every 5 days. After decalcification, pieces were rinsed under running tap water and underwent automated histological processing (Automated tissue processor, Leica Microsystems TP1020®, Nussloch, Germany). After that, pieces were embedded in paraffin and blocks were obtained (Purified Paraffin, Vetec Química Fina®, RJ, Brazil). These blocks were cut with semi-automatic microtome (Hestion®, ERM3000, Daintree Scientific, St. Helens, Australia) in order to obtain sections of 5 µm in width. Cuts were selected according to region of exposure and mounted in histological lamina with hematoxylin and eosin stain technique.
Histological analysis
Histological analyses were performed by two previously trained observers, in blind fashion, using 40×, 100× and 400× magnifications (Optic Microscope Leica Microsystems® ICC50HD, Nussloch, Germany) and image capture system (Las Ez–Leica®, version 2.10, 2012, Solms, Germany). Descriptive analysis was performed, analyzing the following parameters: presence of pulp necrosis, infiltration of inflammatory cells and tertiary dentin formation, which were classified using scores and are described in Table 1, adapted from Suzuki et al. [22].
Table 1.
Scores and parameters analyzed
| Scores | Parameter |
|---|---|
| Necrosis on pulp tissue | |
| 0 | Absence of necrosis |
| 1 | Presence of necrosis on coronary third of the pulp |
| 2 | Presence of necrosis on coronary third and middle |
| 3 | Presence of necrosis on all the pulp |
| Inflammatory cells infiltration | |
| 0 | Absence or occasional presence of inflammatory cells on pulp (none) |
| 1 | Mild/chronic inflammation (mild) |
| 2 | Inflammatory reactions such as micro abscesses or polymorphonuclear leukocytes infiltrates, histiocytes and lymphocytes affecting the coronary pulp up to half of the root pulp (moderate) |
| 3 | Inflammatory reaction affecting all of the pulp tissue (severe) |
| 4 | Unable to evaluate inflammatory cells due to necrotic degeneration |
| Tertiary dentin formation | |
| 0 | Complete formation of the dentinal bridge (complete) |
| 1 | Partial/incomplete dentinal bridge formation, which extends to more than half of the exposure region, but doesn’t completely close the exposure region (partial) |
| 2 | Initial dentinal bridge formation, extending to no more than half of the exposure region (initial) |
| 3 | Without dentinal bridge formation (none) |
Statistical analysis
For the evaluation of variables of this study, all scores were expressed as median±interquartile range. Histological comparisons and sample calculation were based on study by Shinkai et al. [21] and performed with BioStat 5.3 program (Mamirauá Institute, Belém, Pará, Brazil), using Kruskal-Wallis test and Dunn’s post hoc test, with significance level of 0.05. Furthermore, as few animals as possible were used per group, for animal protection.
Results
Seventh day evaluation
In response to calcium hydroxide on the seventh day (group 1), it was observed that the superficial portion of the odontoblastic layer and coronary pulp adjacent cells were disorganized due to the diamond bur action during pulp exposure. Furthermore, necrosis was observed on pulp tissue at cervical third of the pulp in 80 % of the samples, and acute-to-moderate inflammatory infiltrate involving coronary pulp up to half of the root pulp, represented by neutrophil exudation areas. On the deep layers pulp tissue had moderate cellularity with fibroblasts, granulation tissue and hyperemia in the majority of analyzed samples (Figure 4A).
Figure 4.
Representative photomicrographs of the study groups. A) G1: It was noted superficial necrosis on coronary third of root pulp (arrow) while the remainder of root pulp showed light and mild degrees of inflammation, predominantly chronic (asterisk); B) G2: All of the root pulp is necrotic (bidirectional arrow), rendering evaluation of other proposed parameters for pulp condition impossible; C) G3: Discontinuous tertiary dentin formation on coronal root portion (asterisk); D) G4: Superficial necrosis can be noted on coronary third of root pulp (arrow) and absence of mineralized material formation, while the remainder had complete disarray of root pulp (asterisk) (HE, 100×)
On the other hand, pulp response to silk sericin in 7 days (group 2) displayed necrotic degeneration involving all coronary and root pulp in 80% of the cases, rendering qualitative morphological analysis of other parameters impossible (Figure 4B). It can be noted that, in Tables 2 and 3 respectively, group 1 had better score comparing to group 2, regarding pulp tissue necrosis degree (P=0.007) and presence of inflammatory cells infiltration (P=0.008). Concerning tertiary dentin formation at 7 days, there was no dentin barrier formation in all cases, regardless of material used, with no statistical difference between groups for this criterion (P>0.05).
Table 2.
Statistical analysis of presence of necrosis on pulp tissue for the evaluated materials (median ± interquartile range)
| Material/Time | 7 days | Scores | Frequency | 30 days | Scores | Frequency |
|---|---|---|---|---|---|---|
| Calcium Hydroxide | 1 ± 0.0 Aa | 1 | 4 | 3 ± 1.0 Ab | 1 | 0 |
| 2 | 1 | 2 | 2 | |||
| 3 | 0 | 3 | 3 | |||
| Sericin | 3 ± 0.0 Ba | 1 | 0 | 2 ± 1.0 Aa | 1 | 0 |
| 2 | 1 | 2 | 3 | |||
| 3 | 4 | 3 | 2 |
Different upper case letters in the same column mean statistically significant differences, P<0.05. Different lower case letters in the same line mean statistically significant differences, P<0.05
Table 3.
Statistical analysis of inflammatory cells infiltration for the evaluated materials (median±interquartile range)
| Material/Time | 7 days | Scores | Frequency | 30 days | Scores | Frequency |
|---|---|---|---|---|---|---|
| Calcium hydroxide | 2±0.0 Aa | 1 | 1 | 4±1.0 Ab | 1 | 0 |
| 2 | 3 | 2 | 0 | |||
| 3 | 1 | 3 | 2 | |||
| 4 | 0 | 4 | 3 | |||
| Sericin | 4±0.0 Bb | 1 | 0 | 2±1.0 Aa | 1 | 0 |
| 2 | 0 | 2 | 3 | |||
| 3 | 1 | 3 | 1 | |||
| 4 | 4 | 4 | 1 |
Different upper case letters in the same column mean statistically significant differences, P<0.05. Different lower case letters in the same line mean statistically significant differences, P<0.05
Thirtieth day evaluation
Within 30 days, presence of necrosis was observed predominantly on coronary, middle and apical thirds of pulp tissue for the calcium hydroxide group (group 3). Regarding silk sericin (group 4), presence of necrosis was observed mainly
on coronary third and middle of pulp. Underneath necrosis, for both groups, chronic moderate inflammatory infiltrate was observed and, in some cases, extensive, characterized mainly by mononuclear leukocytes, such as lymphocytes and macrophages. Regarding tertiary dentin formation, in only one sample from group 3 it was possible to see partial atubular dentin barrier formation capability, in more than half of the exposure region (Figure 4C), while group 4 did not show any tertiary dentin barrier formation (Figure 4D). In comparison between groups, there was no significant statistical difference for any of the three evaluation criteria (P>0.05).
Comparison between 7 and 30 days
Regarding the occurrence of pulp necrosis on groups that underwent calcium hydroxide treatment (group 1 and group3), it was possible to note that time duration was a spread aggravating factor (P=0.010), since pulp area affected by necrosis was greater in 30 days (Table 2). Concerning inflammatory cells infiltration in the same groups, there was also a greater degree of inflammation (P=0.012) after 30 days (Table 3). Concerning tertiary dentin formation, calcium hydroxide induced dentin barrier formation in 20% of samples in 30 days. It was not observed statistically significant differences among the groups regarding the different time periods (Table 4). Silk sericin had decreased levels of inflammation after 30 days of treatment (P=0.041), even though it did not show histological signs of mineralized material formation. Silk sericin did not show significant statistical difference between 7 and 30 days concerning pulp tissue necrosis and tertiary dentin formation (P>0.05).
Table 4.
Statistical analysis of tertiary dentin formation for the evaluated materials (median±interquartile range)
| Material/Time | 7 days | Scores | Frequency | 30 days | Scores | Frequency |
|---|---|---|---|---|---|---|
| Calcium hydroxide | 3 ± 0.0 Aa | 1 | 0 | 3 ± 0.0 Aa | 1 | 1 |
| 2 | 0 | 2 | 0 | |||
| 3 | 5 | 3 | 4 | |||
| Sericin | 3 ± 0.0 Aa | 1 | 0 | 3 ± 0.0 Aa | 1 | 0 |
| 2 | 0 | 2 | 0 | |||
| 3 | 5 | 3 | 5 |
Different upper case letters in the same column mean statistically significant differences, P<0.05. Different lower case letters in the same line mean statistically significant differences, P<0.05
Discussion
In this study, pulp exposure treatment with lyophilized silk sericin in pure form pointed its pro-inflammatory capacity during initial response and has also shown to be more aggressive to pulp tissue in 7 days of treatment when compared to calcium hydroxide . On the other hand, inflammatory response decreased after 30 days, fact that can be compared to a study by Aramwit et al. [13] which concluded that healing process of wounds showed improvement in treatment after use of silk sericin, demonstrating that inflammatory mediators TNF-α and IL-1β, when the experiment performed with silk sericin, were significantly lower.
Expectations on potential use of silk sericin as biomaterial in odontology, as a complement or even as a new alternative in direct pulp capping, is based on observation of results on this biomaterial application in other tissues, where it presented itself to be very promising [6-10, 13]. Considering that there are no studies using this protein for such end, these are the first morphological and functional data obtained from silk sericin application directly on exposed pulp.
The initial aggressive response may be due to the fact that glass ionomer cement has acid and caustic properties [23] and may have caused injury to pulp, leading to an exacerbated inflammatory response.
For many years, importance of inflammation in pulp healing was underestimated, being considered only as an undesirable effect. However, there are evidences that inflammation is a requirement for tissue healing and pulp regeneration, and immuno-competent cells (monocytes, macrophages and stem cells) migrate towards the crown as part of this process. Due to the capping agent’s high alkalinity, mineralization starts and becomes thicker, while inflammatory processes induce pulp cells proliferation, presenting cells both greater in number and size [24].
Corroborating study by Aramwit et al. [6] and Ersel et al. [9], showed that silk sericin accelerated skin tissue regeneration and supported new cells proliferation, its contact with dentinal pulp. In this study, while not capable of inducing tertiary dentin formation and not suggesting interaction with odontoblasts, showed a lower degree of inflammatory cells infiltration after 30 days of treatment when compared to its initial response, demonstrating improvement during treatment and indicating a reparative response tendency. Since it did not form tertiary dentin, lyophilized silk sericin applied in pure form did not show direct interaction with odontoblasts, although further studies could test its reparative potential when associated to materials that induce hard mineralized tissue formation, contributing to inflammatory response improvement.
Despite its potential, pure silk sericin forms fragile filaments and it becomes difficult to use it as a biomaterial in tissue engineering [25]. Thus, different strategies have been applied in order to increase its physical properties [7]. In fact, improving some properties of the material may have deleterious effects on other properties. Therefore, clinical effectiveness of the modification in a given material in addition to its physical properties, antibacterial activity, sealing ability and biocompatibility should be considered [26]. Additionally, cavity size performed on animal tooth, as well as pulp exposure extension, hinder material insertion and settlement efficiency on desired region.
Calcium hydroxide is a material that holds a long history in clinical use, considered the gold standard for this application, for many years, and still is widely utilized for exposed pulp direct coverage [4, 22]. In this study, direct pulp capping performed with calcium hydroxide showed considerable increase in necrosis incidence and pulp inflammation after 30 days of treatment when compared to its initial response, in 7 days, in addition to showing tertiary dentin formation capacity. This result revealed to be similar to a study reported by Tran et al. [27], who observed a persistent necrotic layer in all groups tested with calcium hydroxide next to exposure region, and capacity for a barrier formation of mineralized dentin under the capping material, often incomplete and predominantly porous.
Tertiary dentin formation in relation to pulp capping with calcium hydroxide after 30 days was noted in only 20% of samples. This percentage could not be more significant due to reduced number of samples, as it is a well-established material in dental practice. Nonetheless, this result displays repair capability under calcium hydroxide activity, as already noted in several similar studies [18, 22, 23, 28].
Conclusion
Based on the results, it was possible to conclude that silk sericin contact with pulp tissue showed improvement in inflammatory response throughout treatment and new cells proliferation. However, silk sericin adhibition in pure form did not show capability for induction of tertiary dentin formation. When compared to calcium hydroxide, initial response to silk sericin displayed higher degree of necrosis and inflammatory cells infiltration, decreasing after 30 days. Therefore, considering all silk sericin properties and benefits extensively related on literature, more researches should be performed, combining it with other similar biomaterials, or currently commercial materials for pulp capping.
Acknowledgment
The authors gratefully acknowledge Coordination for the Improvement of Higher Education Personnel (CAPES) and the Department of Biological Sciences and Health of the Western Paraná State University (UNIOESTE) for the financial support. This work was performed as part of the requirements for Master Degree in Dentistry of Giovani Ceron Hartmann, in UNIOESTE, Cascavel, Paraná, Brazil.
Conflict of Interest:
‘None declared’.
References
- 1.Baume LJ, Holz J. Long term clinical assessment of direct pulp capping. Int Dent J. 1981;31(4)::251–60. [PubMed] [Google Scholar]
- 2.Raedel M, Hartmann A, Bohm S, Konstantinidis I, Priess HW, Walter MH. Outcomes of direct pulp capping: interrogating an insurance database. Int Endod J. 2016;49(11): 1040–7. doi: 10.1111/iej.12564. [DOI] [PubMed] [Google Scholar]
- 3.Glass RL, Zander HA. Pulp Healing. J Dent Res. 1949;28(2):97–107. doi: 10.1177/00220345490280021101. [DOI] [PubMed] [Google Scholar]
- 4.Akhavan A, Arbabzadeh F, Bouzari M, Razavi SM, Davoudi A. Pulp Response following Direct Pulp Capping with Dentin Adhesives and Mineral Trioxide Aggregate; An Animal Study. Iran Endod J. 2017;12(2):226–30. doi: 10.22037/iej.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zakerzadeh A, Esnaashari E, Dadfar S. In Vitro Comparison of Cytotoxicity and Genotoxicity of Three Vital Pulp Capping Materials. Iran Endod J. 2017;12(4):419–25. doi: 10.22037/iej.v12i4.15104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aramwit P, Palapinyo S, Srichana T, Chottanapund S, Muangman P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch Dermatol Res. 2013;305(7):585–94. doi: 10.1007/s00403-013-1371-4. [DOI] [PubMed] [Google Scholar]
- 7.Lamboni L, Gauthier M, Yang G, Wang Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol Adv. 2015;33(8):1855–67. doi: 10.1016/j.biotechadv.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Xie H, Yang W, Chen J, Zhang J, Lu X, Zhao X, Huang K, Li H, Chang P, Wang Z, Wang L. A silk sericin/silicone nerve guidance conduit promotes regeneration of a transected sciatic nerve. Adv Healthc Mater. 2015;4(15):2195–205. doi: 10.1002/adhm.201500355. [DOI] [PubMed] [Google Scholar]
- 9.Ersel M, Uyanikgil Y, Karbek Akarca F, Ozcete E, Altunci YA, Karabey F, Cavusoglu T, Meral A, Yigitturk G, Oyku Cetin E. Effects of Silk Sericin on Incision Wound Healing in a Dorsal Skin Flap Wound Healing Rat Model. Medical science monitor Med Sci Monit. 2016;22:1064–78. doi: 10.12659/MSM.897981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagai N, Ogata F, Deguchi S, Ueno A, Kawasaki N, Ito Y. Combination Ointment Containing Solid Tranilast Nanoparticles and Dissolved Sericin Is Efficacious for Treating Skin Wound-Healing Deficits and Redness in Diabetic Rats. Biol Pharm Bull. 2017;40(4):444–50. doi: 10.1248/bpb.b16-00812. [DOI] [PubMed] [Google Scholar]
- 11.Kato N, Sato S, Yamanaka A, Yamada H, Fuwa N, Nomura M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci Biotechnol Biochem. 1998;62(1):145–7. doi: 10.1271/bbb.62.145. [DOI] [PubMed] [Google Scholar]
- 12.Wu J-H, Wang Z, Xu S-Y. Preparation and characterization of sericin powder extracted from silk industry wastewater. Food Chem. 2007;103(4):1255–62. [Google Scholar]
- 13.Aramwit P, Kanokpanont S, De-Eknamkul W, Srichana T. Monitoring of inflammatory mediators induced by silk sericin. J Biosci Bioeng. 2009;107(5):556–61. doi: 10.1016/j.jbiosc.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Siritientong T, Aramwit P. Characteristics of carboxymethyl cellulose/sericin hydrogels and the influence of molecular weight of carboxymethyl cellulose. 2015. [Google Scholar]
- 15.Jo YN, Um IC. Effects of solvent on the solution properties, structural characteristics and properties of silk sericin. Int J Biol Macromol. 2015;78:287–95. doi: 10.1016/j.ijbiomac.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Song DW, Park YH, Um IC. Effect of residual sericin on the structural characteristics and properties of regenerated silk films. Int J Biol Macromol. 2016;89:273–8. doi: 10.1016/j.ijbiomac.2016.04.073. [DOI] [PubMed] [Google Scholar]
- 17.Ramesan MT, Athira VK, Jayakrishnan P, Gopinatahn C. Preparation, characterization, electrical and antibacterial properties of sericin/poly(vinyl alcohol)/poly(vinyl pyrrolidone) composites. 2016. [Google Scholar]
- 18.Schroder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985;64 :541–8. doi: 10.1177/002203458506400407. [DOI] [PubMed] [Google Scholar]
- 19.Gimenes M R, Silva V, Vieira M, Silva M, Scheer A. High Molecular Sericin from Bombyx mori Cocoons: Extraction and Recovering by Ultrafiltration. Int J Chem Engin App. 2014:266–71. [Google Scholar]
- 20.Kirschneck C, Proff P, Fanghaenel J, Behr M, Wahlmann U, Roemer P. Differentiated analysis of orthodontic tooth movement in rats with an improved rat model and three-dimensional imaging. Ann Anat. 2013;195(6):539–53. doi: 10.1016/j.aanat.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Shinkai K, Taira Y, Kawashima S, Suzuki S, Suzuki M. Histological evaluation of direct pulp capping with all-in-one adhesives in rat teeth. Dent Mater J. 2017;36(3):348–56. doi: 10.4012/dmj.2016-148. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, Taira Y, Kato C, Shinkai K, Katoh Y. Histological evaluation of direct pulp capping of rat pulp with experimentally developed low-viscosity adhesives containing reparative dentin-promoting agents. J Dent. 2016;44:27–36. doi: 10.1016/j.jdent.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Tziafa C, Koliniotou-Koumpia E, Papadimitriou S, Tziafas D. Dentinogenic Activity of Biodentine in Deep Cavities of Miniature Swine Teeth. J Endod. 2015;41(7):1161–6. doi: 10.1016/j.joen.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg M, Njeh A, Uzunoglu E. Is Pulp Inflammation a Prerequisite for Pulp Healing and Regeneration? Mediators Inflamm. 2015;2015:11. doi: 10.1155/2015/347649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal BB, Priya AS, Kundu SC. Novel silk sericin/gelatin 3-D scaffolds and 2-D films: fabrication and characterization for potential tissue engineering applications. Acta Biomater. 2009;5(8):3007–20. doi: 10.1016/j.actbio.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Manochehrifar H, Parirokh M, Kakooei S, Oloomi MM, Asgary S, Eghbal MJ, Mashhadi Abbas F. The Effect of Mineral Trioxide Aggregate Mixed with Chlorhexidine as Direct Pulp Capping Agent in Dogs Teeth: A Histologic Study. Iran Endod J. 2016;11(4):320–4. doi: 10.22037/iej.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, Opsahl Vital S, Chaussain C, Boukpessi T. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res. 2012;91(12):1166–71. doi: 10.1177/0022034512460833. [DOI] [PubMed] [Google Scholar]
- 28.Banava S, Fazlyab M, Heshmat H, Mojtahedzadeh F, Motahhary P. Histological Evaluation of Single and Double-visit Direct Pulp Capping with Different Materials on Sound Human Premolars: A Randomized Controlled Clinical Trial. Iran Endod J. 2015;10(2):82–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Please cite this paper as: Ceron Hartmann G, Costa Brancalhão RM, Chasko Ribeiro LF, Carrinho Ayroza Rangel AL, Giampietro-Brandão C. Histological Evaluation of Direct Pulp Capping with Silk Sericin: A Preliminary Animal Study. Iran Endod J. 2019;14(3):190–6. doi: 10.22037/iej.v14i3.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]





