Abstract
Hypertensive urgencies (HU) and hypertensive emergencies (HE) are challenges for the Emergency Department (ED). A prospective multicentre study is ongoing to characterize patients with acute hypertensive disorders, prevalence of subclinical hypertension-mediated organ damage (HMOD), short- and long-term prognosis; this is a preliminary report. Patients admitted to the ED with symptomatic blood pressure (BP) ≥180/110 mmHg were enrolled. They were managed by ED personnel according to their clinical presentations. Subsequently they underwent clinical evaluation and subclinical HMOD assessment at a Hypertension Centre within 72 h from enrolment. 122 patients were included in this report. Mean age was 60.7±13.9 years, 52.5% were females. 18 (14.8%) patients were diagnosed with HE, 108 (88.5%) with HU. There were no differences in gender, BMI, and cardiovascular comorbidities between groups. At ED discharge, 66.7% and 93.6% (p = 0.003) of HE and HU patients, respectively, had BP < 180/110 mmHg. After 72 h, 34.4% of patients resulted normotensive; 35.2%, 22.1%, and 8.2% had hypertension grade 1, 2, and 3, respectively. Patients with uncontrolled BP at office evaluation had higher vascular HMOD (49.1 vs. 25.9%, p = 0.045). Cardiac (60 vs. 34%, p = 0.049), renal (27.8 vs. 9.6%, p = 0.010) and cerebral (100 vs. 21%, p < 0.001) HMOD was more frequent in HE compared to HU group. HE showed greater cardiac, renal, and cerebral subclinical HMOD, compared to HU. 72-hours BP control is not associated with different HMOD, except for vascular HMOD; therefore, proper comprehensive examination after discharge from the ED could provide added value in cardiovascular risk stratification of such patients.
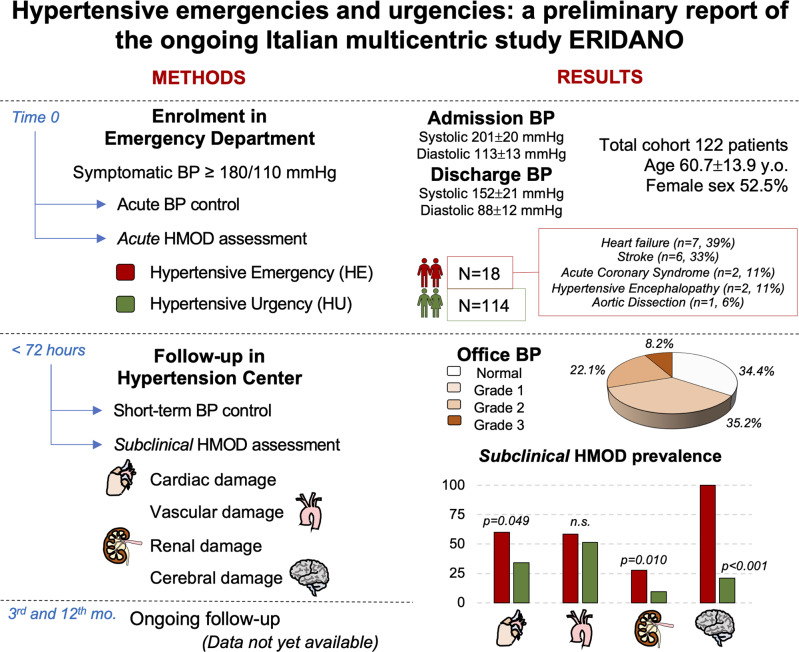
One third of patients with acute blood pressure rise evaluated to the ED resulted normotensive at office evaluation (<72 hours after discharge). Patients with hypertensive emergency showed greater cardiac, renal, and cerebral subclinical HMOD, compared to the patients with hypertensive urgency. BP: blood pressure; HMOD: hypertension-mediated organ damage; y.o.: years old; mo.: months.
Keywords: emergency department, hypertensive emergencies, hypertensive urgencies, short-term blood pressure control, hypertension mediated organ damage
One third of patients with acute blood pressure rise evaluated to the ED resulted normotensive at office evaluation (<72 hours after discharge). Patients with hypertensive emergency showed greater cardiac, renal, and cerebral subclinical HMOD, compared to the patients with hypertensive urgency. BP: blood pressure; HMOD: hypertension-mediated organ damage; y.o.: years old; mo.: months.
Introduction
Acute blood pressure (BP) disorders are a major challenge for the Emergency Department (ED). The prevalence of acute BP disorders considerably differs among studies, even depending on the definition used, but it ranges from 0.24% to 2.4% of ED admissions for hypertensive urgencies (HU) and from 0.08% to 0.76% for hypertensive emergencies (HE) [1]. These prevalences seem comparable across continents [2], although with some differences probably due to ethnic disparities, medication adherence, and insurance status [3]. Although HU do not appear to be associated with short-term adverse outcomes [4, 5], or at least have significantly lower in-hospital mortality compared to HE [6], long-term implications, such as risk of stroke and fatal or non-fatal cardiovascular events, are relevant [7–9].
Despite the significant clinical and epidemiological impact, the management of patients with acute BP disorders is still very uneven among professionals of critical areas, as pointed out by a recent Italian surveys [10, 11]. The lack of good-quality evidence makes it difficult to propose strong recommendations for clinical practice. The therapeutic management is very uneven, especially for HU. The timing of follow-up, when present, is heterogeneous and it is not clear whether a referral to a Hypertension Centre could have a prognostic role compared to standard care.
In order to obtain more and more accurate information on this category of patients, we are conducting the ERIDANO prospective multicenter cohort study on behalf of the Italian Society of Hypertension (SIIA: Società Italiana dell’Ipertensione Arteriosa). The aim of the ERIDANO study is to characterize patients with acute hypertensive disorders, their prevalence of subclinical organ damage and secondary hypertension and their short- and long-term prognosis, by providing referral to a Hypertension Centre immediately after discharge from the ED, as described below.
The present study is intended to be a preliminary, mainly descriptive, report of the first hundred patients enrolled, focusing on the clinical and demographic characteristics, on the management in the ED, on BP control within 72 hours of discharge, and on the prevalence of hypertension-mediated subclinical organ damage (HMOD).
Methods
The current enrolment has involved 6 Italian hospitals, officially starting in Turin, the main center, in January 2020. Enrollment is not to be considered consecutive, as there were many months of interruption, caused by the closure of Hypertension Centers during the four Italian epidemic wave and the commitment of internal medicine, emergency medicine, and cardiology specialists to COVID wards; in addition, the other recruiting centers became active in the first months of the year 2021 or 2022.
Consecutive patients, aged 18 years and over, admitted to the ED with a symptomatic BP rise, defined as systolic BP ≥ 180 mmHg and/or diastolic BP ≥ 110 mmHg associated to at least one symptom consistent with suspected HE as defined by latest guidelines [12], were enrolled. BP measurements were performed according to the current European Society of Hypertension/European Society of Cardiology (ESH/ESC) recommendations [13], with validated automatic sphygmomanometers (e.g., Omron, M10-IT models, Matsusaka, Kyoto, Japan), with patients in the sitting position whenever possible. Three BP measurement were performed, and the mean value was used for subsequent analysis.
Patients with BP rise due to traumatic causes or known neoplastic pain, or with BP rise without any associated symptoms were excluded, as were those who withheld their informed consent.
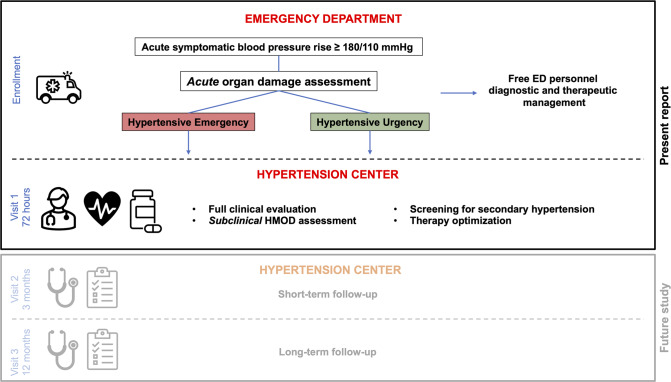
Enrolled patients were managed by the emergency physicians in the ED, according to their clinical presentations, as suggested in the current European position paper [12]. After appropriate work-up, in the presence of acute organ damage (coronary ischemia, acute cardiogenic pulmonary oedema, acute ischemic or hemorrhagic stroke, hypertensive encephalopathy, acute aortic disease, acute kidney injury, relative to known creatinine values in the previous 12 months) as defined by current guidelines [12] (HE), patients were admitted to an appropriate hospital specialist setting; in the absence of acute organ damage (HU), they were discharged after a period of observation. In any case, an evaluation at an ESH Hypertension Excellence Centre was performed within 72 hours of enrolment; this visit was carried out on an outpatient-basis for discharged patients (HU), and on an inpatient-basis for HE patients, still hospitalized in the appropriate specialist setting. Subsequent therapeutic modifications, or indications for further diagnostic investigations, related to the detection of subclinical organ damage (which may be present independently of the acute organ damage), have been left to the discretion of the hypertension specialist, always guided by current guidelines [13]. The presence of subclinical HMOD does not reclassify patients into HE or HU, the definition of which is based on acute clinical damage.
Figure 1 summarizes the study protocol, although data from visit 2 and visit 3 have not yet been considered in the present report.
Fig. 1.
Summary of Eridano Study protocol. ED emergency department, HMOD hypertension mediated organ damage
Subclinical HMOD criteria
Subclinical cardiac HMOD—Echocardiography
Standard two-dimensional transthoracic echocardiographic (TTE) images were acquired by expert accredited staff with commercially available ultrasound machines (e.g., IE33, Phillips Medical Systems, Andover, Massachusetts, USA). Conventional parameters were assessed according to the current guidelines [14]. Left ventricular (LV) mass was estimated. Dubois’ formula was used to calculate body surface area (BSA) and LV mass values were indexed for BSA (LVMi). LV volumes and ejection fraction, and left atrial volume were assessed using Simpson’s Biplane technique from apical two and four-chamber views. LV diastolic function was estimated through the evaluation of left atrial volume, mitral inflow peak systolic velocities of early (E) and late (A) diastolic filling on pulsed-wave Doppler, color-tissue Doppler imaging of the septal and lateral mitral annulus (E’), according to current international recommendations [15].
Alterations of LV mass and geometry, increased left atrial volume, and diastolic dysfunction were considered subclinical cardiac HMOD [13, 16]. LV hypertrophy (LVH) was defined by LVMi > 115 g/m2 in men and > 95 g/m2 in women [13, 14]. Relative wall thickness (RWT) was defined as two-times inferolateral wall thickness divided by the LV diastolic diameter and was used to classify LV remodeling as either concentric (RWT > 0.42) or eccentric (RWT ≤ 0.42). Left atrial enlargement (LAe) was considered as left atrial volume indexed to BSA (LAVi) > 34 ml/m2 [14].
Subclinical vascular HMOD
Arterial stiffness was quantified using carotid-femoral pulse wave velocity (PWV). Pressure waveforms at the carotid and femoral artery were obtained non-invasively by applanation tonometry with validated instruments (e.g., Sphygmocor, AtCor Medical—Sydney, Australia) [17].
Carotid artery imaging assessment was performed by experienced staff using available ultrasound machines, equipped with 4–12 MHz linear-array ultrasound transducer. The common carotid artery (CCA) intima-media thickness (IMT) was detected by validated software (e.g., Q-lab, Philips) on longitudinal bidimensional imaging. When clinically indicated patients underwent further imaging investigation.
PWV > 10 m/s and CCA IMT > 0.9 mm or the presence of carotid plaques (identified by an IMT ≥1.5 mm, or by a focal increase in thickness of 0.5 mm or 50% of the surrounding carotid IMT value) were considered subclinical vascular HMOD [13, 17].
Subclinical renal HMOD
Estimated glomerular filtration rate (eGFR) was assessed with Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula based on serum creatinine measured within 3 months from ED presentation [18]. Moreover, patients underwent microalbuminuria assessment. eGFR <60 ml/min/1.73 m2, urinary albumin/creatinine ratio >30 mg/g, and albuminuria > 30 mg/24 h were considered endpoints of significant renal HMOD [13, 19].
Subclinical cerebral HMOD
When clinically indicated, according to ED presentations, patients underwent brain imaging, either by computed tomography or magnetic resonance imaging. The presence of white matter lesions, microinfarcts (e.g., lacunar infarctions), microbleeds, and brain atrophy identified by experienced radiologists were considered cerebral HMOD [13, 20, 21].
Statistical analysis
Statistical analysis was performed by a dedicated software (R: A Language and Environment for Statistical Computing, v4.0.0 for Mac OSX, R Core Team., Vienna, Austria). Continuous variables were expressed as mean ± standard deviation. Qualitative variables were expressed as absolute values of frequency and percentage values. Normal distribution of variables was tested using the Kolmogorov-Smirnov and residual analysis tests. Differences between independent groups were evaluated using a t-test for continuous variables with normal distribution and the Mann-Whitney or Kruskal-Wallis test for continuous variables with non-normal distribution. Categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. Statistical significance was considered for p values < 0.05.
The present study was firstly approved by the Institutional Review Committee of Turin (Comitato Etico Interaziendale A.O.U. Città della Salute e della Scienza di Torino – A.O. Ordine Mauriziano, CS2/1075), as well as by the local ethics committees of each participating center. All subjects gave their written informed consent.
Results
A total of 113,694 patients were registered in the ED during the months of active enrollment; 1910 (1.7%) admissions were due to acute BP elevation, but only 122 (0.1% of the total) met the inclusion/exclusion criteria. Therefore, a total of 122 patients (52.5% female) with a mean age of 60.7 ± 13.9 years were enrolled until May 2022 and thus included in the present report. A total of 18 patients (14.8%) had acute organ damage at ED presentation (HE), whereas the remaining 104 (85.2%) patients were diagnosed as HU. These data correspond to a prevalence of 0.09% (104/113,694) for HU and 0.02% (18/113,694) for HE.
The acute organ damages detected were heart failure (no. 7, 39%), stroke (no. 6, 33%), acute coronary syndrome (no. 2, 11%), hypertensive encephalopathy (no. 2, 11%), aortic dissection (no. 1, 6%). An eGFR < 60 ml/min/1.73 m2 was found in 5 patients at ED evaluation, but the presence of similar creatinine values in the previous 12 months (available in the informatic system), did not make them categorize as acute kidney injury.
No significant difference emerged between HE and HU groups in terms of gender, BMI, cardiovascular comorbidities (Table 1). Hypertensive therapy ongoing at ED admission is listed in Table 2.
Table 1.
Demographic and clinical characteristics of study population
| Total N = 122 | HE N = 18 | HU N = 104 | p value | |
|---|---|---|---|---|
| Male Sex [no. (%)] | 58 (47.5%) | 9 (50.0%) | 49 (47.1%) | 0.821 |
| Age (y) | 60.7 ± 13.9 | 66.5 ± 15.9 | 60.0 ± 13.5 | 0.134 |
| Height (cm) | 165 ± 10 | 166 ± 9 | 165 ± 11 | 0.644 |
| Weight (kg) | 79.6 ± 19.4 | 78 ± 25 | 79 ± 19 | 0.883 |
| BMI (kg/m2) | 28.9 ± 5.78 | 28.6 ± 7.2 | 28.9 ± 5.6 | 0.826 |
| ED SBP (mmHg) | 201 ± 20 | 205 ± 18 | 200 ± 20 | 0.372 |
| ED DBP (mmHg) | 113 ± 13 | 110 ± 14 | 113 ± 13 | 0.357 |
| Discharge SBP (mmHg) | 152 ± 21 | 155 ± 25 | 151 ± 20 | 0.669 |
| Discharge DBP (mmHg) | 88 ± 12 | 87 ± 14 | 88 ± 12 | 0.820 |
| ED Stay (h) [IQ range] | 7.2 [4.7; 12.8] | 5.6 [4.7; 18.7] | 7.2 [4.7; 12.2] | 0.900 |
| BP < 180/110 at ED discharge [no. (%)] | 96 (78.7%) | 8 (44.4%) | 88 (84.6%) | 0.003 |
| Office SBP (mmHg) | 147 ± 22 | 149 ± 22 | 147 ± 23 | 0.680 |
| Office DBP (mmHg) | 87 ± 15 | 88 ± 15 | 87 ± 16 | 0.746 |
| Difference ED-Office SBP (mmHg) | 54 ± 28 | 56 ± 34 | 53 ± 27 | 0.770 |
| Difference ED-Office DBP (mmHg) | 26 ± 17 | 22 ± 19 | 26 ± 17 | 0.322 |
| Silent medical history [no. (%)] | 20 (16.4%) | 1 (5.6%) | 19 (18.3%) | 0.179 |
| Arterial Hypertension [no. (%)] | 94 (77.0%) | 17 (94.4%) | 77 (74.0%) | 0.057 |
| Hypertension duration (y) [IQ range] | 10.0 [5.0; 18.0] | 15.5 [10.0; 28.5] | 10.0 [5.0; 16.0] | 0.066 |
| Diabetes [no. (%)] | 24 (19.7%) | 4 (22.2%) | 20 (19.2%) | 0.768 |
| Dyslipidemia [no. (%)] | 36 (29.5%) | 8 (44.4%) | 28 (26.9%) | 0.132 |
| CAD [no. (%)] | 15 (12.3%) | 4 (22.2%) | 11 (10.6%) | 0.165 |
| Heart failure [no. (%)] | 5 (4.1%) | 2 (11.1%) | 3 (2.9%) | 0.104 |
| Atrial fibrillation [no. (%)] | 7 (5.7%) | 1 (5.6%) | 6 (5.8%) | 0.971 |
| Previous stroke [no. (%)] | 5 (4.1%) | 2 (11.1%) | 3 (2.9%) | 0.104 |
| CKD [no. (%)] | 8 (6.6%) | 2 (11.1%) | 6 (5.8%) | 0.398 |
BMI body mass index, BP blood pressure, CAD coronary artery disease, CKD chronic kidney disease, ED emergency department, HE hypertensive emergencies, HU hypertensive urgencies, SBP systolic blood pressure, DBP diastolic blood pressure
Table 2.
Ongoing hypertensive therapy and medications of study population at ED admission
| Previous Hypertensive Therapy | Total N = 122 | HE N = 18 | HU N = 104 | p value |
|---|---|---|---|---|
| Previous Hyp therapy [no. (%)] | 85 (69.7%) | 15 (83.3%) | 70 (67.3%) | 0.172 |
| Nr. Previous Hyp drugs [IQ range] | 1.0 [0.0; 2.0] | 1.0 [1.0; 2.0] | 1.0 [0.0; 2.0] | 0.471 |
| Previous Hyp drugs ≥ 3 [no. (%)] | 23 (18.9%) | 2 (11.1%) | 21 (20.2%) | 0.363 |
| ACE-Inhibitors [no. (%)] | 33 (27.0%) | 8 (44.4%) | 25 (24.0%) | 0.072 |
| ARB [no. (%)] | 30 (24.6%) | 1 (5.6%) | 29 (27.9%) | 0.042 |
| CCB [no. (%)] | 27 (22.1%) | 6 (33.3%) | 21 (20.2%) | 0.215 |
| CCB NDH [no. (%)] | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Beta-blockers [no. (%)] | 44 (36.1%) | 7 (38.9%) | 37 (35.6%) | 0.787 |
| Alfa-blockers [no. (%)] | 9 (7.4%) | 0 (0.0%) | 9 (8.7%) | 0.195 |
| Alfa2-agonist [no. (%)] | 2 (1.6%) | 0 (0.0%) | 2 (1.9%) | 0.553 |
| MRA [no. (%)] | 1 (0.8%) | 0 (0.0%) | 1 (1.0%) | 0.676 |
| Thiazides [no. (%)] | 14 (11.5%) | 2 (11.1%) | 12 (11.5%) | 0.958 |
| Loop diuretics [no. (%)] | 8 (6.6%) | 1 (5.6%) | 7 (6.7%) | 0.852 |
| Potassium sparing [no. (%)] | 1 (0.8%) | 0 (0.0%) | 1 (1.0%) | 0.676 |
| Nitrates [no. (%)] | 3 (2.5%) | 1 (5.6%) | 2 (1.9%) | 0.358 |
| Others hyp drugs [no. (%)] | 2 (1.6%) | 1 (5.6%) | 1 (1.0%) | 0.156 |
| Benzodiazepines [no. (%)] | 7 (5.7%) | 1 (5.6%) | 6 (5.8%) | 0.971 |
ACE-Inhibitors inhibitors of angiotensin-converting enzyme, ARB angiotensin II receptor blockers, CCB calcium channel blockers, CCB-NDH non-dihydropyridine CCB, ED emergency department, HE hypertensive emergencies, HU hypertensive urgencies, Hyp hypertension, MRA mineralocorticoid receptor antagonists
At ED presentation mean systolic BP was 201 ± 20 mmHg and mean diastolic BP was 113 ± 13 mmHg, without significant difference between HE and HU patients. The most common clinical presentation was headache (46.7%), followed by chest pain (23.8%), dyspnea (14.8%), and neurological symptoms (6.6%), while other non-specific symptoms were present in 68.9% of patients.
A silent medical history was present in 20 patients (16.4%). Moreover, 94 patients (77%) had previously known arterial hypertension and 85 (69.7%) were on antihypertensive medical therapy, with a median number of medications of 1.0 [IQ range 0.0;2.0]; 23 patients (18.9%) were on ≥3 hypertensive drugs.
Hypertensive therapy and BP control during ED stay
Among patients enrolled, 61.1% and 94.2% of HE and HU group (p < 0.001) received antihypertensive therapy during ED stay (89.3% of total population), with more drugs administered in the latter group (1.0 [0.0;2.0] vs. 2.0 [1.0;2.0] in HE and HU patients, respectively, p = 0.003). A total of 25 patients (24%) of HU group received 3 or more antihypertensive medications. Intravenous antihypertensive drugs were given to 27.8% and 15.4% of patients in HE and HU group (p = 0.198).
The most used class of medication was calcium channel blockers (CCB), administered to 74 patients (60.7%) (22.2% vs. 67.3% in HE and HU group, respectively, p < 0.001), followed by benzodiazepines, administered to 57 patients (46.7%) (16.7% vs. 51.9%, in HE and HU group, respectively, p = 0.006) and ACE-Inhibitors, given to 53 patients (43.4%) (16.7% vs. 48.1%, in HE and HU group, respectively, p = 0.013). The remaining classes of drugs administered during ED stay are listed in Table 3.
Table 3.
Hypertensive therapy and medications administered during ED stay
| Hypertensive therapy administered in ED | Total N = 122 | HE N = 18 | HU N = 104 | p value |
|---|---|---|---|---|
| Hyp therapy in ED [no. (%)] | 109 (89.3%) | 11 (61.1%) | 98 (94.2%) | <0.001 |
| Nr. Hyp drugs in ED [IQ range] | 2.0 [1.0; 2.0] | 1.0 [0.0; 2.0] | 2.0 [1.0; 2.0] | 0.003 |
| Hyp drugs in ED ≥3 [no. (%)] | 25 (20.5%) | 0 (0.0%) | 25 (24.0%) | 0.020 |
| IV Hyp drugs in ED [no. (%)] | 21 (17.2%) | 5 (27.8%) | 16 (15.4%) | 0.198 |
| ACE-Inhibitors [no. (%)] | 53 (43.4%) | 3 (16.7%) | 50 (48.1%) | 0.013 |
| ARB [no. (%)] | 13 (10.7%) | 2 (11.1%) | 11 (10.6%) | 0.946 |
| CCB [no. (%)] | 74 (60.7%) | 4 (22.2%) | 70 (67.3%) | <0.001 |
| CCB NDH [no. (%)] | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Beta-blockers [no. (%)] | 26 (21.3%) | 2 (11.1%) | 24 (23.1%) | 0.252 |
| Alfa-blockers [no. (%)] | 14 (11.5%) | 0 (0.0%) | 14 (13.5%) | 0.098 |
| Alfa2-agonist [no. (%)] | 11 (9.0%) | 1 (5.6%) | 10 (9.6%) | 0.579 |
| MRA [no. (%)] | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Thiazides [no. (%)] | 6 (4.9%) | 0 (0.0%) | 6 (5.8%) | 0.296 |
| Loop diuretics [no. (%)] | 11 (9.0%) | 2 (11.1%) | 9 (8.7%) | 0.737 |
| Potassium sparing [no. (%)] | 1 (0.8%) | 0 (0.0%) | 1 (1.0%) | 0.676 |
| Nitrates [no. (%)] | 8 (6.6%) | 2 (11.1%) | 6 (5.8%) | 0.398 |
| Other vasodilators [no. (%)] | 1 (0.8%) | 0 (0.0%) | 1 (1.0%) | 0.676 |
| Others hyp drugs [no. (%)] | 2 (1.6%) | 1 (5.6%) | 1 (1.0%) | 0.156 |
| Benzodiazepines [no. (%)] | 57 (46.7%) | 3 (16.7%) | 54 (51.9%) | 0.006 |
Abbreviations as in Table 2
After 1 hour from ED admission, 50.0% of HE patients and 76.7% of HU patients had BP values <180/110 mmHg. At the time of ED discharge, these percentages increased to 66.7% of and 93.6%, respectively (p = 0.003) (90.6% of total population), with a median ED stay of 7.2 hours [IQ range 4.7;12.8]. At ED discharge mean systolic BP was 152 ± 21 mmHg and diastolic BP was 88 ± 12 mmHg.
No drugs were significantly associated with the achievement of BP values <180/110 mmHg during ED stay (data not shown).
Office blood pressure control (72 hours after ED discharge)
At 72 hours visit patients had mean systolic BP of 148 ± 22 mmHg (p = 0.037, compared to BP at ED discharge) and diastolic BP of 88 ± 16 (p = 0.944).
BP values <140/90 mmHg were achieved in 42 patients (34.4%) who resulted normotensive at 72 hours visit (22.2% and 36.5% of HE and HU patients, p = 0.238). 43 patients (35.2%) had grade 1 hypertension, 27 (22.1%) had grade 2 hypertension, and 10 (8.2%) had grade 3 hypertension, with no differences between HE and HU patients (p = 0.592).
Patients with uncontrolled BP were more frequently males (56.3% vs. 31.0%, p = 0.008), but there were no other significant differences in terms of age, body size, and cardiovascular comorbidities. Moreover, patients with uncontrolled BP had higher mean PWV (10.1 ± 2.3 vs. 8.9 ± 2.2 m/s, p = 0.017) and higher prevalence of PWV > 10 m/s (49.1 vs. 25.9%, p = 0.045), even after adjusting for heart rate and mean BP (data not shown). Hypertensive therapy prescribed at 72 hours visit is depicted in Table 4.
Table 4.
Hypertensive therapy and medications prescribed at 72 hours visit
| Hypertensive therapy prescribed at 72 h visit | Total N = 122 | HE N = 18 | HU N = 104 | p value |
|---|---|---|---|---|
| Hyp therapy at 72 h [no. (%)] | 105 (86.0%) | 10 (55.6%) | 95 (91.3%) | <0.001 |
| Hyp drugs at 72 h (no) [IQ range] | 3.0 [2.0; 4.0] | 2.0 [0.0; 2.75] | 3.0 [2.0; 4.0] | 0.023 |
| Hyp drugs at 72 h ≥ 3 [no. (%)] | 65 (53.3%) | 5 (27.8%) | 60 (57.7%) | 0.019 |
| ACE-Inhibitors [no. (%)] | 30 (24.6%) | 5 (27.8%) | 25 (24.0%) | 0.734 |
| ARB [no. (%)] | 56 (45.9%) | 4 (22.2%) | 52 (50.0%) | 0.029 |
| CCB [no. (%)] | 85 (69.7%) | 7 (38.9%) | 78 (75%) | 0.002 |
| CCB NDH [no. (%)] | 15 (12.3%) | 0 (0.0%) | 15 (14.4%) | 0.085 |
| Beta-blockers [no. (%)] | 39 (32.0%) | 6 (33.3%) | 33 (31.7%) | 0.893 |
| Alfa-blockers [no. (%)] | 30 (24.6%) | 1 (5.6%) | 29 (27.9%) | 0.042 |
| Alfa2-agonist [no. (%)] | 2 (1.6%) | 0 (0.0%) | 2 (1.9%) | 0.553 |
| MRA [no. (%)] | 8 (6.6%) | 2 (11.1%) | 6 (5.8%) | 0.398 |
| Thiazides [no. (%)] | 25 (20.5%) | 2 (11.1%) | 23 (22.1%) | 0.286 |
| Loop diuretics [no. (%)] | 9 (7.4%) | 3 (16.7%) | 6 (5.8%) | 0.102 |
| Potassium sparing [no. (%)] | 2 (1.6%) | 0 (0.0%) | 2 (1.9%) | 0.553 |
| Nitrates [no. (%)] | 10 (8.2%) | 1 (5.6%) | 9 (8.7%) | 0.658 |
| Others hyp drugs [no. (%)] | 1 (0.8%) | 1 (5.6%) | 0 (0.0%) | 0.016 |
| Benzodiazepines [no. (%)] | 2 (1.6%) | 1 (5.6%) | 1 (1.0%) | 0.156 |
Abbreviations as in Table 2
Hypertension-mediated subclinical organ damage (HMOD) at 72 hours visit
LVH was present in 41 patients (33.6% of total population; 50% and 30.8% of HE and HU patients, respectively, p = 0.0.54). HE group showed higher LVMi compared to HU group (110.9 ± 36.0 vs. 93.0 ± 26.4 g/m2, p = 0.023).
LAe was detected in 26 patients (21.3%); no difference in LAe prevalence was found between HE and HU group (22.2% vs. 21.2%, p = 0.836), but the former group had significant higher LAVi (37.8 ± 17.4 vs. 28.2 ± 10.0, p = 0.014). Systolic and diastolic function was similar between the two groups.
Subclinical vascular HMOD was assessed in 91 patients and was detected in 49 patients (53.9%). Of the 82 patients whose arterial stiffness was tested, 34 (41.5%) had PWV > 10 m/s, and of the 58 patients tested with carotid ultrasound, 25 (43.1%) had CCA IMT > 0.9 mm or carotid plaques. Indices of subclinical vascular HMOD were proved to be comparable between the two groups (Table 5).
Table 5.
Subclinical hypertension mediated organ damage characteristics of study population
| Total N = 122 | HE N = 18 | HU N = 104 | p value | |
|---|---|---|---|---|
| LVMi (g/m2) | 95.5 ± 28.4 | 110.9 ± 36.0 | 93.0 ± 26.4 | 0.023 |
| LVH [no. (%)] | 41 (33.6%) | 9 (50.0%) | 32 (30.8%) | 0.054 |
| EF (%) | 61.3 ± 7.9 | 57.9 ± 5.0 | 61.9 ± 7.0 | 0.067 |
| LAVi (ml/m2) | 29.2 ± 11.2 | 37.8 ± 17.4 | 28.2 ± 10.0 | 0.014 |
| LAe [no. (%)] | 26 (21.3%) | 4 (22.2%) | 22 (21.2%) | 0.836 |
| Ascending aorta (mm) | 34.4 ± 4.9 | 36.2 ± 5.0 | 34.1 ± 4.8 | 0.171 |
| E/E’ ratio | 9.28 ± 4.57 | 9.91 ± 3.34 | 9.21 ± 4.71 | 0.634 |
| E/E’ ratio > 14 [no. (%)] | 12 (9.8%) | 3 (16.7%) | 9 (8.7%) | 0.081 |
| TR max vel (m/s) | 2.32 ± 0.43 | 2.51 ± 0.33 | 2.31 ± 0.44 | 0.380 |
| PWV (m/s)a | 9.71 ± 2.30 | 9.83 ± 1.54 | 9.68 ± 2.41 | 0.847 |
| PWV > 10 m/s [no. (%)]a | 34 (39.5%)a | 4 (33.3%)a | 30 (40.5%)a | 0.536 |
| Abnormal carotid US [no. (%)]b | 25 (43.1%)b | 4 (66.7%)b | 21 (40.4%)b | 0.218 |
| Vascular HMOD [no. (%)]c | 49 (53.8%)c | 7 (46.7%)c | 42 (55.3%)c | 0.542 |
| Renal HMOD [no. (%)] | 15 (12.3%) | 5 (27.8%) | 10 (9.6%) | 0.010 |
| Cerebral HMOD [no. (%)]d | 16 (34.8%)d | 8 (100%)d | 8 (21.1%)d | <0.001 |
E/E’ ratio mean transmitral inflow early wave on pulsed-wave Doppler to mitral annulus (lateral/septal) early wave on tissue-doppler imaging ratio, EF ejection fraction, HMOD hypertension mediated organ damage, LAe left atrial enlargement, LAVi left atrial volume indexed for body surface area, LVH left ventricular hypertrophy, LVMi left ventricular mass indexed for body surface area, PWV pulse wave velocity
aData available for 86 patients (12 patients among HE, 74 patients among HU)
bData available for 58 patients (6 patients among HE, 52 patients among HU)
cData available for 91 patients (15 patients among HE, 76 patients among HU)
dData available for 46 patients (8 patients among HE, 38 patients among HU)
Subclinical renal HMOD was observed in 15 patients (12.3%). HE patients had higher prevalence of renal damage than HU patients (27.8% vs. 9.6%, p = 0.010).
Brain damage was detected in 16 patients (34.8% of 46 patients who underwent brain imaging during ED evaluation), and it was detected in all HE patients who underwent brain imaging (100% vs. 21.1%, p < 0.001).
In summary, subclinical HMOD was detected in 82 patients (67.2% of total population), 100% of HE patients and 61.5% of HU patients (p = 0.001). Patients with detected subclinical HMOD were older than patients without HMOD (64.4 ± 13 vs. 53.3 ± 12 years, p < 0.001), and had more likely history of diabetes (p < 0.001), dyslipidemia (p = 0.042), coronary artery disease (p = 0.021), and chronic kidney disease (p = 0.041). Patients with detected subclinical HMOD were also taking higher median number of hypertensive drugs at ED admission (1.0 [0.0; 1.0] vs. 1.0 [0.0; 2.0], p = 0.004), and had higher mean systolic BP values at ED admission (204 ± 18 vs. 194 ± 20 mmHg, p = 0.007) and at 72 h visit (150 ± 23 vs. 140 ± 19 mmHg, p = 0.016).
Discussion
This report described around the first hundred patients with acute hypertensive disorders enrolled within the Italian multicenter prospective study called Eridano. This study has an ambitious prognostic aim, but, at present, only descriptive data from the first visits have been presented, specifically the ED enrolment and the office evaluation within 72 hours of ED discharge.
Acute hypertensive disorders are serious medical conditions, with a combined prevalence of 1.2% of total admission in the ED, in the most recent meta-analysis on the topic [1]. In the present prospective study, it is difficult to estimate the true prevalence of these conditions, considering the changes in the ED admissions dictated by the COVID-19 pandemic [22, 23]. Our data correspond to a prevalence of 0.09% for HU and 0.02% for HE; much lower prevalences compared to literature data, which could partly account for the truth regarding the lower number of ED admissions during the pandemic, but could also be the result of underestimation of data due to enrollment issue for logistical difficulties that have affected all italian hospitals in recent years. In fact, the overall prevalence of admission for acute BP disorders (1.7%), not considering the inclusion/exclusion criteria of the current study, is similar to the literature data. Also, this large difference could be dictated by the high attention to the presence of symptoms at ED presentation. Most data regarding acute BP disorders consider both symptomatic and asymptomatic patients, while we focused on patients having symptoms consistent with possible acute hypertensive organ damage. This could have led to a low proportion of patients actually included in the study, among the overall patients being registered at ED presentation as having acute BP disorders.
The ratio between HE and HU is similar to those of previous studies [24–30]. Some differences are at least in part explained by the different HE/HU definitions, in terms of BP cut-off or diagnostic coding; in a large retrospective study, the prevalence of HE in the United States between 2006 and 2013 was lower, probably due to the strict definition, based on acute BP elevation together with a diagnosis of acute organ damage based on the ICD-9 code [2]. In a recent review, the prevalence of HE in the Asian population ranges from 0.1 to 1.5% [31].
Our population is younger than the previous Italian multicenter study, whose enrolment was held in 2009, by about 10 years [27], but with similar age of an Asian study from the most recent recruitment [32]. Although we need to increase the sample size to confirm these data, no differences in age, sex and cardiovascular comorbidities are currently present between HE and HU. This seems to disagree with previous findings, in which HE was associated with male sex [24, 27], older age, and comorbidities [29].
Pharmacological management in ED confirms for the umpteenth time the great inconsistency among professionals concerning the treatment of acute BP disorders, as well pointed out by the GEAR project [10]. Frequently, antihypertensive drugs are used with the goal of acutely reducing BP in HU, while there is no benefits to support this practice [4, 33]. In contrast, there are data on the possible damage from rapid BP reduction in patients without organ damage [34].
Although mostly based on expert opinion, there are official recommendations on the treatment of HE [12]; moreover, a reasoned pharmacological approach has recently been proposed, starting from the pathophysiology of HE [35, 36]. Indeed, the major problem seems to be represented by patients with HU, where the greatest discrepancies in treatment approach are found, including the use of intravenous drugs, absolutely not recommended in this context. The current European position paper [12] suggests that HU should be treated in the same way as asymptomatic uncontrolled hypertension, by modifying home therapy without claiming rapid BP reduction in the emergency room. In these patients, oral administration of antihypertensive drugs, aimed at gradual BP reduction over the following days, is the best approach [37–39].
In our cohort, CCBs were the most widely used class; in particular, amlodipine, the most available drug in the class in Italian ED, was used in 99% of cases (73 out of 74 patients); nifedipine was used in only one case. These data are fortunately a marked improvement from the frequent use shown in the survey cited above [10], where 22% of participants (and 23% of those working in the ED) were inclined to use sublingual nifedipine to reduce BP, although its use has been discouraged for years because of possible deleterious effects [40]. Long-acting CCBs are also encouraged in this context because they do not interfere with diagnostics, and consequently allow the search for secondary causes of hypertension when indicated [35].
Captopril remains by far the most widely used drug within the class of ACE inhibitors (31 out of 53 patients treated with this class in our cohort). Compared to nifedipine, captopril has been shown to be equally effective in terms of BP reduction, but with fewer side effects [41]; however, considerations must be taken even with this drug due to the possible sudden hypotension [42].
A special consideration should be given to benzodiazepines, class not officially suggested but widely used in clinical practice, as evidenced by previous studies [10, 24, 29]. Administered in almost half of the cases in our cohort, benzodiazepines are definitely recommended medication in adrenergic hyperactivity BP disorders, such as cocaine abuse [38, 43], but their use outside this context would merit more in-depth studies. Patients with HU treated with benzodiazepines demonstrated greater reductions in systolic BP values, than patients not treated with anxiolytic therapy [44]. In a randomized clinical trial, diazepam demonstrated the same pressor effect as nifedipine and propranolol [45]; in another trial, the same pressor effect of captopril [46]. In the present report, 70% of prescribers considered administering benzodiazepines to reduce an obvious anxiety symptomatology associated with the BP rise, while 30% of prescribers used benzodiazepines for an expected stand-alone antihypertensive effect, independent of anxiety. The marked difference in the proportion of benzodiazepines administration between ED and ambulatory visit (52 vs. 1%) is probably due to the need to counter the anxiety effect on BP during symptomatic ED presentation, not so markedly present at the office evaluation. Likely, patients with acute BP disorders, especially those without acute clinical organ damage, suffer from an overlap of true BP elevation and anxiety effect, that leads to very high BP values. This effect is also probably enhanced by the presence of symptoms. During office evaluation, once the symptoms and the fear for life-threatening situation are over, BP values are less influenced by anxiety, and benzodiazepines prescription is not required anymore.
The fact that not all patients with HE were treated in our cohort is surprising, but this data could be distorted by rapid admission to the intensive or semi-intensive units with treatment initiated outside the ED (indeed, the median ED stay of 7.2 hours is mainly due to HU, in a situation of Italian ED currently characterized by overcrowding and boarding problems). Furthermore, in ischemic strokes (no. 5 in our cohort), the cut-off for starting acute antihypertensive treatment is higher than that of HE diagnosis.
To our knowledge, our study is currently the only one that prospectively and systematically assesses short-term (72 hours) BP control in office setting after ED discharge, except for a small study on 21 hypertensive patients in which 24h-ABPM immediately after discharge from the ED [47]. Approximately 90% of patients in our study were discharged from the ED with BP < 180/110 mmHg, thus no longer meeting the criteria for HU, for those without organ damage; a similar rate has been described in recent studies [48, 49]. In about one-third of the cases, normal office BP was present at 72 hours after ED discharge; similar outcome than that reported, of about 20% at 2 weeks after discharge, in a retrospective study conducted in the Thai population [49], but very different from the previously cited Israeli report in which 17 out of 21 patients remained with a SBP > 180 mmHg 24 hours after ED discharge [47].
The median number of hypertensive drugs prescribed increased from 1.0 [IQR 0.0; 2.0] before ED admission, to 2.0 [IQR 1.0; 2.0] during ED stay, and eventually to 3.0 [IQR 2.0; 4.0] at 72 h visit. These data confirm both the high BP variability in this population and the need for aggressive treatment.
Finally, we presented some data on subclinical HMOD: to our knowledge this is the first study to assess subclinical HMOD in HE and HU patients immediately after ED discharge.
In general, HE patients had worse subclinical HMOD profile than HU patients, particularly cardiac, renal, and cerebral HMOD, while vascular HMOD was comparable. At 72 h visit, patients with uncontrolled BP had worse PWV, suggesting a possible role of aortic stiffness in impeding proper BP control, or possibly grater vascular damage in patients with short-term uncontrolled BP. A recent study showed that HU patients had subclinical HMOD profile midway between patients with asymptomatic grade 3 hypertension and patients with various grade hypertension, matched for office BP [50]. The higher prevalence of subclinical HMOD in HE patients found in the present study underlines that HE patients have worse baseline CV risk profile than HU patients, leading to more severe manifestations of acute BP rise. Moreover, this difference in subclinical HMOD was not observed when comparing patients with controlled and uncontrolled BP at 72 h visit, somehow indicating that some patients could represent a special high-risk population, irrespectively of acute and short-term BP control. Ongoing follow-up is needed to better define this aspect.
Study limitations and future perspectives
It should be stressed first of all that the prevalence data are the result of an estimation calculated on the basis of the months of active enrollment in the various centers and the average visits to the EDs; therefore, these are numbers to be taken with caution because they could represent an underestimation of reality even if a reduction in prevalences could have been expected during COVID-19 pandemic.
As specified, complete follow-up is needed to add prognostic value to the subclinical organ damage in this category of patients. It may be interesting to assess whether early in-depth evaluation at specialized hypertension centers could improve prognosis compared to standard management especially for the category of patients without acute clinical damage.
The present study has a purely descriptive nature, impaired by the small total number and the numerical discrepancy between the two groups analyzed (HE and HU); this must make comparisons interpreted with caution. At the same time, it has the advantages of describing short-term BP control and the investigation of subclinical HMOD immediately after discharge from the ED.
Ad hoc designed studies are needed to suggest appropriate management of HU in the ED, as well as targeted education to ED physicians by hypertension experts. Finally, in addition to cardiovascular drugs, benzodiazepines may be powerful weapons, already long used in clinical practice, to treat these disorders. It would be intriguing to evaluate their real hypotensive potential, perhaps considering the psychological characteristics of each patient. The use of benzodiazepines in these cases may be beneficial for both hypotensive and stress releasing effects, while significant harm is unlikely to result, being careful not to overestimate anxiety/stress effect over BP. Anyway, at present, pending stronger evidence, the results presented do not allow benzodiazepines to be recommended in acute hypertensive disorders, except in cases of associated overt anxiety, which is itself an indication for such therapy.
Conclusions
Acute BP disorders are a major challenge for the ED. The lack of good-quality evidence makes it difficult to propose strong recommendations for clinical practice. In this first report about the ongoing prospective Italian multicenter study ERIDANO, we showed that great inconsistency is present in acute BP disorder management. Up to one third of patients resulted normotensive after 72 h after ED discharge. HE patients showed greater cardiac, renal, and cerebral subclinical HMOD, compared to HU patients. 72 h BP control is not associated with different subclinical HMOD, except for vascular HMOD; therefore, proper comprehensive examination after discharge from the ED could provide added value in cardiovascular risk stratification of such patients.
Acknowledgements
We are grateful to staff physicians and fellows of the Emergency departments and Hypertensive centers for their work. We also thank Michela Algeri, Arianna Ardito, Giordano Bianchi, Carmine De Luca, Saverio Fabbri, Claudio Pascale, Alessandra Piazza, Giuliano Pinna, Giovanni Saccà, Nicole Saxinger, and Maria Tizzani for essential collaboration and useful discussion.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fabrizio Vallelonga, Marco Cesareo.
References
- 1.Astarita A, Covella M, Vallelonga F, Cesareo M, Totaro S, Ventre L, et al. Hypertensive emergencies and urgencies in emergency departments: a systematic review and meta-analysis. J Hypertens. 2020;38:1203–10. doi: 10.1097/HJH.0000000000002372. [DOI] [PubMed] [Google Scholar]
- 2.Janke AT, McNaughton CD, Brody AM, Welch RD, Levy PD. Trends in the incidence of hypertensive emergencies in us emergency departments from 2006 to 2013. J Am Heart Assoc. 2016;5. 10.1161/JAHA.116.004511 [DOI] [PMC free article] [PubMed]
- 3.Van Den Born BJH, Koopmans RP, Groeneveld JO, Van Montfrans GA. Ethnic disparities in the incidence, presentation and complications of malignant hypertension. J Hypertens. 2006;24:2299–304. doi: 10.1097/01.hjh.0000249710.21146.38. [DOI] [PubMed] [Google Scholar]
- 4.Levy PD, Mahn JJ, Miller J, Shelby A, Brody A, Davidson R, et al. Blood pressure treatment and outcomes in hypertensive patients without acute target organ damage: a retrospective cohort. Am J Emerg Med. 2015;33:1219–24. doi: 10.1016/j.ajem.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Patel KK, Young L, Howell EH, Hu B, Rutecki G, Thomas G, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176:981–8. doi: 10.1001/jamainternmed.2016.1509. [DOI] [PubMed] [Google Scholar]
- 6.Guiga H, Decroux C, Michelet P, Loundou A, Cornand D, Silhol F, et al. Hospital and out-of-hospital mortality in 670 hypertensive emergencies and urgencies. J Clin Hypertens. 2017;19:1137–42. doi: 10.1111/jch.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 8.Vlcek M, Bur A, Woisetschläger C, Herkner H, Laggner AN, Hirschl MM. Association between hypertensive urgencies and subsequent cardiovascular events in patients with hypertension. J Hypertens. 2008;26:657–62. doi: 10.1097/HJH.0b013e3282f4e8b6. [DOI] [PubMed] [Google Scholar]
- 9.Paini A, Tarozzi L, Bertacchini F, Aggiusti C, Rosei CA, De Ciuceis C, et al. Cardiovascular prognosis in patients admitted to an emergency department with hypertensive emergencies and urgencies. J Hypertens. 2021;39:2514–20. doi: 10.1097/HJH.0000000000002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saladini F, Mancusi C, Bertacchini F, Spannella F, Maloberti A, Giavarini A, et al. Diagnosis and treatment of hypertensive emergencies and urgencies among Italian emergency and intensive care departments. Results from an Italian survey: Progetto GEAR (Gestione dell’Emergenza e urgenza in ARea critica) Eur J Intern Med. 2020;71:50–56. doi: 10.1016/j.ejim.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Saladini F, Mancusi C, Bertacchini F, Spannella F, Maloberti A, Giavarini A, et al. Differences in Diagnosis and Management of Hypertensive Urgencies and Emergencies According to Italian Doctors from Different Departments Who Deal With Acute Increase in Blood Pressure-Data from Gear (Gestione Dell’emergenza e Urgenza in ARea Critica) St. J Clin Med. 2022;11. 10.3390/jcm11112986 [DOI] [PMC free article] [PubMed]
- 12.Van Den Born BJH, Lip GYH, Brguljan-Hitij J, Cremer A, Segura J, Morales E, et al. ESC Council on hypertension position document on the management of hypertensive emergencies. Eur Hear J Cardiovasc Pharmacother. 2019;5:37–46. doi: 10.1093/ehjcvp/pvy032. [DOI] [PubMed] [Google Scholar]
- 13.Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for themanagement of arterial hypertension. Eur Heart J. 2018;39:3021–104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Victor MA, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, et al. Recommendations on the use of echocardiography in adult hypertension: A report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) Eur Heart J Cardiovasc Imaging. 2015;16:577–605. doi: 10.1093/ehjci/jev076. [DOI] [PubMed] [Google Scholar]
- 17.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung AK, Chang TI, Cushman WC, Furth SL, Hou FF, Ix JH, et al. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99:559–69. doi: 10.1016/j.kint.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth WTJ, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–82. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 21.Boulestreau R, Lucas L, Cremer A, Debeugny S, Rubin S, Gaudissard J, et al. Neurologically asymptomatic patients frequently present cerebral injuries during malignant hypertension: a MRI study. J Hypertens. 2021;39:2463–9. doi: 10.1097/HJH.0000000000002950. [DOI] [PubMed] [Google Scholar]
- 22.Reschen ME, Bowen J, Novak A, Giles M, Singh S, Lasserson D, et al. Impact of the COVID-19 pandemic on emergency department attendances and acute medical admissions. BMC Emerg Med. 2021;21:143. doi: 10.1186/s12873-021-00529-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leontsinis I, Papademetriou V, Chrysohoou C, Kariori M, Dalakouras I, Tolis P, et al. Hypertensive urgencies during the first wave of the COVID-19 pandemic in a tertiary hospital setting: a U-shaped alarming curve. Arch Med Sci. 2022;18:982–90. doi: 10.5114/aoms/141243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvetti M, Paini A, Colonetti E, Tarozzi L, Bertacchini F, Aggiusti C, et al. Hypertensive emergencies and urgencies: A single-centre experience in Northern Italy 2008-2015. J Hypertens. 2020;38:52–58. doi: 10.1097/HJH.0000000000002213. [DOI] [PubMed] [Google Scholar]
- 25.Martin JFV, Higashiama E, Garcia E, Luizon MR, Cipullo JP. Hypertensive crisis profile. Prevalence and clinical presentation. Arq Bras Cardiol. 2004;83. [DOI] [PubMed]
- 26.Vilela-Martin JF, Vaz-De-Melo RO, Kuniyoshi CH, Abdo ANR, Yugar-Toledo JC. Hypertensive crisis: Clinical-epidemiological profile. Hypertens Res. 2011;34:367–71. doi: 10.1038/hr.2010.245. [DOI] [PubMed] [Google Scholar]
- 27.Pinna G, Pascale C, Fornengo P, Arras S, Piras C, Panzarasa P, et al. Hospital admissions for hypertensive crisis in the emergency departments: A large multicenter Italian study. PLoS One. 2014;9. 10.1371/journal.pone.0093542 [DOI] [PMC free article] [PubMed]
- 28.Zampaglione B, Pascale C, Marchisio M, Cavallo-Perin P. Hypertensive urgencies and emergencies: Prevalence and clinical presentation. Hypertension. 1996;27:144–7. doi: 10.1161/01.HYP.27.1.144. [DOI] [PubMed] [Google Scholar]
- 29.Vallelonga F, Carbone F, Benedetto F, Airale L, Totaro S, Leone D, et al. Accuracy of a Symptom-Based Approach to Identify Hypertensive Emergencies in the Emergency Department. J Clin Med. 2020;9:2201. doi: 10.3390/jcm9072201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fragoulis C, Dimitriadis K, Siafi E, Iliakis P, Kasiakogias A, Kalos T, et al. Profile and management of hypertensive urgencies and emergencies in the emergency cardiology department of a tertiary hospital: a 12-month registry. Eur J Prev Cardiol. 2022;29:194–201. doi: 10.1093/eurjpc/zwab159. [DOI] [PubMed] [Google Scholar]
- 31.Kotruchin P, Tangpaisarn T, Mitsungnern T, Sukonthasarn A, Hoshide S, Turana Y, et al. Hypertensive emergencies in Asia: A brief review. J Clin Hypertens. 2022;24:1226–35. doi: 10.1111/jch.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin J-H, Kim BS, Lyu M, Kim H-J, Lee JH, Park J-K, et al. Clinical Characteristics and Predictors of All-Cause Mortality in Patients with Hypertensive Urgency at an Emergency Department. J Clin Med. 2021;10. 10.3390/jcm10194314 [DOI] [PMC free article] [PubMed]
- 33.Jolly H, Freel EM, Isles C Management of hypertensive emergencies and urgencies: narrative review. Postgrad Med J (e-pub ahead of print October 2021. 10.1136/postgradmedj-2021-140899). [DOI] [PubMed]
- 34.Yanturali S, Akay S, Ayrik C, Cevik AA. Adverse events associated with aggressive treatment of increased blood pressure. Int J Clin Pr. 2004;58:517–9. doi: 10.1111/j.1368-5031.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 35.Rossi GP, Rossitto G, Maifredini C, Barchitta A, Bettella A, Cerruti L, et al. Modern Management of Hypertensive Emergencies. High blood Press Cardiovasc Prev J Ital Soc Hypertens. 2022;29:33–40. doi: 10.1007/s40292-021-00487-1. [DOI] [PubMed] [Google Scholar]
- 36.Gosse P, Boulestreau R, Brockers C, Puel C, Rubin S, Cremer A. The pharmacological management of malignant hypertension. J Hypertens. 2020;38:2325–30. doi: 10.1097/HJH.0000000000002547. [DOI] [PubMed] [Google Scholar]
- 37.Muiesan ML, Salvetti M, Amadoro V, di Somma S, Perlini S, Semplicini A, et al. An update on hypertensive emergencies and urgencies. J Cardiovasc Med. 2015;16:372–82. doi: 10.2459/JCM.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 38.Salvetti M, Paini A, Bertacchini F, Stassaldi D, Aggiusti C, Agabiti Rosei C, et al. Acute blood pressure elevation: Therapeutic approach. Pharm Res. 2018;130:180–90. doi: 10.1016/j.phrs.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 39.Miller J, McNaughton C, Joyce K, Binz S, Levy P. Hypertension Management in Emergency Departments. Am J Hypertens. 2020;33:927–34. doi: 10.1093/ajh/hpaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276:1328–31. doi: 10.1001/jama.1996.03540160050032. [DOI] [PubMed] [Google Scholar]
- 41.Kaya A, Tatlisu MA, Kaplan Kaya T, Yildirimturk O, Gungor B, Karatas B, et al. Sublingual vs. Oral Captopril in Hypertensive Crisis. J Emerg Med. 2016;50:108–15. doi: 10.1016/j.jemermed.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Maloberti A, Cassano G, Capsoni N, Gheda S, Magni G, Azin GM, et al. Therapeutic Approach to Hypertension Urgencies and Emergencies in the Emergency Room. High blood Press Cardiovasc Prev J Ital Soc Hypertens. 2018;25:177–89. doi: 10.1007/s40292-018-0261-4. [DOI] [PubMed] [Google Scholar]
- 43.Hollander JE. Cocaine intoxication and hypertension. Ann Emerg Med. 2008;51:S18–20. doi: 10.1016/j.annemergmed.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Paštrović F, Okštajner PK, Vodanović M, Raos D, Jug J, Lovrić Benčić M, et al. The Role of Anxiolytics in Hypertensive Urgency Management. Psychiatr Danub. 2020;32:593–6. [PubMed] [Google Scholar]
- 45.Mansur A, de P, Ramires JA, Avakian SD, de Paula RS, Pileggi F. [Comparison of the effects of diazepam, nifedipine, propranolol and a combination of nifedipine and propranolol, by sublingual administration, in patients with hypertensive crisis] Arq Bras Cardiol. 1991;57:313–7. [PubMed] [Google Scholar]
- 46.Grossman E, Nadler M, Sharabi Y, Thaler M, Shachar A, Shamiss A. Antianxiety treatment in patients with excessive hypertension. Am J Hypertens. 2005;18:1174–7. doi: 10.1016/j.amjhyper.2005.03.728. [DOI] [PubMed] [Google Scholar]
- 47.Rock W, Zbidat K, Schwartz N, Elias M, Minuhin I, Shapira R, et al. Pattern of Blood Pressure Response in Patients With Severe Asymptomatic Hypertension Treated in the Emergency Department. J Clin Hypertens (Greenwich) 2016;18:796–800. doi: 10.1111/jch.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y-T, Liu Y-H, Hsiao Y-L, Chiang H-Y, Chen P-S, Chang S-N, et al. Pharmacological blood pressure control and outcomes in patients with hypertensive crisis discharged from the emergency department. PLoS One. 2021;16:e0251311.. doi: 10.1371/journal.pone.0251311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotruchin P, Mitsungnern T, Ruangsaisong R, Imoun S, Pongchaiyakul C. Hypertensive Urgency Treatment and Outcomes in a Northeast Thai Population: The Results from the Hypertension Registry Program. High Blood Press Cardiovasc Prev. 2018;25:309–15. doi: 10.1007/s40292-018-0272-1. [DOI] [PubMed] [Google Scholar]
- 50.Vallelonga F, Cesareo M, Menon L, Airale L, Leone D, Astarita A, et al. Cardiovascular Hypertension-Mediated Organ Damage in Hypertensive Urgencies and Hypertensive Outpatients. Front Cardiovasc Med. 2022;9:889554.. doi: 10.3389/fcvm.2022.889554. [DOI] [PMC free article] [PubMed] [Google Scholar]