Abstract
Invasive pulmonary aspergillosis (IPA) is one of the most frequent forms of invasive fungal infections (IFI); however, it is often difficult to identify the pathogenic fungal species and to select appropriate treatments for patients with IFI including IPA. Here, we describe the detailed pathophysiology of an autopsy case of severe respiratory failure due to IPA with candidiasis. The patient developed severe respiratory failure after influenza infection and died, and the autopsy revealed a mixed disease of IPA with candidiasis. In this study, in addition to the routine pathological examination, we further examined formalin-fixed paraffin-embedded (FFPE) tissues by scanning electron microscopy (SEM) and partial genomic DNA sequencing. Although optical microscopy alone was insufficient to identify the pathogenic organisms, SEM clearly depicted the characteristic morphology of Aspergillus sp. and Candida sp. as closely overlapping in a nested fashion, providing evidence of mixed infection of both fungal species in a focal site. The technique using FFPE tissue in combination with ultrastructural observation by SEM, elemental analysis by SEM–EDX, and DNA sequencing is promising for analyzing the pathophysiology of IFI.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00795-023-00349-w.
Keywords: Invasive pulmonary aspergillosis, Candidiasis, Mixed infection, FFPE tissue, Calcium oxalate, Scanning electron microscope–energy-dispersive X-ray spectrometry, Direct sequence
Introduction
Invasive fungal infections (IFI) are systemic fungal infections that hematogenously infect various organs and cause tissue destruction. Invasive pulmonary aspergillosis (IPA), which predominantly affects the respiratory system, is the most frequent form of invasive fungal infection. Aspergillus species and Candida species are the most common pathogenic organisms [1, 2]. IPA is well known to develop in immunosuppressed patients including recipients of inherited or acquired immunodeficiency such as patients who have undergone solid organ transplantation or hematopoietic cell transplantation for leukemia [1, 3, 4]. In addition, COVID-19, influenza infection, liver cirrhosis, and chronic obstructive pulmonary disease (COPD) have recently been reported as risks for IPA [4–7].
Here, we report a case of IPA caused by Aspergillus niger (A. niger) after an influenza infection complicated with Candida infection. We performed ultrastructural and elemental analyses of several fungal species and surrounding calcium oxalate crystals by scanning electron microscope (SEM) and SEM–energy-dispersive X-ray spectrometry (SEM–EDX) using formalin-fixed paraffin-embedded (FFPE) specimens. In addition, partial genomic DNA analysis led to the diagnosis of A. niger and Candida albicans (C. albicans) infection.
Clinical findings
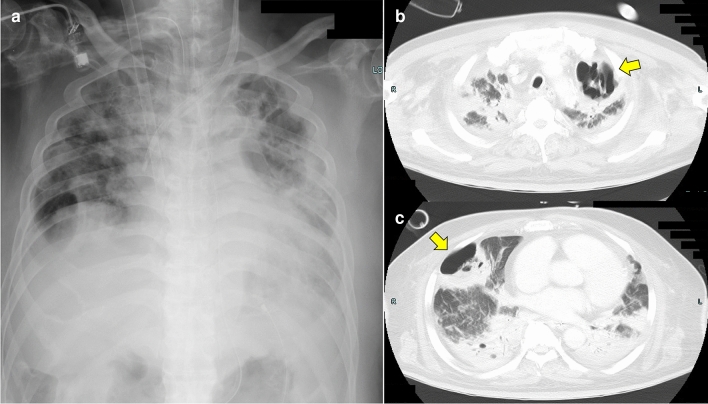
A 60-year-old Japanese man suffered from cold symptoms while traveling in Southeast Asia. The patient visited a hospital in Thailand and was diagnosed with influenza infection. Subsequently, the patient's respiratory condition worsened and he was admitted and treated in the intensive care unit (ICU) of the hospital in Thailand. Since the patient strongly requested to receive treatment in Japan, he returned to Japan while receiving oxygen administration with adequate informed consent. The patient was immediately admitted to our hospital after arriving in Japan, and veno-venous extracorporeal membrane oxygenation was performed to maintain respiratory function. Tazobactam/piperacillin, linezolid, ganciclovir and voriconazole were administered to treat bacterial, fungal, and viral infections. Chest Xp showed bilateral pleural effusions and severe consolidation in the bilateral whole lung fields (Fig. 1a). Chest computed tomography (CT) also showed severe consolidation, pleural effusion, and cavity formation in the bilateral lungs (Fig. 1b, c). The patient was administered dobutamine, noradrenaline, and vasopressin due to cardiopulmonary failure associated with severe inflammation, but his circulatory condition could not be maintained and he died on day 5 of admission. A pathological autopsy was performed to elucidate the causes of severe respiratory failure.
Fig. 1.
Chest Xp and CT. a Bilateral pleural effusions and severe consolidation in the bilateral whole lung fields. b Consolidation in the bilateral upper lobe and a cavitary lesion (arrow) in the left upper lobe. c Consolidation in the bilateral middle lobe and a cavitary lesion (arrow) in the right middle lobe
Autopsy findings
Macroscopic findings
A whitish-black nodule was exposed outside of the right lung (Fig. 2a). The cut surface showed a black nodule with a cavity in the right middle lobe, and there was a similar lesion in the left upper lobe of the lung, being consistent with the lesion observed on chest Xp and CT (Fig. 2b).
Fig. 2.
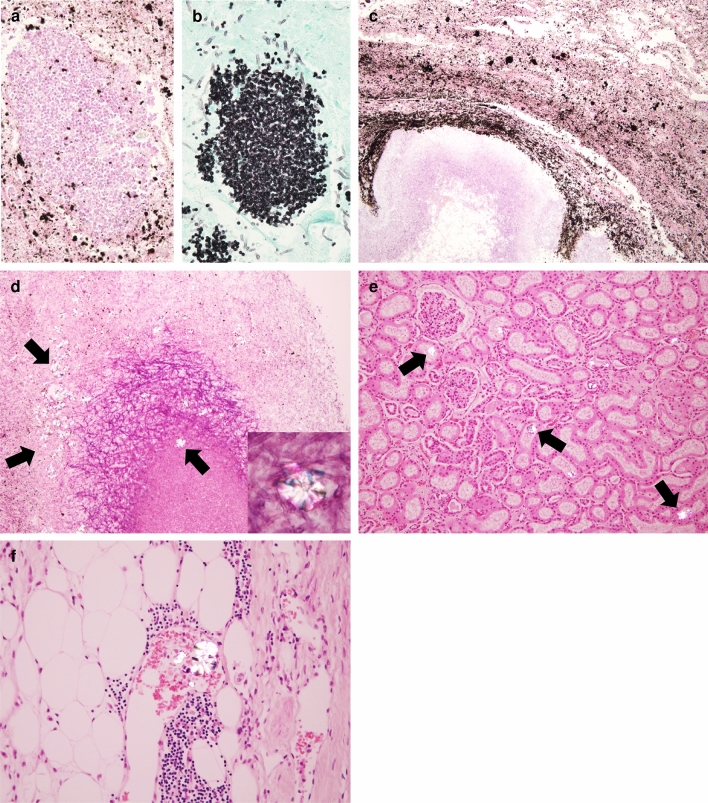
Macroscopic and microscopic findings of the lungs. a A lesion exposed to the pleural surface of the right middle lobe was observed. The lesion showed a round black nodule with a white deposition. b On the cut surface, the black nodular lesion (arrow) of the right middle lobe with a cavitary lesion (left image) and a similar lesion (arrowhead) of the left upper lobe (right image). c The nodular lesions had diffuse hemorrhage and necrotic tissue and severely damaged alveolar structures due to intense inflammation. HE staining, 4x. d Infiltration of inflammatory cells, mainly neutrophils, into the alveoli. HE staining, 20x. e Clusters of fungi in the necrotic tissue in the nodular lesions. HE staining, 4x. f, g Fungi with septate, Y-shaped, acute angle of 45°. f HE staining, 40x. g Grocott staining, 40x. h, i Aspergillus hyphae in a blood vessel (arrowheads). h HE staining, 40x. i Grocott staining, 40x
The nodular lesion in the lung showed destruction of lung structures with severe inflammation, hemorrhage, and necrosis (Fig. 2c). In addition, fungal masses were identified within the nodular lesions (Fig. 2d). There was severe infiltration of inflammatory cells, mainly neutrophils, within the residual alveoli and on the alveolar walls (Fig. 2e). The fungal mass found in the nodular lesions consisted of Y-shaped filamentous fungi that were positive for Grocott staining and branched at approximately 45°, consistent with the characteristics of Aspergillus species (Fig. 2f, g). Aspergillus hyphae were also found in the vessels and vessel walls of the lungs, which lead to the diagnosis of IPA (Fig. 2h, i). Several Grocott stain-positive circular yeast-like fungi were also observed around the Aspergillus lesions, suggesting mixed infection with Aspergillus and Candida species (Fig. 3a, b). Black pigmentation was also observed around the hyphae (Fig. 3c), and numerous colorless crystals were observed not only around the hyphae but also in necrotic and inflamed tissue (Fig. 3d). Polarized light microscopy showed that these needle-like crystals were birefringent and they were found in the pulmonary lesions as well as in the renal tubules and vessels of perirenal adipose tissue (Fig. 3e).
Fig. 3.
Microscopic findings of the lungs and kidneys. a, b A cluster of oval yeast-like fungi in the lesions. a HE staining, 40x. b Grocott staining, 40x. c Numerous black pigmentations around clusters of Aspergillus. HE staining, 10x. d Numerous colorless crystals(arrows) within clusters of Aspergillus and in the necrotic tissue. HE staining, 10x. Polarized light microscopic image (insert) revealed that the crystals were birefringent. HE staining, 40x. e, f Scattered crystals (arrows) in the renal tubules and blood vessels within the renal fatty tissue. e HE staining, 10x. f HE staining, 20x
Ultrastructural and elemental analyses by SEM and SEM–EDX
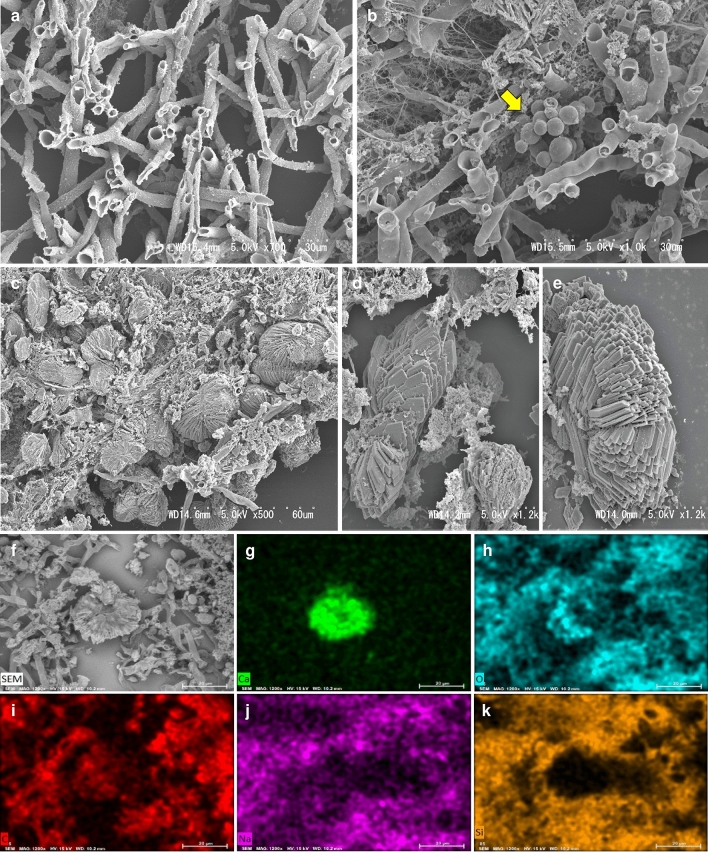
We performed ultrastructural observation of the fungal hyphae and the crystal structure by SEM (HITACHI, S-4300) using FFPE tissue. Around the necrotic tissue of the lung, complex folds of hyphae, which were hollow inside and branched at about 45°, were observed. The widths of the hyphae were relatively constant, with no bends or other irregularities, and there were no nicks in the branched sections. They were consistent with the ultrastructure of Aspergillus species (Fig. 4a). In some nests of Aspergillus hyphae, yeast-like fungi had formed small colonies, and a structure that appeared to be budding was also observed. These features were consistent with the ultrastructure of Candida species (Fig. 4b). Numerous crystals were embedded in Aspergillus hyphae, fibrinous material, and fibrous tissue (500x, Fig. 4c). At higher magnification (1200x), aggregated needle-like crystals and multilayered elongated hexagonal plate-like crystals were also observed (Fig. 4d, e). To investigate the elemental composition of the crystals, we performed SEM–EDX (energy-dispersive X-ray) analysis (HITACHI, TM4000 plus), a method to visualize the abundance of elements present (Fig. 4f–k and Online Resource 1a). Ca (green), O (light blue), and C (red) were highly abundant in the regions of the crystal structure observed in the backscattered electrons (BSE) (Fig. 4f). However, Na (purple) and Si (orange) were not related to the distribution of crystal structures. The SEM–EDX results were consistent with those expected from the chemical formula for calcium oxalate (CaOx), CaC2O4.
Fig. 4.
Analysis of the ultrastructure of the fungi and crystals in the lungs and investigation of constituent elements of the crystals by SEM. a Numerous overlapped hyphae with Y-shape and hollow interior. 700x. b A cluster of oval yeast-like fungi (arrow) embedded within Aspergillus clusters. 1000x. c Crystals composed of the accumulation of needle-like crystals. 500x. d Crystals composed of multi-layers of thin, slender, hexagonal plate-like crystals. 1200x. e Crystals composed of the accumulation of polygonal thin crystals. 1200x. f Crystal in the necrotic tissue of the nodular lesions, used for the constituent elements analysis by SEM–EDX. 1200x. Distribution of Ca (green, g), O (light blue, h), and C (red, i), consistent with the distribution of the crystal. Distribution of Na (purple, j) and Si (orange, k), inconsistent with the distribution of the crystal. g–k: 1200x
Identification of fungi by genetic analysis
Genomic DNA was extracted from a portion of FFPE tissue that contained a large amount of Aspergillus species and also from a portion of tissue containing relatively yeast-like fungal colony-rich area. The DNA samples were subjected to a polymerase chain reaction (PCR) using primers from the internal transcribed spacer 1 (ITS1) region, and the amplified products were confirmed by agarose gel electrophoresis (Fig. 5a and Online Resource 2a). Amplified DNA was purified to determine sequences [8]. We performed a BLAST search and found that the obtained DNA sequence showed a high concordance rate with A. niger and C. albicans (Fig. 5b and Online Resource 2b). The detailed sequence is shown in Online Resources 1b and 2c.
Fig. 5.
a Electrophoresis of amplicons of the fungi’s gene showed a range from 200 to 300 bps, which corresponds to the range of the number of base pairs of ITS1. b A. niger was the top result of the search by BLAST for DNA sequences similar to DNA sequences obtained from the fungi
Discussion
This is the first report on identification of an aspergillosis-responsible species based on ultrastructural analysis (SEM–EDX) and genomic amplicon sequencing using FFPE tissues as a source material. Thanks to recent technical progress, we can examine FFPE tissues by multiple methods beyond traditional pathological staining and observation.
Aspergillus species are the most common cause of systemic fungal infections, followed by Candida species. Aspergillus species are present in the environment, are inhaled from the air, and invade the respiratory tract or lung, causing a variety of infections and allergic diseases [9]. The most severe form of Aspergillus infection is invasive aspergillosis (IA), which affects more than 300,000 people worldwide every year [10]. IPA accounts for more than 90% of cases of IA, and viral infections such as infections with SARS-CoV-2 and influenza viruses have recently been reported to be risks for IPA [7, 11]. Influenza virus infection is known to induce 'influenza-associated pulmonary aspergillosis (IAPA)', which can progress to IPA, and the incidence of IAPA has been increasing [5, 6]. Our patient died of IPA after influenza infection, which is presumed to have occurred via IAPA based on the clinical course of the case. There is about a 10% risk that IPA will complicate severe influenza in ICU management, and the mortality rate is over 50% when it does occur [6].
In this case, thin-sliced sections were prepared from FFPE tissue, and the pathogenic microorganism and crystal structure were identified by ultrastructural analysis using SEM. As a result, a Y-shaped structure with a hollow inside, representing Aspergillus hyphae, was observed. In addition, Candida fungi were incidentally observed to be buried in the numerous Aspergillus hyphae, suggesting that the hyphae may function as a kind of basement for Candida colonization. SEM is useful for considering the etiology of mixed infection as well as identification of minor populations. Sometimes, it is difficult to find other fungi in a large population of a single fungus by Grocott staining using an optical microscope, but it is possible with SEM. Mixed infections with multiple fungi may be misinterpreted as a single fungal infection by current diagnostic methods, and the diagnosis would affect the patient´s survival and outcome. Ultrastructural observation by SEM using FFPE tissue will facilitate the recognition of potentially previously unidentifiable mixed fungal infections and might be helpful in the selection of appropriate antibiotics. In addition, taxonomic analysis of the FFPE tissue-derived fungal DNA amplicon sequence revealed that the main pathogenic organism was A. niger. A number of recently published studies have demonstrated that DNA-based detection of microorganisms from FFPE specimens is a powerful tool for rapid and accurate diagnosis [12]. It would be necessary and useful to identify multiple fungi at the genetic level as well as by morphological analysis.
Furthermore, detailed observation of colorless crystals deposited in the necrotic lung tissue by SEM revealed a structure of thin, elongated, and hexagonal crystals multilayered on each other. The crystals were analyzed by SEM–EDX, a technique for elemental and composition analysis based on the energy spectra of characteristic X-rays, and abundant Ca deposition was confirmed. CaOx crystal deposition and black pigmentation are known to be caused by A. niger, and the morphology of the crystals was determined by SEM to be consistent with calcium oxalate [13, 14]. It has been reported that damage in organs with deposition of CaOx crystals is caused by the product of iron, a surface complex source of the crystals. Iron is considered to be an important transition metal in free radical injury because it is reduced by superoxide ions to ferrous iron, which participates in the Fenton reaction to produce hydroxyl radicals (•OH). CaOx crystals produced by A. niger in the lungs are known to form complexes on their surfaces with ferric iron derived from the body [13, 15]. The combination of histopathological feature identification, fungal DNA sequencing, and ultrastructural observation was used for the first time to clarify the pathogenesis of the present case. IFI are increasing, and it is important to understand the pathophysiology of these diseases through multilayered analysis to provide appropriate treatment. Using FFPE tissue combined with ultrastructural observation by SEM, elemental analysis by SEM–EDX, and DNA sequencing, is a promising method for pathological analysis of IFI.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 a Elemental analysis of backscattered electrons with large peaks that were observed in Ca, O and C. The cross-hair on the inset image indicates the analyzed point. b Detailed sequence of the amplified product of PCR confirmed by electrophoresis. The total number of base pairs was 225 (TIF 2050 KB)
Supplementary file2 a Electrophoresis of amplicons of oval yeast-like fungi‘s gene showed approximately 200 bps (arrow), which corresponds to the range of the number of base pairs of ITS1. b C. albicans was the top result of the search by BLAST for DNA sequences similar to DNA sequences obtained from the fungi. c Detailed sequence of the amplified product of PCR confirmed by electrophoresis. The total number of base pairs was 197 (TIF 1794 KB)
Acknowledgements
The authors would like to thank Hirohisa Okushima and Yuko Hayakawa for the technical assistance with the ultrastructural analysis.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS), KAKENHI Grant Number: JP22K06944 (T.A.)
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Ethical approval
This study was approved by the Ethics Committee of Sapporo Medical University (approval number: 3-1-2).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Togano T, Suzuki Y, Nakamura F, Tse W, Kume H. Epidemiology of visceral mycoses in patients with acute leukemia and myelodysplastic syndrome: analyzing the national autopsy database in Japan. Med Mycol. 2021;59:50–57. doi: 10.1093/mmy/myaa029. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RE, Cahyame-Zuniga L, Leventakos K, Chamilos G, Ben-Ami R, Tamboli P, Tarrand J, Bodey GP, Luna M, Kontoyiannis DP. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses. 2013;56:638–645. doi: 10.1111/myc.12081. [DOI] [PubMed] [Google Scholar]
- 3.Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20:156–174. doi: 10.1183/09059180.00001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taccone FS, Van den Abeele AM, Bulpa P, Misset B, Meersseman W, Cardoso T, Paiva JA, Blasco-Navalpotro M, De Laere E, Dimopoulos G, Rello J, Vogelaers D, Blot SI. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. doi: 10.1186/s13054-014-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson KM. Mechanistic basis of super-infection: influenza-associated invasive pulmonary aspergillosis. J Fungi (Basel) 2022;8:428. doi: 10.3390/jof8050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi C, Shan Q, Xia J, Wang L, Wang L, Qiu L, Xie Y, Lin N, Wang L. Incidence, risk factors and mortality of invasive pulmonary aspergillosis in patients with influenza: a systematic review and meta-analysis. Mycoses. 2022;65:152–163. doi: 10.1111/myc.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shishido AA, Mathew M, Baddley JW. Overview of COVID-19-associated invasive fungal infection. Curr Fungal Infect Rep. 2022;16:87–97. doi: 10.1007/s12281-022-00434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porras-Alfaro A, Liu KL, Kuske CR, Xie G. From genus to phylum: large-subunit and internal transcribed spacer rRNA operon regions show similar classification accuracies influenced by database composition. Appl Environ Microbiol. 2014;80:829–840. doi: 10.1128/AEM.02894-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 10.Global action fund for fungal infections (GAFFI). Priority fungal infections. Available online: https://gaffi.org/why/fungal-disease-frequency/ (Accessed on 20 August 2022).
- 11.Ma X, Zhang S, Xing H, Li H, Chen J, Li H, Jiao M, Shi Q, Xu A, Xing L, Cao W. Invasive pulmonary aspergillosis diagnosis via peripheral blood metagenomic next-generation sequencing. Front Med (Lausanne) 2022;9:751617. doi: 10.3389/fmed.2022.751617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moncada PA, Budvytiene I, Ho DY, Deresinski SC, Montoya JG, Banaei N. Utility of DNA sequencing for direct identification of invasive fungi from fresh and formalin-fixed specimens. Am J Clin Pathol. 2013;140:203–208. doi: 10.1309/AJCPNSU2SDZD9WPW. [DOI] [PubMed] [Google Scholar]
- 13.Pabuççuoğlu U. Aspects of oxalosis associated with aspergillosis in pathology specimens. Pathol Res Pract. 2005;201:363–368. doi: 10.1016/j.prp.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Metzger JB, Garagusi VF, Kerwin DM. Pulmonary oxalosis caused by Aspergillus niger. Am Rev Respir Dis. 1984;129:501–502. doi: 10.1164/arrd.1984.129.3.501. [DOI] [PubMed] [Google Scholar]
- 15.Ghio AJ, Peterseim DS, Roggli VL, Piantadosi CA. Pulmonary oxalate deposition associated with Aspergillus niger infection. An oxidant hypothesis of toxicity. Am Rev Respir Dis. 1992;145:1499–1502. doi: 10.1164/ajrccm/145.6.1499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 a Elemental analysis of backscattered electrons with large peaks that were observed in Ca, O and C. The cross-hair on the inset image indicates the analyzed point. b Detailed sequence of the amplified product of PCR confirmed by electrophoresis. The total number of base pairs was 225 (TIF 2050 KB)
Supplementary file2 a Electrophoresis of amplicons of oval yeast-like fungi‘s gene showed approximately 200 bps (arrow), which corresponds to the range of the number of base pairs of ITS1. b C. albicans was the top result of the search by BLAST for DNA sequences similar to DNA sequences obtained from the fungi. c Detailed sequence of the amplified product of PCR confirmed by electrophoresis. The total number of base pairs was 197 (TIF 1794 KB)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.