Abstract

The molecular shuttling function of rotaxanes can be exploited to design mechanoresponsive reporter molecules. Here, we report a new approach to such rotaxane-based mechanophores, in which the fluorescence resonance energy transfer (FRET) between a donor–acceptor pair is mechanically controlled. A cyclic molecule containing a green-light-emitting FRET donor connected to a red-light-emitting FRET acceptor was threaded onto an axle equipped with a quencher at its center and two stoppers in the peripheral positions. In the force-free state, the green emitter is located near the quencher so that charge transfer interactions or photo-induced electron transfer between the two moieties suppress green emission and prevent the FRET from the green to the red emitter. The mechanophore was covalently incorporated into a linear polyurethane-urea (PUU), and stretchable hydrogels were prepared by swelling this polymer with water. Upon deformation of the PUU hydrogels and under an excitation light that selectively excites the donor, the intensity of the red fluorescence increases, as a result of a force-induced separation of the green emitter from the quencher, which enables the FRET. The switching contrast is much more pronounced in the gels than in dry films, which is due to increased molecular mobility and hydrophobic effects in the hydrogel, which both promote the formation of inclusion complexes between the ring containing the green emitter and the quencher.
Keywords: supramolecular mechanophore, rotaxane, FRET, mechanoresponsive luminescence, hydrogel
Introduction
Mechanochromic responses that are achieved by translating mechanical forces applied to polymeric objects into optical signals allow studying failure and stress-transfer mechanisms and are useful for applications such as pressure sensing, defect monitoring, electronic skins, data encryption, anticounterfeiting features, or tamper-proof packaging materials.1 The incorporation of so-called mechanophores—mechanoresponsive motifs—into these materials is one widely employed design approach to achieve such behavior.2−6 The usefulness of the rotaxane structure7−11 for the design of mechanochromic mechanophores was first recognized by Stoddart and co-workers, who reported that an intramolecular charge transfer complex (CT) was irreversibly dissociated when a tetrahydrofuran (THF) solution of a rotaxane-containing poly(methyl acrylate) was sonicated, leading to the disappearance of the CT absorption.12 We showed more recently that the force-induced shuttling function of rotaxanes can be utilized to prepare mechanophores that show instantly reversible changes in photoluminescence intensity.13−16 The rotaxane-based supramolecular mechanophores we reported are composed of a cyclic molecule equipped with a fluorophore and an axle molecule containing an electronically matched quencher. When no force is applied, the cycle resides near the quencher and the fluorescence is quenched due to intramolecular CT interactions or photo-induced electron transfer (PeT). The two optically active moieties can be pulled apart by applying a force via two handles attached to the axle and the cycle, respectively, and this is accompanied by a turn-on of the fluorescence. Unless the rotaxane’s stoppers are designed to allow for mechanically induced de-threading,16 the process is fully reversible. Thus, rubbery polyurethane elastomers into which the rotaxanes were covalently incorporated display instantly reversible changes of the fluorescence intensity upon being deformed. De Bo and co-workers also reported several rotaxane mechanophores,17,18 including a mechanochromic motif in which cycle and axle molecules interact through hydrogen bonds.19 The motif was designed to display green fluorescence in the absence of force, due to hydrogen bonding between a fluorophore in the cycle and an aminochloromaleimide (ACM) moiety in the axle molecule. The mechanically induced separation of the cycle and the ACM leads to a significant decrease in fluorescence intensity.
Collectively, the above results suggest that mechanochromic responses should also be attainable in rotaxanes featuring other photofunctional groups, whose assembly and separation promote different photophysical effects. Indeed, here we report a rotaxane-based mechanophore in which a fluorescence resonance energy transfer (FRET) is mechanically switched. FRET effects have been widely used to develop photofunctional materials, including supramolecular polymers,20−22 organogels,23,24 fluorescent proteins,25 and fluorescent probes.26 Since the FRET efficiency depends on the distance between donor and acceptor fluorophores and the directions of their transition dipole moments, and because these factors can be controlled by applying mechanical forces, adequately coupled FRET pairs can display mechanochromic behavior.27−30 Besides, combination of mechanophore and FRET has been reported to obtain mechanochromic polymer.31,32 For our rotaxane mechanophores, bulky fluorophores are not suitable for the emitters directly incorporated into cycle structure because the association constant between the quencher and cycle becomes low, resulting in poor contrast upon stretching the films in which the mechanophores are covalently introduced. Indeed, we previously reported a red-light-emitting supramolecular rotaxane mechanophore with a bulky red fluorophore in the cyclic structure, and the polyurethane film containing the mechanophore exhibited a less pronounced fluorescence contrast.15 Introduction of FRET mechanism would make it possible for such nonplanar fluorophores to be involved in rotaxane mechanophores as FRET acceptors without reducing the emission contrast upon stretching because the bulky acceptors don’t need to form π-stacked structure.
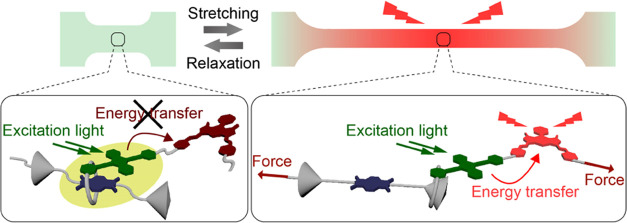
Although external stimuli such as pH changes,33,34 light irradiation,35 and changes of solvent polarity36 have been used to modulate the efficiency of FRET pairs in rotaxanes, mechanical control over this process has, to our best knowledge, not been demonstrated. The rotaxane mechanophore reported here features a green-light-emitting fluorophore in the cyclic molecule, and this donor is connected via a short spacer to a red acceptor fluorophore (Figure 1). The cycle is threaded onto an axle equipped with a quencher at its center and two stoppers in the peripheral positions. In the force-free state, the emitter is located near the quencher so that the CT interactions or PeT between the two moieties suppress green emission and prevent FRET from the green to red emitter. The mechanophore was covalently incorporated into a linear polyurethane-urea (PUU) and stretchable hydrogels were prepared by swelling with water.37,38 Upon deformation of the PUU hydrogels, the intensity of the red fluorescence increases, as a result of the force-induced separation of the emitter from the quencher, which enables the FRET process. Importantly, the ability to tune the emission color of rotaxane mechanophores by simply modifying an existing motif with a FRET acceptor allows one to tune the emission color, which, as we demonstrate, is critical for applications in colored materials.
Figure 1.
Schematic illustration of FRET control by forces on the rotaxane-based supramolecular mechanophore in stretchable hydrogels. In the force-free state (left), the green emission is quenched because of CT complex formation or PeT.
Results and Discussion
9,10-Bis(phenylethynyl)anthracene was selected as the green fluorophore because of its high fluorescence quantum yield and electron-donating propensity,14,39−42 which is pivotal for CT interactions between this fluorophore and 1,4,5,8-naphthalenetetracarboxylic diimide (NpI).43−45 NPI was selected as the quencher owing to the electron-deficient character. A π-extended 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) dye, which also displays a high fluorescence quantum efficiency,46−48 was chosen as the red fluorophore. The rotaxane RotAnBP (Figure 2, left bottom) was designed based on our previously reported rotaxane mechanophores.13−15 The cyclic molecule AnBP (Figure 2, left top) contains the green emitter and a naphthalene group, which are connected via two tetraethylene glycol chains. The red fluorophore is attached to the green fluorophore through a propyl linker so that efficient energy transfer is possible. The axle features an NpI at its center and two tetraphenylmethane units49−51 as stoppers at both ends. RotAnBP was prepared through a 1,3-dipolar cycloaddition click-type reaction52 between alkyne and azide groups of two precursors of the axle molecule in the presence of a high concentration of AnBP (see the Supporting Information for details). The rotaxane mechanophore was characterized by 1H NMR and 13C NMR spectroscopy and high-resolution electrospray ionization mass spectroscopy. A comparison of the 1H NMR spectra of cycle AnBP and rotaxane RotAnBP shows that diagnostic signals associated with aromatic protons of the cyclic structure and the 9,10-bis(phenylethynyl)anthracene residue are shifted up-field (Figure 3), indicating the formation of an inclusion complex between the cycle and NpI. By contrast, signals ascribed to protons of the π-extended BODIPY hardly change. The rotaxane formation is also evidenced by a decrease in the red fluorescence intensity after rotaxane formation, due to the suppression of FRET (see below). The anthracene derivative An (Figure 2, right top) and BODIPY derivative BP (Figure 2, right bottom) were also prepared as the reference compounds.
Figure 2.
Molecular structures of cyclic compound AnBP, rotaxane-based supramolecular mechanophore RotAnBP, and reference compounds containing the green (An) or red (BP) emitter.
Figure 3.
Partial 1H NMR spectra of AnBP (top) and RotAnBP (bottom). The spectra were measured in CDCl3 at r.t.
The optical characteristics of An, BP, AnBP, and RotAnBP were first measured in THF/methanol (1:4, v/v) solutions (c = 1 × 10–5 M). The absorption spectrum of An shows a band with maxima at 450 and 476 nm (Figure 4a, green line). BP has one absorption band in the UV region with a maximum at 369 nm and a second band with well-resolved structures and maxima at 590 and 639 nm (Figure 4a, red line). The data show that it is possible to preferentially excite An at 490 nm, whereas BP can be selectively excited above 500 nm. Under excitation at 410 nm, where the two motifs show a similar extinction (Figure 4a), An and BP solutions exhibit strong green and red fluorescence, respectively. Both fluorescence spectra show well-resolved peaks with maxima at 495 and 522 nm (An, quantum efficiency Φ = 0.84) and 661 and 715 nm (BP, Φ = 0.54), respectively (Figure 4b, green and red solid lines). When excited at 490 nm, BP shows a much weaker fluorescence than An (Figure 4b, green and red dashed lines) because of the lower absorption. By contrast, An does not show any fluorescence and BP displays strong red fluorescence upon excitation at 590 nm (Figure S1, green and red lines).
Figure 4.
Absorption (a, c) and photoluminescence spectra (b, d) of An (green), BP (red), AnBP (blue), and RotAnBP (black) in THF/methanol (1:4, v/v) solutions (c = 1 × 10–5 M). The photoluminescence spectra were recorded with excitation at 410 nm (solid lines) or 490 nm (dashed lines) at r.t.
The absorption spectrum of AnBP shows a superposition of the absorbances of An and BP (Figure 4c, blue line). However, its fluorescence spectrum displays only the red fluorescence band associated with the BODYPY residue, for both, excitation at 410 and 490 nm (Figure 4d, blue solid and dashed lines), indicating efficient energy transfer from the 9,10-bis(phenylethynyl)anthracene donor to the π-extended BODIPY acceptor. The energy transfer efficiency exceeds 99%, as determined by comparing the intensities of the green fluorescence of solutions of An and AnBP. The spectral overlap integral of the donor fluorescence and the acceptor absorption and the Förster distance were calculated to be 5.58 × 1015 M–1 cm–1 nm4 and 67.1 Å, respectively (see the Supporting Information for details). Based on these values, the energy transfer efficiency is greater than 99% when the distance between the donor and acceptor is within 31.2 Å, supporting that efficient energy transfer occurs in AnBP. In a comparison of the red fluorescence intensities of BP and AnBP, the proportions of the red fluorescence attributed to the direct excitation of the red emitter in AnBP are calculated to be ca. 50 and 9% under the excitation lights of 410 and 490 nm, respectively. The FRET process is further supported by the fact that the excitation spectrum of AnBP recorded at an emission wavelength of 660 nm (Figure S2, blue line) shows a broad band between 400 and 500 nm that corresponds to the absorption band of AnBP in the same wavelength region. The overall emission quantum yield Φtotal of AnBP is 0.54 for excitation at 410 nm, with a yield of below 0.01 for the green emitter (ΦAn) and 0.54 for the red emitter (ΦBP). Moreover, the quantum yield of AnBP is hardly affected by the excitation wavelength (Φtotal = 0.54, excitation at 490 nm). These results also indicated highly efficient FRET.
The rotaxane RotAnBP displays a slightly red-shifted absorption band of the green emitter, due to the CT interaction between the 9,10-bis(phenylethynyl)anthracene and NpI (Figure 4c, black line).14 By contrast, the absorption band of the π-extended BODIPY group hardly changes, suggesting that ground-state electronic interactions between the BODIPY moiety and other π-conjugated portions of the rotaxane are absent in solution. As expected, RotAnBP exhibits no green fluorescence and only faint red fluorescence in solution when excited at 490 nm (Φ = 0.01, Figure 4d, black dashed line). The excitation spectrum of RotAnBP (Figure S2, black line) reveals that the FRET contribution to the red emission is negligible and the faint red emission is caused by direct excitation of the π-extended BODIPY dye, although the absorption of the red emitter at 490 nm is weak. These results reflect that the energy transfer from the green-light-emitting donor to the red-light-emitting acceptor is suppressed, due to the CT interaction or PeT between the donor and the quencher. It is noteworthy that even upon excitation at 410 nm (Figure 4d, black solid line) and 590 nm (Figure S1, black line), the fluorescence intensity of RotAnBP is still weaker than that of BP and AnBP although the BODIPY residue is directly and exclusively excited. The emission quantum yield of the red fluorescence originating from RotAnBP is 0.14 when excited at 590 nm, which is much smaller than that of BP (Φ = 0.54). Besides, the emission decay of RotAnBP monitored at 660 nm in THF/methanol is accelerated relative to that of BP and AnBP (Figure S3). These results suggest that the BODIPY residue in the rotaxane in the excited state interacts with other π-conjugated groups, leading to dynamic quenching in solution, presumably due to PeT. In contrast to AnBP, the quantum yield of RotAnBP depends on the excitation wavelength (ΦBP = 0.06, excitation at 410 nm), which supports our conclusion that the FRET is suppressed and red fluorescence is observed only when the BODIPY derivative is directly excited.
Recently, linear PUUs carrying carboxyl groups on the main chain were reported to form hydrogels after swelling with water.37,38 The hydrophobic urea segments aggregate to form strong physical cross-links, which bestow the PUU hydrogels with high extensibility and toughness. Speculating that hydrophobic interactions would increase the association constant between the cycle and the quencher, we elected to investigate the mechanoresponsive behavior of RotAnBP in hydrogels by integrating the new mechanophore into a hydrophilic PUU. This was achieved by the polyaddition reaction between poly(ethylene glycol) (PEG) (Mn = ca. 2000 g/mol), isophorone diisocyanate, isophorone diamine, and 2,2-bis(hydroxymethyl)propionic acid in the presence of 0.004 mol % of RotAnBP (Scheme S1). The reaction was modified from the reported procedures37,38 because RotAnBP was unstable when heated (see the Supporting Information for details). The resulting polymer RotAnBP-PUU is soluble in polar organic solvents, such as methanol, dimethylformamide, and dimethyl sulfoxide and displays a number-average molecular weight of Mn = 48 kg/mol. The successful incorporation of RotAnBP into the polymer was confirmed by the absorption spectrum of RotAnBP-PUU in methanol, which shows the diagnostic signals of the chromophores (Figure S4). However, no peaks corresponding to RotAnBP can be observed in the 1H NMR spectrum of the polymer, on account of its low concentration (Figure S5). The photoluminescence spectrum of a methanol solution of RotAnBP-PUU (Figure S4) shows that the FRET process remains suppressed after incorporation into the polymer.
The thermal properties of RotAnBP-PUU were investigated by thermogravimetric analysis (TGA) (Figure S6a) and differential scanning calorimetric (DSC) measurement (Figure S6b). The TGA scan shows that decomposition sets in at ca. 220 °C, and the DSC trace reveals a weak melting transition at around 15 °C upon heating, which is associated with crystalline PEG domains. These data reflect that the thermal properties of RotAnBP-PUU are similar to a similar PUU that was reported previously.37,38RotAnBP-PUU was processed into thin films with a thickness of 70–100 μm by solvent casting (see the Supporting Information for details). The mechanical properties of the material in the dry state were characterized by dynamic mechanical analysis (DMA) (Figure S7) and uniaxial tensile tests (Figure S8a, Table S1). The DMA traces reveal a glass-transition temperature (Tg) of ca. −40 °C, a rubbery plateau that extends to ca. 80 °C, and a regime in which the storage modulus decreases with increasing temperature, before the samples fail at ca. 180 °C. The tensile tests, which were carried out at r.t., reveal a Young’s modulus of 29.5 ± 7.8 MPa, a tensile strength of 14.5 MPa, and an extensibility of more than 1000%.
RotAnBP-PUU hydrogels were prepared by immersing the dried films in deionized water for 2 h; after this time, an equilibrium state with a water take-up of 70% had been reached (Figure S9). Under ambient conditions, the gels slowly dry and water is released with an initial rate of ca. 0.5% min–1 (Figure S10). Tensile tests (Figure S8b, Table S1) reveal a Young’s modulus of 1.19 ± 0.04 MPa, which is more than an order of magnitude lower than that of the dry films. The hydrogels display an elongation of ca. 1200% and a nominal tensile strength of 5.93 MPa.
The dry RotAnBP-PUU films exhibit moderately weak red fluorescence (Φ = 0.05, excited at 490 nm), although the methanol solution of RotAnBP-PUU hardly fluoresces under the same excitation conditions. Residual emission in the force-free state was also observed for previously reported polyurethanes containing other rotaxane-based mechanophores,15 and appears to be related to residual stresses in the material that cause separation of the green emitter and the quencher in a fraction of the rotaxanes. This enables energy transfer from the excited 9,10-bis(phenylethynyl)anthracene to the π-extended BODIPY. In contrast, the red fluorescence intensity of the RotAnBP-PUU hydrogel becomes much weaker (Φ = 0.02, excited at 490 nm) than that of the dried material, likely on account of an increased cycle-quencher association constant due to hydrophobic interactions and the increased molecular mobility, which both should reduce the fraction of activated mechanophores.
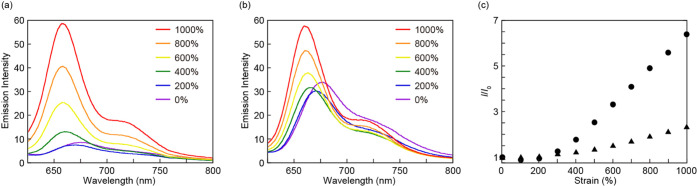
The force-induced shuttling function controls the on/off switching of FRET, as depicted in Figure 1. Upon uniaxial tensile deformation, the RotAnBP-PUU hydrogel initially shows a slight decrease in the red fluorescence intensity due to a decrease in the thickness of the hydrogel, and then exhibits subsequently a considerable increase in the red fluorescence intensity when excited at 490 nm (Figure 5a, Figure S11a, Movies S1). Upon subsequent removal of the force, the fluorescence intensity gradually decreases (Figure S12). This indicates that the increase in the red fluorescence intensity of the hydrogel with excitation at 490 nm is mainly due to the activation of the FRET process. To the best of our knowledge, this is the first report on the on/off switching of the FRET by force on rotaxanes at single molecular level. Even upon excitation at 590 nm, the red fluorescence intensity of the hydrogels increases; however, the attainable contrast or relative increase is smaller than that achieved when exciting the sample at 490 nm (Figure S13). The fluorescence contrast between unstretched and stretched states is improved compared to our previously reported red-emitting rotaxane, in which a BODIPY-based red emitter was directly introduced into cyclic moiety.15
Figure 5.
(a, b) Fluorescence spectra of a RotAnBP-PUU hydrogel (a) and a dried RotAnBP-PUU film (b) recorded upon stretching the samples to the strains indicated. (c) Plots of the relative fluorescence intensity I/I0 of the RotAnBP-PUU hydrogel (circles) and the dried RotAnBP-PUU film (triangles) as a function of strain. All fluorescence spectra were recorded at r.t. with λex = 490 nm. The fluorescence intensities in (c) were recorded at 660 nm.
From a practical viewpoint, the rotaxane RotAnBP functions as a red-emissive supramolecular mechanophore in hydrogels. Red luminescence is useful for multicolor imaging and for developing white-emissive materials. Besides red emission can penetrate into the living tissues deeply, making it favorable for bioimaging. Thus, the development of the red-light-emitting mechanophores is desirable to expand the application of mechanophores. The possibility to readily vary the emission color of a given mechanophore is further useful for applications in colored materials. To demonstrate this, we carried out experiments in which we covered the RotAnBP-PUU hydrogel with a polyurethane film containing a red dye (see Figures S14–S19 and the Supporting Information for details). Gratifyingly, a clear increase in the red fluorescence intensity was observed upon stretching the RotAnBP-PUU hydrogel whether or not the red film cutting light of the wavelength ranging below 600 nm was applied (Figure S18). By contrast, when a reference polyurethane film containing the “parent”, green-light-emitting rotaxane mechanophore without the FRET acceptor16 was stretched, virtually all of the green fluorescence was absorbed by the red dye (Figure S19).
The dry RotAnBP-PUU films also show a mechanoresponse upon stretching (Figure 5b, Figure S11b, Movies S2). However, plots of the relative fluorescence intensities, i.e., the ratio of the fluorescence intensities of the stretched (I) and unstretched (I0) samples recorded at 660 nm (Figure 5c) show that the contrast observed for the RotAnBP-PUU hydrogels is much larger than that in the dry RotAnBP-PUU films, although significantly higher stresses are required to deform the dry films (Figure S8). The difference is caused by the weak fluorescence intensity of the hydrogel in the force-free state and perhaps also a lower fraction of activated mechanophores in the material because of the bulky structure of the ring featuring the FRET pair.
Conclusions
In conclusion, we successfully demonstrated that the mechanically induced molecular shuttling function of rotaxanes can be used to control the on/off switching of a FRET process between a donor–acceptor pair in the reporter molecule. The FRET pair, a green-emissive energy donor and a red-emissive energy acceptor, were connected via a short spacer and attached to the cyclic part of the rotaxane. The photophysical properties of the fluorophores allow the preferred (although not exclusive) excitation of the donor at 490 nm. An efficient FRET process causes the cyclic molecule to emit almost exclusively red light, even if the donor is preferentially excited. Upon rotaxane formation, CT interactions or PeT between the green emitter and NpI residue placed in the axle prevent the FRET so that the red fluorescence becomes weak if the donor is excited. The new rotaxane mechanophore was covalently incorporated into a PUU, which was swelled with water to produce a PUU hydrogel. The hydrogel displays an up to 7-fold increase of the red emission intensity upon mechanical deformation. The contrast between the idle and the mechanically activated state is considerably higher than that observed for the dry polymer, on account of the increased association constant between the cycle and NpI as well as the higher molecular mobility.
The results of the present study suggest that rotaxane mechanophores can be used to control photophysical processes that go beyond FRET, for example, circularly polarized emission, phosphorescence, and so on, allowing access to fascinating and sophisticated supramolecular mechanophores. Furthermore, the data suggest that supramolecular mechanophores may function better in hydrophilic environments and that increasing the molecular mobility improves the supramolecular mechanophore property.
Experimental Section
Materials
All reagents and solvents were purchased from Merck, Kanto Chemical, Tokyo Kasei, or FUJIFILM Wako Pure Chemical Corporation. Anhydrous dimethylacetamide (DMAc) and methanol were used as solvents for the synthesis of polymers and solvent casting, respectively. Telechelic poly(ethylene glycol) (Mn = 2000 g/mol) and 2,2-bis(hydroxymethyl)propionic acid were dried in vacuo at 100 °C for 1 h before use. Isophorone diisocyanate was distilled under a vacuum and stored over molecular sieves at 4 °C. Isophorone diamine was dried over molecular sieves at r.t. for 1 day before use. Deionized water for hydrogel preparation was obtained using a Merck Direct-Q UV5.
Synthesis Procedure
The detailed synthesis procedures to prepare compounds are shown in the Supporting Information (Schemes S1–S4).
Synthesis of Polyurethane-Urea RotAnBP-PUU
Dibutyltin dilaurate (2 drops) was added to a stirred mixture of RotAnBP (6.0 mg, 1.7 μmol), telechelic hydroxy-terminated poly(ethylene glycol) (Mn= 2000 g/mol, 1.0 g, 0.50 mmol), isophorone diisocyanate (167 mg, 0.750 mmol), and 2,2-bis(hydroxymethyl)propionic acid (33.5 mg, 0.250 mmol) in DMAc (4 mL) and the mixture was stirred at r.t. for 6 h (Scheme S5). A solution of isophorone diisocyanate (383 mg, 1.72 mmol) in DMAc (2 mL) was then added. After the reaction mixture was stirred at r.t. for an additional 12 h, a solution of isophorone diamine (255 mg, 1.50 mmol) in DMAc (4 mL) was added dropwise over the course of 2 h and the reaction mixture was stirred at r.t. for another 24 h. Methanol (10 mL) was added, and the reaction mixture was stirred for 30 min. Then, the reaction mixture was poured into a mixture of hexane (400 mL) and ethyl acetate (800 mL). The green precipitate was collected by filtration and dried in vacuo for 12 h at 40 °C to afford RotAnBP-PUU as a green rubbery solid (1.70 g, 92%, Mn = 48 kg/mol, PDI = 2.04).
Preparation of Dried PUU Films and PUU Hydrogels
RotAnBP-PUU (400 mg) was dissolved in methanol (8 mL), and the solution was divided between two square poly(tetrafluoroethylene) molds (51 × 51 × 5.0 mm3). The molds were placed under an inverted funnel to control the evaporation rate. The solvent was evaporated over the course of 12 h under ambient conditions, and the resulting films were further dried in vacuo at 40 °C for 6 h. The green films thus obtained were smooth and transparent with a thickness of 70–100 μm. After fully swelling the films in a large amount of deionized water for 2 h, transparent hydrogels were obtained with a thickness of 110–150 μm.
Acknowledgments
The authors thank the Materials Analysis Division, Open Facility Center, at the Tokyo Institute of Technology for HRMS measurements. This work was supported by JSPS KAKENHI (nos. JP20K21215 and JP22H04531). This work was partially supported by Japan Science Technology Agency (JST), PRESTO (no. JPMJPR17P6) and FOREST (no. JPMJFR201N). This work was also supported by Izumi Science and Technology Foundation, International Polyurethane Technology Foundation, Toshiaki Ogasawara Memorial Foundation, and Shorai Foundation for Science and Technology. Financial support through the National Center of Competence in Research (NCCR) Bio-Inspired Materials, a research instrument of the Swiss National Science Foundation (SNSF), as well as funding from the Adolphe Merkle Foundation are gratefully acknowledged. T.M. acknowledges financial support through a JSPS Research Fellowship for Young Scientists (no. 21J21969).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.2c20904.
The authors declare no competing financial interest.
Supplementary Material
References
- Guo Q.; Zhang X. A Review of Mechanochromic Polymers and Composites: From Material Design Strategy to Advanced Electronics Application. Composites, Part B 2021, 227, 109434 10.1016/j.compositesb.2021.109434. [DOI] [Google Scholar]
- Caruso M. M.; Davis D. A.; Shen Q.; Odom S. A.; Sottos N. R.; White S. R.; Moore J. S. Mechanically-Induced Chemical Changes in Polymeric Materials. Chem. Rev. 2009, 109, 5755–5798. 10.1021/cr9001353. [DOI] [PubMed] [Google Scholar]
- Li J.; Nagamani C.; Moore J. S. Polymer Mechanochemistry: From Destructive to Productive. Acc. Chem. Res. 2015, 48, 2181–2190. 10.1021/acs.accounts.5b00184. [DOI] [PubMed] [Google Scholar]
- De Bo G. Polymer Mechanochemistry and the Emergence of the Mechanophore Concept. Macromolecules 2020, 53, 7615–7617. 10.1021/acs.macromol.0c01683. [DOI] [Google Scholar]
- Traeger H.; Kiebala D. J.; Weder C.; Schrettl S. From Molecules to Polymers—Harnessing Inter- and Intramolecular Interactions to Create Mechanochromic Materials. Macromol. Rapid Commun. 2021, 42, 2000573 10.1002/marc.202000573. [DOI] [PubMed] [Google Scholar]
- He S.; Stratigaki M.; Centeno S. P.; Dreuw A.; Göstl R. Tailoring the Properties of Optical Force Probes for Polymer Mechanochemistry. Chem. – Eur. J. 2021, 27, 15889–15897. 10.1002/chem.202102938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzani V.; Gómez-López M.; Stoddart J. F. Molecular Machines. Acc. Chem. Res. 1998, 31, 405–414. 10.1021/ar970340y. [DOI] [Google Scholar]
- Okumura Y.; Ito K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-links. Adv. Mater. 2001, 13, 485–487. . [DOI] [Google Scholar]
- Arai T.; Jang K.; Koyama Y.; Asai S.; Takata T. Versatile Supramolecular Cross-linker: A Rotaxane Cross-linker That Directly Endows Vinyl Polymers with Movable Cross-links. Chem. – Eur. J. 2013, 19, 5917–5923. 10.1002/chem.201204402. [DOI] [PubMed] [Google Scholar]
- Stoddart J. F. Putting Mechanically Interlocked Molecules (MIMs) to Work in Tomorrow’s World. Angew. Chem., Int. Ed. 2014, 53, 11102–11104. 10.1002/anie.201408043. [DOI] [PubMed] [Google Scholar]
- Erbas-Cakmak S.; Leigh D. A.; McTernan C. T.; Nussbaumer A. L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. 10.1021/acs.chemrev.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll R. S.; Friedman D. C.; Stoddart J. F. Mechanically Interlocked Mechanophores by Living-Radical Polymerization from Rotaxane Initiators. Org. Lett. 2011, 13, 2706–2709. 10.1021/ol200801b. [DOI] [PubMed] [Google Scholar]
- Sagara Y.; Karman M.; Verde-Sesto E.; Matsuo K.; Kim Y.; Tamaoki N.; Weder C. Rotaxanes as Mechanochromic Fluorescent Force Transducers in Polymers. J. Am. Chem. Soc. 2018, 140, 1584–1587. 10.1021/jacs.7b12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y.; Karman M.; Seki A.; Pannipara M.; Tamaoki N.; Weder C. Rotaxane-Based Mechanophores Enable Polymers with Mechanically Switchable White Photoluminescence. ACS Cent. Sci. 2019, 5, 874–881. 10.1021/acscentsci.9b00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T.; Sagara Y.; Traeger H.; Tamaoki N.; Weder C. Mechanoresponsive Behavior of a Polymer-Embedded Red-Light Emitting Rotaxane Mechanophore. ACS Appl. Mater. Interfaces 2019, 11, 24571–24576. 10.1021/acsami.9b06302. [DOI] [PubMed] [Google Scholar]
- Muramatsu T.; Okado Y.; Traeger H.; Schrettl S.; Tamaoki N.; Weder C.; Sagara Y. Rotaxane-Based Dual Function Mechanophores Exhibiting Reversible and Irreversible Responses. J. Am. Chem. Soc. 2021, 143, 9884–9892. 10.1021/jacs.1c03790. [DOI] [PubMed] [Google Scholar]
- Zhang M.; De Bo G. Impact of a Mechanical Bond on the Activation of a Mechanophore. J. Am. Chem. Soc. 2018, 140, 12724–12727. 10.1021/jacs.8b08590. [DOI] [PubMed] [Google Scholar]
- Zhang M.; De Bo G. Mechanical Susceptibility of a Rotaxane. J. Am. Chem. Soc. 2019, 141, 15879–15883. 10.1021/jacs.9b06960. [DOI] [PubMed] [Google Scholar]
- Sandoval-Torrientes R.; Carr T. R.; De Bo G. A Mechanochromic Hydrogen-Bonded Rotaxane. Macromol. Rapid Commun. 2021, 42, 2000447 10.1002/marc.202000447. [DOI] [PubMed] [Google Scholar]
- Praveen V. K.; George S. J.; Varghese R.; Vijayakumar C.; Ajayaghosh A. Self-Assembled π-Nanotapes as Donor Scaffolds for Selective and Thermally Gated Fluorescence Resonance Energy Transfer (FRET). J. Am. Chem. Soc. 2006, 128, 7542–7550. 10.1021/ja0587594. [DOI] [PubMed] [Google Scholar]
- Kim S.; Yoon S.-J.; Park S. Y. Highly Fluorescent Chameleon Nanoparticles and Polymer Films: Multicomponent Organic Systems that Combine FRET and Photochromic Switching. J. Am. Chem. Soc. 2012, 134, 12091–12097. 10.1021/ja3027295. [DOI] [PubMed] [Google Scholar]
- Rajdev P.; Ghosh S. Fluorescence Resonance Energy Transfer (FRET): A Powerful Tool for Probing Amphiphilic Polymer Aggregates and Supramolecular Polymers. J. Phys. Chem. B 2019, 123, 327–342. 10.1021/acs.jpcb.8b09441. [DOI] [PubMed] [Google Scholar]
- Vijayakumar C.; Praveen V. K.; Kartha K. K.; Ajayaghosh A. Excitation Energy Migration in Oligo(p-phenylenevinylene) Based Organogels: Structure-property Relationship and FRET Efficiency. Phys. Chem. Chem. Phys. 2011, 13, 4942–4949. 10.1039/c0cp02110e. [DOI] [PubMed] [Google Scholar]
- Vijayakumar C.; Praveen V. K.; Ajayaghosh A. RGB Emission through Controlled Donor Self-Assembly and Modulation of Excitation Energy Transfer: A Novel Strategy to White-Light-Emitting Organogels. Adv. Mater. 2009, 21, 2059–2063. 10.1002/adma.200802932. [DOI] [Google Scholar]
- Piston D. W.; Kremers G.-J. Fluorescent Protein FRET: the Good, the Bad and the Ugly. Trends Biochem. Sci. 2007, 32, 407–414. 10.1016/j.tibs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Lin W.; Zheng K.; Zhu S. FRET-Based Small-Molecule Fluorescent Probes: Rational Design and Bioimaging Applications. Acc. Chem. Res. 2013, 46, 1462–1473. 10.1021/ar300273v. [DOI] [PubMed] [Google Scholar]
- Grashoff C.; Hoffman B. D.; Brenner M. D.; Zhou R.; Parsons M.; Yang M. T.; McLean M. A.; Sligar S. G.; Chen C. S.; Ha T.; Schwartz M. A. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010, 466, 263–266. 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merindol R.; Delechiave G.; Heinen L.; Catalani L. H.; Walther A. Modular Design of Programmable Mechanofluorescent DNA Hydrogels. Nat. Commun. 2019, 10, 528 10.1038/s41467-019-08428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns N.; Pustelny K.; Bergeron L. M.; Whitehead T. A.; Clark D. S. Mechanical Nanosensor Based on FRET within a Thermosome: Damage-Reporting Polymeric Materials. Angew. Chem., Int. Ed. 2009, 48, 5666–5669. 10.1002/anie.200900554. [DOI] [PubMed] [Google Scholar]
- Ogi S.; Sugiyasu K.; Takeuchi M. Synthesis of a Doubly Strapped Light-Harvesting Porphyrin Bearing Energy Donor Molecules Hanging on to the Straps: An Attempt toward Macroscopic Control over Molecular Conformation that Affects the Efficiency of Fluorescence Resonance Energy Transfer. Bull. Chem. Soc. Jpn. 2011, 84, 40–48. 10.1246/bcsj.20100232. [DOI] [Google Scholar]
- Jia Y.; Wang S.; Wang W.-J.; Li B.-G.; Zhu S. Design and Synthesis of a Well-Controlled Mechanoluminescent Polymer System Based on Fluorescence Resonance Energy Transfer with Spiropyran as a Force-Activated Acceptor and Nitrobenzoxadiazole as a Fluorescent Donor. Macromolecules 2019, 52, 7920–7928. 10.1021/acs.macromol.9b01556. [DOI] [Google Scholar]
- Khang T. M.; Huang R.; Khan A.; Chuang W.-T.; Nhien P. Q.; Cuc T. T. K.; Hue B. T. B.; Wei K.-H.; Li Y.-K.; Lin H.-C. Reversible Ratiometric Mechanochromic Fluorescence Switching in Highly Stretchable Polyurethane Elastomers with Ultratoughness Enhanced by Polyrotaxane. ACS Mater. Lett. 2022, 4, 2537–2546. 10.1021/acsmaterialslett.2c00847. [DOI] [Google Scholar]
- Nhien P. Q.; Cuc T. T. K.; Khang T. M.; Wu C.-H.; Hue B. T. B.; Wu J. I.; Mansel B. W.; Chen H.-L.; Lin H.-C. Highly Efficient Förster Resonance Energy Transfer Modulations of Dual-AIEgens between a Tetraphenylethylene Donor and a Merocyanine Acceptor in Photo-Switchable [2]Rotaxanes and Reversible Photo-Patterning Applications. ACS Appl. Mater. Interfaces 2020, 12, 47921–47938. 10.1021/acsami.0c12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.; Zhang J.; Cao J.; Yao X.; Cao T.; Gong Y.; Zhao C.; Tian H. A Room Temperature Phosphorescence Encoding [2]Rotaxane Molecular Shuttle. Chem. Sci. 2016, 7, 4582–4588. 10.1039/C6SC00769D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Li H.; Li Y.; Liu H.; Wang S.; He X.; Wang N.; Zhu D. Energy Transfer Switching in a Bistable Molecular Machine. Org. Lett. 2005, 7, 4835–4838. 10.1021/ol051567t. [DOI] [PubMed] [Google Scholar]
- Onagi H.; Rebek J. Fluorescence Resonance Energy Transfer Across a Mechanical Bond of a Rotaxane. Chem. Commun. 2005, 36, 4604–4606. 10.1039/b506177f. [DOI] [PubMed] [Google Scholar]
- Yang N.; Yang H.; Shao Z.; Guo M. Ultrastrong and Tough Supramolecular Hydrogels from Multiurea Linkage Segmented Copolymers with Tractable Processablity and Recyclability. Macromol. Rapid Commun. 2017, 38, 1700275 10.1002/marc.201700275. [DOI] [PubMed] [Google Scholar]
- Cao B.-H.; Chen W.; Wei W.-Y.; Chen Y.; Yuan Y. Carbon Dots Intensified Mechanochemiluminescence from Waterborne Polyurethanes as Tunable Force Sensing Materials. Chin. J. Polym. Sci. 2021, 39, 1403–1411. 10.1007/s10118-021-2601-4. [DOI] [Google Scholar]
- Sagara Y.; Weder C.; Tamaoki N. Tuning the Thermo- and Mechanoresponsive Behavior of Luminescent Cyclophanes. RSC Adv. 2016, 6, 80408–80414. 10.1039/C6RA18348D. [DOI] [Google Scholar]
- Levitus M.; Garcia-Garibay M. A. Polarized Electronic Spectroscopy and Photophysical Properties of 9,10-Bis(phenylethynyl)anthracene. J. Phys. Chem. A 2000, 104, 8632–8637. 10.1021/jp001483w. [DOI] [Google Scholar]
- Demeter A. First Steps in Photophysics. I. Fluorescence Yield and Radiative Rate Coefficient of 9,10-Bis(phenylethynyl)anthracene in Paraffins. J. Phys. Chem. A 2014, 118, 9985–9993. 10.1021/jp507626h. [DOI] [PubMed] [Google Scholar]
- Lübtow M.; Helmers I.; Stepanenko V.; Albuquerque R. Q.; Marder T. B.; Fernández G. Self-Assembly of 9,10-Bis(phenylethynyl) Anthracene (BPEA) Derivatives: Influence of π-π and Hydrogen-Bonding Interactions on Aggregate Morphology and Self-Assembly Mechanism. Chem. – Eur. J. 2017, 23, 6198–6205. 10.1002/chem.201605989. [DOI] [PubMed] [Google Scholar]
- Jacquot de Rouville H.-P.; Iehl J.; Bruns C. J.; McGrier P. L.; Frasconi M.; Sarjeant A. A.; Stoddart J. F. A Neutral Naphthalene Diimide [2]Rotaxane. Org. Lett. 2012, 14, 5188–5191. 10.1021/ol3022963. [DOI] [PubMed] [Google Scholar]
- Choudhary U.; Northrop B. H. Rotaxanes and Biofunctionalized Pseudorotaxanes via Thiol-Maleimide Click Chemistry. Org. Lett. 2012, 14, 2082–2085. 10.1021/ol300614z. [DOI] [PubMed] [Google Scholar]
- Hamilton D. G.; Davies J. E.; Prodi L.; Sanders J. K. M. Synthesis, Structure and Photophysics of Neutral π-Associated [2]Catenanes. Chem. – Eur. J. 1998, 4, 608–620. . [DOI] [Google Scholar]
- Loudet A.; Burgess K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- Wang C.; Xia X.; Luo J.; Qian Y. A Novel Near-infrared Styryl-BODIPY Fluorescent Probe for Discrimination of GSH and Its Application in Living Cells. Dyes Pigm. 2018, 152, 85–92. 10.1016/j.dyepig.2018.01.034. [DOI] [Google Scholar]
- Ansteatt S.; Meares A.; Ptaszek M. Amphiphilic Near-IR-Emitting 3,5-Bis(2-Pyrrolylethenyl)BODIPY Derivatives: Synthesis, Characterization, and Comparison with Other (Hetero)Arylethenyl-Substituted BODIPYs. J. Org. Chem. 2021, 86, 8755–8765. 10.1021/acs.joc.1c00586. [DOI] [PubMed] [Google Scholar]
- Gibson H. W.; Lee S.-H.; Engen P. T.; Lecavalier P.; Sze J.; Shen Y. X.; Bheda M. New Triarylmethyl Derivatives: “Blocking Groups” for Rotaxanes and Polyrotaxanes. J. Org. Chem. 1993, 58, 3748–3756. 10.1021/jo00066a030. [DOI] [Google Scholar]
- Dichtel W. R.; Miljanić O. S.; Spruell J. M.; Heath J. R.; Stoddart J. F. Efficient Templated Synthesis of Donor-Acceptor Rotaxanes Using Click Chemistry. J. Am. Chem. Soc. 2006, 128, 10388–10390. 10.1021/ja063127i. [DOI] [PubMed] [Google Scholar]
- Gong C.; Gibson H. W. Synthesis and Characterization of a Polyester/Crown Ether Rotaxane Derived from a Difunctional Blocking Group. Macromolecules 1996, 29, 7029–7033. 10.1021/ma960769p. [DOI] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.