Abstract
Rationale
CC16 is a protein mainly produced by nonciliated bronchial epithelial cells (BECs) that participates in host defense. Reduced CC16 protein concentrations in BAL and serum are associated with asthma susceptibility.
Objectives
Few studies have investigated the relationship between CC16 and asthma progression, and none has focused on BECs. In this study, we sought to determine if CC16 mRNA expression levels in BECs are associated with asthma severity.
Methods
Association analyses between CC16 mRNA expression levels in BECs (242 asthmatics and 69 control subjects) and asthma-related phenotypes in Severe Asthma Research Program were performed using a generalized linear model.
Measurements and Main Results
Low CC16 mRNA expression levels in BECs were significantly associated with asthma susceptibility and asthma severity, high systemic corticosteroids use, high retrospective and prospective asthma exacerbations, and low pulmonary function. Low CC16 mRNA expression levels were significantly associated with high T2 inflammation biomarkers (fractional exhaled nitric oxide and sputum eosinophils). CC16 mRNA expression levels were negatively correlated with expression levels of Th2 genes (IL1RL1, POSTN, SERPINB2, CLCA1, NOS2, and MUC5AC) and positively correlated with expression levels of Th1 and inflammation genes (IL12A and MUC5B). A combination of two nontraditional T2 biomarkers (CC16 and IL-6) revealed four asthma endotypes with different characteristics of T2 inflammation, obesity, and asthma severity.
Conclusions
Our findings indicate that low CC16 mRNA expression levels in BECs are associated with asthma susceptibility, severity, and exacerbations, partially through immunomodulation of T2 inflammation. CC16 is a potential nontraditional T2 biomarker for asthma development and progression.
Keywords: asthma severity, asthma exacerbations, bronchial epithelial cells, CC16, T2 inflammation
At a Glance Commentary
Scientific Knowledge on the Subject
Reduced CC16 protein concentrations in BAL and serum have been associated with asthma susceptibility, however, it has not been investigated in bronchial epithelial cells (BECs), the most relevant cell type in which CC16 is primarily produced. In addition, very few studies have investigated the relationship between CC16 and asthma progression.
What This Study Adds to the Field
This study shows that reduced CC16 mRNA expression levels in BECs are associated with asthma susceptibility and asthma severity. More specifically, among patients with asthma, reduced CC16 mRNA expression levels are associated with decreased lung function, enhanced systemic corticosteroids use, and increased asthma exacerbations. Importantly, reduced CC16 mRNA expression levels are associated with increased T2 biomarkers and increased expression levels of T2 pathway genes. Furthermore, a combination of two nontraditional T2 biomarkers (CC16 mRNA expression levels in BECs and blood IL-6 protein concentrations) reveals four distinct asthma endotypes with different characteristics of T2 inflammation, obesity, and asthma severity. In summary, low CC16 mRNA expression levels in BECs are associated with asthma progression, probably through upregulation of T2 inflammation. Thus, CC16 is a potential nontraditional T2 biomarker for asthma development and progression.
CC16 (club cell secretory protein-16), encoded by the SCGB1A1 gene on chr11q12.3, is predominantly produced by nonciliated bronchial epithelial cells (club cells) (1). CC16 may have antiinflammatory, immunomodulatory, antitoxicant, and antioxidative functions (2, 3). CC16 represents a biomarker for airway epithelium integrity or acute lung epithelium injury (4–7). In population-based studies, low serum CC16 protein concentrations have been associated with decreased lung function, increased airway hyperresponsiveness (AHR), accelerated lung function decline, development of chronic obstructive pulmonary disease (COPD), and lung cancer-driven mortality (8–13). Smoking reduces the number of CC16-producing club cells and CC16 protein concentrations in BAL and serum, and low CC16 protein concentrations are associated with a smoking-related decline in lung function (14–16). In patients with COPD (vs. smoking control subjects), low serum CC16 protein concentrations have been associated with decreased lung function, accelerated lung function decline, emphysema, COPD, and mortality (17–21).
In patients with asthma (vs. nonsmoking control subjects), low CC16 protein concentrations in BAL, serum, and urine have been associated with asthma susceptibility (22–26). Immunohistochemical staining in small airways has shown decreased numbers of CC16-producing club cells and increased numbers of T cells, activated eosinophils, and mast cells in patients with asthma compared with healthy control subjects (27). Previous studies did not find significant differences in CC16 protein concentrations in BAL with asthma severity, but they had small sample sizes (and even smaller numbers of patients with severe asthma) and did not directly address gene expression in BECs (22–23). In this study, we sought to determine if CC16 mRNA expression levels in BECs are associated with asthma severity in the well-characterized SARP (Severe Asthma Research Program) cohort.
Some of the results of these studies have been previously reported in the form of an abstract (28).
Methods
Study Participants
SARP is a currently active multicenter program funded for the last 20 years by the NHLBI (National Heart, Lung, and Blood Institute) involving nonsmokers with mild to severe asthma (enriched for severe asthma on the basis of the ATS–ERS [American Thoracic Society–European Respiratory Society] criteria [29]) and a subset of healthy control subjects. Participants in SARP were comprehensively phenotyped using standard protocols as described previously (30–40) and in the online supplement. Bronchoscopy was performed on a subset of participants (age >12 yr) in the SARP longitudinal cohort (n = 156) and cross-sectional cohort (n = 155) to obtain epithelial cells from brush biopsies for RNA sequencing (RNAseq) and microarray mRNA chip analysis, respectively (without overlapped participants). SARP was approved by the appropriate institutional review board, including informed consent.
Statistical Analysis
Association analysis of CC16 mRNA expression levels and asthma-related phenotypes
RNAseq and mRNA microarray data from bronchial epithelial cells (BECs) in the longitudinal and cross-sectional cohorts were extracted for the SCGB1A1 gene and 16,067 and 19,566 other genes, respectively, as described previously (32, 41–43) and in the online supplement. The RNAseq and microarray expression data have been deposited and can be accessed through dbGaP (phs001446) and GEO (GSE63142 and GSE43696), respectively (32, 41, 43–45). Association analyses of CC16 mRNA expression levels and asthma-related phenotypes were performed using a logistic regression, a linear regression, and a negative binomial model for binary, continuous, and counting variables, respectively, adjusted for age, sex, body mass index (BMI), race, and batch effect using SAS 9.4 software (SAS Institute Inc.). Nominal P value of 0.05 was considered significant.
Association analysis of CC16–IL-6 and asthma-related phenotypes
To increase sample size and power, patients with asthma in the cross-sectional and longitudinal cohorts were merged on the basis of the standardized median value of CC16 mRNA expression levels. In brief, CC16 mRNA expression levels in patients with asthma in the cross-sectional cohort and the longitudinal cohort were standardized by dividing the median of CC16 mRNA expression levels of the nonsmoking healthy control subjects in the cross-sectional cohort (n = 27; median = 18.83) and the longitudinal cohort (n = 42; median = 16.33), respectively. The standardized median value of CC16 mRNA expression levels in patients with asthma in the cross-sectional cohort was used as a cut-off value (0.9684) to categorize patients with asthma into high CC16 (n = 108) and low CC16 (n = 134) groups in the SARP cross-sectional and longitudinal cohorts. Patients with asthma with CC16 mRNA expression levels were further categorized into high IL-6 (n = 50) and low IL-6 (n = 61) groups on the basis of the median value of blood IL-6 protein concentrations in patients with asthma (n = 763; cut-off value = 1.58 pg/ml) in the SARP cross-sectional and longitudinal cohorts (37). Association analyses of asthma-related phenotypes stratified by high and low CC16 or a combination of CC16 mRNA expression levels in BECs and blood IL-6 protein concentrations: HH (high CC16 and high IL-6; n = 19), HL (high CC16 and low IL-6; n = 28), LH (low CC16 and high IL-6; n = 31), and LL (low CC16 and low IL-6; n = 33) were performed using a nonparametric Kruskal-Wallis test and a generalized linear regression model adjusted for age, sex, BMI, race, and batch effect. Nominal P value of 0.05 was considered significant.
Meta-analysis of genes correlated with CC16 mRNA expression levels
Correlation analyses of CC16 mRNA expression levels in BECs with 16,067 and 19,566 genes in the longitudinal and cross-sectional cohorts, respectively, were performed using Spearman correlation as described previously (32). Meta-analysis of Spearman correlation coefficient (rho) was performed in the overlapped 14,233 genes in the SARP cohorts (n = 311) using metacor R package (46). Bonferroni-adjusted P value of 3.513 × 10−6 (0.05/14,233) on the basis of the Olkin-Pratt fixed-effect meta-analytical model was considered significant.
Gene set enrichment analysis of genes significantly correlated with CC16 mRNA expression levels
A total of 801 and 670 genes were positively and negatively correlated with CC16 mRNA expression levels significantly on the basis of meta-analysis, respectively. These 1,471 genes and the SCGB1A1 gene were input into Enrichr software package for gene set enrichment analysis (47). P value and q-value (Benjamini-Hochberg adjusted P value) of the top 10 terms were extracted from GO (Gene Ontology) Biological Process 2021, GO Cellular Component 2021, KEGG (Kyoto Encyclopedia of Genes and Genomes) 2021 Human, Jensen TISSUES, CellMarker Augmented 2021, and dbGAP libraries.
Results
Demographics of SARP Participants with mRNA Expression in BECs
Bronchoscopy was performed on a subset of SARP participants to obtain epithelial cells from brush biopsies for mRNA expression analysis (Table 1). In the longitudinal cohort, RNAseq in BECs was performed in 156 participants (42 healthy control subjects, 52 patients with nonsevere asthma, and 62 patients with severe asthma). In the cross-sectional cohort, microarray mRNA expression in BECs was performed in 155 participants (27 healthy control subjects, 78 patients with nonsevere asthma, and 50 patients with severe asthma). There were no overlapped participants with mRNA expression in BECs in the longitudinal and cross-sectional cohorts.
Table 1.
Demographics (Mean ± SD) of Participants with mRNA Expression Measured in Bronchial Epithelial Cells
| SARP Cross-sectional Cohort Microarray mRNA (n = 155) | Nonsmoking Control Subjects (n = 27) | Nonsmoking Asthma* (n = 128) | Nonsevere Asthma† (n = 78) | Severe Asthma† (n = 50) | P Value‡ (Asthma vs. Control Subjects) | P Value‡ (Severe vs. Nonsevere) |
|---|---|---|---|---|---|---|
| Age at enrollment, yr | 33 ± 13 | 37 ± 13 | 33 ± 12 | 44 ± 11 | 0.19 | <0.0001 |
| Sex, n (%) female | 15 (56) | 86 (67) | 51 (65) | 35 (70) | 0.27 | 0.7 |
| Race (% White/African American/other§) | 70/19/11 | 60/31/9 | 56/32/11 | 65/29/6 | 0.43 | 0.55 |
| Body mass index, kg/m2 | 26 ± 5.2 | 30 ± 6.9 | 29 ± 6.7 | 32 ± 7.0 | 0.0016 | 0.029 |
| Baseline Pre-BD FEV1% predictedǁ | 94 ± 9.1 | 72 ± 22.1 | 82 ± 17 | 56 ± 20 | <0.0001 | <0.0001 |
| Baseline Pre-BD FEV1/FVCǁ | 0.81 ± 0.04 | 0.70 ± 0.12 | 0.75 ± 0.09 | 0.61 ± 0.12 | <0.0001 | <0.0001 |
| SARP Longitudinal Cohort RNAseq (n = 156) | Nonsmoking Control Subjects (n = 42) | Nonsmoking Asthma* (n = 114) | Nonsevere Asthma† (n = 52) | Severe Asthma† (n = 62) | P value‡ (Asthma vs. Control Subjects) | P value‡ (Severe vs. Nonsevere) |
|---|---|---|---|---|---|---|
| Age at enrollment, yr | 41 ± 13 | 41 ± 13 | 37 ± 12 | 44 ± 13 | 0.92 | 0.0032 |
| Sex, n (%) female | 25 (60) | 74 (65) | 34 (65) | 40 (65) | 0.58 | 1 |
| Race (% White/African American/other§) | 69/17/14 | 63/25/12 | 67/21/12 | 60/27/13 | 0.57 | 0.69 |
| Body mass index, kg/m2 | 28 ± 5.5 | 31 ± 8.7 | 30 ± 9.1 | 32 ± 8.3 | 0.033 | 0.025 |
| Baseline Pre-BD FEV1% predictedǁ | 99 ± 12 | 76 ± 19 | 85 ± 15 | 69 ± 20 | <0.0001 | <0.0001 |
| Baseline Pre-BD FEV1/FVCǁ | 0.81 ± 0.04 | 0.70 ± 0.08 | 0.73 ± 0.09 | 0.68 ± 0.10 | <0.0001 | 0.015 |
Definition of abbreviations: Pre-BD = prebronchodilator; SARP = Severe Asthma Research Program.
For the longitudinal cohort, nonsmokers were never-smokers or previous smokers with less than 5 to 10 pack-years of tobacco use, depending on age. For the cross-sectional cohort, nonsmokers were never-smokers or previous smokers with less than 5 pack-years of tobacco use.
Asthma severity (nonsevere or severe asthma) was on the basis of American Thoracic Society–European Respiratory Society criteria (29).
P value was generated using the Kruskal-Wallis test.
Other races include Hispanic, Asian, American Indian, and mixed.
Pre-BD pulmonary function was presented.
Demographics of the longitudinal and cross-sectional cohorts were similar (Table 1). Patients with severe asthma were older than patients with nonsevere asthma. Sex (percentage of females) and race (percentage of White, African American, and other) were not significantly different between healthy control subjects and patients with nonsevere or severe asthma. BMI showed an increasing trend, and baseline prebronchodilator (pre-BD) FEV1% predicted, and FEV1/FVC showed a decreasing trend from healthy control subjects to nonsevere asthma, then to severe asthma.
Low CC16 mRNA Expression Levels in BECs were Associated with Asthma Susceptibility and Asthma Severity
CC16 mRNA expression levels in BECs were significantly higher in healthy control subjects than patients with asthma in the cross-sectional cohort (18.9 vs. 17.9 [log2 transformed] or 2 × difference; P < 0.0001) (Table 2 and Figure 1A) and in the longitudinal cohort (16.3 vs. 15.4 [natural log transformed] or 2.5 × difference; P < 0.0001) (Table 2 and Figure 1B). CC16 mRNA expression levels in BECs were significantly higher in patients with nonsevere asthma than patients with severe asthma in the cross-sectional cohort (18.2 vs. 17.5 or 1.6 × difference; P = 0.005) (Table 2 and Figure 1A) and in the longitudinal cohort (15.6 vs. 15.1 or 1.6 × difference; P = 0.058) (Table 2 and Figure 1B).
Table 2.
Association of CC16 mRNA Expression Levels in Bronchial Epithelial Cells with Asthma Susceptibility and Asthma Severity
| SARP Cross-sectional Cohort Microarray mRNA (n = 155) | Nonsmoking Control Subjects (n = 27) | Nonsmoking Asthma* (n = 128) | OR (P Value)† | Nonsevere Asthma‡ (n = 78) | Severe Asthma‡ (n = 50) | OR (P Value)† |
|---|---|---|---|---|---|---|
| CC16 mRNA expression levels (log2) | 18.9 ± 0.36 | 17.9 ± 1.14 | 0.10 (<0.0001) | 18.2 ± 0.86 | 17.5 ± 1.36 | 0.55 (0.0049) |
| SARP Longitudinal Cohort RNAseq (n = 156) | Nonsmoking Control Subjects (n = 42) | Nonsmoking Asthma* (n = 114) | OR (P value)† | Nonsevere Asthma‡ (n = 52) | Severe Asthma‡ (n = 62) | OR (P value)† |
|---|---|---|---|---|---|---|
| CC16 mRNA expression levels (ln) | 16.3 ± 0.44 | 15.4 ± 1.01 | 0.14 (<0.0001) | 15.6 ± 0.97 | 15.1 ± 1.00 | 0.67 (0.058) |
Definition of abbreviations: ln = natural logarithm; log2 = logarithm base 2; OR = odds ratio; RNAseq = RNA sequencing; SARP = Severe Asthma Research Program.
For the longitudinal cohort, nonsmokers were never-smokers or previous smokers with less than 5 to 10 pack-years of tobacco use, depending on age. For the cross-sectional cohort, nonsmokers were never-smokers or previous smokers with less than 5 pack-years of tobacco use.
OR and P value were generated using a logistic regression model adjusted for age, sex, body mass index, race, and batch effect.
Asthma severity (nonsevere or severe asthma) was on the basis of American Thoracic Society–European Respiratory Society criteria (29). CC16 mRNA expression levels were log2- and ln-transformed in the SARP cross-sectional cohort and longitudinal cohort, respectively.
Figure 1.
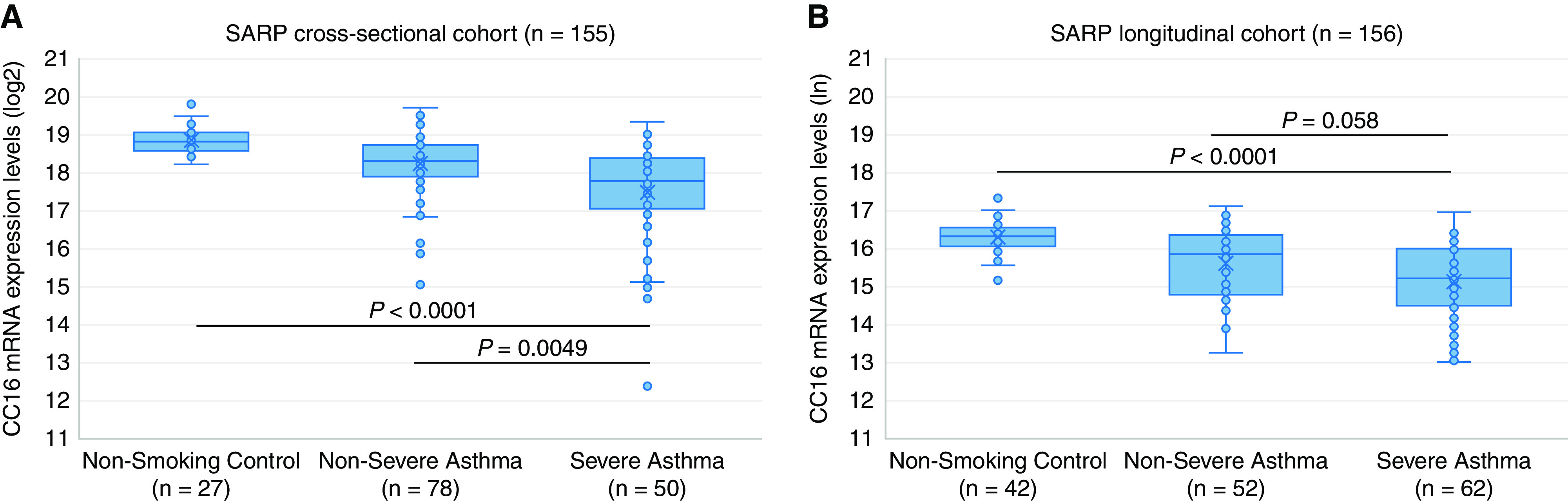
Association of CC16 mRNA expression levels in bronchial epithelial cells with asthma susceptibility and asthma severity in the (A) SARP (Severe Asthma Research Program) cross-sectional cohort and (B) longitudinal cohort. CC16 mRNA expression levels have been logarithm base 2 (log2)- and natural logarithm (ln)-transformed in the (A) SARP cross-sectional cohort and (B) longitudinal cohort, respectively.
Low CC16 mRNA Expression Levels in BECs were Associated with Asthma Exacerbations and Low Lung Function
Reduced CC16 mRNA expression levels in BECs were significantly associated with decreased baseline pre-BD FEV1% predicted and FEV1/FVC in patients with asthma in the cross-sectional cohort (R2 = 0.11; P = 0.002) (Table 3 and Figure 2A) and longitudinal cohort (R2 = 0.16; P = 0.0008) (Table 3 and Figure 2B). CC16 mRNA expression levels in BECs were significantly higher in patients with asthma without exacerbations than with exacerbations in the last 12 months in the cross-sectional cohort (18.2 vs. 17.5 or 1.6 × difference; P = 0.01) (Table 4 and Figure 2C) and longitudinal cohort (15.6 vs. 15.0 or 1.8 × difference; P = 0.007) (Table 4). More importantly, reduced CC16 mRNA expression levels in BECs were significantly associated with an increased number of prospective exacerbations over 3 years of follow-up in the longitudinal cohort (risk ratio = 1.75; P = 0.02) (Table 4 and Figure 2D).
Table 3.
Association of CC16 mRNA Expression Levels in Bronchial Epithelial Cells with Pulmonary Function in Asthmatics
| SARP Cross-sectional Cohort Microarray mRNA (Asthmatics, n = 128) | Baseline Prebronchodilator FEV1% Predicted (n = 128) β (P Value)* |
Baseline Prebronchodilator FEV1/FVC (n = 127) β (P Value)* |
|---|---|---|
| CC16 mRNA expression levels (log2) | 4.99 (0.0021) | 0.031 (0.0005) |
| SARP Longitudinal Cohort RNAseq (Asthmatics, n = 114) | Baseline Prebronchodilator FEV1% Predicted (n = 114) β (P value)* |
Baseline Prebronchodilator FEV1/FVC (n = 114) β (P value)* |
|---|---|---|
| CC16 mRNA expression levels (ln) | 5.69 (0.0008) | 0.032 (0.0003) |
Definition of abbreviations: ln = natural logarithm; log2 = logarithm base 2; RNAseq = RNA sequencing; SARP = Severe Asthma Research Program.
Regression slope (β) and P value were generated using a linear regression model adjusted for age, sex, body mass index, race, and batch effect. CC16 mRNA expression levels were log2- and ln-transformed in the SARP cross-sectional cohort and longitudinal cohort, respectively.
Figure 2.
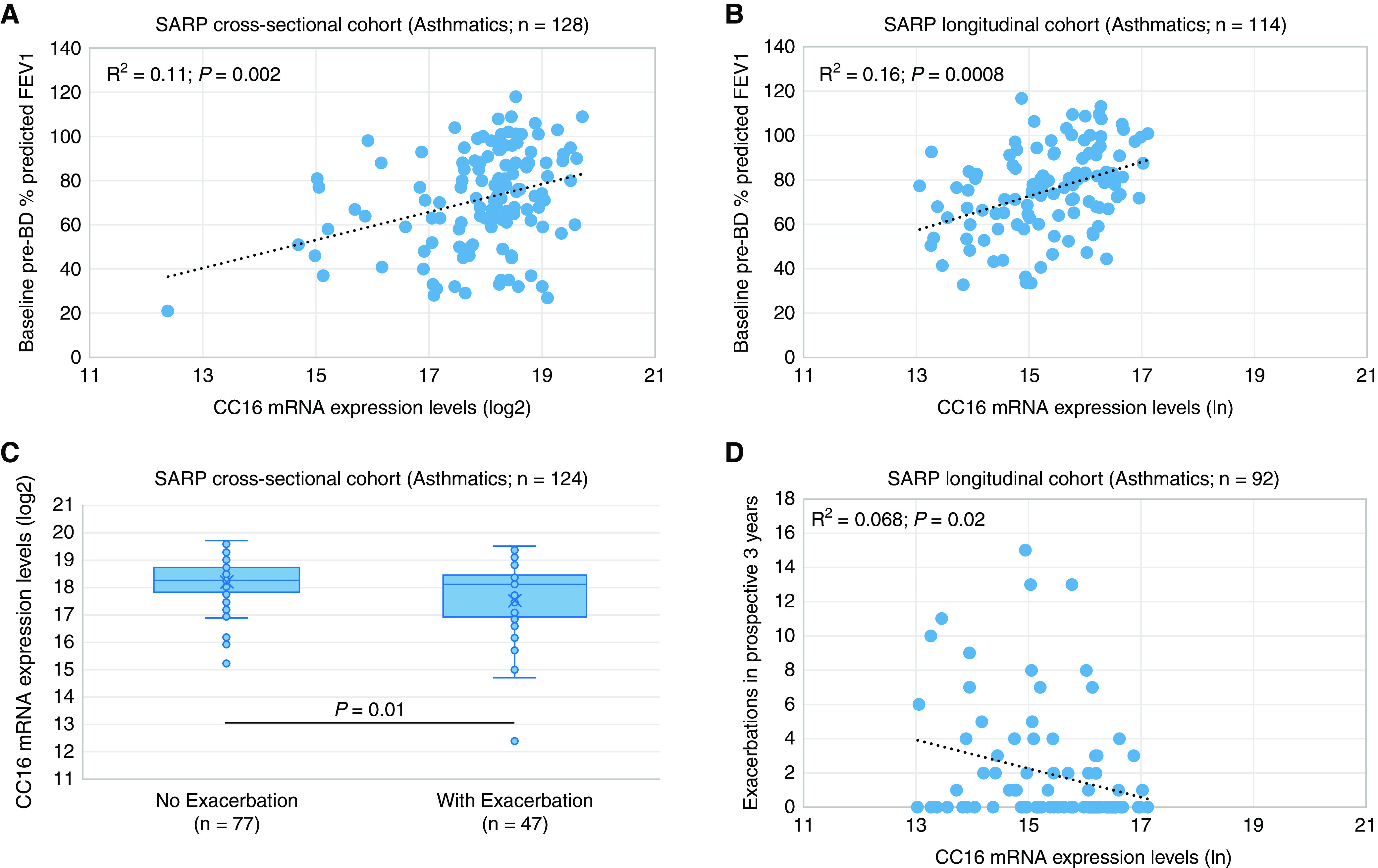
Association of CC16 mRNA expression levels in bronchial epithelial cells with (A and B) baseline FEV1% predicted and (C and D) asthma exacerbations in the SARP (Severe Asthma Research Program) cross-sectional and longitudinal cohorts. CC16 mRNA expression levels have been logarithm base 2 (log2)- and natural logarithm (ln)-transformed in the (A and C) SARP cross-sectional cohort and (B and D) longitudinal cohort, respectively. Pre-BD = prebronchodilator.
Table 4.
Association of CC16 mRNA Expression Levels in Bronchial Epithelial Cells with Asthma Exacerbations in Asthmatics
| SARP Cross-sectional Cohort Microarray mRNA (Asthmatics, n = 128) | Exacerbation (Yes/No) in the Last 12 mo (n = 124) |
Number of exacerbations in the prospective 3 yr (n = 0) RR (P Value)† |
||
|---|---|---|---|---|
| No (n = 77) | Yes (n = 47) | OR (P Value)* | ||
| CC16 mRNA expression levels (log2) | 18.2 ± 0.83 | 17.5 ± 1.46 | 0.62 (0.012) | NA |
| SARP Longitudinal Cohort RNAseq (Asthmatics, n = 114) | Exacerbation (Yes/No) in the Last 12 mo (n = 114) |
Number of exacerbations in the prospective 3 yr (n = 92) RR (P Value)† |
||
|---|---|---|---|---|
| No (n = 63) | Yes (n = 51) | OR (P Value)* | ||
| CC16 mRNA expression levels (ln) | 15.6 ± 0.93 | 15.0 ± 1.02 | 0.55 (0.0067) | 0.57 (0.015) |
Definition of abbreviations: ln = natural logarithm; log2 = logarithm base 2; OR = odds ratio; RNAseq = RNA sequencing; RR = risk ratio; SARP = Severe Asthma Research Program.
OR and P value were generated using a logistic regression model adjusted for age, sex, body mass index, race, and batch effect.
RR and P value were generated using a negative binomial model adjusted for age, sex, body mass index, race, and batch effect. CC16 mRNA expression levels were logarithm base 2- and natural logarithm-transformed in the SARP cross-sectional cohort and longitudinal cohort, respectively.
Low CC16 mRNA Expression Levels in BECs were Associated with High T2 Biomarkers
In the cross-sectional and longitudinal cohorts, reduced CC16 mRNA expression levels in BECs were significantly associated with increased fractional exhaled nitric oxide (FeNO) concentrations (R2 = 0.13; P = 0.0003 and R2 = 0.063; P = 0.002) (Table 5 and Figures 3A and 3D) and sputum percentage eosinophils (R2 = 0.032; P = 0.08 [borderline significant] and R2 = 0.13; P = 0.0009) (Table 5 and Figures 3C and 3F). In contrast, the association of CC16 mRNA expression levels in BECs with blood eosinophils or neutrophils and sputum neutrophils was not consistent in the longitudinal and cross-sectional cohorts (Table 5 and Figure 3B and 3E).
Table 5.
Association of CC16 mRNA Expression Levels in Bronchial Epithelial Cells with T2 AND non-T2 Biomarkers in Asthmatics
| SARP Cross-sectional Cohort Microarray mRNA (Asthmatics, n = 128) | FeNO (n = 104) | Total Serum IgE (n = 103) | Blood Eosinophil Counts (n = 102) | Blood Neutrophil Counts (n = 102) | Sputum % Eosinophils (n = 62) | Sputum % Neutrophils (n = 62) |
|---|---|---|---|---|---|---|
| β (P Value)* | β (P Value)* | β (P Value)* | β (P Value)* | β (P Value)* | β (P Value)* | |
| CC16 mRNA expression levels (log2) | −0.10 (0.0003) | −0.088 (0.11) | 0.11 (0.07) | −0.043 (0.017) | −0.20 (0.084) | −0.028 (0.43) |
| SARP Longitudinal Cohort RNAseq (Asthmatics, n = 114) | FeNO (n = 113) | Total Serum IgE (n = 111) | Blood Eosinophil Counts (n = 114) | Blood Neutrophil Counts (n = 114) | Sputum % Eosinophils (n = 84) | Sputum % Neutrophils (n = 84) |
|---|---|---|---|---|---|---|
| β (P Value)* | β (P Value)* | β (P Value)* | β (P Value)* | β (P Value)* | β (P Value)* | |
| CC16 mRNA expression levels (ln) | −0.10 (0.0022) | −0.080 (0.18) | −0.22 (0.0001) | −0.0009 (0.96) | −0.40 (0.0009) | 0.069 (0.042) |
Definition of abbreviations: FeNO = fractional exhaled nitric oxide; ln = natural logarithm; log2 = logarithm base 2; RNAseq = RNA sequencing; SARP = Severe Asthma Research Program.
Regression slope (β) and P value were generated using a linear regression model adjusted for age, sex, body mass index, race, and batch effect. CC16 mRNA expression levels were log2- and ln-transformed in the SARP cross-sectional cohort and longitudinal cohort, respectively.
Figure 3.
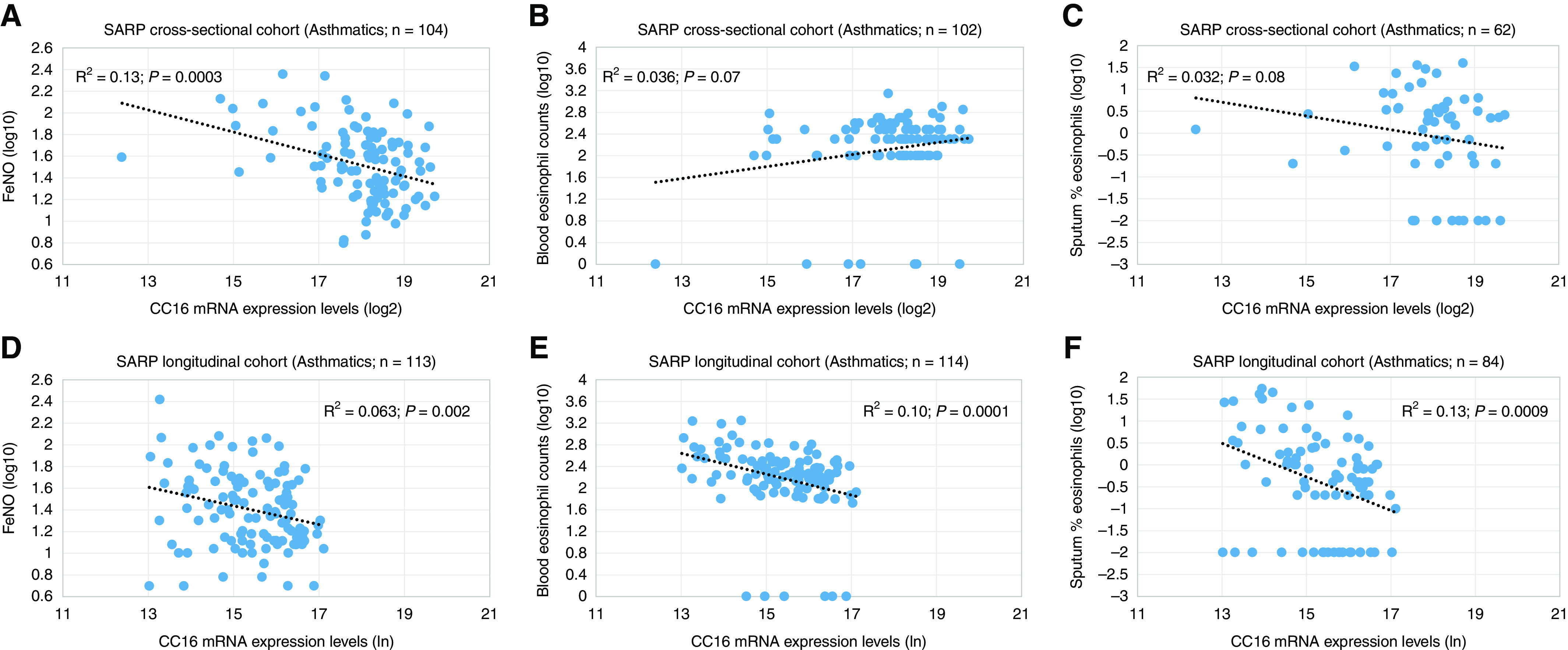
Association of CC16 mRNA expression levels in bronchial epithelial cells with (A and D) fractional exhaled nitric oxide (FeNO) values, (B and E) blood eosinophil counts, and (C and F) sputum percentage eosinophils in the SARP (Severe Asthma Research Program) cross-sectional and longitudinal cohorts. CC16 mRNA expression levels have been logarithm base 2 (log2)- and natural logarithm (ln)-transformed in the (A–C) SARP cross-sectional cohort and (D–F) longitudinal cohort, respectively.
Low CC16 mRNA Expression Levels in BECs were Correlated with Higher mRNA Expression Levels of Th2 Genes
Spearman correlation analyses were performed between CC16 mRNA expression levels and 16,067 genes in the longitudinal cohort (n = 156) or 19,566 genes in the cross-sectional cohort (n = 155). Meta-analysis of Spearman correlation coefficient (rho) was then performed in the overlapping 14,233 genes in the SARP cohorts (n = 311) (46). With Bonferroni correction, 801 and 670 genes were positively and negatively correlated with CC16 mRNA expression levels, respectively (Table E1 in the online supplement). Among a subset of candidate genes selected on the basis of biological relevance, CC16 mRNA expression levels in BECs were negatively correlated with mRNA expression levels of the Th2 pathway and inflammation genes (IL1RL1, IL18R1, POSTN, SERPINB2, CLCA1, NOS2, MUC5AC, and PLA2G4A) (Table 6). CC16 mRNA expression levels in BECs were positively correlated with mRNA expression levels of the Th1 pathway and inflammation genes (IL12A, MUC5B, C3, TLR5, and CXCL6).
Table 6.
Genes with Expression Levels Significantly Correlated with CC16 mRNA Expression Levels in Bronchial Epithelial Cells
| Gene | SARP Cross-sectional Cohort (n = 155) |
SARP Longitudinal Cohort (n = 156) |
Meta-analysis of SARP Cohorts (n = 311) |
|||
|---|---|---|---|---|---|---|
| rho* | P Value* | rho* | P Value* | rho† | P Value† | |
| IL12A | 0.34 | 2.02 × 10−5 | 0.39 | 4.39 × 10−7 | 0.37 | 5.55 × 10−14 |
| MUC5B | 0.47 | 4.26 × 10−10 | 0.47 | 6.05 × 10−10 | 0.48 | 1.53 × 10−27 |
| TLR5 | 0.49 | 1.35 × 10−10 | 0.54 | <2.20 × 10−16 | 0.52 | 3.04 × 10−35 |
| C3 | 0.51 | 1.93 × 10−11 | 0.48 | 2.31 × 10−10 | 0.50 | 3.48 × 10−31 |
| CXCL6 | 0.44 | 9.26 × 10−9 | 0.47 | 1.01 × 10−9 | 0.46 | 2.10 × 10−24 |
| IL18R1 | −0.37 | 1.66 × 10−6 | −0.32 | 4.38 × 10−5 | −0.35 | 1.37 × 10−12 |
| MUC5AC | −0.30 | 1.87 × 10−4 | −0.47 | 5.67 × 10−10 | −0.39 | 6.69 × 10−16 |
| PLA2G4A | −0.44 | 1.31 × 10−8 | −0.37 | 2.54 × 10−6 | −0.40 | 1.07 × 10−17 |
| POSTN | −0.41 | 1.09 × 10−7 | −0.32 | 4.00 × 10−5 | −0.37 | 3.28 × 10−14 |
| SERPINB2 | −0.26 | 1.09 × 10−3 | −0.41 | 1.92 × 10−7 | −0.33 | 1.91 × 10−11 |
| CLCA1 | −0.42 | 6.74 × 10−8 | −0.52 | <2.20 × 10−16 | −0.47 | 1.09 × 10−26 |
| IL1RL1 | −0.39 | 6.36 × 10−7 | −0.44 | 1.07 × 10−8 | −0.42 | 4.55 × 10−19 |
| NOS2 | −0.42 | 7.61 × 10−8 | −0.39 | 7.96 × 10−7 | −0.40 | 1.82 × 10−17 |
Definition of abbreviation: SARP = Severe Asthma Research Program.
A subset of genes was presented in this table, whereas the full table was presented in Table E1.
Correlation coefficient (rho) and P value were generated using a Spearman correlation model in which genes were inverse normalized with age, sex, body mass index, race, batch effect, and asthma status.
Correlation coefficient (rho) and P value were generated on the basis of Olkin-Pratt fixed-effect meta-analytical model using metacor R package (46).
Gene Set Enrichment Analysis of Genes Correlated with CC16 mRNA Expression Levels in BECs
A total of 1,471 genes significantly correlated with CC16 mRNA expression levels in BECs were input into Enrichr software package for gene set enrichment analysis (47). In the GO Biological Process 2021 library (Table E2 and Figure E1A), genes involved in the cytokine-mediated signaling pathway, negative regulation of cell migration, epithelium development, and epithelial cell differentiation were enriched. In the GO Cellular Component 2021 library (Table E3 and Figure E1B), genes involved in the MHC class II protein complex were enriched. In the Jensen TISSUES library (Table E4 and Figure E1C), genes expressed in the lung and esophagus were enriched. In the CellMarker Augmented 2021 library (Table E5 and Figure E1D), genes expressed in secretory cells and type II pneumocytes in the lung were enriched. In the KEGG 2021 Human library (Table E6 and Figure E1E), genes associated with asthma and Th1/Th2 cell differentiation were enriched. In the dbGaP library (Table E7 and Figure E1F), genes associated with asthma were enriched.
Association of High–Low CC16 mRNA Expression Levels in BECs with Asthma Phenotypes
To increase sample size and statistical power, patients with asthma in the cross-sectional and longitudinal cohorts were merged (n = 242) on the basis of the standardized median value of CC16 mRNA expression levels. High CC16 mRNA expression levels in BECs were significantly associated with younger age (P = 0.0003), a higher percentage non-Hispanic White (P = 0.003), and lower BMI (P = 0.03) (Table 7). Consistent with the findings of continuous CC16 mRNA expression levels (Table E8), low CC16 mRNA expression levels were significantly associated with decreased pulmonary function, higher T2 biomarkers, and a greater percentage of exacerbations and ATS–ERS asthma severity (adjusted P < 0.05). In addition, low CC16 mRNA expression levels were significantly associated with greater airway reversibility (percentage change in FEV1% predicted to albuterol: 25 vs. 16; adjusted P = 0.004), greater AHR (PC20 to methacholine: 0.58 vs. 1.5; adjusted P = 0.02), higher ACQ-6 score (asthma control questionnaire-6: 1.61 vs. 0.95; adjusted P = 0.03), and greater percentage of use of high-dose ICS (61% vs. 40%; adjusted P = 0.04) and oral or systemic corticosteroids (48% vs. 28%; adjusted P = 0.02).
Table 7.
Association of High–Low CC16 mRNA Expression Levels in Bronchial Epithelial Cells in Asthmatics with Asthma Phenotypes
| SARP (Asthmatics, n = 242) | CC16 High* (n = 108) |
CC16 Low* (n = 134) |
P Value |
|||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | Unadjusted† | Adjusted‡ | |
| Age at enrollment, yr | 108 | 36 ± 13 | 134 | 42 ± 13 | 0.0003 | NA |
| Age of asthma onset, yr | 106 | 13 ± 13 | 132 | 16 ± 15 | 0.29 | NA |
| Sex, n (%) female | 108 | 76 (70) | 134 | 84 (63) | 0.22 | NA |
| Race (% White/African American/other§) | 103 | 73/22/5 | 130 | 52/32/15 | 0.0026 | NA |
| Body mass index, kg/m2 | 108 | 29 ± 7.3 | 134 | 32 ± 8.1 | 0.025 | NA |
| Baseline Pre-BD FEV1% predictedǁ | 108 | 80 ± 20 | 134 | 69 ± 20 | <0.0001 | 0.0005 |
| Baseline Pre-BD FEV1/FVCǁ | 108 | 0.74 ± 0.11 | 133 | 0.67 ± 0.11 | <0.0001 | <0.0001 |
| Maximal FEV1% predicted | 96 | 91 ± 18 | 124 | 83 ± 20 | 0.0006 | 0.013 |
| Maximal reversibility | 108 | 16 ± 18 | 134 | 25 ± 25 | 0.0001 | 0.0042 |
| PC20 methacholine, mg/ml, median (IQR) | 58 | 1.5 (0.5–3.1) | 49 | 0.58 (0.28–2.0) | 0.0053 | 0.016 |
| Total serum IgE concentrations, IU/ml, median (IQR) | 94 | 99 (29–307) | 120 | 159 (86–336) | 0.042 | 0.024 |
| FeNO, ppb, median (IQR) | 97 | 22 (15–42) | 120 | 36 (19–63) | 0.0024 | 0.0011 |
| Blood eosinophils, cells/μl, median (IQR) | 95 | 183 (100–300) | 121 | 200 (100–374) | 0.024 | 0.082 |
| Blood neutrophils, cells/μl, median (IQR) | 95 | 3,800 (2,900–5,008) | 121 | 3,800 (2,906–5,548) | 0.53 | 0.39 |
| Sputum % eosinophils, median (IQR) | 61 | 0.4 (0.001–2.2) | 85 | 1.2 (0.2–4.4) | 0.011 | 0.019 |
| Sputum % neutrophils, median (IQR) | 61 | 59 (33–78) | 85 | 45 (30–63) | 0.034 | 0.28 |
| Serum or plasma IL-6 concentrations, pg/ml, median (IQR) | 47 | 1.2 (0.68–2.3) | 64 | 1.4 (0.74–3.0) | 0.52 | 0.45 |
| Serum sIL-6R concentrations, ng/ml, median (IQR) | 49 | 37 (30–42) | 73 | 37 (31–47) | 0.21 | 0.10 |
| High-dose ICS (%) | 86 | 40 | 117 | 61 | 0.0043 | 0.040 |
| Oral or systemic CS (%) | 97 | 28 | 124 | 48 | 0.0035 | 0.015 |
| Exacerbations in the last 12 mo, n (%) | 106 | 33 (31) | 132 | 65 (49) | 0.0055 | 0.046 |
| ACQ-6 | 42 | 0.95 ± 1.21 | 69 | 1.61 ± 1.40 | 0.0058 | 0.031 |
| ATS-ERS severe asthma, n (%) | 108 | 37 (34) | 134 | 78 (58) | 0.0003 | 0.0033 |
Definition of abbreviations: ACQ-6 = asthma control questionnaire-6; CS = corticosteroids; ATS-ERS = American Thoracic Society-European Respiratory Society; FeNO = fractional exhaled nitric oxide; ICS = inhaled corticosteroids; Pre-BD = prebronchodilator; SARP = Severe Asthma Research Program.
High and low CC16 mRNA expression levels in asthmatics were categorized using the standardized median value of CC16 mRNA expression levels as described in the Methods section.
Unadjusted P value was generated using Kruskal-Wallis test.
Adjusted P value was generated using a generalized linear model adjusted for age, sex, body mass index, race, and batch effect.
Other races include Hispanic, Asian, American Indian, and mixed.
Pre-BD pulmonary function was presented.
Association of a Combination of CC16 mRNA Expression Levels in BECs and Blood IL-6 Protein Concentrations with Asthma Phenotypes
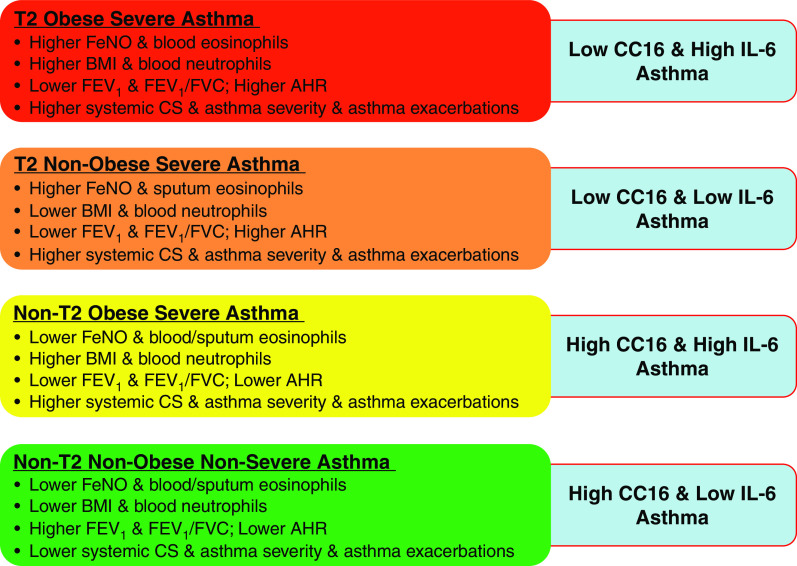
Participants with asthma were further categorized into four groups on the basis of a combination of two nontraditional T2 biomarkers (CC16 mRNA expression levels in BECs and blood IL-6 protein concentrations): HH (high CC16 and high IL-6), HL (high CC16 and low IL-6), LH (low CC16 and high IL-6), and LL (low CC16 and low IL-6) (Table 8 and Figure 4). Patients with asthma in two low CC16 groups (LH and LL) showed higher T2 biomarkers (median FeNO ⩾ 25 ppb), higher AHR, and lower pulmonary function. Patients with asthma in two high IL-6 groups (LH and HH) showed higher BMI (mean BMI ⩾ 30 kg/m2), higher blood neutrophils, and lower pulmonary function, similar to what we have shown in a previous study (37). Patients with asthma in the HL group showed lower T2 biomarkers, lower AHR, and lower BMI. The HL group was a nonsevere asthma group (18% with severe asthma) with higher pulmonary function (baseline pre-BD FEV1% predicted = 87), lower ACQ-6 score (0.67), lower systemic corticosteroids use (21%), and lower asthma exacerbations (18%) in the last 12 months than the other three severe asthma groups (LH, LL, and HH), though not always statistically significant because of relatively small sample size.
Table 8.
Association of a Combination of CC16 mRNA Expression Levels in Bronchial Epithelial Cells and Blood IL-6 Protein Concentrations in Asthmatics with Asthma Phenotypes
| SARP (Asthmatics, n = 111) | HH (n = 19) |
HL (n = 28) |
LH (n = 31) |
LL (n = 33) |
P Value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | Unadjusted* | Adjusted† | |
| Age at enrollment, yr | 19 | 41 ± 11 | 28 | 33 ± 11 | 31 | 44 ± 13 | 33 | 42 ± 11 | 0.004 | NA |
| Age of asthma onset, yr | 19 | 13 ± 12 | 28 | 12 ± 12 | 31 | 17 ± 15 | 33 | 16 ± 12 | 0.49 | NA |
| Sex, n (%) female | 19 | 12 (63) | 28 | 20 (71) | 31 | 26 (84) | 33 | 13 (39) | 0.002 | NA |
| Race (% White/African American/other‡) | 19 | 53/47/0 | 28 | 89/11/0 | 31 | 42/42/16 | 33 | 48/33/18 | 0.002 | NA |
| BMI, kg/m2 | 19 | 35 ± 8.1 | 28 | 27 ± 5.6 | 31 | 36 ± 10 | 33 | 27 ± 5.4 | <0.0001 | NA |
| Obesity (BMI ⩾30 kg/m2), % | 19 | 74 | 28 | 21 | 31 | 77 | 33 | 21 | <0.0001 | NA |
| Baseline Pre-BD FEV1% predicted§ | 19 | 72 ± 19 | 28 | 87 ± 13 | 31 | 72 ± 17 | 33 | 67 ± 22 | 0.0002 | 0.029 |
| Baseline Pre-BD FEV1/FVC§ | 19 | 0.70 ± 0.09 | 28 | 0.75 ± 0.08 | 31 | 0.69 ± 0.08 | 33 | 0.64 ± 0.1 | 0.0004 | 0.0026 |
| Maximal FEV1% predicted | 19 | 84 ± 16 | 28 | 96 ± 14 | 31 | 87 ± 15 | 33 | 83 ± 21 | 0.009 | 0.33 |
| Maximal reversibility | 19 | 20 ± 23 | 28 | 11 ± 9.0 | 31 | 24 ± 21 | 33 | 27 ± 26 | 0.002 | 0.042 |
| PC20 methacholine, mg/ml, median (IQR) | 12 | 1.9 (0.49–3.2) | 15 | 0.99 (0.39–2.4) | 13 | 0.37 (0.17–0.71) | 8 | 0.32 (0.18–0.60) | 0.007 | 0.067 |
| Total serum IgE concentrations, IU/ml, median (IQR) | 19 | 140 (41–374) | 27 | 139 (42–337) | 30 | 168 (69–345) | 32 | 153 (90–335) | 0.97 | 0.99 |
| FeNO, ppb, median (IQR) | 19 | 17 (14–33) | 27 | 22 (14–44) | 28 | 40 (27–66) | 32 | 25 (13–46) | 0.093 | 0.036 |
| Blood eosinophils, cells/μl, median (IQR) | 19 | 180 (68–252) | 27 | 183 (100–300) | 31 | 294 (100–400) | 33 | 150 (100–304) | 0.17 | 0.24 |
| Blood neutrophils, cells/μl, median (IQR) | 19 | 4,402 (3,825–5,280) | 27 | 3,031 (2,300–4,095) | 31 | 4,116 (2,777–5,969) | 33 | 3,658 (3,088–5,044) | 0.02 | 0.060 |
| Sputum % eosinophils, median (IQR) | 17 | 0.8 (0.3–2.6) | 19 | 0.8 (0.1–3.2) | 20 | 0.8 (0.2–7.9) | 24 | 2.0 (0.5–4.0) | 0.52 | 0.62 |
| Sputum % neutrophils, median (IQR) | 17 | 64 (33–88) | 19 | 59 (48–76) | 20 | 46 (32–76) | 24 | 45 (30–69) | 0.51 | 0.89 |
| High-dose ICS (%) | 15 | 47 | 22 | 36 | 29 | 48 | 31 | 52 | 0.74 | 0.89 |
| Oral or systemic CS (%) | 19 | 42 | 28 | 21 | 31 | 42 | 33 | 42 | 0.28 | 0.61 |
| Exacerbations in the last 12 mo, n (%) | 19 | 7 (37) | 28 | 5 (18) | 31 | 14 (45) | 33 | 15 (45) | 0.1 | 0.29 |
| ACQ-6 | 11 | 0.91 ± 1.22 | 21 | 0.67 ± 0.80 | 19 | 1.68 ± 1.45 | 26 | 1.62 ± 1.47 | 0.022 | 0.13 |
| ATS-ERS severe asthma, n (%) | 19 | 7 (37) | 28 | 5 (18) | 31 | 14 (45) | 33 | 16 (48) | 0.07 | 0.55 |
Definition of abbreviations: ACQ-6 = asthma control questionnaire-6; BMI = body mass index; CS = corticosteroids; ATS-ERS = American Thoracic Society-European Respiratory Society; FeNO = fractional exhaled nitric oxide; HH = high CC16 mRNA expression levels in bronchial epithelial cells and high blood IL-6 protein concentrations; HL = high CC16 and low IL-6; ICS = inhaled corticosteroids; LH = low CC16 and high IL-6; LL = low CC16 and low IL-6; Pre-BD = prebronchodilator; SARP = Severe Asthma Research Program.
Unadjusted P value was generated using Kruskal-Wallis test.
Adjusted P value was generated using a generalized linear model adjusted for age, sex, BMI, race, and batch effect.
Other races include Hispanic, Asian, American Indian, and mixed.
Pre-BD pulmonary function was presented.
Figure 4.
Summary of asthma endotypes defined by CC16 mRNA expression levels in bronchial epithelial cells and blood IL-6 protein concentrations. AHR = airway hyperresponsiveness; BMI = body mass index; CS = corticosteroids; FeNO = fractional exhaled nitric oxide.
Discussion
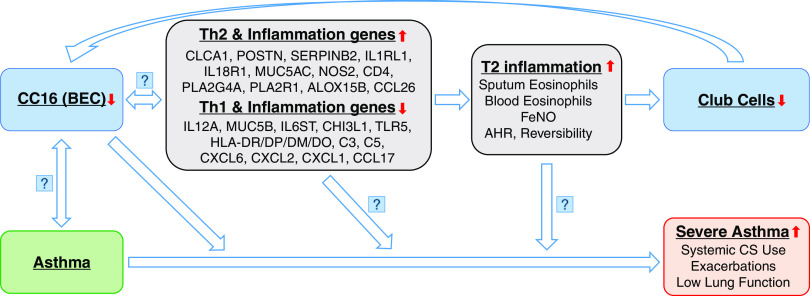
By using mRNA expression in BECs (the most relevant tissue for CC16 expression) in well-characterized SARP cross-sectional and longitudinal cohorts enriched for severe asthma, we have shown that low CC16 mRNA expression levels in BECs are associated with not only asthma susceptibility but also asthma severity. Our findings indicate that, among patients with asthma, reduced CC16 mRNA expression levels in BECs are associated with decreased lung function, enhanced systemic corticosteroids use, and increased retrospective and prospective asthma exacerbations. In addition, reduced CC16 mRNA expression levels are associated with increased T2 biomarkers and increased expression concentrations of Th2 pathway genes. We postulate that low CC16 expression levels in BECs upregulate airway T2 inflammation (Figure 5); chronic T2 inflammation in the airway may alter bronchial epithelium architecture and decrease the number of club cells, which further reduces CC16 expression levels in BECs.
Figure 5.
Asthma progression model with involvement of CC16 and T2 inflammation. AHR = airway hyperresponsiveness; BEC = bronchial epithelial cells; CS = corticosteroids; FeNO = fractional exhaled nitric oxide.
Multiple factors may influence CC16 expression levels. In a population-based cohort, serum CC16 protein concentrations increased from birth to childhood and into the age of 32 years (48). In contrast, high CC16 mRNA expression levels in BECs were associated with younger age in adults with asthma (Tables 7 and E8). In a previous study, high BMI has been associated with low blood CC16 protein concentrations, indicating its potential role in obese asthma (49). In this study, low CC16 mRNA expression levels in BECs were also associated with high BMI in adults with asthma (Table 7). Smoking has shown strong effects on reducing CC16 protein concentrations (14–16), and thus, it should be adjusted for when investigating CC16 in population-based or COPD cohorts (17–21). The SARP cohorts are “nonsmoking” asthma cohorts, including only a few former smokers with a low amount of tobacco use. In this study, smoking status (never smoker vs. former smoker) was not significantly associated with CC16 mRNA expression levels in BECs or asthma severity (Table E9). Adjustment for smoking status in addition to age, sex, BMI, race, and batch effect showed no further effect on the significant association between CC16 mRNA expression levels in BECs and asthma severity.
It is unclear whether reduced CC16 expression levels upregulate expression of Th2 pathway genes, increased expression levels of Th2 pathway genes downregulate CC16 expression, or maybe both (Figure 5). An in vivo study using CC16-deficient mice has shown increased expression of Th2 cytokines (IL-4/-5/-13) and eotaxin and heightened pulmonary eosinophilic inflammation (50). Reconstitution of the CC16 gene in CC16-deficient mice inhibited the expression of Th2 cytokines through inhibition of GATA-3 expression (51). An in vitro study using cord blood cells has found that CC16 inhibits Th2 cell differentiation of human naive neonatal T cells by affecting dendritic cells (52). In this study, lower CC16 mRNA expression levels were associated with higher expression of Th2 pathway genes (CLCA1, POSTN, SERPINB2, IL1RL1, and NOS2) and inflammation genes (PLA2G4A and PLA2R1). Higher CC16 mRNA expression levels were associated with higher expression of the Th1 pathway gene (IL12A), complement pathway genes (C3 and C5), and chemokines (CXCL1, 2, and 6) involved in neutrophil chemoattractant and antimicrobial activity. A gene expression analysis in BECs has identified that mRNA expression of three T2 biomarkers (CLCA1, POSTN, and SERPINB2) is increased in patients with asthma, and expression of these genes can be upregulated by IL-13 or downregulated by corticosteroids treatment (53). The expression of the NOS2 gene (nitric oxide synthase 2) in BECs is the main determinant for FeNO concentrations (54). IL1RL1, the receptor for IL-33, is involved in eosinophilic inflammation (55). In this study, CC16 mRNA expression levels are negatively associated with T2 inflammation biomarkers such as FeNO and sputum eosinophils but are only weakly associated with total serum IgE concentrations (Table 5). Thus, it seems like CC16 can decrease FeNO concentrations through downregulating IL-13 and NOS and decrease sputum eosinophils through downregulating IL-5 and IL1RL1, but less so for total serum IgE concentrations through IL-4.
It is unknown whether reduced CC16 expression levels cause asthma development or asthma leads to reduced CC16 expression levels (Figure 5). An early-life study with a relatively small sample size has shown that low plasma CC16 protein concentrations at 4 months of age do not predict the development of asthma at 3 years of age (56). Larger longitudinal population-based birth cohorts are needed to reveal the relationship between CC16 and asthma development. rs3741240, a single-nucleotide polymorphism located at the 5′ untranslated region of the SCGB1A1 gene, has been consistently associated with serum CC16 protein concentrations (57–58). In this study, the A allele of rs3741240 was also associated with low CC16 mRNA expression levels in BECs in non-Hispanic Whites in the SARP cross-sectional cohort (regression slope β = −0.81; P = 0.007) and longitudinal cohort (β = −0.69; P = 0.04) (Table E10). Several early candidate-gene association analyses with relatively small sample sizes have indicated that the A allele of rs3741240 was associated with asthma susceptibility (59–60); however, this finding was not confirmed by a meta-analysis (61). Although large-scale genome-wide association studies have not found significant associations between genetic variants in CC16 (including rs3741240) and asthma or other obstructive lung diseases (62), candidate gene studies have reported significant associations between rs3741240 and decline in lung function (63–64) and a recent Mendelian randomization study (58) has provided support toward a causal role of CC16 in COPD. Future studies will need to elucidate the nature of the association between low CC16 expression levels and asthma susceptibility.
It is also unknown whether reduced CC16 expression levels cause asthma progression or severe asthma leads to further reduced CC16 expression levels. GINA (Global Initiative for Asthma) guidelines and ATS–ERS define severe asthma as asthma that remains uncontrolled despite optimized treatment with high-dose ICS–LABA (inhaled corticosteroids-long-acting beta-agonists) or that requires high-dose ICS–LABA to prevent it from becoming uncontrolled (29, 65). The GINA definition of severe asthma was derived from the ATS–ERS Task Force definition and represents the same definition of severe asthma. Thus, although GINA scores were not used in SARP, we would expect high GINA scores to also be associated with low CC16 mRNA expression levels in BECs. Because high-dose ICS and oral or systemic corticosteroids use is the major criterion to define severe asthma, it is difficult to dissect the influence of corticosteroids as a causal factor between CC16 mRNA expression levels and asthma severity in the current cross-sectional study design. As expected, high-dose ICS and oral or systemic corticosteroids use were significantly associated with both severe asthma and low CC16 mRNA expression levels in BECs (Table E11). Thus, adjustment for ICS doses in addition to age, sex, BMI, race, and batch effect eliminated the significant association between CC16 mRNA expression levels in BECs and asthma severity, possibly because of overadjustment. Future studies will need to elucidate causality between low CC16 expression levels and asthma severity and the role of corticosteroids use in this relationship.
Biomarkers are biological indicators of a specific pathophysiological process, which can be used to stratify patients, track disease status and progression, and indicate drug effects. At the current stage, blood eosinophils, sputum eosinophils, FeNO, and total serum IgE have been commonly used as biomarkers for T2 asthma (66). In contrast, biomarkers for non-T2 asthma are less well defined. Some potential nontraditional T2 asthma biomarkers are sputum neutrophils, Th1 cytokines, Th17 cytokines, and inflammation biomarkers such as IL-6 (37, 66). Although most patients with asthma have evidence of persistent T2 endotypes, others have evidence that their persistent asthma is related to non-T2 mechanisms (30, 36, 37). Thus, the identification of nontraditional T2 asthma biomarkers is urgently needed. In this study, low CC16 mRNA expression levels are associated with asthma susceptibility, severity and exacerbations, low lung function, and high systemic corticosteroids use. In addition, CC16 mRNA expression levels are not significantly associated with the other two nontraditional T2 biomarkers (sputum neutrophils and blood IL-6 protein concentrations) (Table 7).
To increase sample size and power for investigating the combinatory effect of CC16 and IL-6, patients with asthma in the cross-sectional and longitudinal cohorts were merged. To accommodate the difference between the two mRNA measurement methods (microarray and RNAseq) (Figure 1), CC16 mRNA expression levels in patients with asthma in the cross-sectional and longitudinal cohorts were standardized by dividing the median of CC16 mRNA expression levels of the control subjects without asthma in the cross-sectional and the longitudinal cohorts, respectively. To accommodate the difference between the two SARP cohorts (different proportions of severe asthma) (Table 1), the standardized median value of CC16 mRNA expression levels in patients with asthma in the cross-sectional cohort was used as a cut-off value to categorize patients with asthma into high CC16 and low CC16 groups in the cross-sectional and longitudinal cohorts. As for the high IL-6 and low IL-6 groups, the median value of blood IL-6 protein concentrations in all SARP patients with asthma was used as a cut-off value in the cross-sectional and longitudinal cohorts. In the future, potentially better cut-off values of CC16 and IL-6 could be investigated using sensitivity analysis in the large cohorts.
A combination of two nontraditional T2 biomarkers (CC16 and IL-6) reveals four asthma endotypes (i.e., LH, LL, HH, and HL groups, which represent T2 obese severe asthma, T2 nonobese severe asthma, non-T2 obese severe asthma, and non-T2 nonobese nonsevere asthma, respectively) (Figure 4 and Table 8). In a previous analysis, we have shown that a combination of IL-6 (nontraditional T2 biomarker) and FeNO (T2 biomarker) identified four asthma endotypes (37), including two severe asthma endotypes driven by high IL-6. In this study, a combination of CC16 and IL-6 identified four asthma endotypes, including three severe asthma endotypes driven by both low CC16 and high IL-6. The current analysis further shows the value of understanding two overlapped nontraditional T2 biomarkers and may represent a basis for targeted therapy in the future.
To further dissect CC16 as a nontraditional T2 asthma biomarker, the association of high–low CC16 mRNA expression levels in BECs with asthma severity was analyzed in atopic/nonatopic asthma (defined by positive/negative response to skin prick test in the cross-sectional cohort or specific IgE measurement in the longitudinal cohort) and in early/late onset asthma (age of asthma onset <12 or ⩾12 yr) (Table E12). Asthmatics with low CC16 mRNA expression levels in BECs showed a consistently high percentage of severe asthma in atopic/nonatopic and early/late-onset asthma, which indicate that CC16 is a biomarker less affected by atopic status or age onset of asthma. Thus, CC16 is a consistent nontraditional T2 biomarker for asthma, asthma severity, and exacerbations.
Whether CC16 is truly a nontraditional T2 biomarker or simply a “negative” T2 biomarker is an important question. CC16 mRNA expression levels in BECs were associated with both T2 biomarkers (Tables 5 and E13) and selected gene expression biomarkers (Tables 6 and E14), but the magnitude of these correlations was moderate (0.25 < rho < 0.55) (67). T2 biomarkers (FeNO and sputum eosinophils) (Table E13) as well as genes correlated with CC16 mRNA expression levels (Table 6) were not significantly associated with asthma severity, except for PLA2G4A in the longitudinal cohort (Table E14). In addition, the association between CC16 mRNA expression levels and asthma severity was not significantly affected by adjusting for T2 pathway genes (POSTN, SERPINB2, CLCA1, IL1RL1, and NOS2). Thus, CC16 should be considered a nontraditional T2 biomarker instead of a totally negative T2 biomarker.
There are some important questions that remain to be answered. Gene expression is dependent on the cell type or tissue, time and progression of asthma, and environmental factors, including exposures (32, 42, 43). It is critical that cells are obtained from the appropriate tissue (BECs for CC16) and from participants with the disease being investigated. BECs obtained from brush biopsies are mainly composed of epithelial cells (including ciliated cells, goblet cells, basal cells, and club cells), although there may be a small proportion of inflammatory and immune cells. A flow cytometry study showed that 95–97% of the cells from bronchial brushings were epithelial cells (68). In this study, cell populations were not available for every participant and, thus, were not adjusted. Future expression analyses by single-cell RNAseq of club cells may reveal interesting results. As a potential biomarker, the measurement of serum CC16 protein concentrations is more practical and noninvasive. However, the main source of serum CC16 is the respiratory epithelium because CC16 is a small secretory protein that can passively move across the airway–blood barrier into serum (3). Therefore, measurements of CC16 airway expression or CC16 concentrations in BAL or induced sputum may show stronger associations with asthma than serum CC16, as suggested by results in BAL from previous studies (23).
Conclusions
Our findings indicate low CC16 mRNA expression levels in BECs are associated with asthma susceptibility, severity and exacerbations, low lung function, and high systemic corticosteroids use. Although CC16 mRNA expression levels in BECs correlated with increased expression of Th2 genes and high T2 inflammation biomarkers, the association of CC16 with asthma severity was confirmed after adjustment for T2 pathway genes. Thus, CC16 is a potential nontraditional T2 biomarker for asthma development and progression.
Acknowledgments
Acknowledgment
The authors acknowledge all investigators, staff, and participants in the SARP studies. The following companies provided financial support for study activities at the Coordinating and Clinical Centers beyond the third year of patient follow-up: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi–Genzyme–Regeneron, and TEVA.
Footnotes
Supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grants HL142769 and AI135108. SARP cross-sectional cohort (stage 1 and 2; SARP1-2) was supported by NIH grants HL69116, HL69130, HL69149, HL69155, HL69167, HL69170, HL69174, HL69349, UL1RR024992, M01RR018390, M01RR07122, M01RR03186, HL087665, and HL091762. SARP longitudinal cohort (stage 3; SARP3) was funded by the NHLBI U10 HL109172, HL109168, HL109152, HL109257, HL109146, HL109250, HL109164, and HL109086, and the Clinical and Translational Science Awards (CTSA) Program UL1 TR001102, UL1 TR000427, UL1 TR001420, and UL1 TR002378. SARP longitudinal cohort (stage 4; SARP4) was funded by NIH HL146002. Genetic studies for SARP cross-sectional cohort were funded by NIH HL87665 and Go Grant RC2HL101487. This work is also supported by the NIH grant AI149754. The following companies provided financial support for study activities at the Coordinating and Clinical Centers beyond the third year of patient follow-up: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi–Genzyme–Regeneron, and TEVA. These companies had no role in study design or data analysis, and the only restriction on the funds was that they be used to support the SARP initiative.
Author Contributions: X.L., S.G., J.G.L., M.K., D.A.M., and E.R.B. designed the initial concept. X.L. and H.L. performed the statistical analysis. A.T.H., M.C., L.C.D., S.C.E., J.V.F., B.G., E.I., N.N.J., B.D.L., D.T.M., W.C.M., J.Z., N.K., S.E.W., P.G.W., and E.R.B. collected the data. X.L., S.G., J.G.L., H.L., D.A.M., and E.R.B. wrote the manuscript. All authors reviewed the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202206-1230OC on September 6, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Singh G, Katyal SL, Brown WE, Phillips S, Kennedy AL, Anthony J, et al. Amino-acid and cDNA nucleotide sequences of human Clara cell 10 kDa protein. Biochim Biophys Acta . 1988;950:329–337. doi: 10.1016/0167-4781(88)90129-7. [DOI] [PubMed] [Google Scholar]
- 2. Almuntashiri S, Zhu Y, Han Y, Wang X, Somanath PR, Zhang D. Club cell secreted protein CC16: potential applications in prognosis and therapy for pulmonary diseases. J Clin Med . 2020;9:4039. doi: 10.3390/jcm9124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann N Y Acad Sci . 2000;923:68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x. [DOI] [PubMed] [Google Scholar]
- 4. Jorens PG, Sibille Y, Goulding NJ, van Overveld FJ, Herman AG, Bossaert L, et al. Potential role of Clara cell protein, an endogenous phospholipase A2 inhibitor, in acute lung injury. Eur Respir J . 1995;8:1647–1653. doi: 10.1183/09031936.95.08101647. [DOI] [PubMed] [Google Scholar]
- 5. Hermans C, Petrek M, Kolek V, Weynand B, Pieters T, Lambert M, et al. Serum Clara cell protein (CC16), a marker of the integrity of the air-blood barrier in sarcoidosis. Eur Respir J . 2001;18:507–514. doi: 10.1183/09031936.01.99102601. [DOI] [PubMed] [Google Scholar]
- 6. Bernard A, Nickmilder M, Dumont X. Airway epithelium defects and risks of allergic diseases: multiple associations revealed by a biomarker study among adolescents. Am J Respir Crit Care Med . 2015;191:714–717. doi: 10.1164/rccm.201409-1748LE. [DOI] [PubMed] [Google Scholar]
- 7. Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med . 1999;159:646–678. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- 8. Guerra S, Halonen M, Vasquez MM, Spangenberg A, Stern DA, Morgan WJ, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med . 2015;3:613–620. doi: 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhai J, Insel M, Addison KJ, Stern DA, Pederson W, Dy A, et al. Club cell secretory protein deficiency leads to altered lung function. Am J Respir Crit Care Med . 2019;199:302–312. doi: 10.1164/rccm.201807-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rava M, Tares L, Lavi I, Barreiro E, Zock JP, Ferrer A, et al. Serum levels of Clara cell secretory protein, asthma, and lung function in the adult general population. J Allergy Clin Immunol . 2013;132:230–232. doi: 10.1016/j.jaci.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 11. Stockley RA, Halpin DMG, Celli BR, Singh D. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med . 2019;199:1195–1204. doi: 10.1164/rccm.201810-1860SO. [DOI] [PubMed] [Google Scholar]
- 12. Keene JD, Jacobson S, Kechris K, Kinney GL, Foreman MG, Doerschuk CM, et al. COPDGene and SPIROMICS Investigators ‡ Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med . 2017;195:473–481. doi: 10.1164/rccm.201607-1330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martinez FD. Serum concentrations of club cell secretory protein (Clara) and cancer mortality in adults: a population-based, prospective cohort study. Lancet Respir Med . 2013;1:779–785. doi: 10.1016/S2213-2600(13)70220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shijubo N, Itoh Y, Yamaguchi T, Shibuya Y, Morita Y, Hirasawa M, et al. Serum and BAL Clara cell 10 kDa protein (CC10) levels and CC10-positive bronchiolar cells are decreased in smokers. Eur Respir J . 1997;10:1108–1114. doi: 10.1183/09031936.97.10051108. [DOI] [PubMed] [Google Scholar]
- 15. Bernard AM, Roels HA, Buchet JP, Lauwerys RR. Serum Clara cell protein: an indicator of bronchial cell dysfunction caused by tobacco smoking. Environ Res . 1994;66:96–104. doi: 10.1006/enrs.1994.1047. [DOI] [PubMed] [Google Scholar]
- 16. Lam DC, Kwok HH, Yu WC, Ko FW, Tam CY, Lau AC, et al. CC16 levels correlate with cigarette smoke exposure in bronchial epithelial cells and with lung function decline in smokers. BMC Pulm Med . 2018;18:47. doi: 10.1186/s12890-018-0607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. ECLIPSE Investigators Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med . 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 18. Park HY, Churg A, Wright JL, Li Y, Tam S, Man SF, et al. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2013;188:1413–1419. doi: 10.1164/rccm.201305-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lomas DA, Silverman EK, Edwards LD, Miller BE, Coxson HO, Tal-Singer R, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort Thorax 2008. 63 1058 1063 18757456 [Google Scholar]
- 20. Zemans RL, Jacobson S, Keene J, Kechris K, Miller BE, Tal-Singer R, et al. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res . 2017;18:117. doi: 10.1186/s12931-017-0597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laucho-Contreras ME, Polverino F, Gupta K, Taylor KL, Kelly E, Pinto-Plata V, et al. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J . 2015;45:1544–1556. doi: 10.1183/09031936.00134214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Vyve T, Chanez P, Bernard A, Bousquet J, Godard P, Lauwerijs R, et al. Protein content in bronchoalveolar lavage fluid of patients with asthma and control subjects. J Allergy Clin Immunol . 1995;95:60–68. doi: 10.1016/s0091-6749(95)70153-2. [DOI] [PubMed] [Google Scholar]
- 23. Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martin RJ. Club cell secretory protein in serum and bronchoalveolar lavage of patients with asthma. J Allergy Clin Immunol . 2016;138:932–934.e1. doi: 10.1016/j.jaci.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shijubo N, Itoh Y, Yamaguchi T, Sugaya F, Hirasawa M, Yamada T, et al. Serum levels of Clara cell 10-kDa protein are decreased in patients with asthma. Lung . 1999;177:45–52. doi: 10.1007/pl00007626. [DOI] [PubMed] [Google Scholar]
- 25. Gioldassi XM, Papadimitriou H, Mikraki V, Karamanos NK. Clara cell secretory protein: determination of serum levels by an enzyme immunoassay and its importance as an indicator of bronchial asthma in children. J Pharm Biomed Anal . 2004;34:823–826. doi: 10.1016/S0731-7085(03)00570-3. [DOI] [PubMed] [Google Scholar]
- 26. Ma YN, Wang J, Lee YL, Ren WH, Lv XF, He QC, et al. Association of urine CC16 and lung function and asthma in Chinese children. Allergy Asthma Proc . 2015;36:59–64. doi: 10.2500/aap.2015.36.3853. [DOI] [PubMed] [Google Scholar]
- 27. Shijubo N, Itoh Y, Yamaguchi T, Imada A, Hirasawa M, Yamada T, et al. Clara cell protein-positive epithelial cells are reduced in small airways of asthmatics. Am J Respir Crit Care Med . 1999;160:930–933. doi: 10.1164/ajrccm.160.3.9803113. [DOI] [PubMed] [Google Scholar]
- 28. Li X, Guerra S, Li H, Ledford JG, Busse WW, Erzurum SC, et al. Genomic and transcriptomic analyses of CC16 as a biomarker for asthma. Am J Respir Crit Care Med . 2018;197:A1326. [Google Scholar]
- 29. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J . 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 30. Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program-3 Investigators Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med . 2017;195:302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the severe asthma research program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract . 2018;6:545–554.e4. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Christenson SA, Modena B, Li H, Busse WW, Castro M, et al. NHLBI Severe Asthma Research Program (SARP) Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. J Allergy Clin Immunol . 2021;147:894–909. doi: 10.1016/j.jaci.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. National Heart, Lung, Blood Institute’s Severe Asthma Research Program Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s severe asthma research program. J Allergy Clin Immunol . 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med . 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, et al. NHLBI Severe Asthma Research Program (SARP) Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute severe asthma research program. Am J Respir Crit Care Med . 2012;185:356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med . 2016;4:574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li X, Hastie AT, Peters MC, Hawkins GA, Phipatanakul W, Li H, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program (SARP) Networks Investigation of the relationship between IL-6 and type 2 biomarkers in patients with severe asthma. J Allergy Clin Immunol . 2020;145:430–433. doi: 10.1016/j.jaci.2019.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hawkins GA, Robinson MB, Hastie AT, Li X, Li H, Moore WC, et al. National Heart, Lung, and Blood Institute–sponsored Severe Asthma Research Program (SARP) The IL6R variation Asp(358)Ala is a potential modifier of lung function in subjects with asthma. J Allergy Clin Immunol . 2012;130:510–5.e1. doi: 10.1016/j.jaci.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol . 2013;132:72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hastie AT, Mauger DT, Denlinger LC, Coverstone A, Castro M, Erzurum S, et al. NHLBI SARP 3 Investigators Baseline sputum eosinophil + neutrophil subgroups’ clinical characteristics and longitudinal trajectories for NHLBI severe asthma research program (SARP 3) cohort. J Allergy Clin Immunol . 2020;146:222–226. doi: 10.1016/j.jaci.2020.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kasela S, Ortega VE, Martorella M, Garudadri S, Nguyen J, Ampleford E, et al. NHLBI SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium. Genetic and non-genetic factors affecting the expression of COVID-19-relevant genes in the large airway epithelium. Genome Med . 2021;13:66. doi: 10.1186/s13073-021-00866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li X, Hawkins GA, Moore WC, Hastie AT, Ampleford EJ, Milosevic J, et al. Expression of asthma susceptibility genes in bronchial epithelial cells and bronchial alveolar lavage in the severe asthma research program (SARP) cohort. J Asthma . 2016;53:775–782. doi: 10.3109/02770903.2016.1158268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li X, Hastie AT, Hawkins GA, Moore WC, Ampleford EJ, Milosevic J, et al. eQTL of bronchial epithelial cells and bronchial alveolar lavage deciphers GWAS-identified asthma genes. Allergy . 2015;70:1309–1318. doi: 10.1111/all.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Modena BD, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Wu W, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med . 2014;190:1363–1372. doi: 10.1164/rccm.201406-1099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Modena BD, Bleecker ER, Busse WW, Erzurum SC, Gaston BM, Jarjour NN, et al. Gene expression correlated with severe asthma characteristics reveals heterogeneous mechanisms of severe disease. Am J Respir Crit Care Med . 2017;195:1449–1463. doi: 10.1164/rccm.201607-1407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulze R. Meta-analysis: a comparison of approaches. Gottingen, Germany: Hogrefe & Huber; 2004. [Google Scholar]
- 47. Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res . 2016;44:W90-7. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhai J, Stern DA, Sherrill DL, Spangenberg AL, Wright AL, Morgan WJ, et al. Trajectories and early determinants of circulating CC16 from birth to age 32 years. Am J Respir Crit Care Med . 2018;198:267–270. doi: 10.1164/rccm.201712-2398LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goudarzi H, Kimura H, Kimura H, Makita H, Matsumoto M, Takei N, et al. Effects of obesity on CC16 and their potential role in overweight/obese asthma. Respir Res . 2022;23:174. doi: 10.1186/s12931-022-02038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen LC, Zhang Z, Myers AC, Huang SK. Cutting edge: altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10-kDa protein. J Immunol . 2001;167:3025–3028. doi: 10.4049/jimmunol.167.6.3025. [DOI] [PubMed] [Google Scholar]
- 51. Hung CH, Chen LC, Zhang Z, Chowdhury B, Lee WL, Plunkett B, et al. Regulation of TH2 responses by the pulmonary Clara cell secretory 10-kd protein. J Allergy Clin Immunol . 2004;114:664–670. doi: 10.1016/j.jaci.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 52. Johansson S, Wennergren G, Aberg N, Rudin A. Clara cell 16-kd protein downregulates T(H)2 differentiation of human naive neonatal T cells. J Allergy Clin Immunol . 2007;120:308–314. doi: 10.1016/j.jaci.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 53. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA . 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lane C, Knight D, Burgess S, Franklin P, Horak F, Legg J, et al. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax . 2004;59:757–760. doi: 10.1136/thx.2003.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol . 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johansson SL.2008. http://hdl.handle.net/2077/9679
- 57. Kim DK, Cho MH, Hersh CP, Lomas DA, Miller BE, Kong X, et al. ECLIPSE, ICGN, and COPDGene Investigators Genome-wide association analysis of blood biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2012;186:1238–1247. doi: 10.1164/rccm.201206-1013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Milne S, Li X, Hernandez Cordero AI, Yang CX, Cho MH, Beaty TH, et al. Protective effect of club cell secretory protein (CC-16) on COPD risk and progression: a Mendelian randomisation study. Thorax . 2020;75:934–943. doi: 10.1136/thoraxjnl-2019-214487. [DOI] [PubMed] [Google Scholar]
- 59. Laing IA, Goldblatt J, Eber E, Hayden CM, Rye PJ, Gibson NA, et al. A polymorphism of the CC16 gene is associated with an increased risk of asthma. J Med Genet . 1998;35:463–467. doi: 10.1136/jmg.35.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Laing IA, Hermans C, Bernard A, Burton PR, Goldblatt J, Le Souëf PN. Association between plasma CC16 levels, the A38G polymorphism, and asthma. Am J Respir Crit Care Med . 2000;161:124–127. doi: 10.1164/ajrccm.161.1.9904073. [DOI] [PubMed] [Google Scholar]
- 61. Cheng D, Di H, Xue Z, Zhen G. CC16 gene A38G polymorphism and susceptibility to asthma: an updated meta-analysis. Intern Med . 2015;54:155–162. doi: 10.2169/internalmedicine.54.2979. [DOI] [PubMed] [Google Scholar]
- 62. Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res . 2019;47:D1005–D1012. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhai J, Emond MJ, Spangenberg A, Stern DA, Vasquez MM, Blue EE, et al. Club cell secretory protein and lung function in children with cystic fibrosis J Cyst Fibros 2022;21:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Petersen H, Leng S, Belinsky SA, Miller BE, Tal-Singer R, Owen CA, et al. Low plasma CC16 levels in smokers are associated with a higher risk for chronic bronchitis. Eur Respir J . 2015;46:1501–1503. doi: 10.1183/13993003.00682-2015. [DOI] [PubMed] [Google Scholar]
- 65.Global strategy for asthma management and prevention (GINA) https://ginasthma.org [DOI] [PubMed]
- 66. Li X. Hot topic: precision medicine for asthma-has the time come? Curr Allergy Asthma Rep . 2019;19:45. doi: 10.1007/s11882-019-0881-3. [DOI] [PubMed] [Google Scholar]
- 67. Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med . 2018;18:91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hodge SJ, Hodge GL, Holmes M, Reynolds PN. Flow cytometric characterization of cell populations in bronchoalveolar lavage and bronchial brushings from patients with chronic obstructive pulmonary disease. Cytometry B Clin Cytom . 2004;61:27–34. doi: 10.1002/cyto.b.20020. [DOI] [PubMed] [Google Scholar]