Asthma is a common respiratory disease that chronically affects approximately 330 million people worldwide and 30 million people in the United States (1–3). Despite its high prevalence, asthma is underdiagnosed and undertreated, particularly among patients residing in lower-income communities (4). Most (approximately 96%) of the 250,000 annual asthma deaths occur in low-income countries in which access to health practitioners and effective treatments, particularly maintenance inhalers, is sparse (3, 5). Furthermore, even in high-income countries, racial and ethnic minorities and lower socioeconomic status households continue to disproportionally experience asthma morbidity and mortality (6–9).
Although new knowledge regarding the complex pathophysiology underpinning asthma has led to the recent development of novel therapeutics in severe asthma, most patients with mild/moderate asthma have experienced few, if any, improvements in exacerbation risk and symptom control recently (10–12). To make meaningful progress for most patients with asthma, improvement in access and adherence to the backbone of asthma treatment, an inhaled corticosteroid (ICS)-containing treatment, is required (13–15). Unfortunately, in the United States, high inhaler costs and poor maintenance ICS adherence remain widespread challenges (16–18). The reasons for these challenges are likely numerous, including continuous introduction by pharmaceutical companies of new and expensive inhalers, patient reluctance to use corticosteroid-containing medications when well, and the episodic nature of asthma itself, wherein patients often deem regular maintenance inhaler adherence simply unnecessary.
Rationale for New Inhaler Paradigms in Asthma
Beginning about 20 years ago, asthma researchers started evaluating new inhaler paradigms because of a desire to develop an effective inhaler approach that would better align with many patients’ real-world behaviors and preferences for reliever therapy rather than adherence to maintenance therapy (19). Furthermore, researchers began exploring new inhaler approaches partially because of trials that demonstrated reductions in severe exacerbations with reliever formoterol usage compared with short-acting β2 agonist (SABA) in patients on (20, 21) or not on (20) concomitant maintenance ICS-containing treatment.
Because of subsequent concerns about the risks of long-acting β-agonist (LABA)–only treatment, these studies were followed by studies with combination budesonide–formoterol as a reliever, on top of maintenance budesonide–formoterol (i.e., the first studies of single maintenance and reliever therapy, which is often referred to as SMART [Single Maintenance and Reliever Therapy] or MART [Maintenance and Reliever Therapy] were started). Because of the significant reduction in risk of severe exacerbations with SMART compared with the same or higher-dose ICS or ICS–LABA with reliever SABA (22), a similar concept of reliever-only combination ICS–formoterol usage was subsequently investigated in mild asthma (23–26). These studies demonstrated that reliever usage of ICS–formoterol led to a large reduction in severe exacerbations and emergency department visits/hospitalizations compared with SABA monotherapy (24, 26). Further studies have examined reliever ICS–SABA usage in patients with mild asthma, either in combination (27) or as two separate inhalers (28–30), and recently in patients with moderate–severe asthma (31).
The investigation of new inhaler approaches also followed numerous observational studies demonstrating that SABA overuse is both common and associated with excess asthma morbidity and mortality (32–41). Because β agonists lack antiinflammatory properties, high usage of SABAs alone does not ameliorate airway inflammation. Furthermore, on the basis of some (but not all) studies, even short-term regular use of a SABA without a concurrent ICS results in increased airway inflammation and increased airway hyperresponsiveness because of tachyphylaxis from airway β adrenoceptor downregulation (42–46). Historically, spikes in asthma mortality have been observed after the introduction of inhalers with high-dose adrenergic agents with low β2 selectivity, such as isoproterenol and fenoterol (47). Data from the Salmeterol Multicenter Asthma Research Trial demonstrated that salmeterol (a LABA), uncoupled to an ICS, is associated with an increased mortality risk (47, 48). This finding ultimately resulted in an FDA (U.S. Food and Drug Administration) boxed warning. However, notably, repeated large clinical trials have demonstrated the safety of LABAs when used in combination with an ICS (which has resulted in a clear statement from the FDA of ICS–LABA safety, whereas LABA monotherapy remains discouraged) (49, 50). The findings regarding the risk of β-agonist monotherapy serve in stark contrast to findings with ICS inhalers, in which underuse, rather than overuse, is associated with exacerbations and mortality (35, 36, 51, 52).
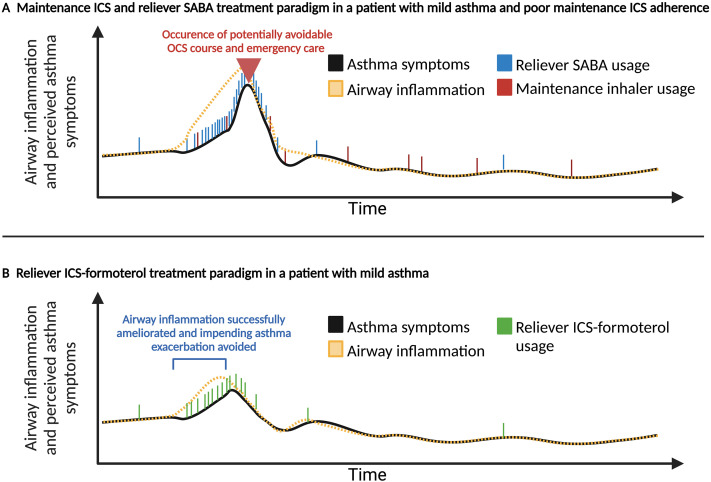
Notably, newly explored strategies that universally couple an ICS with a reliever β agonist are also believed to potentially combat the so-called SABA paradox in asthma (42). The SABA paradox recognizes that adherence to a SABA during episodes of poor asthma control is reinforced by quick symptomatic relief, whereas this behavioral reinforcement does not exist for maintenance ICS inhalers. Thus, during periods of high symptoms (with presumably increasing airway inflammation and risk of impending exacerbation), patients often increase their SABA usage but may or may not choose to use their ICS-containing inhaler (assuming they even have one), which does not ameliorate airway inflammation when theoretically most beneficial (Figure 1) (53–56).
Figure 1.
Hypothetical illustration of asthma symptoms and underlying airway inflammation in two different inhaler paradigms in a representative patient who is nonadherent to maintenance inhaled corticosteroid (ICS)-containing inhaler therapy. (A) The hypothetical first patient has suboptimal maintenance ICS adherence (red dash) throughout time and relies on increasing SABA usage (blue dash) when symptomatic, whereas (B) the second patient is using a reliever inhaler that contains an ICS and formoterol (green dash). As asthma symptoms (black line) and underlying airway inflammation (dashed yellow line) increase, hypothetical patient B uses increasing amounts of ICS (contained in the reliever inhaler) and is able to avert an impending serious asthma exacerbation requiring oral corticosteroids by treatment of airway inflammation. Illustration partially adapted for this manuscript from Larson and colleagues (141). OCS = oral corticosteroid; SABA = short-acting β2 agonist.
Formoterol Is a Unique Long-acting β agonist That Allows for Reliever Usage
When exploring options to combine an ICS with a β agonist for symptom relief, a fundamental requirement is that the β agonist component quickly relieves symptoms. One option to achieve this goal is to instruct patients to actuate a separate ICS inhaler whenever using their reliever SABA. This option, however, requires patients to fill, carry, then actuate two separate inhalers when symptomatic. Combining an ICS with albuterol in a single reliever device is being increasingly explored (31). However, the option that has been most explored involves using formoterol in combination with an ICS as a reliever. Formoterol is a unique LABA that has an onset of action that is similar to albuterol itself (57, 58), provides a longer duration of action, and, perhaps most notably, allows for the potential simplicity of using the same inhaler for both maintenance and reliever usage (i.e., SMART) if indicated (59).
When considering updated asthma approaches, providers should carefully note formoterol’s placement in them. Every study of SMART has used ICS–formoterol, which also has a dose–response relationship that safely allows for repeated inhalations if necessary (60, 61). Other non–formoterol-containing ICS–LABAs have different pharmacologic and posologic properties and have not been explored with SMART. All but one study (61) with as-needed ICS–formoterol has used budesonide–formoterol. A mometasone–formoterol combination is commercially available in the United States; although data about the safety and efficacy of its use in SMART are needed (59, 62), the potential use of mometasone–formoterol in SMART is supported by indirect evidence from the results of studies using budesonide–formoterol or beclomethasone–formoterol.
Reliever-only Use of ICS–Formoterol in Mild Asthma
In adults and adolescents with mild asthma, large randomized controlled trials have shown that using low-dose budesonide–formoterol as a reliever-only treatment reduces the occurrence of severe exacerbations by almost two-thirds compared with SABA monotherapy and reduces severe exacerbations requiring emergency department visits or hospitalizations by one-third compared with traditional maintenance ICS plus reliever SABA therapy (23–28, 63, 64) (Table 1). In the large SYGMA-1 (Symbicort Given as Needed in Mild Asthma) trial, among patients previously taking an ICS or leukotriene receptor antagonist, reliever use of budesonide–formoterol inhaler resulted in 63% fewer severe asthma exacerbations (rate ratio 0.37, 95% confidence interval [CI], 0.25–0.54) than reliever SABA use alone, with a number needed to treat to prevent a severe exacerbation of only 13 (65). Finally, in two pragmatic randomized controlled trials (PRACTICAL [PeRsonalised Asthma Combination Therapy with Inhaled Corticosteroid And fast-onset Long-acting β agonist] and Novel START [Novel Symbicort Turbuhaler Asthma Reliever Therapy]), a reliever-only budesonide–formoterol treatment approach was more beneficial than traditional maintenance ICS plus reliever SABA at preventing severe asthma exacerbations (23, 26).
Table 1.
Representative Randomized Controlled Trials in Mild Asthma of Reliever Inhaled Corticosteroid–Formoterol Therapy versus Traditional Maintenance Inhaled Corticosteroid + Reliever Short-acting β2-agonist Therapy versus Reliever Short-acting β2-agonist Alone Therapy
| Trial Name | Trial Design | Primary Inclusion Criteria | Participants (n) | Treatment Arms | Primary Outcome | Conclusions |
|---|---|---|---|---|---|---|
| SYGMA 1 (NEJM 2018) (24) | Double-blinded, multicenter, parallel-group RCT over 52 wk | ⩾12 yr old, mild asthma, deemed to warrant GINA 2012 step 2 therapy; two strata: asthma uncontrolled on SABA alone or controlled on ICS or LTRA | 3,849 | 1. Twice-daily placebo + reliever SABA 2. Twice-daily placebo + reliever budesonide–formoterol (200/6 μg)* 3. Twice-daily budesonide (200 μg) + reliever SABA |
Weeks with well-controlled asthma: 1. Reliever budesonide–formoterol vs. reliever SABA alone (34.4% vs. 31.1% of wk; OR, 1.14; 95% CI, 1.00–1.30; P = 0.046) 2. Reliever budesonide–formoterol vs. twice-daily budesonide + reliever SABA (34.4% vs. 44.4% of wk; OR, 0.64; 95% CI, 0.57–0.73) |
1. On the basis of weeks with asthma control, reliever budesonide–formoterol was superior to reliever SABA alone but inferior to twice-daily budesonide + reliever SABA therapy. 2. On the basis of the rate of severe exacerbations, reliever budesonide–formoterol was superior to reliever SABA alone but equivalent to maintenance budesonide + reliever SABA therapy. 3. Reliever budesonide–formoterol had lower ICS exposure than maintenance budesonide + reliever SABA therapy. |
| SYGMA 2 (NEJM 2018) (25) | Double-blinded, multicenter, parallel-group RCT over 52 wk | ⩾12 yr old, mild asthma, deemed to warrant GINA 2012 step 2 therapy; two strata: asthma uncontrolled on SABA alone or controlled on ICS or LTRA | 4,215 | 1. Twice-daily placebo + reliever budesonide–formoterol (200/6 μg) only* 2. Twice-daily budesonide (200 μg) + reliever SABA |
Annualized rate of severe exacerbations: Reliever budesonide–formoterol vs. twice-daily budesonide + reliever SABA (0.11 vs. 0.12 severe exacerbations/yr; rate ratio, 0.97; upper one-sided 95% confidence limit, 1.16) |
1. On the basis of the rate of severe exacerbations, reliever budesonide–formoterol was noninferior to maintenance budesonide + reliever SABA. 2. Reliever budesonide–formoterol had lower ICS exposure than maintenance budesonide + reliever SABA therapy. |
| PRACTICAL (Lancet 2019) (23) | Open-label, pragmatic, multicenter, parallel-group RCT over 52 wk | 18–75 yr old, provider-diagnosed asthma, prescribed reliever SABA ± low–moderate maintenance ICS; deemed to warrant GINA 2014 step 2 therapy | 885 | 1. Reliever budesonide–formoterol (200/6 μg) only* 2. Twice-daily budesonide (200 μg) + reliever SABA |
Annualized rate of severe exacerbations: Reliever budesonide–formoterol vs. twice-daily budesonide + reliever SABA (0.12 vs. 0.17 severe exacerbations/yr; relative rate, 0.69; 95% CI, 0.48–1.00; P = 0.049) |
1. In an open-label pragmatic trial, on the basis of the rate of severe exacerbations, reliever budesonide–formoterol only was superior to maintenance budesonide + reliever SABA therapy. 2. Reliever budesonide–formoterol had lower ICS exposure than maintenance budesonide + SABA therapy. |
| Novel START (NEJM 2019) (26) | Open-label, pragmatic, multicenter, parallel-group RCT over 52 wk | 18–75 yr old, provider-diagnosed asthma, prescribed as-needed SABA; deemed to warrant GINA 2014 step 2 therapy | 668 | 1. Twice-daily placebo + reliever SABA 2. Twice-daily placebo + reliever budesonide–formoterol (200/6 μg)* 3. Twice-daily budesonide (200 μg) + reliever SABA |
Annualized rate of exacerbations: 1. Reliever budesonide–formoterol vs. reliever SABA alone (0.20 vs. 0.40 severe exacerbations/yr; relative rate, 0.49; 95% CI, 0.33–0.72) 2. Reliever budesonide–formoterol vs. twice-daily budesonide + reliever SABA (0.20 vs. 0.18 severe exacerbations/yr; relative rate, 1.12; 95% CI, 0.70–1.79) |
1. On the basis of the rate of exacerbations, reliever budesonide–formoterol was superior to SABA alone but equivalent to maintenance budesonide + reliever SABA therapy. 2. On the basis of the rate of severe exacerbations, as-needed budesonide–formoterol was superior to reliever SABA alone and maintenance budesonide + reliever SABA therapy. |
Definition of abbreviations: CI = confidence interval; GINA = Global Strategy for Asthma Management and Prevention; ICS = inhaled corticosteroid inhaler; LTRA = leukotriene receptor antagonist; Novel START = Novel Symbicort Turbuhaler Asthma Reliever Therapy; OR = odds ratio; PRACTICAL = PeRsonalised Asthma Combination Therapy with Inhaled Corticosteroid And fast-onset Long-acting β agonist; RCT = randomized controlled trial; SABA = short-acting β2 agonist; SYGMA = Symbicort Given as Needed in Mild Asthma.
Throughout multiple RCTs totaling approximately 10,000 adolescents and adults with asthma, reliever use of an ICS–formoterol has shown superiority to reliever SABA monotherapy and equivalence (or superiority) with traditional maintenance ICS + reliever SABA therapy.
The 200/6 μg budesonide–formoterol dose used in these trials corresponds to a 160/4.5 μg delivered dose (mass of drug emitted per actuation that is available for inhalation at the mouth); however, each of these trials used a dry powder inhaler that is unavailable in the United States.
In mild asthma, a reliever-only ICS–formoterol approach leads to lower cumulative inhaled corticosteroid exposure than traditional maintenance ICS plus reliever SABA therapy. Nevertheless, using the reliever-only budesonide–formoterol approach (as compared with maintenance ICS and reliever SABA) may lead to more day-to-day asthma symptoms for some patients. However, the difference in asthma symptoms between these approaches on the basis of the ACQ-5 (Asthma Control Questionnaire) was only 0.15 (minimal clinically important difference of the ACQ-5 is 0.50); furthermore, in clinical practice, unlike in the SYGMA studies (24, 25), patients can use ICS–formoterol prophylactically, including before exercise, to prevent symptoms from occurring (63).
SMART in Moderate/Severe Asthma
In moderate/severe asthma, a SMART approach instructs patients to use a combination ICS–formoterol inhaler on a maintenance and reliever basis. One should note that SMART is thus different from the aforementioned concept of reliever-only usage of ICS–formoterol in mild asthma. SMART has shown clear superiority to traditional inhaler management paradigms in numerous randomized trials enrolling more than 20,000 patients over the last 20 years (Table 2) (22, 54, 66–75). A recent meta-analysis showed that simply switching a patient with uncontrolled moderate/severe asthma to SMART (as opposed to stepping up maintenance ICS dosage) resulted in a prolonged time to first severe exacerbation and an approximately 30% lower risk of a future severe exacerbation (hazard ratio, 0.71; 95% CI, 0.52–0.97) (76). Notably, most studies of SMART (and all large studies of reliever-only budesonide–formoterol in mild asthma) used a dry-powder delivery device rather than a U.S.-available metered dose inhaler (MDI), which can affect the airway delivery of active medication (77, 78). However, the safety and efficacy of SMART have also been demonstrated with U.S.-available budesonide–formoterol 160/4.5 μg MDI in one study (70). Although nearly all of the evidence supporting the benefits of SMART only enrolled patients at least 12 years old (60), SMART with ultra-low–dose budesonide–formoterol (80/4.5 μg one puff daily and for symptoms) was investigated in children (age 4–11 yr) and significantly reduced the rate of exacerbations in this population (79).
Table 2.
Representative Randomized Controlled Trials in Moderate-to-Severe Asthma of Maintenance Therapy + Reliever Inhaled Corticosteroid–β-agonist Therapy versus Maintenance Therapy + Reliever β2-agonist Therapy
| Trial Name | Trial Design | Primary Inclusion Criteria | Participants (n) | Treatment Arms | Primary Outcome | Conclusion(s) |
|---|---|---|---|---|---|---|
| SMILE (Lancet 2006) (66) | Double-blinded, multicenter, parallel-group RCT over 12 mo | ⩾12 yr old with asthma and ⩾1 exacerbation in the prior yr, pre-BD FEV1 50–100% with ⩾12% reversibility, received ICS for 3 mo before entry yet remained symptomatic | 3,394 | Budesonide–formoterol (160/4.5 μg) twice-daily + reliever: 1. SABA 2. Formoterol (4.5 μg), or 3. Budesonide–formoterol (160/4.5 μg) |
Time to first severe exacerbation: SMART therapy with reliever budesonide–formoterol resulted in a longer time to first severe exacerbation than twice-daily budesonide–formoterol and reliever SABA (P = 0.005; log-rank test) or reliever formoterol (P = 0.005) |
1. On the basis of time to first exacerbation, SMART therapy was superior to maintenance budesonide–formoterol with reliever SABA or reliever formoterol. 2. SMART therapy with reliever budesonide–formoterol resulted in a 48% reduction in the rate of severe exacerbations compared with the same maintenance therapy with reliever SABA usage. |
| COMPASS (IJCP 2007) (67) | Double-blinded, multicenter, parallel-group RCT over 6 mo | ⩾12 yr old with asthma and ⩾1 exacerbation in the prior yr, pre-BD FEV1 50–100% with ⩾12% reversibility, received ICS for 3 mo before entry yet remained symptomatic | 3,335 | 1. SMART therapy with maintenance budesonide–formoterol (160/4.5 μg) twice daily and as a reliever 2. Budesonide–formoterol (320/9 μg) twice daily and reliever SABA 3. Fluticasone–salmeterol (125/25 μg) twice daily and reliever SABA |
Time to first severe exacerbation: SMART resulted in a longer time to first severe exacerbation than twice-daily budesonide–formoterol and reliever SABA (P = 0.02; log-rank test) or twice-daily fluticasone/salmeterol and reliever SABA (P = 0.003) |
1. On the basis of time to first exacerbation, SMART therapy was superior to maintenance budesonide–formoterol and reliever SABA or fluticasone/salmeterol and reliever SABA. 2. SMART therapy resulted in a 28% reduction (rate ratio, 0.72; 95% CI, 0.57–0.90) and 39% (rate ratio, 0.61; 95% CI, 0.49–0.76) reduction in the rate of severe exacerbations compared with maintenance budesonide–formoterol with reliever SABA or fluticasone–salmeterol with reliever SABA. |
| PREPARE (NEJM 2021) (68, 69) | Open-label, pragmatic, multicenter, parallel-group RCT for 52 wk | 18–75 yr old, self-identified as Black or Latinx, clinician-diagnosed asthma, prescribed ICS ± LABA, uncontrolled on the basis of ACT score | 1,201 | Usual clinical care with maintenance ICS ± LABA and reliever: 1. SABA, or 2. SABA + beclomethasone (80 μg) if using MDI or SABA + beclomethasone (400 μg) if using a nebulizer |
Annualized rate of severe exacerbations: As-needed SABA + beclomethasone resulted in a decreased rate of severe exacerbations vs. SABA alone (0.82 vs. 0.69 severe exacerbations/yr; hazard ratio, 0.85; 95% CI, 0.72–1.00; P = 0.048) |
In an open-label trial in Black and Latinx adults, on the severe exacerbation rate, the addition of an as-needed ICS to usual clinician care was superior to reliever SABA alone. |
| MANDALA (NEJM 2022) (70) | Double-blinded, multicenter, parallel-group RCT over 24 wk | ⩾4 yr old with either a diagnosis of asthma and ⩾1 exacerbation in the prior yr, pre-BD FEV1% 40–90% with ⩾12% reversibility, prescribed medium-to-high dose ICS ± LABA, uncontrolled on the basis of ACQ score | 3,132 | Usual clinical care with maintenance ICS ± LABA and reliever: 1. SABA (albuterol 180 μg) 2. Budesonide–albuterol (80/180 μg)* 3. Budesonide–albuterol (160/180 μg) |
First event of severe exacerbation: Reliever use of high-dose budesonide–albuterol resulted in a decreased rate of severe exacerbations (hazard ratio, 0.74, 95% CI, 0.62–0.89) as compared with albuterol alone, but low-dose budesonide/albuterol did not (hazard ratio, 0.84; 95% CI, 0.71–1.00; P = 0.052) |
Reliever budesonide–albuterol (160/180 μg) resulted in a lower rate of severe exacerbations than as-needed albuterol alone. |
Definition of abbreviations: BD = bronchodilator; CI = confidence interval; COMPASS = effect of budesonide–formoterol maintenance and reliever therapy on asthma exacerbation; ICS = inhaled corticosteroid; LABA = long-acting β2 agonist; MANDALA = as-needed albuterol/budesonide versus albuterol in adults and children aged at least 4 years with moderate-to-severe asthma; MDI = metered dose inhaler; OR = odds ratio; PREPARE = PeRson EmPowered Asthma Relief study; RCT = randomized controlled trial; SABA = short-acting β2 agonist; SMART = Single Maintenance and Reliever Therapy; SMILE = effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomized controlled, double-blind study.
Throughout multiple randomized controlled trials, the reliever use of an ICS with formoterol or albuterol has shown superiority to similar maintenance therapy with reliever SABA therapy. The above represents only a small sample of these results and is not inclusive of all studies of a SMART approach. Systematic reviews and meta-analyses of SMART can be found by Cates and colleagues (142) and Sobieraj and colleagues (22). Notably, the PREPARE and MANDALA trials are not considered SMART but are shown to demonstrate data regarding the result of concomitant ICS and SABA reliever usage when prescribed with a separate maintenance inhaler.
Only low-dose budesonide–albuterol was examined in children under 12 years old.
Asthma Recommendations Call for Preferential Usage of Reliever ICS–Formoterol Inhalers in Mild Asthma (GINA [Global Initiative for Asthma]) and Usage of SMART in Moderate/Severe Asthma (GINA and NAEPP [National Asthma Education and Prevention Program])
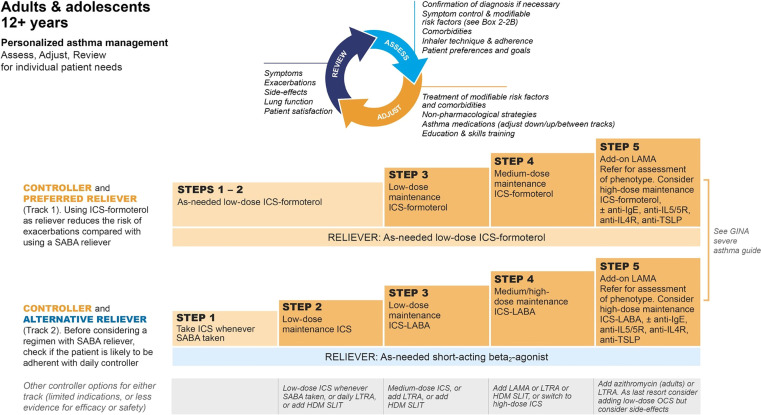
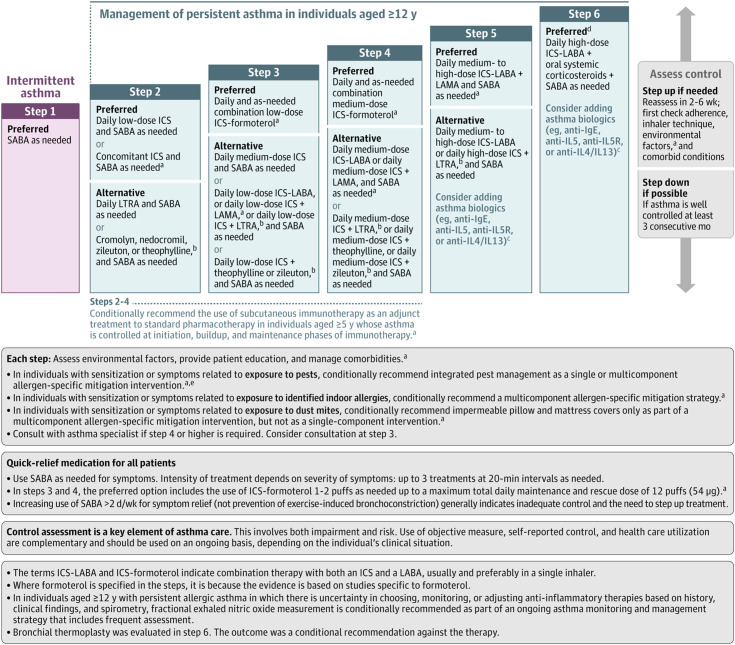
Recognizing new data, committees from both GINA and the NAEPP recently updated their recommendations by deemphasizing reliever β-agonist usage without concomitant ICS (80–82). GINA has moved more aggressively than the NAEPP by preferentially recommending reliever ICS–formoterol usage at steps one and two of therapy, with the addition of maintenance ICS–formoterol SMART in step three, then higher maintenance doses for steps four and five (Figure 2) (62). In its latest iteration, the NAEPP 2020 update did not examine the evidence for reliever-only ICS–formoterol usage or unopposed reliever SABA use at step one of therapy, and so its recommendations at steps one and two were retained in the most recent update (62, 64). However, the NAEPP now alternatively recommends low-dose ICS accompany reliever SABA use at step two and escalating SMART as the preferred option at steps three and four (Figure 3).
Figure 2.
GINA (Global Initiative for Asthma) 2022 recommendations for the management of individuals at least 12 years old with asthma. GINA 2022, reprinted with permission. Available from www.ginasthma.org. HDM = house dust mite; ICS = inhaled corticosteroid; LABA = long-acting β2 agonist; LAMA = long-acting muscarinic antagonist; LTRA = leukotriene receptor antagonist; OCS = oral corticosteroids; SABA = short-acting β2 agonist; SLIT = sublingual immunotherapy; TSLP = thymic stromal lymphopoietin.
Figure 3.
NAEPP (National Asthma Education and Prevention Program) 2020 recommendations for the management of individuals at least 12 years old with asthma. Note that the NAEPP did not address step one or step six management in their update published in 2020 because of prespecified objectives. aNew recommendation on the basis of the 2020 Asthma Guideline Update. bCromolyn, nedocromil, leukotriene receptor antagonists, inhibitors of 5-lipoxygenase (including zileuton and montelukast), and theophylline were not considered for the update. These have limited availability for use in the United States and/or have an increased risk of adverse consequences and need for monitoring that make their use less desirable. The U.S. Food and Drug Administration issued a boxed warning for montelukast in March 2020 because of adverse effects related to serious behavior- and mood-related changes. cThe AHRQ (Agency for Healthcare Research and Quality) systematic reviews that informed the update did not include studies that examined the role of asthma biologics (anti-IgE, anti–IL-5, anti–IL-5R, and anti–IL-4/IL-13). Thus, this report does not contain specific recommendations for the use of biologics in asthma in steps five and six. dData on the use of long-acting muscarinic antagonist therapy in individuals with severe persistent asthma (step six) were not included in the AHRQ systematic review; thus, no recommendations were made. ePests refers to mice and cockroaches, which were specifically examined in the AHRQ systematic review. Figure reproduced with written permission from Cloutier and colleagues (83). ICS = inhaled corticosteroid; LABA = long-acting β2 agonist; LAMA = long-acting muscarinic antagonist; LTRA = leukotriene receptor antagonist; SABA = short-acting β2 agonist.
Although differences between recommendations are highlighted, the GINA and NAEPP recommendations are more similar than discordant. Both committees have rethought inhaler recommendations in asthma altogether while clearly advocating for SMART. Importantly, the NAEPP committee’s objectives were set before most of the reliever ICS–formoterol efficacy data in mild asthma emerged (80, 83, 84). GINA’s decision to recommend against SABA-only therapy in step one is on the basis of evidence of ICS–formoterol efficacy in patients with symptoms as infrequently as twice per month and because starting treatment with SABA may behaviorally train patients to regard it as their main asthma therapy (26, 62, 85).
A Potential Role for Implementation Science
Unfortunately, establishing scientific evidence of efficacy and effectiveness is insufficient to ensure subsequent real-world adoption of interventions (86). One classic example of this reality is the delayed adoption of β-blocker usage in heart failure management. Observations that β blockers improve mortality in heart failure were featured in prominent medical literature dating back to 1979 (87–90). Yet, the FDA did not approve the first β blocker for this indication, carvedilol, until 1997 (91). Moreover, the routine prescription of β blockers for heart failure was still below 40% in 2001 (92, 93). Undoubtedly, a missed opportunity for tangible public health benefits occurred during this 20-year evidence-to-practice gap. The field of implementation science was created to narrow these gaps. Recognizing its importance over the past 2 decades, implementation science has received ever-increasing attention from the NIH (National Institutes of Health) and professional societies, including the ATS (American Thoracic Society) (94, 95).
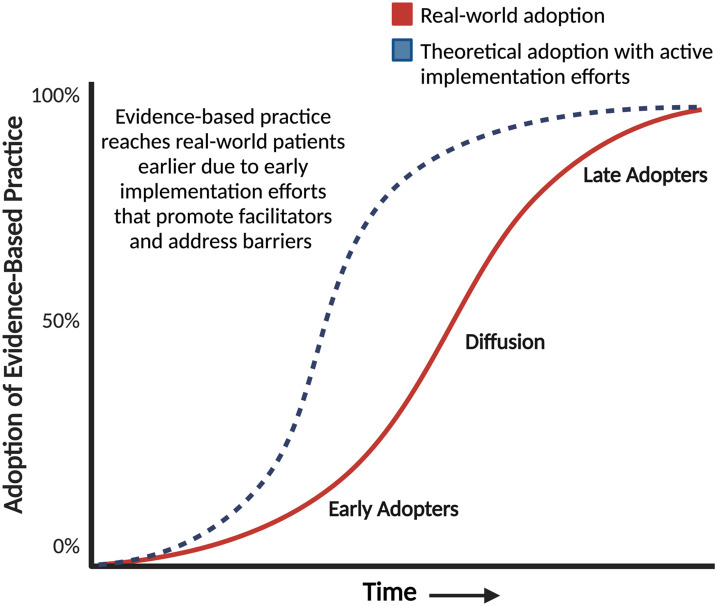
Implementation science attempts to understand the complex variables that cause an evidence-based intervention to be used, or frequently not used, in the real world. The evidence for implementation comes in multiple forms and is complex and dynamic (96). In collaboration with multiple stakeholders, implementation scientists elucidate facilitators and barriers to real-world adoption of evidence-based practices using implementation science frameworks, theories, and models (86). Implementation strategies are then developed to disseminate recommendations and implement evidence-based practices (97). Grounded in part in Diffusion Theory, implementation science is deliberate and proactive in contrast to the passive dissemination of scientific discoveries and guideline recommendations that theoretically follow a slow curvilinear diffusion process (Figure 4) (98).
Figure 4.
Theoretical curvilinear process of the diffusion of innovations. Innovations often spread following an S-shaped curvilinear process wherein a select few adopt an intervention early (early adopters). This is followed by slow diffusion that happens with word-of-mouth communication and positive experiences reinforcing this communication and, finally, adoption by late adopters. Active implementation attempts to understand what facilitators and barriers exist in the real world to shift and expedite the uptake of an evidence-based intervention (i.e., transition the adoption of a practice from the solid red line to the dotted blue line). Figure adapted from Diffusion of Innovation (98).
Significant Real-world Barriers to the Adoption of New Inhaler Approaches Exist
Despite the potential for public health benefits, reliever usage of ICS–formoterol inhalers in asthma is infrequent in the United States, particularly in primary care and emergency department practice, which is where most patients with asthma receive care (99). We postulate on the multiple barriers to the use of reliever ICS–formoterol inhalers and opportunities for implementation research in (Table 3) (59, 77, 80, 100).
Table 3.
Potential Real-world Barriers to the Implementation of Reliever Inhaled Corticosteroid–Formoterol Usage in the United States and Need for Future Research and Action
| Potential Barrier to Implementation of Reliever ICS–Formoterol Inhalers in the United States | Explanation of Potential Barriers and Knowledge Gaps | Future Work Needed |
|---|---|---|
| Provider knowledge | • At least 60% of chronic asthma care occurs in primary care settings. • Specialist and primary care clinician knowledge of the logistics of a reliever-only ICS-formoterol approach and SMART is largely unknown. |
• Better understanding of successful knowledge dissemination strategies in primary care and specialty settings. |
| Discordance between guidelines | • Important differences exist between the GINA and NAEPP recommendations. • The role this discordance plays in a provider’s decision to use ICS–formoterol inhalers on a reliever-only basis is unclear. |
• Dissemination of GINA and NAEPP recommendations. • Expeditious update to NAEPP 2020 update that includes management recommendations at steps one and six. • Consider a more dynamic way to continuously update guidelines, given the increasing rate that evidence emerges. |
| Medico–legal and regulatory issues | • Clinical trials of new inhaler approach are primarily on the basis of dry-powder budesonide–formoterol (not available in the United States). • FDA-labeling of budesonide–formoterol does not include its use as a reliever inhaler. |
• Explore the extent to which these factors influence clinician decision-making. • Encourage FDA approval of budesonide–formoterol for reliever use. |
| Pharmaceutical coverage concerns and cost | • Pharmacy benefits and formulary practices may preferentially cover ICS–LABA inhalers that do not include formoterol and cannot be used as a reliever device and with SMART. • Annual formulary changes may promote the substitution of formoterol-containing ICS–LABAs for non–formoterol-containing combinations. |
• Comparative cost-effectiveness analyses of reliever ICS–formoterol therapy and SMART therapy as compared with traditional management. • Advocate for classifying rapid-onset and non–rapid-onset LABAs into separate pharmacological categories. |
| Patient preference for reliever nebulizers* | • Real-world usage of nebulizers for symptom relief is common in asthma. In patients who prefer nebulizers, instructions on how to or if we should use new inhaler approaches are not clear. | • Clarify what recommendations should be made to patients who prefer nebulizers when using the latest asthma recommendations/guidelines. |
| Integration into asthma action plans | • The use of asthma action plans is recommended. Historically, asthma action plans have not incorporated reliever ICS–formoterol usage or SMART. | • Develop, test, and disseminate model asthma action plans that use reliever-only ICS–formoterol and SMART. |
| Lack of incentives to move toward SMART | • Health systems and payers may not recognize the opportunity to reduce rates of severe asthma exacerbations with SMART. | • Study the effects of health plan or health system interventions to promote the uptake of SMART. • Examine the effects of SMART usage on preventable emergency care, hospitalization, and costs of asthma care on a system level. |
Definition of abbreviations: FDA = U.S. Food and Drug Administration; GINA = Global Initiative for Asthma; ICS = inhaled corticosteroid; LABA = long-acting β2 agonist; NAEPP = National Asthma Education and Prevention Program; SMART = Single Maintenance and Reliever Therapy.
We do not promote nebulizer usage of reliever asthma medications over inhaler usage as some studies have shown that nebulizers are associated with a higher risk of asthma morbidity (143). However, we do recognize that many real-world patients prefer nebulizers (68, 144). As such, in patients who clearly prefer nebulizer usage and in whom a provider is considering SMART or reliever-only ICS–formoterol therapy, optimal patient instructions should be explored and clarified.
Importantly, despite SMART being recommended by the U.S.-based NAEPP guidelines and approved by regulators in more than 120 countries, budesonide–formoterol inhalers are currently not FDA-labeled for reliever usage in the United States, which has cascading effects on prescription decisions (59, 101). Pharmaceutical formulary practices are a particular obstacle for SMART therapy (102). Prescription drug formularies outline the medications available to patients on the basis of their insurance plan; theoretically, formularies should also facilitate evidenced-based prescribing practices by selecting recommended medications for the lowest cost-sharing tier (103, 104). However, in the United States, formularies often consider all ICS–LABAs as interchangeable, and the choice of a preferred ICS–LABA frequently changes for a patient–insurance dyad (105). Consequently, if a clinical provider uses budesonide–formoterol in line with the latest evidence, the prescription may be substituted for another ICS–LABA combination that does not contain the quick-onset formoterol necessary for reliever usage. Furthermore, if a patient uses two puffs twice daily of budesonide–formoterol and the same inhaler on a reliever basis, they could run out of their 120-actuation inhaler before the end of the month when they are eligible for a refill.
These barriers, if not quickly addressed, delay the implementation of evidence-based practices in the United States. They also run counter to patient preferences. Patients with asthma have endorsed that the latest inhaler recommendations are more intuitive while also fostering a greater sense of self-efficacy (30, 106). Simply put, failure to think about implementation, promote facilitators, and address real-world barriers to the newest asthma recommendations will result in preventable asthma morbidity in the United States over the coming years.
A Bold Consideration to Expand ICS–Formoterol Access and Reduce Inhaler Cost: A Prescription (Rx)–to–Over-the-counter (OTC) Transition
As the asthma treatment paradigm shifts toward more widespread use of ICS–formoterol inhalers, ensuring that these inhalers are affordable and readily accessible will be critical to improving outcomes and asthma-related healthcare disparities. In the United States, changing budesonide–formoterol inhalers (e.g., budesonide–formoterol 160/4.5 μg MDI one puff for symptoms for adults/adolescents and 80/4.5 μg one puff for symptoms for children) from Rx-only access to OTC status (Rx-to-OTC) is one course of action that should be seriously considered and has the potential to decrease inhaler cost and decrease real-world usage of SABA monotherapy (107, 108). Although the pros and cons of an Rx-to-OTC transition for other inhalers could also be considered, we chose budesonide–formoterol as our exemplar because there are substantial clinical trial data supporting its safety and efficacy, which has led to it being the preferred reliever inhaler at all steps by GINA and two steps by NAEPP. Furthermore, budesonide–formoterol is now available as a generic option in the United States, and its use as an OTC medication would not lead to unopposed SABA use if used as a reliever. There is a substantial risk of any inhaler that contains a β agonist alone being available OTC because it facilitates real-world use of β-agonist monotherapy.
In the United States, the only currently approved OTC inhaler for asthma contains a β agonist alone (inhaled epinephrine; marketed as Primatene MIST). The recent reapproval of this epinephrine inhaler as an OTC device occurred despite concerns from the ATS and other health organizations (109). Proponents of this move noted that the OTC status of this inhaler made “access to [a] life-saving medication…affordable, convenient, and [provided] a safety net for medically marginalized and underinsured populations” (109). Given the association of uncoupled β-agonist use with increased mortality (particularly for those like epinephrine that have low β2 selectivity) (110) and the greater safety and efficacy data surrounding reliever use of ICS–formoterol, continuing to provide aerosolized epinephrine as a safety net for patients with poor access to medications is arguably unethical (111, 112).
Why is inhaled epinephrine the only reliever medication available OTC in the United States, whereas superior inhalers are not available OTC? The answer is partially a historical accident because of inhaled epinephrine’s marketing before modern FDA regulatory oversight (113). However, this unique designation is also likely attributable to pharmaceutical companies’ financial self-interests (108). Amphastar Pharmaceuticals Inc. earned $73 million in net revenue from Primatene MIST sales in 2021 (114). This monetary reward may have driven Amphastar’s persistent effort to obtain FDA approval to be the only OTC asthma inhaler despite the rejection of its original application in 2014 (109). As large as Amphastar’s financial gains are, they are trivial compared with the potential financial losses other pharmaceutical companies could suffer if the billion-dollar market for current Rx-only inhalers were disrupted by an Rx-to-OTC switch (115).
Rx-to-OTC switches generally reduce drug prices, which would be critical if OTC placement of budesonide–formoterol were to improve medication access for patients with fewer financial resources (116). For example, the Rx-to-OTC switch of the second-generation antihistamine loratadine reduced its price by 99% in the early 2000s (117). Although currently stymied by legal processes, the FDA recently approved the first generic budesonide–formoterol inhaler (118–120). More generic budesonide–formoterol inhalers are likely to emerge, which may be hastened by OTC status and new competition (116). The current price of a budesonide–formoterol inhaler in the United States is $307 (121). The price of Primatene MIST is $29 (121, 122). Typically, branded medication prices fall 30% after the first generic competitor enters the market, 50% after the second, and 95% after the sixth (123). With adequate generic competition, it is not unreasonable to expect that the price of budesonide–formoterol could substantially decrease and make it a highly accessible OTC option in the future.
Neither the presence of a suboptimal alternative medication that has OTC labeling nor a desire to improve access to an evidence-based inhaler are sufficient reasons to advocate for a controversial Rx-to-OTC switch of budesonide–formoterol. Rx-to-OTC switches require careful and serious deliberation. Some studies have demonstrated that patients have the maximum symptom control, with a low exacerbation risk, when they maintain greater than 80% adherence to a maintenance ICS-containing regimen supplemented by infrequent reliever SABA use (124, 125). However, this ideal strategy depends on unrealistic assumptions about patient behavior. Decades of adherence research have demonstrated that high-maintenance inhaler adherence is not achievable in the real world except among a small group of highly motivated patients (126–129).
Another concern is that a potential Rx-to-OTC switch for a new inhaler would lead to patients’ inappropriate self-diagnoses and harmful self-management. However, provider control over inhaler prescriptions has not prevented over 25% of patients from using amounts of SABA associated with mortality (38). When prescribed a maintenance inhaler and SABA, patients can easily choose which Rx to fill, and clinicians cannot prevent patients from preferentially filling their SABA Rx. For this reason, prescribing separate maintenance and SABA reliever therapy is often illusory. If forward-thinking action were taken, making budesonide–formoterol inhalers the only OTC option in the United States, then theoretically, patients’ ICS exposure would increase, whereas SABA monotherapy use would decrease. An Rx-to-OTC switch of budesonide–formoterol is not a solution that would cure all issues. The point of this proposal is simply that an OTC ICS–formoterol inhaler is much safer than the current OTC option, may go a long way to improve overreliance on SABAs alone, may drive down inhaler cost, and would provide a more acceptable safety net for patients, which makes it worthy of serious consideration.
A Call to Action
We believe it is time to rethink how the typical patient with asthma is managed in the United States. Expediting the real-world adoption of reliever ICS–formoterol inhalers (either as a reliever-only option in mild asthma or as part of SMART) should be prioritized. Using proven implementation science methodologies, researchers should engage patients, clinicians, pharmacists, insurance companies, policymakers, and other stakeholders to discern the real-world facilitators and barriers to using reliever-only ICS–formoterol regimens and SMART. For example, templates for asthma action plans should be updated with critical feedback from clinicians and patients. This work has already been done in some countries but needs to be adapted for the United States and other locations (59). In addition, the FDA should relabel budesonide–formoterol as both a maintenance and reliever therapy in the United States now. This action would bring the U.S. labeling of budesonide–formoterol in line with more than 120 other countries and be concordant with recommendations from both GINA and the NIH-sponsored NAEPP guidelines.
The most obvious route for seeking new budesonide–formoterol labeling (as a reliever and for OTC dispensing) is by the initiation of the process from the drug manufacturers; however, if this is not in their financial interests, then other sponsors (e.g., third-party payers, patient advocacy organizations, or professional organizations such as ATS) could request labeling changes (130, 131). Furthermore, although rarely attempted, the FDA could force a switch through its regulatory powers. There are, of course, both benefits and risks to extrapolating data on the efficacy of dry-powder ICS–formoterol formulations to U.S.-specific formulations. If regulators believe more studies are needed with U.S.-specific ICS–formoterol devices before relabeling, then those studies should be organized and completed expeditiously. As it stands, our collective delay in relabeling ICS–formoterol inhalers as both a maintenance and reliever device is likely depriving patients of greater access to an evidence-based inhaler approach that would benefit many.
There is accumulating data on the use of a combination ICS–SABA as a reliever, but this should not delay the needed action on budesonide–formoterol. The recent MANDALA (as-needed albuterol/budesonide vs. albuterol in adults and children aged at least 4 years with moderate-to-severe asthma) trial demonstrated benefits of reliever budesonide–albuterol in moderate-to-severe asthma (31), and PREPARE (PeRson EmPowered Asthma Relief) showed the benefits of providing separate ICS for use whenever SABA is taken (68, 69). In November 2022, the FDA Pulmonary–Allergy Drugs Advisory Committee recommended approval of budesonide–albuterol for as-needed treatment or prevention of bronchoconstriction in adults but not for adolescents or children (132). Both the potential lack of FDA labeling for budesonide–albuterol as a reliever in adolescents and children, as well as the paucity of data on reliever-only budesonide–formoterol therapy and SMART in children under 12 years, should prompt pediatric-specific trials in these areas.
Although a potential combination ICS–SABA reliever is preferential to a SABA-only reliever, differences between budesonide–formoterol and budesonide–albuterol should be noted. First, in mild asthma, data for the use of reliever ICS–SABA therapy while not part of a strategy with a maintenance therapy is sparse, with only 235 adults and 174 children/adolescents having been randomized to this therapy to date (27–30, 133). Moreover, importantly, in moderate-to-severe persistent asthma, SMART (wherein the same inhaler can be used conveniently as both a maintenance and reliever device) is only currently possible with an ICS–formoterol device.
To stimulate this work, the ATS should play an active role, including the formation of a working group to tackle these proposals. We also believe an update to the NAEPP 2020 guidelines is urgently needed because of the emergence of significant new data since its last iteration (84). The most recent NAEPP guidelines are on the basis of studies published through October 2018, and do not include the four paradigm-shifting studies of reliever-only ICS–formoterol conducted in almost 10,000 adults and adolescents with mild asthma (23–26).
As we consider future work, we should acknowledge that there are significant healthcare disparities in asthma, and inhaler costs are too high (111). If we do not explicitly integrate a health equity lens into our upcoming efforts, we should expect our latest evidence-based inhaler approaches to reach patients from higher socioeconomic statuses first, whereas lower-income patients continue to use suboptimal inhaler paradigms. Prescription of biological therapeutics in asthma is already revealing uneven access, with providers more likely to prescribe these medications to higher-income White patients (134, 135). Frameworks for implementation efforts that explicitly consider equity outcomes exist and have been used in other fields (136–139).
Finally, all strategies to increase inhaler access while driving down cost, however bold, should be on the table. Changing ICS–formoterol inhalers to OTC should be treated as a serious option. At present, the official ATS position opposes making prescription-only inhalers, particularly SABAs, available OTC. Providing OTC options for inhalers was seriously considered by the FDA back in 2012 (140). However, a lot has changed since the last time the ATS and FDA considered an Rx-to-OTC change for inhalers. Today, a suboptimal epinephrine inhaler is available OTC, ICS-containing inhaler costs remain unrelentingly high, and new data has emerged on an efficacious reliever ICS–formoterol option. These facts should prompt a re-examination of the advantages and disadvantages of an Rx-to-OTC change for ICS–formoterol inhalers.
Conclusions
A new paradigm for treating patients with asthma is emerging wherein combination ICS–formoterol inhalers are simpler, more effective, and a safer alternative to SABA inhalers for reliever therapy. For the public to benefit from new inhaler approaches, it will be necessary to ensure these new approaches are accessible and affordable. We believe now is the time to take the next steps toward implementation while learning why clinicians are, or are not, following the latest asthma recommendations. One bold option to consider is transitioning budesonide–formoterol to OTC status. Powerful opposition to these proposed changes is likely to be encountered because of deontological imperatives within the medical profession as well as financial self-interests in the U.S. health system. Nonetheless, we currently have an opportunity to radically change how we treat asthma and reduce some of the preventable burdens our patients face.
Footnotes
Supported by grants from the American Lung Association (ALA) (Early Career Investigator Award) and the NIH (KL2-TR002346 [J.G.K.] and UL1-TR002345). The content is solely the responsibility of the authors and does not represent the official views of either the NIH or the ALA.
Author Contributions: J.G.K., J.K.G., M.C., and F.D.M. conceived this article, with M.C. and F.D.M. serving as senior authors. J.G.K. and J.K.G. wrote the first draft. All authors revised the draft critically for intellectual content and provided approval of the final manuscript version in its entirety.
Originally Published in Press as DOI: 10.1164/rccm.202209-1729PP on December 20, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet . 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pate CA, Zahran HS, Qin X, Johnson C, Hummelman E, Malilay J. Asthma surveillance—United States, 2006-2018. MMWR Surveill Summ . 2021;70:1–32. doi: 10.15585/mmwr.ss7005a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Enilari O, Sinha S. The global impact of asthma in adult populations. Ann Glob Health . 2019;85:2. doi: 10.5334/aogh.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz AA, Stelmach R, Ponte EV. Asthma prevalence and severity in low-resource communities. Curr Opin Allergy Clin Immunol . 2017;17:188–193. doi: 10.1097/ACI.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 5. Mortimer K, Reddel HK, Pitrez PM, Bateman ED. Asthma management in low and middle income countries: case for change. Eur Respir J . 2022;60:2103179. doi: 10.1183/13993003.03179-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris KM. Mapping inequality: childhood asthma and environmental injustice, a case study of St. Louis, Missouri. Soc Sci Med . 2019;230:91–110. doi: 10.1016/j.socscimed.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 7. Fitzpatrick AM, Gillespie SE, Mauger DT, Phillips BR, Bleecker ER, Israel E, et al. Racial disparities in asthma-related health care use in the National Heart, Lung, and Blood Institute’s severe asthma research program. J Allergy Clin Immunol . 2019;143:2052–2061. doi: 10.1016/j.jaci.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaffney AW, Hawks L, Bor D, White AC, Woolhandler S, McCormick D, et al. National trends and disparities in health care access and coverage among adults with asthma and COPD: 1997-2018. Chest . 2021;159:2173–2182. doi: 10.1016/j.chest.2021.01.035. [DOI] [PubMed] [Google Scholar]
- 9. Bose S, Madrigano J, Hansel NN. When health disparities hit home: redlining practices, air pollution, and asthma. Am J Respir Crit Care Med . 2022;206:803–804. doi: 10.1164/rccm.202206-1063ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sullivan PW, Ghushchyan V, Kavati A, Navaratnam P, Friedman HS, Ortiz B. Trends in asthma control, treatment, health care utilization, and expenditures among children in the United States by place of residence: 2003-2014. J Allergy Clin Immunol Pract . 2019;7:1835–1842.e1832. doi: 10.1016/j.jaip.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 11. Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol . 2020;42:5–15. doi: 10.1007/s00281-020-00785-1. [DOI] [PubMed] [Google Scholar]
- 12. Pennington E, Yaqoob ZJ, Al-Kindi SG, Zein J. Trends in asthma mortality in the United States: 1999 to 2015. Am J Respir Crit Care Med . 2019;199:1575–1577. doi: 10.1164/rccm.201810-1844LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Byrne P, Fabbri LM, Pavord ID, Papi A, Petruzzelli S, Lange P. Asthma progression and mortality: the role of inhaled corticosteroids. Eur Respir J . 2019;54:1900491. doi: 10.1183/13993003.00491-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dima AL, van Ganse E, Stadler G, de Bruin M, ASTRO-LAB group Does adherence to inhaled corticosteroids predict asthma-related outcomes over time? A cohort study. Eur Respir J . 2019;54:1900901. doi: 10.1183/13993003.00901-2019. [DOI] [PubMed] [Google Scholar]
- 15. Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J . 2015;45:396–407. doi: 10.1183/09031936.00075614. [DOI] [PubMed] [Google Scholar]
- 16. Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev . 2017;4:CD012226. doi: 10.1002/14651858.CD012226.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med . 2013;107:1481–1490. doi: 10.1016/j.rmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 18. Thomas A, Haddad I, Hoskere G. Trends in inhaler prescriptions and associated cost in the United States from 2014 to 2018: an analysis from the Medicare part D database. Cureus . 2021;13:e13498. doi: 10.7759/cureus.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaplan A, Mitchell PD, Cave AJ, Gagnon R, Foran V, Ellis AK. Effective asthma management: is it time to let the air out of SABA? J Clin Med . 2020;9:921. doi: 10.3390/jcm9040921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pauwels RA, Sears MR, Campbell M, Villasante C, Huang S, Lindh A, et al. RELIEF Study investigators Formoterol as relief medication in asthma: a worldwide safety and effectiveness trial. Eur Respir J . 2003;22:787–794. doi: 10.1183/09031936.03.00055803. [DOI] [PubMed] [Google Scholar]
- 21. Tattersfield AE, Löfdahl CG, Postma DS, Eivindson A, Schreurs AG, Rasidakis A, et al. Comparison of formoterol and terbutaline for as-needed treatment of asthma: a randomised trial. Lancet . 2001;357:257–261. doi: 10.1016/S0140-6736(00)03611-4. [DOI] [PubMed] [Google Scholar]
- 22. Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA . 2018;319:1485–1496. doi: 10.1001/jama.2018.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, et al. PRACTICAL study team Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet . 2019;394:919–928. doi: 10.1016/S0140-6736(19)31948-8. [DOI] [PubMed] [Google Scholar]
- 24. O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med . 2018;378:1865–1876. doi: 10.1056/NEJMoa1715274. [DOI] [PubMed] [Google Scholar]
- 25. Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med . 2018;378:1877–1887. doi: 10.1056/NEJMoa1715275. [DOI] [PubMed] [Google Scholar]
- 26. Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, et al. Novel START Study Team Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med . 2019;380:2020–2030. doi: 10.1056/NEJMoa1901963. [DOI] [PubMed] [Google Scholar]
- 27. Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, et al. BEST Study Group Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med . 2007;356:2040–2052. doi: 10.1056/NEJMoa063861. [DOI] [PubMed] [Google Scholar]
- 28. Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet . 2011;377:650–657. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA . 2012;308:987–997. doi: 10.1001/2012.jama.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sumino K, Bacharier LB, Taylor J, Chadwick-Mansker K, Curtis V, Nash A, et al. A pragmatic trial of symptom-based inhaled corticosteroid use in African-American children with mild asthma. J Allergy Clin Immunol Pract . 2020;8:176–185.e2. doi: 10.1016/j.jaip.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 31. Papi A, Chipps BE, Beasley R, Panettieri RA, Jr, Israel E, Cooper M, et al. Albuterol-budesonide fixed-dose combination rescue inhaler for asthma. N Engl J Med . 2022;386:2071–2083. doi: 10.1056/NEJMoa2203163. [DOI] [PubMed] [Google Scholar]
- 32. Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med . 2006;144:904–912. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- 33. Rodrigo GJ, Moral VP, Marcos LG, Castro-Rodriguez JA. Safety of regular use of long-acting beta agonists as monotherapy or added to inhaled corticosteroids in asthma. A systematic review. Pulm Pharmacol Ther . 2009;22:9–19. doi: 10.1016/j.pupt.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 34. Pearce N, Hensley MJ. Epidemiologic studies of beta agonists and asthma deaths. Epidemiol Rev . 1998;20:173–186. doi: 10.1093/oxfordjournals.epirev.a017979. [DOI] [PubMed] [Google Scholar]
- 35. Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA . 1997;277:887–891. [PubMed] [Google Scholar]
- 36. Rachelefsky G. Inhaled corticosteroids and asthma control in children: assessing impairment and risk. Pediatrics . 2009;123:353–366. doi: 10.1542/peds.2007-3273. [DOI] [PubMed] [Google Scholar]
- 37. O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW, START Investigators Group Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med . 2009;179:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 38. Stanford RH, Shah MB, D’Souza AO, Dhamane AD, Schatz M. Short-acting beta-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol . 2012;109:403–407. doi: 10.1016/j.anai.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 39. Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med . 1994;149:604–610. doi: 10.1164/ajrccm.149.3.8118625. [DOI] [PubMed] [Google Scholar]
- 40. Sears MR, Taylor DR, Print CG, Lake DC, Li QQ, Flannery EM, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet . 1990;336:1391–1396. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- 41. Suissa S, Blais L, Ernst P. Patterns of increasing beta-agonist use and the risk of fatal or near-fatal asthma. Eur Respir J . 1994;7:1602–1609. doi: 10.1183/09031936.94.07091602. [DOI] [PubMed] [Google Scholar]
- 42. O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J . 2017;50:1701103. doi: 10.1183/13993003.01103-2017. [DOI] [PubMed] [Google Scholar]
- 43. Aldridge RE, Hancox RJ, Robin Taylor D, Cowan JO, Winn MC, Frampton CM, et al. Effects of terbutaline and budesonide on sputum cells and bronchial hyperresponsiveness in asthma. Am J Respir Crit Care Med . 2000;161:1459–1464. doi: 10.1164/ajrccm.161.5.9906052. [DOI] [PubMed] [Google Scholar]
- 44. Hancox RJ, Cowan JO, Flannery EM, Herbison GP, McLachlan CR, Taylor DR. Bronchodilator tolerance and rebound bronchoconstriction during regular inhaled beta-agonist treatment. Respir Med . 2000;94:767–771. doi: 10.1053/rmed.2000.0820. [DOI] [PubMed] [Google Scholar]
- 45. Cockcroft DW, Swystun VA. Functional antagonism: tolerance produced by inhaled beta 2 agonists. Thorax . 1996;51:1051–1056. doi: 10.1136/thx.51.10.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drazen JM, Israel E, Boushey HA, Chinchilli VM, Fahy JV, Fish JE, et al. Asthma Clinical Research Network Comparison of regularly scheduled with as-needed use of albuterol in mild asthma. N Engl J Med . 1996;335:841–847. doi: 10.1056/NEJM199609193351202. [DOI] [PubMed] [Google Scholar]
- 47. Silva D, Jacinto T. Inhaled β2-agonists in asthma management: an evolving story. Breathe (Sheff) . 2016;12:375–377. doi: 10.1183/20734735.017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, SMART Study Group The salmeterol multicenter asthma research trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest . 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 49. Busse WW, Bateman ED, Caplan AL, Kelly HW, O’Byrne PM, Rabe KF, et al. Combined analysis of asthma safety trials of long-acting β2-agonists. N Engl J Med . 2018;378:2497–2505. doi: 10.1056/NEJMoa1716868. [DOI] [PubMed] [Google Scholar]
- 50.FDA Drug Safety Communication. 2018. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-finds-no-significant-increase-risk-serious-asthma-outcomes?utm_campaign=Long-Acting%20Beta%20agonists%20%28LABAs%29%20and%20Inhaled%20Corticosteroids%20%28ICS%29&utm_medium=email&utm_source=Eloqua&elqTrackId=de90a40b47ac46f49cbfedb9752d9a88&elq=62c18bc18584487f8d6f06c9c1554121&elqaid=1864&elqat=1&elqCampaig
- 51. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. START Investigators Group Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet . 2003;361:1071–1076. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 52. Dusser D, Montani D, Chanez P, de Blic J, Delacourt C, Deschildre A, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy . 2007;62:591–604. doi: 10.1111/j.1398-9995.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- 53. Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med . 2006;6:13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med . 2007;101:2437–2446. doi: 10.1016/j.rmed.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 55. Buhl R, Kuna P, Peters MJ, Andersson TL, Naya IP, Peterson S, et al. The effect of budesonide/formoterol maintenance and reliever therapy on the risk of severe asthma exacerbations following episodes of high reliever use: an exploratory analysis of two randomised, controlled studies with comparisons to standard therapy. Respir Res . 2012;13:59. doi: 10.1186/1465-9921-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zheng J, Gustafson P, et al. Effect of a single day of increased as-needed budesonide-formoterol use on short-term risk of severe exacerbations in patients with mild asthma: a post-hoc analysis of the SYGMA 1 study. Lancet Respir Med . 2021;9:149–158. doi: 10.1016/S2213-2600(20)30416-1. [DOI] [PubMed] [Google Scholar]
- 57. Balanag VM, Yunus F, Yang PC, Jorup C. Efficacy and safety of budesonide/formoterol compared with salbutamol in the treatment of acute asthma. Pulm Pharmacol Ther . 2006;19:139–147. doi: 10.1016/j.pupt.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 58. Jonkers RE, Bantje TA, Aalbers R. Onset of relief of dyspnoea with budesonide/formoterol or salbutamol following methacholine-induced severe bronchoconstriction in adults with asthma: a double-blind, placebo-controlled study. Respir Res . 2006;7:141. doi: 10.1186/1465-9921-7-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reddel HK, Bateman ED, Schatz M, Krishnan JA, Cloutier MM. A practical guide to implementing SMART in asthma management. J Allergy Clin Immunol Pract . 2022;10:S31–S38. doi: 10.1016/j.jaip.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 60. Sobieraj DM, Baker WL, Nguyen E, Weeda ER, Coleman CI, White CM, et al. Association of inhaled corticosteroids and long-acting muscarinic antagonists with asthma control in patients with uncontrolled, persistent asthma: a systematic review and meta-analysis. JAMA . 2018;319:1473–1484. doi: 10.1001/jama.2018.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Papi A, Corradi M, Pigeon-Francisco C, Baronio R, Siergiejko Z, Petruzzelli S, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med . 2013;1:23–31. doi: 10.1016/S2213-2600(13)70012-2. [DOI] [PubMed] [Google Scholar]
- 62. Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Eur Respir J . 2021;59:2102730. doi: 10.1183/13993003.02730-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lazarinis N, Jørgensen L, Ekström T, Bjermer L, Dahlén B, Pullerits T, et al. Combination of budesonide/formoterol on demand improves asthma control by reducing exercise-induced bronchoconstriction. Thorax . 2014;69:130–136. doi: 10.1136/thoraxjnl-2013-203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Reddel HK, Busse WW, Pedersen S, Tan WC, Chen YZ, Jorup C, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the START study. Lancet . 2017;389:157–166. doi: 10.1016/S0140-6736(16)31399-X. [DOI] [PubMed] [Google Scholar]
- 65. Bateman ED, O’Byrne PM, FitzGerald JM, Barnes PJ, Zheng J, Lamarca R, et al. Positioning as-needed budesonide-formoterol for mild asthma: effect of prestudy treatment in pooled analysis of SYGMA 1 and 2. Ann Am Thorac Soc . 2021;18:2007–2017. doi: 10.1513/AnnalsATS.202011-1386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet . 2006;368:744–753. doi: 10.1016/S0140-6736(06)69284-2. [DOI] [PubMed] [Google Scholar]
- 67. Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract . 2007;61:725–736. doi: 10.1111/j.1742-1241.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Israel E, Cardet JC, Carroll JK, Fuhlbrigge AL, She L, Rockhold FW, et al. Reliever-triggered inhaled glucocorticoid in Black and Latinx adults with asthma. N Engl J Med . 2022;386:1505–1518. doi: 10.1056/NEJMoa2118813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Israel E, Cardet JC, Carroll JK, Fuhlbrigge AL, Pace WD, Maher NE, et al. A randomized, open-label, pragmatic study to assess reliever-triggered inhaled corticosteroid in African American/Black and Hispanic/Latinx adults with asthma: design and methods of the PREPARE trial. Contemp Clin Trials . 2021;101:106246. doi: 10.1016/j.cct.2020.106246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Patel M, Pilcher J, Pritchard A, Perrin K, Travers J, Shaw D, et al. SMART Study Group Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med . 2013;1:32–42. doi: 10.1016/S2213-2600(13)70007-9. [DOI] [PubMed] [Google Scholar]
- 71. Atienza T, Aquino T, Fernández M, Boonsawat W, Kawai M, Kudo T, et al. Budesonide/formoterol maintenance and reliever therapy via Turbuhaler versus fixed-dose budesonide/formoterol plus terbutaline in patients with asthma: phase III study results. Respirology . 2013;18:354–363. doi: 10.1111/resp.12009. [DOI] [PubMed] [Google Scholar]
- 72. O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med . 2005;171:129–136. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 73. Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin . 2004;20:1403–1418. doi: 10.1185/030079904X2051. [DOI] [PubMed] [Google Scholar]
- 74. Vogelmeier C, D’Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J . 2005;26:819–828. doi: 10.1183/09031936.05.00028305. [DOI] [PubMed] [Google Scholar]
- 75. Kew KM, Karner C, Mindus SM, Ferrara G. Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database Syst Rev . 2013;2013:CD009019. doi: 10.1002/14651858.CD009019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Beasley R, Harrison T, Peterson S, Gustafson P, Hamblin A, Bengtsson T, et al. Evaluation of budesonide-formoterol for maintenance and reliever therapy among patients with poorly controlled asthma: a systematic review and meta-analysis. JAMA Netw Open . 2022;5:e220615. doi: 10.1001/jamanetworkopen.2022.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hendeles L, Blake KV, Galbreath A. A single inhaler combining a corticosteroid and long-acting beta-2 agonist for maintenance with additional doses for reliever therapy (SMART): obstacles for asthma patients in the USA. Pediatr Allergy Immunol Pulmonol . 2021;34:73–75. doi: 10.1089/ped.2021.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thorsson L, Edsbäcker S, Conradson TB. Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J . 1994;7:1839–1844. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 79. Bisgaard H, Le Roux P, Bjåmer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest . 2006;130:1733–1743. doi: 10.1378/chest.130.6.1733. [DOI] [PubMed] [Google Scholar]
- 80. Chipps BE, Murphy KR, Oppenheimer J. 2020 NAEPP guidelines update and GINA 2021-asthma care differences, overlap, and challenges. J Allergy Clin Immunol Pract . 2022;10:S19–S30. doi: 10.1016/j.jaip.2021.10.032. [DOI] [PubMed] [Google Scholar]
- 81. Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Am J Respir Crit Care Med . 2022;205:17–35. doi: 10.1164/rccm.202109-2205PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, et al. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC) 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program coordinating committee expert panel working Group. J Allergy Clin Immunol . 2020;146:1217–1270. doi: 10.1016/j.jaci.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cloutier MM, Dixon AE, Krishnan JA, Lemanske RF, Jr, Pace W, Schatz M. Managing asthma in adolescents and adults: 2020 asthma guideline update from the National Asthma Education and Prevention Program. JAMA . 2020;324:2301–2317. doi: 10.1001/jama.2020.21974. [DOI] [PubMed] [Google Scholar]
- 84. Krishnan JA, Cloutier MM, Schatz M. National Asthma Education and Prevention Program 2020 guideline update: where do we go from here? Am J Respir Crit Care Med . 2021;203:164–167. doi: 10.1164/rccm.202011-4236ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Reddel HK, FitzGerald JM, Bateman ED, Bacharier LB, Becker A, Brusselle G, et al. GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J . 2019;53:1901046. doi: 10.1183/13993003.01046-2019. [DOI] [PubMed] [Google Scholar]
- 86.Brownson RC, Colditz GA, Proctor EK. Dissemination and implementation research in health: translating science to practice. Oxford, UK; New York, NY: Oxford University Press; 2012. [Google Scholar]
- 87. Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet . 1979;1:1374–1376. doi: 10.1016/s0140-6736(79)92010-5. [DOI] [PubMed] [Google Scholar]
- 88. Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Beneficial effects of long-term beta-blockade in congestive cardiomyopathy. Br Heart J . 1980;44:117–133. doi: 10.1136/hrt.44.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wetzel RC. Beta-adrenergic blockade in congestive cardiomyopathy. Lancet . 1980;1:1031. [PubMed] [Google Scholar]
- 90. Engelmeier RS, O’Connell JB, Walsh R, Rad N, Scanlon PJ, Gunnar RM. Improvement in symptoms and exercise tolerance by metoprolol in patients with dilated cardiomyopathy: a double-blind, randomized, placebo-controlled trial. Circulation . 1985;72:536–546. doi: 10.1161/01.cir.72.3.536. [DOI] [PubMed] [Google Scholar]
- 91. Fisher LD, Moyé LA. Carvedilol and the Food and Drug Administration approval process: an introduction. Control Clin Trials . 1999;20:1–15. doi: 10.1016/s0197-2456(98)00052-x. [DOI] [PubMed] [Google Scholar]
- 92. Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation . 2003;107:1570–1575. doi: 10.1161/01.CIR.0000065187.80707.18. [DOI] [PubMed] [Google Scholar]
- 93. De Groote P, Isnard R, Assyag P, Clerson P, Ducardonnet A, Galinier M, et al. Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur J Heart Fail . 2007;9:1205–1211. doi: 10.1016/j.ejheart.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 94. Weiss CH, Krishnan JA, Au DH, Bender BG, Carson SS, Cattamanchi A, et al. ATS Ad Hoc Committee on Implementation Science An Official American Thoracic Society research statement: implementation science in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med . 2016;194:1015–1025. doi: 10.1164/rccm.201608-1690ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health . 2012;102:1274–1281. doi: 10.2105/AJPH.2012.300755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brownson RC, Shelton RC, Geng EH, Glasgow RE. Revisiting concepts of evidence in implementation science. Implement Sci . 2022;17:26. doi: 10.1186/s13012-022-01201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Powell BJ, McMillen JC, Proctor EK, Carpenter CR, Griffey RT, Bunger AC, et al. A compilation of strategies for implementing clinical innovations in health and mental health. Med Care Res Rev . 2012;69:123–157. doi: 10.1177/1077558711430690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rogers EM. Diffusion of innovations. New York, NY: Free Press; 2003. [Google Scholar]
- 99. Cho EY, Oh KJ, Rhee CK, Yoo KH, Kim BY, Bae HW, et al. Comparison of clinical characteristics and management of asthma by types of health care in South Korea. J Thorac Dis . 2018;10:3269–3276. doi: 10.21037/jtd.2018.05.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Norris MR, Modi S, Al-Shaikhly T. SMART—is it practical in the United States? Curr Opin Pulm Med . 2022;28:245–250. doi: 10.1097/MCP.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 101.U.S. Food and Drug Administration. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021929s013lbl.pdf
- 102. Gilbert I, Aslam Mahmood A, Devane K, Tan L. Association of nonmedical switches in inhaled respiratory medications with disruptions in care: a retrospective prescription claims database analysis. Pulm Ther . 2021;7:189–201. doi: 10.1007/s41030-021-00147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Régnier SA. How does drug coverage vary by insurance type? Analysis of argue formularies in the United States. Am J Manag Care . 2014;20:322–331. [PubMed] [Google Scholar]
- 104. The formulary system: brief history and 1960s perspective. Excerpted from mirror to hospital pharmacy. 1964. Am J Hosp Pharm . 1986;43:2838–2839. [PubMed] [Google Scholar]
- 105. Usmani OS, Bosnic-Anticevich S, Dekhuijzen R, Lavorini F, Bell J, Stjepanovic N, et al. Real-world impact of non-clinical inhaler regimen switches on asthma or COPD: a systematic review. J Allergy Clin Immunol Pract . 2022;10:2624–2637. doi: 10.1016/j.jaip.2022.05.039. [DOI] [PubMed] [Google Scholar]
- 106. Baggott C, Reddel HK, Hardy J, Sparks J, Holliday M, Corin A, et al. Patient preferences for symptom-driven or regular preventer treatment in mild to moderate asthma: findings from the PRACTICAL study, a randomised clinical trial. Eur Respir J . 2020;55:1902073. doi: 10.1183/13993003.02073-2019. [DOI] [PubMed] [Google Scholar]
- 107. Gerald JK, Wechsler ME, Martinez FD. Asthma medications should be available for over-the-counter use: pro. Ann Am Thorac Soc . 2014;11:969–974. doi: 10.1513/AnnalsATS.201404-139OI. [DOI] [PubMed] [Google Scholar]
- 108. Feldman WB, Avorn J, Kesselheim AS. Switching to over-the-counter availability of rescue inhalers for asthma. JAMA . 2022;327:1021–1022. doi: 10.1001/jama.2022.1160. [DOI] [PubMed] [Google Scholar]
- 109. Sadreameli SC, Brigham EP, Patel A. The surprising reintroduction of Primatene Mist in the United States. Ann Am Thorac Soc . 2019;16:1234–1236. doi: 10.1513/AnnalsATS.201902-164HP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Barisione G, Baroffio M, Crimi E, Brusasco V. Beta-adrenergic agonists. Pharmaceuticals (Basel) . 2010;3:1016–1044. doi: 10.3390/ph3041016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Forno E, Celedón JC. Health disparities in asthma. Am J Respir Crit Care Med . 2012;185:1033–1035. doi: 10.1164/rccm.201202-0350ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Plint AC, Osmond MH, Klassen TP. The efficacy of nebulized racemic epinephrine in children with acute asthma: a randomized, double-blind trial. Acad Emerg Med . 2000;7:1097–1103. doi: 10.1111/j.1553-2712.2000.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 113. Blake K, Raissy H. Nonprescription epinephrine metered-dose inhaler: to be or not to be. Pediatr Allergy Immunol Pulmonol . 2014;27:143–146. doi: 10.1089/ped.2014.0399. [DOI] [PubMed] [Google Scholar]
- 114.Amphastar Pharmaceuticals Inc United Security and Exchange Comission, Form 10-K Rancho Cucamonga, CA: Amphastar Pharmaceuticals; 2021. [accessed 2022 Sep 1]. Available from: https://ir.amphastar.com/websites/amphastar/English/3210/us-sec-filing.html?shortDesc=Annual%20Report&format=convpdf&secFilingId=e545c22e-f69c-4031-ac47-7321f020e481 [Google Scholar]
- 115. Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, et al. US health care spending by payer and health condition, 1996-2016. JAMA . 2020;323:863–884. doi: 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mahecha LA. Rx-to-OTC switches: trends and factors underlying success. Nat Rev Drug Discov . 2006;5:380–385. doi: 10.1038/nrd2028. [DOI] [PubMed] [Google Scholar]
- 117.Kittinger P, Herrick D. National Center for Policy Analysis; 2005. http://www.ncpathinktank.org/pub/ba524 [Google Scholar]
- 118.U.S. Food and Drug Administration. 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-generic-symbicort-treat-asthma-and-copd
- 119. Rubin R. First Symbicort generic gains approval. JAMA . 2022;327:1439. doi: 10.1001/jama.2022.5095. [DOI] [PubMed] [Google Scholar]
- 120.United States Court of Appeals for the Federal Circuit. 2021. https://cafc.uscourts.gov/opinions-orders/21-1729.OPINION.12-8-2021_1875979.pdf
- 121.GoodRx. 2022. https://www.goodrx.com/symbicort
- 122.Amazon.com. 2022. https://www.amazon.com/Primatene-Inhaler-Temporary-Relief-Intermittent/dp/B07N141L57/
- 123.U.S. Food and Drug Administration. 2019. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/generic-competition-and-drug-prices
- 124. Singh D, Garcia G, Maneechotesuwan K, Daley-Yates P, Irusen E, Aggarwal B, et al. Correction to: new versus old: the impact of changing patterns of inhaled corticosteroid prescribing and dosing regimens in asthma management. Adv Ther . 2022;39:3866–3867. doi: 10.1007/s12325-022-02209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Singh D, Garcia G, Maneechotesuwan K, Daley-Yates P, Irusen E, Aggarwal B, et al. New versus old: the impact of changing patterns of inhaled corticosteroid prescribing and dosing regimens in asthma management. Adv Ther . 2022;39:1895–1914. doi: 10.1007/s12325-022-02092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev . 2014;2014:CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hardtstock F, Maywald U, Timmermann H, Unmüßig V, Müller S, Wilke T, et al. Extent of non-adherence and non-persistence in asthma patients: analysis of a large claims data set. J Asthma . 2022;59:829–839. doi: 10.1080/02770903.2021.1871738. [DOI] [PubMed] [Google Scholar]
- 128. Blake KV. Improving adherence to asthma medications: current knowledge and future perspectives. Curr Opin Pulm Med . 2017;23:62–70. doi: 10.1097/MCP.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 129. Davidsen JR, Søndergaard J, Hallas J, Siersted HC, Knudsen TB, Lykkegaard J, et al. Impact of socioeconomic status on the use of inhaled corticosteroids in young adult asthmatics. Respir Med . 2011;105:683–690. doi: 10.1016/j.rmed.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 130. Holbein ME. Understanding FDA regulatory requirements for investigational new drug applications for sponsor-investigators. J Investig Med . 2009;57:688–694. doi: 10.231/JIM.0b013e3181afdb26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karst K. FDA Law Blog; 2010. https://www.thefdalawblog.com/2010/02/has-fda-already-resolved-one-critical-issue-concerning-forced-rxtootc-switches/ [Google Scholar]
- 132.Bassett MT. MedPage Today; 2022. https://www.medpagetoday.com/pulmonology/asthma/101633 [Google Scholar]
- 133. O’Byrne PM, Reddel HK, Beasley R. The management of mild asthma. Eur Respir J . 2021;57:2003051. doi: 10.1183/13993003.03051-2020. [DOI] [PubMed] [Google Scholar]
- 134. Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA. Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract . 2020;8:549–554.e1. doi: 10.1016/j.jaip.2019.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Akenroye AT, Heyward J, Keet C, Alexander GC. Lower use of biologics for the treatment of asthma in publicly insured individuals. J Allergy Clin Immunol Pract . 2021;9:3969–3976. doi: 10.1016/j.jaip.2021.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Woodward EN, Singh RS, Ndebele-Ngwenya P, Melgar Castillo A, Dickson KS, Kirchner JE. A more practical guide to incorporating health equity domains in implementation determinant frameworks. Implement Sci Commun . 2021;2:61. doi: 10.1186/s43058-021-00146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Brownson RC, Kumanyika SK, Kreuter MW, Haire-Joshu D. Implementation science should give higher priority to health equity. Implement Sci . 2021;16:28. doi: 10.1186/s13012-021-01097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Odeny B. Closing the health equity gap: a role for implementation science? PLoS Med . 2021;18:e1003762. doi: 10.1371/journal.pmed.1003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Baumann AA, Cabassa LJ. Reframing implementation science to address inequities in healthcare delivery. BMC Health Serv Res . 2020;20:190. doi: 10.1186/s12913-020-4975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Food and Drug Administration. 2012. https://www.federalregister.gov/documents/2012/02/28/2012-4597/using-innovative-technologies-and-other-conditions-of-safe-use-to-expand-which-drug-products-can-be
- 141. Larsson K, Kankaanranta H, Janson C, Lehtimäki L, Ställberg B, Løkke A, et al. Bringing asthma care into the twenty-first century. NPJ Prim Care Respir Med . 2020;30:25. doi: 10.1038/s41533-020-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cates CJ, Karner C. Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database Syst Rev . 2013:CD007313. doi: 10.1002/14651858.CD007313.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Paris J, Peterson EL, Wells K, Pladevall M, Burchard EG, Choudhry S, et al. Relationship between recent short-acting beta-agonist use and subsequent asthma exacerbations. Ann Allergy Asthma Immunol . 2008;101:482–487. doi: 10.1016/S1081-1206(10)60286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Apter A, Carroll JK, Cardet JC, Cohen R, Colon-Moya AD, Ericson B, et al. Nebulizer use by Black and Latinx adults with moderate to severe asthma. J Allergy Clin Immunol Pract . 2022;10:517–524.e2. doi: 10.1016/j.jaip.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]