Abstract
Objective:
Apathy is a common and disabling symptom after stroke with no proven treatments. Selective serotonin reuptake inhibitors are widely used to treat depressive symptoms post-stroke but whether they reduce apathetic symptoms is unknown. We determined the effect of fluoxetine on post-stroke apathy in a post hoc analysis of the EFFECTS (Efficacy oF Fluoxetine—a randomized Controlled Trial in Stroke) trial.
Methods:
EFFECTS enrolled patients ⩾18 years between 2 and 15 days after stroke onset. Participants were randomly assigned to receive oral fluoxetine 20 mg once daily or matching placebo for 6 months. The Montgomery–Åsberg Depression Rating Scale (MADRS) was administered at baseline and 6 months. Individual items on this scale were divided into those reflecting symptoms of apathy and depression. Symptoms were compared between fluoxetine and placebo groups.
Results:
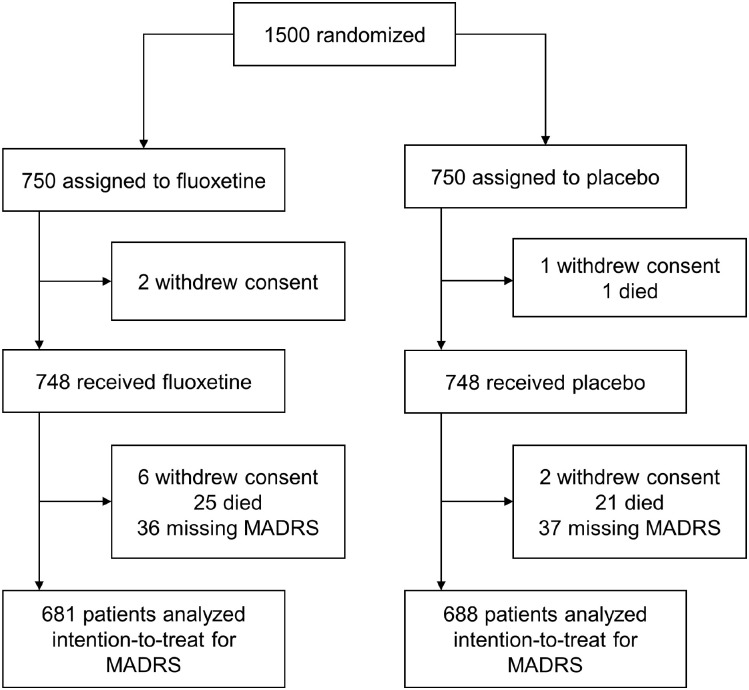
Of 1500 participants enrolled, complete MADRS data were available for 1369. The modified intention-to-treat population included 681 patients in the fluoxetine group and 688 in the placebo group. Confirmatory factor analysis revealed that apathetic, depressive, and anhedonic symptoms were dissociable. Apathy scores increased in both fluoxetine and placebo groups (both p ⩽ 0.00001). In contrast, fluoxetine was associated with a reduction in depressive scores (p = 0.002)
Conclusion:
Post-stroke apathetic and depressive symptoms respond differently to fluoxetine treatment. Our analysis suggests fluoxetine is ineffective in preventing post-stroke apathy.
Keywords: Apathy, depression, SSRI, clinical, Stroke, cognition
Introduction
Apathy occurs in one out of three patients after stroke.1 It describes a reduction in goal-directed activity in the cognitive, behavioral, emotional, or social domains of a patient’s life. Despite its frequency, apathy is clinically under-recognized, and there are no proven drug treatment approaches.
Antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), are often used to treat post-stroke depression. Whether SSRIs are also effective in apathy remains uncertain.1 Despite shared symptoms, post-stroke apathy and depression are dissociable syndromes. Negative emotionality is a key characteristic of depression that distinguishes it from apathy. Depressed patients may present with pessimism and hopelessness, while those with apathy show lack of emotional distress. Recent studies suggest apathy and depression have different neuroanatomical correlates with white matter track damage and subsequent complex network disruption underlying apathy, but not depression.1,2 This suggests they might respond differently to therapeutic interventions.
We determined whether fluoxetine reduced apathy in a post hoc analysis of data from the Efficacy oF Fluoxetine, a randomized Controlled Trial in Stroke (EFFECTS) trial.
Methods
Participants
EFFECTS was a randomized, double-blind, placebo-controlled clinical trial conducted in 35 hospital centers in Sweden.3 Eligible participants were adults (age ⩾18 years) with a diagnosis of acute stroke within the previous 2–15 days, brain imaging consistent with ischemic or hemorrhagic stroke, and persisting neurological deficit. Exclusion criteria included depression or antidepressant use; fluoxetine contraindication; unlikely to be available for follow-up; unlikely to survive until follow-up; enrollment in another clinical trial of an investigational medical product or device; women if pregnant, breast-feeding, or of child-bearing age and not using contraception. Apathy was not an exclusion criteria.
Participants were randomized via a secure, centralized web-based system using a minimization algorithm that assigned participants to fluoxetine 20 mg once daily (od) or placebo one od for 6 months in a 1:1 ratio.3 Placebo capsules were visually identical to fluoxetine capsules. Patients were followed-up at 6 months by postal questionnaire or telephone from the trial coordinating center. If patients were unable to complete questionnaires, assistance was sought from their next of kin or carer. The participant, care provider, investigator, and outcome assessor remained masked to allocated trial treatment until completion of the study. The design, methods, and primary results have been published.3
EFFECTS was approved by a medical ethics committee in Stockholm (reference 2013/1265-31/2) and the Swedish Medical Agency (reference 5.1-2014-43006). The study was registered in the EU Clinical Trials Register (clinicaltrialsregister.eu; EudraCT no. 2011-006130-16) and at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT02683213). All participants provided written informed consent.
Clinical measures and confirmatory factor analysis
Stroke severity was quantified using the National Institutes of Health Stroke Scale (NIHSS).4 Apathy and depression were assessed using the Montgomery–Åsberg Depression Rating Scale (MADRS).5 The MADRS has 10 items, each graded from 0 (no symptoms) to 6 (most severe symptoms). Item 7 on the MADRS (lassitude) assesses difficulty in starting and completing everyday tasks, and was determined to be a measure of apathy. Item 8 (inability to feel) assesses anhedonia, and given theoretical overlap with apathy,6 was investigated separately to other depressive symptoms. The remaining eight items were used as a measure of depression. We conducted confirmatory factor analysis (CFA) to validate our use of these measures.
CFA quantitatively tests whether a hypothesized scale structure matches the observed data.7 In the context of this study, we tested the hypothesis that separating the MADRS into apathy and depression subscales would yield a better fit to the observed data compared to taking all the items on the MADRS to represent a unitary depressive construct. Previous studies have found support for a multi-factorial structure of the MADRS that included apathy in stroke patients,8 but not in Parkinson’s disease.9 Importantly, CFA alone does not assess whether patients have apathy or depression per se, but rather identify whether patterns of responding on certain items are correlated.
We tested this by comparing nested models that used the same variables, but different hypothesized factor structures.10 The initial baseline model was a one-factor model of depression, where all items on the MADRS loaded onto a single depression construct. The second model was a three-factor model that separately assessed apathy, depression, and anhedonia. Anhedonia was separated from other symptoms of depression due to its theoretical overlap with apathy. Apathy was assessed using item 7, anhedonia using item 8, and depression using the remaining items on the MADRS. Models were compared using the Akaike information criterion (AIC) and Bayesian information criterion (BIC). For both measures, lower values indicate better fitting models, with a difference of 10 being indicative of a significantly different model.11
All models were fitted using default arguments to the cfa() function in laavan 0.6-7.12 Although the Likert-type responses on the MADRS suggest that items should be treated as ordinal variables, simulation studies have shown that maximum likelihood estimation yields similar results to categorical estimation methods when applied to variables with six to seven categories.13 As items on the MADRS were graded on a 7-point scale, we opted to treat items as continuous variables and use maximum likelihood to estimate CFA models.
Statistical analyses
Statistics were calculated using R 4.0.4. All tests were two-tailed with ɑ = 0.05. As this was a post hoc analysis, no power calculations were conducted; power calculations for the main trial have been published.7
Mann–Whitney U and Wilcoxon signed-rank tests were used to compare apathy and depression between-groups (fluoxetine vs placebo) and within-groups (baseline to 6 months). Analyses were repeated in the following pre-specified subgroups: age (⩽70 or >70), sex, stroke type (ischemic or hemorrhagic), ischemic stroke subtype classified using modified Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria,14 NIHSS (⩽5 or >5), and medication adherence (taken 7 days a week or not). p values across all subgroup analyses were corrected for multiple comparisons using the false discovery rate (FDR).
To further validate longitudinal comparisons, patients were grouped into whether they had apathy symptoms (i.e. item 7 score >0) at baseline and at 6 months. McNemar’s test was applied to the resulting 2 × 2 contingency table to determine if the proportion of patients endorsing apathy symptoms changed after fluoxetine treatment. This test was conducted in both fluoxetine and placebo groups. This analysis was also repeated for anhedonia symptoms (item 8 score >0).
Data availability statement
Anonymized data supporting the findings of the trial are available to researchers upon reasonable request to the corresponding author (EL; erik.lundstrom@neuro.uu.se) following receipt of a written application and proposal for use of the data, approval by the EFFECTS trial Steering Committee, and establishment of a data sharing agreement.
Results
Population characteristics
Recruitment started 20 October 2014 and ended 28 June 2019. One thousand five-hundred participants were randomized to fluoxetine (n = 750) or placebo (n = 750). Of these, 1369 had complete MADRS scores at 6 months (fluoxetine = 681 and placebo = 688) and were included in the modified intention-to-treat (ITT) analysis (Figure 1). Excluded patients were older and had higher NIHSS scores (Supplementary Table 1). Baseline characteristics were well balanced between both groups, although the fluoxetine group showed higher apathy scores at baseline (Table 1).
Figure 1.
Consort flow diagram.
Table 1.
Baseline characteristics of the modified intention-to-treat population in EFFECTS.
| Fluoxetine (n = 681) | Placebo (n = 688) | p | |
|---|---|---|---|
| Age, mean (SD) | 70 (11.2) | 71.4 (10.4) | 0.37 |
| Sex, female, n (%) | 270 (39.6) | 253 (36.8) | 0.32 |
| NIHSS, median (IQR) | 3.0 (2–6) | 3.0 (2–6) | 0.61 |
| Stroke type | 0.55 | ||
| Ischemic, n (%) | 599 (88.0) | 595 (86.5) | |
| Hemorrhagic, n (%) | 82 (12.0) | 91 (13.2) | |
| Ischemic stroke cause* | 0.27 | ||
| Large artery disease, n (%) | 95 (14.0) | 81 (11.8) | |
| Small-vessel disease, n (%) | 199 (29.2) | 196 (28.5) | |
| Cardioembolism, n (%) | 120 (17.6) | 134 (19.5) | |
| Other, n (%) | 25 (3.7) | 14 (2.0) | |
| Unknown or uncertain, n (%) | 164 (24.1) | 172 (25.0) | |
| MADRS variables | |||
| Total score, mean (SD) | 2.8 (3.6) | 2.7 (3.0) | 0.87 |
| Apathy, mean (SD) | 0.4 (0.8) | 0.3 (0.7) | 0.007 |
| Depression, mean (SD) | 2.1 (2.7) | 2.1 (2.3) | 0.71 |
| Anhedonia, mean (SD) | 0.1 (0.4) | 0.1 (0.3) | 0.28 |
NIHSS: National Institute of Health Stroke Scale; MADRS: Montgomery–Åsberg Depression Rating Scale.
Assessed using modified TOAST criteria.
CFA
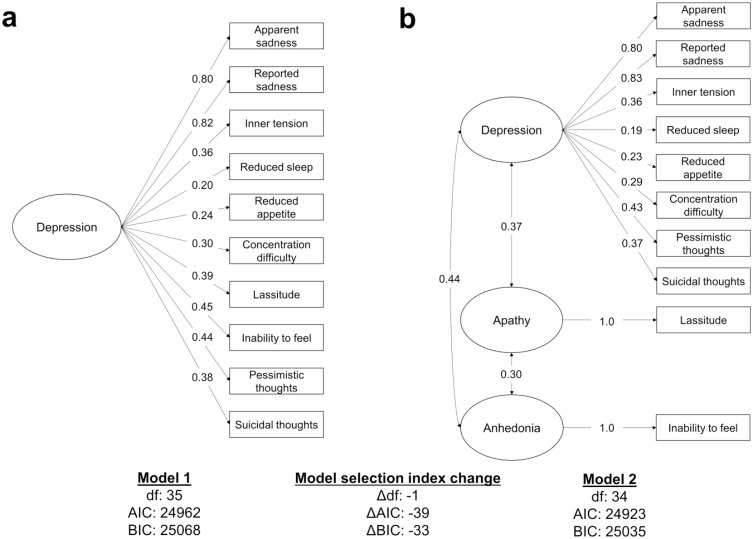
Comparison of CFA models revealed that a three-factor model that separately assessed apathetic, depressive, and anhedonic symptoms was a better fit to baseline MADRS data than a single-factor depression model (Figure 2). The three-factor model demonstrated lower AIC and BIC values compared to the one-factor model, with differences greater than 30, indicating that assessing the symptoms separately better explained the observed data. All factors showed moderately strong inter-factor correlations (β = 0.30–0.44).
Figure 2.
Confirmatory factor analysis of the Montgomery–Åsberg Depression Rating Scale. (a) One-factor model of depression and (b) three-factor model assessing apathy, depression, and anhedonia. For both indices, lower values indicate a better fit to the data, with differences over 10 being considered significant. The three-factor model demonstrated lower AIC and BIC values, suggesting it was a better fit to the data. Single-headed arrows indicate the factor loadings while double-headed arrows indicate the correlations. All paths are significant at p < 0.05. AIC: Akaike information criterion; BIC: Bayesian information criterion.
Comparisons between fluoxetine and placebo
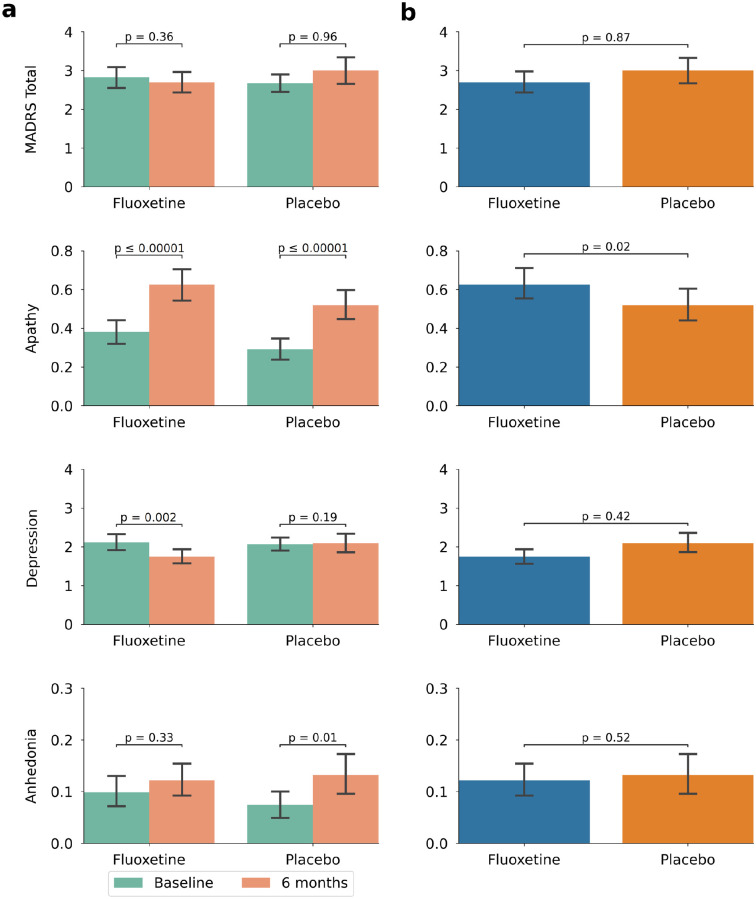
Depression scores decreased in the fluoxetine group (baseline = 2.11 (2.67); 6 months = 1.75 (2.41), p = 0.002) but not in the placebo group (baseline = 2.07 (2.34); 6 months = 2.09 (3.14), p = 0.19). In contrast, apathetic scores increased in both fluoxetine (baseline = 0.38 (0.84); 6 months = 0.63 (1.08)) and placebo (baseline = 0.29 (0.75); 6 months = 0.52 (1.03)) groups (both p ⩽ 0.00001). There appeared to be a trend toward increasing anhedonia in both groups (fluoxetine baseline = 0.10 (0.39), 6 months = 0.12 (0.41); placebo baseline = 0.07 (0.34), 6 months = 0.13 (0.50)), but this was only significant in the placebo group (p = 0.01). There was no change in total MADRS score between baseline and 6 months in either group.
We analyzed subgroups to determine factors related to change in depressive and apathetic scores between baseline and 6 months. Results were largely consistent with main longitudinal results (Table 2 and Supplementary Table 2). Apathetic symptoms increased in most subgroups except the following: female (fluoxetine), hemorrhagic stroke (placebo), ischemic stroke patients with large artery disease, cryptogenic, or other strokes (fluoxetine and placebo), NIHSS >5 (placebo), and non-adherent (placebo). Decreased depressive symptoms in patients on fluoxetine were observed in the following subgroups: age ⩽70 years, ischemic stroke, ischemic stroke patients with small-vessel disease (SVD), and NIHSS ⩽5.
Table 2.
Longitudinal subgroup comparisons within fluoxetine and placebo groups in EFFECTS.
| Fluoxetine | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | 6 months | PFDR | n | Baseline | 6 months | PFDR | ||
| Age | |||||||||
| >70 | Total | 405 | 2.66 | 2.82 | 0.85 | 383 | 2.72 | 2.95 | 0.61 |
| Apathy | 405 | 0.38 | 0.62 | 0.0008 | 383 | 0.3 | 0.49 | 0.03 | |
| Depression | 405 | 1.99 | 1.84 | 0.45 | 383 | 2.12 | 2.12 | 0.36 | |
| Anhedonia | 405 | 0.09 | 0.13 | 0.28 | 383 | 0.08 | 0.11 | 0.58 | |
| ⩽70 | Total | 276 | 3.07 | 2.51 | 0.18 | 303 | 2.61 | 3.08 | 0.61 |
| Apathy | 276 | 0.38 | 0.63 | 0.007 | 303 | 0.29 | 0.56 | 0.003 | |
| Depression | 276 | 2.29 | 1.61 | 0.008 | 303 | 2.01 | 2.07 | 0.86 | |
| Anhedonia | 276 | 0.12 | 0.11 | 0.86 | 303 | 0.07 | 0.16 | 0.04 | |
| Sex | |||||||||
| Female | Total | 270 | 3.07 | 2.79 | 0.45 | 253 | 2.48 | 2.83 | 0.66 |
| Apathy | 270 | 0.4 | 0.54 | 0.15 | 253 | 0.25 | 0.51 | 0.009 | |
| Depression | 270 | 2.3 | 1.87 | 0.08 | 253 | 1.88 | 1.94 | 0.92 | |
| Anhedonia | 270 | 0.11 | 0.13 | 0.85 | 253 | 0.1 | 0.15 | 0.56 | |
| Male | Total | 411 | 2.66 | 2.63 | 0.93 | 433 | 2.79 | 3.11 | 0.83 |
| Apathy | 411 | 0.37 | 0.68 | 0.00002 | 433 | 0.32 | 0.53 | 0.009 | |
| Depression | 411 | 1.99 | 1.67 | 0.1 | 433 | 2.18 | 2.19 | 0.37 | |
| Anhedonia | 411 | 0.09 | 0.12 | 0.58 | 433 | 0.06 | 0.12 | 0.08 | |
| Stroke type | |||||||||
| Ischemic | Total | 599 | 2.82 | 2.64 | 0.61 | 595 | 2.62 | 2.92 | 0.92 |
| Apathy | 599 | 0.39 | 0.62 | 0.0002 | 595 | 0.28 | 0.5 | 0.0003 | |
| Depression | 599 | 2.1 | 1.7 | 0.02 | 595 | 2.05 | 2.05 | 0.41 | |
| Anhedonia | 599 | 0.1 | 0.13 | 0.56 | 595 | 0.07 | 0.13 | 0.06 | |
| Hemorrhagic | Total | 82 | 2.88 | 3.12 | 0.85 | 91 | 3.04 | 3.54 | 0.83 |
| Apathy | 82 | 0.34 | 0.7 | 0.03 | 91 | 0.41 | 0.63 | 0.28 | |
| Depression | 82 | 2.22 | 2.07 | 0.56 | 91 | 2.24 | 2.38 | 0.93 | |
| Anhedonia | 82 | 0.09 | 0.1 | 1 | 91 | 0.11 | 0.16 | 0.73 | |
| Ischemic stroke type | |||||||||
| Large artery disease | Total | 95 | 2.56 | 3.08 | 0.18 | 81 | 2.91 | 3.73 | 0.56 |
| Apathy | 95 | 0.36 | 0.68 | 0.09 | 81 | 0.37 | 0.56 | 0.53 | |
| Depression | 95 | 1.95 | 1.93 | 0.94 | 81 | 2.27 | 2.62 | 0.85 | |
| Anhedonia | 95 | 0.07 | 0.22 | 0.19 | 81 | 0.07 | 0.25 | 0.16 | |
| Small-vessel disease | Total | 199 | 2.88 | 2.61 | 0.41 | 196 | 2.07 | 2.64 | 0.25 |
| Apathy | 199 | 0.39 | 0.65 | 0.02 | 196 | 0.22 | 0.45 | 0.04 | |
| Depression | 199 | 2.21 | 1.69 | 0.04 | 196 | 1.66 | 1.9 | 0.67 | |
| Anhedonia | 199 | 0.1 | 0.1 | 1 | 196 | 0.04 | 0.11 | 0.09 | |
| Cardioembolism | Total | 120 | 2.71 | 2.19 | 0.28 | 134 | 2.87 | 3.37 | 0.79 |
| Apathy | 120 | 0.29 | 0.58 | 0.01 | 134 | 0.32 | 0.6 | 0.03 | |
| Depression | 120 | 2.02 | 1.39 | 0.06 | 134 | 2.17 | 2.34 | 0.83 | |
| Anhedonia | 120 | 0.12 | 0.07 | 0.61 | 134 | 0.12 | 0.15 | 0.85 | |
| Other | Total | 25 | 2.8 | 3.04 | 0.99 | 14 | 4.14 | 2.93 | 0.45 |
| Apathy | 25 | 0.16 | 0.48 | 0.32 | 14 | 0.14 | 0.07 | 1 | |
| Depression | 25 | 2.44 | 2.32 | 0.85 | 14 | 3.36 | 2.43 | 0.61 | |
| Anhedonia | 25 | 0.12 | 0.08 | 0.93 | 14 | 0.14 | 0 | 0.58 | |
| Unknown | Total | 164 | 2.52 | 2.61 | 0.88 | 172 | 2.77 | 2.42 | 0.04 |
| Apathy | 164 | 0.4 | 0.52 | 0.33 | 172 | 0.28 | 0.44 | 0.2 | |
| Depression | 164 | 1.79 | 1.76 | 0.99 | 172 | 2.15 | 1.67 | 0.009 | |
| Anhedonia | 164 | 0.08 | 0.12 | 0.53 | 172 | 0.08 | 0.08 | 0.97 | |
| NIHSS | |||||||||
| 5 | Total | 211 | 2.38 | 2.7 | 0.56 | 188 | 2.74 | 2.77 | 0.85 |
| Apathy | 211 | 0.3 | 0.7 | 0.0002 | 188 | 0.32 | 0.45 | 0.34 | |
| Depression | 211 | 1.84 | 1.66 | 0.53 | 188 | 2.05 | 1.95 | 0.61 | |
| Anhedonia | 211 | 0.05 | 0.12 | 0.2 | 188 | 0.1 | 0.12 | 0.85 | |
| ⩽5 | Total | 470 | 3.02 | 2.69 | 0.2 | 498 | 2.65 | 3.09 | 0.93 |
| Apathy | 470 | 0.42 | 0.59 | 0.009 | 498 | 0.28 | 0.55 | 0.0003 | |
| Depression | 470 | 2.23 | 1.79 | 0.02 | 498 | 2.08 | 2.15 | 0.56 | |
| Anhedonia | 470 | 0.12 | 0.12 | 0.98 | 498 | 0.07 | 0.14 | 0.03 | |
| Medication adherence | |||||||||
| Adherent | Total | 542 | 2.78 | 2.56 | 0.41 | 550 | 2.74 | 3.2 | 0.86 |
| Apathy | 542 | 0.39 | 0.62 | 0.0003 | 550 | 0.3 | 0.56 | 0.0002 | |
| Depression | 542 | 2.07 | 1.63 | 0.007 | 550 | 2.13 | 2.21 | 0.56 | |
| Anhedonia | 542 | 0.1 | 0.12 | 0.66 | 550 | 0.08 | 0.15 | 0.05 | |
| Not adherent | Total | 138 | 3.01 | 3.22 | 0.85 | 135 | 2.42 | 2.24 | 0.67 |
| Apathy | 138 | 0.36 | 0.62 | 0.01 | 135 | 0.28 | 0.36 | 0.58 | |
| Depression | 138 | 2.28 | 2.23 | 0.94 | 135 | 1.87 | 1.65 | 0.62 | |
| Anhedonia | 138 | 0.09 | 0.12 | 0.79 | 135 | 0.05 | 0.07 | 0.86 | |
FDR: false discovery rate; NIHSS: National Institutes of Health Stroke Scale.
Between-group comparisons between fluoxetine and placebo at 6 months revealed no differences in MADRS total score, depressive, or anhedonic symptoms, although apathetic symptoms were elevated in the fluoxetine group (Figure 3(b)). Due to these negative treatment effects, no between-group subgroup analysis was performed.
Figure 3.
Results in the modified intention-to-treat population. All measures were derived from the Montgomery–Åsberg Depression Rating Scale. Note that scale axes have been truncated for clarity; the maximum scores are 6 for apathy and 48 for depression and 6 for anhedonia. (a) Within-group comparisons between baseline and 6 months. Total score did not change in either group. Apathetic symptoms increase in both groups, depressive symptoms decrease in the fluoxetine group, and anhedonic symptoms increase in the placebo group. (b) Comparisons between the fluoxetine and placebo groups at 6 months, with the fluoxetine group showing higher apathetic symptoms.
Comparison of proportions
In the fluoxetine group, 7.5% of patients had apathy (defined as item 7 score <1) at baseline and follow-up, 14.4% had apathy at baseline, but not at follow-up, 16.3% had no apathy at baseline, but had apathy at follow-up, and 61.8% had no apathy at baseline or follow-up. The proportion of patients having apathy symptoms did not change in the fluoxetine group (McNemar χ2 = 0.69, p = 0.41). In the placebo group, 7.6% of patients had apathy at baseline and follow-up, 9.6% had apathy at baseline, but not follow-up, 21.4% had no apathy at baseline, but had apathy at follow-up, and 61.4% had no apathy at baseline or follow-up. The proportion of patients having apathy symptoms increased in the placebo group (McNemar χ2 = 30.05, p ⩽ 0.00001).
In the fluoxetine group, 0.7% of patients had anhedonia at baseline and follow-up, 5.9% had anhedonia at baseline, but not at follow-up, 7.6% had no anhedonia at baseline, but did at follow-up, and 85.8% had no anhedonia at baseline or follow-up. Patients endorsing anhedonia did not change in the fluoxetine group (McNemar χ2 = 1.32, p = 0.25). In the placebo group, 0.9% of patients had anhedonia at baseline and follow-up, 6.1% had anhedonia at baseline, but not at follow-up, 6.7% had no anhedonia at baseline, but did at follow-up, and 86.3% had no anhedonia at baseline or follow-up. Patients endorsing anhedonia did not change in the fluoxetine group (McNemar χ2 = 0.10, p = 0.75).
Discussion
In a post hoc analysis of a large randomized controlled trial, apathetic and depressive symptoms responded differently to fluoxetine treatment post-stroke. Apathetic symptoms increased in a similar fashion over time in both the fluoxetine and placebo groups. In contrast, depressive symptoms reduced from baseline to 6 months in the fluoxetine, but not placebo, group. These findings suggest that fluoxetine reduces post-stroke depressive symptoms, but do not alter the time course of apathetic symptoms post-stroke. These differences were not observed when examining total MADRS score, highlighting the importance of dissociating apathetic and depressive symptoms when examining treatment effects.
Apathetic symptoms increased over time in both groups consistent with some studies reporting increased apathy over time following stroke.15 In addition, there was an increase in the proportion of patients reporting apathy symptoms after 6 months in the placebo group. This was not observed in the fluoxetine group, although this may be explained by that group having higher rates of apathy at baseline. These results suggest that post-stroke patients experience a greater burden of motivational deficits over time, although overall severity may be mild. Research on 1 year trajectories suggests that the majority of post-stroke patients can be categorized into high (7%), moderate (33%), or low/no (50%) levels of apathy from the acute phase, which remains stable for up to a year.16 A small group of patients will spontaneously improve (7%) or worsen (7%).14 Over the course of 5 years following stroke, the prevalence of apathy was reported to increase by about 10%.15 Theoretical work suggests that increases in post-stroke apathy can be explained by anterograde neurodegeneration, which can propagate from the initial infarct to large-scale brain networks underlying motivation.17 These networks may also be affected by ischemic white matter disease,2,18 which was supported by our finding that apathy increased in the SVD subgroup.
Decreases in depressive symptoms in the fluoxetine group were observed in patients with age ⩽70 years, SVD, and NIHSS ⩽5, suggesting that fluoxetine may be more effective in treating depressive symptoms in stroke patients with less severe disease. Why this may be the case is unclear. One explanation could be that the pathogenesis and development of depressive symptoms are different in SVD compared to other stroke types, and that these are more amenable to SSRI treatment. Regardless of the reasons, future treatment studies should replicate and further investigate the possibility that fluoxetine can alleviate depressive symptoms in participants with mild disease. It is important to emphasize that these claims pertain to symptomatic, rather than syndromic, depression (e.g. major depression). All patients enrolled in EFFECTS were free from depression upon entry to the trial. Therefore, the decrease in depressive symptoms observed in the fluoxetine group should be interpreted as a decrease in subthreshold depressive symptomatology, rather than the treatment of a pre-existing depressive disorder.
The depressive construct we investigated excluded anhedonia. Research suggests that SSRIs can ameliorate depressive symptomatology in general, but specific effects for treating anhedonia are mixed.19 Our results supported this, with anhedonic symptoms not differing between groups or decreasing in the fluoxetine group over time. We did, however, observe an increase in anhedonia in the placebo group. Theoretical work suggests that apathy and anhedonia may increase over time due to similar pathophysiological mechanisms.6 If this is the case, then it is possible that fluoxetine prevented an increase in anhedonia in patients who received it.
A strength of this study is that it used a large randomized controlled trail data set. A limitation is that it was a post hoc analysis. Rather than using individual scales for apathy and depression, both measures had to be derived from the MADRS. Our results should therefore be replicated in future studies, which can be designed around assessing fluoxetine-related changes in apathy and depression as a primary outcome. Three large trials have examined the effect of fluoxetine post-stroke, but only the EFFECTS trial had data collected which allowed comparison of fluoxetine on apathetic and depressive symptoms.20 This study also has a number of limitations primarily related to the tools we had available to assess apathy. Apathy and anhedonia were only assessed using a single item. This limits our conclusions to symptomatic apathy and anhedonia, rather than broader syndromes. Item 7 of the MADRS, which we used to assess apathy, primarily measures slowness or difficulty initiating activities and therefore is best seen as an index of behavioral apathy, but is not a good indicator of the motivation aspect of apathy. The use of single items may also reduce sensitivity to detect effect; of note, depressive symptoms were in contrast assessed using eight items. Furthermore, we cannot make conclusions about the apathy severity from the one item we used. Another related limitation is that recent studies have suggested apathy is a multidimensional concept, including different facets, such as cognitive, behavioral, and emotional.21 The use of a single item for apathy did not allow us to examine differences on particular dimensions of apathy. In addition, previous work examining a three-item measure of apathy derived from the Geriatric Depression Scale-15 revealed that the measure had low sensitivity and high specificity.22 This suggests that the use of a single-item ad hoc measure of apathy in this study may have led to an underestimation of the prevalence of apathy in our sample.
In conclusion, we have shown that fluoxetine has differential effects on post-stroke apathetic and depressive symptoms in a post hoc analysis of a large randomized trial data set. An increase in apathetic symptoms was observed in both groups, suggesting that fluoxetine is ineffective in treating motivational deficits. In contrast, depressive symptoms decreased in the fluoxetine group, suggesting a possible treatment effect. Our preliminary results suggest that alternative strategies, both pharmacological and behavioral, for treating apathy after stroke need to be developed.
Supplemental Material
Supplemental material, sj-docx-1-wso-10.1177_17474930221124760 for Does fluoxetine reduce apathetic and depressive symptoms after stroke? An analysis of the Efficacy oF Fluoxetine—a randomized Controlled Trial in Stroke trial data set by Jonathan Tay, Björn Mårtensson, Hugh S Markus and Erik Lundström in International Journal of Stroke
Acknowledgments
The authors thank Graeme Hankey, Osvaldo Almeida, Maree Hackett, and Qilong Yi for helpful discussions.
Footnotes
Author contributions: J.T. and H.S.M. contributed to the conception and design of the study. J.T. contributed to the analysis of data. E.L. contributed to the acquisition of the data. J.T. and H.S.M. contributed to writing the first draft. All authors contributed to the revising draft of paper.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: IJS EIC.
EFFECTS writing committee: Erik Lundström, Eva Isaksson, Per Näsman, Per Wester, Björn Mårtensson, Bo Norrving, Håkan Wallén, Jörgen Borg, Martin Dennis, Gillian Mead, Graeme Hankey, Maree Hackett, and Katharina Sunnerhagen.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is funded by a Stroke Association Priority Programme Grant (2015-02). This research was supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the author(s) and not necessarily those of the NIHR or Department of Health and Social Care. EFFECTS has received funding from the Swedish Research Council, the Swedish Heart-Lung Foundation (2013-0496 and 2016-0245), the Swedish Brain Foundation (FO2017-0115), the Swedish Society of Medicine (ID 692921), the King Gustav V and Queen Victoria’s Foundation of Freemasons, and the Swedish Stroke Association (STROKE-Riksförbundet).
ORCID iDs: Jonathan Tay  https://orcid.org/0000-0003-0598-0004
https://orcid.org/0000-0003-0598-0004
Hugh S Markus  https://orcid.org/0000-0002-9794-5996
https://orcid.org/0000-0002-9794-5996
Supplemental material: Supplemental material for this article is available online.
References
- 1. Tay J, Morris RG, Markus HS. Apathy after stroke: diagnosis, mechanisms, consequences, and treatment. Int J Stroke 2021; 16: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hollocks MJ, Lawrence AJ, Brookes RL, et al. Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain 2015; 138: 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. EFFECTS Trial Collaboration. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2020; 19: 661–669. [DOI] [PubMed] [Google Scholar]
- 4. Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 5. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]
- 6. Husain M, Roiser JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci 2018; 19: 470–484. [DOI] [PubMed] [Google Scholar]
- 7. Brown TA, Moore MT. Confirmatory factor analysis. In: Hoyle R. (ed). Handbook of structural equation modeling. New York: The Guilford Press, 2012, pp.361–379. [Google Scholar]
- 8. Kanellopoulos D, Wilkins V, Avari J, et al. Dimensions of poststroke depression and neuropsychological deficits in older adults. Am J Geriatr Psychiatry 2020; 28: 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ketharanathan T, Hanwella R, Weerasundera R, de Silva VA. Diagnostic validity and factor analysis of Montgomery–Asberg Depression Rating Scale in Parkinson disease population. J Geriatr Psychiatry Neurol 2016; 29: 115–119. [DOI] [PubMed] [Google Scholar]
- 10. Klein RB. Measurement models and confirmatory factor analysis. In: Kline R. (ed.) Principles and practice of structural equation modeling. New York: The Guilford Press, 2011, pp.230–264. [Google Scholar]
- 11. Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 2004; 33: 261–304. [Google Scholar]
- 12. Rosseel Y. lavaan: an R package for structural equation modeling and more. J Stat Softw 2012; 48: 1–36. [Google Scholar]
- 13. Rhemtulla M, Brosseau-Liard PÉ, Savalei V. When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychol Methods 2012; 17: 354–373. [DOI] [PubMed] [Google Scholar]
- 14. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 15. Brodaty H, Liu Z, Withall A, Sachdev PS. The longitudinal course of post-stroke apathy over five years. J Neuropsychiatry Clin Neurosci 2013; 25: 283–291. [DOI] [PubMed] [Google Scholar]
- 16. Mayo NE, Fellows LK, Scott SC, Cameron J, Wood-Dauphinee S. A longitudinal view of apathy and its impact after stroke. Stroke 2009; 40: 3299–3307. [DOI] [PubMed] [Google Scholar]
- 17. Tay J, Lisiecka-Ford DM, Hollocks MJ, et al. Network neuroscience of apathy in cerebrovascular disease. Prog Neurobiol 2020; 188: 101785. [DOI] [PubMed] [Google Scholar]
- 18. Tay J, Tuladhar AM, Hollocks MJ, et al. Apathy is associated with large-scale white matter network disruption in small vessel disease. Neurology 2019; 92: e1157–e1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao B, Zhu J, Zuckerman H, et al. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 2019; 92: 109–117. [DOI] [PubMed] [Google Scholar]
- 20. Mead GE, Legg L, Tilney R, et al. Fluoxetine for stroke recovery: meta-analysis of randomized controlled trials. Int J Stroke 2020; 15: 365–376. [DOI] [PubMed] [Google Scholar]
- 21. Myhre P, Radakovic R, Ford C. Validation of the self-rated Dimensional Apathy Scale in community stroke survivors. J Neurol Sci 2022; 434: 120103. [DOI] [PubMed] [Google Scholar]
- 22. Bertens AS, Moonen JE, de Waal MW, et al. Validity of the three apathy items of the Geriatric Depression Scale (GDS-3A) in measuring apathy in older persons. Int J Geriatr Psychiatry 2017; 32: 421–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-wso-10.1177_17474930221124760 for Does fluoxetine reduce apathetic and depressive symptoms after stroke? An analysis of the Efficacy oF Fluoxetine—a randomized Controlled Trial in Stroke trial data set by Jonathan Tay, Björn Mårtensson, Hugh S Markus and Erik Lundström in International Journal of Stroke
Data Availability Statement
Anonymized data supporting the findings of the trial are available to researchers upon reasonable request to the corresponding author (EL; erik.lundstrom@neuro.uu.se) following receipt of a written application and proposal for use of the data, approval by the EFFECTS trial Steering Committee, and establishment of a data sharing agreement.