Abstract
Purpose of Review:
Magnesium is an essential mineral for bone metabolism, but little is known about how magnesium intake alters fracture risk. We conducted a narrative review to better understand how magnesium intake, through supplementation, diet, or altering the concentration of dialysate magnesium, affects mineral bone disease and the risk of fracture in individuals across the spectrum of kidney disease.
Sources of Information:
Peer-reviewed clinical trials and observational studies.
Methods:
We searched for relevant articles in MEDLINE and EMBASE databases. The methodologic quality of clinical trials was assessed using a modified version of the Downs and Black criteria checklist.
Key Findings:
The role of magnesium intake in fracture prevention is unclear in both the general population and in patients receiving maintenance dialysis. In those with normal kidney function, 2 meta-analyses showed higher bone mineral density in those with higher dietary magnesium, whereas 1 systematic review showed no effect on fracture risk. In patients receiving maintenance hemodialysis or peritoneal dialysis, a higher concentration of dialysate magnesium is associated with a lower concentration of parathyroid hormone, but little is known about other bone-related outcomes. In 2 observational studies of patients receiving hemodialysis, a higher concentration of serum magnesium was associated with a lower risk of hip fracture.
Limitations:
This narrative review included only articles written in English. Observed effects of magnesium intake in the general population may not be applicable to those with chronic kidney disease particularly in those receiving dialysis.
Keywords: mineral bone disease, fracture, hemodialysis, magnesium, parathyroid horomone
Abrégé
Justification:
Le magnésium est un minéral essentiel pour le métabolisme osseux, mais on en sait peu sur la façon dont un apport en magnésium modifie le risque de fracture. Nous avons procédé à un examen narratif afin de mieux comprendre comment les maladies liées à la densité minérale osseuse et le risque de fracture sont affectés par un apport en magnésium (supplémentation, régime alimentaire ou modification de la concentration de dialysat de magnésium) chez les personnes atteintes d’insuffisance rénale.
Sources:
Essais cliniques et études observationnelles examinés par des pairs.
Méthodologie:
Nous avons répertorié les articles pertinents dans les bases de données MEDLINE et EMBASE. Une version modifiée des critères de contrôle de la qualité des études de Downs et Black a servi à évaluer la qualité méthodologique des essais cliniques retenus.
Principaux résultats:
Le rôle d’un apport en magnésium dans la prévention des fractures n’est pas clair, tant dans la population générale que chez les patients sous dialyse d’entretien. Chez les personnes ayant une fonction rénale normale, deux méta-analyses ont montré que les personnes dont le régime alimentaire est riche en magnésium présentent une densité minérale osseuse plus élevée; alors qu’une revue systématique n’a montré aucun effet sur le risque de fracture. Chez les patients sous hémodialyse d’entretien ou dialyse péritonéale, une concentration plus élevée de dialysat de magnésium est associée à une plus faible concentration d’hormone parathyroïdienne, mais on en sait peu sur les autres effets liés aux os. Dans deux études observationnelles portant sur des patients sous hémodialyse, une concentration plus élevée de magnésium sérique a été associée à un risque plus faible de fracture de la hanche.
Limites:
Cet examen narratif ne comprend que des articles rédigés en anglais. Il est possible que les effets d’un apport en magnésium observés dans la population générale ne puissent s’appliquer aux personnes atteintes d’une néphropathie chronique, en particulier aux personnes sous dialyse.
Introduction
Patients receiving maintenance dialysis are 5 times more likely to suffer a skeletal fracture than people in the general population.1 This corresponds to an overall fracture rate of 10 to 25 per 1000 patient-years, with those who are older, female, or with a prior fracture being at higher risk.2 -5 For example, 1 in 10 women aged 65 years and older will experience a fracture within 3 years of starting dialysis.2 Patients receiving maintenance dialysis who fracture are also twice as likely to die compared with those who do not, with 50% of those dying within the 1 year of experiencing a fracture.4,6,7
The reasons for the higher fracture risk in patients receiving dialysis are multifactorial. Beyond age-related osteoporotic changes, there are an array of metabolic effects of kidney disease on bone (commonly referred to as mineral bone disorder) which lead to changes in bone turnover and mineralization, resulting in lower bone quality.1,8 These changes are often exacerbated by comorbidities common in patients receiving dialysis such as diabetes and frailty, which independently increase the risk of falls and fracture (Figure 1).9 -12 There has been no significant decrease in the incidence of fracture in patients receiving dialysis in the United States in recent years, despite a clinical emphasis on improved control of bone disease through dietary changes and medications that alter concentrations of serum parathyroid hormone (PTH), calcium, and phosphate.13,14
Figure 1.
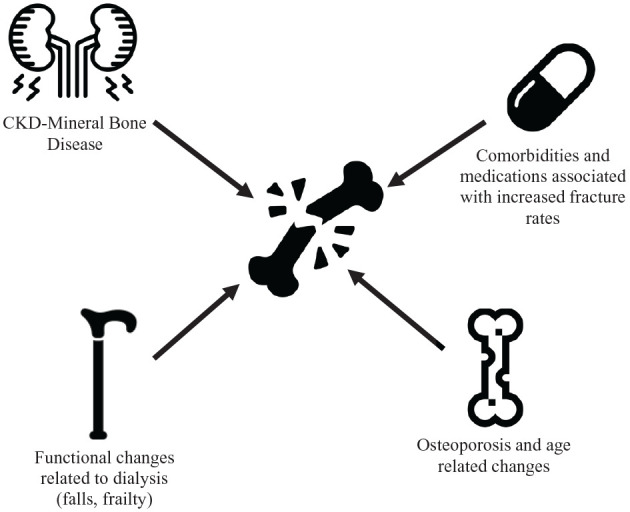
Multifactorial etiology of increased fracture risk in individuals with CKD.
Note. CKD = chronic kidney disease.
Magnesium is the fourth most abundant cation in the body and is primarily stored in bone where it incorporates directly into the hydroxyapatite crystal and contributes to the regulation of bone turnover and homeostasis.15 In the general population, higher magnesium intake may associate with higher bone density.16,17 As patients approach kidney failure, there is less magnesium excretion by the kidney, resulting in higher bone magnesium concentrations.18 -22 Once patients start dialysis, serum and bone magnesium concentrations can be affected by the concentration of dialysate magnesium, presenting the potential to modify fracture risk through alterations in dialysate composition.23,24 Little is known about how these changes affect bone health and fracture risk, but in one large observational study of patients receiving hemodialysis, a higher concentration of serum magnesium was associated with a lower risk of hip fracture.25
In 2022, we launched a large-scale, pragmatic, cluster-randomized clinical trial to assess the effect of a higher versus lower concentration of hemodialysate magnesium on mortality, cardiovascular health, and patient-reported muscle cramps (Dial-Mag Canada, clinicaltrials.gov/ct2/show/NCT04079582). This trial also provides the opportunity to examine the effects of different concentrations of dialysate magnesium on the risk of skeletal fracture and serum markers of mineral and bone disorder in patients receiving in-center hemodialysis. We undertook this narrative review to inform the planning and design of a trial substudy investigating the effect of dialysate magnesium concentration on bone health. The primary objective of this review is to summarize what is known about the effects of magnesium intake on fracture risk across the spectrum of kidney function with a focus on the dialysis population. We also summarized what is known about the effects of different dialysate magnesium concentrations on bone health. This review will focus on 5 areas:
Magnesium homeostasis and its role in bone formation.
The role of magnesium in bone health in adults without chronic kidney disease (CKD).
The effect of magnesium on fracture risk in adults without CKD.
The role of magnesium in bone health in adults receiving dialysis.
The effect of magnesium on fracture risk in adults receiving dialysis.
Methods
A study author (ACC) searched the MEDLINE and EMBASE databases (search strategies are provided in Supplemental Appendix 1). Additional articles were found by searching the reference lists of relevant articles and by using the cited-by search function in Google Scholar. Only English language sources were included.
The methodological quality of clinical trials was assessed by a study author (ACC) using a modified version of the Downs and Black criteria checklist (summarized in Supplemental Appendix 2).26 This checklist contains 27 criteria to evaluate both randomized and nonrandomized trials. This scale assesses the completeness and clarity of study reporting, internal validity (eg, bias and confounding), external validity, and statistical power. The tool was modified slightly for use in our review. Specifically, the scoring for question 27 dealing with statistical power was simplified to a choice of awarding either 1 or 0 points depending on whether there was sufficient power to detect a clinically important effect. On the modified scale, we gave all included studies a score from 0 to 28, and grouped into the following 4 quality levels: excellent (26-28), good (20-25), fair (15-19), and poor (≤14). Results of the methodologic assessment can be seen in Supplemental Appendix 3.
Review
Magnesium Homeostasis, Measurement, and Supplementation
Total body magnesium is determined by the balance between intake and excretion, with dietary magnesium being found in foods such as leafy greens, legumes, seeds, and nuts. The recommended daily allowance of dietary magnesium in Canadian men and women is 420 and 320 mg, respectively; however, up to 50% of the general North American population consumes less than this.27 -30
Magnesium supplementation is commonly oral, although its use is limited by poor intestinal absorption of large doses, as well as diarrhea which may prompt clinicians to use intravenous formulations.31 Approximately 30%-50% of ingested magnesium is absorbed, primarily in the small bowel.22,30,31 While the amount absorbed can range from 20% to 80% in the context of magnesium excess or deficiency, respectively, magnesium balance is predominantly regulated by the kidney. In the setting of normal kidney function, 70%-80% of magnesium (approximately 2 g/d) is filtered through the glomerulus, and 95% is reabsorbed, predominantly in the loop of Henle.22,32
Beyond intake and renal function, medications may also affect magnesium balance. Metformin, a commonly prescribed medication in patients with diabetes, may contribute to magnesium deficiency, and diuretics can also potentiate urinary magnesium loss.33,34 Similarly, proton pump inhibitors are associated with a 3-fold higher rate of hypomagnesemia.22,35
The best way to ascertain magnesium status remains unknown. Magnesium is stored primarily intracellularly, with less than 1% of the body’s stores contained in serum, casting doubt on serum magnesium as a reliable marker for total body magnesium status.36 Although a higher serum concentration of magnesium associates with higher bone magnesium content, there is little correlation between serum magnesium and its concentration in other intracellular compartments, which house a large proportion of total body magnesium, such as erythrocytes, lymphocytes, and muscle.37,38 Measurements of intracellular magnesium, such as the concentration of erythrocyte magnesium, are described in the literature as an alternative assessment of total body magnesium; however, these methods lack robust validation and have not been adopted into general practice.36
Magnesium and Bone Health
Within bone, magnesium is found in 2 distinct areas: cortical and trabecular bone. Cortical magnesium is found on the surface of hydroxyapatite crystals where it is readily exchanged with serum in response to changes in extracellular magnesium18,20 explaining the strong correlation between the concentration of magnesium in serum and bone.18,20
Magnesium affects bone in 2 ways: directly through incorporation into bone microstructure, and indirectly through PTH and 1,25-OH vitamin D. Both magnesium deficiency and excess can be deleterious to bone health, yet the optimal amount of magnesium in bone is unknown. Current Canadian and European osteoporosis guidelines do not discuss magnesium supplementation, and American guidelines only recommend supplementation in only in those who have risk factors for magnesium deficiency.39 -41
Direct effects of magnesium on bone
Magnesium is incorporated into hydroxyapatite crystals through the displacement of calcium. This alters the size and shape of the crystals and results in imperfections, which can paradoxically lead to stronger bone.21,42 Patients with magnesium deficiency and less magnesium incorporation have larger, more perfect hydroxyapatite crystals on bone biopsy, which results in more fragile and brittle bone.21,42 Magnesium deficiency also leads to increased osteoclast and decreased osteoblast activity, resulting in increased bone reabsorption and lower bone density.42
Conversely, in situations where there is an excessive amount of magnesium in bone, hydroxyapatite crystals are smaller and more imperfect, but there is also a higher volume of unmineralized tissue resulting in lower quality bone. Of note, bone biopsies in patients with excess bone magnesium are typically taken from patients with advanced CKD, so the exact contribution of magnesium excess compared with other metabolic derangements of CKD is unclear.19,21,43 In vitro, a high magnesium environment also appears to decrease osteoblast activity.44
Indirect effects on bone
Magnesium plays an indirect role in bone metabolism by regulating PTH and vitamin D synthesis and response. In the general population, hypomagnesemia is a well-established risk factor for hypocalcemia through PTH resistance and decreased PTH secretion. PTH resistance is due to decreased levels of cyclic adenosine 3,5 monophosphate, which is required for PTH signaling.45,46 While well described, the mechanism for decreased PTH secretion is not clear.38,46
Magnesium deficiency may also lead to lower levels of activated vitamin D, through both PTH resistance and because magnesium is a cofactor for multiple proteins in the vitamin D activation pathway.29,47 However, supplementing magnesium in patients who are deficient has not reliably been shown to increase 1,25 dihydroxy vitamin D levels.29
A high concentration of serum magnesium has been shown to suppress PTH levels, on occasion more potently than calcium; however, the reason for this has not been as well explained.48 -52 The effect of magnesium excess on vitamin D activation has not been well studied.
The Role of Magnesium in Bone Health in Adults Without Kidney Disease
In our review of the literature, we found no interventional studies assessing the effects of magnesium intake on fractures in the general population. We reviewed interventional studies of magnesium supplementation on surrogate outcomes of fracture, and observational studies with fracture as an outcome. We found 8 interventional studies meeting our criteria, 3 of which were graded as poor quality, 3 of which were fair, and 2 of which were good quality. We found no difference in findings between higher and lower quality studies. The results of these studies are summarized in Table 1 and full details of the studies can be found in Supplemental Appendix 3.
Table 1.
Summary of the Evidence of the Effect of Magnesium on Parameters of Bone Health.
| Magnesium | Result | Studies found |
|---|---|---|
| Patients receiving dialysis | ||
| Higher (vs lower) concentration of dialysate magnesium | Lower concentration of serum PTH | Three small prospective cohorts Two randomized controlled trials37,58 -61 |
| No change in BMD | One small quasi-experimental study84 | |
| Oral magnesium supplementation | No change in the concentration of serum PTH | Two small prospective cohorts63,64 |
| Lower concentration of serum PTH | Two small randomized controlled trials and one meta-analysis62,85,86 | |
| No change in bone mineral density or histomorphometric parameters on bone biopsy | Two small prospective cohorts63,64 | |
| Higher (vs lower) concentration of serum magnesium | Lower fracture risk | Two observational cohorts one retro25,91 |
| Lower concentration of serum PTH | Five cross-sectional cohorts48 -52 | |
| Lower bone density or bone mineral content | Two small cohorts89,90 | |
| General population | ||
| Oral magnesium supplementation | Higher bone mineral density | Three studies69 -71 |
| Lower concentration of serum PTH | Two small cohorts65,66 | |
| No change in the concentration of serum PTH | Two small cohorts67,68 | |
| Higher concentration of serum PTH | One cohort38 | |
| Higher (vs lower) amount of dietary magnesium | No change in fracture risk | Systematic review of 12 observational studies17 |
| Increase in hip bone mineral density | Two meta-analyses of 12 and 11 observational studies16,17 | |
Note. PTH = parathyroid hormone; BMD = bone mineral density.
The effect of magnesium on bone biochemistry and histomorphometry
Magnesium supplementation and bone biochemistry
Five small studies (sample size ranging from 14 to 67 patients) reported conflicting results on the short-term effect of magnesium supplementation on bone turnover markers.38,65 -68 A nonrandomized, controlled study of postmenopausal women found that short-term magnesium supplementation decreased the concentration of serum PTH and urinary deoxypyridinoline, and increased the concentration of serum osteocalcin, suggesting there was overall lower bone turnover.65 Another nonrandomized, controlled study of supplementation in young healthy men also demonstrated a decrease in serum PTH but showed a decrease in serum osteocalcin levels compared with controls.66
In contrast, a placebo-controlled trial of magnesium-bicarbonate supplemented spring water in postmenopausal women found no changes in markers of bone turnover, including concentrations of serum osteocalcin, urinary hydroxyproline, or N-telopeptide.67 Another randomized, placebo-controlled study of young females also found that magnesium supplementation had no effect on concentrations of PTH or osteocalcin, or on urinary pyridinoline or deoxypyridinoline.68
Finally, one cohort studying magnesium supplementation in patients with gluten-sensitive enteropathy and a low level of erythrocyte magnesium found magnesium supplementation resulted in a 25% increase in serum PTH values with no change in the serum concentration of osteocalcin. This finding contradicts similar studies done in patients receiving dialysis (discussed below) and suggests that the effect of magnesium on bone turnover may depend on baseline magnesium status.38 Of these 5 studies, 2 mentioned an increased incidence of diarrhea in those taking supplementation, while 3 did not discuss side effects at all.35,47 -50
Magnesium supplementation and bone density
Three studies showed magnesium supplementation increased bone mineral density, although the methodological quality of all studies was poor (see Supplemental Appendix 3 for quality assessment). In a nonrandomized trial of 54 postmenopausal women, those supplemented with 100 to 300 mg of elemental magnesium in the form of magnesium hydroxide had an increase in bone mineral density over 2 years compared with those who did not receive supplementation. However, the 2 groups differed significantly on baseline characteristics, and 68% of participants were lost to follow-up.69
Two small studies examined the effect of combination oral nutritional supplements containing magnesium on bone density. A nonrandomized study of 26 postmenopausal women receiving an oral supplement of 600 mg of elemental magnesium and 50% of the RDA (recommended dietary allowance) of calcium, showed supplementation was associated with a 10% increase in bone density over 6 to 12 months compared with dietary advice alone.70 Similarly, a retrospective cohort of 77 predominantly postmenopausal women examined a nutritional supplement containing 25 mg of elemental magnesium in addition to vitamin D, strontium, vitamin K, and docosahexaenoic acid. The result was a modest increase in bone density after 1 year of treatment.71 However, it is difficult to ascertain the independent effect of magnesium supplementation on bone density with the multiple supplements provided.
The effect of magnesium on fracture risk in adults without CKD
Dietary magnesium and fracture risk
Seven observational cohort studies examined the association between magnesium intake and fracture risk. The magnitude and direction of the observed effects are inconsistent, imprecise, and limited by confounding.
Three large cohort studies found a possible association between increased dietary magnesium intake and decreased fracture risk, although in 2 studies, this did not persist after adjustment.72 -74 In a retrospective cohort from Norway, men and women with the highest (vs lowest) tertile concentration of magnesium in drinking water had a 20% and 10% relative risk reduction in hip fracture, respectively.72 This association was maintained after adjustment for demographic factors as well as water pH and calcium content; however, the contribution of drinking water to total dietary magnesium intake was very small (0.2% in men and 0.3% in women).72 Another prospective cohort of 3765 Italian patients with osteoarthritis found the highest (vs lowest) quintile of dietary magnesium intake (as measured by a food frequency questionnaire) was potentially protective against osteoporotic fracture (hazard ratio [HR]: 0.47, 95% confidence interval [CI]: 0.21-1.00 in men, HR: 0.38, 95% CI: 0.17-0.82 in women). However, these results were not robust as no association was seen when magnesium intake was modeled as a continuous variable (as opposed to the quintile categories).73 Finally, a Japanese ecological study comparing regional dietary patterns and hip fracture also found that the association between higher magnesium intake and lower rates of fracture did not persist after adjustment for other regional dietary factors including calcium, vitamin D, and vitamin K.74
In contrast, 2 other cohort studies found no effect of dietary magnesium on fracture risk.75,76 In a prospective cohort study of Finnish men followed over 25 years, dietary magnesium intake did not associate with fracture risk; however, the lowest (vs highest) quartile of serum magnesium concentration was associated with a higher risk of fracture after adjustment for demographic variables (HR: 1.80, 95% CI: 1.10-2.94).75 Similarly, another European cohort study found no association between magnesium intake and fracture, but a higher serum magnesium concentration was found to be potentially protective against fracture in men. In this study, however, there was no association between dietary magnesium intake and the serum concentration of magnesium, illustrating the limitation of equating dietary intake with serum concentration.76
The Women’s Health Initiative noted a possible association between higher magnesium intake and increased fracture.77 More than 70 000 women were prospectively followed over an average of 7.6 years and the highest (vs lowest) quartile of dietary magnesium intake was associated with an increase in bone mineral density. However, patients in the highest quartile of dietary magnesium intake had a higher rate of arm and wrist fracture, which the authors speculated was related to the increased rate of falls also observed in this group.77
Finally, a case-control study of patients with a history of hip fracture also found those in the highest quartile of dietary magnesium intake had almost a 3-fold higher risk of hip fracture compared with those in the lowest quartile. However, this analysis adjusted for only a few demographic variables and did not adjust for other differences in dietary intake, which may have confounded the results.78
In a systematic review and meta-analysis (12 studies including some mentioned above), there was no overall association between magnesium intake and risk of fracture, and the between-study heterogeneity was large (I2 > 70%).17 There was a weak trend toward higher dietary magnesium being associated with higher bone density in the hip, lumbar spine, and forearm; however, these results were limited by small study sizes and not statistically significant.17 A second recent meta-analysis showed that a higher magnesium intake was associated with an increase in hip bone mineral density (beta coefficient: 0.03, 95% CI: 0.01-0.06), but there was inadequate information to assess the association with bone mineral density at other sites or with fracture risk.16
Supplemental magnesium and fracture risk
The association between supplemental magnesium intake and fracture rates is difficult to evaluate as magnesium supplements are often purchased over-the-counter and difficult to retrospectively quantify. In a recently published observational cohort study of >50 000 older adults in Taiwan, those prescribed magnesium oxide (as either an antacid or a laxative) had a higher risk of hip fracture than controls matched on age, sex, comorbidities, and medication use: adjusted HR: 1.66, 95% CI: 1.55-1.81.79 This association persisted when analyzed based on indication (laxative or antacid).79
Two case reports suggest that excessive magnesium supplementation may result in osteomalacia and fracture. In both cases, patients were taking more than 4 to 7 g of elemental magnesium per day and developed multiple femoral stress fractures that healed with cessation of the supplements.80,81
Conclusions based on evidence in adults without CKD
In the general population, there is a lack of high-quality evidence on the effect of magnesium intake on bone health. The effect of magnesium on bone turnover may vary with baseline magnesium status. Supplementation may potentially increase bone mineral density; however, more research is needed to understand its impact on fracture outcomes.
Magnesium Homeostasis in Adults With Kidney Disease
In patients with CKD, the fractional excretion of magnesium is increased to compensate for a decrease in the glomerular filtration rate (GFR). Although individuals with a GFR below 30 mL/min are predisposed to hypermagnesemia, elevated serum magnesium concentration is rare in those with a GFR greater than 10 mL/min, in the setting of normal magnesium intake.22,32
Patients with CKD, however, are also at risk of magnesium deficiency for several reasons. Dietary restriction of potassium and phosphate-rich foods such as fruits, vegetables, and unprocessed cereals inadvertently limits magnesium intake.22,76,53 -55 One study of patients receiving dialysis in the Canary Islands found that only 2% met the RDA of magnesium.55
In addition to diuretics and proton pump inhibitors discussed earlier, other commonly prescribed medications such as calcium-based phosphate binders and the novel potassium binder patiromer can result in magnesium deficiency through decreased intestinal absorption.22,29,35,33,56,82
In patients receiving hemodialysis or peritoneal dialysis, a low concentration of dialysate magnesium can result in net magnesium loss. For example, a dialysate magnesium concentration of 0.5 mmol/L has been shown to lower total body magnesium over time.23 Furthermore, the number of patients who develop hypermagnesemia (defined by a serum magnesium level greater than the lab upper limit of normal; typically a value of ~1.0 mmol/L) is strongly correlated with the concentration of dialysate magnesium.24 In Canada, the dialysate magnesium concentration used in routine practice ranges from 0.375 to 0.75 mmol/L. Rates of hypermagnesemia ranged around 50% to 75% in patients receiving peritoneal or hemodialysis with a dialysate magnesium concentration of 0.75 to 1.0 mmol/L. Serum magnesium concentrations typically remain below 2.0 mmol/L and we did not find any reported cases of symptomatic hypermagnesemia.25,48,50 It is important to note that the optimal concentration of serum magnesium in patients receiving maintenance dialysis has not been well established, and moderate hypermagnesemia (vs normal serum magnesium) was associated with a lower risk of mortality and fractures in several studies.25,51,57
Elevated bone magnesium levels have been widely observed in bone biopsies of patients with advanced CKD both receiving and not receiving dialysis. When compared with patients without kidney disease, patients with CKD had a 66% increase in the magnesium content of both cortical and trabecular bone.18 -21 The clinical effect on fracture risk is unknown, given the complexity of bone disease in patients with CKD.
The Role of Magnesium in Bone Health in Adults Receiving Hemodialysis
The effect of magnesium in individuals with CKD is primarily limited to small studies looking at its effect on surrogate markers of fracture risk in patients receiving dialysis. While some bone biopsy studies included uremic patients who may not be receiving dialysis, we did not find any evidence specifically patients with predialysis CKD. We found 12 interventional studies, 4 of which were considered poor quality, 3 of which were fair, and 5 of which were good quality. We found no difference in findings between higher and lower quality studies. Results are summarized in Table 1 and full details are available in Appendix 3.
Magnesium on bone biochemistry and histomorphometry
Dialysate magnesium
Interventional data on the effect of the concentration of dialysate magnesium on bone health are limited to small studies focused on surrogate markers of fracture risk including PTH and bone biopsy results. Study results are summarized in Table 1 and full details are available in Supplemental Appendix 3.
We found 5 studies examining the effect of the concentration of dialysate magnesium on serum PTH, all of which showed that increasing dialysate magnesium led to reductions in PTH.37,58 -61 A randomized trial of 22 patients receiving hemodialysis found that increasing the dialysate magnesium concentration from 0.2 to 0.75 mmol/L led to a 20% reduction in the concentration of serum PTH.37 Similar reductions in PTH concentration with higher hemodialysate magnesium were found in a randomized trial of 59 patients comparing concentrations of 0.5 and 1.0 mmol/L.61 A post hoc analysis of this trial also showed an increase in bone-specific alkaline phosphatase and a decrease in tartrate-resistant acid-phosphate 5b concentrations in the higher dialysate magnesium concentration group, suggestive of decreased bone resorption and increased bone formation.83 In a nonrandomized crossover trial of 26 hemodialysis patients, increasing the dialysate magnesium concentration from 0.25 to 1.25 mmol/L resulted in a 35% to 40% reduction in the concentration of serum PTH, along with small reductions in the serum concentration of calcium and phosphate (4% and 10%, respectively).60
Similarly, another observational cohort study also found that lowering the concentration of dialysate magnesium from 0.5 to 0.25 mmol/L was associated with a rise in the concentration of serum PTH, with a larger increase seen in those with a lower baseline PTH.59 Finally, a cohort study of 20 patients receiving hemodialysis found increasing the concentration of dialysate magnesium from 0.75 to 1.5 mmol/L was associated with a 23% decline in the concentration of serum PTH.58 One small nonrandomized study of 40 patients suggested that a dialysate magnesium concentration of 0.875 mmol/L could be associated with a lower serum PTH compared with a dialysate magnesium concentration of 0.35 mmol/L but this was not statistically significant.84 There was also no change in bone mineral density.84
Although studies have consistently suggested that increasing the concentration of dialysate magnesium is associated with a reduction in the concentration of serum PTH, it is unclear whether this association translates into lower fracture risk (or potentiates low turnover bone disease). In fact, a small study of 6 patients using serial bone biopsy suggested that lowering the concentration of dialysate magnesium from 0.5 to 0.25 mmol/L for 1 year resulted in a drop in bone magnesium content, and resulted in less osteomalacia and fewer mineralization defects on bone biopsy.43
The above studies suggest that altering dialysate magnesium is an effective way to alter serum and total body magnesium and may affect bone turnover. However, the ideal dialysate, serum, or bone magnesium concentration for the prevention of fracture remains unknown.
Magnesium supplementation in dialysis
We found 4 studies examining the effect of magnesium supplementation on markers of bone health in patients receiving dialysis. In 2 studies, oral magnesium salts used as phosphate binders showed little effect on the concentration of serum PTH or other markers of bone health. A pilot study of 7 patients used magnesium/calcium carbonate as a phosphate binder over 18 months (700 mg of elemental magnesium daily) and a dialysate magnesium concentration of 0.375 mmol/L. While phosphate concentrations remained on target, there was no change in the concentration of serum PTH or vertebral bone mineral density over time.63 Another study switched patients from aluminum hydroxide to magnesium hydroxide as phosphate binders (average 1086 mg of elemental magnesium per day) while concurrently decreasing the concentration of dialysate magnesium from 0.75 to 0.375 mmol/L. This resulted in a 50% increase in the concentration of serum magnesium (0.96-1.56 mmol/L) but no change in the concentration of serum PTH or change in histomorphometric parameters on bone biopsy.64
Two randomized clinical trials examined oral magnesium explicitly used as supplementation in patients receiving maintenance hemodialysis with a dialysate magnesium concentration of 0.5 mmol/L. In the first, 47 patients were randomized 3:1 to receive either magnesium citrate (98 mg of elemental magnesium every other day) or calcium acetate. A 45% reduction in serum PTH after 2 months was observed in the magnesium citrate group; however, the final PTH concentration was not statistically different from those receiving calcium acetate.62 In the second trial, patients were randomized to receive magnesium oxide (250 mg of elemental magnesium) 3 times weekly or placebo. A 22% decrease in the concentration of serum PTH after 6 months (P = .02) was observed in the magnesium group, whereas no change was observed in the placebo group.85 A meta-analysis of these 2 trials showed a weighted mean decrement in the concentration of PTH 25 pmol/L with magnesium supplementation, which was statistically significant with low heterogeneity.86 In these trials, 3% to 4% of individuals discontinued their supplementation due to significant diarrhea.62,85
Serum magnesium
We found several observational studies evaluating the association between serum magnesium concentration and serum markers of CKD-mineral bone disease in patients receiving dialysis. These results must be interpreted with caution, as the concentration of serum magnesium is a poor marker of total body magnesium and is heavily influenced by other factors that affect fracture risk, including nutritional and inflammatory status.36,87,88
Five cross-sectional studies of patients receiving dialysis have shown that a higher concentration of serum magnesium (vs lower) associates with lower concentration of serum PTH. Two small studies (41 and 110 patients receiving hemodialysis) showed a moderate-to-strong inverse correlation between the concentration of serum magnesium and serum PTH (r = −.61 and r = −0.48, respectively) despite a large proportion (59% and 73%) of the patients being hypermagnesemic (>1.0 or 1.03 mmol/L, respectively).49,50 Similarly, in a large cross-sectional study of more than 140 000 patients receiving maintenance hemodialysis in Japan, those with the lowest serum magnesium concentration (<1.11 mmol/L) had a higher concentration of serum PTH than those with intermediate or higher concentrations of serum magnesium (>1.28 mmol/L).51
The inverse association between the serum concentrations of magnesium and serum PTH has also been shown in patients undergoing continuous ambulatory peritoneal dialysis (CAPD). A cross-sectional study of patients receiving a dialysate magnesium concentration of 0.25 mmol/L had a 26% prevalence of hypermagnesemia (>1.1 mmol/L). Serum magnesium was weakly inversely associated with serum PTH, but only in a subgroup of those with a relatively suppressed baseline PTH concentration of less than 32 pmol/L.52 A similar study of 51 patients receiving CAPD using a higher concentration of dialysate magnesium (0.75 mmol/L) observed a 59% incidence of hypermagnesemia (>1.01 mmol/L) and found that a higher concentration of serum magnesium was moderately associated with a lower concentration of serum PTH (r = −0.70), regardless of the degree of baseline PTH elevation.48
Bone magnesium content
One study examining bone biopsies of 153 patients receiving hemodialysis or peritoneal dialysis found that patients with higher bone magnesium content had lower microhardness and mineralization on biopsy than those with lower bone magnesium content.19 In contrast to the above studies assessing serum magnesium and PTH concentrations, there was a weak but statistically significant positive correlation between higher bone magnesium and a higher concentration of serum PTH (a coefficient of determination: R2 = .0724; P = .001), but serum magnesium was not assessed.19
Serum magnesium and bone density in patients receiving dialysis
Two studies have shown that a higher concentration of serum magnesium associates with lower bone density. A cross-sectional study of 25 men receiving chronic dialysis found that low femoral bone density was independently associated with hypermagnesemia, along with hypoalbuminemia, a higher concentration of serum alkaline phosphatase and serum aluminum.89 Another study of serial bone mineral content measurements in a cohort of 93 patients receiving maintenance dialysis similarly found low bone mineral content was associated with a high concentration of serum magnesium. Serum magnesium did not appear to affect the rate of bone mineral content loss.90
Effect of magnesium on fracture risk in adults with CKD
We did not find any interventional or observational studies examining the effect of magnesium intake on fracture risk in adults with CKD including those using dialysis. One retrospective cohort of more than 100 000 patients receiving dialysis in Japan found that a lower (vs higher) concentration of serum magnesium was associated with a higher 2-year risk of hip fracture. In this study, the highest quartile of magnesium concentration was above the laboratory upper limit of normal.25 After adjusting for relevant confounders, the risk ratio of hip fracture in the lowest quartile of participants (serum magnesium ≤0.95 mmol/L) compared with the highest quartile (serum magnesium ≥1.19 mmol/L) was 1.23 (95% CI: 1.06-1.44).25 The population attributable risk of hip fracture from low magnesium for the lowest 3 magnesium quartiles (ie, serum magnesium <1.19 mmol/L) was 14% suggesting a role for mild hypermagnesemia in protecting against hip fracture.25 Similarly, a smaller retrospective cohort of 358 hemodialysis patients found a serum magnesium <1.03 mmol/L was associated with a 2.3-fold higher risk of fracture (95% CI: 1.03-5.17) compared with those with a serum magnesium ≥1.03.91
Conclusions Based on Evidence From Studies in Adults With Kidney Disease
In individuals with CKD, renal magnesium excretion is decreased, however they also have many risk factors for magnesium deficiency. The effect of magnesium intake or supplementation on fracture risk in patients receiving dialysis has mixed evidence (effects summarized in Table 1). A higher concentration of dialysate and serum magnesium consistently associates with a lower concentration of serum PTH in small, short-term studies. However, it is unknown whether this translates into a lower risk of fracture or potentiates low-turnover bone disease. There is also mixed evidence around the effect of magnesium supplementation on markers of bone turnover and bone density. In future research, it will be important to examine the effect of magnesium on fracture risk, as well as consequences of hyperparathyroidism such as cinacalcet or parathyroidectomy rates.
Limitations
We limited our search to English language articles. We were unable to find any applicable studies in those with advanced kidney disease not yet receiving dialysis. Given the differences in physiology, conclusions in the general population may not be applicable to those receiving dialysis.
Conclusion
Overall, there is limited high-quality evidence regarding the role of magnesium in fracture prevention. In the general population, magnesium supplementation may increase bone density; however, there is no evidence that this alters fracture risk. In those with dialysis-dependent CKD, increased magnesium intake may decrease bone turnover and lower PTH but the effect on fracture risk remains unknown. Mildly elevated serum magnesium has also been associated with a lower risk of fracture than those with normal serum magnesium but this relationship may be affected by other factors such as nutritional intake and inflammatory status. With the current evidence, limited by surrogate outcomes and sometimes poor quality, there is no clear guidance for magnesium supplementation across the CKD spectrum. Given that individuals with kidney disease have an increased risk of fracture and risk factors predisposing to hypomagnesemia, adjusting the dialysate magnesium concentration is an attractive method of altering total body magnesium while avoiding side effects associated with supplementation or the concomitant potassium and phosphate loads associated with increased dietary intake. This review informs a substudy of a larger trial of dialysate magnesium which will focus specifically on the effect of dialysate magnesium concentration on fracture risk and consequences of hyperparathyroidism.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581231154183 for Magnesium and Fracture Risk in the General Population and Patients Receiving Dialysis: A Narrative Review by Andrea C. Cowan, Kristin K. Clemens, Jessica M. Sontrop, Stephanie N. Dixon, Lauren Killin, Sierra Anderson, Rey R. Acedillo, Amit Bagga, Clara Bohm, Pierre Antoine Brown, Brenden Cote, Varun Dev, Claire Harris, Swapnil Hiremath, Mercedeh Kiaii, Eduardo Lacson Jr, Amber O. Molnar, Matthew J. Oliver, Malvinder S. Parmar, Jennifer M. McRae, Bharat Nathoo, Kathleen Quinn, Nikhil Shah, Samuel A. Silver, Daniel J. Tascona, Stephanie Thompson, Robert H. Ting, Marcello Tonelli, Hans Vorster, Davinder B. Wadehra, Ron Wald, Myles Wolf and Amit X. Garg in Canadian Journal of Kidney Health and Disease
Acknowledgments
We would like to thank Alla Iansavitchene for her assistance in developing the search strategy for the literature review.
Footnotes
Ethics Approval and Consent to Participate: No patient consent or ethics approval was required for this review.
Consent for Publication: All authors provided consent to the publication of this research.
Availability of Data and Materials: There is no data or raw materials available for this review.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Cowan is supported by Physicians Services Incorporated, Lawson Health Research Institute, Schulich School of Medicine and Dentisry and the Western University Department of Medicine. Dr. Garg supported by the Dr. Adam Linton Chair in Kidney Health Analytics.
ORCID iDs: Andrea C. Cowan  https://orcid.org/0000-0002-9480-969X
https://orcid.org/0000-0002-9480-969X
Jessica M. Sontrop  https://orcid.org/0000-0001-7784-2028
https://orcid.org/0000-0001-7784-2028
Stephanie N. Dixon  https://orcid.org/0000-0002-7566-6574
https://orcid.org/0000-0002-7566-6574
Pierre Antoine Brown  https://orcid.org/0000-0002-0283-4530
https://orcid.org/0000-0002-0283-4530
Swapnil Hiremath  https://orcid.org/0000-0003-0010-3416
https://orcid.org/0000-0003-0010-3416
Amber O. Molnar  https://orcid.org/0000-0003-4549-0202
https://orcid.org/0000-0003-4549-0202
Malvinder S. Parmar  https://orcid.org/0000-0003-3182-0530
https://orcid.org/0000-0003-3182-0530
Samuel A. Silver  https://orcid.org/0000-0002-1843-6131
https://orcid.org/0000-0002-1843-6131
Stephanie Thompson  https://orcid.org/0000-0003-3109-6837
https://orcid.org/0000-0003-3109-6837
Amit X. Garg  https://orcid.org/0000-0003-3398-3114
https://orcid.org/0000-0003-3398-3114
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Moe SM, Nickolas TL. Fractures in patients with CKD: time for action. Clin J Am Soc Nephrol. 2016;11(11):1929-1931. doi: 10.2215/CJN.09500916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naylor KL, McArthur E, Leslie WD, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014;86(4):810-818. doi: 10.1038/ki.2013.547. [DOI] [PubMed] [Google Scholar]
- 3. Iseri K, Carrero JJ, Evans M, et al. Major fractures after initiation of dialysis: incidence, predictors and association with mortality. Bone. 2020;133:115242. doi: 10.1016/j.bone.2020.115242. [DOI] [PubMed] [Google Scholar]
- 4. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 5. Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70(7):1358-1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 6. Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis. 2004;44(4):672-679. doi: 10.1053/j.ajkd.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 7. Nitsch D, Mylne A, Roderick PJ, Smeeth L, Hubbard R, Fletcher A. Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol Dial Transplant. 2009;24(5):1539-1544. doi: 10.1093/ndt/gfn678. [DOI] [PubMed] [Google Scholar]
- 8. Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO chronic kidney disease–mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. 2017;92(1):26-36. doi: 10.1016/j.kint.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 9. Wu PY, Chao C, ter Chan DC, Huang JW, Hung KY. Contributors, risk associates, and complications of frailty in patients with chronic kidney disease: a scoping review. Ther Adv Chronic Dis. 2019;10:2040622319880382. doi: 10.1177/2040622319880382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18(4):427-444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 11. Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62(7):744-751. https://academic.oup.com/biomedgerontology/article/62/7/744/581910. Accessed January 27, 2023. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz A, v Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls a prospective study. Diabetes Care. 2002;25(10):1749-1754. [DOI] [PubMed] [Google Scholar]
- 13. Wagner J, Jhaveri KD, Rosen L, Sunday S, Mathew AT, Fishbane S. Increased bone fractures among elderly United States hemodialysis patients. Nephrol Dial Transplant. 2014;29(1):146-151. doi: 10.1093/ndt/gft352. [DOI] [PubMed] [Google Scholar]
- 14. Beaubrun AC, Kilpatrick RD, Freburger JK, Bradbury BD, Wang L, Brookhart MA. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol. 2013;24(9):1461-1469. doi: 10.1681/ASN.2012090916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aydin H. Magnesium supplementation and bone. In: Watson R, Preedy V, Zibadi S, eds. Magnesium in Human Health and Disease. Totowa, NJ: Humana Press Inc.; 2013:149-157. doi: 10.1007/978-1-62703-044-1_10. [DOI] [Google Scholar]
- 16. Groenendijk I, van Delft M, Versloot P, van Loon LJC, de Groot LCPGM. Impact of magnesium on bone health in older adults: a systematic review and meta-analysis. Bone. 2022;154:116233. doi: 10.1016/j.bone.2021.116233. [DOI] [PubMed] [Google Scholar]
- 17. Farsinejad-Marj M, Saneei P, Esmaillzadeh A. Dietary magnesium intake, bone mineral density and risk of fracture: a systematic review and meta-analysis. Osteoporos Int. 2016;27(4):1389-1399. doi: 10.1007/s00198-015-3400-y. [DOI] [PubMed] [Google Scholar]
- 18. Contiguglia SR, Alfrey AC, Miller N, Butkus D. Total body magnesium excess in chronic renal failure. Lancet. 1972;299(7764):1300-1302. doi: 10.1016/S0140-6736(72)91032-X. [DOI] [PubMed] [Google Scholar]
- 19. Ng AHM, Hercz G, Kandel R, Grynpas MD. Association between fluoride, magnesium, aluminum and bone quality in renal osteodystrophy. Bone. 2004;34(1):216-224. doi: 10.1016/j.bone.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 20. Alfrey AC, Miller NL. Bone magnesium pools in uremia. J Clin Invest. 1973;52(12):3019-3027. doi: 10.1172/JCI107500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen L, Kitzes R. Infrared spectroscopy and magnesium content of bone mineral in osteoporotic women. Isr J Med Sci. 1982;17(12):1123-1125. https://europepmc.org/article/med/7327911. Accessed September 24, 2020. [PubMed] [Google Scholar]
- 22. Navarro-González JF, Mora-Fernández C, García-Pérez J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial. 2009;22(1):37-44. doi: 10.1111/j.1525-139X.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 23. Leenders NHJ, van Ittersum FJ, Hoekstra T, Hoenderop JGJ, Vervloet MG. Routine hemodialysis induces a decline in plasma magnesium concentration in most patients: a prospective observational cohort study. Sci Rep. 2018;8(1):1-9. doi: 10.1038/s41598-018-28629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saha H, Harmoinen A, Pietilä K, Mörsky P, Pasternack A. Measurement of serum ionized versus total levels of magnesium and calcium in hemodialysis patients. Clin Nephrol. 1996;46(5):326-331. https://pubmed-ncbi-nlm-nih-gov.proxy1.lib.uwo.ca/8953122/. Accessed November 16, 2020. [PubMed] [Google Scholar]
- 25. Sakaguchi Y, Hamano T, Wada A, Hoshino J, Masakane I. Magnesium and risk of hip fracture among patients undergoing hemodialysis. J Am Soc Nephrol. 2018;29(3):991-999. doi: 10.1681/ASN.2017080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Canada.ca. Dietary reference intakes tables. Date unknown. https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/dietary-reference-intakes/tables.html. Accessed November 16, 2020.
- 28. Cahill F, Shahidi M, Shea J, et al. High dietary magnesium intake is associated with low insulin resistance in the Newfoundland population. PLoS ONE. 2013;8(3):e58278. doi: 10.1371/journal.pone.0058278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosanoff A, Dai Q, Shapses SA. Essential nutrient interactions: does low or suboptimal magnesium status interact with vitamin D and/or calcium status? Adv Nutr. 2016;7(1):25-43. doi: 10.3945/an.115.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quamme GA. Recent developments in intestinal magnesium absorption. Curr Opin Gasteroenterol. 2008;24(2):230-235. doi: 10.1097/MOG.0b013e3282f37b59. [DOI] [PubMed] [Google Scholar]
- 31. Schuchardt JP, Hahn A. Intestinal absorption and factors influencing bioavailability of magnesium-an update. Curr Nutr Food Sci. 2017;13(4):260-278. doi: 10.2174/1573401313666170427162740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van de Wal-Visscher ER, Kooman JP, van der Sande FM. Magnesium in chronic kidney disease: should we care? Blood Purif. 2018;45(1-3):173-178. doi: 10.1159/000485212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cunningham J, Rodríguez M, Messa P. Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clin Kidney J. 2012;5(suppl 1):i39-i51. doi: 10.1093/ndtplus/sfr166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bouras H, Roig SR, Kurstjens S, et al. Metformin regulates TRPM6, a potential explanation for magnesium imbalance in type 2 diabetes patients. Can J Physiol Pharmacol. 2020;98(6):400-411. doi: 10.1139/cjpp-2019-0570. [DOI] [PubMed] [Google Scholar]
- 35. Srinutta T, Chewcharat A, Takkavatakarn K, et al. Proton pump inhibitors and hypomagnesemia: a meta-analysis of observational studies. Medicine. 2019;98(44):e17788. doi: 10.1097/MD.0000000000017788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Workinger JL, Doyle RP, Bortz J. Challenges in the diagnosis of magnesium status. Nutrients. 2018;10(9):1202. doi: 10.3390/nu10091202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nilsson P, Johansson SG, Danielson BG. Magnesium studies in hemodialysis patients before and after treatment with low dialysate magnesium. Nephron. 1984;37(1):25-29. doi: 10.1159/000183202. [DOI] [PubMed] [Google Scholar]
- 38. Rude RK, Olerich M. Magnesium deficiency: possible role in osteoporosis associated with gluten-sensitive enteropathy. Osteoporos Int. 1996;6(6):453-461. doi: 10.1007/BF01629578. [DOI] [PubMed] [Google Scholar]
- 39. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(suppl 1):1-46. doi: 10.4158/GL-2020-0524SUPPL. [DOI] [PubMed] [Google Scholar]
- 40. Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporo Int. 2019;30(1):3-44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595-1622. doi: 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- 42. Castiglioni S, Cazzaniga A, Albisetti W, Maier JAM. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013;5(8):3022-3033. doi: 10.3390/nu5083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gonella M, Ballanti P, della Rocca C, et al. Improved bone morphology by normalizing serum magnesium in chronically hemodialyzed patients. Miner Electrolyte Metab. 1988;14(4):240-245. https://pubmed-ncbi-nlm-nih-gov.proxy1.lib.uwo.ca/3211092/. Accessed January 27, 2023. [PubMed] [Google Scholar]
- 44. Leidi M, Dellera F, Mariotti M, Maier JAM. High magnesium inhibits human osteoblast differentiation in vitro. Magnes Res. 2011;24(1):1-6. doi: 10.1684/mrh.2011.0271. [DOI] [PubMed] [Google Scholar]
- 45. Pironi L, Malucelli E, Guidetti M, et al. The complex relationship between magnesium and serum parathyroid hormone: a study in patients with chronic intestinal failure. Magnes Res. 2009;22(1):37-43. doi: 10.1684/mrh.2009.0158. [DOI] [PubMed] [Google Scholar]
- 46. Freitag JJ, Martin KJ, Conrades MB, et al. Evidence for skeletal resistance to parathyroid hormone in magnesium deficiency. Studies in isolated perfused bone. J Clin Invest. 1979;64(5):1238-1244. doi: 10.1172/JCI109578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rude RK, Adams JS, Ryzen E, et al. Low serum concentrations of 1,25-dihydroxyvitamin D in human magnesium deficiency. J Clin Endocrinol Metab. 1985;61(5):933-940. doi: 10.1210/jcem-61-5-933. [DOI] [PubMed] [Google Scholar]
- 48. Navarro JF, Mora C, Macia M, Garcia J. Serum magnesium concentration is an independent predictor of parathyroid hormone levels in peritoneal dialysis patients. Perit Dial Int. 1999;19(5):455-461. doi: 10.1177/089686089901900509. [DOI] [PubMed] [Google Scholar]
- 49. Navarro JF, Mora C, Jimenez A, Torres A, Macia M, Garcia J. Relationship between serum magnesium and parathyroid hormone levels in hemodialysis patients. Am J Kidney Dis. 1999;34(1):43-48. doi: 10.1016/S0272-6386(99)70106-X. [DOI] [PubMed] [Google Scholar]
- 50. Navarro JF, Macía ML, Gallego E, et al. Serum magnesium concentration and PTH levels. Is long-term chronic hypermagnesemia a risk factor for adynamic bone disease? Scand J Urol Nephrol. 1997;31(3):275-280. doi: 10.3109/00365599709070348. [DOI] [PubMed] [Google Scholar]
- 51. Sakaguchi Y, Fujii N, Shoji T, et al. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: a cohort study. PLoS ONE. 2014;9(12):e116273. doi: 10.1371/journal.pone.0116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cho MS, Lee KS, Lee YK, et al. Relationship between the serum parathyroid hormone and magnesium levels in continuous ambulatory peritoneal dialysis (CAPD) patients using low magnesium peritoneal dialysate. Korean J Intern Med. 2002;17(2):114-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brink EJ, Beynen AC. Nutrition and magnesium absorption: a review. Prog Food Nutr Sci. 1992;16(2):125-162. [PubMed] [Google Scholar]
- 54. Ryder KM, Shorr RI, Bush AJ, et al. Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects. J Am Geriatr Soc. 2005;53(11):1875-1880. doi: 10.1111/j.1532-5415.2005.53561.x. [DOI] [PubMed] [Google Scholar]
- 55. Luis D, Zlatkis K, Comenge B, et al. Dietary quality and adherence to dietary recommendations in patients undergoing hemodialysis. J Ren Nutr. 2016;26(3):190-195. doi: 10.1053/j.jrn.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 56. Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211-221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 57. Xiong J, He T, Wang M, et al. Serum magnesium, mortality, and cardiovascular disease in chronic kidney disease and end-stage renal disease patients: a systematic review and meta-analysis. J Nephrol. 2019;32(5):791-802. doi: 10.1007/s40620-019-00601-6. [DOI] [PubMed] [Google Scholar]
- 58. Mcgonigle R, Weston M, Keenan J, Jackson D, Parsons V. Effect of hypermagnesemia on circulating plasma parathyroid hormone in patients on regular hemodialysis therapy. Magnesium. 1984;3(1):1-7. [PubMed] [Google Scholar]
- 59. Parsons V, Papapoulos SE, Weston MJ, Tomlinson S, O’Riordan JLH. The long-term effect of lowering dialysate magnesium on circulating parathyroid hormone in patients on regular haemodialysis therapy. Acta Endocrinol (Copenh). 1980;93(4):455-460. doi: 10.1530/acta.0.0930455. [DOI] [PubMed] [Google Scholar]
- 60. Pletka P, Bernstein DS, Hampers CL, Merrill JP, Sherwood LM. Relationship between magnesium and secondary hyperparathyroidism during long-term hemodialysis. Metabolism. 1974;23(7):619-630. doi: 10.1016/S0026-0495(74)80021-1. [DOI] [PubMed] [Google Scholar]
- 61. Bressendorff I, Hansen D, Schou M, Pasch A, Brandi L. The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease: a randomized, controlled clinical trial. Clin J Am Soc Nephrol. 2018;13(9):1373-1380. doi: 10.2215/CJN.13921217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Turgut F, Kanbay M, Metin MR, Uz E, Akcay A, Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008;40(4):1075-1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 63. Spiegel DM, Farmer B. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int. 2009;13(4):453-459. doi: 10.1111/j.1542-4758.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 64. Morinière PH, Vinatier I, Westeel PF, et al. Magnesium hydroxide as a complementary aluminium-free phosphate binder to moderate doses of oral calcium in uraemic patients on chronic haemodialysis: lack of deleterious effect on bone mineralisation. Nephrol Dial Transplant. 1988;3(5):651-656. doi: 10.1093/oxfordjournals.ndt.a091722. [DOI] [PubMed] [Google Scholar]
- 65. Aydin H, Deyneli O, Yavuz D, et al. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol Trace Elem Res. 2010;133(2):136-143. doi: 10.1007/s12011-009-8416-8. [DOI] [PubMed] [Google Scholar]
- 66. Dimai HP, Porta S, Wirnsberger G, et al. Daily Oral magnesium supplementation suppresses bone turnover in young adult males. J Clin Endocrinol Metab. 1998;83(8):2742-2748. doi: 10.1210/jcem.83.8.5015. [DOI] [PubMed] [Google Scholar]
- 67. Day RO, Liauw W, Tozer LMR, McElduff P, Beckett RJ, Williams KM. A double-blind, placebo-controlled study of the short term effects of a spring water supplemented with magnesium bicarbonate on acid/base balance, bone metabolism and cardiovascular risk factors in postmenopausal women. BMC Res Notes. 2010;3(1):180. doi: 10.1186/1756-0500-3-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Doyle L, Flynn A, Cashman K. The effect of magnesium supplementation on biochemical markers of bone metabolism or blood pressure in healthy young adult females. Eur J Clin Nutr. 1999;53(4):255. doi: 10.1038/sj.ejcn.1600714. [DOI] [PubMed] [Google Scholar]
- 69. Stendig-Lindberg G, Tepper R, Leichter I. Trabecular bone density in a two year controlled trial of peroral magnesium in osteoporosis. Magnes Res. 1993;6(2):155-163. [PubMed] [Google Scholar]
- 70. Abraham GE, Grewal H. A total dietary program emphasizing magnesium instead of calcium: effect on the mineral density of calcaneous bone in postmenopausal women on hormonal therapy. J Reprod Med. 1990;35(5):503-507. https://europepmc.org/article/med/2352244. Accessed September 21, 2020. [PubMed] [Google Scholar]
- 71. Genuis SJ, Bouchard TP. Combination of micronutrients for bone (COMB) study: bone density after micronutrient intervention. J Environ Public Health. 2012;2012:354151. doi: 10.1155/2012/354151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dahl C, Søgaard AJ, Tell GS, et al. Nationwide data on municipal drinking water and hip fracture: could calcium and magnesium be protective? a NOREPOS study. Bone. 2013;57(1):84-91. doi: 10.1016/j.bone.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 73. Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117(11):1570-1576. doi: 10.1017/S0007114517001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yaegashi Y, Onoda T, Tanno K, Kuribayashi T, Sakata K, Orimo H. Association of hip fracture incidence and intake of calcium, magnesium, vitamin D, and vitamin K. Eur J Epidemiol. 2008;23(3):219-225. doi: 10.1007/s10654-008-9225-7. [DOI] [PubMed] [Google Scholar]
- 75. Kunutsor SK, Whitehouse MR, Blom AW, Laukkanen JA. Low serum magnesium levels are associated with increased risk of fractures: a long-term prospective cohort study. Eur J Epidemiol. 2017;32(7):593-603. doi: 10.1007/s10654-017-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hayhoe RPG, Lentjes MAH, Luben RN, Khaw KT, Welch AA. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am J Clin Nutr. 2015;102(2):376-384. doi: 10.3945/ajcn.114.102723. [DOI] [PubMed] [Google Scholar]
- 77. Orchard TS, Larson JC, Alghothani N, et al. Magnesium intake, bone mineral density, and fractures: results from the Women’s Health Initiative Observational Study. Am J Clin Nutr. 2014;99(4):926-933. doi: 10.3945/ajcn.113.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Michaëlsson K, Holmberg L, Mallmin H, et al. Diet and hip fracture risk: a case-control study. Int J Epidemiol. 1995;24(4):771-782. doi: 10.1093/ije/24.4.771. [DOI] [PubMed] [Google Scholar]
- 79. Wu YY, Chang CL, Wang JH, Wei WT. Magnesium oxide and hip fracture in the elderly: a population-based retrospective cohort analysis. Osteoporos Int. 2020;31(7):1231-1238. doi: 10.1007/s00198-020-05278-3. [DOI] [PubMed] [Google Scholar]
- 80. Sivas F, Günesen O, Ozoran K, Alemdaroğlu E. Osteomalacia from Mg-containing antacid: a case report of bilateral hip fracture. Rheumatol Int. 2007;27(7):679-681. doi: 10.1007/s00296-006-0273-6. [DOI] [PubMed] [Google Scholar]
- 81. Neumann L, Jensen BG. Osteomalacia from al and mg antacids: report of a case of bilateral hip fracture. Acta Orthop Scand. 1989;60(3):361-362. doi: 10.3109/17453678909149294. [DOI] [PubMed] [Google Scholar]
- 82. Fusaro M, D’Arrigo G, Pitino A, et al. Increased risk of bone fractures in hemodialysis patients treated with proton pump inhibitors in real world: results from the dialysis outcomes and practice patterns study (DOPPS). J Bone Miner Res. 2019;34(12):2238-2245. doi: 10.1002/jbmr.3842. [DOI] [PubMed] [Google Scholar]
- 83. Bressendorff I, Hansen D, Pasch A, et al. The effect of increasing dialysate magnesium on calciprotein particles, inflammation and bone markers: post hoc analysis from a randomized controlled clinical trial. Nephrol Dial Transplant. 2021;36(4):713-721. doi: 10.1093/ndt/gfz234. [DOI] [PubMed] [Google Scholar]
- 84. Srisuwarn P, Sethakarun S, Nongnuch A, et al. Dialysate magnesium and coronary artery calcification, bone mineral density, and cramping in maintenance hemodialysis: a quasi-experimental study. Kidney Med. 2022;4(2):100374. doi: 10.1016/j.xkme.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mortazavi M, Moeinzadeh F, Saadatnia M, Shahidi S, McGee JC, Minagar A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: a double-blind, randomized, placebo-controlled trial. Eur Neurol. 2013;69(5):309-316. doi: 10.1159/000346427. [DOI] [PubMed] [Google Scholar]
- 86. Guo G, Zhou J, Xu T, et al. Effect of magnesium supplementation on chronic kidney disease-mineral and bone disorder in hemodialysis patients: a meta-analysis of randomized controlled trials. J Ren Nutr. 2022;32:102-111. doi: 10.1053/j.jrn.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 87. Fein P, Suda V, Borawsky C, et al. Relationship of serum magnesium to body composition and inflammation in peritoneal dialysis patients. Adv Perit Dial. 2010;26:112-115. [PubMed] [Google Scholar]
- 88. Pakfetrat M, Malekmakan L, Roozbeh J, Haghpanah S. Magnesium and its relationship to C-reactive protein among hemodialysis patients. Magnes Res. 2008;21(3):167-170. doi: 10.1684/mrh.2008.0145. [DOI] [PubMed] [Google Scholar]
- 89. Eisenberg B, Tzamaloukas AH, Murata GH, Elliott TM, Jackson JE. Factors affecting bone mineral density in elderly men receiving chronic in-center hemodialysis. 1991;16:30-36. doi: 10.1097/00003072-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 90. Heaf JG, Nielsen LP, Mogensen NB. Use of bone mineral content determination in the evaluation of osteodystrophy among hemodialysis patients. Nephron. 1983;35(2):103-107. doi: 10.1159/000183056. [DOI] [PubMed] [Google Scholar]
- 91. Hori M, Yasuda K, Takahashi H, Yamazaki C, Morozumi K, Maruyama S. Impact of serum magnesium and bone mineral density on systemic fractures in chronic hemodialysis patients. PLoS ONE. 2021;16(5):e0251912. doi: 10.1371/journal.pone.0251912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581231154183 for Magnesium and Fracture Risk in the General Population and Patients Receiving Dialysis: A Narrative Review by Andrea C. Cowan, Kristin K. Clemens, Jessica M. Sontrop, Stephanie N. Dixon, Lauren Killin, Sierra Anderson, Rey R. Acedillo, Amit Bagga, Clara Bohm, Pierre Antoine Brown, Brenden Cote, Varun Dev, Claire Harris, Swapnil Hiremath, Mercedeh Kiaii, Eduardo Lacson Jr, Amber O. Molnar, Matthew J. Oliver, Malvinder S. Parmar, Jennifer M. McRae, Bharat Nathoo, Kathleen Quinn, Nikhil Shah, Samuel A. Silver, Daniel J. Tascona, Stephanie Thompson, Robert H. Ting, Marcello Tonelli, Hans Vorster, Davinder B. Wadehra, Ron Wald, Myles Wolf and Amit X. Garg in Canadian Journal of Kidney Health and Disease


