Abstract
Background:
There is increasing evidence of the association between chronic low-grade inflammation and severe mental illness (SMI). The objective of our study was to assess serum cytokine levels (SCLs) at admission and discharge in a true-to-life-setting population of inpatients with major depression (MD), bipolar disorder (BD), and schizophrenia (Sz), as well as of healthy controls.
Methods:
We considered MD, BD, and Sz to be SMIs. We evaluated 206 inpatients [MD, N = 92; BD, N = 26; mania (Ma), N = 44; Sz, N = 44). Generalized estimating equations were used to analyze variations in SCL [interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin (IL)-2, IL-4, IL-6, IL-10, and IL-17] at hospital admission and discharge. Results of 100 healthy controls were compared with those of SMI patients at both time points. We evaluated patients’ improvement during in-hospital treatment in terms of general psychiatric symptoms, global clinical impression, functionality, and manic and depressive symptoms with validated scales.
Results:
In all, 68.9% of patients completed the study. Overall, SMI inpatients had higher SCL when compared with controls regardless of diagnosis. There was a significant decrease in Brief Psychiatric Rating Scale (BPRS) and Clinical Global Impression–Severity Scale (CGI-S) scores, and an increase in Global Assessment of Functioning (GAF) scores for all disorders evaluated (p < 0.001), as well as a significant decrease in HDRS-17 scores among MD inpatients (p < 0.001) and in YMRS scores among Ma inpatients (p < 0.001). IL-2 and IL-6 levels decreased significantly between admission and discharge only among MD inpatients (p = 0.002 and p = 0.03, respectively). We found no further statistically significant changes in SCL among the remaining disorders (BD, Ma, and Sz). There was no significant decrease in IFN-γ (p = 0.64), TNF-α (p = 0.87), IL-4 (p = 0.21), IL-10 (p = 0.88), and IL-17 (p = 0.71) levels in any of the evaluated diagnoses.
Conclusion:
MD inpatients had a decrease in IL-2 and IL-6 levels during hospitalization, which was accompanied by clinical improvement. No associations were found for the remaining SMIs (BD, Ma, and Sz).
Keywords: cytokines, inflammation, major depression
Background
People with severe mental illness (SMI), including major depression (MD), bipolar disorder (BD), and schizophrenia (Sz), have a mortality rate two to three times higher than that of the general population,1–3 shortening their life expectancy by 10–20 years.4,5 Despite clinical differences, there is increasing evidence that psychiatric disorders share common genetic,6 biological, and psychosocial determinants. For example, a New Zealand complete birth cohort examined the structure of psychopathology, considering dimensionality, persistence, co-occurrence, and sequential comorbidity of mental disorders across 20 years, from adolescence to midlife. In that study, psychiatric disorders were explained with a dimension of general psychopathology called p factor. The p factor can help to understand a component of psychopathology related to low functioning that cannot be captured by our current classification system [Diagnostic and Statistical Manual of Mental Disorders (DSM)], which, in addition to other factors, is limited by biological validity. This study proposes transdiagnostic approaches to mental disorders.7
In the search for new therapeutic targets to treat different SMIs, there is a growing interest on the role of inflammation and the immune system in the pathogenesis of these disorders.8,9 There is significant preclinical and clinical evidence that the immune system contributes to pathological expression of SMI.10–14 The relationship between mental illness and the immune system can be attributed to hyperactivity of the hypothalamus–pituitary–adrenal axis as a result of anomalous feedback inhibition of endogenous glucocorticoids.15 Then, a complex cascade of pro- and anti-inflammatory cytokines can trigger an immune dysregulation resulting in different psychiatric symptoms. Cytokines are key signaling molecules of the immune system that exert effects on the peripheral and central nervous system. They are produced by immune and nonimmune cells and exert their effects by binding to specific receptors on several target cells. Cytokines are major regulators of acute and chronic inflammation, a complex but vital biological response that affects all organ systems. Cytokines help coordinate the function of the innate and adaptive components of the immune system, in addition to a number of other physiological processes throughout the body.9,16 With advances in molecular and genetic biology, associations were identified between genes involved in the regulation of the immune system and increased risk of MD, BD, and Sz.17–21 These diseases are also associated with abnormalities in the number of immune cells, inflammatory markers, and antibody titers.22
Over the last few years, several meta-analyses were designed to assess the association of inflammatory markers with mental illness, including studies on MD,10,14 BD,11,23–25 and Sz.12,26 Publications including these data involve different aspects of inflammation and mental illness. There are publications addressing clinical,27 postmortem,24 blood marker,9 cerebrospinal marker,26 diagnostic,28 and treatment29 aspects. Different inflammatory markers have been studied, including interleukins (ILs), tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ). However, this variety of published studies makes understanding complex. For example, these studies generally assess each psychiatric disorder separately and crosswise. To our knowledge, there is no study evaluating main SMIs (MD, BD, and Sz) in the same sample controlling for heterogeneity of settings, recruitment methods, and geographical origins of the sample.
To fill this gap and try to better understand the association of SMI with the inflammatory system, our study aimed to: (1) compare levels of different kinds of cytokines in patients with MD, BD, and Sz from an SMI population of inpatients in a ‘true-to-life’ setting; (2) assess levels of different kinds of cytokines at admission and discharge; (3) compare them with healthy controls; and (4) evaluate clinical improvement. With these data, we can compare serum cytokine levels (SCLs) between different diagnoses and with a healthy population as well as estimate whether there is an influence of usual treatment on the inflammatory process of these patients.
Methods
We performed a prospective cohort study in which we included inpatients at a psychiatric unit of a tertiary care hospital (Hospital de Clínicas de Porto Alegre, southern Brazil) who were examined for eligibility upon admission. We invited all patients 18 years or older who were admitted between June 2011 and December 2013. Patients were included if they met both criteria of the National Institute of Mental Health (NIMH) for SMI or had a Global Assessment of Functioning (GAF) score ⩽50 (at initial assessment).30 The two major NIMH criteria are the following: (1) duration, characterized as ‘prolonged illness’ and ‘long-term treatment’ with a history of mental illness or treatment for 2 years or more and (2) disability, which includes dangerous or disruptive social behavior, moderate impairment in work and nonwork activities, and mild impairment in the performance of activities of daily living and in meeting basic needs.30,31 Within 72 h of hospitalization, the following clinical assessments were conducted: the Mini-International Neuropsychiatric Interview (MINI), a structured interview based on DSM, 5th edition (DSM-5) criteria;32 the Clinical Global Impression–Severity Scale (CGI-S),33 a 7-point scale that measures the severity of the disease perceived by the clinician; the Brief Psychiatric Rating Scale (BPRS),34 which measures general psychiatric symptoms, such as depression, anxiety, hallucinations, and unusual behavior; the GAF,35 which measures symptoms and functioning, the Hamilton Depression Rating Scale 17-item version (HDRS-17), which measures depressive symptoms and Young Mania Rating Scale (YMRS) to access manic symptoms. Clinical and sociodemographic evaluations were performed by psychiatrists and psychiatry residents, and blood samples were taken 24 h after admission. Seventy-two hours before discharge, inpatients were reassessed, and blood samples were collected again. Patients with insufficient communication skills to participate in the interview or provide written informed consent were excluded.
We excluded patients with the following criteria: hospital stay <7 days; drug or alcohol addiction or dependence as the main diagnosis; pregnancy or breastfeeding; acute or chronic infectious, autoimmune, neoplastic, or endocrine disease; or occurrence of myocardial infarction or other major cardiovascular disorders in the past 6 months.
One hundred healthy blood donors were invited to participate as a healthy control group. A psychiatrist not affiliated with the hematologic center conducted the same structured interviews using MINI, and if the subjects met the diagnostic criteria for any psychiatric disorder, they were excluded from the study. Controls were also excluded if they were using any psychiatric medications at the time of the interview. Inflammatory marker measurements were conducted after screening the participants’ medical histories, physical examinations, and laboratory tests in the Brazilian hematologic center of the Federal University of Rio Grande do Sul.
Evaluation of serum cytokines
We collected venous blood samples (10 ml) by venipuncture into an anti-coagulant-free tube. The samples were centrifuged at 4000 g for 10 min, and serum was collected and stored at −80°C. Serum cytokine concentrations were determined by flow cytometry using the BD™ cytometric bead array T-helper cell 1 (Th1)/Th2/Th17 Human Cytokine Kit (BD Biosciences, San Diego, CA, USA). This kit allows the discrimination of the anti-inflammatory cytokines IL-4 and IL-10, and the pro-inflammatory cytokines IL-2, IL-6, TNFα, IFN-γ, and IL-17. We used a FACSCalibur flow cytometer (BD Biosciences) for sample processing and data analyses. The results were generated in graphical and tabular formats using the FCAP Array™ cytometric bead array analysis software (BD Biosciences).
Statistical analysis
The sample size was based on a previous observational study whose subjects were recruited at the same place as ours.36 The normality of data distribution was examined by the Shapiro–Wilk test, and the assumption was satisfactory when the p value was >0.05. As for baseline sociodemographic data, continuous variables were compared between groups by Mann–Whitney U test (cases and controls) and Kruskal–Wallis test, followed by Dunn post hoc test (SMI groups). We used chi-square test for categorical variables. Statistical significance was achieved if p value <0.05. Kruskal–Wallis test, followed by Dunn post hoc test, was also used for comparing between-group SCL at admission and discharge.
Generalized estimating equations (GEEs) were used for longitudinal data analyses. GEEs can be used in models with non-normally distributed and unbalanced error data (i.e. when data are missing).32 In this study, two GEEs were performed. The first analysis evaluated SCL at admission and discharge in SMI patients controlling for subgroup [MD, BD, mania (Ma), and Sz], gender, age, weight, and length of stay. The second analysis compared SCL between SMI patients and controls at both time points. As the control group was assessed a single time, their SCL values were repeated in the analyses. Age, gender, and weight were also controlled for in this second analysis. P values <0.05 defined statistical significance.
All analyses were performed using the Statistical Package for the Social Sciences, version 18, for Windows (SPSS®, Chicago, IL, USA).
Results
Sociodemographic and clinical characteristics of the total SMI patient sample, its diagnostic subgroups, and the characteristics of the control sample are shown in Table 1. There were no significant differences between diagnostic patient subgroups regarding age, years of study, ethnicity, and smoking status. However, there were significant differences between subgroups regarding sex proportion (χ² = 18,36; p < 0.001), previous admissions (H = 35.55; p < 0.001), age of onset (H = 47.11; p < 0.001), and length of stay (H = 10.59; p = 0.01). There was no statistically significant correlation between Caucasians and non-Caucasians.
Table 1.
Patient demographic characteristics.
| SMI N = 206 |
Controls N = 100 |
U/χ²a | P | SMI | H/χ²b | p | Post hoc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MD N = 92 |
BD N = 26 |
Ma N = 44 |
Sz N = 44 |
||||||||
| Sex, F | 137 (53.3) | 44 (44) | 2.49 | 0.11 | 60 (63.2) | 15 (55.6) | 33 (70.2) | 24 (34.8) | 18.36 | <0.001 | Sz < (MD = BD) < Ma |
| Age, years | 42 (31–52) | 31 (25–42) | 7948.5 | < 0.001 | 42 (33–54) | 46 (40–59) | 39 (28–56) | 38 (28–47) | 7.96 | 0.05 | = |
| Years of study | 11 (6–12) | 11 (11–15) | 12 160 | < 0.001 | 11 (7–12) | 10 (7–12) | 11 (8–12) | 8 (5–11) | 4.99 | 0.17 | = |
| Ethnicity, Caucasian | 166 (80.6) | 83 (83) | 0.001 | 0.97 | 75 (78.9) | 25 (92.6) | 37 (78.7) | 59 (85.5) | 3.77 | 0.29 | = |
| Smoker, yes | 70 (27.2) | 18 (18) | 5.71 | 0.02 | 23 (24.2) | 11 (40.7) | 14 (29.8) | 17 (24.6) | 2.46 | 0.48 | = |
| Previous admissions, n | 2 (0–5) | – | – | – | 1 (0–3) | 10 (7–12) | 3 (1–5) | 4 (2–9) | 33.55 | <0.001 | MD < (Ma = Sz) = BD |
| Age of onset, years | 26 (20–39) | – | – | – | 31 (24–45) | 32 (26–42) | 23 (17–33) | 21 (18–23) | 47.11 | <0.001 | (Ma = Sz) < (MD = BD) |
| LOS, days | 30.03 (20.62) | – | – | – | 23 (16–32) | 28 (20–37) | 25 (19–35) | 31 (18–52) | 10.59 | 0.01 | (MD < Sz) = Ma = BD |
BD, bipolar depression; LOS, length of stay; Ma, mania; MD, major depression; SMI, severe mental illness; Sz, schizophrenia.
Mann–Whitney U test for continuous variables or χ² test for categorical variables.
Kruskal–Wallis H test for continuous variables or χ² for categorical variables. Values are shown as median (interquartile range) or n (%).
Sz patients were predominantly male, were younger at disease onset, and had a longer length of stay. BD patients were older and had a considerably higher number of previous psychiatric admissions compared with other subgroups. MD patients, in turn, had fewer previous psychiatric admissions and a shorter length of stay.
When healthy controls and SMI patients were compared (Table 1), the control group was found to be younger (U = 7.94; p < 0.001) and to have a smaller proportion of female individuals (χ2 = 2.49; p = 0.11).
The body mass index (BMI) mean of the population studied was 27.59 (median = 25.8).
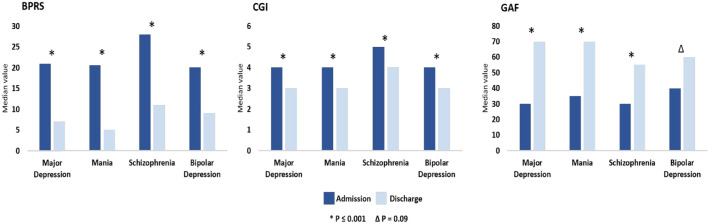
Table 2 shows median SCL values in SMI patients at admission and discharge as well as in controls. At discharge, there was a retention of 68.9% of patients. There was a significant reduction in BPRS and CGI scores, and an increase in GAF scores for all groups assessed (p < 0.001). There was a significant reduction in HDRS-17 scores among MD patients (p < 0.001) and in YMRS scores among Ma patients (p < 0.001) (Figure 1). Compared with controls, SMI patients were found to have a higher overall SCL (regardless of diagnosis), with a significance level at <0.05.
Table 2.
Median serum cytokine levels among patients with severe mental illness and controls..
| Major depression (MD) (n = 92) |
Bipolar depression (BD) (n = 26) |
Mania (Ma) (n = 44) |
Schizophrenia (Sz) (n = 44) |
Controls (n = 100) |
p value | Post hoc | |
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||
| TNF-α (pg/ml) Admission Discharge |
1.91 (1.51–2.35) 1.74 (1.46–2.52) |
2.11 (1.76–2.27) 2.24 (1.62–2.63) |
2.0 (1.59–2.42) 1.72 (1.23–2.45) |
1.80 (1.36–2.29) 1.86 (1.37–2.32) |
1.77 (1.37–1.14) |
0.05 0.22 |
– – |
| IFN-γ (pg/ml) Admission Discharge |
1.50 (1.12–1.9) 1.46 (1.1–1.86) |
1.28 (0.89–1.71) 1.27 (1.13–1.63) |
1.48 (1.14–1.68) 1.77 (1.32–2.19) |
1.52 (1.16–1.86) 1.44 (1.01–1.84) |
1.52 (1.17–1.79) |
0.52 0.3 |
– – |
| IL-2 (pg/ml) Admission Discharge |
1.07 (0.86–1.32) 0.89 (0.72–1.25) |
0.99 (0.79–1.3) 0.88 (0.75–1.29) |
1.09 (0.8–1.49) 0.88 (0.72–1.34) |
0.97 (0.8–1.25) 0.86 (0.69–1.54) |
0.81 (0.67–1) |
<0.001
0.164 |
(C = BD = Sz) < (MD = Ma) - |
| IL-4 (pg/ml) Admission Discharge |
0.52 (0.41–0.73) 0.54 (0.39–0.79) |
0.47 (0.35–0.6) 0.5 (0.4–0.67) |
0.54 (0.37–0.76) 0.58 (0.48–0.76) |
0.52 (0.38–0.62) 0.51 (0.38–0.71) |
0.48 (0.37–0.59) |
0.2 0.1 |
– – |
| IL-6 (pg/ml) Admission Discharge |
2.64 (1.4–6.62) 1.82 (1.03–3.24) |
2.15 (1.38–3.77) 2.38 (1.2–5.66) |
3.23 (1.88–6.56) 1.91 (0.88–5.59) |
2.39 (1.12–4.31) 1.94 (1.01–2.79) |
0.7 (0.48–1.22) |
<0.001
<0.001 |
C < (MD = BD = Ma = Sz) C < (MD = BD = Ma = Sz) |
| IL-10 (pg/ml) Admission Discharge |
0.92 (0.67–1.12) 0.83 (0.62–1.14) |
0.86 (0.57–1.13) 0.83 (0.62–1.04) |
0.98 (0.72–1.52) 0.98 (0.75–1.43) |
1.01 (0.76–1.55) 0.97 (0.67–1.86) |
0.52 (0.42–0.7) |
<0.001
<0.001 |
C < (MD = BD = Ma = Sz) C < (MD = BD = Ma = Sz) |
| IL-17(pg/ml) Admission Discharge |
55.5 (39.4–77.3) 50.1 (28.5–63.5) |
50.5 (29–58.1) 48.2 (33.9–61.4) |
57.5 (44.5–75.7) 57.86 (36.4–82) |
51.3 (24.6–68.8) 45.2 (23.3–63.6) |
26.6 (20.4–44.8) |
<0.001
<0.001 |
C < (MD = BD = Ma = Sz) C < (MD = BD = Ma = Sz) |
IFN-γ, interferon gamma; IQR, interquartile range; IL, interleukin; TNF-α, tumor necrosis factor alpha.
The significance of bold values p<0.05.
Figure 1.
Clinical improvement in different patient subgroups.
BPRS, Brief Psychiatric Rating Scale; CGI, Clinical Global Impression–Severity Scale; GAF, Global Assessment of Functioning.
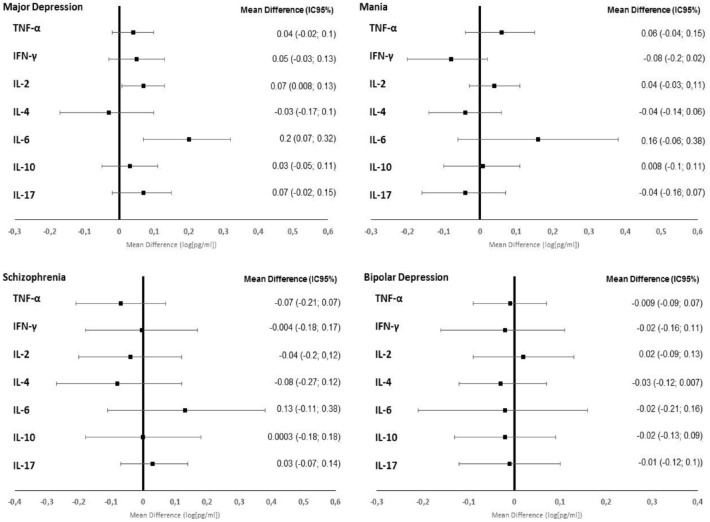
There was no significant reduction in the levels of IFN-γ, TNF-α, IL-4, IL-10, and IL-17 (p = 0.71) in any of the subgroups. IL-6 and IL-2 levels decreased significantly between admission and discharge only among MD patients (p = 0.002 and p = 0.03, respectively). However, IL-2 was not different compared with controls at discharge, while IL-6 was still significantly lower among controls at discharge. Among the other subgroups (Sz, Ma, and BD), there was no statistically significant change in SCL (p < 0.05) (Figure 2).
Figure 2.
Serum cytokine level alterations in different patient subgroups.
CI, confidence interval; IFN-γ, interferon gamma; IQR, interquartile range; IL, interleukin, TNF-α: tumor necrosis factor alpha.
Discussion
The three main findings of this study are as follows: (1) there was an increase in SCL in all subgroups (MD, BD, Ma, and Sz) compared with healthy controls and there was no statistical difference between the different subgroups; (2) there was a reduction in IL-2 and IL-6 in the MD subgroup at the end of hospital treatment; and (3) all inpatients with SMI (MD, BD, Ma, and Sz) showed clinical improvement at the end of hospital treatment.
We believe that this is the first naturalistic study to evaluate a single sample of SMI inpatients with different diagnoses and compare it with healthy controls. In addition, we evaluated the interference of hospital treatment in serum inflammatory markers.
Although the published studies do not group different disorders together in the same sample, a 2016 meta-analysis evaluated 114 studies that included patients with Sz, BD, and MD in the acute and chronic phases. This meta-analysis showed that IL-6 and TNF levels, for example, increased in the acute phase of Sz, Ma, and MD compared with healthy controls. After treatment, IL-6 levels decreased significantly in Sz and MD. In chronic patients, IL-6 levels were significantly increased for Sz, euthymic (but not depressed) BD, and MD compared with healthy controls. Overall, there were similarities in the pattern of cytokine changes in Sz, BD, and MD during the acute and chronic phases of the disease and in the common underlying pathways for immune dysfunction.9 Our study showed that all patients admitted with SMI had higher levels of IL-2, IL-6, IL-10, and IL-17 than controls at admission and higher levels of IL-6, IL-10, and IL-17 at discharge. These findings, added to those found in the existing literature, suggest a common pathophysiological interface among SMIs, with the inflammatory theory as a possible explanation to the intersection of these different disorders.
In the subgroup of MD patients, there was a reduction in IL-2 and IL-6 levels with hospital treatment, accompanied by clinical improvement. Although our study did not assess which intervention specifically alters the levels of inflammatory markers in this subgroup, we believe that the improvement in the inflammatory pattern is a response to treatment. A 2017 meta-analysis involving 82 studies (3212 depressed patients and 2798 healthy controls) evaluated inflammatory markers and depression, and also found that depressed patients had high levels of IL-6 and other cytokines.37 Moreover, another meta-analysis published by the same author showed that antidepressant treatment significantly decreased peripheral levels of IL-6 in addition to other cytokines.38
Direct evidence of inflammation in depression comes from meta-analyses of cross-sectional studies of inflammatory markers in depression, which showed increased concentrations of different cytokines.10,39 Other evidence for a role of inflammation in psychiatric disorders comes from treatment studies: meta-analyses of clinical trials indicate that anti-inflammatory drugs may have antidepressant effects.40,41 However, some studies have shown the lack of effectiveness of anti-inflammatory drugs in depression and suggested that this variability may be due to heterogeneous inflammatory changes among patients with depression.42 Some clinical trials testing specific inflammatory cytokines, such as IL-6, suggested their contribution to the pathogenesis of a type of ‘inflamed depression’.43 Therefore, identifying the profile of the patient with ‘inflamed depression’ and which cytokines are altered can help find specific treatments for depression, since a large portion of patients with depression is known to be refractory to the usual treatments. The decrease in IL-2 and IL-6 levels associated with clinical improvement in severely depressed patients, while they were hospitalized, was the main finding of our study. This information may be important so that new specific interventions in this inflammatory pathway can be studied in this population.
A 2019 meta-analysis evaluated the effectiveness of immunomodulatory drugs for depressive symptoms that are comorbidly associated with inflammatory disorders. Anti-IL-6 antibodies had effects on depressive symptoms. There are no clinical trials that tested the effect of these medications on the clinical population of depressed patients.44
A preliminary study conducted in China studied the relationship of inflammatory markers as a predictor of ketamine response as a rapid antidepressant agent in patients with MD resistant to conventional treatment. This study showed that IL-6 levels were significantly higher in the responder group than those in the non-responder group, which could be a useful predictive biomarker of treatment.45
The present study has two main limitations. First, the samples are of different sizes, with the MD subgroup being larger. This may have influenced the reported finding to be statistically significant. Second, we did not investigate which factor is specifically associated with a decrease in IL-2 and IL-6 levels in MD patients. However, as patients had clinical improvement, this suggests that usual treatment interferes with the inflammatory pathway even though it is not based on an anti-inflammatory agent. Finally, several ILs have been tested for various disorders, which could lead to the possibility of an alpha error. Even so, we decided not to use any specific correction for multiple comparisons in statistical analysis, in view of the damage that correction for multiple statistical tests can contribute to psychiatric research.46
Conclusion
Patients with MD experienced a reduction in IL-2 and IL-6 levels during hospital treatment, which was accompanied by clinical improvement. This association was not found in the remaining SMIs (BD, Ma, and Sz).
This study corroborates the hypothesis that SMI patients experience inflammatory changes. Our findings may contribute to the improvement of treatment strategies and provide such patients with better adaptation skills, which in turn will improve their quality of life.
Acknowledgments
The authors kindly thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Fundação de Amparo à Pesquisa do Estado do RS, and Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre for their support.
Footnotes
ORCID iDs: Antonio Augusto Schmitt Junior  https://orcid.org/0000-0002-8386-5725
https://orcid.org/0000-0002-8386-5725
Lucas Primo de Carvalho Alves  https://orcid.org/0000-0003-4387-224X
https://orcid.org/0000-0003-4387-224X
Contributor Information
Antonio Augusto Schmitt Junior, Department of Psychiatry, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil; Center for Clinical Research, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, Brazil; Graduate Program in Psychiatry and Behavioral Sciences, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; Innovations and Interventions for Quality of Life (I-QoL), Universidade Federal do Rio Grande do Sul, Hospital de Clínicas de Porto Alegre, Postgraduate Programin Medical Sciences: Psychiatry, Avenue Ramiro Barcellos, 2350, Porto Alegre, RS CEP 90035-903, Brazil.
Lucas Primo de Carvalho Alves, Innovations and Interventions for Quality of Life (I-QoL), Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
Barbara Larissa Padilha, Center for Clinical Research, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, Brazil; Innovations and Interventions for Quality of Life (I-QoL), Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; School of Medicine, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil.
Neusa Sica da Rocha, Department of Psychiatry, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil; Center for Clinical and Experimental Research, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, Brazil; Graduate Program in Psychiatry and Behavioral Sciences, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; Innovations and Interventions for Quality of Life (I-QoL), Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; School of Medicine, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil.
Declarations
Ethics approval and consent to participate: The Scientific Committee and Research Ethics Committee from the Hospital de Clínicas de Porto Alegre approved the study (approval number: 10-0265). All subjects included signed an informed consent form.
Consent for publication: All subjects included signed an informed consent form.
Author contributions: Antonio Augusto Schmitt Junior: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Lucas Primo de Carvalho Alves: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing – review & editing.
Barbara Larissa Padilha: Investigation; Writing – review & editing.
Neusa Sica da Rocha: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by CAPES (Coordenação de Aperfeiçoamento Profissional de Nível Superior): no. 530/2010, CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FIPE/HCPA (Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre): no. 10-0265, PPG-Psiq/FAMED/UFRGS (Programa de Pós-Graduação em Psiquiatria da Faculdade de Medicina da Universidade Federal do Rio Grande do Sul), and FAPERGS (Fundo de Amparo à Pesquisa do Estado do Rio Grande do Sul). The abstract of this study was accepted for poster publishing in the Lancet Summit event, on 2018. This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico under Grants 102212/2020-0 and 303652/2019-5; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior under Code 001; Fundação de Amparo à Pesquisa do Estado do RS under Grant 19/251-0001930-0; and Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data materials: Not applicable.
References
- 1. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 2015; 14: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reininghaus U, Dutta R, Dazzan P, et al. Mortality in schizophrenia and other psychoses: a 10-year follow-up of the ÆsOP first-episode cohort. Schizophrenia Bulletin 2015; 41: 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Osborn DPJ, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database. Arch Gen Psych 2007; 64: 242–249. [DOI] [PubMed] [Google Scholar]
- 4. Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE 2011; 6: e19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ (Online) 2013; 346: 7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anttila V, Bulik-Sullivan B, Finucane HK, et al. Analysis of shared heritability in common disorders of the brain. Science 2018; 360: 6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caspi A, Houts RM, Belsky DW, et al. The p factor : one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci 2015; 2: 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehav Rev 2014; 42: 93–115. [DOI] [PubMed] [Google Scholar]
- 9. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 2016; 21: 1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–457. [DOI] [PubMed] [Google Scholar]
- 11. Modabbernia A, Taslimi S, Brietzke E, et al. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry 2013; 74: 15–25. [DOI] [PubMed] [Google Scholar]
- 12. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biological Psychiatry 2008; 63: 801–808. [DOI] [PubMed] [Google Scholar]
- 13. Mørch RH, Dieset I, Faerden A, et al. Persistent increase in TNF and IL-1 markers in severe mental disorders suggests trait-related inflammation: a one year follow-up study. Acta Psychiatr Scand 2017; 136: 400–408. [DOI] [PubMed] [Google Scholar]
- 14. Osimo EF, Pillinger T, Rodriguez IM, et al. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun 2020; 87: 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev 2013; 37: 2331–2371. [DOI] [PubMed] [Google Scholar]
- 16. Ingman WV, Robertson SA. The essential roles of TGFB1 in reproduction. Cytokine Growth Factor Rev 2009; 20: 233–239. [DOI] [PubMed] [Google Scholar]
- 17. O’dushlaine C, Rossin L, Lee PH, et al. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci 2015; 18: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460: 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barbosa IG, Machado-Vieira R, Soares JC, et al. The immunology of bipolar disorder. Neuroimmunomodulation 2014; 21: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Müller N. Immunology of major depression. Neuroimmunomodulation 2014; 21: 123–130. [DOI] [PubMed] [Google Scholar]
- 22. Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol 2013; 8: 900–920. [DOI] [PubMed] [Google Scholar]
- 23. Misiak B, Bartoli F, Carrà G, et al. Chemokine alterations in bipolar disorder: a systematic review and meta-analysis. Brain Behav Immun 2020; 88: 870–877. [DOI] [PubMed] [Google Scholar]
- 24. Giridharan VV, Sayana P, Pinjari OF, et al. Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review. Mol Psychiatry 2020; 25: 94–113. [DOI] [PubMed] [Google Scholar]
- 25. Castaño-Ramírez OM, Sepúlveda-Arias JC, Duica K, et al. Inflammatory markers in the staging of bipolar disorder: a systematic review of the literature. Rev Colomb Psiquiatr (Engl Ed) 2018; 47: 119–128. [DOI] [PubMed] [Google Scholar]
- 26. Gallego JA, Blanco EA, Husain-Krautter S, et al. Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: new data and an updated meta-analysis. Schizophr Res 2018; 202: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anjum S, Qusar MMAS, Shahriar M, et al. Altered serum interleukin-7 and interleukin-10 are associated with drug-free major depressive disorder. Ther Adv Psychopharmacol 2020; 10: 2045125320916655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ortiz-Domínguez A, Hernández ME, Berlanga C, et al. Immune variations in bipolar disorder: phasic differences. Bipolar Disord 2007; 9: 596–602. [DOI] [PubMed] [Google Scholar]
- 29. Haroon E, Daguanno AW, Woolwine BJ, et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 2018; 95: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parabiaghi A, Bonetto C, Ruggeri M, et al. Severe and persistent mental illness: a useful definition for prioritizing community-based mental health service interventions. Soc Psychiatry Psychiatr Epidemiol 2006; 41: 457–463. [DOI] [PubMed] [Google Scholar]
- 31. Ruggeri M, Leese M, Thornicroft G, et al. Definition and prevalence of severe and persistent mental illness. Br J Psychiatry 2000; 177: 149–155. [DOI] [PubMed] [Google Scholar]
- 32. Amorim P. Mini international neuropsychiatric interview (MINI): validation of a short structured diagnostic psychiatric interview. Revista Brasileira Psiquiatria 2002; 22: 106–115. [Google Scholar]
- 33. Guy W. ECDEU assessment manual for psychopharmacology. Clin Global Impress 1976; 60, https://ia800306.us.archive.org/35/items/ecdeuassessmentm1933guyw/ecdeuassessmentm1933guyw.pdf [Google Scholar]
- 34. Elkis H, Romano F. Translation and adaption of the Brief Psychiatric Rating Scale-anchored version (BPRS-A). J Bras Psiquiatr 1996; 45: 43–49. [Google Scholar]
- 35. Endicott J. The global assessment scale. Arch Gen Psych 1976; 33: 766. [DOI] [PubMed] [Google Scholar]
- 36. Nuernberg GL. Desfechos clínicos e BDNF em pacientes com doença mental grave durante internação psiquiátrica em hospital geral 2016, https://lume.ufrgs.br/bitstream/handle/10183/148111/001000570.pdf?sequence=1&isAllowed=y
- 37. Köhler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 2017; 135: 373–387. [DOI] [PubMed] [Google Scholar]
- 38. Köhler CA, Freitas TH, Stubbs B, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol 2018; 55: 4195–4206. [DOI] [PubMed] [Google Scholar]
- 39. Haapakoski R, Mathieu J, Ebmeier KP, et al. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 2015; 49: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Köhler OE, Benros M, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014; 71: 1381–1391. [DOI] [PubMed] [Google Scholar]
- 41. Kappelmann N, Lewis G, Dantzer R, et al. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 2018; 23: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khandaker GM, Oltean BP, Kaser M, et al. Protocol for the insight study: a randomised controlled trial of singledose tocilizumab in patients with depression and low-grade inflammation. BMJ Open 2018; 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wittenberg GM, Stylianou A, Zhang Y, et al. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry 2020; 25: 1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang JJ, Wang N, Yang C, et al. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol Psychiatry 2015; 77: e19–e20. [DOI] [PubMed] [Google Scholar]
- 46. Primo de Carvalho Alves L, Sica da Rocha N. The harm of adjusting for multiple statistical testing in psychiatric research. Acta Psychiatr Scand 2019; 140: 586–588. [DOI] [PubMed] [Google Scholar]