Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is one of the causes of severe hyperbilirubinemia, prolonged jaundice, and bilirubin-induced encephalopathy in neonates. In a randomized controlled trial, we evaluated the effect of oral ursodeoxycholic acid (UDCA) on indirect hyperbilirubinemia in G6PD-deficient neonates requiring phototherapy. Intervention group I (N = 45; received phototherapy and 10 mg/kg/day UDCA), Intervention group II (N = 40; received phototherapy and 20 mg/kg/day UDCA), and a control group (N = 49; received phototherapy and placebo). Levels of total serum bilirubin (TSB) in all 3 groups decreased significantly over time (P = .001) but the level of TSB at different hours after admission and the duration of phototherapy did not differ significantly between the 3 groups. After discharge, the 2 intervention groups had a significantly lower rate of readmission than the control group (P = .001). No significant difference was observed between the 10 and 20 mg/kg/day groups. Further evaluation is recommended, especially in terms of the pharmacokinetics of UDCA in neonates.
Trial registration number: IRCT20091201002801N4, prospectively registered on 2019-06-1
Keywords: glucose-6-phosphate dehydrogenase deficiency, hyperbilirubinemia, jaundice, newborn, ursodeoxycholic acid
Introduction
Hyperbilirubinemia is one of the most prevalent health issues in neonates that in severe form is associated with the development of brain damage and results in disability in affected neonates.1,2 Although serious complications of neonatal hyperbilirubinemia are less observed in recent years with appropriate therapeutic interventions, severe neonatal hyperbilirubinemia secondary to reduced glucose-6-phosphate dehydrogenase (G6PD) enzyme activity is still complicated by bilirubin-induced encephalopathy.3 There are no definite findings compatible with hemolysis such as reticulocytosis or anemia in most cases of neonatal hyperbilirubinemia caused by G6PD deficiency. Hyperbilirubinemia in G6PD deficient neonates is thought to be mainly due to reduced hepatic conjugation or excretion of bilirubin.4-6
Although phototherapy is the main treatment used for reducing bilirubin in neonates, using adjuvant therapies which reduce hyperbilirubinemia and the duration of phototherapy can be effective. To date, several drugs such as D-penicillamine, activated charcoal, phenobarbital, clofibrate, metalloporphyrin, and bile salts have been used for the treatment of neonatal indirect hyperbilirubinemia.7
Ursodeoxycholic acid (UDCA) is a bile acid used to treat cholestatic liver disease. UDCA has been reported to have anti-apoptotic activity and protect the liver against oxidative stress. It also reduces enteral absorption of bilirubin and increases bile flow and excretion of bilirubin from stool.8
Efficacy and safety of UDCA have been already confirmed in adults.9,10 In addition, it has been used in children with liver cholestasis and the post-operative management of infants with biliary atresia without recording side effects.11,12 There are fewer studies on the use of UDCA in the neonatal period, but its role in the treatment of neonatal cholestasis has been confirmed.13 An in-vitro study mimicking severe neonatal hyperbilirubinemia also showed that UDCA had a preventive effect on the disruption of the blood-brain barrier by unconjugated bilirubin.14 In recent years, several clinical trials have been carried out to investigate the effect of UDCA on neonatal indirect hyperbilirubinemia. These studies showed that UDCA is an adjuvant drug to phototherapy that reduces the bilirubin level and shortens the duration of phototherapy.7,9,12 Some studies have previously evaluated the effect of different doses of UDCA on neonatal hyperbilirubinemia, however, information on the exact dose of the drug for the treatment of neonatal jaundice is scarce.9,15 In addition, most of these studies have been performed on neonates without risk factors for jaundice. G6PD deficiency as a cause of severe hyperbilirubinemia is highly prevalent in the Middle East.16
Iran is among the countries with a high prevalence of G6PD deficiency and has the highest prevalence in the northern and southern provinces of Iran.17 Alteration in the oxidant-antioxidant profile is also known to occur in neonatal jaundice and antioxidant mechanisms may become insufficient in cases of glucose 6-phosphate dehydrogenase (G6PD) deficiency,18 and the UDCA may protect the liver against oxidative stress.
Therefore, this study aims to evaluate the effect of 2 different doses of UDCA on total serum bilirubin levels in neonates with G6PD deficiency complicated by indirect hyperbilirubinemia in northern Iran.
Methods
Study Design
This study was carried out as a single-center blinded randomized controlled clinical trial.
Study Setting and Duration
This study was conducted in the neonatal ward of Boo-Ali Sina hospital in Sari in northern Iran from September 2019 to March 2022.
Participants
Two to fourteen-day-old neonates with significant clinical icterus and unconjugated hyperbilirubinemia who required phototherapy according to the standard curve19 and had G6PD deficiency in the initial evaluation.
Inclusion Criteria
Full-term neonates, birth weight 2500 to 4000 g, exclusive breastfeeding, age over 48 hours, and direct bilirubin <1.5 mg/dl
Exclusion Criteria
Septicemia, direct hyperbilirubinemia, ABO or Rh incompatibility, major congenital anomalies, need for exchange transfusion at the time of hospitalization, previous use of any drug such as phenobarbital or herbal medicine, history of previous phototherapy or exchange transfusion, significant evidence of hemolysis such as reticulocyte count ≥5%, spherocytosis or elliptocytosis in peripheral blood smear and infants of diabetic mothers.
This clinical trial was conducted with a parallel design. Neonates were randomly assigned into 3 groups by permuted block randomization (block size 4 which guaranteed allocation concealment). Randomization was done by an online random number generator in a sealed envelope.
The neonates in the intervention group I received both phototherapy and 10 mg/kg/day oral UDCA.
The neonates in intervention group II received both phototherapy and 20 mg/kg/day oral UDCA.
The neonates in the control group received both phototherapy and the placebo (including an equal volume of sterile water).
In this study, UDCA 300 mg capsule (KoushanPharmed Company, Tehran, Iran) was used and 300 mg of the drug was dissolved in 60 cc sterile water (Shahid Ghazi pharmaceutical company, Tabriz, Iran) then estimated drug was used for babies as cc/kg as a single daily dose till discharge. Both control and intervention groups received continuous phototherapy using daylight fluorescent lamps (Tosan Company, Tehran, Iran) in an Air Shields unit. The phototherapy unit was located 30 to 35 cm from the neonates and the neonates’ eyes and genitalia were covered. The lamps were changed after 1500 hours concerning the manufacturer’s instruction.
The duration of phototherapy was measured by the timer of the phototherapy unit. Bilirubin levels were measured by the Diazo method. The sample size based on the previous study7 for the mean level of total serum bilirubin (our main outcome) and considering α = .05, β = .2, and d = 0.95 was estimated to be 49 for each group.
Analysis of the data was performed by SPSS24 using 2 independent samples Fisher’s exact test and repeated measure ANOVA (which was used to compare the mean of total serum bilirubin level). Bonferroni test was used for the pairwise comparison of bilirubin levels at different hours and the time required for phototherapy and post-discharge readmission. The significance level was considered less than .05.
Blinding
In this study, parents and outcome assessors (the attending neonatologist and the resident of the team) were both blind to the type of medication given to babies in each group. The drug was prepared and used as an open-labeled by a nurse who was not blind. Upon admission, blood samples were taken from all neonates for measurement of TSB (including direct and indirect bilirubin), CBC, PBS as well as other routine laboratory data. G6PD deficiency was detected by rapid fluorescent spot testing and quantitative spectrometry. Laboratory data were prepared in less than 2 hours, and when the baby was diagnosed with G6PD deficiency, parents were asked to give informed consent. Then, the characteristics of each neonate were recorded in a questionnaire.
TSB levels were assessed 12 hours after admission and then every 24 hours until discharge. Babies were discharged when the TSB was less than 10 mg/dl under simple conventional phototherapy. If any side effects were observed, they would be documented. We followed all patients up to 28 days of age in our outpatient follow-up clinic and recorded cases requiring readmission due to hyperbilirubinemia according to phototherapy guidelines.19
Endpoints
The primary outcome in this study is the mean TSB levels at 12, 24, 48, and 72 hours after admission. The secondary outcome includes the percentage reduction in TSB 24 hours after admission, the duration of phototherapy, the duration of hospitalization, the need for exchange transfusion, and the need for readmission due to hyperbilirubinemia after discharge. If the discharge of the baby was delayed after the recovery of jaundice due to other complications, the baby was excluded from the study. The follow-up period was the end of the neonatal period (28 days).
Ethical Consideration
This study was approved by the ethics committee and Institutional Review Board of Mazandaran University of Medical Sciences (reference: IR.MAZUMS.REC.1398.222). All parents of neonates completed a written informed consent form.
Results
In this study, 134 newborns were enrolled and randomly assigned into 3 groups and analyzed. The flow chart of patients’ recruitment is shown in Figure 1.
Figure 1.
Diagram showing the flow of participants through the trial.
The baseline characteristics for each of the 3 groups are shown in Table 1. As shown in Table 1, the baseline variables are equal among the 3 groups and had no significant differences.
Table 1.
The Baseline Characteristics for each of the 3 Groups.
| Variables | Intervention group I (N = 45) | Intervention group II (N = 40) | Control group (N = 49) | P-value |
|---|---|---|---|---|
| Sex (Male) | 38 (84.4) | 34 (85) | 40 (81.6) | .89 |
| n (%) | ||||
| Age (day) | 4.29 ± 2.74 | 3.80 ± 2.24 | 4 ± 2.93 | .69 |
| Mean ± SD | ||||
| Weight (g) | 3180 ± 398 | 3082 ± 419 | 3177 ± 422 | .46 |
| Mean ± SD | ||||
| Admission bilirubin (mg/dl) | 16.80 ± 3.33 | 15.35 ± 2.75 | 16.08 ± 4.24 | .17 |
| Mean ± SD | ||||
| Admission hemoglobin (g/dl) | 15.73 ± 1.91 | 16.19 ± 2.72 | 15.09 ± 2.11 | .07 |
| Mean ± SD | ||||
| Reticulocyte count (%) | 2.56 ± 2.03 | 3 ± 2.06 | 3.07 ± 1.85 | .16 |
| Mean ± SD |
Table 2 shows the TSB levels 12, 24, 48, and 72 hours after admission. The results revealed that bilirubin levels in all 3 groups decreased significantly (P = .001) over time (Figure 2) but based on univariate analysis of variance, no significant difference was observed between the 3 groups for TSB levels in 12, 24, 48, and 72 hours after admission (Table 2).
Table 2.
Total Serum Bilirubin Levels in Different Hours After Admission Among 3 Groups.
| TSB (mg/dl) | Intervention group I | Intervention group II | Control group | P-value |
|---|---|---|---|---|
| Time | ||||
| 12 h after admission | 13.62 ± 2.75 | 12.60 ± 3.10 | 13.64 ± 3.35 | .21 |
| 24 h after admission | 10.68 ± 1.95 | 10.02 ± 2.32 | 10.62 ± 2.55 | .35 |
| 48 h after admission | 9.10 ± 1.74 | 8.70 ± 1.60 | 9.20 ± 2.22 | .43 |
| 72 h after admission | 8.56 ± 1.44 | 7.99 ± 1.23 | 8.71 ± 2.44 | .15 |
Figure 2.
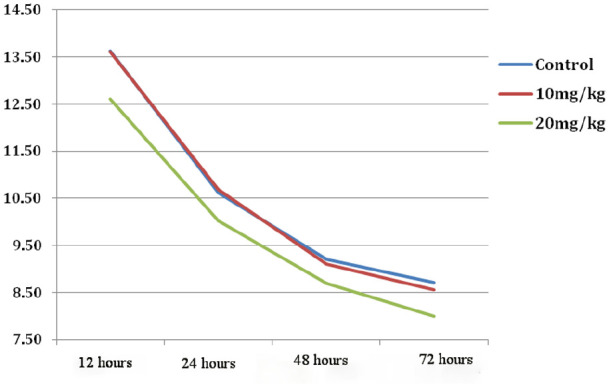
Time trend of total serum bilirubin changes among 3 groups.
In addition, the percentage of decrease in total serum bilirubin level 24 hours after admission compared to baseline bilirubin showed no significant difference between the 3 groups [Group I: 34.51 ± 12.99, Group II: 34.87 ± 14.93, Group III: 32.53 ± 15.14 (P = .70)].
No significant differences were found between the 3 groups in the total time required for phototherapy, hospitalization days, and the need for exchange transfusion (Table 3).
Table 3.
Comparison of Secondary Outcomes Between 3 Groups.
| Outcome | Intervention group I (N = 45) | Intervention group II (N = 40) | Control group (N = 49) | P-value |
|---|---|---|---|---|
| Duration required for phototherapy (h) | 87.07 ± 25.77 | 90.98 ± 17.12 | 91.03 ± 45.14 | .08 |
| Mean ± SD | ||||
| Need to exchange transfusion, n(%) | 0 | 0 | 3 (6.1) | .11 |
| Hospitalization time (days) | 4.40 ± 0.93 | 3.95 ± 0.78 | 4.63 ± 1.87 | .06 |
| Mean ± SD | ||||
| Readmission after discharge, n (%) | 0 | 4 (10) | 15 (30.6) | .001 |
As Table 3 shows, in the follow-up of neonates, it was observed that readmission after discharge was significantly different between the 3 groups and had a higher rate in the control group. Results of the Bonferroni test showed no significant difference between the 10 and 20 mg/kg/day groups in this regard.
No side effects were observed in the 3 study groups.
Discussion
This study investigated the effect of 2 different doses of UDCA in G6PD deficient neonates with indirect hyperbilirubinemia who required admission and phototherapy. To the best of our knowledge, the present study is the second study of its kind in the world that has been conducted in Iran, a region with a high prevalence of G6PD deficiency. Results showed that although UDCA did not affect TSB levels during hospitalization, it significantly reduced post-discharge readmission. No significant difference was found between the 2 different doses of UDCA in terms of reduced readmission.
The association between severe neonatal indirect hyperbilirubinemia and G6PD deficiency has already been proven.3 Because high levels of bilirubin may be toxic to the developing brain of newborn infants, reducing unconjugated bilirubin in neonates receiving phototherapy with medication is an interesting approach to treating neonatal hyperbilirubinemia. In this regard, UDCA is promising in both in vitro and in vivo research.20 A study evaluated the efficacy of treatment of unconjugated hyperbilirubinemia with oral bile salts in Gun rats and the results supported the feasibility of oral bile salt therapy in neonates with unconjugated hyperbilirubinemia by stimulating unconjugated bilirubin turnover and its fecal excretion.21 A recent study showed that therapeutic bile acids effectively reduce plasma and brain bilirubin and highlight their potential in the treatment of neonatal hyperbilirubinemia.22
Some previous studies have reported the effect of UDCA on the reduction of unconjugated bilirubin in neonatal hyperbilirubinemia.7,12,23,24 Honar et al7 reported that the addition of UDCA to phototherapy had a positive effect on indirect neonatal hyperbilirubinemia and could reduce the hours required for phototherapy and hospitalization, and similar results have been reported in 2 other studies.12,25 Most of these previous studies were carried out on neonates with indirect hyperbilirubinemia without other underlying diseases or risk factors such as G6PD deficiency. On the other hand, Akefi et al26 in a study similar to ours showed that UDCA did not reduce the duration of phototherapy and the length of hospital stay of neonates with hyperbilirubinemia. They concluded that although the combination of UDCA with phototherapy may enhance serum total bilirubin reduction, this effect is not clinically applicable.
A recent study, contrary to our study, showed that adding 10 mg/kg UDCA to phototherapy in infants with G6PD deficiency could significantly reduce serum bilirubin levels and reduce phototherapy time and hospital stay. However, the limitations of this study were the small sample size, and the authors recommended further studies with large sample sizes and different doses of UDCA.27 To the best of our knowledge, our study is the second one that exclusively investigated the effect of UDCA on the reduction of bilirubin in neonates with unconjugated hyperbilirubinemia who at the same time were G6PD deficient. Our study was performed on a larger sample size and we also evaluated 2 different doses. Unlike the first study, our study did not show a significant decrease in total serum bilirubin level compared to the control group and no change was observed on hospitalization days.
Iran is one of the countries with a high prevalence of G6PD deficiency.16 Newborns with G6PD deficiency are at risk for hyperbilirubinemia and sequelae such as kernicterus. The overall prevalence of G6PD deficiency in Iranian newborns with jaundice is reported to be 7%.28 Our results showed that although UDCA can be effective in reducing unconjugated hyperbilirubinemia, it may not be very useful for neonates with risk factors such as G6PD deficiency. One possible cause could be the jaundice mechanism in G6PD enzyme-deficient newborns. In most cases of neonatal hyperbilirubinemia due to G6PD deficiency, there are no findings consistent with hemolysis. Decreased bilirubin conjugation and decreased bilirubin glucuronidation in liver cells have been suspected as contributing factors.4-6 However, the main function of UDCA is to increase intestinal excretion and fecal disposal of bilirubin.27
The results of the present study showed that the need for exchange transfusion did not change between the 3 groups. No previous studies on this subject have been assessed and studies with larger sample sizes are necessary to evaluate this clinical outcome.
Interestingly, in our study, the need for readmission for jaundice decreased, but no difference was observed between the 2 doses. In the latest 2022 neonatal jaundice management guideline, G6PD deficiency is introduced as one of the most common risk factors for prolonged jaundice and readmission in neonates with hyperbilirubinemia, especially in areas where G6PD deficiency is common.29,30 Therefore, the results obtained in our study can show the useful role of UDCA in reducing readmission. However, this requires knowledge of the half-life and pharmacokinetics of UDCA. The elimination half-life of UDCA in adults is approximately 3 to 6 days, and steady-state concentrations are usually reached within 3 weeks of starting treatment.31 UDCA pharmacokinetic studies in the neonatal population are often limited, so the dose and half-life of the drug are based on evidence from adult studies.32
None of the patients experienced any complications during our study. Previous studies have confirmed UDCA tolerance and safety in neonates.26,27
Since most limited previous studies have examined the efficacy of UDCA on neonatal jaundice by eliminating risk factors such as G6PD deficiency or ABO incompatibility, the use of UDCA in neonates with clinical risk factors appears to require more extensive research. Because it may only be effective if there is no risk factor for jaundice.
One of the strengths of this study is a relatively large sample size and we included 134 neonates in the study. In addition, we examined 2 different doses in neonates with hyperbilirubinemia and G6PD deficiency.
Limitations
Our study has some limitations: At first, due to the lack of facilities, we could not study the pharmacokinetics of UDCA. Second, we conducted the study in a single center. Therefore, for better conclusions, it is recommended to conduct a study on neonates of different gestational ages with larger sample sizes.
Conclusion
A lower or higher dose of UDCA in combination with phototherapy in neonates with risk factors such as G6PD deficiency cannot reduce serum total bilirubin and the duration of phototherapy or hospital stay but it reduces the rate of readmission. Therefore, further studies to evaluate the half-life and pharmacokinetics of UDCA in these patients are necessary.
Acknowledgments
The authors would like to thank Mehrangiz Mokhtarpour and Sedigheh Gerayili (nurses of the neonatal ward) for their assistance in the study and for charting the documents.
Footnotes
Author Contributions: RF is responsible for designing and conducting the study and developing the first draft manuscript. EK collected clinical data and followed patients. MN supervised the study and edited the manuscript. ANG is responsible for data interpretation and analysis. All authors agreed on the final submitted version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project resulted from the residency thesis of Elham Keyhanian and was supported by a grant (1398.222) from Mazandaran University of Medical Sciences.
Ethical Approval: This research has been performed following the principles stated in the Declaration of Helsinki. Ethical approval was obtained for this study from the research ethics committee and Institutional Review Board of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1398.222). The trial was prospectively registered in the Iranian Registry of Clinical Trials (IRCT20091201002801N4) which is a primary registry in the WHO Registry Network (https://en.irct.ir/trial/39858).
Consent Form: Informed consent was obtained from patients’ guardians.
Data Availability: All data generated or analyzed during this study are included in this published article.
ORCID iD: Roya Farhadi  https://orcid.org/0000-0002-6525-527X
https://orcid.org/0000-0002-6525-527X
References
- 1. Thielemans L, Trip-Hoving M, Landier J, et al. Indirect neonatal hyperbilirubinemia in hospitalized neonates on the Thai-Myanmar border: a review of neonatal medical records from 2009 to 2014. BMC Pediatr. 2018;18(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage–mechanisms and management approaches. New Engl J Med. 2013;369(21):2021-2030. [DOI] [PubMed] [Google Scholar]
- 3. Isa HM, Mohamed MS, Mohamed AM, Abdulla A, Abdulla F. Neonatal indirect hyperbilirubinemia and glucose-6-phosphate dehydrogenase deficiency. Korean J Pediatr. 2017;60(4):106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khodashenas E, Kalani-Moghaddam F, Araghi Z, Khodaparast M, Yazdani Z. Glucose-6-phosphate dehydrogenase deficiency and neonatal hyperbilirubinemia. Iran J Neonatol. 2015;6(3):28-31. [Google Scholar]
- 5. Cunningham AD, Hwang S, Mochly-Rosen D. Glucose-6-phosphate dehydrogenase deficiency and the need for a novel treatment to prevent Kernicterus. Clin Perinatol. 2016;43(2):341-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. M Abo El Fotoh WM, Rizk MS. Prevalence of glucose-6-phosphate dehydrogenase deficiency in jaundiced Egyptian neonates. J Matern Fetal Neonatal Med. 2016;29(23):3834-3837. [DOI] [PubMed] [Google Scholar]
- 7. Honar N, GhashghaeiSaadi E, Saki F, Pishva N, Shakibazad N, Hosseini Teshnizi S. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62(1):97-100. [DOI] [PubMed] [Google Scholar]
- 8. Kotb MA. Molecular mechanisms of ursodeoxycholic acid toxicity & side effects: ursodeoxycholic acid freezes regeneration & induces hibernation mode. Int J Mol Sci. 2012;13(7):8882-8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jafari S, Khan KA, Bhatnagar S, Srivastava G, Nanda C, Chandra A. Role of ursodeoxycholic acid in neonates with indirect hyperbilirubinemia-an open labelled randomised control trial. Int J Contemp Pediatr. 2018;5(2):432-435. [Google Scholar]
- 10. Akazawa T, Uchida Y, Miyauchi E, Tachikawa M, Ohtsuki S, Terasaki T. High expression of UGT1A1/1A6 in monkey small intestine: comparison of protein expression levels of cytochromes P450, UDP-glucuronosyltransferases, and transporters in small intestine of cynomolgus monkey and human. Mol Pharm. 2018;15(1):127-140. [DOI] [PubMed] [Google Scholar]
- 11. Poddar U, Bhattacharya A, Thapa BR, Mittal BR, Singh K. Ursodeoxycholic acid-augmented hepatobiliary scintigraphy in the evaluation of neonatal jaundice. J Nucl Med. 2004;45(9):1488-1492. [PubMed] [Google Scholar]
- 12. Hasan AM, Abdulrahman A, Husain RH. Effect of ursodeoxycholic acid in lowering neonatal indirect hyperbilirubinemia: a randomized controlled trial. Merit Res J Med Med Sci. 2015;3(9):402-405. [Google Scholar]
- 13. Lewis T, Kuye S, Sherman A. Ursodeoxycholic acid versus phenobarbital for cholestasis in the Neonatal Intensive Care Unit. BMC Pediatr. 2018;18(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmela I, Correia L, Silva RFM, et al. Hydrophilic bile acids protect human blood-brain barrier endothelial cells from disruption by unconjugated bilirubin: an in vitro study. Front Neurosci. 2015;9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gharehbaghi MM, Sani AM, Refeey M. Evaluating the effects of different doses of ursodeoxycholic acid on neonatal jaundice. Turk J Pediatr. 2020;62(3):424-430. [DOI] [PubMed] [Google Scholar]
- 16. Moosazadeh M, Amiresmaili M, Aliramezany M. Prevalence of G6PD deficiency in Iran, a mata-analysis. Acta Med Iran. 2014;52(4):256-264. [PubMed] [Google Scholar]
- 17. Zahedpasha Y, AhmadpourKachouri M, AkhavanNiaki H, Farhadi R. Comparison of molecular mutations of G6PD deficiency gene between icteric and nonicteric neonates. Int J Mol Cell Med. 2013;2(1):14-20. [PMC free article] [PubMed] [Google Scholar]
- 18. Raicevic S, Eventov-Friedman S, Bolevich S, et al. Correlation between oxidative stress and G6PD activity in neonatal jaundice. Mol Cell Biochem. 2014;395(1–2):273-279. [DOI] [PubMed] [Google Scholar]
- 19. Lissauer T, Fanaroff AA, Miall L, et al. Neonatology at a Glance, 19th ed. John Wiley & Sons; 2020. [Google Scholar]
- 20. Ullah S, Rahman K, Hedayati M. Hyperbilirubinemia in neonates: types, causes, clinical examinations, preventive measures and treatments: a narrative review article. Iran J Public Health. 2016;45(5):558-568. [PMC free article] [PubMed] [Google Scholar]
- 21. Cuperus FJ, Hafkamp AM, Havinga R, et al. Effective treatment of unconjugated hyperbilirubinemia with oral bile salts in Gunn rats. Gastroenterology. 2009;136(2):673-682.e1. [DOI] [PubMed] [Google Scholar]
- 22. van der Schoor LWE, Verkade HJ, Bertolini A, et al. Potential of therapeutic bile acids in the treatment of neonatal hyperbilirubinemia. Sci Rep. 2021;11(1):11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shahramian I, Tabrizian K, Ostadrahimi P, Afshari M, Soleymanifar M, Bazi A. Therapeutic effects of ursodeoxycholic acid in neonatal indirect hyperbilirubinemia: a randomized double-blind clinical trial. Arch Anesthesiol Crit Care. 2019;5(3):99-103. [Google Scholar]
- 24. Dobryansky D, Bonetska L, Nosova I, et al. Clinical efficacy of domestic preparation of ursodeoxycholic acid in comprehensive treatment of hyperbilirubinemia in premature infants. Childs Health. 2022;2014:45-50. [Google Scholar]
- 25. Ughasoro MD, Adimorah GN, Chukwudi NK, Nnakenyi ID, Iloh KK, Udemba CE. Reductive effect of ursodeoxycholic acid on bilirubin levels in neonates on phototherapy. Clin Exp Gastroenterol. 2019;12:349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akefi R, Hashemi SM, Alinejad S, Almasi-Hashiani A. The effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy: a randomized clinical trial. J Matern Fetal Neonatal Med. 2022;35:4075-4080. [DOI] [PubMed] [Google Scholar]
- 27. Rezaie M, Gholami R, Jafari M, Haghighinejad H. Evaluating the effect of ursodeoxycholic acid on total bilirubin of neonates with glucose-6-phosphate dehydrogenase deficiency complicated by indirect hyperbilirubinaemia. J Paediatr Child Health. 2021;57(8):1175-1181. [DOI] [PubMed] [Google Scholar]
- 28. Javadi M, Deravi S, Zarei S, Mahdavi N, Ranjbaran M. Prevalence of G6PD deficiency in Iranian neonates with jaundice: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022;35:5813-5820. [DOI] [PubMed] [Google Scholar]
- 29. Al-Omran A, Al-Abdi S, Al-Salam Z. Readmission for neonatal hyperbilirubinemia in an area with a high prevalence of glucose-6-phosphate dehydrogenase deficiency: a hospital-based retrospective study. J Neonatal Perinat Med. 2017;10(2):181-189. [DOI] [PubMed] [Google Scholar]
- 30. Kemper AR, Newman TB, Slaughter JL, et al. Clinical practice guideline revision: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2022;150(3):e2022058859. [DOI] [PubMed] [Google Scholar]
- 31. Book ML. Use of ursodiol in infants and children. Pediatr Pharmacol. 2009;15:2. [Google Scholar]
- 32. Gordi T, Baillie R, Vuong le T, et al. Pharmacokinetic analysis of 14C-ursodiol in newborn infants using accelerator mass spectrometry. J Clin Pharmacol. 2014;54(9):1031-1037. [DOI] [PubMed] [Google Scholar]



