Summary
Background:
The literature evaluating multi-component interventions for long-term weight loss in adolescents with intellectual disabilities (ID) is extremely limited.
Objectives:
To compare the effectiveness of two delivery strategies, face-to-face (FTF) or remote delivery (RD), and two diets, enhanced Stop Light diet (eSLD) or conventional diet (CD) on weight change across 12 and 18 months. in response to an 18 months. weight management intervention (6 months Weight loss/12 months. Weight maintenance) in adolescents with ID.
Methods:
Adolescents with ID were randomized to one of three arms: FTF /CD, RD/CD, RD/eSLD and asked to attend individual education sessions with a health educator which were delivered during FTF home visits or remotely using video conferencing. The CD followed the US dietary guidelines. The eSLD utilized the Stop Light guide and was enhanced with portion-controlled meals. Participants were also asked to increase their physical activity (PA) and to self-monitor diet, PA and body weight across the 18-month. intervention.
Results:
Weight was obtained from 92(84%) and 89(81%) randomized adolescents at 12 and 18 months, respectively. Weight change across 12 months. Differed significantly by diet (RD/eSLD: −7.0% vs. RD/CD: −1.1%, p = 0.002) but not by delivery strategy (FTF/CD: +1.1% vs. RD/CD: −1.1%, p = 0.21). Weight change across 18 months. Was minimal in all intervention arms and did not differ by diet (RD/eSLD: −2.6% vs. RD/CD: −0.5%; p = 0.28) or delivery strategy (FTF/CD: +1.6% vs. RD/CD: −0.5%; p = 0.47).
Conclusions:
Additional research is required to identify effective strategies to improve long-term weight loss in adolescents with ID.
Keywords: autism, body composition, down syndrome, physical activity, weight loss, youth
1 |. INTRODUCTION
The American Association on Intellectual and Developmental Disabilities defines intellectual disability (ID) as a developmental disability identified prior to age 22 characterized by significant limitations in both intellectual functioning (IQ ≤75) and adaptive behaviours, which include conceptual skills, e.g., language and literacy, money, time etc., social skills, e.g. interpersonal skills, social responsibility, self-esteem, ability to follow rules etc., and practical skills, e.g. activities of daily living, occupational skills, health care, safety, use of money etc.1 Data from the National Health Interview Survey indicates that ~1.4% of children/ adolescents age 8–17 years in the U.S. are diagnosed with ID.2 ID co-occurs or is fully comorbid with other developmental disabilities including, but not limited, to autism spectrum disorder, Down syndrome, cerebral palsy, fetal alcohol syndrome, etc.1
The risk of obesity (BMI ≥95th percentile) in adolescents with ID (age ≥ 11–18 years) is 1.8 times greater than their typically developing peers.3 The prevalence of overweight/obesity is especially high in adolescents with Down Syndrome and autism spectrum disorder.4–8 The elevated obesity risk in adolescents with ID may be associated with lower levels of physical activity (PA),9 lower lean body mass,10 impaired motor skills,11 the use of medications associated with weight gain,12 and the higher rates of food selectivity (i.e., consumption of an abnormally limited variety of foods).13 Overweight in adolescence increases the probability of developing obesity and other chronic conditions including hypertension, type 2 diabetes, gallbladder disease and increased mortality in adulthood.14–17
An extensive literature describing multicomponent interventions have consistently demonstrated clinically relevant weight loss for up to 12 months in typically developing children and adolescents with overweight and obesity.18,19 In contrast, the literature relative to the impact of multicomponent interventions on long-term weight loss in youth with ID is limited to two chart reviews (n = 115, n = 74) which reported on weight change over 12 months in participants in an ongoing special needs weight management clinic at a children’s hospital.20,21 and one randomized trial conducted in a university setting which reported weight change across 18 months (6 months weight loss, 6 months maintenance, 6 months no-contact follow-up) in 24 youth with ID.22
The current report examines the effect of two important intervention components, strategies for reducing energy intake and strategies for intervention delivery on weight change across 12 and 18 months in response to a multi-component weight management intervention (6 months weight loss/12 months weight maintenance) in in a large sample (n = 110) of adolescents and young adults (age ~16 years) with mild-to-moderate ID and overweight and/or obesity. This trial, which was approved by the University’s Institutional Review Board and registered on clinical trials.gov (NCT02561754), was conducted in the local metropolitan area from November 2015 to May 2021.
The rationale, design, methods and results for weight loss at 6 months, the primary aim of this trial, have been described in detail in previous publications.23,24 Briefly, participants were randomized to one of three intervention arms: Individual face-to-face (FTF) delivery with a conventional meal plan diet (FTF/CD), individual remote delivery with a conventional meal plan diet (RD/CD), and individual remote delivery with an enhanced Stop Light Diet (RD/eSLD). The RD arms were delivered using FaceTime™ on an iPad® provided by the trial (Apple Inc., Cupertino, CA) while the FTF arm was delivered during a home visit. Participants randomized to the eSLD arm were asked to follow the Stop Light Diet,25 which categorizes foods by energy content: green (low energy-consume freely), yellow (moderate energy-consume in moderation), and red (high energy-consume sparingly). The SLD was enhanced by encouraging the consumption of high volume, low energy portion-controlled entrées and shakes (HMR Weight Management Services Corp, Boston, MA) and fruits and vegetables. Participants randomized to the CD arms were asked to follow a nutritionally balanced, reduced energy diet which followed the recommendations found on the USDA website ChooseMyPlate.gov26 and the Dietary Guidelines for Americans.27 During weight loss (0–6 months) energy intake in both the CD and eSLD arms was prescribed at 500–700 kcal/day below estimated total daily energy expenditure obtained using the Dietary Reference Intake equation for total daily energy expenditure for overweight boys/girls.28 The portion-controlled entrées and shakes associated with the eSLD were provided by the trial and shipped to participants homes during the weight loss intervention. During weight loss, participants and a parent were asked to attend 30–45 min education/behavioural counselling sessions with a health educator twice per month. Participants were also asked to increase their moderate-to-vigorous physical activity (MVPA) to 60 min/day at least 5 days/week29 and to increase their daily steps by 10% each week from their current level until reaching a goal of 10 000 steps/day and to self-monitor diet, MVPA and steps across the 18 month intervention. Weight loss at 6 months was clinically relevant and significantly greater in the eSLD compared with the CD arms when both interventions were delivered remotely: RD/eSLD (−5.0 ± 5.9 kg; −6.4%) vs. RD/CD (−1.8 ± 4.0 kg; −2.4%, p = 0.01). However, 6-month weight loss in the CD arms was minimal and did not differ by delivery strategy: FTF/CD (−0.3 ± 5.0 kg; −0.2%) vs. RD/CD (−1.8 ± 4.0 kg; −2.4% p = 0.20).
2 |. METHODS
2.1 |. Participant eligibility
2.1.1 |. Inclusion
Age 13–21 years with mild-to-moderate ID (IQ 40–74), as verified by a primary care physician, body mass index (BMI) ≥ 85th percentile on CDC growth charts (age ≤ 19 years) or BMI ≥25 kg/m2 (age > 19 years), or waist circumference to height ratio >0.5 which indicates excess central adiposity in children and adolescents30–33 and is commonly observed in youth with Down Syndrome,34 sufficient functional ability to understand directions, communicate through spoken language, living at home with a parent or guardian, and internet access in the home.
2.1.2 |. Exclusion
Type 1 diabetes, or Type 2 diabetes treated with insulin, Prader-Willi Syndrome, participation in a weight management program involving diet and physical activity in the past 6 months, eating disorders, serious food allergies, consuming special diets, or the inability to participate in MVPA. To enhance the generalizability of our findings, individuals who used medications for prevalent conditions associated with obesity or other medications commonly prescribed for individuals with ID were allowed to participate. Clearance from a primary care physician was required for all participants.
2.2 |. Recruitment/randomization
Participants were recruited through local community programs serving adolescents with ID and using print and web advertisements in the target area. Parental consent, adolescent assent and written physician clearance were obtained prior to randomization. Randomization was stratified by BMI (25.0–29.9 kg/m2 vs. ≥30 kg/m2) for participants age ≥ 19 years or BMI percentile (<95th percentile vs ≥95th percentile) for participants age < 19 years. Randomization sequences were computer generated by the project statistician to achieve equal allocation to the RD/CD, RD/eSLD, and FTF/CD arms.
2.3 |. Weight maintenance intervention (7–18 months)
Immediately following completion of the previously described 6-month weight loss intervention,24 participants began a 12-month maintenance intervention continuing in the intervention arm assigned at baseline. Both the weight loss and maintenance interventions were based on the behavioural principles of the Social Cognitive Theory, which considers the influence of individual experiences, the actions of others, and environmental factors on individual health behaviours.35
2.3.1 |. Role of the parent
Adolescents were required to designate one parent to serve as the primary family contact across the 18-month trial. The parent was asked to attend all behavioural sessions to familiarize themselves with both the diet and physical activity recommendations and the behavioural strategies incorporated in the intervention. The parent was asked to provide support and encouragement, while aiding in following the prescribed diet, promoting physical activity, and self-monitoring of diet and physical activity, if necessary.
2.3.2 |. Diet
Recommended energy intake at the initiation of weight maintenance was estimated using the Dietary Reference Intake equation for total daily energy expenditure for overweight boys/girls,28 based on participant weight at 6 months with consideration for growth and development and adjusted as required based on observed changes in weight across the weight maintenance intervention. Participants in the eSLD arm were encouraged to continue using the eSLD, i.e., a minimum of two entrées (200–270 kcal each), two shakes (~100 kcal each), five one-cup servings of fruits and vegetables each day, and lower energy foods (green/yellow) from a chart/pictures of foods that were colour-coded based on the SLD system. During weight maintenance participants were asked to purchase low calorie entrées and shakes from a list of these items that are readily available at most grocery stores, developed by the health educators. This contrasted with the weight loss intervention where the entrées and shakes recommended for the eSLD arm were provided by the trial and shipped to the participant’s homes every other week. Participants randomized to the CD arms were asked to continue using a CD as recommended during weight loss; however, suggested servings of grains, proteins, fruits and vegetables, dairy, and fats were recalculated based on their energy needs for weight maintenance.
2.3.3 |. Physical activity
Participants were asked to continue the physical activity recommendations prescribed for weight loss (0–6 months), i.e., 60 min./day of MVPA least 5 days/week29 and 10 000 steps/day.
2.3.4 |. Education/behavioural counselling
Individual education/behavioural counselling sessions (30–45 min) specifically developed for adolescents with ID were delivered to the adolescent and a parent by a trained health educator twice each month during the first 6 months of weight maintenance (7–12 months), the same session frequency used during weight loss, and once each month during the final 6 months of weight maintenance (13–18 months). COVID-19 restrictions prohibited FTF contacts with participants between March and June 2020. Therefore, during this period all sessions with participants in the FTF arm were conducted by telephone. Participants who were uncomfortable with attending FTF meetings following the lifting of the COVID-19 restrictions (n = 10) were allowed to continue with telephone meetings from July 2020 through the completion of the trial (May 2021). The content and duration of the education/behavioural counselling sessions were identical in all three intervention arms and included strategies focused on weight maintenance, e.g., making healthy choices when eating out, healthy eating in social situations, resistance training, and maintaining motivation for MVPA etc. Health educator feedback and counselling relative to participants self-monitored dietary intake, MVPA, and body weight were provided during each session.
2.3.5 |. Self-monitoring
Daily self-monitoring of MVPA and steps in the RD arms was completed using Fitbit® Charge HR wireless activity monitors (Google LLC, Mountain View, CA). Self-monitoring of diet in the RD arms was completed using the Lose it! app on the iPad®. To provide feedback regarding weight change participants in the RD arms weighed during each FaceTime™ session using a calibrated digital wireless scale (Model WS-30; Withings Inc., Cambridge, MA). The FTF arm completed daily self-monitoring of dietary intake, pedometer steps (Omron HJ-320;Healthcare Inc, Lake Forest, IL), and self-reported minutes of MVPA using pencil and paper records designed specifically for adolescents with ID. To provide feedback regarding weight change in the FTF arm participants were weighed on a calibrated digital scale (Model #PS6600; Belfour, Saukville, WI) during each education/behavioural counselling session. Self-monitoring of body weight was not possible for participants in the FTF arm who completed behavioural sessions during weight maintenance by telephone (n = 10) as necessitated by COVID-19 restrictions prohibiting FTF meetings. All self-monitoring data were available to health educators to provide feedback to participants during education/behavioural counselling sessions and to assess compliance with the self-monitoring protocols.
2.3.6 |. Intervention fidelity
Health educators were randomly assigned participants in each of the three intervention arms at baseline to diminish the potential for health educator bias. All health educator/participant sessions were audio recorded. Intervention fidelity was assessed by comparing recordings with a check list of content to be delivered. The study protocol required health educators who failed to deliver at least 80% of the scheduled content to receive additional training from the investigative team. One health educator fell below the 80% criteria for one behavioural session.
2.3.7 |. Incentives
We provided modest incentives to motivate participants to meet their goals in accordance with positive behavioural support programs which have been used successfully in schools, community organizations and by parents to promote behaviour change in individuals with ID. Participants were allowed to keep their iPad®. Participants also received $2 for each week they completed self-monitoring for diet and MVPA on at least 5 of 7 days. All participants and parents received gift cards ($15 participants, $10 parents) for completing outcome assessments at 12 and 18 months.
2.4 |. Outcome assessments
2.4.1 |. Anthropometrics
Weight, height, and waist circumference were assessed during FTF home visits at 12 and 18 months by trained staff blinded to intervention arm. Weight was measured, in duplicate, to the nearest 0.1 kg using a calibrated digital scale (Model #PS6600, Belfour, Saukville, WI) with participants wearing shorts and a t-shirt. Standing height was measured in duplicate with a portable stadiometer (Model #IP0955, Invicta Plastics Limited, Leicester, UK). Waist circumference was measured using the procedures described by Lohman et al.36 Three measurements were obtained with the outcome recorded as the average of the closest 2 measures. During COVID-19 restrictions, which prohibited FTF participant contact, body weight was assessed using the following protocol. Research staff delivered the calibrated digital scale to participant’s homes in a sanitized box. Staff, who remained in their vehicle, phoned participants, and directed them to retrieve and set-up the scale on a firm, non-carpeted surface in their home. Participants were asked to step on the scale and take a cell-phone photo of the scales digital display and text the photo to the research staff. Participants subsequently returned the scale to the box and placed it outside the home for retrieval. We were unable to develop workable protocols for no contact assessments of waist circumference and height which resulted in missing data for waist circumference and BMI (kg/m2) at 18 months for 7 participants (FTF/CD = 4, RC/CD = 2, RD/eSLD = 1).
2.4.2 |. Process outcomes
The percentage of behavioural sessions attended, and the percentage of days participants provided self-monitoring data for diet, MVPA and steps were calculated from records maintained by the health educators across the intervention.
2.4.3 |. Intervention costs
We assessed the cost of both intervention delivery and equipment/supplies required to conduct the 18-month intervention. Delivery costs included health educator time required for preparation and delivery of behavioural sessions and travel costs associated with delivery of the FTF intervention. Equipment/supplies included iPads®, Fitbits™, pedometers, wireless scales, and self-monitoring sheets. Delivery costs were obtained from health educator records while equipment/supply costs were obtained from receipts of purchases.
2.5 |. Analysis
This trial was powered to detect differences in weight change across 6 months between two dietary (RD/eSLD vs. RD/CD) and intervention delivery strategies (FTF/CD vs. RD/CD). The comparison of weight change between dietary and delivery strategies across 12 and 18 months, as presented herein, were unpowered secondary analyses.
2.5.1 |. Choice of outcome
We are aware of the controversy regarding the best measure to describe change in adiposity for use in adolescents, e.g., weight (kg), BMI (kg/m2), BMI z-score, or percent of the 95th percentile.37–40 BMI percentile and associated outcomes, i.e., BMI Z-score, and percent at or above the 95th percentile calculations were unavailable for the 15 participants 19 years of age or older (17% of the sample). Thus, we selected change in body weight (kg) as our outcome measure to include all participants in the analysis. To account for any increases in height over the intervention period we also compared changes in BMI (kg/m2) and percent change in BMI between intervention arms.22
2.5.2 |. Analytic strategy
Sample characteristics and outcomes were summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Separate two sample t-tests were used to compare the RD/CD and RD/eSLD arms (diet effect) and RD/CD and FTF/CD arms (delivery strategy effect) across 12 and 18 months. Analysis of weight change (kg) from baseline to 18 months, was based on intent-to-treat principles with multiple imputation, followed by a completer’s only analysis. SAS Proc MI was used to create five imputed data sets for weight change across 18 months by modelling missing data as a function of baseline weight, intervention arm and age. All other analyses were performed using data from completers only per the study design (Ref). Between arm differences for change in BMI, percent change in BMI, waist circumference, and intervention costs across 18 months were also evaluated using two-sample t-tests. We used correlations (continuous variables) or two-sample t-tests (categorical variables) to evaluate the impact of the following on weight change across 18 months: demographics (age, sex, race, Down syndrome diagnosis), process outcomes (percentage of behavioural sessions attended, percentage of days participants provided self-monitoring data for diet and MVPA), and COVID-19 restrictions (completed the intervention before or after March 2020). All analyses were conducted using SAS 9.4 (Cary, NC).
3 |. RESULTS
3.1 |. Participants
Adolescents with ID (n = 110) were randomized to the FTF/CD (n = 36), RD/CD (n = 39) or RD/eSLD (n = 35) arms. Body weight at 12 months was obtained from 32 (89%), 34 (87%) and 26 (74%) participants randomized at baseline to the FTF/CD, RD/CD, and RD/eSLD arms respectively (Figure 1). Body weight at 18 months was obtained from 30 (83%), 32 (82%), and 26 (74%) participants randomized at baseline to the FTF/CD, RD/CD, and RD/eSLD arms respectively (Figure 1). At baseline participants were ~16 years of age, ~53% female, and ~81% non-Hispanic white. Additionally, ~48% were diagnosed with Down Syndrome and ~28% were diagnosed with autism spectrum disorder (Table 1).
FIGURE 1.
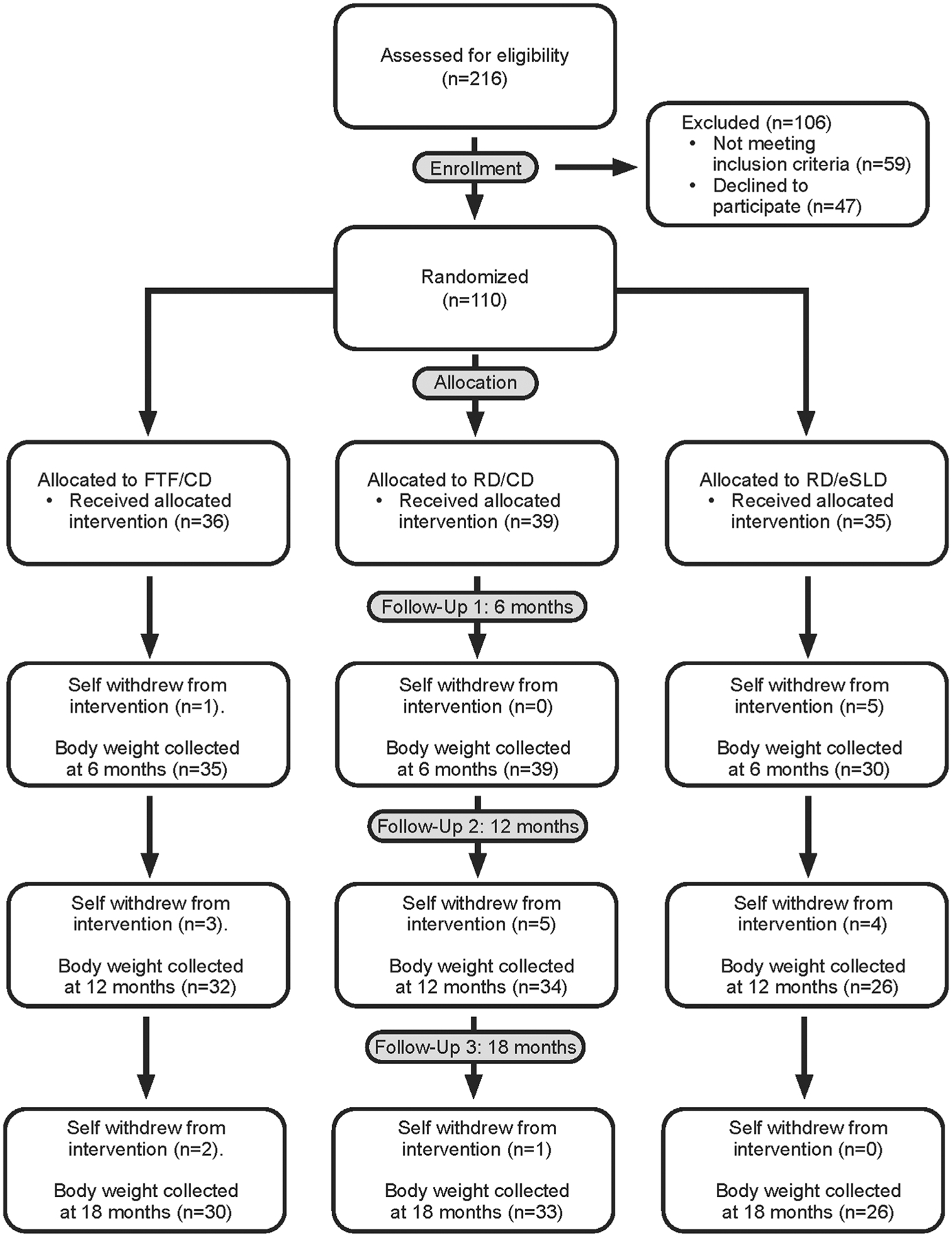
Consort diagram
TABLE 1.
Baseline characteristics of adolescents with intellectual disabilities by intervention arm
| FTF/CDa (n = 36) M ± SD / % (n) |
RD/CDb (n = 39) M ± SD / % (n) |
RD/eSLDc (n = 35) M ± SD / % (n) |
|
|---|---|---|---|
| Age (years) | 16.3 ± 2.7 | 15.6 ± 1.7 | 16.7 ± 2.5 |
| Sex | |||
| Male | 56% (20) | 39% (15) | 49% (17) |
| Female | 44% (16) | 62% (24) | 51% (18) |
| Race | |||
| White | 83% (30) | 97% (38) | 83% (29) |
| Black | 8% (3) | 0% (0) | 11% (4) |
| Two or More Races | 8% (3) | 3% (1) | 6% (2) |
| Ethnicity | |||
| Not Hispanic/Latino | 94% (34) | 95% (37) | 89% (31) |
| Hispanic/ Latino | 6% (2) | 5% (2) | 11% (4) |
| Diagnosis | |||
| Autism Spectrum Disorder | 42% (15) | 36% (14) | 37% (13) |
| Down Syndrome | 47% (17) | 54% (21) | 43% (15) |
| Other | 11% (4) | 10% (4) | 20% (7) |
| Weight (kg) | 88.4 ± 29.5 | 74.9 ± 16.5 | 83.6 ± 26.4 |
| BMI (kg/m2) | 34.1 ± 8.3 | 31.3 ± 5.8 | 32.7 ± 7.1 |
| BMI percentiled | 96% ± 6% | 95% ± 6% | 96% ± 4% |
| Waist circumference(cm) | 98.3 ± 17.1 | 90.5 ± 11.3 | 94.4 ± 15.3 |
RD/CD = Remote Delivery/Conventional Diet.
RD/eSLD = Remote Delivery/Enhanced Stop Light Diet.
FTF/CD=Face-to-face Delivery/Conventional Diet.
Calculated for participants age ≤19 year (FTF/CD: n = 30, RD/CD: n = 38, RD/eSLD: n = 27).
3.2 |. Weight change
3.2.1 |. Effect of diet (RD/CD vs. RD/eSLD) (Tables 2 and 3, Figures 2 and 3)
TABLE 2.
Change in weight, BMI, BMI percentile, and waist circumference across 12 and 18 months in adolescents with intellectual disabilities
| FTF/CDa | RD/CDb | RD/eSLDc | ||||||
|---|---|---|---|---|---|---|---|---|
| n | M (SD) | n | M (SD) | n | M (SD) | Delivery p-value | Diet p-value | |
| Across 12-months (0–12 months) | ||||||||
| Δ Weight (kg) | 32 | +1.3 (8.2) | 34 | −0.7 (4.6) | 26 | −5.3 (6.3) | 0.21 | 0.002 |
| Δ Weight (%) | 32 | +1.1 (9.1) | 34 | −1.1 (6.4) | 26 | −7.0 (7.8) | 0.25 | 0.002 |
| Δ BMI (kg/m2) | 23 | −0.5 (2.4) | 31 | −0.7 (2.1) | 22 | −2.2 (2.7) | 0.71 | 0.03 |
| Δ BMI (%) | 23 | −1.6 (7.4) | 31 | −2.6 (7.2) | 22 | −7.4 (8.6) | 0.59 | 0.03 |
| Δ Waist Circumference (cm) | 27 | −1.6 (6.2) | 26 | −0.8 (4.2) | 19 | −5.5 (5.3) | 0.56 | 0.002 |
| Across 18-months (0–18 months) | ||||||||
| Δ Weight (kg) | 30 | +1.4 (9.7) | 33 | −0.2 (6.6) | 26 | −2.2 (7.4) | 0.47 | 0.28 |
| Δ Weight (%) | 30 | +1.6 (12.3) | 33 | −0.5 (8.7) | 26 | −2.6 (10.5) | 0.41 | 0.42 |
| Δ BMI (kg/m2) | 26 | −0.1 (3.2) | 31 | −0.6 (2.5) | 25 | −1.3 (2.7) | 0.54 | 0.29 |
| Δ BMI (%) | 26 | −0.2 (10.1) | 31 | −2.1 (8.3) | 25 | −4.4 (8.9) | 0.45 | 0.32 |
| Δ Waist Circumference (cm) | 26 | −0.5 (6.6) | 31 | −1.1 (5.7) | 23 | −3.2 (5.9) | 0.75 | 0.19 |
All p-values are based on a completers only analysis.
FTF/CD=Face-to-Face Delivery/Conventional Diet.
RD/CD = Remote Delivery/Conventional Diet.
RD/eSLD = Remote Delivery/Enhanced Stop Light Diet.
TABLE 3.
Categories of weight change in adolescents with intellectual disabilities across 18-months
| FTF/CDa | RD/CDb | RD/eSLDc | ||||
|---|---|---|---|---|---|---|
| n | % | N | % | n | % | |
| Weight loss | ||||||
| 0 – <3% | 5 | 16.7 | 6 | 18.2 | 5 | 19.2 |
| 3 – <5% | 1 | 3.3 | 6 | 18.2 | 2 | 7.7 |
| ≥5% | 10 | 33.3 | 9 | 27.3 | 9 | 34.6 |
| Weight gain | ||||||
| 0 – <3% | 4 | 13.3 | 3 | 9.1 | 4 | 15.4 |
| ≥3% | 10 | 33.3 | 9 | 27.3 | 6 | 23.1 |
FTF/CD=Face-to-Face Delivery/Conventional Diet.
RD/CD = Remote Delivery/Conventional Diet.
RD/eSLD = Remote Delivery/Enhanced Stop Light Diet.
FIGURE 2.
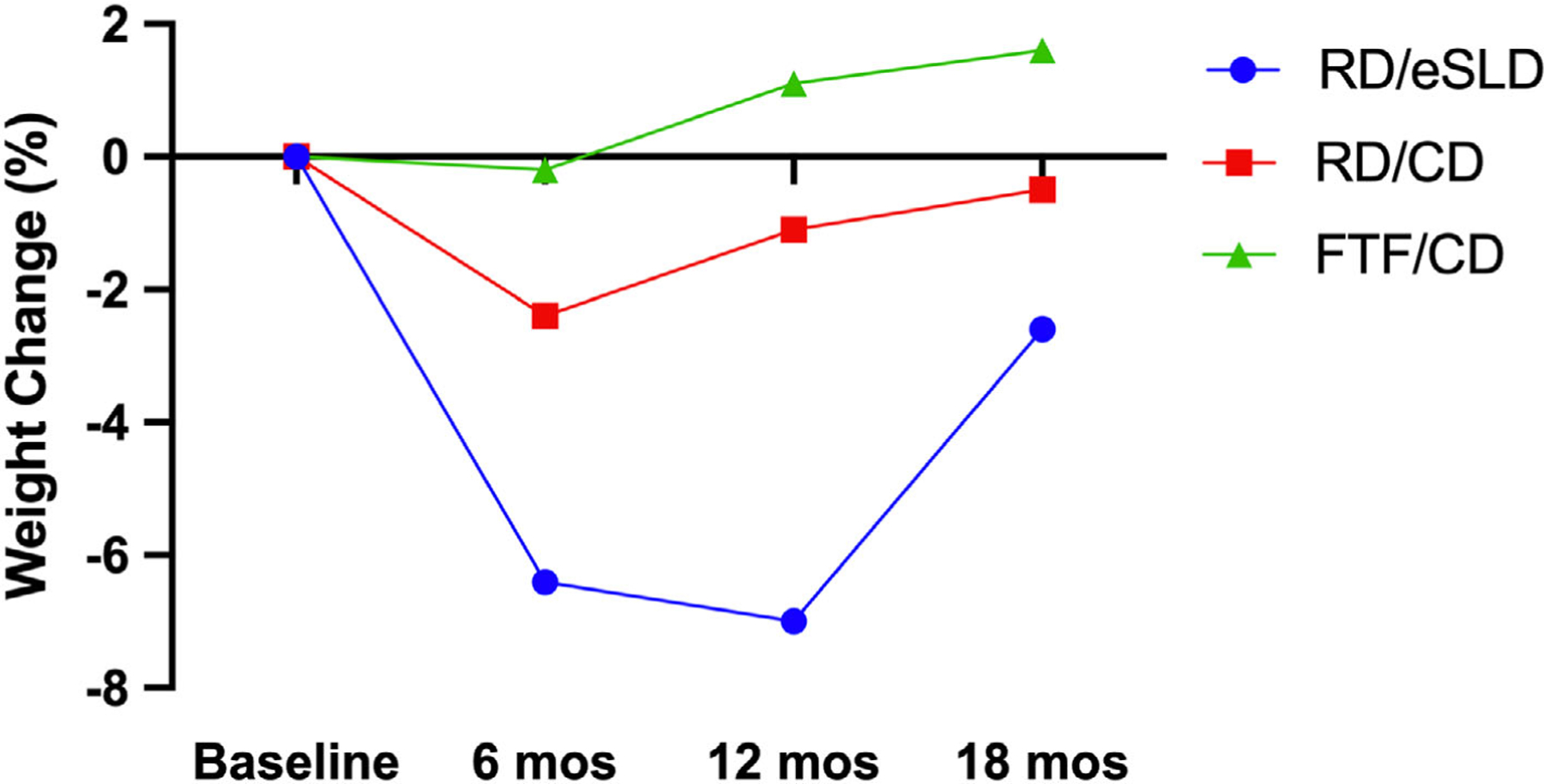
Percent change in weight in adolescents with intellectual disabilities across 12 and 18-months. FTF/CD = Face-to-face Delivery/Conventional Diet, RD/CD = Remote Delivery/Conventional Diet, RD/eSLD = Remote Delivery/Enhanced Stop Light Diet
FIGURE 3.
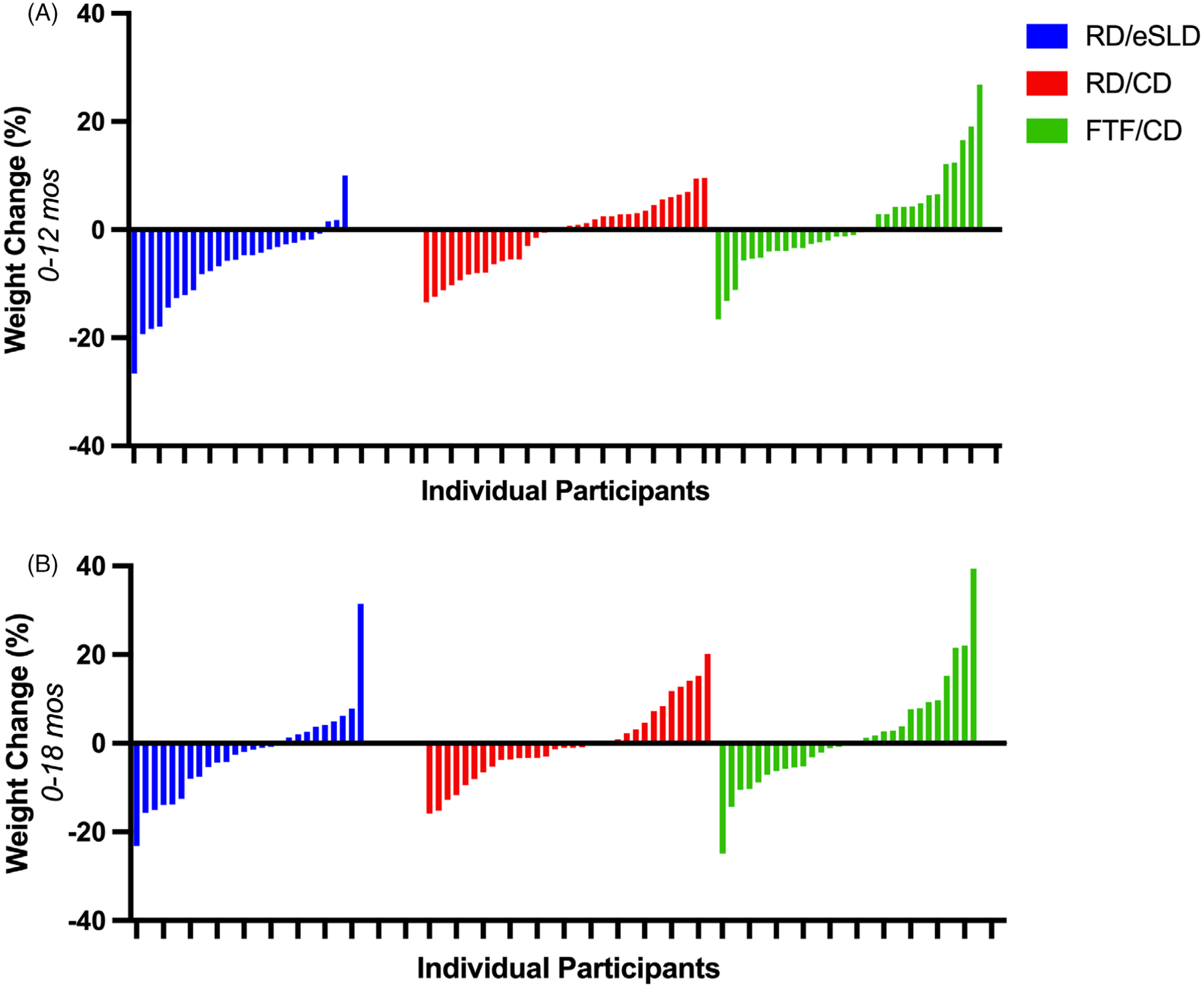
Individual data for percent weight change in adolescents with intellectual disabilities across 12 and 18-months. Panel A: 0–12 months, Panel B: 0–18 months. FTF/CD = Face-to-Face Delivery/Conventional Diet, RD/CD = Remote Delivery/Conventional Diet, RD/eSLD = Remote Delivery/Enhanced Stop Light Diet
Mean weight loss across 12 months was clinically relevant and significantly greater when using the eSLD (−5.3 ± 6.3 kg; − 7.0%) compared with the CD (−0.7 ± 4.6 kg; −1.1%, p = 0.002) when both interventions were delivered remotely (Figure 2, Table 2). Similarly, reductions in BMI (p = 0.03), percent change in BMI (p = 0.03), and waist circumference (p = 0.002) across 12 months were significantly greater in the RD/eSLD compared with the RD/CD arm. In contrast to our results across 12 months, mean weight loss across 18 months was minimal and did not differ significantly between the eSLD and CD arms (RD/eSLD: −2.2 ± 7.4 kg, −2.6% vs. RD/CD: −0.2 ± 6.6 kg, −0.5%, p = 0.0.37 imputation, p = 0.28 completers only). Similarly, there were no significant differences between the RD/CD and RD/eSLD arms for change in BMI, percent change in BMI, or waist circumference across 18-months. The proportion of participants achieving ≥5% weight loss (RD/eSLD = 34.6%, RD/CD = 27.3%) and the proportion of participants gaining ≥3% of baseline weight (RD/eSLD = 23.1%, RD/CD = 27.3%) were similar in the CD or eSLD arms across 18 months. Process measures across the 18-month intervention including the percentage of educational/behavioural counselling sessions attended (RD/CD = 71.1%, RD/eSLD = 71.4%, p = 0.97) and the percentage of days of self-monitoring of diet (RD/CD = 60.6%, RD/eSLD = 60.7%, p = 0.99) and MVPA /steps(RD/CD = 62.3%, RD/eSLD = 62.4%, p = 0.99) did not differ by dietary strategy.
3.2.2 |. Effect of intervention delivery (FTF/CD vs. RD/CD) (Tables 2 and 3, Figures 2 and 3)
Mean weight change using a CD delivered FTF or remotely was minimal across both 12 and 18 months and did not differ significantly between the FTF and RD arms: 12 months (FTF/CD: +1.3 ± 8.2 kg; +1.1% vs. RD/CD: −0.7 ± 4.6 kg; −1.1%, p = 0.21); 18 months (FTF/CD: +1.4 ± 9.7 kg, +1.6% vs. RD/CD = −0.2 ± 6.6 kg, −0.5%, p = 0.43 imputation, p = 0.47 completers only). Similarly, there were no significant differences using a CD delivered FTF or remotely for change in BMI, percent change in BMI, or waist circumference across 12 and 18 months. Across 18-months, the proportion of participants achieving ≥5% weight loss (FTF/CD = 33.3%, RD/CD = 27.3%) and the proportion of participants gaining ≥3% over their baseline weight (FTF/CD = 33.3%, RD/CD = 27.3%) were similar using the CD delivered either FTF or remotely. Process measures across the 18-month intervention including the percentage of education/behavioural counselling sessions attended (FTF/CD = 75.9%, RD/CD = 71.1%, p = 0.37), and the percentage of days of self-monitoring of diet (FTF/CD = 67.1%, RD/CD = 60.6%, p = 0.39) or MVPA/steps (FTF/CD = 66.3%, RD/CD = 62.3%, p = 0.58) did not differ significantly between FTF or remote delivery.
3.2.3 |. Factors associated with weight change across 18 months
Higher participant age (r = 0.42, p < 0.001) and a higher percentage of days participants tracked MVPA/steps (r = 0.29, p = 0.006) were associated with greater weight loss across 18 months. Weight loss across 18-months was not significantly associated with the percentage of behavioural sessions attended (r = 0.19, p = 0.08) or the percentage of days participants tracked dietary intake (r = 0.20, p = 0.06). Additionally, no significant differences in weight change across 18 months were observed between males (+1.4 ± 9.8 kg) and females (−1.7 ± 5.8 kg, p = 0.07), participants with (−1.0 ± 6.5 kg) or without Down syndrome (+0.5 ± 9.2 kg, p = 0.37), race categorized as white (−0.8 ± 6.8 kg) or other (+3.6 ± 14.2 kg, p = 0.34) or completing the intervention prior to (+0.6 ± 7.9 kg) or during the COVID-19 pandemic restrictions (−0.7 ± 8.2 kg, p = 0.46).
3.3 |. Cost
As expected, the additional costs associated with the time and health educator travel required for FTF delivery resulted in a total per person cost for delivering the 18-month intervention ($1600, $54/session) that was significantly higher than delivering the intervention remotely ($750, $25/session, p < 0.001). However, the cost of equipment required to conduct the remote intervention, i.e., iPads®, Fitbits™, wireless scales ($580), far exceeds the cost of the pedometers and self-monitoring sheets used with the FTF intervention ($25). Thus, the total per person cost for delivering the FTF intervention in the context of this trial ($1625, $54/session) was $295 higher than the cost of RD ($1330, $44/session). Additionally, the cost of the RD arms included provision of iPads® (~$300) which were required to comply with University IRB requirements to protect the privacy of participant in clinical trials. However, most families currently own a desktop, laptop, or tablet, computer, or a smart phone. Thus, in clinical practice additional cost savings in delivering the education/behavioural counselling sessions could be achieved by using a no-cost video conferencing platforms such as Zoom® on these devices.
4 |. DISCUSSION
We examined the effect of both diet and delivery strategy on weight change across 12 and 18-months in response to a multicomponent weight management intervention (6 months weight loss, 12 months maintenance) in adolescents with mild-to-moderate ID and overweight/obesity. We observed weight loss across 12 months that was clinically relevant and significantly greater using the eSLD (−7.0%) compared with minimal weight loss using a CD (−1.1%) when both interventions were delivered remotely, and non-significant differences in weight change across 12 months when using a CD delivered either remotely (−1.1%) or FTF (+1.1%). However, weight change across 18-months was minimal in all intervention arms (range −2.6% to +1.6%) and did not differ by diet (eSLD vs. CD) or delivery strategy (FTF vs. RD).
The clinically relevant weight loss (≥5%) across 12 months observed in the RD/eSLD arm in this trial (−7.0%, 45% ≥ 5% weight loss) was encouraging. Unfortunately, weight regain in the RD/eSLD arm during the final 6 months of the maintenance intervention (+3.3 kg, +4.8%) reduced mean weight loss across 18 months to −2.6% (35% ≥ 5% weight loss). We speculate that the reduction in participant contract from two to one education/behavioural counselling session per month during the final 6 months of the maintenance intervention may be associated with the observed weight regain. However, we are unaware of previous trials that have evaluated the impact of contact frequency or changes in contact frequency on weight loss maintenance in adolescents with ID, topics worthy of additional investigation.
We are unaware of other trials that have compared the effectiveness an eSLD and CD for long-term weight management in adolescents with ID; however, our research group previously reported results from trial that compared long-term weight loss (6 months weight loss, 12 months maintenance) between an eSLD and a CD in adults with ID.41 Participants (age ~37 years) with overweight/obesity (BMI = ~36 kg/m2) were randomized to an eSLD (n = 77) or CD (n = 72) arm in the context of a multicomponent weight management intervention, which in addition to reduced energy intake, included increased MVPA, self-monitoring (diet, weight, MVPA) and education/behavioural counselling delivered during monthly FTF home visits. In contrast with the results from the current trial in adolescents where weight loss across 18-months was minimal in all intervention arms (−2.6 to +1.1%) with no significant differences between the eSLD and CD arms, in the adult trial we observed clinically relevant weight loss in both the eSLD (−6.7%, ~57% ≥ 5% weight loss) and CD arms (−6.4%, ~49% ≥ 5% weight loss) with no significant between arms differences (p = 0.94). Additionally, participants in the eSLD arm in the adult trial were able to maintain weight loss across the 12-month maintenance intervention in contrast to the current trial in adolescents who maintained weight loss across the first 6 months and regained a large portion of the weight loss across the final 6 months of the weight maintenance intervention. As described previously, the frequency of educational/behavioural counselling sessions in the adolescent trial was reduced from two to one session per month during the last 6 months of the maintenance intervention, while session frequency in the adult trial was consistent (monthly) across 12-months. This provides some evidence to support our hypothesis that the change in the frequency of education/behavioural sessions may be associated with the weight regain observed in the RD/eSDL arm over the last 6 months in the adolescent trial.
In the current trial we observed minimal mean weight change across 6 (−1%), 12 (0%), and 18 months (−0.6%) in our two intervention arms that used a CD. However, other reports that have included more intensive interventions than those used in the current trial have demonstrated the effectiveness of a CD for long-term weight management in adolescents with ID. For example, a recent publication from Bandini et al.22 reported 18-month weight loss of −6.1 kg in response to a family-based intervention (6 months weight loss, 6 months maintenance, 6 months no contact follow-up) in a small sample of youth with ID (n = 24, 56% Down syndrome, age 14–22 years) who were randomized to the weight management intervention or wait-lost control. The intervention included three 45-minute in-person group sessions per month (2–5 participants and parent) and one monthly 45-minute session with participants and parents separately during weight loss and 2 sessions per month during weight maintenance which alternated between 90 min in-person group sessions (participants and parent) and 30-min individual sessions targeting behavioural training for parents. In contrast, our intervention included 30–45-min individual sessions with participants and parents twice per month during weight loss and the first 6 months of maintenance and one session per month during the final 6 months of maintenance. However, none of our intervention content was directed specifically to the parent. We simply asked parents to familiarize themselves with our recommendations for diet, physical activity and self-monitoring and the behavioural strategies incorporated in the intervention, and to provide participant support and encouragement. Two reports on the impact of participation in a multidisciplinary special needs weight management clinic at a children’s hospital in youth (age ~11–12 years) with primarily intellectual disabilities (n = 115)21 and with autism spectrum disorder (n = 74)20 have also shown significant reductions in BMI z-score,21 BMI% of the 95th percentile20 over 12 months of treatment using a CD in combination with intensive behavioural therapy. These clinics were intensive and included extensive involvement of a multi-disciplinary team of health professionals including child psychologists, nurse practitioners/paediatricians, dietitians, and occupational therapists, Thus, the limited available information suggests that a CD may be effective for long-term weight loss in adolescents with ID when used in the framework of an intensive intervention, However, the burden and costs associated delivering an intervention of sufficient intensity, and the level of parental involvement and the professional expertise required to achieve clinically relevant weight loss greatly limits the potential for widespread implementation and reach of interventions using a CD for long-term weight management in this population.
Data from the current trial and other weight management trials completed by our group suggests that the frequency of education/behavioural counselling required to achieve clinically relevant weight loss may differ between individuals with ID and typically developing/developed individuals. For example, in the current trial we observed clinically relevant weight loss across 12-months in the RD/eSLD arm (−7.0%, 45% ≥ 5% weight loss) than included twice monthly 45-min education/behavioural counselling sessions, or ~18 contact hours across the 12-month intervention. This is considerably less than the ≥26 contact hours over 12 months to improve weight status in typically developing children and adolescents as recommended by the US Preventive Services Task Force Recommendation Statement on Screening for Obesity in Children and Adolescents.18 Additionally, results from a completed (n = 150)41 trial and preliminary results from an ongoing weight management trial from our research group in adults with ID42 (n = 120) suggest that clinically relevant 6-month weight loss using an eSLD (~ −7 to 8%) that was successfully maintained across a 12 month maintenance intervention (~ −5 to 7%) can be achieved using monthly 45–60-min education/behavioural counselling sessions across 18 months. The monthly education/behavioural counselling sessions during weight loss (0–6 months), i.e., 6 sessions across 6 months are fewer than the ≥14 sessions over 6 months recommended for weight loss, while the monthly sessions are consistent with current weight maintenance guidelines for typically developed adults.43
Weight change across 18-months in the current trial did not differ significantly between interventions using a CD delivered FTF or remotely to individual adolescents with ID and a parent. We are unaware of previous trials which have compared the effectiveness of FTF and remote delivery for long-term weight loss in adolescents with ID. However, preliminary results from an on-going trial conducted by our research group indicates similar 18-month weight loss in adults with ID (n = 120) who were randomized to an intervention using an eSLD (6 months weight loss, 12 months maintenance) delivered to the adult with ID and a caregiver/study partner during FTF home visits (−3.7%) or remotely using Zoom® (−5.1%).42 Some evidence in typically developing children and adolescents with overweight/obesity suggests that weight loss achieved with remotely delivered behavioural interventions is minimal (~0.10 change in BMI z-scores)44 and that a combination of FTF and remotely delivered sessions may be required to elicit clinically relevant weight loss.45,46 However, the significant weight loss across 12 months in both the RD/eSLD arm of the current trial in adolescents, and our on-going trial in adults with ID, suggests that remote delivery may be an effective strategy for long-term weight management in individuals with ID.
We observed clinically relevant weight loss (≥5%) across 12 months in the RD/eSLD arm in this trial (−7.0%, 45% ≥ 5% weight loss). However, weight regain observed in the eSLD arm across the final 6 months of the weight maintenance intervention was disappointing and suggests the need for additional trials designed to evaluate strategies to achieve longer term weight loss maintenance in adolescents with ID. Several aspects of the RD/eSLD intervention utilized in the current trial that achieved clinically relevant weight loss across 12 months suggest the potential for the widespread implementation and reach of this approach for use with adolescents with ID if successful strategies to improve longer-xterm weight loss maintenance using the RD/eSLD can be identified. These aspects include the use of the eSLD delivered remotely to individual adolescents and a parent, minimal parental involvement, and a reasonable frequency of educational/behavioural counselling sessions. The eSLD is easier to understand and implement and the inclusion of commercially available portion-controlled entrées and shakes make it easier to adhere to specific energy and nutrient recommendations compared with a CD. Remote delivery eliminates the need for parents to provide transportation to the intervention site, and from the provider perspective, remote delivery eliminates the travel costs associated with conducting individual FTF home-visits and allows the intervention to be delivered to participants regardless of their geographic location. The limited attention span and the need for individualized instruction in adolescents with ID favours the use of individual versus group-based interventions. Parents of adolescents with ID face emotional and physical challenges that exceed those of parents caring for typically developing adolescents thus, parental involvement was limited to attending education/behavioural counselling sessions and providing support and encouragement to their adolescent. Finally, our approach includes a reasonable level of participant contact i.e., 30-to-45- minute education/behavioural counselling sessions twice monthly over 12 months.
Strengths of this trial include a randomized design that allowed for the comparison of two important intervention components, i.e., strategies for reducing energy intake and intervention delivery, an intervention tailored to the cognitive abilities of adolescents with ID, intervention delivery by health educators trained and supervised by a member of the investigative team to ensure intervention fidelity, assignment of the same health educator to participants in all 3 arms to reduce the potential for health educator bias, a large sample size (n = 110) high participant retention across 18-months (81%), and reasonable behavioural session attendance (73%) and compliance with the self-monitoring protocols for both diet (63%) and MVPA (63%). The primary weakness in this trial, as described in more detail in our previous publication on weight loss at 6 months,29 was the inability to obtain data of sufficient quality and quantity to evaluate participant compliance with both our dietary, i.e., energy intake, consumption of fruits and vegetables, entrées, and low-calorie shakes, and physical activity recommendations. Additionally, our results are based on a sample of adolescents with mild-to-moderate ID and overweight/obesity living at home with a parent, who volunteered and were incentivized to participate in a weight management trial. Thus, these results may not be generalizable to adolescents with more severe ID, those living in group homes or other living arrangements, or outside of the context of a research trial.
In summary, the results of this study demonstrated minimal weight change across 18 months in adolescents with ID and overweight/obesity. Weight change did not differ by the type of diet used to reduce energy intake (eSLD or CD) or by delivery system (FTF or RD). The clinically relevant 12-month weight loss observed with the RD/eSLD arm in this trial was encouraging and suggests the potential for the widespread implementation and reach of the RD/eSLD approach for longer term weight management in adolescents with ID. However, prior to implementation, additional trials will be required to evaluate strategies to minimize weight regain after 12 months using the RD/eSLD intervention.
ACKNOWLEDGEMENTS
This study was funded by the National Institutes of Child Health and Development (R01HD079642). We would like to acknowledge Ms. Jessica Danon, Mr. Joseph Sherman, Mr. Andrew Collie, Ms. Rachel Foster, Ms. Lauren Keller, Ms. Kirstin Reitmeier, Ms. Randi Grey, and Ms. Leah Osborne for their assistance with data collection and delivery of the intervention.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: HD079642
CONFLICT OF INTEREST
Drs. Ptomey, Washburn, Donnelly, Sullivan, Goetz, Mayo, and Lee as well as Mr. Honas and Ms. Rice all received funding from National Institutes of Child Health and Development (R01HD079642) to conduct this trial. Drs. Ptomey and Donnelly received personal fees from Healthy Weight Research Network for Children with Autism Spectrum Disorder and Developmental Disabilities, which is outside the submitted work and unrelated to the project.
Abbreviations:
- CD
Conventional Diet
- eSLD
Enhanced Stop Light Diet
- FTF
Face to Face
- ID
Intellectual Disabilities
- MVPA
Moderate-to-vigorous physical activity
- RD
Remote Delivery
Footnotes
Clinical Trials Number: NCT02561754.
REFERENCES
- 1.Schalock RL, Luckasson R, Tasse MJ. Intellectual Disability: Definition, Diagnosis, Classsification, and Systems of Supports. 12th ed. American Association on Intellectual and Developmental Disabilities; 2021. [DOI] [PubMed] [Google Scholar]
- 2.Zablotsky B, Black LI, Blumberg SJ. Estimated prevalence of children with diagnosed developmental disabilities in the United States, 2014–2016. NCHS Data Brief. 2017;291:1–8. [PubMed] [Google Scholar]
- 3.Maïano C, Hue O, Morin AJ, Moullec G. Prevalence of overweight and obesity among children and adolescents with intellectual disabilities: a systematic review and meta-analysis. Obes Rev. 2016;17(7): 599–611. [DOI] [PubMed] [Google Scholar]
- 4.Grondhuis SN, Aman MG. Overweight and obesity in youth with developmental disabilities: a call to action. J Intellect Disabil Res. 2014; 58(9):787–799. [DOI] [PubMed] [Google Scholar]
- 5.Bertapelli F, Pitetti K, Agiovlasitis S, Guerra-Junior G. Overweight and obesity in children and adolescents with down syndrome-prevalence, determinants, consequences, and interventions: a literature review. Res Dev Disabil. 2016;57:181–192. [DOI] [PubMed] [Google Scholar]
- 6.Criado KK, Sharp WG, McCracken CE, et al. Overweight and obese status in children with autism spectrum disorder and disruptive behavior. Autism. 2018;22(4):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Healy S, Aigner CJ, Haegele JA. Prevalence of overweight and obesity among US youth with autism spectrum disorder. Autism. 2019;23(4): 1046–1050. [DOI] [PubMed] [Google Scholar]
- 8.OS M, OS C, Gibson L, Leo J, Carty C. The prevalence of obesity in children and young people with down syndrome. J Appl Res Intellect Disabil. 2018;31(6):1225–1229. [DOI] [PubMed] [Google Scholar]
- 9.Einarsson I, Ólafsson Á, Hinriksdóttir G, Jóhannsson E, Daly D, Arngrímsson S. Differences in physical activity among youth with and without intellectual disability. Med Sci Sports Exerc. 2015;47(2): 411–418. [DOI] [PubMed] [Google Scholar]
- 10.Magge SN, Zemel BS, Pipan ME, Gidding SS, Kelly A. Cardiometabolic risk and body composition in youth with down syndrome. Pediatrics. 2019;144(2):e20190137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blomqvist S, Olsson J, Wallin L, Wester A, Rehn B. Adolescents with intellectual disability have reduced postural balance and muscle performance in trunk and lower limbs compared to peers without intellectual disability. Res Dev Disabil. 2013;34(1):198–206. [DOI] [PubMed] [Google Scholar]
- 12.McQuire C, Hassiotis A, Harrison B, Pilling S. Pharmacological interventions for challenging behaviour in children with intellectual disabilities: a systematic review and meta-analysis. BMC Psychiatry. 2015; 15:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rancaño KM, Bandini LG, Curtin C, Eliasziw M, Odoms-Young A, Must A. Gender and racial/ethnic differences in food selectivity in children with intellectual disabilities. J Appl Res Intellect Disabil. 2021;34(6):1511–1520. [DOI] [PubMed] [Google Scholar]
- 14.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: the Bogalusa heart study. Pediatrics. 2005;115(1):22–27. [DOI] [PubMed] [Google Scholar]
- 15.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993; 119(7):655–660. [DOI] [PubMed] [Google Scholar]
- 16.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26(4):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer JF, Larsen SB, Blond K, Damsgaard CT, Bjerregaard LG, Baker JL. Associations between body mass index and height during childhood and adolescence and the risk of coronary heart disease in adulthood: a systematic review and meta-analysis. Obes Rev. 2021; 22(9):e13276. [DOI] [PubMed] [Google Scholar]
- 18.Grossman DC, Bibbins-Domingo K, Curry SJ, et al. Screening for obesity in children and adolescents: US preventive services task force recommendation statement. JAMA. 2017;317(23):2417–2426. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight Management in Children and Adolescents: evidence report and systematic review for the US preventive services task force. JAMA. 2017;317(23):2427–2444. [DOI] [PubMed] [Google Scholar]
- 20.Killian HJ, Pallotto IK, Sweeney BR, Dreyer Gillette ML. Weight management outcomes of youth with autism spectrum disorder seeking treatment from a multidisciplinary team. J Autism Dev Disord. 2022; 52(2):791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pona AA, Dreyer Gillette ML, Odar Stough C, Gerling JK, Sweeney BR. Long-term outcomes of a multidisciplinary weight management intervention for youth with disabilities. Child Obes. 2017; 13(6):455–461. [DOI] [PubMed] [Google Scholar]
- 22.Bandini LG, Eliasziw M, Dittrich GA, et al. A family-based weight loss randomized controlled trial for youth with intellectual disabilities. Pediatr Obes. 2021;16:e12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly J, Ptomey L, Goetz J, et al. Weight management for adolescents with intellectual and developmental disabilities: rationale and design for an 18 month randomized trial. Contemp Clin Trials. 2016;51:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ptomey LT, Washburn RA, Goetz JR, et al. Weight loss interventions for adolescents with intellectual disabilities: an RCT. Pediatrics. 2021; 148(3):e2021050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein L, Squires S. The Stoplight Diet for Children: An Eight-Week Program for Parents and Children. Little Brown & co; 1988. [Google Scholar]
- 26.U.S. Department of Agriculture. Choose MyPlate. www.choosemyplate.gov
- 27.Dietary Guidelines Advisory Committee. Report of the dietary guidelines advisory committee on the dietary guidelines for Americans, 2010, to the secretary of agriculture and the secretary of health and human services. Agricult Res Serv 2010. [Google Scholar]
- 28.Institute of Medicine. Dietary reference intakes for energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). 2002. [DOI] [PubMed]
- 29.United States Department of Health and Human Services. Physical activity guidelines advisory committee report to the Secretary of Health and Human Services. 2008. http:\\www.health.gov/PAGuidelines/committeereport.aspx
- 30.Mokha JS, Srinivasan SR, Dasmahapatra P, et al. Utility of waist-to-height ratio in assessing the status of central obesity and related cardiometabolic risk profile among normal weight and overweight/obese children: the Bogalusa heart study. BMC Pediatr. 2010;10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nambiar S, Hughes I, Davies PS. Developing waist-to-height ratio cut-offs to define overweight and obesity in children and adolescents. Public Health Nutr. 2010;13(10):1566–1574. [DOI] [PubMed] [Google Scholar]
- 32.Nambiar S, Truby H, Abbott RA, Davies PS. Validating the waist-height ratio and developing centiles for use amongst children and adolescents. Acta Paediatr. 2009;98(1):148–152. [DOI] [PubMed] [Google Scholar]
- 33.Garnett SP, Baur LA, Cowell CT. Waist-to-height ratio: a simple option for determining excess central adiposity in young people. Int J Obes. 2008;32(6):1028–1030. [DOI] [PubMed] [Google Scholar]
- 34.González-Agüero A, Ara I, Moreno LA, Vicente-Rodríguez G, Casajús JA. Fat and lean masses in youths with down syndrome: gender differences. Res Dev Disabil. 2011;32(5):1685–1693. [DOI] [PubMed] [Google Scholar]
- 35.Bandura A Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52:1–16. [DOI] [PubMed] [Google Scholar]
- 36.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Hum Kinet Books; 1988. [Google Scholar]
- 37.Kelly AS, Daniels SR. Rethinking the use of body mass index z-score in children and adolescents with severe obesity: time to kick it to the curb? J Pediatr. 2017;188:7–8. [DOI] [PubMed] [Google Scholar]
- 38.Hunt LP, Ford A, Sabin MA, Crowne EC, Shield JP. Clinical measures of adiposity and percentage fat loss: which measure most accurately reflects fat loss and what should we aim for? Arch Dis Child. 2007; 92(5):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59(3):419–425. [DOI] [PubMed] [Google Scholar]
- 40.Wei R, Ogden CL, Parsons VL, Freedman DS, Hales CM. A method for calculating BMI z-scores and percentiles above the 95(th) percentile of the CDC growth charts. Ann Hum Biol. 2020;47(6):514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(Suppl 1):82–96. [DOI] [PubMed] [Google Scholar]
- 42.Ptomey LT, Washburn RA, Mayo MS, et al. Remote delivery of weight management for adults with intellectual and developmental disabilities: rationale and design for a 24month randomized trial. Contemp Clin Trials. 2018;73:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen MD, Ryan DH, Donato KA, et al. Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association task force on practice guidelines. Based on a systematic review from the the obesity expert panel, 2013. Obesity. 2014;22(S2):S5–S39. [DOI] [PubMed] [Google Scholar]
- 44.Moorman EL, Koskela-Staples NC, Mathai BB, Fedele DA, Janicke DM. Pediatric obesity treatment via telehealth: current evidence and future directions. Curr Obes Rep. 2021;10(3):371–384. [DOI] [PubMed] [Google Scholar]
- 45.DeSilva S, Vaidya SS. The application of telemedicine to pediatric obesity: lessons from the past decade. Telemed e-Health. 2021;27(2): 159–166. [DOI] [PubMed] [Google Scholar]
- 46.Whitley A, Yahia N. Efficacy of clinic-based telehealth vs. face-to-face interventions for obesity treatment in children and adolescents in the United States and Canada: a systematic review. Childhood Obes. 2021;17(5):299–310. [DOI] [PubMed] [Google Scholar]


