ABSTRACT
Streptococcus dysgalactiae subspecies dysgalactiae (SDSD), also known as Lancefield group C streptococcus, is a pathogen found in animals. It is known to cause pyogenic infections in animals and is one of the most common pathogens that can cause mastitis in cattle. Very few reports of SDSD causing human diseases to have been reported in the literature, but we report a case of community-acquired meningitis and pyogenic ventriculitis caused by SDSD. This report is the first case of SDSD causing a central nervous system (CNS) infection in humans and aims to raise awareness about the role of SDSD in CNS infections. It also seeks to promote the recognition of this bacteria as a potential cause of invasive diseases.
Keywords: Streptococcus dysgalactiae subspecies dysgalactiae, Acute ventriculitis, Community-acquired meningitis
INTRODUCTION
Streptococcus dysgalactiae subspecies dysgalactiae (SDSD) is an animal pathogen, known to cause pyogenic infections in animals and is one of the most common pathogens that can cause bovine mastitis [1-3]. Very few reports of SDSD causing human diseases to have been reported in the literature [4-9]. We report case of pyogenic ventriculitis (PV) and meningitis caused by SDSD and aim to raise awareness of its role as a causative pathogen of endovascular and central nervous system infections.
CASE REPORT
After three weeks of evolution, a 53-year-old man with any know comorbidities presented to the emergency department (ED) with fever and floating horizontal binocular diplopia.
The patient was a construction worker responsible for transporting hazardous materials. He did not take any medication daily except painkillers sporadically. He consumed alcohol with main meals (about 1 to 2 glasses of wine) and one pack of cigarettes daily since he was 16. There was no recent travel history, and he denied insect or animal bites or contact with rodents. And the consumption of unpasteurized dairy products and risky behavior. The patient did not have any dental caries and had not undergone any recent dental procedure.
On admission, he was lethargic and febrile with a temperature of 39.4°C, tachycardic with a heart rate of 110 bpm, blood pressure of 90/70 mmHg and peripheral saturations of 97% on room air. He had no murmurs on cardiac auscultation or cutaneous lesion. Aggressive fluid therapy and antipyretic were started. Neurological examination revealed floating horizontal binocular diplopia, right central facial paresis, and left hemiparesis, with hypoesthesia and hyporeflexia. He also had signs of meningeal irritation with positive Brudzinski and Kernig signs.
CT scan with contrast was performed, which did not show changes in the brain parenchyma, suggesting acute or expansive vascular lesions (Figure 1).
Figure 1.
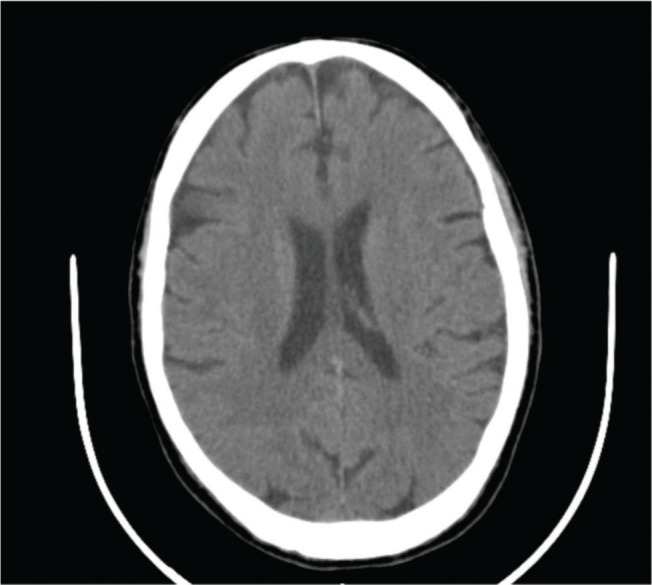
Axial section of cranioencephalic CT without evidence of alterations in the brain parenchyma, suggestive of acute or expansive vascular lesions.
Laboratory investigation identified leukocytosis (15x103/μL) with neutrophilia 94.2% (reference value 40-75%), creatinine of 0.69 mg/dL and C-reactive protein of 19.21 mg/dl (reference values <0.50 mg/dL). Given the suspicion of acute meningitis, antibiotic therapy with dexamethasone, ceftriaxone, vancomycin and ampicillin were initiated, in addition to the collection of cultures.
After two days of hospitalization, the patient maintained the neurological deficits and an MRI was performed. It described the presence of a recent right paramedian medullary ischemic lesion, predominantly anterior, focused without mass effect (Figure 2); cortical linear enhancement in the right posterior, posterior convexity, pre and postcentral with some restriction of diffusion suggestive of a focused meningeal infectious process (Figure 3) and sedimentation of purulent material in the posterior slope of both lateral ventricles (Figure 4).
Figure 2.
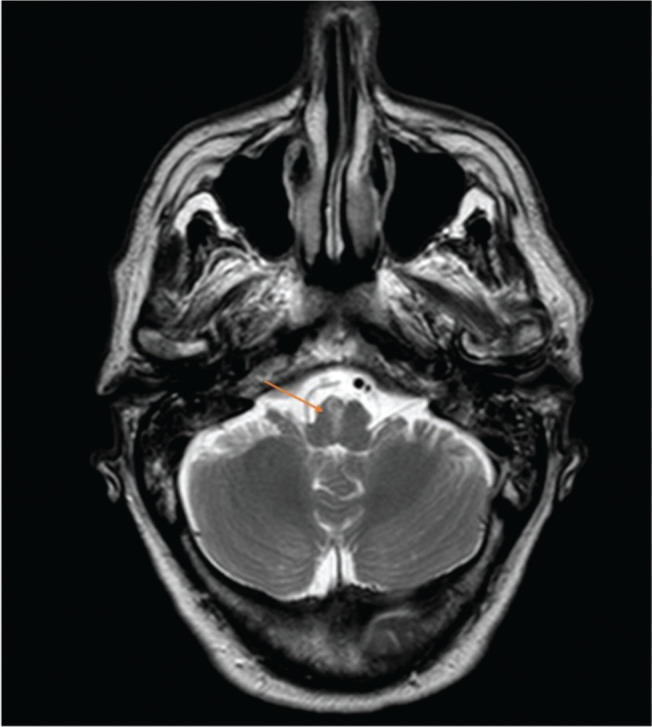
Axial sections of cranial MRI and orange arrow indicates right paramedian medullary ischemic lesion, predominantly anterior.
Figure 3.
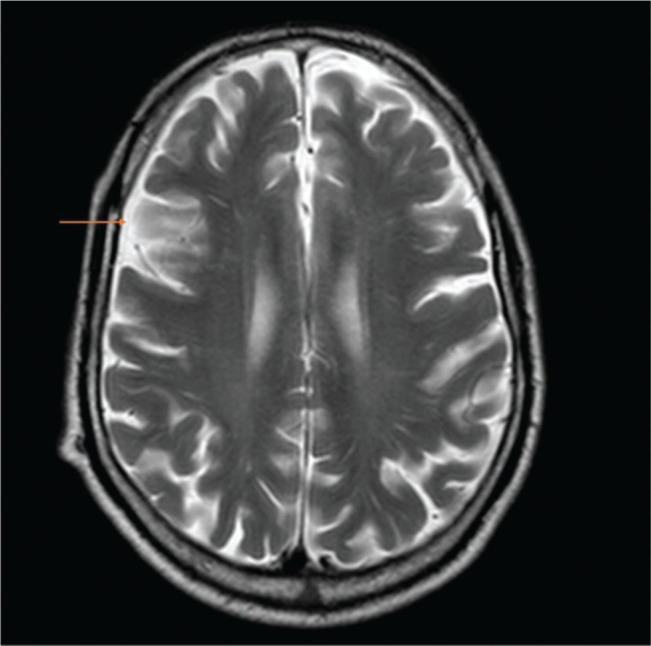
Axial sections of cranial MRI and orange arrow indicates cortical linear enhancement in the right posterior suggestive of a focused meningeal infectious process.
Figure 4.
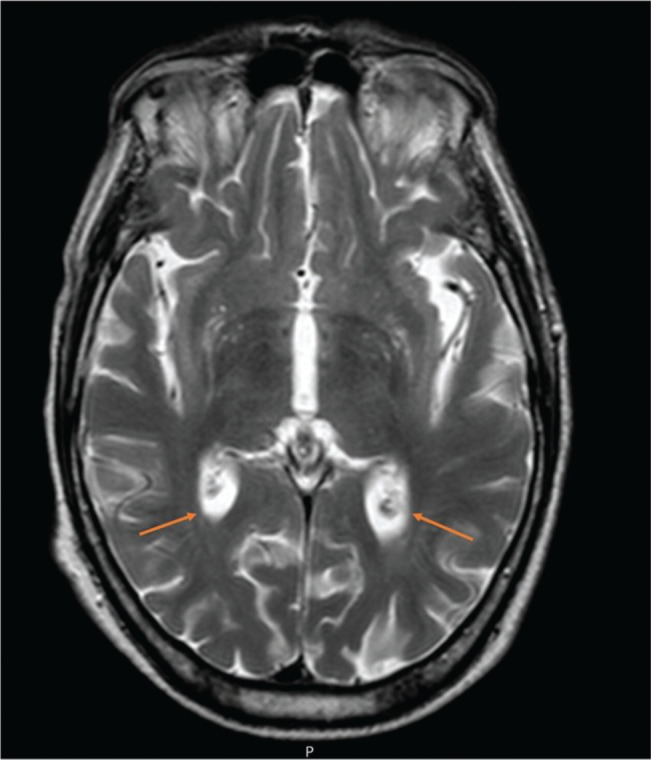
Axial sections of cranial MRI and orange arrows indicate sedimentation of purulent material in the posterior slope of both lateral ventricles.
Acute ischemic changes appear to result from small vessel involvement secondary to the infectious process.
Blood and cerebrospinal fluid cultures isolated Streptococcus dysgalactiae subspecies dysgalactiae with sensitivity to penicillin, vancomycin, and fluoroquinolones. Transesophageal echocardiography ruled out endocarditis as a source of septic emboli.
Given acute meningitis caused by the SNSD with an acute right paramedian ischemic lesion and ventriculitis due to the involvement of small vessels, we decided to maintain ceftriaxone and vancomycin. We maintained the antibiotic regimen for six weeks, during which the patient underwent physical rehabilitation. On the 30th day, the MRI was repeated and showed that the purulent foci were significantly smaller.
After a long and arduous rehabilitation, the patient regained his autonomy six months after discharge without any neurological sequelae.
DISCUSSION
Streptococcus dysgalactiae (SD) has two main subspecies: SDSD and Streptococcus dysgalactiae subspecies equisimilis (SDSE). The former expresses Lancefield group C or L antigen while the latter has Lancefield group C or G antigen [1]. Most human infections belonging to SD have been associated with SDSE, including pharyngitis, skin and soft tissue infections, bacteremia, and endocarditis.
SDSD is a significant cause of bovine mastitis and has been classified as an animal-only pathogen, though rare human infections have been reported. Recent human infections indicate that this subspecies’ range may expand due to acquiring virulence genes that increase its pathogenicity [2].
Studies have shown that the SDSD strains isolated from a cow’s milk with mastitis can carry genes from a human pathogen. In vitro and in vivo studies have shown that these strains can interact with human respiratory cell lines. They also exhibited the ability to cause invasive zebrafish infections and mortality [3].
Although the infection capabilities of the SDSD strains were not as strong as those of the human Streptococcus pyogenes, the results of these studies suggest that these strains can infect zebrafish, a vertebrate infectious model, emerging as pathogens with zoonotic capability [2,3].
As a result, it can spread from animals to humans, particularly in immunocompromised people and those working in the fishing or animal industries [4]. Septic shock [4,5], infective endocarditis [6,7], cellulitis [8,9], and neonatal meningitis [10] have all been reported as a result of SDSD infection.
Our patient was not immunosuppressed and denied consuming unpasteurized milk, raw meat or even contact with animals. However, given the professional context of handling and transporting construction materials, there was probably a gateway to contact with the microorganism.
Acute meningitis (AM) is an inflammatory disease of the spinal cord and the brain. It is a medical emergency, and immediate steps must be taken to establish the cause and effective therapy. The major causes of community-acquired bacterial meningitis in adults over 50 years are Streptococcus pneumoniae, Neisseria meningitidis and Listeria monocytogenes [9]. Acute complications include cerebral oedema, ventriculitis, arterial stroke, and empyema [10]. Furthermore, we reported two of these complications in our case. Ventriculitis is a rare intracranial infection characterized by suppurative fluid in the ventricles. The clinic includes fever, headache, altered mental status, and neck rigidity. Diagnosis is confirmed by cerebrospinal fluid (CSF) analysis and magnetic resonance imaging (MRI) demonstrating pus in the ventricles, ependymal thickening, or intraventricular locations. Ventriculitis most commonly occurs as a complication of external ventricular drains or in patients with ventricular shunts. Only a few cases of primary ventriculitis have been reported associated with community-acquired bacterial meningitis [11-13]. Pathological studies of patients who died from bacterial meningitis have shown that the ventricular fluid usually turned cloudy by the end of the first week of the infection. Nowadays, the incidence of this complication is considered very low. However, in the case reported, there was a three-week period with symptoms. The patient did not seek medical services at the time, which probably contributed to this condition’s development [14].
MRI including gadolinium-enhanced sequences is the best imaging, particularly in T2 FLAIR sequences, to reveal periventricular hyperintensity, an ependymal enhancement and irregular intraventricular debris layering in the occipital horns suggestive of ventriculitis [15]. MRI is preferred to a CT scan, which lacks sensitivity. Thus, MRI should be considered in those patients who fail to improve despite appropriate antibiotic therapy, like in our case [16].
The selection of antibiotics and duration of treatment may be controversial. Although there is not enough evidence supporting the long-term use of antibiotics in treating complicated ventriculitis, a course of 6 to 12 weeks is a good option. Therefore, considering the results of the SDSD antibiotic sensitivity, we chose a 6-week course of antibiotic therapy with ceftriaxone and vancomycin. According to current guidelines, our patient was given dexamethasone which has beneficial potential of preventing neurological sequelae and hearing loss in adults with purulent bacterial meningitis [17].
CONCLUSION
Streptococcus dysgalactiae subspecies dysgalactiae can cause cross-infection from animals to humans and it should be recognized as a pathological agent capable of causing severe infections in multiple organs and systems. Its presentation depends on the site of initial infection, but from all published cases, it intervenes in medical emergencies in humans of all ages. Immunosuppression and contact with infected animals should always be considered. Still, they may not be identified, leading to even more incredible difficulty in managing the infection by SDSD until its microbiological identification.
This article describes the first human case of pyogenic ventriculitis and meningitis caused by SDSD. Our goal was to promote the recognition of this bacteria as a potential cause of invasive diseases.
Acknowledgement
None to declare.
Financial Disclosure or Funding
This is a self-financed manuscript without funding source.
Conflict of Interest
The authors do not have any conflict of interest.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
REFERENCES
- 1.Vandamme P, Pot B, Falsen E, et al. Taxonomic study of lancefield streptococcal groups C, G, and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov [published correction appears in Int J Syst Bacteriol. 1997; 47(2):605] Int J Syst Bacteriol. 1996;46(3):774–781. doi: 10.1099/00207713-46-3-774. [DOI] [PubMed] [Google Scholar]
- 2.Alves-Barroco C, Roma-Rodrigues C, Raposo LR, et al. Streptococcus dysgalactiae subsp. dysgalactiae isolated from milk of the bovine udder as emerging pathogens: In vitro and in vivo infection of human cells and zebrafish as biological models. Microbiologyopen. 2019;8(1):e00623. doi: 10.1002/mbo3.623. Epub 2018 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves-Barroco C, Caço J, Roma-Rodrigues C, et al. New Insights on Streptococcus dysgalactiae subsp. dysgalactiae Isolates. Front Microbiol. 2021;12:686413. doi: 10.3389/fmicb.2021.686413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan B, Pillai V, Ayyan SM, et al. Streptococcus dysgalactiae Subspecies dysgalactiae Infection Presenting With Septic Shock. Cureus. 2021;13(1):e12465. doi: 10.7759/cureus.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordal S, Glambek M, Oppegaard O, et al. New tricks from an old cow: infective endocarditis caused by Streptococcus dysgalactiae subsp. dysgalactiae. J Clin Microbiol. 2015;53:731–734. doi: 10.1128/JCM.02437-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh TH, Sng LH, Yuen SM, et al. Streptococcal cellulitis following preparation of fresh raw seafood. Zoonoses Public Health. 2009;56(4):206–208. doi: 10.1111/j.1863-2378.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 7.Park MJ, Eun IS, Jung CY, et al. Streptococcus dysgalactiae subspecies dysgalactiae infection after total knee arthroplasty: a case report. Knee Surg Relat Res. 2012;24(2):120–123. doi: 10.5792/ksrr.2012.24.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im G, Park Y, Kim M, et al. A case of neonatal meningitis caused by Streptococcus dysgalactiae subspecies dysgalactiae and Herpes simplex virus. Pediatr Infect Vaccine. 2019;26:194–198. doi: 10.14776/piv.2019.26.e17. [DOI] [Google Scholar]
- 9.Erdem H, Ozturk-Engin D, Cag Y, et al. Central nervous system infections in the absence of cerebrospinal fluid pleocytosis. Int J Infect Dis. 2017;65:107–109. doi: 10.1016/j.ijid.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 10.de Vries LS, Volpe J. Bacterial and fungal intracranial infections. In: Volpe J, Inder T, Darras B, et al., editors. Volpe’s Neurology of the Newborn. 6th Ed. Elsevier, Philadelphia; 2017. p. 1050. [Google Scholar]
- 11.Adhikari P, Antala D, Pyakuryal B, et al. Community-Acquired Meningitis Complicated by Pyogenic Ventriculitis: A Case Report. Cureus. 2022;14(4):e23907. doi: 10.7759/cureus.23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyairi I, Causey KT, DeVincenzo JP, et al. Group B streptococcal ventriculitis: a report of three cases and literature review. Pediatr Neurol. 2006;34(5):395–399. doi: 10.1016/j.pediatrneurol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Salmon JH. Ventriculitis complicating meningitis. Am J Dis Child. 1972;124(1):35–40. doi: 10.1001/archpedi.1972.02110130037005. [DOI] [PubMed] [Google Scholar]
- 14.Adams RD, Kubik CS, Bonner FJ. The clinical and pathological aspects of influenzal meningitis. Arch Pediatr (N Y) 1948;65(8):408–441. [PubMed] [Google Scholar]
- 15.Ziai WC, Lewin JJ., 3rd Update in the diagnosis and management of central nervous system infections. Neurol Clin. 2008;26(2):427–468. doi: 10.1016/j.ncl.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Gronthoud F, Hassan I, Newton P. Primary pyogenic ventriculitis caused by Neisseria meningitidis: case report and review of the literature. JMM Case Rep. 2017;4(1):e005078. doi: 10.1099/jmmcr.0.005078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwer MC, McIntyre P, Prasad K, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2015;2015(9):CD004405. doi: 10.1002/14651858.CD004405.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]


