Abstract
Background
Some patients with systemic autoimmune rheumatic disease and immunosuppression might still be at risk of severe COVID-19. The effect of outpatient SARS-CoV-2 treatments on COVID-19 outcomes among patients with systemic autoimmune rheumatic disease is unclear. We aimed to evaluate temporal trends, severe outcomes, and COVID-19 rebound among patients with systemic autoimmune rheumatic disease and COVID-19 who received outpatient SARS-CoV-2 treatment compared with those who did not receive outpatient treatment.
Methods
We did a retrospective cohort study at Mass General Brigham Integrated Health Care System, Boston, MA, USA. We included patients aged 18 years or older with a pre-existing systemic autoimmune rheumatic disease, who had COVID-19 onset between Jan 23 and May 30, 2022. We identified COVID-19 by positive PCR or antigen test (index date defined as the date of first positive test) and systemic autoimmune rheumatic diseases using diagnosis codes and immunomodulator prescription. Outpatient SARS-CoV-2 treatments were confirmed by medical record review. The primary outcome was severe COVID-19, defined as hospitalisation or death within 30 days after the index date. COVID-19 rebound was defined as documentation of a negative SARS-CoV-2 test after treatment followed by a newly positive test. The association of outpatient SARS-CoV-2 treatment versus no outpatient treatment with severe COVID-19 outcomes was assessed using multivariable logistic regression.
Findings
Between Jan 23 and May 30, 2022, 704 patients were identified and included in our analysis (mean age 58·4 years [SD 15·9]; 536 [76%] were female and 168 [24%] were male, 590 [84%] were White and 39 [6%] were Black, and 347 [49%] had rheumatoid arthritis). Outpatient SARS-CoV-2 treatments increased in frequency over calendar time (p<0·0001). A total of 426 (61%) of 704 patients received outpatient treatment (307 [44%] with nirmatrelvir–ritonavir, 105 [15%] with monoclonal antibodies, five [1%] with molnupiravir, three [<1%] with remdesivir, and six [1%] with combination treatment). There were nine (2·1%) hospitalisations or deaths among 426 patients who received outpatient treatment compared with 49 (17·6%) among 278 who did not receive outpatient treatment (odds ratio [adjusted for age, sex, race, comorbidities, and kidney function] 0·12, 95% CI 0·05–0·25). 25 (7·9%) of 318 patients who received oral outpatient treatment had documented COVID-19 rebound.
Interpretation
Outpatient treatment was associated with lower odds of severe COVID-19 outcomes compared with no outpatient treatment. These findings highlight the importance of outpatient SARS-CoV-2 treatment for patients with systemic autoimmune rheumatic disease and COVID-19 and the need for further research on COVID-19 rebound.
Funding
None.
Introduction
Outpatient SARS-CoV-2 treatment options include monoclonal antibodies, remdesivir, and oral medications such as nirmatrelvir–ritonavir and molnupiravir.1, 2, 3, 4 For patients with systemic autoimmune rheumatic disease, effective COVID-19 treatments are important because altered immunity and immunosuppression might affect vaccine response5, 6 and COVID-19 severity.7 COVID-19 rebound is a complication after treatment, characterised by recurrence of symptoms and test positivity after regimen completion.8, 9, 10, 11 However, there is a paucity of data on outcomes with and without outpatient SARS-CoV-2 treatment among patients with systemic autoimmune rheumatic disease and COVID-19, and on the prevalence of rebound COVID-19. The trials showing efficacy of outpatient SARS-CoV-2 treatments were done before the emergence of contemporary viral variants and the introduction of COVID-19 vaccines.1, 2, 3, 4 Also, although these trials focused on people at high risk of progressing to severe COVID-19, people who were immunosuppressed, such as those with systemic autoimmune rheumatic disease, were infrequently enrolled (eg, 0% in the nirmatrelvir–ritonavir trial and 4% in the outpatient remdesivir trial).1, 2, 3, 4 Thus, the potential benefit of outpatient SARS-CoV-2 treatment among patients with systemic autoimmune rheumatic diseases and immunosuppression who develop COVID-19 after vaccination and during an omicron-dominant period is unclear.
Research in context.
Evidence before this study
We searched PubMed on Dec 2, 2022, for articles published from database inception to Dec 2, 2022, with no language restrictions, using the terms: ((“rheumatic” or “immunosuppressed”) AND “COVID-19” AND (“outpatient” OR “nirmatrelvir” OR “molnupiravir”) NOT ((review) OR (editorial) OR “case report”)). The search yielded 86 articles. Many of the articles pertained to vaccine response rather than SARS-CoV-2 infection. A single-centre study in 2022 investigated breakthrough COVID-19 among patients with immune-mediated inflammatory diseases who had received B-cell-depleting therapy. The authors found that outpatient treatment with monoclonal antibodies was associated with lower risk of severe COVID-19 compared with no outpatient treatment, but this analysis was performed before the SARS-CoV-2 omicron variant was dominant and before oral outpatient treatment options were available. A large study in Israel using administrative data found that only 4737 (2·6%) of 180 351 patients at high risk of severe COVID-19 in early 2022 were treated with nirmatrelvir–ritonavir, and this treatment was associated with lower risk of severe COVID-19, but the study did not focus on patients with systemic autoimmune rheumatic disease and immunosuppression. We found no studies that investigated COVID-19 rebound (recurrence of symptoms and test positivity after regimen completion) after oral outpatient SARS-CoV-2 treatment.
Added value of this study
To our knowledge, this is one of the first studies to evaluate outpatient SARS-CoV-2 treatments among patients with systemic autoimmune rheumatic disease that includes oral outpatient treatment options and quantifies the prevalence of COVID-19 rebound. Even among a cohort of patients with rheumatic disease and immunosuppression who were mostly vaccinated against SARS-CoV-2, outpatient treatment was associated with substantially reduced odds of severe COVID-19 outcomes compared with no outpatient treatment. At least 8% of patients with systemic autoimmune rheumatic disease who received oral outpatient treatment had COVID-19 rebound.
Implications of all the available evidence
These results should encourage clinicians to prescribe—and patients with systemic autoimmune rheumatic disease and COVID-19 to seek—prompt outpatient SARS-CoV-2 treatment. This research provides an early estimate of the prevalence of COVID-19 rebound after oral outpatient SARS-CoV-2 treatment, which could be used to quantify this risk to clinicians and patients with systemic autoimmune rheumatic disease and encourage future research.
We aimed to evaluate outpatient SARS-CoV-2 treatments and outcomes, including COVID-19 rebound, in patients with systemic autoimmune rheumatic diseases and COVID-19. First, we examined temporal trends and the proportion of patients receiving SARS-CoV-2 outpatient treatment (monoclonal antibodies, oral medications, or remdesivir). Second, we compared severe COVID-19 outcomes among patients who received outpatient SARS-CoV-2 treatment with those who did not receive outpatient treatment. Third, we examined the prevalence of COVID-19 rebound among patients with systemic autoimmune rheumatic disease who received oral outpatient SARS-CoV-2 treatment.
Methods
Study design and participants
We did a retrospective cohort study at Mass General Brigham Integrated Health Care System, Boston, MA, USA. Mass General Brigham is a multicentre health-care system that includes 14 hospitals and primary care or specialty outpatient centres. We included patients aged 18 years or older with a pre-existing systemic autoimmune rheumatic disease who had COVID-19 onset between Jan 23 and May 30, 2022. This study was approved by the Mass General Brigham Institutional Review Board. Patient informed consent was not required for this retrospective study.
We identified patients with COVID-19 using an electronic query of the Mass General Brigham Research Patient Data Registry, which gathers data from the electronic health record. We identified COVID-19 as a positive SARS-CoV-2 PCR or antigen test, or a positive COVID-19 flag in the electronic health record, or both. At Mass General Brigham, a positive COVID-19 flag indicates a confirmed diagnosis of COVID-19 and captures patients with a positive test outside of the Mass General Brigham health-care system, including home rapid antigen assay reported to health-care providers or clinics and when ordering or administering outpatient treatments. The index date was defined as the date of the first positive test or flag within the study dates.
From this cohort of patients with COVID-19, we identified patients who had a pre-existing systemic autoimmune rheumatic disease at onset of COVID-19. We previously described identification of systemic autoimmune rheumatic diseases at Mass General Brigham using administrative data for COVID-19 studies in detail, validated with a 90% positive predictive value.12 Briefly, systemic autoimmune rheumatic diseases were defined as at least two International Classification of Diseases (ICD)-10 codes (appendix p 2) for a systemic autoimmune rheumatic disease within 2 years before the index date and separated by at least 30 days, and prescription of a disease-modifying antirheumatic drug within 12 months before the index date or a glucocorticoid prescription within 6 months before the index date (appendix pp 3–4). Patients with osteoarthritis, fibromyalgia, or crystalline arthritis only (or a combination thereof) were not included.
Procedures
The primary exposure variable of the study was any outpatient SARS-CoV-2 treatment versus no outpatient treatment. The decision to prescribe or not prescribe outpatient therapy was likely to be multifactorial, reflecting both patient and health-care provider factors, which could include insurance coverage, geographical location relative to treatment availability, delays in time to notifying the health-care provider of infection, contraindications to treatment, and socioeconomic status factors contributing to access to care, among others. A single factor to determine why a patient did not receive outpatient treatment could not be identified in this retrospective observational study. Secondary exposures were specific treatments. Because we aimed to investigate oral outpatient treatment options, we only analysed a time period when these were available locally. Nirmatrelvir–ritonavir received emergency authorisation from the US Food and Drug Administration on Dec 22, 2021, for patients at high risk of severe COVID-19; molnupiravir received authorisation on Dec 23, 2021. The start of this study was on Jan 23, 2022, when these treatments were first prescribed.
We performed manual medical record review of all identified patients to accurately classify outpatient SARS-CoV-2 treatments or verify no outpatient treatment. If a patient received more than one therapy in the outpatient setting, they were classified as having received combination treatment.
Outcomes
The primary outcome was severe COVID-19, defined as hospitalisation or death within 30 days after the index date. This outcome was identified using an electronic query, as in previous studies.13, 14, 15, 16 In a sensitivity analysis, we required that the outcome occurred at least 1 day after the index date, because some patients might have been unable to receive outpatient treatment in time to prevent hospitalisation. Also, some patients might have been incidentally found to have COVID-19 while hospitalised for other reasons and been ineligible for outpatient therapy. In another sensitivity analysis, we limited the outcomes to hospitalisations or deaths that were due to COVID-19, either primarily or as a contributor. We further quantified the number of patients who had COVID-19 pneumonia, and those who died after COVID-19.
Among patients who received oral outpatient SARS-CoV-2 treatment, we performed medical record review to identify patients who had COVID-19 rebound documented in the electronic health record. As in a previous study,10 COVID-19 rebound was defined as: completion of oral outpatient regimen, documentation of negative then newly positive SARS-CoV-2 tests within 7 days of regimen completion, and recurrence in COVID-19 symptoms (after improvement in most or all symptoms) within 7 days of regimen completion. Patients who had little or no improvement in symptoms throughout follow-up were not considered to have COVID-19 rebound. Patients with prolonged viral shedding17 without a negative test in the interim were not considered to have rebound COVID-19.
We classified each patient's systemic autoimmune rheumatic disease diagnosis using ICD-10 codes, as described previously.12 Immunomodulatory medications were identified using prescription data preceding the index date. For conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), we separately considered the most recent prescriptions. For CD20 inhibitors, we classified exposure if last received within 1 year before the index date, due to lengthy effects that can impact vaccine response and COVID-19 severity.16, 18, 19
We used an electronic query to identify most covariates. Demographic factors were age, sex, race (White, Black, Asian, other, or unknown), and ethnicity (Hispanic or Latinx, or non-Hispanic). We measured area-level social deprivation using median household income by zip code area. Lifestyle factors were BMI (continuous) and smoking status (never, past, current, or missing). We calculated the Charlson Comorbidity Index20 from ICD-10 codes in the 1 year before the index date. We also identified individual components of the Charlson Comorbidity Index as well as interstitial lung disease, which has been previously associated with worse COVID-19 outcomes.21 The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation without the race multiplier. We categorised eGFR as less than 30 mL/min/1·73 m2 (severely decreased kidney function), 30 mL/min/1·73 m2 to less than 60 mL/min/1·73 m2 (moderately decreased kidney function), or 60 mL/min/1·73 m2 or greater (normal kidney function), because this affects nirmatrelvir–ritonavir dosing and eligibility.
COVID-19 vaccine types and dates were extracted from patients' electronic health record. Vaccination status was classified as unvaccinated, partially vaccinated, two doses of mRNA vaccine or one dose of adenovirus vaccine, or additional vaccine doses.15 For patients initially classified as unvaccinated or partially vaccinated, we reviewed their medical records to accurately classify vaccination status. For patients who had received vaccines, we also calculated time from last dose to the index date, because immunity wanes over time.22 Electronic query identified previous COVID-19 before the current episode. Medical record review determined tixagevimab–cilgavimab use.23
Statistical analysis
We plotted the total number of COVID-19 cases per calendar week in the study period and subdivided this by outpatient treatment received (nirmatrelvir–ritonavir, molnupiravir, monoclonal antibodies, remdesivir, combination treatment, or no outpatient treatment). We used the χ2 test to calculate the p value for trend of the proportion of patients who received outpatient treatment across the ordinal variable of calendar week. We reported baseline characteristics using descriptive statistics according to outpatient treatment exposure status.
The primary analysis compared any outpatient SARS-CoV-2 treatment versus no outpatient treatment for the outcome of severe COVID-19. We used an unadjusted logistic regression model to calculate the odds ratios (ORs) and 95% CIs for severe COVID-19. In the multivariable model, we included age, Charlson Comorbidity Index, eGFR, and race (White or non-White) as possible confounders. We used directed acyclic graphs (appendix p 5) to choose these as potential confounders that might influence both the decision to prescribe outpatient treatment as well as the risk of severe COVID-19. As there were relatively few outcomes in the group who received outpatient treatment, we also performed an analysis using Poisson regression to obtain risk ratios (RRs) to negate sparse-data bias.24, 25
We performed similar analyses for other comparisons of outpatient treatments for risk of severe COVID-19: nirmatrelvir–ritonavir versus no outpatient treatment, monoclonal antibodies versus no outpatient treatment, nirmatrelvir–ritonavir versus no outpatient treatment and all other treatments, monoclonal antibodies versus no outpatient treatment and all other treatments, and nirmatrelvir–ritonavir versus monoclonal antibodies. We were unable to investigate molnupiravir, outpatient remdesivir, or combination treatment use because few patients received these therapies. We also performed several sensitivity analyses. First, we only considered severe COVID-19 outcomes that occurred at least 1 day after the index date. Second, we only considered severe COVID-19 outcomes that occurred at least 1 day after the index date up to day 14. Third, we performed medical record review of all severe outcomes masked to outpatient treatment status and excluded those felt to be unrelated to COVID-19 after adjudication by two study physicians (eg, admission for labour and delivery, scheduled procedures, wound infection, and nosocomial infections after admission).
We also performed subgroup analyses for COVID-19 severity for the following comparisons that each had adequate sample size: any outpatient SARS-CoV-2 treatment versus no outpatient treatment, nirmatrelvir–ritonavir versus no outpatient treatment, and monoclonal antibodies versus no outpatient treatment. We analysed participants in subgroups by age (<65 years vs ≥65 years), sex (male vs female), dichotomised Charlson Comorbidity Index (0–1 vs ≥2), eGFR (<30 mL/min/1·73 m2 vs ≥30 mL/min/1·73 m2), vaccination status (unvaccinated, two mRNA vaccines or 1 adenovirus vaccine, or additional doses [none were partially vaccinated]), and time since last vaccine dose (≤6 months vs >6 months). We reported the numbers of outcomes and total number of participants in each subgroup and multivariable ORs and 95% CIs in forest plots. We also performed subgroup analyses among patients with rheumatoid arthritis as well as among patients who were receiving the most prevalent immunomodulatory medications (methotrexate and hydroxychloroquine) in our cohort.
For COVID-19 rebound, we reported the number of confirmed cases over the denominator of patients who received either nirmatrelvir–ritonavir, or molnupiravir (either as monotherapy or in combination with other medications, such as monoclonal antibodies). We reported descriptive statistics of baseline characteristics of these patients.
We considered a two-sided p value of less than 0·05 as significant in all analyses. All analyses were performed using SAS (version 9.4).
Role of the funding source
There was no funding source for this study.
Results
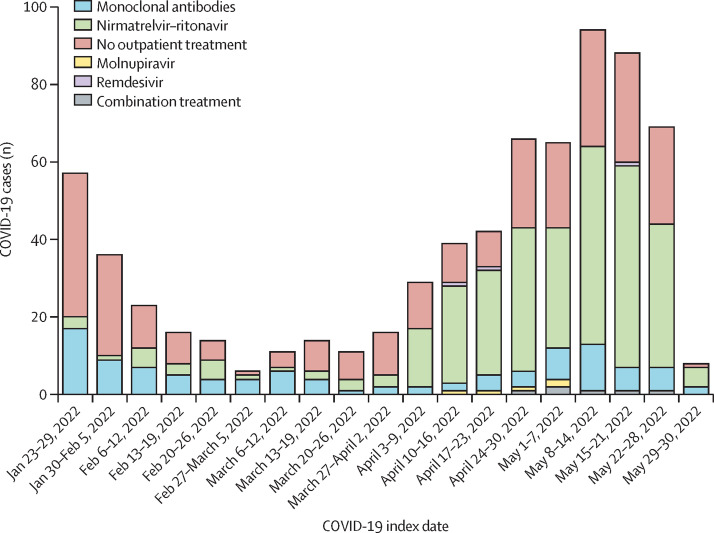
Between Jan 23 and May 30, 2022, 704 patients were identified and included in our analysis (mean age 58·4 years [SD 15·9]; 536 [76%] were female and 168 [24%] were male, 590 [84%] were White and 39 [6%] were Black, and 677 [96%] had received a SARS-CoV-2 vaccine; table 1 ). The proportion of patients who received outpatient SARS-CoV-2 treatment increased over calendar time (figure 1 ); 20 (35%) of 57 patients received outpatient treatment during the first week of the study compared with 44 (65%) of 68 during the last full week of the study (p for trend <0·0001 across all calendar weeks).
Table 1.
Baseline characteristics by outpatient SARS-CoV-2 treatment status
|
Outpatient treatment |
No outpatient treatment (n=278) | ||||
|---|---|---|---|---|---|
| Any treatment (n=426)* | Nirmatrelvir–ritonavir (n=307) | Monoclonal antibodies (n=105) | |||
| Demographics | |||||
| Age, years | 58·3 (15·6) | 57·1 (14·9) | 61·2 (17·5) | 58·7 (16·4) | |
| Sex | |||||
| Female | 331 (78%) | 235 (77%) | 83 (79%) | 205 (74%) | |
| Male | 95 (22%) | 72 (23%) | 22 (21%) | 73 (26%) | |
| Race | |||||
| Asian | 11 (3%) | 9 (3%) | 1 (1%) | 9 (3%) | |
| Black or African American | 19 (4%) | 15 (5%) | 4 (4%) | 20 (7%) | |
| Other | 20 (5%) | 16 (5%) | 4 (4%) | 16 (6%) | |
| White | 367 (86%) | 259 (84%) | 95 (90%) | 223 (80%) | |
| Unknown | 9 (2%) | 8 (3%) | 1 (1%) | 10 (4%) | |
| Hispanic or Latinx ethnicity | 5 (1%) | 4 (1%) | 1 (1%) | 2 (1%) | |
| Zip code area median household income, US$ | 93 125 (77 411–113 509) | 93 889 (78 077–115 533) | 89 742 (70 252–108 876) | 84 847 (65 386–103 978) | |
| BMI, kg/m2 | 28·1 (7·4) | 27·7 (7·3) | 28·9 (7·4) | 27·0 (8·3) | |
| Smoking status | |||||
| Never | 260 (61%) | 202 (66%) | 50 (48%) | 152 (55%) | |
| Past | 146 (34%) | 92 (30%) | 48 (46%) | 98 (35%) | |
| Current | 15 (4%) | 9 (3%) | 6 (6%) | 16 (6%) | |
| Unknown | 5 (1%) | 4 (1%) | 1 (1%) | 12 (4%) | |
| Comorbidities | |||||
| Charlson Comorbidity Index | 1 (1–3) | 1 (1–2) | 2 (1–5) | 2 (1–4) | |
| Charlson Comorbidity Index category | |||||
| 0 | 56 (13%) | 47 (15%) | 8 (8%) | 44 (16%) | |
| 1 | 180 (42%) | 151 (49%) | 25 (24%) | 92 (33%) | |
| 2 | 75 (18%) | 51 (17%) | 22 (21%) | 41 (15%) | |
| ≥3 | 115 (27%) | 58 (19%) | 50 (48%) | 101 (36%) | |
| Individual comorbidities | |||||
| Hypertension | 167 (39%) | 103 (34%) | 57 (54%) | 134 (48%) | |
| Asthma | 68 (16%) | 43 (14%) | 22 (21%) | 44 (16%) | |
| Cancer excluding non-melanoma skin cancer | 57 (13%) | 34 (11%) | 21 (20%) | 49 (18%) | |
| Coronary artery disease | 53 (12%) | 23 (7%) | 26 (25%) | 45 (16%) | |
| Chronic kidney disease | 51 (12%) | 28 (9%) | 20 (19%) | 44 (16%) | |
| Diabetes | 43 (10%) | 19 (6%) | 19 (18%) | 47 (17%) | |
| Heart failure | 26 (6%) | 4 (1%) | 19 (18%) | 38 (14%) | |
| Chronic obstructive pulmonary disease | 20 (5%) | 4 (1%) | 15 (14%) | 24 (9%) | |
| Interstitial lung disease | 20 (5%) | 9 (3%) | 11 (10%) | 22 (8%) | |
| Median eGFR, mL/min/1·73 m2 | 86 (71–101) | 88 (77–101) | 80 (64–97) | 87·5 (70–100) | |
| Categorical eGFR, mL/min/1·73 m2 | |||||
| ≥60 | 378 (89%) | 284 (93%) | 86 (82%) | 236 (85%) | |
| ≥30 to <60 | 42 (10%) | 23 (7%) | 14 (13%) | 33 (12%) | |
| <30 | 6 (1%) | 0 | 5 (5%) | 9 (3%) | |
| Previous immunity to SARS-CoV-2 | |||||
| Vaccination status | |||||
| Unvaccinated | 9 (2%) | 8 (3%) | 1 (1%) | 18 (6%) | |
| Partially vaccinated | 0 | 0 | 0 | 0 | |
| Two mRNA vaccine doses or one adenovirus vaccine dose | 56 (13%) | 41 (13%) | 13 (12%) | 56 (20%) | |
| Additional doses | 361 (85%) | 258 (84%) | 91 (87%) | 204 (73%) | |
| Tixagevimab–cilgavimab use | 8 (2%) | 5 (2%) | 3 (3%) | 4 (1%) | |
| Previous SARS-CoV-2 infection | 8 (2%) | 8 (3%) | 0 | 2 (1%) | |
Data are mean (SD), n (%), or median (IQR). Percentages might not sum to 100% due to rounding. Zip code area median household income was missing for six patients. eGFR=estimated glomerular filtration rate.
Characteristics of other outpatient treatments (five patients received molnupiravir, three received remdesivir, and six received combination treatment [four received nirmatrelvir–ritonavir and monoclonal antibodies and two received molnupiravir and monoclonal antibodies]) are shown in the appendix (pp 6–7).
Figure 1.
COVID-19 cases over calendar time among patients with systemic autoimmune rheumatic disease by outpatient SARS-CoV-2 treatment received (n=704)
Note that the week of May 29, 2022, only includes 2 days.
A total of 426 (61%) of 704 patients received any outpatient SARS-CoV-2 treatment (307 [44%] with nirmatrelvir–ritonavir, 105 [15%] with monoclonal antibodies, five [1%] with molnupiravir, three [<1%] with remdesivir, and six [1%] with combination treatment [four with nirmatrelvir–ritonavir and monoclonal antibodies; two with molnupiravir and monoclonal antibodies]; table 1, appendix pp 6–7). A total of 278 (39%) patients received no outpatient SARS-CoV-2 treatment (table 1). Patients who received outpatient treatment were more likely to be female (331 [78%] of 426 patients vs 205 [74%] of 278) and White (367 [86%] vs 223 [80%]), less likely to have severe kidney impairment (six [1%] vs nine [3%]), and less likely to be unvaccinated (nine [2%] vs 18 [6%]) than those who received no outpatient treatment.
347 (49%) of 704 patients had rheumatoid arthritis, 113 (16%) had psoriatic arthritis, and 87 (12%) had systemic lupus erythematosus (table 2 ). 484 (69%) of 704 patients used conventional synthetic DMARDs, most frequently methotrexate (232 [33%]) and hydroxychloroquine (214 [30%]). 258 (37%) patients used biologic DMARDs, most frequently tumour necrosis factor inhibitors (144 [20%]). Characteristics of patients that used other outpatient treatments are shown in the appendix (pp 6–7).
Table 2.
Rheumatic disease characteristics at COVID-19 onset by outpatient SARS-CoV-2 treatment status
|
Outpatient treatment |
No outpatient treatment (n=278) | ||||
|---|---|---|---|---|---|
| Any treatment (n=426)* | Nirmatrelvir–ritonavir (n=307) | Monoclonal antibodies (n=105) | |||
| Rheumatic disease diagnosis | |||||
| Rheumatoid arthritis | 212 (50%) | 156 (51%) | 52 (50%) | 135 (49%) | |
| Psoriatic arthritis | 72 (17%) | 62 (20%) | 9 (9%) | 41 (15%) | |
| Systemic lupus erythematosus | 54 (13%) | 37 (12%) | 13 (12%) | 33 (12%) | |
| Giant cell arteritis or polymyalgia rheumatica | 28 (7%) | 13 (4%) | 14 (13%) | 17 (6%) | |
| Sjögren's syndrome | 12 (3%) | 7 (2%) | 4 (4%) | 12 (4%) | |
| ANCA-associated vasculitis and other miscellaneous vasculitis | 15 (4%) | 9 (3%) | 5 (5%) | 5 (2%) | |
| Systemic sclerosis | 6 (1%) | 4 (1%) | 2 (2%) | 11 (4%) | |
| Axial spondyloarthritis | 8 (2%) | 5 (2%) | 2 (2%) | 3 (1%) | |
| Mixed connective tissue disease | 5 (1%) | 2 (1%) | 2 (2%) | 6 (2%) | |
| Antiphospholipid syndrome | 3 (1%) | 3 (1%) | 0 | 3 (1%) | |
| Behçet's disease | 3 (1%) | 3 (1%) | 0 | 3 (2%) | |
| Takayasu arteritis | 2 (<1%) | 1 (<1%) | 1 (1%) | 1 (<1%) | |
| Idiopathic inflammatory myositis | 0 | 0 | 0 | 1 (<1%) | |
| Juvenile idiopathic arthritis | 1 (<1%) | 1 (<1%) | 0 | 0 | |
| Multiple primary rheumatic diseases | 5 (1%) | 4 (1%) | 1 (1%) | 7 (3%) | |
| Immunomodulatory medications | |||||
| Oral glucocorticoid | 18 (4%) | 6 (2%) | 12 (11%) | 33 (12%) | |
| Any conventional synthetic DMARDs† | 284 (67%) | 205 (67%) | 71 (68%) | 200 (72%) | |
| Methotrexate | 138 (32%) | 106 (35%) | 32 (30%) | 94 (34%) | |
| Hydroxychloroquine | 117 (27%) | 84 (27%) | 26 (25%) | 97 (35%) | |
| Mycophenolate mofetil or mycophenolic acid | 27 (6%) | 14 (5%) | 12 (11%) | 20 (7%) | |
| Leflunomide | 31 (7%) | 23 (7%) | 7 (7%) | 11 (4%) | |
| Sulfasalazine | 18 (4%) | 15 (5%) | 3 (3%) | 17 (6%) | |
| Tacrolimus | 22 (5%) | 9 (3%) | 12 (11%) | 12 (4%) | |
| Azathioprine | 14 (3%) | 8 (3%) | 5 (5%) | 9 (3%) | |
| Cyclosporine | 9 (2%) | 6 (2%) | 2 (2%) | 8 (3%) | |
| Cyclophosphamide | 4 (1%) | 4 (1%) | 0 | 1 (<1%) | |
| Apremilast | 3 (1%) | 2 (1%) | 1 (1%) | 0 | |
| Any biologic DMARDs† | 180 (42%) | 133 (43%) | 39 (37%) | 78 (28%) | |
| TNF inhibitor | 104 (24%) | 86 (28%) | 15 (14%) | 40 (14%) | |
| CD20 inhibitor | 26 (6%) | 13 (4%) | 10 (10%) | 12 (4%) | |
| IL-6 receptor inhibitor | 17 (4%) | 10 (3%) | 5 (5%) | 5 (2%) | |
| IL-17 inhibitor | 14 (3%) | 13 (4%) | 1 (1%) | 6 (2%) | |
| CTLA-4 immunoglobulin | 11 (3%) | 8 (3%) | 3 (3%) | 9 (3%) | |
| IL-23 inhibitor | 2 (<1%) | 1 (<1%) | 1 (1%) | 3 (1%) | |
| B-cell activating factor inhibitor | 3 (1%) | 1 (<1%) | 2 (2%) | 1 (<1%) | |
| IL-12 and IL-23 inhibitor | 2 (<1%) | 1 (<1%) | 1 (1%) | 1 (<1%) | |
| IL-5 inhibitor | 1 (<1%) | 0 | 1 (1%) | 2 (1%) | |
| IL-1 inhibitor | 2 (<1%) | 1 (<1%) | 1 (1%) | 0 | |
| Targeted synthetic DMARD | |||||
| JAK inhibitor | 11 (3%) | 8 (3%) | 3 (3%) | 5 (2%) | |
Data are n (%). ANCA=antineutrophil cytoplasmic antibodies. CTLA-4=cytotoxic T-lymphocyte-associated protein 4. DMARD=disease-modifying antirheumatic drug. IL=interleukin. JAK=Janus kinase. TNF=tumour necrosis factor.
Characteristics of other outpatient treatments (five patients received molnupiravir, three received remdesivir, and six received combination treatment [four received nirmatrelvir–ritonavir and monoclonal antibodies and two received molnupiravir and monoclonal antibodies]) are shown in the appendix (pp 6–7).
Some patients were on more than one medication.
Among 704 patients, a total of 58 (8·2%) hospitalisations and three (0·4%) deaths occurred within 30 days of the COVID-19 index date (table 3 ). The composite primary outcome of hospitalisation or death (severe COVID-19) within 30 days of the COVID-19 index date occurred in 58 (8·2%) patients. Of the patients who received outpatient SARS-CoV-2 treatment, nine (2·1%; including one death) of 426 had severe COVID-19 compared with 49 (17·6%; two deaths) of 278 patients who did not receive outpatient treatment. Of 307 patients treated with nirmatrelvir–ritonavir, four (1·3%; one death) had severe COVID-19 outcomes. Of 105 patients treated with monoclonal antibodies, five (4·8%) had hospitalisations. No severe COVID-19 outcomes occurred among the 14 patients who received molnupiravir, remdesivir, or combination treatment. Among 27 unvaccinated patients, two (7·4%) had severe COVID-19 outcomes; neither of these patients had received outpatient treatment.
Table 3.
COVID-19 outcomes within 30 days after the index date by outpatient SARS-CoV-2 treatment status
| All patients (n=704) |
Outpatient treatment |
No outpatient treatment (n=278) | |||
|---|---|---|---|---|---|
| Any outpatient treatment (n=426)* | Nirmatrelvir–ritonavir (n=307) | Monoclonal antibodies (n=105) | |||
| Hospitalisation | 58 (8·2%) | 9 (2·1%) | 4 (1·3%) | 5 (4·8%) | 49 (17·6%) |
| Death | 3 (0·4%) | 1 (0·2%) | 1 (0·3%) | 0 | 2 (0·7%) |
| Severe COVID-19 (hospitalisation or death) | 58 (8·2%) | 9 (2·1%) | 4 (1·3%) | 5 (4·8%) | 49 (17·6%) |
| COVID-19 rebound† | NA | NA | 24/311‡ (7·7%) | NA | NA |
Data are n (%) or n/N (%). NA=not applicable.
There were no severe COVID-19 outcomes among patients who received other outpatient treatments (five patients received molnupiravir, three received remdesivir, and six received combination treatment [four received nirmatrelvir–ritonavir and monoclonal antibodies and two received molnupiravir and monoclonal antibodies]).
Within 7 days after completion of the oral outpatient regimen.
The denominator for COVID-19 rebound also includes four patients who received nimatrelvir–ritonavir in combination with monoclonal antibodies; there was also one COVID-19 rebound case among seven (14·3%) patients who received molnupiravir.
Of the three deaths, one was in an older man (who did not receive outpatient treatment) with giant cell arteritis treated with prednisone and methotrexate who was admitted to hospital with COVID-19 pneumonia and progressed to multi-organ failure; one was in an older man (who did not receive outpatient treatment) with metastatic prostate cancer and psoriatic arthritis treated with etanercept who was admitted to hospital with COVID-19 pneumonia, generalised weakness, and fatigue without substantial recovery; and one was in an older woman (who received outpatient treatment with nirmatrelvir–ritonavir) with rheumatoid arthritis treated with methotrexate who was admitted to hospital with fever and severe abdominal pain, found to have peritoneal carcinomatosis, and died after intestinal perforation.
Of the patients with severe COVID-19 outcomes in the primary analysis, 46 (79%) of 58 were adjudicated to have COVID-19 as either a primary or partial contributor to the hospitalisation, and 29 (63%) of 46 had COVID-19 pneumonia as the primary reason for hospitalisation.
After adjustment for age, Charlson Comorbidity Index, eGFR, and race, patients who received any outpatient SARS-CoV-2 treatment had an adjusted OR for severe COVID-19 of 0·12 (95% CI 0·05–0·25) compared with those who did not receive outpatient treatment (table 4 ). The results of the Poisson regression were similar to the primary analyses (adjusted RR 0·16, 95% CI 0·08–0·32; appendix p 8).
Table 4.
ORs for severe COVID-19 (hospitalisation or death) within 30 days after the index date
| Unadjusted | Multivariable model 1* | Multivariable model 2† | |
|---|---|---|---|
| Primary analysis | |||
| Any outpatient treatment vs no outpatient treatment | 0·10 (0·05–0·21) | 0·12 (0·05–0·25) | 0·13 (0·06–0·28) |
| Secondary analyses | |||
| Nirmatrelvir–ritonavir vs no outpatient treatment | 0·06 (0·02–0·17) | 0·08 (0·03–0·24) | 0·09 (0·03–0·27) |
| Monoclonal antibodies vs no outpatient treatment | 0·23 (0·09–0·60) | 0·20 (0·07–0·54) | 0·21 (0·08–0·57) |
| Nirmatrelvir–ritonavir vs no outpatient treatment and all other treatments | 0·08 (0·03–0·23) | 0·12 (0·04–0·34) | 0·13 (0·04–0·37) |
| Monoclonal antibodies vs no outpatient treatment and all other treatments | 0·52 (0·20–1·32) | 0·35 (0·13–0·97) | 0·35 (0·13–0·99) |
| Nirmatrelvir–ritonavir vs monoclonal antibodies | 0·26 (0·07–1·00) | 0·46 (0·11–1·97) | 0·43 (0·10–1·86) |
Data are OR (95% CI). OR=odds ratio.
Model 1 was adjusted for continuous age, continuous Charlson Comorbidity Index, continuous estimated glomerular filtration rate, and race.
Model 2 was adjusted for the covariates listed in Model 1 and zip code area median household income.
In the secondary analyses, nirmatrelvir–ritonavir (adjusted OR 0·08, 95% CI 0·03–0·24) and monoclonal antibodies (0·20, 0·07–0·54) were each associated with lower odds of severe COVID-19 compared with no outpatient treatment. Patients who received nirmatrelvir–ritonavir had an adjusted OR for severe COVID-19 of 0·46 (95% CI 0·11–1·97) compared with those who received monoclonal antibodies (table 4).
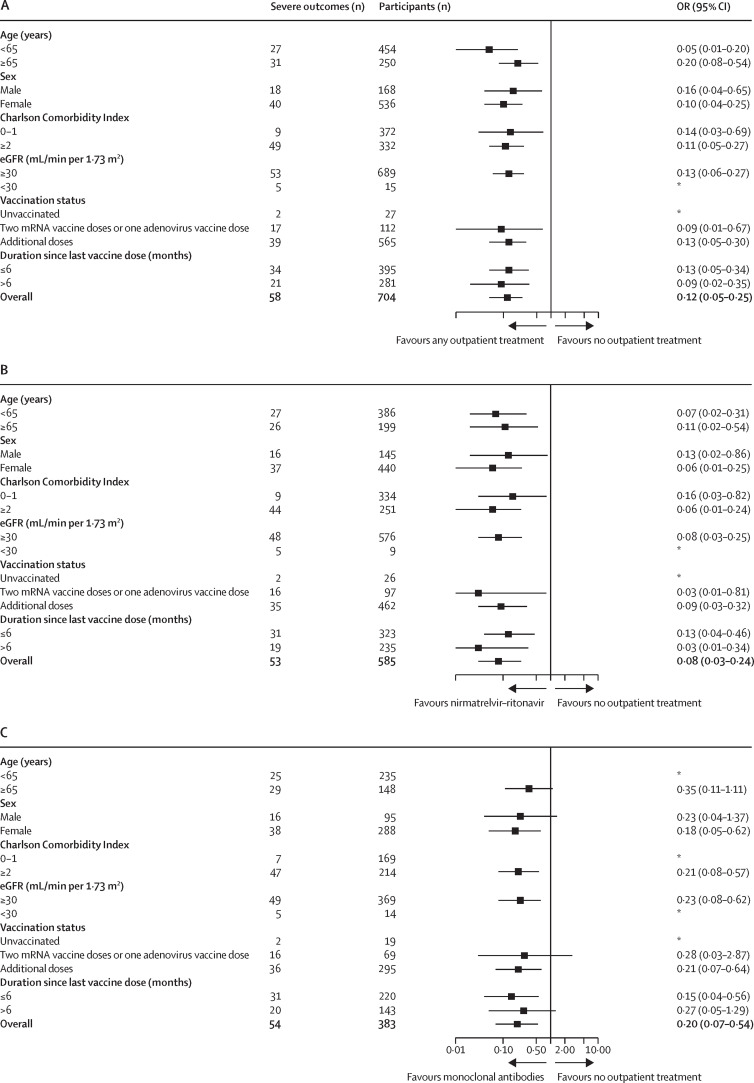
Findings from our primary analysis remained robust across all subgroup analyses (figure 2 ). Results were similar among the subgroup of patients with rheumatoid arthritis and patients receiving specific immunomodulatory medications (ie, methotrexate and hydroxychloroquine; appendix p 9).
Figure 2.
Forest plots of subgroup analyses for odds of severe COVID-19 outcomes (hospitalisation or death) within 30 days after the index date
(A) Any outpatient SARS-CoV-2 treatment versus no outpatient treatment. (B) Nirmatrelvir–ritonavir versus no outpatient treatment. (C) Monoclonal antibodies versus no outpatient treatment. All ORs are adjusted for continuous age, continuous CCI, continuous eGFR, and race. eGFR=estimated glomerular filtration rate. OR=odds ratio. *Model did not converge due to few outcomes.
In the first sensitivity analysis that considered only severe COVID-19 outcomes that occurred at least 1 day after the index date, severe COVID-19 outcomes occurred in 38 (5·4%) of 704 patients (nine [2·1%] of 426 who received any outpatient SARS-CoV-2 treatment vs 29 [10·4%] of 278 who did not receive outpatient treatment; adjusted OR 0·22, 95% CI 0·10–0·48; appendix p 10). In the second sensitivity analysis that considered only severe COVID-19 outcomes that occurred at least 1 day after the index date and up to day 14, severe COVID-19 outcomes occurred in 34 (4·8%) of 704 patients (nine [2·1%] of 426 who received any outpatient SARS-CoV-2 treatment vs 25 [9·0%] of 278 who did not receive outpatient treatment; adjusted OR 0·28, 95% CI 0·12–0·62; appendix p 11). In the third sensitivity analysis, we excluded 12 of the 58 severe COVID-19 outcomes adjudicated to be unrelated to COVID-19 (appendix p 12); patients who received any outpatient SARS-CoV-2 treatment had an adjusted OR for severe COVID-19 outcomes of 0·13 (95% CI 0·05–0·29) compared with those who received no outpatient treatment (appendix p 12).
25 (7·9%) of 318 patients who received oral outpatient SARS-CoV-2 treatment had documented COVID-19 rebound. Among 311 patients who received nirmatrelvir–ritonavir, 24 (7·7%) had COVID-19 rebound. Among seven patients who received molnupiravir, one (14·3%) had COVID-19 rebound. Characteristics of patients who had COVID-19 rebound are shown in the appendix (pp 13–15).
Discussion
In this contemporary cohort of patients with systemic autoimmune rheumatic disease and COVID-19, outpatient SARS-CoV-2 treatment with antivirals or monoclonal antibodies was associated with substantially lower odds of severe COVID-19 outcomes compared with no outpatient treatment. Outpatient COVID-19 treatment increased in frequency over the study period; the most common outpatient treatments were nirmatrelvir–ritonavir and monoclonal antibodies. Among patients who received oral outpatient treatment, the prevalence of COVID-19 rebound was 7·9%, which was likely to be a conservative estimate because of the requirement of electronic health record documentation. These findings highlight the importance of early outpatient treatment in this vulnerable population, even among patients who have been vaccinated, and emphasise the need to further investigate COVID-19 rebound in patients with systemic autoimmune rheumatic disease.
Despite advances in prevention and associated improvements in outcomes, patients with systemic autoimmune rheumatic disease remain at increased risk of SARS-CoV-2 infection, severe COVID-19 outcomes, and prolonged symptom duration, especially those who are receiving B-cell-depleting therapy or who have comorbid conditions, such as interstitial lung disease.16, 19, 21 Although vaccination reduces risk of severe outcomes, patients with rheumatoid arthritis have increased risk of SARS-CoV-2 infection and severe outcomes compared with the general population.26 Thus, even with improving COVID-19 outcomes, some patients with systemic autoimmune rheumatic disease remain vulnerable to poor COVID-19 outcomes.27, 28 A large study in Israel using administrative data investigated nirmatrelvir–ritonavir use among patients at high risk of severe COVID-19 early in 2022 and found that few patients received nirmatrelvir–ritonavir, but those who did had lower risk of severe COVID-19 than those who did not receive this treatment. However, the study was not focused on patients with immune-mediated inflammatory diseases who were at risk of severe COVID-19 due to immunosuppression.29 In this context, our novel findings regarding the substantially lower odds of severe COVID-19 associated with outpatient SARS-CoV-2 treatment are an important reminder to clinicians to consider early outpatient treatment for patients with systemic autoimmune rheumatic disease and COVID-19. We observed a strong association between outpatient treatment and lower risk of severe COVID-19 (adjusted OR 0·12). This result is similar to the pivotal clinical trial for nirmatrelvir–ritonavir that observed a RR of 0·11 for severe COVID-19 compared with placebo.3 To further evaluate the potential effect of sparse-data bias, we did a sensitivity analysis using Poisson regression and confirmed the robustness of our results. Importantly, our findings persisted across all subgroups examined, including in younger (<65 years) patients and those who remained unvaccinated during this study period characterised by predominance of the highly contagious omicron SARS-CoV-2 variants. Most patients in the outpatient treatment group in our study received nirmatrelvir–ritonavir and monoclonal antibodies; few received molnupiravir (1%), remdesivir (<1%), or combination treatment (1%). Whether similar patterns of benefit will be observed in other centres or with receipt of these less frequently used treatments requires further investigation.
COVID-19 rebound is characterised by re-emergence of test positivity and symptoms after completion of oral outpatient SARS-CoV-2 treatments.30 Rebound might have societal effects related to extension of isolation along with health effects and reduced quality of life from prolonged viral infection. The exact mechanisms of COVID-19 rebound are unknown, but it might reflect incomplete viral eradication at the completion of oral treatment. People with systemic autoimmune rheumatic disease have altered underlying immunity and are immunosuppressed, with some known to have prolonged viral shedding,17 so it is possible that patients with immunosuppression could have higher risk of COVID-19 rebound. We found that 7·9% of patients with systemic autoimmune rheumatic disease who received oral outpatient SARS-CoV-2 treatments had documented COVID-19 rebound. Because our study was retrospective, and we required documentation of recurrent positive test results and symptoms to confirm rebound cases, 7·9% is likely to be an underestimate of the true incidence of COVID-19 rebound. Notably, no patients in our study who had documented COVID-19 rebound were subsequently hospitalised, which is reassuring. Overall, this finding highlights the need for further research on COVID-19 in this vulnerable population, including prospective ascertainment of COVID-19 rebound, possible relationships with severe COVID-1931 and long-COVID, and consideration of longer courses of oral treatment regimens.
Monoclonal antibodies were the first outpatient treatment shown to be effective in preventing severe COVID-19 among patients at high risk.1 Pre-exposure prophylaxis with monoclonal antibodies might also reduce severe outcomes, and among patients with systemic autoimmune rheumatic disease who received B-cell-depleting therapy, monoclonal antibodies might be effective, even after vaccination.23, 32 Our study adds to the literature by investigating all patients with systemic autoimmune rheumatic disease, not only those at highest risk of severe outcomes due to B-cell depletion. Compared with no outpatient treatment, monoclonal antibodies were associated with substantially lower odds of severe COVID-19. These patients might have had high clinical suspicion to progress to severe COVID-19. Indeed, patients with systemic autoimmune rheumatic disease who received monoclonal antibodies had more comorbidities and worse kidney function than those who did not receive outpatient treatment. Even with oral options available, many clinicians and patients might choose to receive monoclonal antibodies, due to contraindications for the use of nirmatrelvir–ritonavir or to avoid COVID-19 rebound after oral medications. In the analysis that compared nirmatrelvir–ritonavir with monoclonal antibodies, there was no significant difference between these treatments, which suggests that they might be similarly effective. Our findings were robust across many subgroups and sensitivity analyses that shortened the window for severe COVID-19 outcomes and excluded some severe outcomes that might not have been related to COVID-19 or where COVID-19 was diagnosed after hospitalisation. We had access to individual medical record data and were able to provide clinical detail on the severe COVID-19 outcomes and confirm that the majority of hospitalisations in this analysis were due to COVID-19, rather than an incidental diagnosis.
This study has some limitations. First, the sample was from a single geographical area with a high COVID-19 vaccination coverage, so might not be generalisable to other settings. However, we still detected significant differences in severe COVID-19 risk that might be even more pronounced in populations with lower vaccination coverage. Second, we might not have identified all COVID-19 cases, particularly in those who used home antigen tests and did not inform their health-care provider of the results. Thus, the true denominator of patients with systemic autoimmune rheumatic disease and COVID-19 might be larger than our study reported. However, people at high risk of severe COVID-19 might be more likely to seek testing and treatment, which might have therefore biased our findings towards the null. Third, some of the severe COVID-19 outcomes might have been due to incidentally diagnosed COVID-19 from screening during hospitalisations for other reasons or could have been nosocomial infections. Our findings remained robust in a sensitivity analysis that required a time separation between the index date and outcome. Fourth, it is possible that the results might have been affected by unmeasured confounding that might include specific contraindications to nirmatrelvir–ritonavir, social determinants of health, and access to care. However, in the additional multivariable analysis that adjusted for area-level household income, the results were similar. Fifth, although the results were robust across many subgroups and sensitivity analyses, we performed many comparisons and some subgroups were small, so these results should be considered exploratory and interpreted with caution. Although we were able to detect associations, the precision of effect size estimates was low due to relatively few outcomes, resulting in wide 95% CIs, so the magnitude of the associations should be interpreted with caution. Although we attempted to correct for possible sparse-data bias by also calculating RRs for analyses in which point estimates for ORs were far from the null, this still might not have completely overcome this issue due to few outcomes in the group who received outpatient treatment. Finally, we relied on medical documentation to identify cases of COVID-19 rebound. It is possible that some patients had COVID-19 rebound and it was not documented. Therefore, we presented only descriptive results, and this estimate should be viewed as conservative.
In conclusion, we found that outpatient SARS-CoV-2 treatment was associated with reduced odds of severe COVID-19 outcomes compared with no outpatient treatment. Over time, more patients with systemic autoimmune rheumatic disease received outpatient SARS-CoV-2 treatment, mostly with nirmatrelvir–ritonavir or monoclonal antibodies. The proportion of patients who received outpatient treatment who had confirmed COVID-19 rebound was at least 7·9%, a conservative estimate due to the stringent definition we used that required documentation. These findings should encourage outpatient SARS-CoV-2 treatment among patients with systemic autoimmune rheumatic disease.
Data sharing
Data are available upon request to the corresponding author with appropriate institutional review board approval.
Declaration of interests
NJP reports consulting fees from FVC Health. MEW reports research support from Bristol Myers Squibb, Sanofi, Eli Lilly, Amgen; consulting fees from Abbvie, Aclaris, Amgen, Bristol Myers Squibb, Corevitas, EQRx, Genosco, GlaxoSmithKline, Gilead, Horizon, Janssen, Johnson & Johnson, Eli Lilly, Pfizer, Roche, Sanofi, Scipher, Set Point, and Tremeau; and stock options in Can-Fite, Inmediz, and Scipher, unrelated to this work. ZSW reports research support from Bristol Myers Squibb and Principia Sanofi; consulting fees from Zenas Biopharma, Visterra Otsuka, Horizon, Sanofi, Shionogi, Viela Bio, and MedPace; and has participated on advisory boards for Sanofi and Horizon. JAS reports research support from Bristol Myers Squibb; and consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
NJP is funded by the Rheumatology Research Foundation (Scientist Development Award). YK is funded by the National Institutes of Health Ruth L Kirschstein Institutional National Research Service Award (grant number T32 AR007530). ZSW is funded by National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers K23 AR073334 and R03 AR078938), and the Rheumatology Research Foundation (K Supplement). JAS is funded by National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers R01 AR077607, P30 AR070253, and P30 AR072577), the R Bruce and Joan M Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. The funders had no role in the decision to publish or in the preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centres, or the National Institutes of Health.
Acknowledgments
Contributors
GQ, ZSW, and JAS designed the study, were responsible for the acquisition, analysis, and interpretation of the data, and drafted and revised the manuscript. XW was involved in the analysis and interpretation of the data. All authors were involved in the data acquisition, interpretation, and revision of the manuscript. ZSW and JAS are joint senior authors. GQ, XW, ZSW, and JAS directly accessed and verified the underlying data reported in the manuscript. All authors approved the final version of the manuscript, had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med. 2021;385:e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 7.Conway R, Grimshaw AA, Konig MF, et al. SARS-CoV-2 infection and COVID-19 outcomes in rheumatic diseases: a systematic literature review and meta-analysis. Arthritis Rheumatol. 2022;74:766–775. doi: 10.1002/art.42030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charness ME, Gupta K, Stack G, et al. Rebound of SARS-CoV-2 infection after nirmatrelvir–ritonavir treatment. N Engl J Med. 2022;387:1045–1047. doi: 10.1056/NEJMc2206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulson JM, Adams A, Gray LA, Evans A. COVID-19 “rebound” associated with nirmatrelvir/ritonavir pre-hospital therapy. J Infect. 2022;85:436–480. doi: 10.1016/j.jinf.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranganath N, O'Horo JC, Challener DW, et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease-2019 in high-risk persons. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac481. published online June 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson AS, Caubel P, Rusnak JM. Nirmatrelvir–ritonavir and viral load rebound in COVID-19. N Engl J Med. 2022;387:1047–1049. doi: 10.1056/NEJMc2205944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel NJ, Wang X, Fu X, et al. Factors associated with COVID-19 breakthrough infection among vaccinated patients with rheumatic diseases: a cohort study. Semin Arthritis Rheum. 2022;58 doi: 10.1016/j.semarthrit.2022.152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. 2020;79:1156–1162. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu TY, D'Silva KM, Patel NJ, et al. Laboratory trends, hyperinflammation, and clinical outcomes for patients with a systemic rheumatic disease admitted to hospital for COVID-19: a retrospective, comparative cohort study. Lancet Rheumatol. 2021;3:e638–e647. doi: 10.1016/S2665-9913(21)00140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawano Y, Patel NJ, Wang X, et al. Temporal trends in COVID-19 outcomes among patients with systemic autoimmune rheumatic diseases: from the first wave through the initial omicron wave. Ann Rheum Dis. 2022;81:1742–1749. doi: 10.1136/ard-2022-222954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel NJ, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among recipients of anti-CD20 monoclonal antibodies for immune-mediated diseases: a comparative cohort study. ACR Open Rheumatol. 2022;4:238–246. doi: 10.1002/acr2.11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2022;4:e177–e187. doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Figueroa-Parra G, Gilbert EL, Valenzuela-Almada MO, et al. Risk of severe COVID-19 outcomes associated with rheumatoid arthritis and phenotypic subgroups: a retrospective, comparative, multicentre cohort study. Lancet Rheumatol. 2022;4:e765–e774. doi: 10.1016/S2665-9913(22)00227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furer V, Eviatar T, Freund T, et al. Immunogenicity induced by two and three doses of the BNT162b2 mRNA vaccine in patients with autoimmune inflammatory rheumatic diseases and immunocompetent controls: a longitudinal multicentre study. Ann Rheum Dis. 2022;81:1594–1602. doi: 10.1136/ard-2022-222550. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese C, Kirchner E, Villa-Forte A, et al. Early experience with tixagevimab/cilgavimab pre-exposure prophylaxis in patients with immune-mediated inflammatory disease undergoing B cell depleting therapy and those with inborn errors of humoral immunity. RMD Open. 2022;8 doi: 10.1136/rmdopen-2022-002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Wallace ZS, Sparks JA, et al. Risk of COVID-19 among unvaccinated and vaccinated patients with rheumatoid arthritis: a general population study. Arthritis Care Res (Hoboken) 2022 doi: 10.1002/acr.25028. published online Sept 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Iorio M, Cook CE, Vanni KMM, et al. DMARD disruption, rheumatic disease flare, and prolonged COVID-19 symptom duration after acute COVID-19 among patients with rheumatic disease: a prospective study. Semin Arthritis Rheum. 2022;55 doi: 10.1016/j.semarthrit.2022.152025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiIorio M, Kennedy K, Liew JW, et al. Prolonged COVID-19 symptom duration in people with systemic autoimmune rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2022;8 doi: 10.1136/rmdopen-2022-002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac443. published online June 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boucau J, Uddin R, Marino C, et al. Characterization of virologic rebound following nirmatrelvir–ritonavir treatment for COVID-19. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac512. published online June 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malden DE, Hong V, Lewin BJ, et al. Hospitalization and emergency department encounters for COVID-19 after paxlovid treatment—California, December 2021–May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:830–833. doi: 10.15585/mmwr.mm7125e2. [DOI] [PubMed] [Google Scholar]
- 32.Calabrese CM, Kirchner E, Husni EM, et al. Breakthrough SARS-CoV-2 infections in patients with immune-mediated disease undergoing B cell-depleting therapy: a retrospective cohort analysis. Arthritis Rheumatol. 2022;74:1906–1915. doi: 10.1002/art.42287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to the corresponding author with appropriate institutional review board approval.