Abstract
Migraine is a complex brain disorder explained by the interaction of genetic and environmental factors. In monogenic migraines, including familial hemiplegic migraine and migraine with aura associated with hereditary small-vessel disorders, the identified genes code for proteins expressed in neurons, glial cells, or vessels, all of which increase susceptibility to cortical spreading depression. The study of monogenic migraines has shown that the neurovascular unit plays a prominent role in migraine. Genome-wide association studies have identified numerous susceptibility variants that each result in only a small increase in overall migraine risk. The more than 180 known variants belong to several complex networks of “pro-migraine” molecular abnormalities, which are mainly neuronal or vascular. Genetics has also highlighted the importance of shared genetic factors between migraine and its major co-morbidities, including depression and high blood pressure. Further studies are still needed to map all of the susceptibility loci for migraine and then to understand how these genomic variants lead to migraine cell phenotypes.
Keywords: Migraine, Familial hemiplegic migraine, Genetics, Genome-wide association studies, Polygenic
Migraine, a complex genetic condition
The goal of genetics is to identify key proteins in order to better understand the pathophysiology of a disease, to define new therapeutic targets and to find diagnostic biomarkers. Migraine is a highly disabling, complex brain disorder with a strong familial aggregation. Twin and family studies conducted in the 1990s demonstrated the existence of hereditary factors in migraine [1, 2]. In these studies, the estimated heritability of migraine ranged from 35% to 60%. In population-based studies, the relative risk of migraine for a first-degree relative of an index case was 1.5- to 4-fold compared with the general population [3]. The risk was higher for relatives of cases with higher pain scores and attack frequency, early age of onset, and migraine with aura (MwA).
More recent studies estimate the heritability of migraine to be about 42%. They also reinforce the idea that migraine is a complex disease resulting from interactions between genes and the environment, interactions between genes themselves, and as yet unknown factors [3]. Heritability is higher in MwA than in migraine without aura (MO) [4].
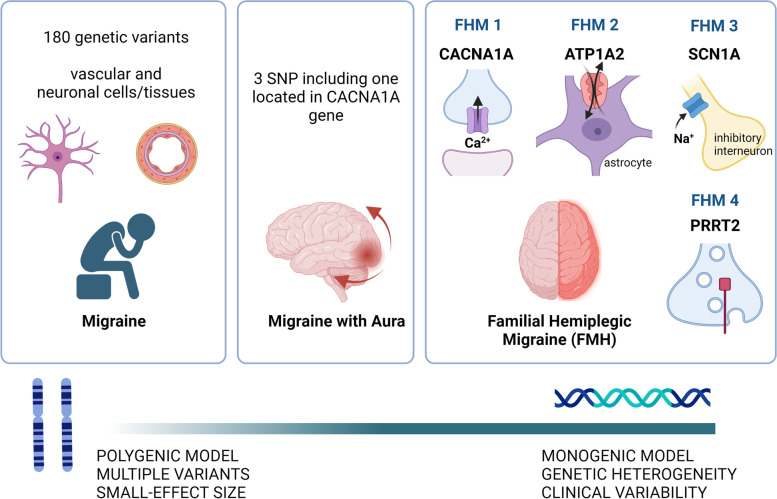
Migraine is predominantly polygenic, with multiple genetic variants, each with a minor-effect size, accumulating to lead to the disease. A portion of MwA cases could be explained by the conjunction of a small number of genetic variants with moderate effect size, or by a single variant with a major functional effect as in monogenic migraines [5] (Fig. 1). In these much rarer disorders, a pathogenic mutation in a single gene is sufficient to produce the disease with almost complete penetrance. The classical example of monogenic migraine is familial hemiplegic migraine (FHM), which is inherited in an autosomal dominant fashion [6]. Migraines can also be part of the clinical spectrum of other hereditary neurological conditions, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Other examples will be discussed further in this paper.
Fig. 1.
Overview of migraine and genetics. An overview of the complex genetic architecture of migraine, from polygenic model on the left, to monogenic model on the right. Created with BioRender.com
Familial hemiplegic migraine (FHM), a monogenic form of migraine
Genetic heterogeneity and clinical variability
Hemiplegic migraine (HM) is a rare disease with an estimated prevalence of 0.01% in the general population [6, 7]. Familial HM, diagnosed when at least one first- or second-degree relative also has HM, accounts for two-third of the cases. Sporadic HM (SHM), diagnosed in the absence of family history, accounts for one-third of cases. HM attacks begin during youth (mean age of onset 12–17 years old), and comprise motor weakness during the aura, always associated with at least one other symptom of typical aura (visual, sensory, speech and language) and often with brainstem aura symptoms (70%) [8, 9]. The frequency of attacks varies from more than one per week to a few over the course of a lifetime, with an average of 3 to 4 per year [10]. Duration of HM aura is often longer than that of typical aura (several days to weeks) [11–13]. Severe attacks with confusion, coma, fever, seizures and reversible brain edema may occur [14–17], sometimes triggered by mild head trauma [18–20]. HM can be pure or associated with a combination of early-onset epilepsy, cerebellar ataxia, learning disabilities, and/or mental retardation, which may begin before or after HM onset [21–25].
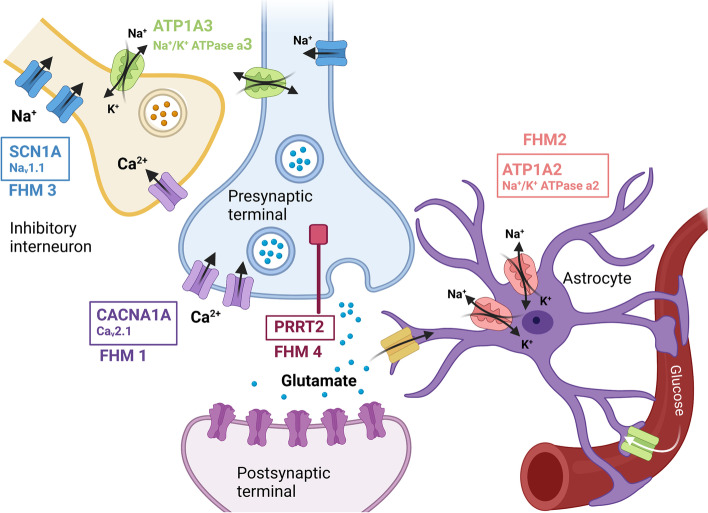
FHM is primarily a monogenic disorder, with an autosomal dominant pattern of inheritance and high penetrance; 70–90% of individuals with a pathogenic mutation clinically express the disease. FHM is genetically heterogeneous and is subdivided into FHM1, FHM2 and FHM3, based on the presence of mutations in the CACNA1A, ATP1A2 and SCN1A genes, respectively [6, 26–28] (Table 1, Fig. 2). The PRRT2 gene should be added to the main FHM genes because recent data have shown that PRRT2 is involved at least as frequently as SCN1A [29, 30]. For convenience, we will therefore refer to FHM4 for HM associated with PRRT2 mutations. Other genes have been reported in a small number of cases and families, and additional data are needed before they can be considered causal (Table 2).
Table 1.
The four major genes implicated in familial hemiplegic migraine
| Genes | Approximate number of cases and families reported in the literaturea | Percentage of affected subjects in four cohorts: a. Sutherland et al, 2020 [29] b. Pelzer et al, 2018 [21] c. Riant et al 2022 [30] d. Hiekkala et al, 2018 [31] |
Nature of causative mutations | Protein encoded and role | Involvement of the gene in other conditions [32] | |
|---|---|---|---|---|---|---|
| FHM1: CACNA1A | > 600 cases | 150 families |
a. 16/203 (7%) b. 107/? c. 26/697 (3.7%) d. 1/293 (0.34%) |
Missense mutations (Gain of function) Rare large exonic deletion or deletion in 5′ non coding end promoter |
Alpha-1 subunit of neuronal Cav2.1 (P/Q type) voltage-gated calcium channels ➔ Control of neuronal excitability at the presynaptic level of glutamatergic synapsis |
Episodic ataxia type 2 (loss of function) Spinocerebellar ataxia type 6 (SCA6) Lennox-Gastaut syndrome or Dravet syndrome, autism spectrum disorder (gain and loss of function) Possibly involved in Benign paroxysmal positional vertigo Dyskinesia Rett syndrome Paroxysmal tonic upward gaze |
| FHM2: ATP1A2 | > 900 cases | 160 families |
a. 20/203 (10%) b. 75/? c. 44/697 (6.3%) d. 2/293 (0.68%) |
Missense mutations Rare small deletions or truncating mutations, frameshift |
Catalytic alpha-2 subunit of the glial and neuronal ATP-dependent trans membrane Na+/K+-pump ➔ Clearance of extracellular K+ and production of a Na+ gradient necessary for glutamate reuptake |
Alternating hemiplegia of childhood Rapid-onset dystonia parkinsonim Cerebellar ataxia-areflexia-progressive optic atrophy Severe childhood epilepsies, encephalopathy and polymicrogyria Mental retardation |
| FHM3: SCN1A | > 120 cases | 20 families |
a. 4/203 (1.7%) b. 26/? c. 15/697 (2.1%) d. 0/293 |
Missense mutations (gain of function) |
Alpha-1 subunit of neuronal Nav1.1 voltage-gated sodium channels ➔ Propagation of action potentials of cortical neurons, especially in GABAergic inhibitory interneurons |
Early onset epileptic encephalopathies (cryptogenic generalized epilepsy, cryptogenic focal epilepsy, myoclonic–astatic epilepsy, Lennox-Gastaut syndrome, infantile spasm, Rasmussen encephalitis |
| FMH4: PRRT2 | > 120 cases | 40 families |
a. 5/203 (2.2%) b. 1/47 (2.1%) c. 30/697 (3.5%) d. not screened |
Missense mutations |
Pre-synaptic proline-rich transmembrane protein ➔ Interaction with the synaptosomal-associated protein 25 (SNAP25), suggesting a role in the fusion of synaptic vesicles to the plasma membrane |
Paroxysmal kinesigenic dyskinesia Benign familial infantile epilepsy Infantile convulsions with choreoathetosis Episodic ataxia Paroxysmal torticollis Intellectual disability |
aBased on research on Pubmed using keywords “name of the gene (example: CACNA1A)” and “migraine” or “name of the gene” and “headache”. Articles in other language than English, focusing on mice studies or other conditions than migraine were excluded. Each case where family history was not detailed was considered as sporadic and did not count as one family
Fig. 2.
Genetics of familial hemiplegic migraine (FHM). Glutamatergic synapse of the central nervous system with proteins encoded by genes involved in familial hemiplegic migraine and their functional roles. Created with BioRender.com
Table 2.
Other genes potentially implicated in familial hemiplegic migraine (FHM)
| Genes potentially implicated | Number of cases and families reported in literature | Percentage of affected subjects in the cohort by Sutherland et al, 2020 [29] | Protein encoded and role | Involvement of the gene in other conditions | References | |
|---|---|---|---|---|---|---|
| PKND | 4 | 2 | 0,4% |
PKND protein ➔ Interaction with proteins of the synaptic termini to modulate the release of neurotransmitters |
Main gene for paroxysmal non-kinesigenic dyskinesia | [33, 34] |
| SLC4A4 | 15 | 11 | 3,9% |
Na + −HCO3 NBCe1 cotransporter ➔ Expressed in astrocytes, regulation of synaptic pH and neurotransmission |
Renal tubular acidosis | [35–37] |
| ATP1A3 | 3 | 3 | 0,9% |
α3 subunit of the Na+/K + -ATPase pump ➔ Maintain of electrochemical gradients across neuronal membranes and regulation of excitability at inhibitory synapses |
Alternating hemiplegia of childhood | [38, 39] |
| SLC1A3 | 4 | 4 | 0,4% |
EAAT1 transporter ➔ Capture of glutamate into astrocytes |
Episodic ataxia type 6 | [40–43] |
| SLC2A1 | 6 | 6 | 1,3% |
Glucose transporter GLUT1 (or EAAT2) ➔ Entry of glucose into the brain across the blood-brain barrier |
Paroxysmal exercise-induced dyskinesia, De Vivo disease and GLUT1 deficiency syndrome | [33, 44–47] |
| ATP1A4 | 15 | 13 | 5.2% | Sodium/potassium-transporting ATPase subunit alpha-4 |
Charcot-Marie-Tooth Disease, Axonal, Type 2Dd Alternating hemiplegia of childhood |
[29, 48] |
Clinical studies of patients with mutations in the four major FHM genes have shown that attacks of HM are similar regardless of the gene involved, and that prolonged auras with confusion are possible in all FHM types. The association of HM with epilepsy is present in 7% of overall HM patients [7, 49], but seems more frequent in FHM2 [50].
Conversely, different mutations in the same gene can influence the phenotype. In FHM1, the two mutations most commonly involved in severe attacks with coma and fever are T666M and S218L [17]. Moreover, the nature of the mutated gene also influences the spectrum of manifestations associated with HM attacks [6]. Febrile comas are frequent in FHM1 (up to 30%), possible in FHM2 (up to 15%), and have not been described in FHM3 and FHM4 [17, 51]. Cerebellar ataxia is common in FHM1 [52–56]; a phenomenon of repeated transient blindness was observed only in FHM3 [57]; mental retardation has been described in FHM1, 2 and 4 [22–24]; and finally, the association of HM with paroxysmal dyskinesia or hypersomnia is suggestive of FHM4 [30]. Finally, there is great variability in HM attacks and associated manifestations between individuals who carry different mutations in the same gene, and even between affected family members who carry the same mutation. This variability suggests that other genetic or environmental factors can modulate the clinical phenotype [26].
FHM1 and CACNA1A mutations
CACNA1A, localized on 19p13, was the first identified HM gene [58]. It encodes the main α1 pore-forming subunit of the neuronal voltage-gated calcium channels CaV2.1 or P/Q. These channels are expressed in synaptic endings in the brain and the cerebellar, and play a role in controlling neurotransmitter release. More than 25 CACNA1A mutations have been identified in FHM1. Most are missense mutations resulting in a gain of function, which increases Ca2+ influx, glutamatergic neurotransmission and neuronal excitability [59].
There are two transgenic FHM1 knock-in (KI) mouse models [60, 61]. KI mice for the R192Q mutation, which causes pure FHM1, show no clinical abnormalities. KI mice for the S218L mutation, which causes very severe FHM1, show cerebellar ataxia, transient hemiparesis and epilepsy. FHM1-KI mice exhibit increased CaV2.1 currents and neurotransmitter release, loss of balance between excitatory and inhibitory cortical neurotransmissions, enhanced cortical excitatory transmission in visual cortex [62] and increased susceptibility to cortical spreading depression (CSD). These transgenic mice have also been shown to exhibit head pain [63], increased trigeminal activity, tissue anoxia during prolonged aura, increased sensitivity to cerebral ischemia, and altered trigeminal nociception mediated by CGRP [64].
The CACNA1A gene is also mutated in other neurological disorders. Episodic ataxia type 2 (EA2), characterized by paroxysmal ataxia, dizziness and nausea, is associated with CACNA1A mutations responsible for loss of function and decreased Ca2+ influx [65]. Spinocerebellar ataxia type 6 (SCA6), characterized by progressive cerebellar ataxia, is caused by an expansion of a CAG repeat in the terminal portion of CACNA1A, which results in toxic degeneration of cerebellar Purkinje cells [16].
FHM2 and ATP1A2 mutations
The ATP1A2 gene on 1q23.2 encodes the α2 isoform of the catalytic subunit of the A1A2 ATP-dependent transmembrane pump (α2 Na+/K+-ATPase) [66]. In the CNS of adults, this pump is primarily expressed in astrocytes, where it provides extracellular K+ clearance and produces a Na+ gradient necessary for glutamate reuptake from the synaptic cleft. More than 80 ATP1A2 mutations have been identified in FHM2. Missense mutations are the most common, but small deletions, a stop-codon altering mutation, and an exonic duplication have also been reported. These mutations result in a variable loss of function of the α2 Na+/K+-ATPase pump. The mutated pumps are reported to have lower glutamate uptake, slowing down recovery from neuronal excitation and promoting excitatory cortical transmission, thereby facilitating the initiation of CSD waves. There are several models of FHM2-KI transgenic mice. Heterozygous transgenic mice show no clinical abnormalities but have increased susceptibility to CSD [67, 68]. Mice with partially knock-out (KO) of ATP1A2 also show increased susceptibility to CSD [69]. Another mouse model with complete KO of ATP1A2 in astrocytes showed episodic paralysis and spontaneous waves of CSD with decreased EEG activity [70]. These animals had abnormalities in brain metabolism with increased serine and glycine. A serine- and glycine-free diet suppressed attacks of paralysis in these mutants.
FHM3 and SCN1A mutations
SCN1A on 2q24.3 encodes the α1 subunit forming the pore of NaV1.1 channels [71]. These voltage-dependent neuronal sodium channels are involved in the genesis and propagation of action potentials in cortical neurons, particularly in GABAergic inhibitory interneurons [72]. SCN1A was already known as an epilepsy gene with over 100 missense and nonsense mutations identified in various forms of childhood epilepsies. A dozen SCN1A mutations, mainly missense variants leading to a gain of function, have been identified in FHM3. Their functional consequences are complex [73, 74]. The mouse model carrying the L1649Q variant showed an increased susceptibility to CSD. The L1649Q mutation results in a defect in Na + channel inactivation with increased Na + currents and hyperactivity of inhibitory interneurons.
FHM4 and PRRT2 mutations
PRRT2 encodes the PRRT2 protein, which plays an important role in brain development, synapse formation, and neurotransmitter release. PRRT2 is expressed in presynaptic terminals and interacts with proteins of the exocytosis complex. Mutations in PRRT2 have now been identified in several dozen cases of FHM4, two-thirds of which have pure FHM, and one-third of which have FHM associated with epilepsy, mental retardation or dyskinesia [21, 75–80]. Mutations in PRRT2 are also associated with several other neurological diseases, including benign familial infantile epilepsy (BFIE), infantile seizure syndrome with choreoathetosis (ICCA) and paroxysmal kinesigenic dyskinesia (PKD) [81, 82].
The different mutations in PRRT2 (point duplication, small deletions, missense, total deletions) all induce a loss of function leading to haploinsufficiency. A given PRRT2 mutation can be associated with several diseases. Indeed, the c.649dupC mutation is common in FHM4, but is also the main causative mutation in PKD and BFIE.
PRRT2-KO mice exhibit paroxysmal abnormal movements upon acquisition of locomotion, develop abnormal audiogenic motor behaviors in adulthood, and have a lowered seizure threshold [83]. Their excitatory hippocampal neurons display increased excitability. Human and murine homozygous KO-PRRT2 neurons in culture express overactive NaV1.2 and NaV1.6 channels, indicating that PRRT2 inhibits voltage-gated sodium channels.
Further experiments are needed to understand the factors underlying the great phenotypical variability associated with PRRT2 mutations, and the potential influence of modifier genes or of the non-mutated allele.
Other potential FHM genes
Mutations in several other genes have been identified in HM (Table 2) [29]. All these genes were already known to be involved in other inherited diseases. In a large cohort of index HM cases from New-Zealand and Australia, analysis of potential new HM genes increased the diagnosis rate from 21% to 27,8% (PKND 0,4%; SLC4A4 3,9%; ATP1A3 0,9%; SLC1A3 0,4%; SLC2A1 1,3%) [29]. Analysis of other large cohorts of index cases, as well as functional studies assessing CSD in animal models would be important to confirm that these genes actually cause FHM.
Genetic architecture of hemiplegic migraine
Among index cases suspected of having HM and referred for genetic diagnosis, a minority has a mutation in one of the four major genes, 15% in a French cohort of 697 patients [78], and 21% in a New Zealand and Australian cohort of 230 patients [29]. These two independent studies yielded similar results, with the most frequent mutations found in the ATP1A2 gene (6,3–10%) followed by CACNA1A (3,7–7%), PRRT2 (2,2-3,5%) and finally, SCN1A (1,7 -2,1%). In contrast, a Dutch study of a cohort of 301 patients found higher rates of mutations in major genes: CACNA1A in 107/301 (35.5%), ATP1A2 in 75/301 (24.9%), SCN1A in 26/301 (8.6%), and PRRT2 in 1 /47 (2.1%) [21]. In addition, only three mutations were identified in a Finnish cohort of 293 HM patients: one in CACNA1A (0.34%) and two in ATP1A 2(0.68%) [31]. The PRRT2 gene was not screened in the Finnish study [31]. These differences could be due to different recruitment methods of the cohorts.
In typical HM cases in whom there are no mutations in the four main genes, additional single-gene variants may be identified by future systematic studies, such as exome studies, and full genome sequencing. New variants with large-effect sizes are expected to be involved in only a small proportion of familial and sporadic cases. In other cases of HM, the inheritance may be polygenic, involving multiple variants with each a small-effect size, or oligogenic, with a combination of one or few variants with a moderate-effect size with or without multiple pro-migraine variants of small-effect size.
SHM, diagnosed in the absence of any affected relative, can result from a de novo mutation of one of the FHM genes in a subject whose two parents do not have a mutation [47, 84–88]. These de novo mutations can be passed to offspring, transforming SHM into FHM. SHM can also result from mutations in known FHM genes with low penetrance, mosaicism in the transmitting parent, or pathogenic variants in as yet unknown genes. Other SHM types might have a different mode of inheritance, either recessive with compound heterozygotes or polygenic [26]. Finally, environmental and psychosocial factors including exposure to stress, psychological and physical trauma, abuse, or negative life events may also play an important role in SHM. According to the US military personnel HM cohort, the incidence of HM was zero from 1997 to 2007, and then steadily increased, with a 25-fold increase in new cases between 2008 and 2017 [89].
Links between FHM and the common varieties of migraine
FHM is a model of hereditary severe migraine with aura. Some authors have suggested that the mechanisms of FHM, namely increased sensitivity to CSD, may be involved in common migraine, with and without aura. Danish studies have shown that the risk of migraine with typical aura (eg without motor deficit) was increased in individuals with FHM compared with the general population, whereas the risk of MO was similar [90, 91]. Thus, FHM is a major model for migraine aura associated with cortical excitability, with subsequent headache triggered by CSD, and FHM genes do not play a major role in the genetics of the common varieties of migraine [92, 93]. A recent study has identified a polymorphism in the FHM1 CACNA1A gene as a susceptibility locus for common varieties of migraine, among 122 other loci [94].
Other monogenic varieties of migraine
Monogenic migraine with aura and TRESK mutations
TRESK is a two-pore K+ channel responsible for maintaining membrane excitability. By a free flow of K+ ions, it contributes to the formation of leakage currents in the trigeminal ganglion and dorsal root ganglia. It is therefore though to play a role in pain processing mechanisms. Mice with a functional knock-out of TRESK show a ‘painful’ behavioural phenotype, and exhibit hyperexcitability of the dorsal root and trigeminal ganglia. In addition, in the trigeminal ganglion, TRESK expression is restricted to nociceptive neurons [5, 95].
A frameshift mutation in KCNK18, which encodes the TRESK channel, was described in a large family with visual MwA following autosomal dominant inheritance [96]. All family members with migraine carried the p.(F139Wfs*24) mutation, which has been shown to exert a dominant negative effect resulting in complete loss of TRESK function and increased neuronal excitability [97].
The causal link between TRESK mutations and migraine has been called into question by the discovery of another mutation with a dominant negative effect, C110R, in individuals without migraine [98]. However, further research revealed that the p.(F139Wfs*24) variant introduces an alternative start codon that shortens the TRESK protein and damages its function resulting in hyperexcitability of nociceptors. Such effects were not observable for C110R [97, 99]. Another missense mutation in the KCNK18 gene (W101R) was identified in a 12-year-old male with migraine with brainstem aura and intellectual disability. This variant was inherited from his mother, who had migraine with aura [100]. Further investigations revealed impaired TRESK channel function associated with this variant [101]. In another study, pharmacological inhibition of TRESK influenced the response to capsaicin, a TRPV1 receptor agonist, and resulted in increased CGRP release and meningeal blood flow [102]. These data suggested that MwA can be caused by a rare genetic variant inducing a major functional effect.
A study of 200,000 exome-sequenced UK Biobank participants conducted in 2022 found the frameshift variant p.(F139Wfs*24) in 196 (0.10%) of the 193,433 participants classified as controls and in 10 (0.14%) of the 7194 migraine cases (p = 0.33) [103]. The authors concluded that KCNK18 should no longer be regarded as being involved in migraine etiology. A major limitation of this study is that the clinical status of healthy controls was not assessed in details. This may imply that a proportion of the 193,433 participants may in fact have been affected by migraine, as mis- and underdiagnosis of migraine and migraine aura is highly common [104]. Studies of cohorts of patients with a firm diagnosis of MwA and well characterized healthy controls are needed to further elucidate the role of KCNK18 and its product, TRESK in migraine.
Familial advanced sleep-phase syndrome (FASP), migraine and CSNK1D mutations
FASPS, which causes an extreme tendency to wake-up early in the morning, can be caused by mutation in a circadian clock gene, CSNK1D, which codes for casein kinase 1 delta (CKIδ). In the two large families with a CKIδ mutation, the sleep disorder was associated with migraine [105]. Transgenic mice expressing T44A variant of CSNK1D displayed a high propensity for nitroglycerin-induced mechanical hyperalgesia, and a reduced threshold for CSD. These findings suggest that migraine may be caused by a mutation in a gene that encodes neither an ion channel nor a protein involved in glutamate signaling. In addition, the link between migraine and FASPS is consistent with the known role of hypothalamus in migraine [106, 107].
ROSAH syndrome, migraine and ALPK1 mutations
ROSAH syndrome (retinal dystrophy, optic nerve edema, splenomegaly, anhidrosis, migraine headache) is an autosomal dominant condition caused by a missense mutation in the ALPK1 gene which was identified in five families. ALPK1 encodes Alpha Kinase 1, which plays a role in inflammation, cellular trafficking, and possibly also affects CGRP activity [26, 108]. Its role in migraine is unknown.
Monogenic cerebral vasculopathies and migraine
CADASIL (Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) is the commonest type of familial cerebral small-vessel disease responsible for recurrent lacunar stroke, leading to dementia and premature death [27, 109]. CADASIL is caused by NOTCH3 mutations, which cause progressive destruction of vascular smooth muscle cells [110]. Migraine is very common in CADASIL (up to 75%), mainly migraine with typical aura, but also hemiplegic migraine or migraine without aura. Migraine is often the first manifestation of the disease, 15 to 20 years before cerebral infarctions [111, 112]. Mutant mice, either knock-in for a CADASIL mutation or NOTCH3-knock-out, have shown increased sensitivity to CSD [113].
Retinal vasculopathy with cerebral leukodystrophy (RVCL) caused by mutations in TREX1, and disorders due to COL4A1 and COL4A2 mutations are other small-vessel diseases that frequently involve migraines [27].
The study of these conditions, especially CADASIL, showed that gene expressed only in vessels could be implicated in migraine, which was later on confirmed by studies in polygenic migraines.
Susceptibility genes for migraine with aura and migraine with aura
Genome-wide association studies (GWAS)
Identification of gene variants involved in migraine has proven difficult, and there have been 30 years of studies without significant results. Because MO and MwA display strong familial aggregation, which may suggest Mendelian inheritance, the initial hope was that the techniques used successfully in FHM would identify the genes for the most common migraines. Initial studies showed that the FHM genes were not involved in the common varieties of MO and MwA. Linkage analysis studies identified dozens of loci that were presented as possibly containing genes involved in migraine, but these were never discovered [5, 114].
Researchers turned to genome-wide association study (GWAS), which examines millions of polymorphisms called SNPs (single nucleotide polymorphisms) in very large cohorts of patients and healthy controls. Each SNP is a variation in the genetic code at a single DNA base pair. More than 100 million SNPs exist in the human genome, and 4–5 million SNPs are distributed throughout an individual genome [115]. A GWAS identifies SNPs that are significantly associated with the disease of interest, by assessing differences in allele frequencies between large numbers of patients and controls. For each SNP, the level of significance is very difficult to reach (5 × 10− 8) because of the multiplicity of tests performed.
In 2010, the first GWAS identified a single migraine susceptibility locus [116] (Table 3) Over the past decade, the International Headache Genetics Consortium (IHGC; www.headachegenetics.org/) has conducted several migraine GWAS, and with increasing sample sizes, the number of associated genetic variants has progressively increased [117–119]. The 2016 migraine GWAS, including 59,674 migraine sufferers and 316,078 controls, identified 38 distinct genomic loci associated with migraine [120]. Tissue expression enrichment analyzes clearly demonstrated the enrichment in genes involved in arterial and smooth-muscle function [120, 121]. The other pathways identified were the neuronal pathway [122], and the pathway related to homeostasis of iron ions and other metals [120].
Table 3.
Genome wide association studies in migraine from 2010 to 2022
| Article | Population | Number of SNPs tested | Number of loci identified, P < 5 × 10−8 |
Number of new loci, P < 5 × 10− 8 |
Genes or nearest genes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Origin | Migraine (n) | MO (n) | MwA + MO (n) | MwA only (n) | Controls (n) | |||||
| Antilla et al., 2010 [116] | Europeans | 2748 | 17 | 2142 | 589 | 10,747 | 429,912 |
1 12a |
1 12a |
Intergenic between MTDH/AEG-1 and PGCP MTDH/AEG-1 PGCP, SMYD3, INSIG2, TRPM8, MYLK4, ZNF311, NAV2, COG3, SGCZ, AQP9.a |
| Ligthart et al., 2011 [123] | Europeans | 2446 | NA | NA | NA | 8534 | 2,394,913 |
0 10a |
0 10a |
- NGFR, AGBL1, MACC1, LIPG, AGA, KIF20B, BMP2, IGLL1, TSPAN2, KDM4C.a |
| Chasman et al., 2012 [117] | American women with European ancestry | 5122 | 1826 | 1177 | 18,108 | 2,608,509 |
0 7b |
0 7b |
- PRDM16, TRPM8, SEPT7, C8orf79, LRP1.b |
|
| Freilinger et al., 2012 [124] | Europeans | 2326 | 2326 | 0 | 0 | 4580 | Approximately 1.4 M |
1 12a |
1 12a |
MEF2D MEF2D, HFM1, RABGAP1L, MARCH4, TGFBR2, RGS12, PHACTR1, FHL5, ADAM28, ASTN2, INA, MMP17.a |
| Anttila et al., 2013 [118] | European descent | 23,285 | 7107 | 5118 | 95,425 | Approximately 2.3 M | 12 | 5 | AJAP1, TSPAN2, FHL5, c7orf10, MMP16, PRDM16, MEF2D, TRPM8, TGFBR2, PHACTR1, ASTN2, LRP1. | |
| Gormley et al., 2016 [120] | European descent | 59,674 | 8348 | 6332 | 316,078 | 8,045,569 | 38 | 28 | LRP1/STAT6/SDR9C7, PRDM16, FHL5/UFL1, TSPAN2/NGF, TRPM8/HJURP, PHACTR1, MEF2D, SLC24A3, FGF6, C7orf10, PLCE1, KCNK5, ASTN2, MRVI1, HPSE2, CFDP1, RNF213, NRP1, SPR149, JAG1, REST/SPINK2, ZCCHC14, HEY2/NCOA7, WSCD1/NLRP1, GJA1, TGFBR2, ITPK1, ADAMTSL4/ECM1, CCM2L/HCK, YAP1, MED14/USP9X, DOCK4/IMMP2L, CARF, ARMS2/HTRA1, IGSF9B, MPPED2, NOTCH4. | |
| Chen et al., 2017 [125] | Asians (Han Chinese) | 1005 | 1005 | 0 | 0 | 1053 | 642,832 |
0 4c |
0 4c |
- DLG2, GFRA1, UPP2, GPR39.c |
| Chang et al., 2018 [126] | African-Americans | 380 | 123 | 40 | 2129 | 522,471 | 1 | 1 | Near NMUR2 and GLRA1 | |
| Choquet et al., 2021 [127] | Multiethnic (East Asian, African American, Hispanic/Latino, European descent) | 88,526 | NA | NA | NA | 841,795 | Over 10 M | 79 | 45 | JAG1, LEPR, REST, HEY2, NGF, TGFB1, HOXB3, LRP1, PLCE1, HOXB2, HOXB6, YAP1, POLR2A, AMBRA1, TJP2, EP300, LRP4, ARHGAP1, ECM1, HOXB5, HOXB1, MRVI1, ZC3H7B, HTRA1, RPRD2, ZCCHC14, STAT6, MAPKAPK2, C7orf10, KCNK5, C20orf112, ZBTB4, POU4F1, C14orf37, IQCK, C1orf51, LINC00472, BTBD16, MPPED2, YRDC, PRPF3, ARID4A, GPRC5B, THADA, ZNF408, TMEM51, CHRNB1, CHST6, MTF1, MEF2D, INPP5B, CKAP5, RNF213, DGKZ, TSPAN2, CFDP1, KIAA0586, FHL5, ATG13, TIMM9, F2, PSMA3, GPR26, PLEKHA1, TMEM170A, PHB, SLC24A3, L3MBTL2, TARS2, DYRK3, MRPS21, FXN, CHRM4, TMEM91, CHADL, MANEAL, HARBI1, RANGAP1, IER3IP1, UFL1, C1orf122, TRPM8, PHACTR1, ACTR10, NCOA7, C11orf49, PRDM16, SLC45A1-RERE, TMEM51, TSPAN2-NGF, MSL3P1-TRPM8, MIR4791-EFHB, LINC00472-RIMS1, GJA1-HSF2, FXN, ASTN2-LOC100128505, GATA3-SFTA1P, MRGPRE-ZNF195, CALCB, FGF23-FGF6, LRP1-MIR1228, B3GNTL1-METRNL, LINC00310-KCNE2. |
| Hautakangas et al., 2022 [94] | Europeans | 102,084 | 15,055 | 14,624 | 771,257 | 10,843,197 | 123 | 86 | PRDM16, CAMTA1, TMEM51, INPP5B, MACF1, C1orf87, LEPR, RP4-598G3.1, TGFBR3, TSPAN2, ADAMTSL4, MEF2D, RABGAP1L, PLA2G4A, MAPKAPK2, KIF26B, THADA, ANKRD36C, ZEB2, AC064865.1, RNU6-546P, MYO3B, HOXD10, CARF, TRPM8, TGFBR2, ATRIP, HNRNPA3P8, CADM2, C3orf38, ITGB5, GPR149, SEC63P2, SPINK2, ANKDD1B, SSBP2, ZNF474, SNX24, POU4F3, TIGD6|HMGXB3, NKX2–5, NSD1, PHACTR1, PRL, IER3, EHMT2, KCNK5, KRT19P1, FHL5, REV3L, GJA1, PCMT1, SUGCT, MLXIPL, TSPAN12, PTK2B, RP11-573 J24.1, NFIB, RP11-373A6.1, TJP2, ZNF462, ASTN2, EHMT1, RNA5SP299, MLLT10, PLCE1, HPSE2, CNNM2, RBM20, HTRA1, GPR26, INPP5A, MRGPRE, MRVI1, INSC, MPPED2, AMBRA1, RAB3IL1, RBM14-RBM4|RBM4, YAP1, SPATA19, FGF6, PDZRN4, LRP1, ATP2B1, RP11-690 J15.1, NCOR2, LRCH1, RNF219-AS1, COL4A1, RP11-384 J4.2, LRFN5, ARID4A, DLST, IFT43, ITPK1, SERPINA1, ABHD17C, HMOX2, CFDP1, ZCCHC14, SMG6, ZBTB4, HOXB3, RP11-81 K2.1, MRC2, TBC1D16, RNF213, RBBP8, SKOR2, FECH, CACNA1A, SUGP1, B9D2|TMEM91, JAG1, SLC24A3, C20orf112, ZMYND8, MRPS6, RUNX1, AC006547.14, FAM47A, MED14 | |
MO Migraine without aura, MwA Migraine with Aura, M Millions, NA Non Applicable, SNPs Single Nucleotide Polymorphisms
aSignificant association with p-values ≤5 × 10− 5
bSignificant association with p-values ≤5 × 10− 6
cSignificant association with p-values ≤5 × 10− 4
In a third recent migraine GWAS from 2021, 79 independent loci were significantly associated with migraine [127]. Of note, this was an ethnically diverse study that included adult individuals (28,852 cases vs. 525,717 controls) from East Asian, African American, and Hispanic/Latino descent.
The most recent migraine GWAS published in 2022 by Hautakangas et al. [94] included 102,084 cases and 771,257 controls and identified 123 distinct loci associated with migraine, of which 86 were novel compared to the 2016 GWAS. Additional analyses even increased the number of independent SNPs to 167. Enrichment analyses in the 2022 migraine GWAS clearly pointed to both vascular and central nervous system tissues and cell types. The newly identified loci involve genes encoding known migraine drug targets, namely calcitonin gene-related peptide (CGRP, encoded by CALCA/CALCB),) and serotonin 1F receptor (HTR1F). The former is the target for CGRP antibodies, and the latter for ditans. In addition, an analysis of about 30,000 patients from the 2022 GWAS with a precise diagnosis of the type of migraine (eg, MO or MwA) showed that three risk variants were specific for MwA (including a SNP in CACNA1A the FHM1 gene), two were specific for MO and nine were associated with both types.
Given that some risk loci were found in the Hautakangas 2022 GWAS only, some in the Choquet 2021 GWAS only, and some in both studies, there are now about 180 migraine risk loci.
In addition to these large GWAS including mainly cases with European ancestries, other smaller GWAS conducted in Asia replicated some of the results obtained in European cases and yielded other new SNPs [128]. Another GWAS study conducted in Asian population identified eight novel susceptibility loci correlated with age of migraine onset [129].
Altogether, migraine GWAS have identified more than 180 low-effect-size genetic variants all across the genome, with enrichment in vascular and neuronal cells/tissues, confirming that migraine is a polygenic neurovascular disorder. Recent GWAS demonstrated that all migraine varieties share common molecular mechanisms, and that MO and MwA have specific genetic risk factors and distinct mechanisms. Due to the large size of the samples, clinical data in GWAS were limited and did not permit to study other migraine varieties, such as “pure” MwA (patients having only attacks of MwA and never MO) or chronic MO.
Future studies will have to determine which are the causal genes modified by the SNPs, which are mostly located in non-coding regions, and do not necessarily affect the closest gene. A first challenging step will be to select the list of most-likely causal genes based on GWAS results. For example, the intronic SNP rs9349379 near PHACTR1 is a proven risk loci for migraine, coronary artery disease, fibromuscular dysplasia, hypertension and cervical artery dissection [130]. A functional study showed that this SNP had no effect on PHACTR1 but on the gene encoding endothelin-1 (ET-1; EDN1), a strong vascular smooth muscle cells constrictor located 600 kB upstream of the risk SNP [130]. More recent data suggested that SNP rs9349379 may in fact regulate the expression of PHACTR1, and not EDN1, and that PHACTR1 could have a role in arterial compliance [131]. This debate on a single SNP shows that enormous amounts of experiments will be necessary to study the 180 migraine SNPs.
In addition, some variants not identified by the mean of GWAS could also be implicated in migraine susceptibility through gene-gene interactions. A case-control study suggested that synergetic effects between a variant in NRXN2, coding a component of the synaptic vesicle machinery, and two other genes, GABRE and CASK, were associated with migraine [132].
Polygenic risk score and genetic architecture of migraine
The almost 200 variants identified by the latest GWAS each explain only a small fraction of the genetic risk, and their sum do not explain the full heritability of migraine. The Polygenic Risk Score (PRS) assesses the individual genetic risk of migraine as the sum of all SNPs and alleles that increase the risk of migraine carried by an individual. The PRS may be used to analyze the genetic links between the different primary headaches and the different forms of migraine, and between migraine and its comorbidities. The PRS can also assess pharmacogenetic effects [5].
In a study of 1589 migraine sufferers from the Finnish population, familial cases had a significantly higher PRS than non-familial cases [133]. The genetic burden was higher in MwA and FHM compared to MO, and was associated with an earlier age of onset of migraine. These data show that migraine is primarily due to an accumulation of minor variants that produce a favorable (pro-migraine) genetic background, and not to highly deleterious single gene mutations. Noteworthy, a recent study using a PRS derived from 38,872 variants associated with migraine in 8602 subjects in Finland showed a correlation between PRS and migraine diagnoses according to ICHD3 criteria [134]. Non-headache, non-migraine headache, probable migraine, migraine headache, migraine with typical visual aura and hemiplegic migraine formed a continuum along the increasing PRS, which paves the way for the potential concept of genetic classifications [134].
The known small-effect-size pro-migraine variants do not explain the full heritability of migraine. The “missing heritability” may be explained by several hypothesis. First, there are probably hundreds of other small-effect-size variants, which increase the risk of migraine but fall below the required levels of significance in GWAS. Second, persons with multiple disease associated SNPs may have an additive effect conferring a greater overall risk. Third, technical limitations in short read sequencing and Sanger analyses may account for part of this “missing heritability”. Finally, variants with minor or moderate-effect-size probably explain a part of the missing heritability, and cannot be identified by GWAS. A study sequenced the genomic areas associated with migraine in a large cohort of patients and identified four rare variants altering regulatory areas close to four variants discovered by GWAS [135]. Another study analyzed RNA sequencing using a coexpression network of aorta, trigeminal ganglion and visual cortex, combined with a whole sequence genoming. The authors identified a ‘gene module’, a set of coexpressed genes, in the visual cortex that had increased mutations in migraine. Pathway analysis of this module revealed association with hormonal signaling, Alzheimer’s disease, serotonin receptors and heterotrimeric G protein signaling pathway. Noteworthy, mutations in two genes involved in glutamate signalling, CACNA1B and ATXN1, were found in several migraine families [136]. Using whole-exome sequencing in small populations, new SNPs have been associated with responsiveness to verapamil as a preventive therapy [137], and neurological outcome, including migraine, after head trauma [138]. Finally, “private” large-size-effect variants may be identified by chance, such as in the very rare families carrying mutations of KCNK18 or CSNK1D. In these families, the strong penetrance of the migraine phenotype could result from the cosegregation of the “private” large-size-effect mutation and a pro-migraine genetic background due to a high PRS, whereas most carriers of the same rare variant would not express a migraine phenotype thanks to a non-permissive genetic background. Some authors have even hypothesized that FHM would not be truly autosomal dominant but the result of a rare mutation on a pro-migraine background.
The genetics of migraine thus seems very complex, based on the interaction of hundreds of common small-effect-size variants with rare variants affecting regulatory areas, and with possible “private” moderate to large-effect-size variants.
Shared genetic background of migraine and its comorbid diseases and traits
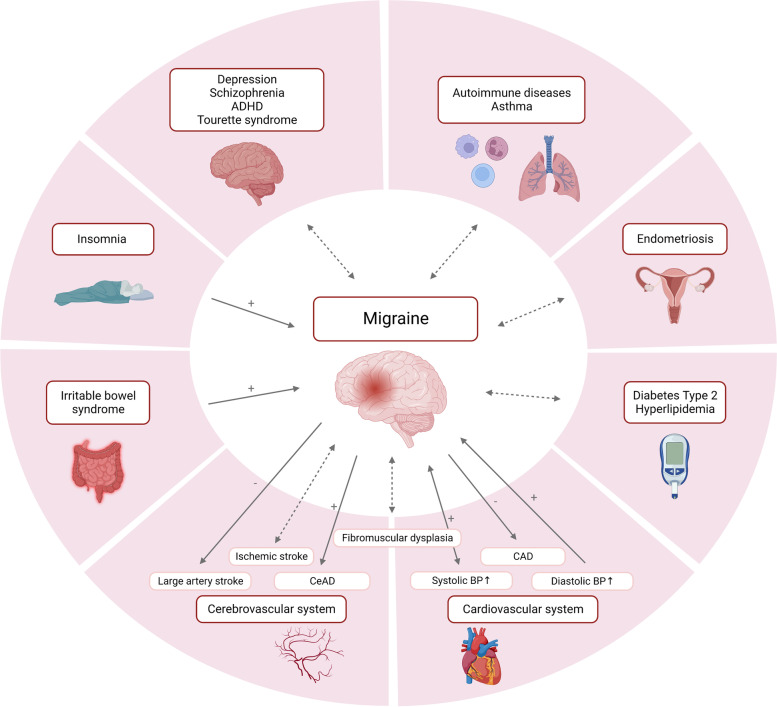
The comorbidities of migraine are diseases whose prevalence is increased in migraine sufferers compared to controls and for which certain pathological mechanisms could be shared (Fig. 3). Genetics is a tool to explore some of the common mechanisms, firstly by identification of genes associated with both conditions (GWAS), secondly, by estimation of the genetic correlation, namely the proportion of variance the two conditions share due to genetics (genetic correlation studies), and finally, by evaluation of causal relationships between two conditions by using genetic variants as proxies of an exposure (Mendelian randomization [MR] studies) [5, 139].
Fig. 3.
Shared genetic background of migraine and its comorbid diseases. Genetic relation of migraine and some of its clinically most relevant comorbidities. Dotted arrow: Genetic association or correlation as demonstrated by GWAS or genetic correlation studies. Solid arrow: Causal association of genetic variants as demonstrated by Mendelian randomization studies. +, liability to one disease increases risk for the comorbidity; −, liability to one disease decreases risk for the comorbidity; ADHD, attention deficit hyperactivity disorder; BP, blood pressure; CAD, coronary artery disease; CeAD, cervical artery dissection. Created with BioRender.com
With regard to genetic studies of associations and correlations, several large studies based on the comparison of GWAS data have shown the existence of a shared genetic susceptibility between migraine and various disorders, including psychiatric disorders [140, 141], ischemic stroke [142], coronary artery disease [143], hypertension [144, 145], sleep disorders [146], and also endometriosis [147], fibromuscular dysplasia [148], type 2 diabetes, hyperlipidemia, autoimmune diseases, asthma, other respiratory conditions [144], restless legs syndrome [149] and hemostatic profile [150]. In contrast, one study found no causal link between genetic susceptibility to migraine and Alzheimer’s disease, intelligence, and brain size [151].
Mendelian randomization studies have provided evidence of causal associations between genetic variants predisposing for migraine and those predisposing for some of the above-mentioned conditions. Findings of Mendelian randomization studies on migraine and comorbid conditions are summarized in Table 4.
Table 4.
Mendelian randomization studies for causal associations of genetic variants predisposing for migraine and its comorbid disorders and traits
| Comorbidity/Trait | Number of patients included | Direction of causality examined | Main finding(s) | Reference | |
|---|---|---|---|---|---|
| Migraine | Comorbidity/Trait | ||||
| Cardio−/vascular comorbidities | |||||
| Ischemic Stroke |
GERA cohort, UK Biobank and International Headache Genetics Consortium: 85,726 migraine cases and 803,292 controls |
MEGASTROKE consortium: 40,585 cases and 406,111 controls | One-directional | No causal associations of migraine and ischemic stroke | Shu et al. [152] |
| Stroke (ischemic and hemorrhagic) |
International Headache Genetics Consortium: Any migraine: 59,674 cases and 316,078 controls MwA: 6332 cases and 144,883 controls MO: 8348 cases and 139,622 controls |
MEGASTROKE consortium: All stroke: 40,585 cases and 406,111 controls Ischemic stroke: 34,217 cases and 406,111 controls Hemorrhagic stroke: 3223 cases and 3725 controls |
One-directional |
No causal association of migraine and stroke, ischemic stroke, and hemorrhagic stroke Migraine liability possibly associated with decreased large-artery atherosclerosis risk |
Lee et al. [153] |
| Stroke and Cervical Artery Dissection |
International Headache Genetics Consortium: Any migraine: 59,674 cases and 316,078 controls MwA: 6332 cases and 144,883 controls MO: 8348 cases and 139,622 controls |
MEGASTROKE consortium: CeAD: 1393 cases and 14,416 controls All stroke: 40,585 cases and 406,111 controls Ischemic stroke: 34,217 cases and 406,111 controls Large artery stroke: 4373 cases and 297,290 controls Cardioembolic stroke: 7193 cases and 355,68 controls Small vessel stroke: 5386 cases and 343,560 controls |
One-directional |
Migraine liability associated with increased risk for CeAD Migraine liability associated with decreased risk of large artery stroke |
Daghlas et al. [154] |
| Coronary artery disease and atrial fibrillation | International Headache Genetics Consortium: 59,674 migraine cases and 316,078 controls |
Coronary artery disease: 76,014 cases and 264,785 controls Myocardial infarction: 43,676 cases and 128,199 controls Angina: 10,618 cases and 326,065 controls Atrial fibrillation: 60,620 and 970,216 |
One-directional | Migraine liability associated with decreased risk of coronary artery disease, myocardial infarction, and angina, but not on atrial fibrillation | Daghlas et al. [143] |
| Blood pressure | International Headache Genetics Consortium: 59,674 cases and 316,078 controls | 757,601 | Bidirectional |
Genetically predicted elevated DBP and SBP, and decreased PP associated with increased migraine risk Migraine liability associated with increased SBP and PP, but not DBP |
Guo et al. [145] |
| Forty-seven traits from the UK Biobank | International Headache Genetics Consortium: 59,674 cases and 316,078 controls |
UK Biobank and a combined meta-analysis: for DBP n > 1 million individuals |
One-directional | Genetically predicted elevated DBP associated with increased migraine risk | Siewert et al. [144] |
| Cardiovascular risk factors and lifestyle | |||||
| Alcohol, coffee consumption, smoking |
7759 cases and 504,902 controls FinnGen study: 6687 cases and 144,780 controls UK Biobank: 1072 cases and 360,122 controls |
Bidirectional |
Genetically predicted alcohol and coffee consumption inversely associated with migraine risk, smoking initiation associated with increased migraine risk Migraine liability inversely associated with alcohol consumption, not associated with coffee consumption or smoking initiation |
Yuan et al. [155] | |
| Coffee consumption |
MwA: 6332 cases and 144,883 controls MO: 8348 cases and 139,622 controls |
UK Biobank: 375,833 participants |
One-directional | Genetically predicted increase of coffee consumption not associated with the risk of any migraine, MwA or MO | Chen et al. [156] |
| Smoking | HUNT study: 7522 cases and 35,342 controls | One-directional | No causal relationship between smoking intensity and migraine | Johnsen et al. [157] | |
| Brain morphometry and cognition | |||||
| Cognition and brain volume | International Headache Genetics Consortium: 59,674 migraine cases and 316,078 controls |
Alzheimer’s disease: 71,880 cases and 383,378 controls Measure of general intelligence: 269,867 subjects Measure of intracranial volume: 11,373 subjects Seven subcortical brain volumes ∼ 13,000 subjects |
One-directional | Migraine liability not associated with Alzheimer’s disease, intelligence, or any brain volume measures | Daghlas et al. [151] |
| Brain morphometry |
International Headache Genetics Consortium: 59,674 migraine patients vs 316,078 controls |
ENIGMA and CHARGE consortia, UK biobank, ABCD cohort: 66,000 participants | Bidirectional |
Genetically determined smaller total brain, hippocampal and ventral diencephalon volume possibly associated with increased migraine risk Migraine liability associated with larger volume of the amygdala |
Mitchell et al. [158] |
| Sleep disorders | |||||
| Insomnia |
finn-a-G6_MIGRAINE: 3650 migraine cases and 83,167 controls UK Biobank: 13,597 migraine cases and 449,336 controls |
UK Biobank: 462,341 participants |
Bidirectional |
Genetically determined insomnia significantly associated with increased migraine risk Migraine liability not associated with insomnia risk |
Chu et al. [159] |
| Sleep disturbances | International Headache Genetics Consortium: 59,674 cases and 316,078 controls |
UK Biobank: ≥ 237,627 participants |
Bidirectional |
Difficulty awakening and liability to insomnia symptoms associated with increased risk of migraine Migraine liability slightly associated with increased napping frequency |
Daghlas et al. [146] |
| Gastroenterological conditions | |||||
| Irritable bowel syndrome |
UK Biobank: 1072 cases and 360,122 controls |
1121 cases and 360,073 controls | One-directional | Genetically determined irritable bowel syndrome associated with increased migraine risk | Chen et al. [160] |
| Gynecological conditions | |||||
| Endometriosis | International Headache Genetics Consortium: 59,674 migraine cases and 316,078 controls | 17,054 cases (all stages of endometriosis) and 191,858 controls | One-directional | No causal association | Adewuyi et al. [147] |
| Laboratory parameters | |||||
| Clinical chemistry tests (HDL-C, LDL-C, TG, iron, diseases of liver) | 29,209 cases and 172,931 controls | Chemistry test values and data on medically relevant disorders from 23,986 to 452,264 participants | One-directional | No causal associations | Tanha et al. [161] |
| Seventy-nine metabolic traits | International Headache Genetics Consortium: 59,674 migraine cases and 316,078 controls | From 6263 to 24,925 | Bidirectional | Genetically predicted shorter length of fatty acids and higher level of a lysophosphatidylethanolamine, LPE(20:4) associated with increased migraine risk | Tanha et al. [162] |
| Nineteen lipoprotein subfractions | International Headache Genetics Consortium: 54,552 cases and 297,970 controls | 47,713 participants | Bidirectional | Lipoprotein subfractions not causally related with migraine | Guo et al. [163] |
| Serum calcium | International Headache Consortium: 23,285 cases and 95,425 controls | 39,400 participants | One-directional | Genetically predicted increase in serum calcium levels associated with increased migraine liability | Yin et al. [164] |
| Twelve blood-based measures of hemostasis | International Headache Genetics Consortium: 59,674 cases and 316,078 controls |
CHARGE Consortium Hemostasis Working Group: n = 2583 to n = 120,246 |
Bidirectional |
Increased FVIII, vWF, and phosphorylated fibrinopeptide A and decreased fibrinogen associated with increased migraine risk (especially MA) Migraine liability not associated with measures of hemostasis |
Guo et al. [150] |
| Vitamine D Levels |
GWAS 1 (Hautakangas et al.): 48,975 cases and 540,381 controls GWAS 2 (Choquet et al.): 28,852 cases and 525,717 controls GWAS 3 (FinnGen): 10,536 cases and 208,845 controls |
UK Biobank 417,580 participants |
Bidirectional |
Genetically determined increase in circulating vitamin D levels associated with a decreased migraine risk Migraine not associated with vitamin D levels |
Niu et al. [165] |
| Circulating Insulin-Like Growth Factor 1 Levels |
GWAS 1 (FinnGen): 10,536 cases and 208,845 controls GWAS 2 (Choquet et al.): 28,852 cases and 554,569 controls |
UK Biobank 363,228 participants |
Bidirectional |
Genetically determined increase in IGF1 levels associated with decreased risk of migraine Migraine not associated with IGF1 levels |
Abuduxukuer et al. [166] |
| DNA methylation | International Headache Genetics Consortium: 59,674 cases and 316,078 controls | DNA methylation quantitative trait loci from whole blood, n = 639 and fetal brain, n = 166 | One-directional | Not provided | Hannon et al. [167] |
| Socioeconomic outcomes | |||||
| School absence and educational attainment |
Avon Longitudinal Study of Parents and Children: 6113 participants |
One-directional | Migraine liability not associated with school absence or educational attainment in childhood and adolescence | Hughes et al. [168] | |
|
Nineteen social and socioeconomic outcomes |
UK Biobank: 10,603 cases, 326,394 controls |
One-directional | Migraine liability associated with reduced chance of having a weekly leisure or social activity | Harrison et al. [169] | |
Other recent genetic findings
Genetics of headache
A 2018 British GWAS studied 74,461 individuals who had had a headache interfering with daily activities in the previous month and 149,312 controls [170]. The majority of patients probably had tension headache and less often migraine. This study identified 28 headache susceptibility loci of which 14 had already been identified by GWAS in migraine, and 14 were new. The majority of the potential headache genes were neuronal and not vascular. This study also found a shared genetic background between the headache phenotype and many psychological traits associated with vulnerability to depression and negative emotions, highlighting the importance of links between psychiatric conditions and painful conditions.
Another recent GWAS including 2084 Taiwanese patients and 11,822 age- and sex-matched controls identified two loci, rs10493859 in TGFBR3 and rs13312779 in FGF23, both functionally relevant to vascular function and migraine, to be significantly associated with self-reported headache [171].
Until recently, studies on genetics of Cluster headache (CH) have been dominated by candidate gene studies with conflicting findings [172, 173]. A first Italian GWAS on 99 patients and 360 controls identified ADCYAP1R1 and MME gene variants as possibly associated with susceptibility for CH [174]. These findings were not replicated in a larger Swedish cohort [175]. In 2021, two GWAS out of which one included Dutch cases (n = 840) and controls (n = 1457) and Norwegian cases (n = 144) and controls (n = 1800) [176], and the second one UK cases (n = 852) and controls (n = 5614) and Swedish cases (n = 591) and controls (n = 1134) [177] independently identified four risk loci for CH on chromosome 1, chromosome 2 (two loci), and chromosome 6, respectively. Subsequently, a meta-analysis of both studies analyzing 8,039,373 variants confirmed a significant association of all 8 index variants (in the 4 loci) and identified three additional loci with genome-wide significance on chromosomes 7, 10 and 19. The nearest genes to the loci on chromosome 2 and 6 are MERTK and UFL1/FHL5, respectively. Interestingly, as stated in Table 3, UFL1/FHL5 has previously been identified as a migraine risk locus [120].
Genetics of chronic migraine
Chronic migraine is the most disabling form of migraine, and causes for migraine chronification remain incompletely understood. In order to identify genetic variants contributing to migraine chronification, a comparison of patients with chronic migraine and patients with episodic migraine is necessary, whereas most studies attempting to find genetic risk factors have compared chronic migraineurs to healthy controls. Recent studies comparing chronic and episodic migraine have found genetic variants in the TRPM8 gene [178], the TRPV1 gene [179], and HLA class I alleles [180] to be associated with chronic migraine.
Previous to these studies, a candidate gene-association study examined 144 SNPs from 48 candidate genes in patients with chronic or high-frequency episodic migraine compared to healthy controls, and did not reveal any significant findings [181].
The first study assessing whole-genome sequencing data in patients with chronic compared to episodic migraine did not show any significant difference [182].
Further studies are needed to determine the proportion of genetic and environmental factors in chronic migraine.
Genetics factors underlying treatment response
Genetic factors strongly influence the absorption, distribution, metabolism and excretion of drugs. Studies addressing genetic factors underlying treatment response to triptans have described GNB3 C825T gene polymorphism to be associated with a better response to triptans in CH patients, and polymorphisms in the PRDM16, SLC6A4 and DRD2 genes to be associated with a better, inconsistent and worse response to triptans in migraine patients, respectively [183]. Another study showed that a polygenic risk score doubling the risk of migraine was associated with a better response to triptans [184].
Recently, whole-exome sequencing in a discovery cohort of migraine patients treated by verapamil (definitive responders n = 21 and definitive non-responders n = 14), followed by genotyping in a confirmation cohort (n = 185), identified 13 SNP, which were highly correlated with the changes in the number of migraine days [137].
In the future, determining the genetic profile of an individual could allow the choice of treatments with the best profile of efficacy and tolerance [5].
Mitochondrial DNA and migraine
Mitochondrial dysfunction has been suspected to contribute to migraine pathophysiology, since migraine-like headache is a clinical feature of several mitochondrial diseases [185–187], presumable mitochondrial biomarkers have been found to be elevated in migraine patients [188], and several studies have reported mitochondrial DNA (mtDNA) candidate variants possibly associated with migraine [189, 190]. However, the first GWAS assessing 775 mitochondrial DNA variants in 4021 migraine sufferers and 14,288 controls found no migraine-associated variants, ruling out the mitochondrial hypothesis suggested by older studies [191]. Limitations discussed by the authors were the diagnosis of migraine based on a questionnaire covering symptoms during the past 12 months instead of a clinical interview, and the absence of consideration of heteroplasmic variation, copy-number variations and epigenetic changes.
Conclusion
Genetics of migraine have made significant progress over the past 15 years [5, 28, 114]. The study of monogenic migraines identified key proteins of the susceptibility to CSD and helped to better appreciate the links between migraine and vascular disorders. GWAS have identified multiple susceptibility genes revealing several complex networks of “pro-migraine” molecular abnormalities, mainly neuronal and vascular (Fig. 1). Genetics has also underscored the importance of genetic factors shared between migraine and its major co-morbidities including depression and high blood pressure. Very large-scale studies are still needed to map all of the susceptibility loci to migraine and then to understand how these genomic variants lead to migraine cell phenotypes. Ultimately, the main pathophysiological mechanism in a given patient, neuronal or vascular or otherwise, could be determined through its genetic risk profile. Pharmacogenetics could help predict the therapeutic response and thus help prescribe the treatment with the best safety and efficacy profile.
Disclosures
Lou Grangeon has received travel and accommodation expenses from Lundbeck, not related to the submitted work.
Kristin Sophie Lange has received travel and accommodation expenses from Acticor Biotech and TEVA GmbH, not related to the submitted work.
Wietse Wiels is a fundamental research scholar of the Flanders Scientific Research Fund (FWO).
Anne Ducros had received personal fees for board membership and/or speaking from Allergan/Abbvie, Lilly, Lundbeck, Novartis, Pfizer, Teva, is associate editor of Cephalalgia and past-president of the French Headache Society, and has received research grants for her institution (CHU de Montpellier) from the Programme Hospitalier de Recherche Clinique (PHRC) and from Pfizer.
Authors’ contributions
LG, KSL,CD and AD designed the outline, wrote the manuscript and the tables, corrected, formatted and led the review. MWP, DO, KM, WW, PM, FF collaborated to the literature search, the drafting of the tables and the revision of the manuscript. All authors reviewed the manuscript. The authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lou Grangeon and Kristin Sophie Lange contributed equally to this work.
Contributor Information
Lou Grangeon, Email: lou.grangeon@chu-rouen.fr.
Kristin Sophie Lange, Email: kristin-sophie.lange@charite.de.
Marta Waliszewska-Prosół, Email: marta.waliszewska@gmail.com.
Dilara Onan, Email: dilaraonan@gmail.com.
Karol Marschollek, Email: karol.marschollek@gmail.com.
Wietse Wiels, Email: wietse.wiels@vub.be.
Petr Mikulenka, Email: pmikulenka@gmail.com.
Fatemeh Farham, Email: ffarham@yahoo.com.
Cédric Gollion, Email: gollion.c@chu-toulouse.fr.
Anne Ducros, Email: a-ducros@chu-montpellier.fr.
References
- 1.Russell MB, Hilden J, Sørensen SA, et al. Familial occurrence of migraine without aura and migraine with aura. Neurology. 1993;43:1369–1373. doi: 10.1212/WNL.43.7.1369. [DOI] [PubMed] [Google Scholar]
- 2.Russell MB, Olesen J. Increased familial risk and evidence of genetic factor in migraine. BMJ. 1995;311:541–544. doi: 10.1136/bmj.311.7004.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polderman TJC, Benyamin B, de Leeuw CA, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. 2015;47:702–709. doi: 10.1038/ng.3285. [DOI] [PubMed] [Google Scholar]
- 4.Russell MB, Ulrich V, Gervil M, et al. Migraine without aura and migraine with aura are distinct disorders. A population-based twin survey. Headache. 2002;42:332–336. doi: 10.1046/j.1526-4610.2002.02102.x. [DOI] [PubMed] [Google Scholar]
- 5.Cader MZ. The genetics of migraine and the path to precision medicine. Prog Brain Res. 2020;255:403–418. doi: 10.1016/bs.pbr.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011;10:457–470. doi: 10.1016/S1474-4422(11)70048-5. [DOI] [PubMed] [Google Scholar]
- 7.Thomsen LL, Eriksen MK, Roemer SF, et al. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain J Neurol. 2002;125:1379–1391. doi: 10.1093/brain/awf132. [DOI] [PubMed] [Google Scholar]
- 8.Headache Classification Committee of the International Hedache Society (IHS). (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38:1–211 [DOI] [PubMed]
- 9.Lykke Thomsen L, Kirchmann Eriksen M, Faerch Romer S, et al. An epidemiological survey of hemiplegic migraine. Cephalalgia Int J Headache. 2002;22:361–375. doi: 10.1046/j.1468-2982.2002.00371.x. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen LL, Ostergaard E, Olesen J, et al. Evidence for a separate type of migraine with aura: sporadic hemiplegic migraine. Neurology. 2003;60:595–601. doi: 10.1212/01.WNL.0000046524.25369.7D. [DOI] [PubMed] [Google Scholar]
- 11.Lai T-H, Hong C-T. Prolonged symptoms in sporadic hemiplegic migraine: aura or migrainous infarction? Acta Neurol Taiwanica. 2012;21:129–132. [PubMed] [Google Scholar]
- 12.Kumar G, Topper L, Maytal J. Familial hemiplegic migraine with prolonged aura and multimodality imaging: a case report. Headache. 2009;49:139–142. doi: 10.1111/j.1526-4610.2008.01180.x. [DOI] [PubMed] [Google Scholar]
- 13.Saleh C, Pierquin G, Beyenburg S. Hemiplegic migraine presenting with prolonged somnolence: a case report. Case Rep Neurol. 2016;8:204–210. doi: 10.1159/000448473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelzer N, Blom D, Stam A, et al. Recurrent coma and fever in familial hemiplegic migraine type 2. A prospective 15-year follow-up of a large family with a novel ATP1A2 mutation. Cephalalgia. 2017;37:737–755. doi: 10.1177/0333102416651284. [DOI] [PubMed] [Google Scholar]
- 15.Echenne B, Ducros A, Rivier F, et al. Recurrent episodes of coma: an unusual phenotype of familial hemiplegic migraine with linkage to chromosome 1. Neuropediatrics. 1999;30:214–217. doi: 10.1055/s-2007-973493. [DOI] [PubMed] [Google Scholar]
- 16.Indelicato E, Boesch S (2021) From genotype to phenotype: expanding the clinical Spectrum of CACNA1A variants in the era of next generation sequencing. Front Neurol 12. Epub ahead of print March 2. 10.3389/fneur.2021.639994 [DOI] [PMC free article] [PubMed]
- 17.Ducros A, Denier C, Joutel A, et al. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med. 2001;345:17–24. doi: 10.1056/NEJM200107053450103. [DOI] [PubMed] [Google Scholar]
- 18.Hauge AW, Kirchmann M, Olesen J. Characterization of consistent triggers of migraine with aura. Cephalalgia Int J Headache. 2011;31:416–438. doi: 10.1177/0333102410382795. [DOI] [PubMed] [Google Scholar]
- 19.Toldo I, Brunello F, Morao V et al (2019) First Attack and Clinical Presentation of Hemiplegic Migraine in Pediatric Age: A Multicenter Retrospective Study and Literature Review. Front Neurol 10. Epub ahead of print October 15. 10.3389/fneur.2019.01079 [DOI] [PMC free article] [PubMed]
- 20.Hansen JM, Hauge AW, Ashina M, et al. Trigger factors for familial hemiplegic migraine. Cephalalgia Int J Headache. 2011;31:1274–1281. doi: 10.1177/0333102411415878. [DOI] [PubMed] [Google Scholar]
- 21.Pelzer N, Haan J, Stam AH, et al. Clinical spectrum of hemiplegic migraine and chances of finding a pathogenic mutation. Neurology. 2018;90:e575–e582. doi: 10.1212/WNL.0000000000004966. [DOI] [PubMed] [Google Scholar]
- 22.Hommersom MP, van Prooije TH, Pennings M et al (2021. Epub ahead of print November 22) The complexities of CACNA1A in clinical neurogenetics. J Neurol. 10.1007/s00415-021-10897-9 [DOI] [PubMed]
- 23.Humbertclaude V, Riant F, Krams B, et al. Cognitive impairment in children with CACNA1A mutations. Dev Med Child Neurol. 2020;62:330–337. doi: 10.1111/dmcn.14261. [DOI] [PubMed] [Google Scholar]
- 24.Guerin AA, Feigenbaum A, Donner EJ, et al. Stepwise developmental regression associated with novel CACNA1A mutation. Pediatr Neurol. 2008;39:363–364. doi: 10.1016/j.pediatrneurol.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Vanmolkot KRJ, Stroink H, Koenderink JB, et al. Severe episodic neurological deficits and permanent mental retardation in a child with a novel FHM2 ATP1A2 mutation. Ann Neurol. 2006;59:310–314. doi: 10.1002/ana.20760. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland HG, Albury CL, Griffiths LR. Advances in genetics of migraine. J Headache Pain. 2019;20:1–20. doi: 10.1186/s10194-019-1017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Boer I, Terwindt GM, van den Maagdenberg AM. Genetics of migraine aura: an update. J Headache Pain. 2020;21:64. doi: 10.1186/s10194-020-01125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bron C, Sutherland HG, Griffiths LR. Exploring the hereditary nature of migraine. Neuropsychiatr Dis Treat. 2021;17:1183–1194. doi: 10.2147/NDT.S282562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutherland HG, Maksemous N, Albury CL, et al. Comprehensive exonic sequencing of hemiplegic migraine-related genes in a cohort of suspected probands identifies known and potential pathogenic variants. Cells. 2020;9:2368. doi: 10.3390/cells9112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riant F, Roos C, Roubertie A, et al. Hemiplegic migraine associated with PRRT2 variations: a clinical and genetic study. Neurology. 2022;98:e51–e61. doi: 10.1212/WNL.0000000000012947. [DOI] [PubMed] [Google Scholar]
- 31.Hiekkala ME, Vuola P, Artto V, et al. The contribution of CACNA1A, ATP1A2 and SCN1A mutations in hemiplegic migraine: a clinical and genetic study in Finnish migraine families. Cephalalgia. 2018;38:1849–1863. doi: 10.1177/0333102418761041. [DOI] [PubMed] [Google Scholar]
- 32.Amberger J, Bocchini CA, Scott AF, et al. McKusick’s online Mendelian inheritance in man (OMIM) Nucleic Acids Res. 2009;37:D793–D796. doi: 10.1093/nar/gkn665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardiner AR, Jaffer F, Dale RC, et al. The clinical and genetic heterogeneity of paroxysmal dyskinesias. Brain J Neurol. 2015;138:3567–3580. doi: 10.1093/brain/awv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H-Y, Xu Y, Huang Y, et al. The gene for paroxysmal non-kinesigenic dyskinesia encodes an enzyme in a stress response pathway. Hum Mol Genet. 2004;13:3161–3170. doi: 10.1093/hmg/ddh330. [DOI] [PubMed] [Google Scholar]
- 35.Igarashi T, Inatomi J, Sekine T, et al. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki M, Van Paesschen W, Stalmans I, et al. Defective membrane expression of the Na(+)-HCO(3)(−) cotransporter NBCe1 is associated with familial migraine. Proc Natl Acad Sci U S A. 2010;107:15963–15968. doi: 10.1073/pnas.1008705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil-Perotín S, Jaijo T, Verdú AG, et al. Epilepsy, status epilepticus, and hemiplegic migraine coexisting with a novel SLC4A4 mutation. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2021;42:3647–3654. doi: 10.1007/s10072-020-04961-x. [DOI] [PubMed] [Google Scholar]
- 38.Potic A, Nmezi B, Padiath QS. CAPOS syndrome and hemiplegic migraine in a novel pedigree with the specific ATP1A3 mutation. J Neurol Sci. 2015;358:453–456. doi: 10.1016/j.jns.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Heinzen EL, Swoboda KJ, Hitomi Y, et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet. 2012;44:1030–1034. doi: 10.1038/ng.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Vries B, Mamsa H, Stam AH, et al. Episodic ataxia associated with EAAT1 mutation C186S affecting glutamate reuptake. Arch Neurol. 2009;66:97–101. doi: 10.1001/archneurol.2008.535. [DOI] [PubMed] [Google Scholar]
- 41.Jen JC, Wan J, Palos TP, et al. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology. 2005;65:529–534. doi: 10.1212/01.WNL.0000172638.58172.5a. [DOI] [PubMed] [Google Scholar]
- 42.Kovermann P, Hessel M, Kortzak D, et al. Impaired K(+) binding to glial glutamate transporter EAAT1 in migraine. Sci Rep. 2017;7:13913. doi: 10.1038/s41598-017-14176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paucar M, Granberg T, Lagerstedt-Robinson K, et al. SLC1A3 variant associated with hemiplegic migraine and acetazolamide-responsive MRS changes. Neurol Genet. 2020;6:e474. doi: 10.1212/NXG.0000000000000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gburek-Augustat J, Heinze A, Abou Jamra R, et al. Hemiplegic migraine in glut1 deficiency syndrome and paroxysmal dyskinesia at ketogenic diet induction: case report and literature review. Mov Disord Clin Pract. 2020;7:965–970. doi: 10.1002/mdc3.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourque DK, Cordeiro D, Nimmo GAM, et al. Phenotypic and genotypic Spectrum of glucose Transporter-1 deficiency syndrome. Can J Neurol Sci J Can Sci Neurol. 2021;48:826–830. doi: 10.1017/cjn.2021.3. [DOI] [PubMed] [Google Scholar]
- 46.Mohammad SS, Coman D, Calvert S. Glucose transporter 1 deficiency syndrome and hemiplegic migraines as a dominant presenting clinical feature. J Paediatr Child Health. 2014;50:1025–1026. doi: 10.1111/jpc.12613. [DOI] [PubMed] [Google Scholar]
- 47.Weller CM, Leen WG, Neville BGR, et al. A novel SLC2A1 mutation linking hemiplegic migraine with alternating hemiplegia of childhood. Cephalalgia Int J Headache. 2015;35:10–15. doi: 10.1177/0333102414532379. [DOI] [PubMed] [Google Scholar]
- 48.Coppola G, Pastorino GMG, Vetri L et al (2020) Familial Hemiplegic Migraine with an ATP1A4 Mutation: Clinical Spectrum and Carbamazepine Efficacy. Brain Sci 10. Epub ahead of print June 15. 10.3390/brainsci10060372 [DOI] [PMC free article] [PubMed]
- 49.Hasırcı Bayır BR, Tutkavul K, Eser M, et al. Epilepsy in patients with familial hemiplegic migraine. Seizure. 2021;88:87–94. doi: 10.1016/j.seizure.2021.03.028. [DOI] [PubMed] [Google Scholar]
- 50.Friedrich T, Tavraz NN, Junghans C (2016) ATP1A2 mutations in migraine: seeing through the facets of an ion pump onto the neurobiology of disease. Front Physiol 7. Epub ahead of print June 21. 10.3389/fphys.2016.00239 [DOI] [PMC free article] [PubMed]
- 51.Jurkat-Rott K, Freilinger T, Dreier JP, et al. Variability of familial hemiplegic migraine with novel A1A2 Na+/K+-ATPase variants. Neurology. 2004;62:1857–1861. doi: 10.1212/01.WNL.0000127310.11526.FD. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki M, Fujiwara K, Tsubuku T, et al. Time course of downbeat positioning nystagmus in familial hemiplegic migraine type 1 treated with acetazolamide. J Neurol Sci. 2016;368:206–208. doi: 10.1016/j.jns.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 53.Battistini S, Stenirri S, Piatti M, et al. A new CACNA1A gene mutation in acetazolamide-responsive familial hemiplegic migraine and ataxia. Neurology. 1999;53:38–43. doi: 10.1212/WNL.53.1.38. [DOI] [PubMed] [Google Scholar]
- 54.Terwindt GM, Ophoff RA, van Eijk R, et al. Involvement of the CACNA1A gene containing region on 19p13 in migraine with and without aura. Neurology. 2001;56:1028–1032. doi: 10.1212/WNL.56.8.1028. [DOI] [PubMed] [Google Scholar]
- 55.Yu W, Horowitz SH. Familial hemiplegic migraine and its abortive therapy with intravenous verapamil. Neurology. 2001;57:1732–1733. doi: 10.1212/WNL.57.9.1732. [DOI] [PubMed] [Google Scholar]
- 56.Elliott MA, Peroutka SJ, Welch S, et al. Familial hemiplegic migraine, nystagmus, and cerebellar atrophy. Ann Neurol. 1996;39:100–106. doi: 10.1002/ana.410390115. [DOI] [PubMed] [Google Scholar]
- 57.Le Fort D, Safran AB, Picard F, et al. Elicited repetitive daily blindness: a new familial disorder related to migraine and epilepsy. Neurology. 2004;63:348–350. doi: 10.1212/01.WNL.0000130251.59422.B4. [DOI] [PubMed] [Google Scholar]
- 58.Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/S0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 59.Tottene A, Fellin T, Pagnutti S, et al. Familial hemiplegic migraine mutations increase ca(2+) influx through single human CaV2.1 channels and decrease maximal CaV2.1 current density in neurons. Proc Natl Acad Sci U S A. 2002;99:13284–13289. doi: 10.1073/pnas.192242399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–710. doi: 10.1016/S0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 61.van den Maagdenberg AMJM, Pizzorusso T, Kaja S, et al. High cortical spreading depression susceptibility and migraine-associated symptoms in ca(v)2.1 S218L mice. Ann Neurol. 2010;67:85–98. doi: 10.1002/ana.21815. [DOI] [PubMed] [Google Scholar]
- 62.Meneghetti N, Cerri C, Vannini E, et al. Synaptic alterations in visual cortex reshape contrast-dependent gamma oscillations and inhibition-excitation ratio in a genetic mouse model of migraine. J Headache Pain. 2022;23:125. doi: 10.1186/s10194-022-01495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chanda ML, Tuttle AH, Baran I, et al. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain. 2013;154:1254–1262. doi: 10.1016/j.pain.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 64.Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65–80. doi: 10.1016/S1474-4422(14)70220-0. [DOI] [PubMed] [Google Scholar]
- 65.Sintas Vives C, Carreño O, Fernández Castillo N, et al. Mutation spectrum in the CACNA1A gene in 49 patients with episodic ataxia. Sci Rep. 2017;7:2514. doi: 10.1038/s41598-017-02554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Fusco M, Marconi R, Silvestri L, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003;33:192–196. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- 67.Leo L, Gherardini L, Barone V, et al. Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 2011;7:e1002129. doi: 10.1371/journal.pgen.1002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capuani C, Melone M, Tottene A, et al. Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. EMBO Mol Med. 2016;8:967–986. doi: 10.15252/emmm.201505944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unekawa M, Ikeda K, Tomita Y, et al. Enhanced susceptibility to cortical spreading depression in two types of Na(+),K(+)-ATPase α2 subunit-deficient mice as a model of familial hemiplegic migraine 2. Cephalalgia Int J Headache. 2018;38:1515–1524. doi: 10.1177/0333102417738249. [DOI] [PubMed] [Google Scholar]
- 70.Smith SE, Chen X, Brier LM, et al. Astrocyte deletion of α2-Na/K ATPase triggers episodic motor paralysis in mice via a metabolic pathway. Nat Commun. 2020;11:6164. doi: 10.1038/s41467-020-19915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dichgans M, Freilinger T, Eckstein G, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet Lond Engl. 2005;366:371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- 72.Martin MS, Dutt K, Papale LA, et al. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010;285:9823–9834. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cestele S, Scalmani P, Rusconi R, et al. Self-limited Hyperexcitability: functional effect of a familial hemiplegic migraine mutation of the Nav1.1 (SCN1A) Na+ channel. J Neurosci. 2008;28:7273–7283. doi: 10.1523/JNEUROSCI.4453-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bertelli S, Barbieri R, Pusch M, et al. Gain of function of sporadic/familial hemiplegic migraine-causing SCN1A mutations: use of an optimized cDNA. Cephalalgia Int J Headache. 2019;39:477–488. doi: 10.1177/0333102418788336. [DOI] [PubMed] [Google Scholar]
- 75.Cloarec R, Bruneau N, Rudolf G, et al. PRRT2 links infantile convulsions and paroxysmal dyskinesia with migraine. Neurology. 2012;79:2097–2103. doi: 10.1212/WNL.0b013e3182752c46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gardiner AR, Bhatia KP, Stamelou M, et al. PRRT2 gene mutations: from paroxysmal dyskinesia to episodic ataxia and hemiplegic migraine. Neurology. 2012;79:2115–2121. doi: 10.1212/WNL.0b013e3182752c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marini C, Conti V, Mei D, et al. PRRT2 mutations in familial infantile seizures, paroxysmal dyskinesia, and hemiplegic migraine. Neurology. 2012;79:2109–2114. doi: 10.1212/WNL.0b013e3182752ca2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riant F, Roze E, Barbance C, et al. PRRT2 mutations cause hemiplegic migraine. Neurology. 2012;79:2122–2124. doi: 10.1212/WNL.0b013e3182752cb8. [DOI] [PubMed] [Google Scholar]
- 79.Dale RC, Gardiner A, Antony J, et al. Familial PRRT2 mutation with heterogeneous paroxysmal disorders including paroxysmal torticollis and hemiplegic migraine. Dev Med Child Neurol. 2012;54:958–960. doi: 10.1111/j.1469-8749.2012.04394.x. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki-Muromoto S, Kosaki R, Kosaki K, et al. Familial hemiplegic migraine with a PRRT2 mutation: phenotypic variations and carbamazepine efficacy. Brain and Development. 2020;42:293–297. doi: 10.1016/j.braindev.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 81.Ebrahimi-Fakhari D, Saffari A, Westenberger A, et al. The evolving spectrum of PRRT2-associated paroxysmal diseases. Brain J Neurol. 2015;138:3476–3495. doi: 10.1093/brain/awv317. [DOI] [PubMed] [Google Scholar]
- 82.Yang L, You C, Qiu S, et al. Novel and de novo point and large microdeletion mutation in PRRT2-related epilepsy. Brain Behav. 2020;10:e01597. doi: 10.1002/brb3.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michetti C, Castroflorio E, Marchionni I, et al. The PRRT2 knockout mouse recapitulates the neurological diseases associated with PRRT2 mutations. Neurobiol Dis. 2017;99:66–83. doi: 10.1016/j.nbd.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riant F, Ducros A, Ploton C, et al. De novo mutations in ATP1A2 and CACNA1A are frequent in early-onset sporadic hemiplegic migraine. Neurology. 2010;75:967–972. doi: 10.1212/WNL.0b013e3181f25e8f. [DOI] [PubMed] [Google Scholar]
- 85.Jen JC, Klein A, Boltshauser E, et al. Prolonged hemiplegic episodes in children due to mutations in ATP1A2. J Neurol Neurosurg Psychiatry. 2007;78:523–526. doi: 10.1136/jnnp.2006.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Sanctis S, Grieco GS, Breda L, et al. Prolonged sporadic hemiplegic migraine associated with a novel de novo missense ATP1A2 gene mutation. Headache. 2011;51:447–450. doi: 10.1111/j.1526-4610.2010.01793.x. [DOI] [PubMed] [Google Scholar]
- 87.Pelzer N, Hoogeveen ES, Ferrari MD, et al. Brain atrophy following hemiplegic migraine attacks. Cephalalgia. 2018;38:1199–1202. doi: 10.1177/0333102417723569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stubberud A, O’Connor E, Tronvik E, et al. R1352Q CACNA1A variant in a patient with sporadic hemiplegic migraine, Ataxia, seizures and cerebral Oedema: a case report. Case Rep Neurol. 2021;13:123–130. doi: 10.1159/000512275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moore BA, Hale WJ, Nabity PS, et al. A retrospective, epidemiological review of hemiplegic migraines in a military population. Mil Med. 2019;184:781–787. doi: 10.1093/milmed/usz040. [DOI] [PubMed] [Google Scholar]
- 90.Thomsen LL, Ostergaard E, Romer SF, et al. Sporadic hemiplegic migraine is an aetiologically heterogeneous disorder. Cephalalgia Int J Headache. 2003;23:921–928. doi: 10.1046/j.1468-2982.2003.00614.x. [DOI] [PubMed] [Google Scholar]
- 91.Thomsen LL, Olesen J, Russell MB. Increased risk of migraine with typical aura in probands with familial hemiplegic migraine and their relatives. Eur J Neurol. 2003;10:421–427. doi: 10.1046/j.1468-1331.2003.00621.x. [DOI] [PubMed] [Google Scholar]
- 92.Wieser T, Mueller C, Evers S, et al. Absence of known familial hemiplegic migraine (FHM) mutations in the CACNA1A gene in patients with common migraine: implications for genetic testing. Clin Chem Lab Med. 2003;41:272–275. doi: 10.1515/CCLM.2003.042. [DOI] [PubMed] [Google Scholar]
- 93.Meamar R, Ostadsharif M, Saadatnia M, et al. Mutation analysis of CACNA1A gene in Iranian migrainous and review literatures. J Res Med Sci Off J Isfahan Univ Med Sci. 2013;18:S6–S10. [PMC free article] [PubMed] [Google Scholar]
- 94.Hautakangas H, Winsvold BS, Ruotsalainen SE, et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet. 2022;54:152–160. doi: 10.1038/s41588-021-00990-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weir GA, Pettingill P, Wu Y, et al. The role of TRESK in discrete sensory neuron populations and somatosensory processing. Front Mol Neurosci. 2019;12:170. doi: 10.3389/fnmol.2019.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lafrenière RG, Cader MZ, Poulin J-F, et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat Med. 2010;16:1157–1160. doi: 10.1038/nm.2216. [DOI] [PubMed] [Google Scholar]
- 97.Pettingill P, Weir GA, Wei T, et al. A causal role for TRESK loss of function in migraine mechanisms. Brain J Neurol. 2019;142:3852–3867. doi: 10.1093/brain/awz342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andres-Enguix I, Shang L, Stansfeld PJ, et al. Functional analysis of missense variants in the TRESK (KCNK18) K channel. Sci Rep. 2012;2:237. doi: 10.1038/srep00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Royal P, Andres-Bilbe A, Ávalos Prado P, et al. Migraine-associated TRESK mutations increase neuronal excitability through alternative translation initiation and inhibition of TREK. Neuron. 2019;101:232–245.e6. doi: 10.1016/j.neuron.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 100.Han JY, Jang JH, Park J, et al. Targeted next-generation sequencing of Korean patients with developmental delay and/or intellectual disability. Front Pediatr. 2018;6:391. doi: 10.3389/fped.2018.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Imbrici P, Nematian-Ardestani E, Hasan S, et al. Altered functional properties of a missense variant in the TRESK K(+) channel (KCNK18) associated with migraine and intellectual disability. Pflugers Arch. 2020;472:923–930. doi: 10.1007/s00424-020-02382-5. [DOI] [PubMed] [Google Scholar]
- 102.Lengyel M, Hajdu D, Dobolyi A, et al. TRESK background potassium channel modifies the TRPV1-mediated nociceptor excitability in sensory neurons. Cephalalgia Int J Headache. 2021;41:827–838. doi: 10.1177/0333102421989261. [DOI] [PubMed] [Google Scholar]
- 103.Markel KA, Curtis D. Study of variants in genes implicated in rare familial migraine syndromes and their association with migraine in 200,000 exome-sequenced UK biobank participants. Ann Hum Genet. 2022;86:353–360. doi: 10.1111/ahg.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diamond ML. The role of concomitant headache types and non-headache co-morbidities in the underdiagnosis of migraine. Neurology. 2002;58:S3–S9. doi: 10.1212/WNL.58.9_suppl_6.S3. [DOI] [PubMed] [Google Scholar]
- 105.Brennan KC, Bates EA, Shapiro RE, et al. Casein kinase iδ mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5:183ra56. doi: 10.1126/scitranslmed.3005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011;12:570–584. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- 107.Alstadhaug K, Salvesen R, Bekkelund S. 24-hour distribution of migraine attacks. Headache. 2008;48:95–100. doi: 10.1111/j.1526-4610.2007.00779.x. [DOI] [PubMed] [Google Scholar]
- 108.Williams LB, Javed A, Sabri A, et al. ALPK1 missense pathogenic variant in five families leads to ROSAH syndrome, an ocular multisystem autosomal dominant disorder. Genet Med Off J Am Coll Med Genet. 2019;21:2103–2115. doi: 10.1038/s41436-019-0476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- 110.Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 111.Tan RYY, Markus HS. CADASIL: migraine, encephalopathy, Stroke and Their Inter-Relationships. PloS One. 2016;11:e0157613. doi: 10.1371/journal.pone.0157613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Adib-Samii P, Brice G, Martin RJ, et al. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke. 2010;41:630–634. doi: 10.1161/STROKEAHA.109.568402. [DOI] [PubMed] [Google Scholar]
- 113.Eikermann-Haerter K, Yuzawa I, Dilekoz E, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy syndrome mutations increase susceptibility to spreading depression. Ann Neurol. 2011;69:413–418. doi: 10.1002/ana.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ducros A. Genetics of migraine. Rev Neurol (Paris) 2021;177:801–808. doi: 10.1016/j.neurol.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 115.Auton A, Brooks LD, Durbin RM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Anttila V, Stefansson H, Kallela M, et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat Genet. 2010;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chasman DI, Schürks M, Anttila V, et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anttila V, Winsvold BS, Gormley P, et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45:912–917. doi: 10.1038/ng.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Freilinger T, Bohe M, Wegener B, et al. Expansion of the phenotypic Spectrum of the CACNA1A T666M mutation: a family with familial hemiplegic migraine type 1, Cerebellar Atrophy and Mental Retardation. Cephalalgia. 2008;28:403–407. doi: 10.1111/j.1468-2982.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 120.Gormley P, Anttila V, Winsvold BS, et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet. 2016;48:856–866. doi: 10.1038/ng.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Finucane HK, Reshef YA, Anttila V, et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat Genet. 2018;50:621–629. doi: 10.1038/s41588-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eising E, Huisman SMH, Mahfouz A, et al. Gene co-expression analysis identifies brain regions and cell types involved in migraine pathophysiology: a GWAS-based study using the Allen human brain atlas. Hum Genet. 2016;135:425–439. doi: 10.1007/s00439-016-1638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ligthart L, de Vries B, Smith AV, et al. Meta-analysis of genome-wide association for migraine in six population-based European cohorts. Eur J Hum Genet EJHG. 2011;19:901–907. doi: 10.1038/ejhg.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Freilinger T, Anttila V, de Vries B, et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet. 2012;44:777–782. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen S-P, Fuh J-L, Chung M-Y, et al. Genome-wide association study identifies novel susceptibility loci for migraine in Han Chinese resided in Taiwan. Cephalalgia Int J Headache. 2018;38:466–475. doi: 10.1177/0333102417695105. [DOI] [PubMed] [Google Scholar]
- 126.Chang X, Pellegrino R, Garifallou J, et al. Common variants at 5q33.1 predispose to migraine in African-American children. J Med Genet. 2018;55:831–836. doi: 10.1136/jmedgenet-2018-105359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choquet H, Yin J, Jacobson AS, et al. New and sex-specific migraine susceptibility loci identified from a multiethnic genome-wide meta-analysis. Commun Biol. 2021;4:864. doi: 10.1038/s42003-021-02356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang Z, Zhao L, Zhang X, et al. Common variants in KCNK5 and FHL5 genes contributed to the susceptibility of migraine without aura in Han Chinese population. Sci Rep. 2021;11:6807. doi: 10.1038/s41598-021-86374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsai C-K, Liang C-S, Lin G-Y, et al. Identifying genetic variants for age of migraine onset in a Han Chinese population in Taiwan. J Headache Pain. 2021;22:89. doi: 10.1186/s10194-021-01301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gupta RM, Hadaya J, Trehan A, et al. A genetic variant associated with five vascular diseases is a distal regulator of Endothelin-1 gene expression. Cell. 2017;170:522–533.e15. doi: 10.1016/j.cell.2017.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Codina-Fauteux V-A, Beaudoin M, Lalonde S, et al. PHACTR1 splicing isoforms and eQTLs in atherosclerosis-relevant human cells. BMC Med Genet. 2018;19:97. doi: 10.1186/s12881-018-0616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alves-Ferreira M, Quintas M, Sequeiros J, et al. A genetic interaction of NRXN2 with GABRE, SYT1 and CASK in migraine patients: a case-control study. J Headache Pain. 2021;22:57. doi: 10.1186/s10194-021-01266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gormley P, Kurki MI, Hiekkala ME, et al. Common variant burden contributes to the familial aggregation of migraine in 1,589 families. Neuron. 2018;98:743–753.e4. doi: 10.1016/j.neuron.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Häppölä P, Gormley P, Nuottamo ME, et al. Polygenic risk provides biological validity for the ICHD-3 criteria among Finnish migraine families. Cephalalgia Int J Headache. 2022;42:345–356. doi: 10.1177/03331024211045651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Techlo TR, Rasmussen AH, Møller PL, et al. Familial analysis reveals rare risk variants for migraine in regulatory regions. Neurogenetics. 2020;21:149–157. doi: 10.1007/s10048-020-00606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rasmussen AH, Kogelman LJA, Kristensen DM, et al. Functional gene networks reveal distinct mechanisms segregating in migraine families. Brain J Neurol. 2020;143:2945–2956. doi: 10.1093/brain/awaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cutrer FM, Moyer AM, Atkinson EJ, et al. Genetic variants related to successful migraine prophylaxis with verapamil. Mol Genet Genomic Med. 2021;9:e1680. doi: 10.1002/mgg3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ibrahim O, Sutherland HG, Maksemous N, et al. Exploring neuronal vulnerability to head trauma using a whole exome approach. J Neurotrauma. 2020;37:1870–1879. doi: 10.1089/neu.2019.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kraft P, Chen H, Lindström S. The use of genetic correlation and Mendelian randomization studies to increase our understanding of relationships between complex traits. Curr Epidemiol Rep. 2020;7:104–112. doi: 10.1007/s40471-020-00233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang Y, Zhao H, Boomsma DI, et al. Molecular genetic overlap between migraine and major depressive disorder. Eur J Hum Genet EJHG. 2018;26:1202–1216. doi: 10.1038/s41431-018-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bahrami S, Hindley G, Winsvold BS, et al. Dissecting the shared genetic basis of migraine and mental disorders using novel statistical tools. Brain J Neurol. 2022;145:142–153. doi: 10.1093/brain/awab267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malik R, Freilinger T, Winsvold BS, et al. Shared genetic basis for migraine and ischemic stroke: a genome-wide analysis of common variants. Neurology. 2015;84:2132–2145. doi: 10.1212/WNL.0000000000001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Daghlas I, Guo Y, Chasman DI. Effect of genetic liability to migraine on coronary artery disease and atrial fibrillation: a Mendelian randomization study. Eur J Neurol. 2020;27:550–556. doi: 10.1111/ene.14111. [DOI] [PubMed] [Google Scholar]
- 144.Siewert KM, Klarin D, Damrauer SM, et al. Cross-trait analyses with migraine reveal widespread pleiotropy and suggest a vascular component to migraine headache. Int J Epidemiol. 2020;49:1022–1031. doi: 10.1093/ije/dyaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo Y, Rist PM, Daghlas I, et al. A genome-wide cross-phenotype meta-analysis of the association of blood pressure with migraine. Nat Commun. 2020;11:3368. doi: 10.1038/s41467-020-17002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Daghlas I, Vgontzas A, Guo Y, et al. Habitual sleep disturbances and migraine: a Mendelian randomization study. Ann Clin Transl Neurol. 2020;7:2370–2380. doi: 10.1002/acn3.51228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Adewuyi EO, Sapkota Y, Iec IEC et al (2020) Shared Molecular Genetic Mechanisms Underlie Endometriosis and Migraine Comorbidity. Genes 11. Epub ahead of print February 29. 10.3390/genes11030268 [DOI] [PMC free article] [PubMed]
- 148.Georges A, Yang M-L, Berrandou T-E, et al. Genetic investigation of fibromuscular dysplasia identifies risk loci and shared genetics with common cardiovascular diseases. Nat Commun. 2021;12:6031. doi: 10.1038/s41467-021-26174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang Y-J, Fann CS-J, Fuh J-L, et al. Genome-wide analysis identified novel susceptible genes of restless legs syndrome in migraineurs. J Headache Pain. 2022;23:39. doi: 10.1186/s10194-022-01409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guo Y, Rist PM, Sabater-Lleal M, et al. Association between hemostatic profile and migraine: a Mendelian randomization analysis. Neurology. 2021;96:e2481–e2487. doi: 10.1212/WNL.0000000000011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Daghlas I, Rist PM, Chasman DI. Effect of genetic liability to migraine on cognition and brain volume: a Mendelian randomization study. Cephalalgia Int J Headache. 2020;40:998–1002. doi: 10.1177/0333102420916751. [DOI] [PubMed] [Google Scholar]
- 152.Shu M-J, Li J-R, Zhu Y-C, et al. Migraine and ischemic stroke: a Mendelian randomization study. Neurol Ther. 2022;11:237–246. doi: 10.1007/s40120-021-00310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee K-J, Lee SJ, Bae H-J, et al. Exploring the causal inference of migraine on stroke: a Mendelian randomization study. Eur J Neurol. 2022;29:335–338. doi: 10.1111/ene.15101. [DOI] [PubMed] [Google Scholar]
- 154.Daghals I, Sargurupremraj M, Danning R, et al. Migraine, stroke, and cervical arterial dissection: shared genetics for a triad of brain disorders with vascular involvement. Neurol Genet. 2022;8:e653. doi: 10.1212/NXG.0000000000000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yuan S, Daghlas I, Larsson SC. Alcohol, coffee consumption, and smoking in relation to migraine: a bidirectional Mendelian randomization study. Pain. 2022;163:e342–e348. doi: 10.1097/j.pain.0000000000002360. [DOI] [PubMed] [Google Scholar]
- 156.Chen H, Zhang H, Zheng L. No causal association between coffee consumption and risk of migraine: a Mendelian randomization study. Front Genet. 2022;13:792313. doi: 10.3389/fgene.2022.792313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johnsen MB, Winsvold BS, Børte S, et al. The causal role of smoking on the risk of headache. A Mendelian randomization analysis in the HUNT study. Eur J Neurol. 2018;25:1148–e102. doi: 10.1111/ene.13675. [DOI] [PubMed] [Google Scholar]
- 158.Mitchell BL, Diaz-Torres S, Bivol S, et al. Elucidating the relationship between migraine risk and brain structure using genetic data. Brain J Neurol. 2022;145:3214–3224. doi: 10.1093/brain/awac105. [DOI] [PubMed] [Google Scholar]
- 159.Chu S, Wu Z, Wu Z, et al. Association between insomnia and migraine risk: a case-control and bidirectional Mendelian randomization study. Pharmacogenomics Pers Med. 2021;14:971–976. doi: 10.2147/PGPM.S305780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen J, Chen X, Xie Y, et al. Irritable bowel syndrome and migraine: evidence from Mendelian randomization analysis in the UK biobank. Expert Rev Gastroenterol Hepatol. 2021;15:1233–1239. doi: 10.1080/17474124.2021.1949290. [DOI] [PubMed] [Google Scholar]
- 161.Tanha HM, Martin NG, Whitfield JB, et al. Association and genetic overlap between clinical chemistry tests and migraine. Cephalalgia Int J Headache. 2021;41:1208–1221. doi: 10.1177/03331024211018131. [DOI] [PubMed] [Google Scholar]
- 162.Tanha HM, Sathyanarayanan A, Nyholt DR. Genetic overlap and causality between blood metabolites and migraine. Am J Hum Genet. 2021;108:2086–2098. doi: 10.1016/j.ajhg.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guo Y, Daghlas I, Gormley P, et al. Phenotypic and genotypic associations between migraine and lipoprotein subfractions. Neurology. 2021;97:e2223–e2235. doi: 10.1212/WNL.0000000000012919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yin P, Anttila V, Siewert KM, et al. Serum calcium and risk of migraine: a Mendelian randomization study. Hum Mol Genet. 2017;26:820–828. doi: 10.1093/hmg/ddw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Niu P-P, Wang X, Xu Y-M. Higher circulating vitamin D levels are associated with decreased migraine risk: a Mendelian randomization study. Front Nutr. 2022;9:907789. doi: 10.3389/fnut.2022.907789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abuduxukuer R, Niu P-P, Guo Z-N, et al. Circulating insulin-like growth factor 1 levels and migraine risk: a Mendelian randomization study. Neurol Ther. 2022;11:1677–1689. doi: 10.1007/s40120-022-00398-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hannon E, Weedon M, Bray N, et al. Pleiotropic effects of trait-associated genetic variation on DNA methylation: utility for refining GWAS loci. Am J Hum Genet. 2017;100:954–959. doi: 10.1016/j.ajhg.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hughes A, Wade KH, Dickson M, et al. Common health conditions in childhood and adolescence, school absence, and educational attainment: Mendelian randomization study. NPJ Sci Learn. 2021;6:1. doi: 10.1038/s41539-020-00080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Harrison S, Davies AR, Dickson M, et al. The causal effects of health conditions and risk factors on social and socioeconomic outcomes: Mendelian randomization in UK biobank. Int J Epidemiol. 2020;49:1661–1681. doi: 10.1093/ije/dyaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meng W, Adams MJ, Hebert HL, et al. A genome-wide association study finds genetic associations with broadly-defined headache in UK biobank (N=223,773) EBioMedicine. 2018;28:180–186. doi: 10.1016/j.ebiom.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tsao Y-C, Wang S-J, Hsu C-L, et al. Genome-wide association study reveals susceptibility loci for self-reported headache in a large community-based Asian population. Cephalalgia Int J Headache. 2022;42:229–238. doi: 10.1177/03331024211037269. [DOI] [PubMed] [Google Scholar]
- 172.Cargnin S, Sances G, Shin JI, et al. Gene polymorphism association studies in cluster headache: a field synopsis and systematic meta-analyses. Headache. 2021;61:1060–1076. doi: 10.1111/head.14168. [DOI] [PubMed] [Google Scholar]
- 173.Gibson KF, Santos AD, Lund N, et al. Genetics of cluster headache. Cephalalgia Int J Headache. 2019;39:1298–1312. doi: 10.1177/0333102418815503. [DOI] [PubMed] [Google Scholar]
- 174.Bacchelli E, Cainazzo MM, Cameli C, et al. A genome-wide analysis in cluster headache points to neprilysin and PACAP receptor gene variants. J Headache Pain. 2016;17:114. doi: 10.1186/s10194-016-0705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ran C, Fourier C, Michalska JM, et al. Screening of genetic variants in ADCYAP1R1, MME and 14q21 in a Swedish cluster headache cohort. J Headache Pain. 2017;18:88. doi: 10.1186/s10194-017-0798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Harder AVE, Winsvold BS, Noordam R, et al. Genetic susceptibility loci in Genomewide association study of cluster headache. Ann Neurol. 2021;90:203–216. doi: 10.1002/ana.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O’Connor E, Fourier C, Ran C, et al. Genome-wide association study identifies risk loci for cluster headache. Ann Neurol. 2021;90:193–202. doi: 10.1002/ana.26150. [DOI] [PubMed] [Google Scholar]
- 178.Ling Y-H, Chen S-P, Fann CS-J, et al. TRPM8 genetic variant is associated with chronic migraine and allodynia. J Headache Pain. 2019;20:115. doi: 10.1186/s10194-019-1064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yakubova A, Davidyuk Y, Tohka J, et al. Searching for predictors of migraine Chronification: a pilot study of 1911A>G polymorphism of TRPV1 gene in episodic versus chronic migraine. J Mol Neurosci MN. 2021;71:618–624. doi: 10.1007/s12031-020-01683-9. [DOI] [PubMed] [Google Scholar]
- 180.Huang C, Chen S-P, Huang Y-H, et al. HLA class I alleles are associated with clinic-based migraine and increased risks of chronic migraine and medication overuse. Cephalalgia Int J Headache. 2020;40:493–502. doi: 10.1177/0333102420902228. [DOI] [PubMed] [Google Scholar]
- 181.Louter MA, Fernandez-Morales J, de Vries B, et al. Candidate-gene association study searching for genetic factors involved in migraine chronification. Cephalalgia Int J Headache. 2015;35:500–507. doi: 10.1177/0333102414547141. [DOI] [PubMed] [Google Scholar]
- 182.Chalmer MA, Rasmussen AH, Kogelman LJA, et al. Chronic migraine: genetics or environment? Eur J Neurol. 2021;28:1726–1736. doi: 10.1111/ene.14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Belyaeva II, Subbotina AG, Eremenko II, et al. Pharmacogenetics in primary headache disorders. Front Pharmacol. 2021;12:820214. doi: 10.3389/fphar.2021.820214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kogelman LJA, Esserlind A-L, Francke Christensen A, et al. Migraine polygenic risk score associates with efficacy of migraine-specific drugs. Neurol Genet. 2019;5:e364. doi: 10.1212/NXG.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Guo S, Esserlind A-L, Andersson Z, et al. Prevalence of migraine in persons with the 3243A>G mutation in mitochondrial DNA. Eur J Neurol. 2016;23:175–181. doi: 10.1111/ene.12832. [DOI] [PubMed] [Google Scholar]
- 186.Terrin A, Bello L, Valentino ML, et al. The relevance of migraine in the clinical spectrum of mitochondrial disorders. Sci Rep. 2022;12:4222. doi: 10.1038/s41598-022-08206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kraya T, Deschauer M, Joshi PR, et al. Prevalence of headache in patients with mitochondrial disease: a cross-sectional study. Headache. 2018;58:45–52. doi: 10.1111/head.13219. [DOI] [PubMed] [Google Scholar]
- 188.Burow P, Haselier M, Naegel S et al (2021) The mitochondrial biomarkers FGF-21 and GDF-15 in patients with episodic and chronic migraine. Cells 10. Epub ahead of print September 18. 10.3390/cells10092471 [DOI] [PMC free article] [PubMed]
- 189.Zaki EA, Freilinger T, Klopstock T, et al. Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia Int J Headache. 2009;29:719–728. doi: 10.1111/j.1468-2982.2008.01793.x. [DOI] [PubMed] [Google Scholar]
- 190.Finnilä S, Autere J, Lehtovirta M, et al. Increased risk of sensorineural hearing loss and migraine in patients with a rare mitochondrial DNA variant 4336A>G in tRNAGln. J Med Genet. 2001;38:400–405. doi: 10.1136/jmg.38.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Børte S, Zwart J-A, Skogholt AH, et al. Mitochondrial genome-wide association study of migraine - the HUNT study. Cephalalgia Int J Headache. 2020;40:625–634. doi: 10.1177/0333102420906835. [DOI] [PMC free article] [PubMed] [Google Scholar]