Abstract
Purpose
Denmark has a high consumption of prescribed opioids, and many citizens with chronic non-cancer pain (CNCP). Therefore, we aimed to characterize and assess epidemiological risk factors associated with long-term non-cancer opioid use among Danish citizens.
Patients and Methods
We conducted a longitudinal, retrospective, observational, register-based study using nationwide databases containing essential medical, healthcare, and socio-economic information. Statistical analysis, including backward stepwise logistic regression analysis, was used to explain long-term opioid use by individuals filling at least one prescription for an opioid product N02AA01–N02AX06 during 01/01/2004–31/12/2017, follow-up until the end of 2018.
Results
The analyzed cohort contained N=1,683,713 non-cancer opioid users, of which 979,666 were classified with CNCP diagnosis using ICD-10 codes. Long-term opioid use was predicted by a mean of 1,583.30 and a median of 300 oral morphine equivalent mg (OMEQ) per day during the first year, together with divorced, age group 40–53 years, retirement, receiving social welfare or unemployment ≥6 months. In addition, living in Northern Jutland, co-medications such as beta-blockers, anti-diabetics, anti-rheumatics, and minor surgery ≤90 days before inclusion. Protective variables were an education level of secondary school or higher, children living at home, household income of middle or highest tertile, opioid doses in either the 2nd or 3rd quartile OMEQ, male, the oldest age group, living in the Capital Region or Zealand, co-medication lipid-lowering, one comorbidity, heart failure, surgeries ≤90 days before the index: lips/teeth/jaw/mouth/throat, heart/vessels, elbow/forearm, hip/thigh, knee/lower leg/ankle/foot.
Conclusion
Long-term opioid users differ epidemiologically from those using opioids for a shorter period. The study findings are essential for future recommendations revision in Denmark and comparable countries.
Keywords: epidemiology, cohort, purchase of medication, risk factors, oral morphine equivalent milligrams, OMEQ
Plain Language Summary
This 15-year follow-up nationwide registry study on associations of long-term opioid use among non-cancer individuals contributes to the ongoing “opioid epidemic” discourse. Denmark is a smaller country with 5.8 million inhabitants, a population suitable for longitudinal observational investigation due to the national registry system comprising information on all citizens. Therefore, the study used data on purchasing prescribed medication, admissions, hospital diagnoses, socio-demographics, and economics to describe the characteristics of a cancer-free population of new opioid users during 2004–2017. As a result, risk factors predicting or protecting from prolonged opioid use are described for a population of 1,683,713 individuals aged 16–110, of which more than every other (979,666) had a chronic non-cancer pain diagnosis. The total cohort and the cohort of those with CNCP were analyzed, and we found that retirement or receiving social welfare highly predicted long-term opioid use applicable for both cohorts. In addition, receiving medical treatment with beta-blockers or anti-rheumatics predicted the risk of long-term opioid use for the total cohort. In contrast, treatment with anti-diabetics was a significant risk factor for both cohorts. Furthermore, being divorced, age 40–53 years, and having had minor surgery were found to have adverse effects in the analysis of the entire cohort. The two cohort analyses showed some differences, mainly according to protective factors. In addition, simultaneously, protective factors such as the highest educational level, having children living at home and being male were statistically highly significant. The study points toward important risk factors worth attention in clinical guidelines.
Introduction
Chronic non-cancer pain (CNCP)1 is a highly prevalent worldwide public health challenge; in Europe, it affects approximately 20% of all adults.2 It has been assessed that 1.3 million Danish citizens live with CNCP, and about 6–7000 new cases of CNCP are added yearly,3,4 representing a significant amount of a relatively small population of 5.8 million inhabitants.5 Notably, CNCP affects more than one-fifth of the Danish population. Additionally, Denmark is notable for the high consumption of prescribed opioids compared to other Nordic countries.6–9
Given the international discussion of opioid use for the long-term management of CNCP,8,10–15 the awareness of addictive behaviours, long-term use, premature death,16–18 and the impact on healthcare costs,3 the associations of prescribed opioid use among CNCP patients in Denmark still have not been fully elucidated. In particular, the demographics of long-term use can provide a meaningful context for understanding potential targeted areas to improve and develop the existing treatment and prevention of high-risk, long-term opioid use. Accordingly, we conducted a retrospective longitudinal population-based study using the Danish National Registries.19 These centralized, comprehensive nationwide databases containing essential medical, healthcare, and socio-economic information are ideal for analyzing patterns of opioid use and characteristics of opioid users.
We hypothesized that if people fill opioid prescriptions for an extended period above 6 months, they will differ in demographics, socio-economics, comorbid conditions, and co-medication from those filling opioid prescriptions for a shorter period. Potential confirmation of this hypothesis may lead to the alteration of strategies targeting the prevention of long-term opioid use, particularly among individuals with CNCP diagnoses.
The aim was to obtain epidemiological characteristics of Danish citizens’ non-cancer opioid use and secondarily aspects of opioid use among citizens with CNCP diagnoses and estimate possible risk factors of long-term opioid use.
Material and Methods
The National Registries
The current study is a national register-based cohort study with data access approved by Statistics Denmark (permit 705989)20 using microdata from the Danish National Registries,19,21 which holds information on the approximately 5.8 million citizens of Denmark.5
We had access to the prescribed purchase of medication, admissions, in- and outpatient diagnoses, socio-demographic and economic information (eg, income, education, family, housing, emigration), and information on death from the Danish National Patient Registry, the Danish National Prescription Registry, the Danish Civil Registration System, and the Danish Register of Causes of Death.
Data are linked by personal identification numbers providing the opportunity to collate information from the different databases on an individual level and without revealing the person’s identity to the researchers when using the Danish Civil Registration System.21–25
Population and Definitions
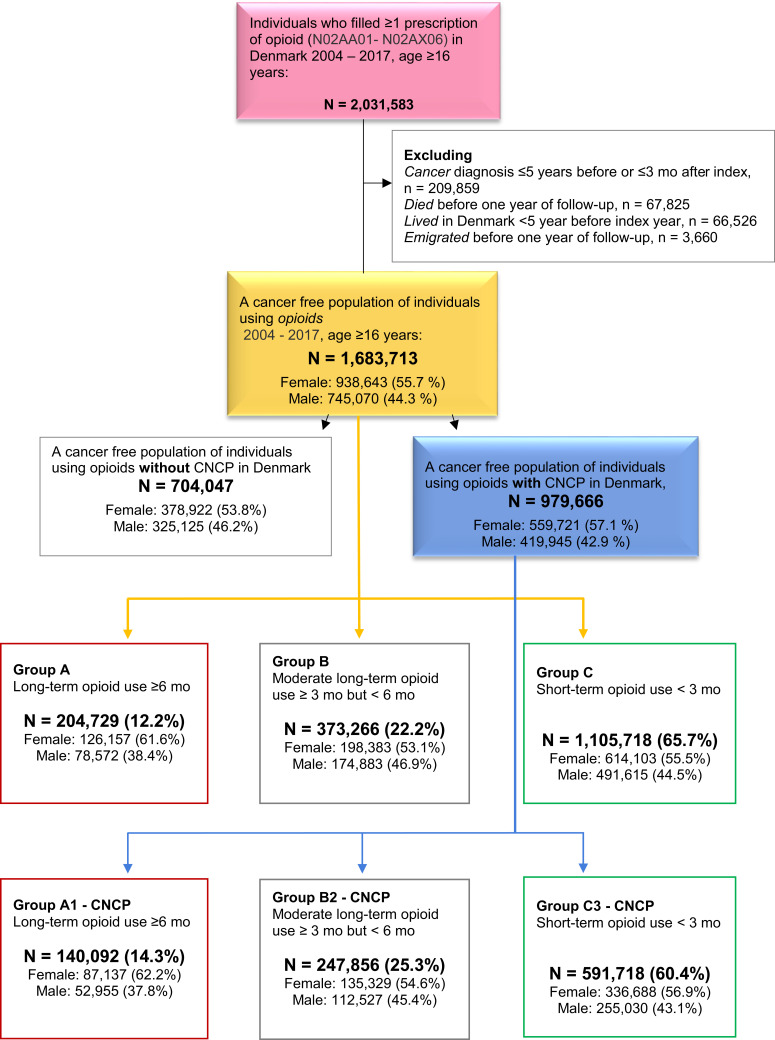
The cohort consists of new opioid users aged 16 years or older who redeemed at least one prescription for an opioid product in the period 01/01/2004-31/12/2017, leading to N = 2,031,583 (Figure 1); follow-up for a minimum of 1 year after the last filled opioid prescription or to December 2018. Treatment initiation is defined as no purchased coverage for an opioid product in the previous 90 days.26,27 Participants are included at the first filled prescription for an opioid product using the Anatomical Therapeutic Chemical Classification code (ATC) N02AA01–N02AX06. Any opioid products in any dispensed available form from the Danish pharmacies are included in the study (Table 1). Taxes finance the health care system in Denmark, and all citizens have free access to healthcare along with some drug costs reimbursement, providing for the National Prescription Registry’s full coverage of the population.
Figure 1.
A cancer-free population of opioid users and chronic non-cancer pain (CNCP).
Table 1.
Baseline Characteristics of Opioid Use and Pain-Intensive Diseases in Denmark, Age ≥16 in Denmark 2004–2017
| Total, N 1,683,713 | Male, N = 745,070 (44.3%) N (%Within Sex/Total) | Female, N = 938,643 (55.7%) N (%Within Sex/Total) | P ♂ / ♀a | |
|---|---|---|---|---|
| Age at inclusion (mean 53.64, median 53.00) | ||||
| 1st quartile (16–39 years) | 427,970 (25.4%) | 195,027 (26.2/11.6) | 232,943 (24.8/13.8) | 0.000 |
| 2nd quartile (40–53 years) | 416,917 (24.8%) | 199,895 (26.8/11.9) | 217,022 (23.1/12.9) | 0.000 |
| 3rd quartile (54–68 years) | 430,311 (25.6%) | 205,801 (27.6/12.2) | 224,510 (23.9/13.3) | 0.000 |
| 4th quartile (69–110 years) | 408,514 (24.3%) | 144,347 (19.4/8.6) | 264,167 (28.1/15.7) | 0.000 |
| ≤21 years | 57,595 (3.4%) | 23,591 (3.2/1.4) | 34,004 (3.6/2.0) | 0.000 |
| 80+ years | 160,310 (9.5%) | 45,124 (6.1/2.7) | 115,186 (12.3/6.8) | 0.000 |
| CNCP (yes) | 979,666 (58.2) | 419,945 (56.4/24.9) | 559,721 (59.6/33.2) | 0.000 |
| Pain-intensive diagnosis/CNCP | ||||
| Back/spine pain | 149,543 (8.9) | 71,831 (9.6/4.3) | 77,712 (8.4/4.6) | 0.000 |
| Headache | 22,837 (1.4) | 7,616 (1.0/0.5) | 15,221 (1.6/0.9) | 0.000 |
| Neuropathic pain | 48,561 (2.9) | 20,769 (2.8/1.2) | 27,792 (3.0/1.7) | 0.000 |
| Non-specific/other pain cond. | 85,935 (5.1) | 35,782 (4.8/2.1) | 50,153 (5.3/3.0) | 0.000 |
| Spondylopathies | 31,330 (1.9) | 14,769 (2.0/0.9) | 16,561 (1.8/1.0) | 0.000 |
| Osteoporosis | 29,865 (1.8) | 5,171 (0.7/0.3) | 24,694 (2.6/1.5) | 0.000 |
| Disorders of muscles | 22,639 (1.3) | 7,752 (1.0/0.5) | 14,887 (1.6/0.9) | 0.000 |
| Multimorbid (ulcer/skin) | 13,096 (0.8) | 6,508 (0.9/0.4) | 6,588 (0.7/0.4) | 0.000 |
| Fibromyalgia | 38,576 (2.0) | 13,602 (1.8/0.8) | 19,974 (2.1/1.2) | 0.000 |
| Complex regional pain syndrome | 525 (0.0) | 159 (0.0/0.0) | 366 (0.0/0.0) | 0.000 |
| Long-term opioid use: filled ≥1 prescription in ≥6 months | 204,729 (12.2) | 78,572 (10.5/4.7) | 126,157 (13.4/7.5) | 0.000 |
| Moderate long-term opioid use: filled ≥1 prescription in ≥3 but <6 months | 373,266 (22.2) | 174,883 (23.5/10.4) | 198,383 (21.1/11.8) | 0.000 |
| Short-term opioid use: filled ≥1 prescription in <3 separate months | 1,105,718 (65.7) | 491,615 (66.0/29.2) | 614,103 (65.4/36.5) | 0.000 |
| High potent opioids | 261,971 (15.6) | 123,394 (16.6/7.3) | 138,577 (14.8/8.2) | 0.000 |
| N02AA01 morphine | 70,319 (4.2) | 34,245 (4.6/2.0) | 36,074 (3.8/2.1) | 0.000 |
| Oral (tablet/capsule) | 34,328 | 18,106 | 16,222 | |
| Oral sustained-release (tablet/capsule) | 34,930 | 15,618 | 19,312 | |
| Oral (drops/sublingual tablet) | 51 | 22 | 29 | |
| Injection/infusion | 677 | 375 | 302 | |
| Suppositories | 333 | 124 | 209 | |
| N02AA03 hydromorphone | 65 (0.0) | 30 (0.0/0.0) | 35 (0.0/0.0) | 0.000 |
| Oral capsule hard | 14 | 8 | 6 | |
| Oral sustained-release (tablet/capsule) | 51 | 22 | 29 | |
| N02AA04 nicomorphine | 6373 (0.4) | 2748 (0.4/0.2) | 3625 (0.4/0.2) | 0.000 |
| Oral (tablet/capsule) | 4339 | 2077 | 2262 | |
| Suppositories | 1748 | 555 | 1193 | |
| Injection/infusion | 286 | 116 | 170 | |
| N02AA05 oxycodone | 79,222 (4.7) | 39,739 (5.3/2.4) | 39,483 (4.2/2.3) | 0.000 |
| Oral (tablet/capsule) | 3,556 | 1782 | 1774 | |
| Oral (drops/sublingual tablet) | 487 | 243 | 244 | |
| Oral sustained-release (tablet/capsule) | 33,545 | 17,129 | 16,416 | |
| Oral capsule hard | 41,620 | 20,580 | 21,040 | |
| Injection/infusion | 14 | 5 | 9 | |
| N02AA55 oxycodone/naloxone | 128 (0.0) | 59 (0.0/0.0) | 69 (0.0/0.0) | 0.000 |
| Oral sustained-release (tablet/capsule) | 128 | 59 | 69 | |
| N02AB02 pethidine | 10,295 (0.6) | 3184 (0.4/0.2) | 7111 (0.8/0.4) | 0.000 |
| Oral (tablet/capsule) | 3187 | 1028 | 2159 | |
| Oral (drops/sublingual tablet) | 1 | 0 | 1 | |
| Suppositories | 6524 | 1958 | 4568 | |
| Injection/infusion | 583 | 198 | 385 | |
| N02AB03 fentanyl | 3235 (0.2) | 1060 (0.1/0.1) | 2175 (0.2/0.1) | 0.000 |
| Oral (drops/sublingual tablet) | 5 | 2 | 2 | |
| Transdermal | 3230 | 1057 | 2173 | |
| N02AE01 buprenorphine | 21,985 (1.3) | 9120 (1.2/0.5) | 12,865 (1.4/0.8) | 0.000 |
| Oral (drops/sublingual tablet) | 14,540 | 7165 | 7375 | |
| Transdermal | 7433 | 1947 | 5486 | |
| Injection/infusion | 12 | 8 | 4 | |
| N02AG02 ketobemidone/antispasmodics | 66,987 (4.0) | 31,824 (4.3/1.9) | 35,163 (3.7/2.1) | 0.000 |
| Oral (tablet/capsule) | 55,749 | 27,589 | 28,160 | |
| Suppositories | 10,823 | 4078 | 6745 | |
| Injection/infusion | 415 | 157 | 258 | |
| N02AX06 tapentadol | 211 (0.0) | 91 (0.0/0.0) | 120 (0.0/0.0) | 0.000 |
| Oral (tablet/capsule) | 7 | 2 | 5 | |
| Oral sustained-release (tablet/capsule) | 204 | 89 | 115 | |
| N02AG02 ketogan | 2404 (0.1) | 965 (0.1/0.1) | 1439 (0.2/0.1) | |
| Oral sustained-release (tablet/capsule) | 2397 | 963 | 1434 | |
| Oral (drops/sublingual tablet) | 7 | 2 | 5 | |
| Low potent opioids - total | 1,124,283 (66.8) | 506,298 (60.0/30.1) | 617,985 (65.8/36.7) | 0.000 |
| N02AA79 codeine/psycholeptics | 297,459 (17.7) | 115,378 (15.5/6.9) | 182,081 (19.4/10.8) | |
| Oral (tablet/capsule) | 297,459 | 115,378 | 182,081 | |
| N02AX02 tramadol | 1,110,606 (66.0) | 502,282 (67.4/29.8) | 608,324 (64.8/36.1) | 0.000 |
| Oral (tablet/capsule) | 106,830 | 49,725 | 57,105 | |
| Oral sustained-release (tablet/capsule) | 68,766 | 31,367 | 37,399 | |
| Oral capsule hard | 903,653 | 408,574 | 495,079 | |
| Oral (drops/sublingual tablet) | 576 | 151 | 425 | |
| Effervescent tablet | 26,605 | 11,367 | 15,238 | |
| Suppositories | 4140 | 1083 | 3057 | |
| Injection/infusion | 36 | 15 | 21 | |
| N02AD01 pentazocine | 747 (0.0) | 329 (0.0/0.0) | 418 (0.0/ 0.0) | 0.909 |
| Oral (tablet/capsule) | 695 | 313 | 382 | |
| Suppositories | 43 | 11 | 32 | |
| Injection/infusion | 9 | 5 | 4 | |
| N02AC04 Dextropropoxyphene | 13,677 (0.8) | 4016 (0.5/0.2) | 9661 (1.0/ 0.6) | 0.000 |
| Oral (tablet/capsule) | 9451 | 2772 | 6679 | |
| Oral sustained-release (tablet/capsule) | 2099 | 670 | 1429 | |
| Oral capsule hard | 2127 | 574 | 1553 | |
| Charlson index (numbers of comorbidity) | ||||
| 0 (ref) | 1,430,788 (85.0) | 627,028 (84.2/37.2) | 803,760 (85.6/47.7) | 0.000 |
| 1 | 160,858 (9.6) | 72,553 (9.7/4.3) | 88,305 (9.4/5.2) | 0.000 |
| 2 | 54,431 (3.2) | 24,770 (3.3/1.5) | 29,661 (3.2/1.8) | 0.000 |
| 3+ | 37,636 (2.2) | 20,719 (2.8/1.2) | 16,917 (1.8/1.0) | 0.000 |
| The first month, n opioid prescriptions | ||||
| 1 | 1,220,931 (72.5) | 533,277 (71.6/31.7) | 687,654 (73.3/40.8) | 0.000 |
| 2–3 | 399,360 (23.7) | 181,741 (24.4/10.8) | 217,619 (23.2/12.9) | 0.000 |
| 4–9 | 62,682 (3.7) | 29,701 (4.0/1.8) | 32,981 (3.5/2.0) | 0.000 |
| 10–38 | 740 (0.0) | 351 (0.0/0.0) | 389 (0.0/0.0) | 0.000 |
| Three months, n opioid prescriptions | ||||
| 1 | 1,083,142 (64.3) | 479,584 (64.4/28.5) | 603,558 (64.3/35.8) | 0.371 |
| 2–3 | 410,154 (24.4) | 183,931 (24.7/10.9) | 226,222 (24.1/13.4) | 0.000 |
| 4–9 | 177,612 (10.5) | 75,695 (10.2/4.5) | 101,917 (10.9/6.1) | 0.000 |
| 10–118 | 12,805 (0.8) | 5859 (0.8/0.3) | 6946 (0.7/0.4) | 0.001 |
| Six months, n opioid prescriptions | ||||
| 1 | 1,083,142 (64.3) | 479,584 (64.4/28.5) | 603,558 (64.3/35.8) | 0.371 |
| 2–3 | 338,547 (20.1) | 157,812 (21.2/9.4) | 180,735 (19.3/10.7) | 0.000 |
| 4–9 | 213,683 (12.7) | 87,559 (11.8/5.2) | 126,124 (13.4/7.5) | 0.000 |
| 10–202 | 48,341 (2.9) | 20,115 (2.7/1.2) | 28,226 (13.0/1.7) | 0.000 |
| One year, n opioid prescriptions | ||||
| 1 | 1,083,142 (64.3) | 479,584 (64.4/28.5) | 603,558 (64.3/35.8) | 0.371 |
| 2–3 | 333,600 (19.8) | 156,024 (20.9/9.3) | 177,576 (18.9/10.5) | 0.000 |
| 4–9 | 156,787 (9.3) | 67,605 (9.1/4.0) | 89,182 (9.5/5.3) | 0.000 |
| 10–311 | 110,184 (6.5) | 41,857 (5.6/2.5) | 68,327 (7.3/4.1) | 0.000 |
| Opioid dose in mg OMEQ/day | ||||
| Up to 1 month (50–80,640) | 1,683,713 (100) | |||
| Mean (552.37) | ||||
| 1st quartile (50 < 200) | 810,364 (48.1) | 347,044 (46.6/20.6) | 463,320 (49.4/27.5) | 0.000 |
| 2nd quartile (200 < 211) | 42,782 (2.5) | 20,258 (2.7/1.2) | 22,524 (2.4/1.3) | 0.000 |
| 3rd quartile (211 < 700) | 422,939 (25.1) | 193,185 (25.9/11.5) | 229,754 (24.5/13.6) | 0.000 |
| 4th quartile (700–80,640) | 407,628 (24.2) | 184,583 (24.8/11.0) | 223,045 (23.8/13.2) | 0.000 |
| Up to 3 months (50–142,590) | ||||
| Mean (817.03) | ||||
| 1st quartile (50 < 200) | 752,746 (44.7) | 324,428 (43.5/19.3) | 428,318 (45.6/25.4) | 0.000 |
| 2nd quartile (200 < 300) | 82,075 (4.9) | 38,875 (4.9/2.2) | 45,200 (4.8/2.7) | 0.000 |
| 3rd quartile (300 < 1000) | 424,612 (25.2) | 196,906 (26.4/11.7) | 227,706 (24.3/13.5) | 0.000 |
| 4th quartile (1000–142,590) | 424,280 (25.2) | 186,861 (25.1/11.1) | 237,419 (25.3/14.1) | 0.000 |
| Up to 6 months (50–283,711) | ||||
| Mean (1,114.26) | ||||
| 1st quartile (50 < 200) | 751,641 (44.6) | 324,136 (43.5/19.3) | 427,505 (45.5/25.4) | 0.000 |
| 2nd quartile (200 < 300) | 78,751 (4.7) | 35,592 (4.8/2.1) | 43,159 (4.6/2.6) | 0.000 |
| 3rd quartile (300 < 1000) | 401,920 (23.9) | 188,192 (25.3/11.2) | 213,728 (22.8/12.7) | 0.000 |
| 4th quartile (1000–283,711) | 451,401 (26.8) | 197,150 (26.5/11.7) | 254,251 (27.1/15.1) | 0.000 |
| Up to 1 year (50–402,000) | ||||
| Mean (1,583.30) | ||||
| 1st quartile (50 < 200) | 751,595 (44.6) | 324,126 (43.5/19.3) | 427,469 (45.5/25.4) | 0.000 |
| 2nd quartile (200 < 300) | 78,536 (4.7) | 35,541 (4.8/2.1) | 42,995 (4.6/2.6) | 0.000 |
| 3rd quartile (300 < 1,000) | 396,370 (23.5) | 186,162 (25.0/11.1) | 210,208 (22.4/12.5) | 0.000 |
| 4th quartile (1,000–402,000) | 457,212 (27.2) | 199,241 (26.7/11.8) | 257,971 (27.5/15.3) | 0.000 |
| Comorbidity | ||||
| Arthritic diseases | 350,747 (20.8) | 148,588 (19.9/8.8) | 202,159 (21.5/12.0) | 0.000 |
| Diabetes | 50,988 (3.0) | 27,442 (3.7/1.6) | 23,546 (2.5/1.4) | 0.000 |
| Pulmonary disease | 67,110 (4.0) | 29,942 (4.0/1.8) | 37,168 (4.0/2.2) | 0.052 |
| Hemiplegia | 499 (0.0) | 244 (0.0/0.0) | 255 (0.0/0.0) | 0.037 |
| Dementia | 7524 (0.4) | 2859 (0.4/0.2) | 4665 (0.5/0.3) | 0.000 |
| Heart failure | 182,668 (10.8) | 91,461 (12.3/5.4) | 91,207 (9.7/5.4) | 0.000 |
| Fracture ≤90 days before the index | ||||
| Spine | 14,714 (0.9) | 7739 (1.0/0.5) | 6975 (0.7/0.4) | 0.000 |
| Hip | 39,803 (2.4) | 11,852 (1.6/0.7) | 27,951 (3.0/1.7) | 0.000 |
| Forearm | 42,295 (2.5) | 11,843 (1.6/0.7) | 30,452 (3.2/1.8) | 0.000 |
| Humerus | 28,444 (1.7) | 8312 (1.1/0.5) | 20,132 (2.1/1.2) | 0.000 |
| Any fracture | 244,930 (14.5) | 110,302 (14.8/6.6) | 134,628 (14.3/8.0) | 0.000 |
| Surgery ≤90 days before the index | ||||
| Skull/intracranial | 2161 (0.1) | 1036 (0.1/0.1) | 1125 (0.1/0.1) | 0.001 |
| Spinal cord/nerve root | 6444 (0.4) | 3495 (0.5/0.2) | 2949 (0.3/0.2) | 0.000 |
| Peripheral nerves | 2688 (0.2) | 1373 (0.2/0.1) | 1315 (0.1/0.1) | 0.000 |
| The autonomic nervous system | 83 (0.0) | 28 (0.0/0.0) | 55 (0.0/0.0) | 0.054 |
| Other or reoperation, nervous system | 119 (0.0) | 63 (0.0/0.0) | 56 (0.0/0.0) | 0.056 |
| Endocrine organs | 413 (0.0) | 85 (0.0/0.0) | 328 (0.0/0.0) | 0.000 |
| Ear, nose or larynx | 2770 (0.2) | 1638 (0.2/0.1) | 1132 (0.1/0.1) | 0.000 |
| Lips, teeth, jaw, mouth or throat | 10,450 (0.6) | 4662 (0.6/0.3) | 5788 (0.6/0.3) | 0.456 |
| Heart/large vessels in thorax | 10,204 (0.6) | 7619 (1.0/0.5) | 2585 (0.3/0.2) | 0.000 |
| Peripheral vessels/lymphatic | 6489 (0.4) | 3444 (0.5/0.2) | 3045 (0.3/0.2) | 0.000 |
| Respiratory system, thorax, mediastinum or diaphragma | 6696 (0.4) | 4593 (0.6/0.3) | 2103 (0.2/0.1) | 0.000 |
| Digestive organs or spleen | 26,541 (1.6) | 13,273 (1.8/0.8) | 13,268 (1.4/0.8) | 0.000 |
| Urin, male genitalia | 6226 (0.4) | 4635 (0.6/0.3) | 1,591 (0.2/0.1) | 0.000 |
| Female genitalia | 10,633 (0.6) | 8 (0.0/0.0) | 10,625 (1.1/0.6) | 0.000 |
| Obstetric surgery | 4015 (0.2) | 0 (0.0/0.0) | 4015 (0.4/0.2) | 0.000 |
| Minorb | 102,284 (6.1) | 50,209 (6.7/3.0) | 52,075 (5.5/3.1) | 0.000 |
| Back or neck | 5784 (0.3) | 3113 (0.4/0.2) | 2671 (0.3/0.2) | 0.000 |
| Shoulder or upper arm | 19,904 (1.2) | 10,886 (1.5/0.6) | 9018 (1.0/0.5) | 0.000 |
| Elbow or forearm | 14,862 (0.9) | 5543 (0.7/0.3) | 9319 (1.0/0.6) | 0.000 |
| Wrist or hand | 10,698 (0.6) | 6805 (0.9/0.4) | 3893 (0.4/0.2) | 0.000 |
| Pelvis | 2386 (0.1) | 1188 (0.2/0.1) | 1198 (0.1/0.1) | 0.000 |
| Hip or thigh | 47,369 (2.8) | 19,485 (2.6/1.2) | 27,884 (3.0/1.7) | 0.000 |
| Knees, lower legs, ankle or foot | 71,069 (4.2) | 34,805 (4.7/2.1) | 36,264 (3.9/2.2) | 0.000 |
| Number of drugs (co-medication) | ||||
| 0 (ref) | 303,349 (18.0) | 147,384 (19.8/8.8) | 155,965 (16.6/9.3) | 0.000 |
| 1–3 | 784,044 (46.6) | 355,688 (47.7/21.1) | 428,356 (45.6/25.4) | 0.000 |
| 4–9 | 490,410 (29.1) | 195,333 (26.2/11.6) | 295,077 (31.4/17.5) | 0.000 |
| 10+ | 105,910 (6.3) | 46,665 (6.3/2.8) | 59,245 (6.3/3.5) | 0.197 |
| Type of co-medication | ||||
| Anti-hypertension | 17,797 (1.1) | 10,382 (1.4/0.6) | 7415 (0.8/0.4) | 0.000 |
| Anti-coagulation AC | 241,512 (14.3) | 119,215 (16.0/7.1) | 122,297 (13.0/7.3) | 0.000 |
| ACE inhibitor | 181,395 (10.8) | 90,348 (12.1/5.4) | 91,047 (9.7/5.4) | 0.000 |
| Ischemic heart disease | 2995 (0.2) | 1148 (0.2/0.1) | 1847 (0.2/0.1) | 0.000 |
| Antiarrhythmics | 62,323 (3.7) | 31,720 (4.3/1.9) | 30,603 (3.3/1.8) | 0.000 |
| AT2 antagonists | 114,219 (6.8) | 49,106 (6.6/2.9) | 65,113 (6.9/3.9) | 0.000 |
| Beta-blockers | 299,854 (17.8) | 131,900 (17.7/7.8) | 167,954 (17.9/10.0) | 0.001 |
| Anti-diabetics | 82,869 (4.9) | 43,121 (5.8/2.6) | 39,748 (4.2/2.4) | 0.000 |
| Lipid-lowering | 181,294 (10.8) | 92,006 (12.3/5.5) | 89,288 (9.5/5.3) | 0.000 |
| Prednisolone | 155,653 (9.2) | 63,386 (8.5/3.8) | 92,267 (9.8/5.5) | 0.000 |
| Immunosuppressants | 18,609 (1.1) | 6598 (0.9/0.4) | 12,011 (1.3/0.7) | 0.000 |
| Anti-rheumatics | 804,663 (47.8) | 339,703 (45.6/20.2) | 464,960 (49.5/27.6) | 0.000 |
| Joint and muscular pain | 40,368 (2.4) | 13,555 (1.8/0.8) | 26,813 (2.9/1.6) | 0.000 |
| Anti-epileptics | 52,046 (3.1) | 22,784 (3.1/1.4) | 29,262 (3.1/1.7) | 0.027 |
| Parkinson medications | 19,618 (1.2) | 7,997 (1.1/0.5) | 11,621 (1.2/0.7) | 0.000 |
| Other antidepressants | 254,015 (15.1) | 86,218 (11.6/5.1) | 167,797 (17.9/10.0) | 0.000 |
| SSRI | 193,627 (11.5) | 64,047 (8.6/3.8) | 129,580 (13.8/7.7) | 0.000 |
| Region | ||||
| Capital | 466,088 (27.7) | 199,304 (26.7/11.8) | 266,784 (28.4/15.8) | 0.000 |
| Zealand | 261,371 (15.5) | 116,962 (15.7/6.9) | 144,409 (15.4/8.6) | 0.000 |
| Southern Denmark | 377,785 (22.4) | 168,787 (22.7/10.0) | 208,998 (22.3/12.4) | 0.000 |
| Central Jutland | 381,532 (22.7) | 171,923 (23.1/10.2) | 209,609 (22.3/12.4) | 0.000 |
| Northern Jutland | 195,579 (11.6) | 87,252 (11.7/5.2) | 108,327 (11.5/6.4) | 0.000 |
| Unknown | 1358 (0.1) | 842 (0.1/0.1) | 516 (0.1/0.0) | 0.000 |
| Education | ||||
| Primary school (7–10 y) | 571,220 (33.9) | 234,089 (31.4/13.9) | 337,131 (35.9/20.0) | 0.000 |
| Secondary school (11–12 y) | 707,700 (42.0) | 365,653 (49.1/21.7) | 342,047 (36.4/20.3) | 0.000 |
| Bachelor’s degree or higher (13+ y) | 289,764 (17.2) | 110,413 (14.8/6.6) | 179,351 (19.1/10.7) | 0.000 |
| Unknown total | 115,029 (6.8) | 34,915 (4.7/2.1) | 80,114 (8.5/4.8) | 0.000 |
| ≤21 years unknown | 312 (0.0) | 213 (0.0/0.0) | 99 (0.0/0.0) | 0.000 |
| Age 80+ unknown | 87,266 (5.2) | 20,526 (2.8/1.2) | 66,740 (7.1/4.0) | 0.000 |
| Marital status/living conditions | ||||
| Married | 869,639 (51.7) | 417,623 (56.1/24.8) | 452,016 (48.2/26.8) | 0.000 |
| Widowed | 200,696 (11.9) | 36,199 (4.9/2.1) | 164,497 (17.5/9.8) | 0.000 |
| Divorced | 209,765 (12.5) | 85,011 (11.4/5.0) | 124,754 (13.3/7.4) | 0.000 |
| Single (unmarried) | 402,255 (23.9) | 205,395 (27.6/12.2) | 196,860 (21.0/11.7) | 0.000 |
| Unknown marital status | 1358 (0.1) | 842 (0.1/0.1) | 516 (0.1/0.0) | 0.000 |
| Household income* | ||||
| Lowest tertile (≤199,999 Dkr) | 233,653 (13.9) | 89,963 (12.1/5.3) | 143,690 (15.3/8.5) | 0.000 |
| Middle tertile (≥200,000 but ≤400,000 Dkr) | 620,824 (36.9) | 278,321 (37.4/16.5) | 342,503 (36.5/20.3) | 0.000 |
| Highest tertile (>400,000 Dkr) | 819,331 (48.7) | 372,108 (49.9/22.1) | 447,223 (47.6/26.6) | 0.000 |
| Unknown total | 9905 (0.6) | 4678 (0.6/0.3) | 5227 (0.6/0.3) | 0.000 |
| ≤21 years/unknown | 588 (0.0) | 150 (0.0/0.0) | 438 (0.0/0.0) | 0.000 |
| Employment/income source | ||||
| Employed | 826,538 (49.1) | 419,403 (56.3/24.9) | 407,135 (43.4/24.2) | 0.000 |
| Retired | 645,146 (46.6) | 240,372 (40.2/17.4) | 404,774 (51.4/29.2) | 0.000 |
| Social welfare | 97,453 (5.8) | 36,959 (5.0/2.2) | 60,494 (6.4/3.6) | 0.000 |
| Other (eg, students or not registered) | 114,576 (6.8) | 48,336 (6.5/2.9) | 66,240 (7.1./3.9) | 0.000 |
| Unemployed ≥ 6 monthsc | 35,928 (2.1) | 17,113 (2.3/1.0) | 18,815 (2.0/1.1) | 0.000 |
| Living area/Municipality | ||||
| Capital area 1,500,000 | 318,717 (18.9) | 134,537 (18.1/8.0) | 184,180 (19.6/10.9) | 0.000 |
| Larger city ≥100,000 <1,500,000 | 154,227 (9.2) | 65,514 (8.8/3.9) | 88,713 (9.5/5.3) | 0.000 |
| City 20,000–99,999 | 317,065 (18.8) | 136,582 (18.3/8.1) | 180,483 (19./10.7) | 0.000 |
| Small city 1000–19,999 | 518,363 (30.8) | 224,423 (30.1/13.3) | 293,940 (31.3/17.5) | 0.000 |
| Countryside or a village ≤999 | 366,735 (21.8) | 179,737 (24.1/10.7) | 186,998 (19.9/11.1) | 0.000 |
| Unknown | 8606 (0.5) | 4277 (0.6/0.3) | 4329 (0.5/0.3) | 0.000 |
| Children living at home (<25 years) | ||||
| 0 | 1,147,217 (68.1) | 508,258 (68.2/30.2) | 638,959 (68.1/37.9) | 0.047 |
| 1 | 217,009 (12.9) | 94,330 (12.7/5.6) | 122,679 (13.1/7.3) | 0.000 |
| 2 | 223,757 (13.3) | 99,182 (13.3/5.9) | 124,575 (13.3/7.4) | 0.448 |
| 3+ | 94,341 (5.6) | 42,442 (5.7/2.5) | 51,899 (5.5./3.1) | 0.000 |
| Country of origin | ||||
| Denmark | 1,566,478 (93.1) | 691,922 (92.9/41.1) | 875,995 (93.3/52.1) | 0.000 |
| EU-28 | 29,451 (1.7) | 12,261 (1.6/0.7) | 17,190 (1.8/1.0) | 0.000 |
| Europe outside EU-28 | 20,546 (1.2) | 8506 (1.1/0.5) | 12,040 (1.3/0.7) | 0.000 |
| Turkey | 16,478 (1.0) | 7734 (1.0/0.5) | 8744 (0.9/0.5) | 0.000 |
| Africa | 8578 (0.5) | 4347 (0.6/0.3) | 4231 (0.4/0.3) | 0.000 |
| North America | 1951 (0.1) | 872 (0.1/0.1) | 1079 (0.1/0.1) | 0.000 |
| South and Central America | 1981 (0.1) | 641 (0.1/0.0) | 1340 (0.1/0.1) | 0.000 |
| Asia | 31,439 (1.9) | 16,104 (2.2/1.0) | 15,335 (1.6/0.9) | 0.000 |
| Oceania | 262 (0.0) | 139 (0.0/0.0) | 123 (0.0/0.0) | 0.000 |
| Pakistan | 4655 (0.3) | 2288 (0.3/0.1) | 2367 (0.3/0.1) | 0.000 |
| Stateless | 258 (0.0) | 146 (0.0/0.0) | 112 (0.0/0.0) | 0.000 |
| Unknown | 197 (0.0) | 110 (0.0/0.0) | 87 (0.0/0.0) | 0.000 |
| Generation of immigration | ||||
| Danish | 1,674,978 (99.5) | 740,678 (99.4/44.0) | 934,300 (99.5/55.5) | 0.000 |
| First generation | 7872 (0.5) | 4000 (0.5/0.2) | 3872 (0.4/0.2) | 0.000 |
| Second generation | 79 (0.0) | 36 (0.0/0.0) | 43 (0.0/0.0) | 0.000 |
| Third generation | 93 (0.0) | 49 (0.0/0.0) | 44 (0.0/0.0) | 0.000 |
| Fourth generation | 687 (0.0) | 304 (0.0/0.0) | 383 (0.0/0.0) | 0.000 |
| Unknown | 4 (0.0) | 3 (0.0/0.0) | 1 (0.0/0.0) | 0.000 |
Notes: aSex: ♂ male, ♀ female. bMinor: eye, breast, skin, minor surgical procedures, endoscopies, procedures during surgery, tissue withdrawals for transplantation. cUnemployed ≥6 months: a category extracted from the other income categories.
It has been found that high or low opioid doses may not predict opioid resumption within 1 year after an interdisciplinary pain rehabilitation program.28 Accordingly, we divided the opioid products into high- versus low-potency opioids, using the oral morphine equivalent mg (OMEQ) for analyses.29–31 Consumption of opioids can be measured in defined daily doses (DDDs) or mg OMEQ. Regarding pain treatment, DDD calculations of the number of opioids used may be ambiguous.8,31 Jarlbaek empathizes: “for example, 1 DDD of codeine is 100 mg codeine, and 1 DDD of morphine is 100 mg morphine. Morphine is around ten times as potent as codeine, implying that 10 DDDs codeine is considered equipotent to 1 DDD of morphine”.8 Specifically, this means that 1 DDD morphine equals 100 mg OMEQ. All opioid products in the current study have been converted to a consistent potency level and are thus reported using mg OMEQ. The Danish Clinical Guideline recommends a high degree of caution in using opioids for CNCP treatment with a maximum of 100 mg morphine equivalents per day (equals 100 mg OMEQ) when in combination with consultations with a specialist in the treatment of patients with chronic pain conditions.31
In the study, long-term opioid use ≥6 months, moderate opioid use ≥3 but <6 months, and short-term opioid use <3 months following the definitions suggested by the Danish Health and Medicines Authority and used by other researchers.8,29,32
ICD-10 specific pain-intensive diagnoses are used as the definition of CNCP3 (Table 1). The allocation to the CNCP group was based on a CNCP diagnosis ≤1 year before or ≤5 years after the index, thus linking the first filled opioid prescription to a CNCP diagnosis.
We excluded individuals with a cancer diagnosis ≤5 years before or ≤3 months after the first filled prescription for an opioid product in the period; a cancer diagnosis is specified as at least one of the ICD10 codes: C00-C11, C13-C15, C17, C20-C22, C24, C25, C30-C32, C34, C37-C41, C45-C49, C52, C55, C64-C66, C70-C72, C74-C83, C85, C88, C90, C92, D00-D02, D38, D42, D43, D46, D47, D90, Z51. The cancer-free cohort studied consists of 1,683,713 individuals living in Denmark from 1999 to 2018 (Figure 1).
The Definition of Continued Use
Gaps of more than 90 days (3 months) were considered non-continued use.26,27 Patients were allowed to change to other opioid drugs without impact on the continued use estimate as long as the gap in treatment did not exceed 90 days. Patients are included from their first treatment period and appear only once in the cohort. A sensitivity analysis was conducted, allowing a treatment gap of 120 days (4 months).
Demographic and Socio-Economic Data
In the analyses of the socio-economic data, we used data from the year before the first prescription (inclusion). Details on the family composition were available from 1/1/1999. A few exceptions involved the address/municipality, available from 1/1/2005, and the registered total family income from 1/1/2004. Consequently, for the included patients in 2004 and 2005, housing data for 2005 and family income for 2004 were used.
Age at inclusion was divided into quartiles. Further, the completed educational level was classified into three categories: primary school ≤10 years, secondary school >10 years, and bachelor’s degree or higher. Family income (total, annual) was divided into tertiles using blocks of 50,000 Danish kroner (€ 6723/$7071). In Denmark, citizens on sick leave or unemployed are granted sickness or unemployment benefits, which are government-funded initiatives of financial compensation for a period.34 When disabled but having some ability to work, the individual may apply for rehabilitation benefits related to supported employment. If unable to work, applying for a disability pension before a regular pension at 67 years is possible. Accordingly, we categorized employment status as employed (self-employed, co-working spouse, employee owner of a business, employee), retired (retired owner of a business, retired, voluntary early retirement), social welfare (employee with social welfare, social welfare), and unemployed ≥6 months. The categories from the registries are followed in all other demographic data.
Statistical analyses, including descriptive logistic regression analyses of participants, are allocated in the three pre-defined non-overlapping outcome groups described above.
Statistical Analyses
Baseline characteristics (Table 1) are described using descriptive statistics. We used multiple logistic regression analysis and chose exclusively (independent/explanatory) biological and demographic variables for models A and B. Thus, in model A, predictors directly driven by the hypothesis were prioritized and mutually adjusted: education level, children at home, marital status, municipality, household income, and opioid dose. In model B, sex and age were included. Model C (the final result) was mutually adjusted for all other significant or borderline significant predictors selected using stepwise analyses with a critical P < 0.20. Thus, the explanatory variables chosen for entry into model C were those of models A and B, plus a frugality subset of additional predictors selected by stepwise backward regression on the maximum model in the analyses of risk groups (the dependent variables). This backward stepwise logistic regression analysis was computed on the opioid users more broadly (Table 2) and as a secondary analysis, including the CNCP sample only (Table 3). The dependent variable in these analyses was long-term opioid use.
Table 2.
Analysis of Long-Term Opioid Use ≥6 Months (Group A) versus Moderate and Short-Term Use (Group B+C)
| Model A Socioeconomicsa HR, Mutually Adjusted | Model B Socioeconomics, Sex, and Ageb HR, Mutually Adjusted | Model C Socioeconomics, Sex, Age, and Major Comorbid Conditionsc HR, Mutually Adjusted | |
|---|---|---|---|
| Education | |||
| Primary school (ref) | 1 | 1 | 1 |
| Secondary school | 0.85 (0.81–0.89)*** | 0.86 (0.81–0.90)*** | 0.92 (0.87–0.97)** |
| Bachelor’s degree or higher | 0.73 (0.68–0.79)*** | 0.72 (0.67–0.78)*** | 0.80 (0.74–0.86)*** |
| Unknown | 1.10 (1.00–1.21)* | 1.12 (1.02–1.23)* | 1.07 (0.97–1.18) |
| Living conditions | |||
| Children at home (<25 years), yes | 0.92 (0.87–0.97)** | 0.86 (0.0.80–0.91)*** | 0.86 (0.82–0.92)*** |
| Marital status | |||
| Married | 1 | 1 | 1 |
| Widowed | 0.98 (0.90–1.06) | 0.95 (0.87–1.03) | 0.93 (0.86–1.01) |
| Divorced | 1.13 (1.06–1.21)*** | 1.10 (1.03–1.18)** | 1.07 (1.00–1.15)* |
| Single (unmarried) | 0.95 (0.90–1.01) | 0.95 (0.88–1.02) | 0.95 (0.89–1.02) |
| Unknown marital status | 0.11 (0.02–0.68)* | 0.11 (0.02–0.72)** | 0.11 (0.02–0.71)* |
| Living area/Municipality | |||
| Capital area 1,500,000 | 0.65 (0.47–0.89)** | 0.64 (0.47–0.88)** | 0.74 (0.53–1.02) |
| Larger city ≥100,000 <1,500,000 | 0.86 (0.62–1.19) | 0.85 (0.62–1.18) | 0.79 (0.57–1.09) |
| City 20,000–99,999 | 0.87 (0.64–1.20) | 0.87 (0.63–1.19) | 0.85 (0.61–1.16) |
| Small city 1000–19,999 | 0.82 (0.60–1.12) | 0.81 (0.59–1.12) | 0.80 (0.59–1.11) |
| Countryside or a village ≤999 | 0.85 (0.62–1.17) | 0.86 (0.62–1.17) | 0.88 (0.60–1.14) |
| Household income | |||
| Lowest sextile (≤150.000 Dkr) | 1.02 (0.88–1.19) | 1.02 (0.87–1.18) | 1.04 (0.89–1.22) |
| Lowest tertile (≤199.999 Dkr) (ref) | 1 | 1 | 1 |
| Middle tertile (≥200,000 but ≤400,000 Dkr) | 0.88 (0.82–0.94)*** | 0.88 (0.81–0.94)*** | 0.92 (0.85–0.98)* |
| Highest tertile (>400,000 Dkr) | 0.80 (0.75–0.86)*** | 0.80 (0.7–086)*** | 0.88 (0.81–0.95)** |
| Unknown | 1.06 (0.78–1.42) | 1.03 (0.77–1.39) | 1.05 (0.78–1.42) |
| Opioid dose in mg OMEQ/day | |||
| Up to 1 month (50–80,640), Mean (552.37) (ref) | 1 | 1 | 1 |
| Up to 3 months (50–142,590), Mean (817.03) | 0.97 (0.97–0.97)*** | 0.97 (0.97–0.97)*** | 0.97 (0.97–0.97)*** |
| Up to 6 months (50–283,711), Mean (1114.26) | 0.87 (0.83–0.92)*** | 0.88 (0. 83–0.92)*** | 0.88 (0.83–0.93)*** |
| Up to 1 year (50–402,000), Mean (1583.30) | 1.18 (1.12–1.24)*** | 1.18 (1.12–1.24)*** | 1.18 (1.11–1.24)*** |
| Sex | |||
| Male | 0.83 (0.80–0.87)*** | 0.86 (0.82–0.90)*** | |
| Age at inclusion | |||
| 1st quartile (16–39 years) ref. | 1 | 1 | |
| 2nd quartile (40–53 years) | 1.12 (1.04–1.20)** | 1.11 (1.03–1.19)** | |
| 3rd quartile (54–68 years) | 0.96 (0.88–1.04) | 0.93 (0.85–1.02) | |
| 4th quartile (69–110 years) | 0.95 (0.87–1.04) | 0.84 (0.75–0.95)** | |
| Employment/income source | |||
| Employed | 1.02 (0.90–1.16) | ||
| Retired | 1.46 (1.27–1.68)*** | ||
| Social welfare | 1.58 (1.37–1.82)*** | ||
| Unemployed ≥6 monthsa | 1.27 (1.05–1.53)* | ||
| Region of municipality | |||
| Capital | 0.79 (0.71–0.87)*** | ||
| Zealand | 0.88 (0.80–0.96)** | ||
| Southern Denmark | 0.95 (0.88–1.03) | ||
| Central Jutland | 0.96 (0.89–1.04) | ||
| Northern Jutland | 1.08 (1.01–1.16)* | ||
| Number of drugs (co-medication) | |||
| 0 (ref) | 1 | ||
| 1–3 | 0.94 (0.87–1.01) | ||
| 4–9 | 0.93 (0.84–1.03) | ||
| 10+ | 0.97 (0.82–1.15) | ||
| Type of co-medication | |||
| Anti-hypertension | 0.94 (0.75–1.18) | ||
| Anti-coagulation AC | 1.01 (0.93–1.10) | ||
| ACE inhibitor | 0.92 (0.85–1.01) | ||
| Ischemic heart disease | 0.66 (0.36–1.19) | ||
| Antiarrhythmics | 1.11 (0.98–1.27) | ||
| AT2 antagonists | 0.96 (0.87–1.06) | ||
| Beta-blockers | 1.08 (1.00–1.17)* | ||
| Anti-diabetics | 1.15 (1.1.00–1.32)* | ||
| Lipid-lowering | 0.80 (0.73–0.88)*** | ||
| Prednisolone | 1.01 (0.93–1.10) | ||
| Immunosuppressants | 0.79 (0.62–1.01) | ||
| Anti-rheumatics | 1.07 (1.01–1.13)* | ||
| Joint and muscular pain | 1.12 (0.98–1.29) | ||
| Anti-epileptics | 0.89 (0.78–1.02) | ||
| Parkinson medications | 0.99 (0.81–1.21) | ||
| Other antidepressants | 1.04 (0.92–1.17) | ||
| SSRI | 1.01 (0.89–1.15) | ||
| Charlson index (numbers of comorbidity) | |||
| 0 (ref) | 1 | ||
| 1 | 0.90 (0.81–0.99)* | ||
| 2 | 0.89 (0.78–1.03) | ||
| 3+ | 0.89 (0.73–1.08) | ||
| Comobidity | |||
| Diabetes | 1.01 (0.83–1.23) | ||
| Pulmonary disease | 1.00 (0.87–1.14) | ||
| Hemiplegia | 0.36 (0.06–2.15) | ||
| Dementia | 0.79 (0.53–1.18) | ||
| Heart failure | 0.91 (0.83–1.00)* | ||
| Fracture ≤90 days before index | |||
| Spine | 0.93 (0.72–1.19) | ||
| Hip | 0.92 (0.76–1.10) | ||
| Forearm | 0.97 (0.81–1.15) | ||
| Humerus | 1.00 (0.84–1.18) | ||
| Any fracture | 0.99 (0.91–1.07) | ||
| Surgery ≤90 days before index | |||
| Skull/intracranial | 0.62 (0.30–1.30) | ||
| Spinal cord/nerve root | 0.77 (0.52–1.16) | ||
| Peripheral nerves | 0.95 (0.53–1.72) | ||
| The autonomic nervous system | 0.45 (0.02–8.55) | ||
| Endocrine organs | 0.75 (0.14–4.05) | ||
| Ear, nose or larynx | 1.24 (0.72–2.13) | ||
| Lips, teeth, jaw, mouth or throat | 0.50 (0.32–0.76)** | ||
| Heart/large vessels in thorax | 0.72 (0.51–1.00)* | ||
| Peripheral vessels/lymphatic | 0.97 (0.68–1.39) | ||
| Resp. sys., thorax, mediastinum or diaphragma | 0.69 (0.47–1.03) | ||
| Digestive organs or spleen | 0.84 (0.69–1.02) | ||
| Urin, male genitalia | 1.07 (0.74–1.55) | ||
| Female genitalia | 0.83 (0.62–1.12) | ||
| Obstetric surgery | 0.60 (0.33–1.07) | ||
| Minorb | 1.12 (1.02–1.23)* | ||
| Back or neck | 1.08 (0.72–1.61) | ||
| Shoulder or upper arm | 0.84 (0.67–1.05) | ||
| Elbow or forearm | 0.67 (0.50–091)** | ||
| Wrist or hand | 0.90 (0.66–1.22) | ||
| Pelvis | 1.02 (0.57–185) | ||
| Hip or thigh | 0.77 (0.65–0.90)** | ||
| Knees, lower legs, ankle or foot | 0.87 (0.77–0.97)* |
Notes: aUnemployed ≥ 6 months: a category extracted from the other income categories. bMinor: eye, breast, skin, minor surgical procedures, endoscopies, procedures during surgery, tissue withdrawals for transplantation. cMajor comorbid conditions: diabetes, pulmonary disease, hemiplegia, dementia, heart failure. *p<0.05, **p<0.01, ***p<0.001.
Abbreviation: HR, hazard ratio.
Table 3.
Analysis of CNCP Individuals and Predictors of Long-Term Opioid Use ≥6 Months (Group A1) versus Moderate to Short-Term Opioid Use (Group B1+C1)
| Model A Socioeconomicsa HR, Mutually Adjusted | Model B Socioeconomics, Sex, and Ageb HR, Mutually Adjusted | Model C Socioeconomics, Sex, Age, and Major Comorbid Conditionsc HR, Mutually Adjusted | |
|---|---|---|---|
| Education | |||
| Primary school (ref) | 1 | 1 | 1 |
| Secondary school | 0.90 (0.85–0.96)** | 0.91 (0.86–0.97)** | 0.95 (0.90–1.02) |
| Bachelor’s degree or higher | 0.79 (0.73–87)*** | 0.78 (0.72–85)*** | 0.85 (0.78–0.93)*** |
| Unknown | 1.09 (0.97–1.21) | 1.11 (0.99–1.24) | 1.08 (0.96–1.21) |
| Living conditions | |||
| Children at home (<25 years), yes | 0.92 (0.86–0.98)* | 0.85 (0.78–91)*** | 0.84 (0.78–0.91)*** |
| Marital status | |||
| Married | 1 | 1 | 1 |
| Widowed | 0.93 (0.85–1.02) | 0.92 (0.84–1.01) | 0.92 (0.84–1.01) |
| Divorced | 1.09 (1.00–1.18)* | 1.05 (0.97–1.14) | 1.04 (0.96–1.13) |
| Single (unmarried) | 0.95 (0.89–1.02) | 0.92 (0.85–1.00) | 0.95 (0.87–1.03) |
| Unknown marital status | 0.30 (0.04–2.27) | 0.33 (0.04–2.50) | 0.33 (0.04–2.53) |
| Living area/Municipality | |||
| Capital area 1,500,000 | 0.74 (0.49–1.11) | 0.74 (0.49–1.10) | 0.84 (0.56–1.26) |
| Larger city ≥100,000 <1,500,000 | 0.98 (0.65–1.48) | 0.98 (0.65–1.47) | 0.90 (0.60–1.36) |
| City 20,000–99,999 | 1.01 (0.67–1.51) | 1.00 (0.67–1.50) | 0.96 (0.64–1.45) |
| Small city 1000–19,999 | 0.94 (0.63–1.40) | 0.94 (0.62–1.40) | 0.91 (0.61–1.37) |
| Countryside or a village ≤999 | 0.99 (0.66–1.48) | 0.99 (0.66–1.49) | 0.95 (0.64–1.43) |
| Household income | |||
| Lowest sextile (≤150.000 Dkr) | 1.11 (0.93–1.33) | 1.09 (0.91–1.31) | 1.11 (0.92–1.34) |
| Lowest tertile (≤199.999 Dkr) (ref) | 1 | 1 | 1 |
| Middle tertile (≥200,000 but ≤400,000 Dkr) | 0.91 (0.83–0.99)* | 0.90 (0.83–0.98)* | 0.93 (0.85–1.01) |
| Highest tertile (>400,000 Dkr) | 0.85 (0.78–0.93)*** | 0.84 (0.77–0.92)*** | 0.90 (0.83–0.99)* |
| Unknown | 1.18 (0.84–1.67) | 1.14 (0.80–1.60) | 1.14 (0.81–1.61) |
| Opioid dose in mg OMEQ/day | |||
| 0–1 month (50–80,640), Mean (552.37) | 1 | 1 | 1 |
| 0–3 months (50–142,590), Mean (817.03) | 0.97 (0.97–0.97)*** | 0.97 (0.97–0.97)*** | 0.97 (0.97–0.97)*** |
| 0–6 months (50–283,711), Mean (1114.26) | 0.88 (0.82–0.94)*** | 0.88 (0.83–94)*** | 0.88 (0.83–0.94)*** |
| 0–12 months (50–402,000), Mean (1583.30) | 1.17 (1.10–1.25)*** | 1.17 (1.09–1.25)*** | 1.17 (1.09–1.24)*** |
| Sex | |||
| Male | 0.85 (0.80–0.90)*** | 0.87 (0.83–93)*** | |
| Age at inclusion, CNCP | |||
| 1st quartile (16–41 years) (ref) | 1 | 1 | |
| 2nd quartile (42–50 years) | 1.08 (0.99–1.18) | 1.09 (1.00–1.18) | |
| 3rd quartile (51–75 years) | 0.91 (0.82–1.01) | 0.92 (0.83–1.02) | |
| 4th quartile (76–110 years) | 0.90 (0.80–1.00)* | 0.85 (0.74–0.97)* | |
| Employment/Income source | |||
| Employed | 1.03 (0.88–1.19) | ||
| Retired | 1.33 (1.1.13–1.57)** | ||
| Social welfare | 1.44 (1.22–1.71)*** | ||
| Unemployed ≥ 6 monthsa | 1.20 (0.96–1.51) | ||
| Region of municipality | |||
| Capital | 0.79 (0.70–0.90)*** | ||
| Zealand | 0.92 (0.83–1.03) | ||
| Southern Denmark | 0.97 (0.88–1.06) | ||
| Central Jutland | 0.97 (0.88–1.07) | ||
| Northern Jutland | 1.06 (0.97–1.15) | ||
| Number of drugs (co-medication) | |||
| 0 (ref) | 1 | ||
| 1–3 | 0.91 (0.83–1.00)* | ||
| 4–9 | 0.87 (0.77–0.98)* | ||
| 10+ | 0.93 (0.77–1.13) | ||
| Type of co-medication | |||
| Anti-hypertension | 0.87 (0.66–1.13) | ||
| Anti-coagulation AC | 1.06 (0.96–1.16) | ||
| ACE inhibitor | 0.94 (0.85–1.03) | ||
| Ischemic heart disease | 0.59 (0.29–1.20) | ||
| Antiarrhythmics | 1.10 (0.95–1.27) | ||
| AT2 antagonists | 1.01 (0.90–1.13) | ||
| Beta-blockers | 1.06 (0.97–1.15) | ||
| Anti-diabetics | 1.20 (1.03–1.41)* | ||
| Lipid-lowering | 0.84 (0.76–93)** | ||
| Prednisolone | 1.01 (0.92–1.10) | ||
| Immunosuppressants | 0.80 (0.62–1.04) | ||
| Anti-rheumatics | 1.07 (1.00–1.14) | ||
| Joint and muscular pain | 1.13 (0.97–1.32) | ||
| Anti-epileptics | 0.91 (0.78–1.06) | ||
| Parkinson medications | 1.00 (0.80–1.26) | ||
| Other antidepressants | 1.06 (0.92–1.21) | ||
| SSRI | 0.98 (0.85–1.14) | ||
| Charlson index (numbers of comorbidity) | |||
| 0 (ref) | 1 | ||
| 1 | 0.87 (0.78–0.97)** | ||
| 2 | 0.92 (0.78–1.07) | ||
| 3+ | 0.90 (0.72–1.11) | ||
| Comobidity | |||
| Diabetes | 0.99 (0.85–1.15) | ||
| Pulmonary disease | 0.20 (0.02–2.09) | ||
| Hemiplegia | 0.77 (0.48–1.21) | ||
| Dementia | 0.92 (0.83–1.02) | ||
| Heart failure | 0.80 (0.19–3.30) | ||
| Fracture ≤90 days before the index | |||
| Spine | 0.95 (0.75–1.21) | ||
| Hip | 0.99 (0.83–1.18) | ||
| Forearm | 0.98 (0.83–1.16) | ||
| Humerus | 0.95 (0.81–1.12) | ||
| Any fracture | 0.88 (0.81–0.95)** | ||
| Surgery ≤90 days before the index | |||
| Skull/intracranial | 0.61 (0.26–1.42) | ||
| Spinal cord/nerve root | 0.75 (0.51–1.10) | ||
| Peripheral nerves | 0.88 (0.49–1.59) | ||
| Autonomic nervous system | 0.56 (0.04–8.86) | ||
| Endocrine organs | 1.13 (0.21–6.07) | ||
| Ear, nose or larynx | 1.32 (0.73–2.39) | ||
| Lips, teeth, jaw, mouth or throat | 0.64 (0.40–1.05) | ||
| Heart/large vessels in thorax | 0.88 (0.60–1.30) | ||
| Peripheral vessels/lymphatic | 0.94 (0.61–1.44) | ||
| Resp. sys., thorax, mediastinum or diaphragma | 0.91 (0.59–1.40) | ||
| Digestive organs or spleen | 1.00 (0.79–1.26) | ||
| Urin, male genitalia | 1.04 (0.66–1.65) | ||
| Female genitalia | 0.79 (0.55–1.14) | ||
| Obstetric surgery | 0.91 (0.46–1.80) | ||
| Minorb | 1.04 (0.94–1.16) | ||
| Back or neck | 0.99 (0.67–1.44) | ||
| Shoulder or upper arm | 0.77 (0.62–0.96)* | ||
| Elbow or forearm | 0.68 (0.51–0.90)** | ||
| Wrist or hand | 0.85 (0.63–1.16) | ||
| Pelvis | 1.06 (0.60–1.88) | ||
| Hip or thigh | 0.75 (0.64–0.87)*** | ||
| Knees, lower legs, ankle or foot | 0.82 (0.74–0.92)** |
Notes: aUnemployed ≥6 months: a category extracted from the other income categories. bMinor: eye, breast, skin, minor surgical procedures, endoscopies, procedures during surgery, tissue withdrawals for transplantation. cMajor comorbid conditions: diabetes, pulmonary disease, hemiplegia, dementia, heart failure. *p<0.05, **p<0.01, ***p<0.001.
Abbreviation: HR, hazard ratio.
The analyses addressed the associations with sex, age, fracture, surgery, co-medication, and comorbidity (ICD-10 codes from hospital contacts: in- or outpatient contacts) between 1/1/1977 and the date of the first prescription of opioids. Various other factors (such as the source of income, education, municipality, and demographics) are described.
Ethics
Data processing is performed via Statistics Denmark (permit 705989),20 and data are analyzed using a secure, encrypted connection, which secures the blinding of participants’ identities. According to Danish law, register-based research does not require ethics committee approval. However, the study was performed following the tenets of the Helsinki Declaration.35
Results
The cohort comprised 2,031,583 adult individuals (Figure 1). The final cancer-free population for analyses consisted of 1,683,713 individuals aged 16–110 years, 55.7% female, with a median age of 53.00 years; the exclusion of participants consisted of 209,859 individuals due to cancer diagnosis ≤5 years before or ≤3 months after the index, 67,825 died before 1 year of follow-up, 66,526 had lived in Denmark for less than 5 years before the index, and 3660 emigrated before 1 year of follow-up. The baseline characteristics of the study cohort are comprehensively described in Table 1. Briefly, 979,666 (58.2%) had a CNCP diagnosis, and 204,729 individuals were categorized as long-term opioid users (filled >1 prescription in ≥6 months) distributed among 13.4% of all females and 10.5% of all males, within the group of CNCP long-term opioid users accounted for 140,092 individuals (14.3% of the CNCP group). The three most common CNCP diagnoses were back/spine pain, non-specific/other pain conditions, and fibromyalgia.
Each individual filled several opioid prescriptions, especially during the first months of treatment.
A larger proportion of 85% of the cohort had no records of hospital-treated comorbidities at in- or outpatient clinics, although only 18% had no record of co-medication, and 35.4% had records of 4+ co-medications the year before the index. Notable co-medications prescribed the year before the index include anti-rheumatics for almost half the cohort, n = 804,663 (47.8%); beta-blockers for 17.8% and antidepressants for 26.6% of the cohort. A smaller sample of the cohort had undergone surgery ≤90 days before the index, mainly minor surgery accounting (6.1%), knees, lower legs, ankle or foot (4.2%), hip or thigh (2.8%), digestive organs or spleen (1.6%), shoulder or upper arm (1.2%). Further, 244,930 (14.5%) of the cohort had experienced a fracture ≤90 days before the index (Table 1).
The educational level was described by primary school education representing 33.9%, secondary school education at 42%, a bachelor’s degree or higher at 17.2%, and 6.8% with unknown educational levels, mainly youngsters ≤21 years and elderly 80+ years. Danish regions were represented in the study population by the Capital Region as the largest with 27.7%, and the smallest was the Region of Northern Jutland with 11.6%; Denmark was the country of origin, comprising 93.1% of the cohort, and 99.5% were registered with Danish generations of immigration.
Outcome Groups and Opioid Grouping
We pre-defined three non-overlapping risk groups (the dependent variables) based on purchased coverage for an opioid product (Figure 1): Group A, long-term opioid use: filled >1 prescription in ≥6 months, representing 13.4% of all females and 10.5% of all males. Individuals with CNCP accounted for 68.4% (n=140,092) of group A in the analysis named group A1. Group B, moderate long-term opioid use: filled ≥1 prescription in ≥3 but <6 months; 21.1% of all females and 23.5% of all males. CNCP comprised 66.4% (n=247,856) of the group B individuals in the analysis named group B1. Group C, Short-term opioid use: filled ≥1 prescription in <3 months. Group C was the largest group, and CNCP n=591,718 accounted for 53.5% of the group C individuals in the analysis named group C1.
Accordingly, the cancer-free cohort comprised a majority of individuals with CNCP.
For analyses, we grouped opioid use into tertiles based on filled opioid dose in mg OMEQ per day, accordingly, up to 1 month, up to 3 months, up to 6 months and up to 1 year. The content of the opioid groups is elaborated in Table 4.
Table 4.
Purchase of Opioids
| Dose in mg OMEQ Per Day | |||||
| Up to 1 Month | Up to 3 Months | Up to 6 Months | Up to 1 Year | ||
| Total cohort N=1,683,713 | Mg OMEQ intervals | 50–80,640 | 50–142,590 | 50–283,711 | 50–402,000 |
| Mean | 552.37 | 817.03 | 1114.26 | 1583.30 | |
| Median | 210.04 | 300.00 | 300.00 | 300.00 | |
| 1st quartile | 50<200 | 50<200 | 50<200 | 50<200 | |
| 2nd quartile | 200<211 | 200<300 | 200<300 | 200<300 | |
| 3rd quartile | 211<700 | 300<1000 | 300<1000.02 | 300<1000.02 | |
| 4th quartile | 700–80,640 | 1000–142,590 | 1000.02–283,710.38 | 1000.02–402,000 | |
| Highest daily dose | 80,640:30 days = 2688 | 142,590:91 days = 1567 | 283,710:182 days = 1559 | 402,000:365 days = 1101 | |
| Total cohort N=1,683,713 | Mg OMEQ | 50–80,640 | 50–142,590 | 50–283,711 | 50–402,000 |
| Mean | 552.37 | 817.03 | 1114.26 | 1583.30 | |
| Median | 210.04 | 300.00 | 300.00 | 300.00 | |
| 1st quartile | 50<200 | 50<200 | 50<200 | 50<200 | |
| 2nd quartile | 200<211 | 200<300 | 200<300 | 200<300 | |
| 3rd quartile | 211<700 | 300<1000 | 300<1000.02 | 300<1000.02 | |
| 4th quartile | 700–80,640 | 1000–142,590 | 1000.02–283,710.38 | 1000.02–402,000 | |
| Female n=938,643 (55.7%) | Up to 1 month | Up to 3 months | Up to 6 months | Up to 1 year | |
| Mean, OMEQ | 540.64 | 817.55 | 1138.42 | 1655.15 | |
| Median, OMEQ | 200.00 | 266.68 | 299.98 | 299.98 | |
| Male n=745,070 (44.3%) | Up to 1 month | Up to 3 months | Up to 6 months | Up to 1 year | |
| Mean, OMEQ | 567.14 | 816.36 | 1083.83 | 1492.77 | |
| Median, OMEQ | 250.00 | 333.33 | 333.33 | 333.33 | |
| OMEQ: oral morphine equivalents - 1 mg morphine equivalent to 1 mg OMEQ | |||||
| Prescriptions | |||||
| First Month | Number of Prescriptions | Total, N = 1,683,713 (%) | Male, n=745,070 | Female, n=938,643 | No. of Prescriptions Per Day |
| 1 | 1,220,931 (72.5) | 533,277 | 687,654 | 0.33–2.26 | |
| 2–3 | 399,360 (23.7) | 181,741 | 217,619 | ||
| 4–9 | 62,682 (3.7) | 29,701 | 32,981 | ||
| 10–38 | 740 (0.0) | 351 | 389 | ||
| Three months | 1 | 1,083,142 (64.3) | 479,584 | 603,558 | 0.33–1.31 |
| 2–3 | 410,154 (24.4) | 183,931 | 226,222 | ||
| 4–9 | 177,612 (10.5) | 75,695 | 101,917 | ||
| 10–118 | 12,805 (0.8) | 5859 | 6946 | ||
| Six months | 1 | 1,083,142 (64.3) | 479,584 | 603,558 | 0.33–1.11 |
| 2–3 | 338,547 (20.1) | 157,812 | 180,735 | ||
| 4–9 | 213,683 (12.7) | 87,559 | 126,124 | ||
| 10–202 | 48,341 (2.9) | 20,115 | 28,226 | ||
| One year | 1 | 1,083,142 (64.3) | 479,584 | 603,558 | 0.33–0.85 |
| 2–3 | 333,600 (19.8) | 156,024 | 177,576 | ||
| 4–9 | 156,787 (9.3) | 67,605 | 89,182 | ||
| 10–311 | 110,184 (6.5) | 41,857 | 68,327 | ||
Characteristics of Overall Long-Term Opioid Users
In the multiple logistic regression analysis of long-term opioid use among 1,683,713 cancer-free individuals, we compared (group A) with those who used opioids for less than 6 months (groups B + C); the results are presented in Table 2.
By the above definition, long-term opioid use was experienced by 12.2% of the cohort. Essential characteristics of individuals who continued opioid use for 6 months or longer were found to be associated with factors predicting increased risk, such as being divorced and having purchased prescriptions of opioid doses of a mean of 1583.30 OMEQ/day for up to 1 year. Additional characteristics included belong to the age group of 40 to 53 years, having an employment/income from retirement or receiving social welfare, and unemployment up to 6 months before the index. A further characteristic was living in the Northern Jutland region. Receiving co-medication treatment with beta-blockers, anti-diabetics, and anti-rheumatics; together with having undergone minor surgeries up to 90 days before the index was associated with an increased risk of long-term use.
Characteristics associated with decreased risk of long-term opioid use were having completed secondary school, or a bachelor’s degree or higher education, having children living at home, unknown marital status, and household income in the middle or highest tertile. Having purchased prescriptions for opioid doses of a mean of 817.03 for up to 3 months or 1114.26 OMEQ/day for up to 6 months was another characteristic of decreased risk. Likewise were male sex, belong to the oldest age group (69–110 years), and living in the regions of the Capital or Zealand. Still further decreased risk included receiving co-medication treatment with lipid-lowering medication; having one comorbidity, and the comorbidity heart failure. Also, predicting decreased risk of long-term opioid use were surgeries up to 90 days before the index for lips, teeth, jaw, mouth or throat, heart/large vessels in thorax, elbow or forearm, hip or thigh, knees/lower legs/ankle or foot.
Characteristics of CNCP Individuals with Repeated Opioid Use
Table 3 presents the multiple logistic regression results of long-term opioid use among 979,666 CNCP cancer-free individuals. We compared (group A1) long-term users with those who used opioids for less than 6 months (outcome groups B1 + C1).
Long-term opioid use was exhibited by 14.3% of the CNCP population accounting for 140,092 individuals (group A1). Characteristics associated with an increased risk of continued opioid use are predicted by a mean of 1583.30 and a median of 300 OMEQ per day for up to 1 year. Additionally, being retired or receiving social welfare and co-medication treatment with anti-diabetic medication the year before the index correlates with an increased risk for long-term opioid use.
We found the factors associated with decreased risk of long-term opioid use to be completed bachelor’s degree or higher educational levels, having children at home, and household income at the highest tertile. In addition, a prescribed filled opioid dose of a mean of 817.03 for up to 3 months, or 1114.26 OMEQ/day for up to 6 months was also associated with decreased risk of long-term opioid use. Other protective characteristics include being male, belonging to the oldest age group (76–110 years), and living in the Capital Region. Additionally, having either 1–3 or 4–9 co-medications or co-medication treatment with lipid-lowering and one comorbidity were all negatively associated with the risk of long-term opioid use. Finally, experiencing a fracture up to 90 days before the index and surgeries up to 90 days before the index (such as shoulder or upper arm, elbow or forearm, hip or thigh, and knees/lower legs/ankle or foot) were likewise negatively associated with the risk of long-term opioid use.
Additionally, an overview of differences between the risk factors for long-term opioid use for the non-cancer population in total (N=1,683,713) and CNCP individuals (n=979,666) is shown in Table 5.
Table 5.
Overview of Statistically Significant Predictors of Increased and Decreased Risk of Long-Term Opioid Use
| Opioid Users in Total (Table 2) | CNCP Opioid Users (Table 3) | |
|---|---|---|
| Increased risk factors | Marital status | |
| Divorced* | ||
| Opioid dose in mg OMEQ/day | Opioid dose in mg OMEQ/day | |
| Up to 1 year (50–402,000), Mean (1583.30)*** | Up to 1 year (50–402,000), Mean (1583.30)*** | |
| Age at inclusion | ||
| 2nd quartile (40–53 years)** | ||
| Employment/income source | Employment/income source | |
| Retired*** | Retired** | |
| Social welfare*** | Social welfare*** | |
| Unemployed ≥6 monthsa* | ||
| Region of municipality | ||
| Northern Jutland* | ||
| Type of co-medication | Type of co-medication | |
| Beta-blockers* | Anti-diabetics* | |
| Anti-diabetics* | ||
| Anti-rheumatics* | ||
| Surgery ≤90 days before the index | ||
| Minorb* | ||
| Decreased risk factors | Education | Education |
| Secondary school** | Bachelor’s degree or higher*** | |
| Bachelor’s degree or higher** | ||
| Living conditions | Living conditions | |
| Children at home (<25 years), yes*** | Children at home (<25 years), yes*** | |
| Marital status | ||
| Unknown marital status* | ||
| Household income | Household income | |
| Middle tertile (≥200,000 but ≤400,000 Dkr)* | Highest tertile (>400,000 Dkr)* | |
| Highest tertile (>400,000 Dkr)** | ||
| Opioid dose in mg OMEQ/day | Opioid dose in mg OMEQ/day | |
| Up to 3 months (50–142,590), Mean (817.03)*** | Up to 3 months (50–142,590), Mean (817.03)*** | |
| Up to 6 months (50–283,711), Mean (1114.26)*** | Up to 6 months (50–283,711), Mean (1114.26)*** | |
| Sex | Sex | |
| Male*** | Male*** | |
| Age at inclusion | Age at inclusion, CNCP | |
| 4th quartile (69–110 years)** | 4th quartile (76–110 years)* | |
| Region of municipality | Region of municipality | |
| Capital*** | Capital*** | |
| Zealand** | ||
| Number of drugs (co-medication) | ||
| 1–3* | ||
| 4–9* | ||
| Type of co-medication | Type of co-medication | |
| Lipid-lowering*** | Lipid-lowering** | |
| Charlson index (numbers of comorbidity) | Charlson index (numbers of comorbidity) | |
| 1* | 1** | |
| Comorbidity | ||
| Heart failure* | ||
| Fracture ≤90 days before the index | ||
| Any fracture** | ||
| Surgery ≤90 days before the index | Surgery ≤90 days before the index | |
| Lips, teeth, jaw, mouth or throat** | Shoulder or upper arm* | |
| Heart/large vessels in thorax* | ||
| Elbow or forearm** | Elbow or forearm** | |
| Hip or thigh** | Hip or thigh*** | |
| Knees, lower legs, ankle or foot* | Knees, lower legs, ankle or foot** |
Notes: aUnemployed ≥6 months: a category extracted from the other income categories. bMinor: eye, breast, skin, minor surgical procedures, endoscopies, procedures during surgery, tissue withdrawals for transplantation *p<0.05, **p<0.01, ***p<0.001.
Discussion
In this study, we report epidemiological characteristics concerning disease and treatment-related characteristics, including socio-economic and demographic factors predicting the risk of long-term opioid use in a cancer-free cohort (Table 2) and among individuals with CNCP (Table 3).
We identified some differences between the group of long-term opioid users and the group of individuals filling opioid prescriptions for less than 6 months. The findings are consistent with the study hypothesis that people using opioids for more than 6 months differ from those using opioids for a shorter period.
Our main objective was to investigate long-term opioid use among Danish citizens. Thus, opioid doses were evident, and we did find purchases of high doses of opioids. The mean opioid doses of the new users during the first year was 1583.30 OMEQ/day (Table 4); this looks pretty high, but we have to point out that over half of the cohort had a CNCP diagnosis (58.2%). Adapting to the therapeutic opioid dose and product sufficient for the individual may take time, often leading to testing different products, quantities, and combinations, thus not consuming all the purchased medication. Additionally, in a similar study from Finland, the researchers found a yearly mean of opioid purchase of 1940–2583 OMEQ and a median of 270–360 OMEQ during 2009–2017;43
in our study, we found the median of the first year of therapy to be 300 OMEQ. Another clear indication that CNCP patients experience alterations in finding the therapeutic level of opioids is the number of prescriptions filled (Table 4). Therefore, it is not unusual for a CNCP patient to have several prescriptions active simultaneously; one prescription typically supplies at least 2–4 weeks of usage or longer until the next consultation.
Compared to earlier studies on opioid use in Scandinavia, we find opioid use may have declined in Denmark. For example, in 2014, Denmark had an annual average purchase of 6361 OMEQ per user,8 which is significantly higher than our study’s mean doses of 1583.30 OMEQ. Noting that some differences in inclusion criteria may explain the significant difference, considerations are, among others, that only new users were included in our study, and we calculated only up to the first year of purchase for opioid products, and excluding individuals with cancer diagnosis. Additionally, a considerable increase in opioid use of 22.7% in Denmark was found when comparing the years 2004–2006 and 2014–2016.6 The Danish Health Authority has focused on reducing opioid consumption among CNCP patients.31
Nationwide patient databases comprising data on opioid use and demographic and socio-economic information are rare outside Nordic countries. Thus, comparing with other European countries is somewhat tricky and mainly based on statistical studies of drug sales. For example, a mixed-methods public health review and national database study in England found that 5.61 million CNCP patients filled a prescription for an opioid product, and 1.17 million were estimated to use opioids for a minimum of 1 year. For the 10 years, 2008–2018, opioid use was found to increase in England.44 Increased opioid use was likewise found in studies from Germany,45 the Netherlands,46 and France,47 using opioid prescription data linked with clinical information.
We used OMEQ for reporting the merged opioid dose for the individual. We have reported opioid use in OMEQ in four intervals during the first year of treatment and found this approach helpful in comparison with prior findings.8,9,30,43,48 Thus, we did not compute high and low potent opioid products in the logistic regression analyses of long-term opioid use, but extensive tramadol use is reported elsewhere.33
Denmark is a small country with some income, education, and access to health care inequalities. We demonstrated that opioid users living in Northern Jutland were at increased risk of long-term opioid use. The region of Northern Jutland has a history of a high level of opioid use, even though this has decreased in the latter years.27 Additionally, we found living in the Capital region was associated with a reduced risk in the analyses of the total population and for CNCP specifically, as was living in Zealand generally. Since we did control for age, education, income, and city size/countryside in our analyses, we could speculate that these findings may be caused by unequal access to healthcare and specialized treatment. On the other hand, Northern Jutland and Zealand regions both struggle with a shortage of physicians and long distances to specialized outpatient/hospital treatment, and thus should not differ; the current study cannot identify the causal explanation.
The fact that being retired or receiving social welfare predicts an increased risk of long-term opioid use may be a contradictory or self-reinforcing fact since, for instance, prolonged post-surgical pain or other CNCP diagnosis often lead to temporary or permanent loss of employment,3 thus the need for social benefits or retirement.
It is emphasized that concomitant medication of beta-blockers, anti-rheumatics, and antidiabetics correlates with an increased risk of long-term opioid use, which may be a picture of the prevailing multimorbid situation of opioid users, perhaps in particular regarding diabetes,36 worth noting in future opioid precaution guidelines.
Besides some well-known factors that statistically reduce the risk of long-term opioid use (education, income, male sex), we found it interesting that children living at home are a statistically highly significant (p<0.001) factor in reduced risk of long-term opioid use (Table 5). Children living at home up to age 25 were prevalent in 535,107 (31.8%) of the cohort (Table 1). This protective factor can be seen in the broader picture of the CNCP parents’ concerns about the long-term consequences and well-being of their children’s upbringing affected by the parents’ CNCP, which was found in a recent qualitative study.37 In addition, CNCP parents’ concerns are justified, as a growing body of epidemiological and clinical research has shown that parental CNCP is a solid link to explaining long-term pain and pain-related disability in childhood and adolescence.38–40 Therefore, we consider it essential that therapists increase focus on the CNCP patients who have children at home; besides children being a protective factor for long-term opioid use, attention should be drawn to whether other actions are necessary, such as specific support targeting the child or the parenthood.
Interestingly, we find a negative correlation between long-term opioid use and several orthopaedic surgeries: shoulder/upper arm; elbow/forearm; hip/thigh; and knees/lower legs/ankle/foot, together with other surgeries: lips/teeth/jaw/mouth/throat and heart/vessels. We find this a positive effect of the increased focus on tapering off opioid treatment post-operatively.31 Internationally, a broad overview is given in a systematic review comprising 35 studies.32 In contrast to our finding, the male sex to be a protective factor on long-term opioid use, Pagé et al (2020) found no consistent differences between sex, fracture, or heart failure. The researchers primarily found the risk of moderate and long-term post-surgical opioid use to be associated with household income (in correspondence with our study), pre-surgical use of tobacco, antidepressants, and opioids.32 To manage the risks of long-term post-surgical opioid therapy, some studies have focused on developing and implementing screening tools to prevent long-term opioid use after surgery.41,42
The current study is an example of one of the primary deficiencies faced in conducting register-based studies. As discussed, we have the information on the purchased opioids, but we do not know to what extent the opioids were used; as a well-known consequence, this may lead to a potential bias of overestimating opioid use, as mirrored in our study. In addition, the study provides no information concerning the treatment effect on pain reduction or adverse effects. In contrast, in the current study, the potential risk of underestimation is also present, mainly addressing co-medications, since we have no information on hospital and institutional delivered medication, nor medication purchased from abroad or on the Internet (illicit use). Moreover, information on comorbidities does not include diagnoses from general practitioners but relies instead solely on in- and outpatient hospital treatment, although information on all filled prescriptions does, to some extent, rectify this lack of knowledge. The comprehensive nationwide study addressing all citizens aged 16+ using opioids in the period 01/12/2004–31/12/2017 can be considered a considerable strength, as well, because of the ability to include population-based information on socio-economics, demographic and health. The results are deemed applicable to other Western countries, particularly Nordic ones.
Conclusion
The study showed widespread use of opioids, indicating a continued need for increased attention generally, especially for CNCP, with a specific focus on individuals with diabetes and treatment with high opioid doses. Health professionals should also draw attention to parents using opioids. The study also identifies inequality among opioid users in different regions of Denmark. These findings comprise recommendations for consideration in future clinical guideline updates.
Acknowledgments
The first author received generous funding from The Novo Nordisk Foundation, an independent organization without any involvement in the study. Research grant no. NNF16OC0023012. This funding body had no role in decisions of the study design, analysis, and interpretation of the data, nor the writing of the manuscript.
Disclosure
Prof. Dr Bo Abrahamsen reports grants, personal fees from UCB, grants from Novartis, personal fees from Amgen, grants from Kyowa-Kirin, grants, personal fees from Pharmacosmos, outside the submitted work. The authors report no conflicts of interest in this work.
References
- 1.Treede R-D, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. 2019;160(1):19–27. doi: 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 2.Breivik H, Eisenberg E, O’Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13(1):1229. doi: 10.1186/1471-2458-13-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen JB, Gustavsson L. Socio-economic consequences of pain intensive diseases in Denmark; 2011. Available from: https://pure.vive.dk/ws/files/2050946/dsi-3112.pdf. Accessed February 8, 2023.
- 4.Foreningen af kroniske smertepatienter [The association of chronic pain patients]; 2017. Available from: https://faks.dk/. Accessed February 8, 2023.
- 5.Statistics Denmark. Danmark i tal 2019 [Denmark in numbers 2019]; 2020. Available from: https://www.dst.dk/Site/Dst/Udgivelser/GetPubFile.aspx?id=28921&sid=dkital2019. Accessed September 12, 2020.
- 6.Bosetti C, Santucci C, Radrezza S, Erthal J, Berterame S, Corli O. Trends in the consumption of opioids for the treatment of severe pain in Europe, 1990–2016. Eur J Pain. 2019;23(4):697–707. doi: 10.1002/ejp.1337 [DOI] [PubMed] [Google Scholar]
- 7.Hamunen K, Paakkari P, Kalso E. Trends in opioid consumption in the Nordic countries 2002–2006. Eur J Pain. 2009;13(9):954–962. doi: 10.1016/j.ejpain.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 8.Jarlbaek L. Opioid prescribing habits differ between Denmark, Sweden and Norway – and they change over time. Scand J Pain. 2019;19(3):491–499. doi: 10.1515/sjpain-2018-0342 [DOI] [PubMed] [Google Scholar]
- 9.Muller AE, Clausen T, Sjøgren P, Odsbu I, Skurtveit S. Prescribed opioid analgesic use developments in three Nordic countries, 2006–2017. Scand J Pain. 2019;19(2):345–353. doi: 10.1515/sjpain-2018-0307 [DOI] [PubMed] [Google Scholar]
- 10.Breivik H. Opioids in chronic non-cancer pain, indications and controversies. Eur J Pain. 2005;9(2):127–130. doi: 10.1016/j.ejpain.2004.05.013 [DOI] [PubMed] [Google Scholar]
- 11.Busse JW, Mahmood H, Maqbool B, et al. Characteristics of patients receiving long-term opioid therapy for chronic noncancer pain: a cross-sectional survey of patients attending the pain management centre at Hamilton General Hospital, Hamilton, Ontario. CMAJ Open. 2015;3(3):E324–30. doi: 10.9778/cmajo.20140126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamunen K. Opioids in chronic pain – primum non nocere. Scand J Pain. 2017;17(1):152–153. doi: 10.1016/j.sjpain.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 13.Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;2010(1):Cd006605. doi: 10.1002/14651858.CD006605.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breivik H. Much needed information to the general public about risks of opioid use disorder in patients appropriately prescribed long-term opioids for chronic non-cancer pain. Eur J Pain. 2022;26(3):555–556. doi: 10.1002/ejp.1908 [DOI] [PubMed] [Google Scholar]
- 15.Breivik H, Stubhaug A. Burden of disease is often aggravated by opioid treatment of chronic pain patients: etiology and prevention. Pain. 2014;155(12):2441–2443. doi: 10.1016/j.pain.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 16.Hah JM, Sturgeon JA, Zocca J, Sharifzadeh Y, Mackey SC. Factors associated with prescription opioid misuse in a cross-sectional cohort of patients with chronic non-cancer pain. J Pain Res. 2017;10:979–987. doi: 10.2147/jpr.S131979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse: part 1. Pain Physician. 2017;2(20;2):S93–s109. doi: 10.36076/ppj.2017.s109 [DOI] [PubMed] [Google Scholar]
- 18.Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20(2s):S111–s133. [PubMed] [Google Scholar]
- 19.Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7_suppl):12–16. doi: 10.1177/1403494811399956 [DOI] [PubMed] [Google Scholar]
- 20.StatBank Denmark. Statistics-Denmark. Available from: http://www.statistikbanken.dk/statbank5a/default.asp?w=1280. Accessed February 8, 2023.
- 21.Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7_suppl):38–41. doi: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 22.Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39(7_suppl):26–29. doi: 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 23.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient Register. Scand J Public Health. 2011;39(7_suppl):30–33. doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 24.Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7_suppl):22–25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 25.Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish civil registration system. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 26.Andrews JS, Wu N, Chen S-Y, Yu YU, Peng X, Novick D. Real-world treatment patterns and opioid use in chronic low back pain patients initiating duloxetine versus standard of care. J Pain Res. 2013;6:825–835. doi: 10.2147/jpr.S50323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.[The Danish Health Authority] Sundhedsstyrelsen. Kortlaegning af opioidforbruget i Danmark – med fokus paa patienter med kroniske non-maligne smerter [Mapping opioid consumption in Denmark – focusing on patients with chronic non-malignant pain]; 2016. Available from: https://www.sst.dk/da/Feeds/~/media/69913B827BAA4850A9A70E0CF76CB305.ashx. Accessed February 8, 2023.
- 28.Huffman KL, Rush TE, Fan Y, et al. Sustained improvements in pain, mood, function and opioid use post interdisciplinary pain rehabilitation in patients weaned from high and low dose chronic opioid therapy. Pain. 2017;158(7):1380–1394. doi: 10.1097/j.pain.0000000000000907 [DOI] [PubMed] [Google Scholar]
- 29.Svendsen K, Borchgrevink P, Fredheim O, Hamunen K, Mellbye A, Dale O. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med. 2011;25(7):725–732. doi: 10.1177/0269216311398300 [DOI] [PubMed] [Google Scholar]
- 30.Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733–737. doi: 10.1002/pds.3945 [DOI] [PubMed] [Google Scholar]
- 31.[The Danish Health Authority] Sundhedsstyrelsen. Opioidbehandling af kroniske non-maligne smerter, National Klinisk Retningslinje [Opioid treatment of chronic non-malignant pain, National Clinical Guideline]. Quick guide in English, National clinical guideline on opioid treatment of chronic non-malignant pain; 2018. Available from: https://www.sst.dk/da/udgivelser/2018/nkr-opioidbehandling-af-kroniske-non-maligne-smerter. Accessed February 8, 2023.
- 32.Pagé MG, Kudrina I, Zomahoun HTV, et al. A systematic review of the relative frequency and risk factors for prolonged opioid prescription following surgery and trauma among adults. Ann Surg. 2020;271(5):845–854. doi: 10.1097/sla.0000000000003403 [DOI] [PubMed] [Google Scholar]
- 33.Hansen CA, Ernst MT, Stougaard M, Abrahamsen B. Tramadol prescribed use in general and chronic noncancer pain: a nationwide register-based cohort study of all patients above 16 years. Scand J Pain. 2020;20(1):109–124. doi: 10.1515/sjpain-2019-0114 [DOI] [PubMed] [Google Scholar]
- 34.European Commission. Your social security rights in Denmark; 2022. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjfqqujsPv7AhXEYPEDHaBzBWoQFnoECAoQAQ&url=https%3A%2F%2Fec.europa.eu%2Fsocial%2FBlobServlet%3FdocId%3D13746%26langId%3Den&usg=AOvVaw0FlwTIYjxlTg-I43WD7fjE. Accessed February 8, 2023.
- 35.World-Medical-Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 36.Carstensen B, Rønn PF, Jørgensen ME. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996–2016. BMJ Open Diabetes Res Care. 2020;8(1):1. doi: 10.1136/bmjdrc-2019-001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roenne PF, Horn NS, Hansen CA. Involvement of relatives in chronic non-malignant pain rehabilitation at multidisciplinary pain centres: part one – the patient perspective. Scand J Pain. 2021;21(1):81–94. doi: 10.1515/sjpain-2019-0162 [DOI] [PubMed] [Google Scholar]
- 38.Stone AL, Wilson AC. Transmission of risk from parents with chronic pain to offspring: an integrative conceptual model. Pain. 2016;157(12):2628–2639. doi: 10.1097/j.pain.0000000000000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins KS, Birnie KA, Chambers CT, et al. Offspring of parents with chronic pain: a systematic review and meta-analysis of pain, health, psychological, and family outcomes. Pain. 2015;156(11):2256–2266. doi: 10.1097/j.pain.0000000000000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beveridge JK, Neville A, Wilson AC, Noel M. Intergenerational examination of pain and posttraumatic stress disorder symptoms among youth with chronic pain and their parents. PAIN Reports. 2018;3(Suppl 1):e667. doi: 10.1097/pr9.0000000000000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyles CC, Hevesi M, Trousdale ER, et al. The 2018 Chitranjan S. Ranawat, MD Award: developing and implementing a novel institutional guideline strategy reduced postoperative opioid prescribing after TKA and THA. Clin Orthop Relat Res. 2019;477(1):104–113. doi: 10.1007/s11999.0000000000000292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Fatemi P, Medress Z, et al. A predictive-modeling based screening tool for prolonged opioid use after surgical management of low back and lower extremity pain. Spine J. 2020;20(8):1184–1195. doi: 10.1016/j.spinee.2020.05.098 [DOI] [PubMed] [Google Scholar]
- 43.Keto J, Heiskanen T, Hamunen K, Kalliomäki M-L, Linna M. Opioid trends in Finland: a register-based nationwide follow-up study. Sci Rep. 2022;12(1):7261. doi: 10.1038/s41598-022-10788-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marsden J, White M, Annand F, et al. Medicines associated with dependence or withdrawal: a mixed-methods public health review and national database study in England. Lancet Psychiatry. 2019;6(11):935–950. doi: 10.1016/s2215-0366(19)30331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosner B, Neicun J, Yang JC, Roman-Urrestarazu A, Cheungpasitporn W. Opioid prescription patterns in Germany and the global opioid epidemic: systematic review of available evidence. PLoS One. 2019;14(8):e0221153. doi: 10.1371/journal.pone.0221153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalkman GA, Kramers C, van Dongen RT, van den Brink W, Schellekens A. Trends in use and misuse of opioids in the Netherlands: a retrospective, multi-source database study. Lancet Public Health. 2019;4(10):e498–e505. doi: 10.1016/s2468-2667(19)30128-8 [DOI] [PubMed] [Google Scholar]
- 47.Chenaf C, Kaboré J-L, Delorme J, et al. Prescription opioid analgesic use in France: trends and impact on morbidity-mortality. Eur J Pain. 2019;23(1):124–134. doi: 10.1002/ejp.1291 [DOI] [PubMed] [Google Scholar]
- 48.Svendsen K, Fredheim OM, Romundstad P, Borchgrevink PC, Skurtveit S. Persistent opioid use and socio-economic factors: a population-based study in Norway. Acta Anaesthesiol Scand. 2014;58(4):437–445. doi: 10.1111/aas.12281 [DOI] [PubMed] [Google Scholar]