Abstract
Purpose:
To assess the fluoroquinolone resistance pattern and trends among bacterial isolates from ocular infections over a 16-year period and explore alternative antibiotics in fluoroquinolone-resistant strains.
Methods:
In this retrospective, longitudinal study, the microbiology laboratory records of patients with different ocular infections diagnosed at an eye institute in central India from 2005–2020 were reviewed to determine the pattern of fluoroquinolone (ciprofloxacin, ofloxacin, gatifloxacin, and moxifloxacin) resistance. Antibiotic susceptibility testing was done using the Kirby–Bauer disc diffusion method.
Results:
In 725 Gram-positive bacteria, the resistance of ciprofloxacin, ofloxacin, gatifloxacin, and moxifloxacin was 55.9% (95% confidence interval [CI]: 52.2 – 59.6), 42.7% (95% CI: 39.0 – 46.4), 47.6% (95% CI: 43.9 – 51.3), and 45.6% (95% CI: 41.7–49.5), respectively. In 266 Gram-negative bacteria, the resistance of ciprofloxacin, ofloxacin, gatifloxacin, and moxifloxacin was 57.9% (95% CI: 51.9 – 63.9), 56.0% (95% CI: 49.7 – 62.1), 59.9% (95% CI: 53.8 – 66.0), and 74.3% (95% CI: 68.3 – 80.2), respectively. A declining trend in resistance to ciprofloxacin (P < 0.001), ofloxacin (P < 0.001), and moxifloxacin (P < 0.001) was seen in Gram-positive bacteria, whereas a reduction in resistance to only moxifloxacin (P = 0.04) was seen in Gram-negative bacteria. In fluoroquinolone-resistant Gram-positive bacteria, cefuroxime exhibited the highest susceptibility, whereas in fluoroquinolone-resistant Gram-negative bacteria, colistin exhibited the highest susceptibility.
Conclusion:
Fluoroquinolone resistance was high among bacteria from ocular infections in central India, but a declining trend in resistance to some of the fluoroquinolones was observed in recent times. Cefuroxime and colistin emerged as alternatives in fluoroquinolone-resistant Gram-positive and Gram-negative bacterial infections, respectively.
Keywords: Antibiotic resistance, bacteria, endophthalmitis, fluoroquinolone, keratitis
Fluoroquinolones have become the most preferred class of antibiotics among ophthalmologists to treat and prevent ocular infections because of their broad antibacterial activity, good ocular penetration, and wide availability as commercial formulations.[1] However, the extensive use of fluoroquinolones has also resulted in widespread bacterial resistance. Within a decade of their introduction in ophthalmology, emerging resistance to ciprofloxacin by S. aureus and P. aeruginosa began to be reported in the ophthalmic literature from all parts of the world.[2,3,4,5]
The pattern of antibiotic resistance varies globally and can differ even within a country due to multiple factors such as local antibiotic policies and usage, patient characteristics, origin of the strains, and clinical settings.[6] Local antibiotic surveillance and susceptibility studies provide the clinician with useful information to choose effective antibiotics to combat and prevent infection. Likewise, the susceptibility pattern with fluoroquinolones may also vary over large geographic areas, and trends in resistance can either be stable or change with time. Whereas some studies have reported that fluoroquinolone resistance patterns have been stable for the last decade,[7,8,9,10,11] other studies[12,13,14] have reported increasing bacterial resistance to both second- and fourth-generation fluoroquinolones. The aim of this study was to analyze the longitudinal trends in antibiotic susceptibility patterns of ocular bacteria to fluoroquinolones in central India, from where no data has been previously published, and also identify alternative antibiotics in fluoroquinolone-resistant bacterial strains. The findings of this study will not only provide insight into the current status of the usefulness of different fluoroquinolones in ocular infections but also guide treatment choices in infections caused by fluoroquinolone-resistant bacteria.
Methods
This study was a retrospective review of the laboratory records of all consecutive cases of bacterial ocular infections that presented between January 2005 and December 2020 at a tertiary eye-care institute in central India. The institute’s ethics committee granted approval for the study. The study included only those patients from whom the bacteria were isolated and an antibiotic susceptibility report was available.
The samples were from bacterial conjunctivitis, microbial keratitis, endophthalmitis, eviscerated contents, lacrimal sac abscesses, and lid abscesses. All samples were carefully collected to prevent contamination. Sterile, cotton-tipped swabs were used to collect conjunctival swabs or lacrimal discharges and inoculated directly on 5% sheep blood agar. Corneal scrapings were obtained at the slit-lamp using a sterile disposable number 15 surgical blade on a Bard–Parker handle. Separate blades were used for preparing slides for direct microscopy for 10% potassium hydroxide mount, Gram’s and Giemsa stain, and for directly inoculating the scraping materials in various media. Undiluted vitreous samples were obtained by dry vitrectomy in patients with suspected endophthalmitis and sent to the laboratory in sealed, sterile disposable syringes. The various media used were 5% sheep blood agar, chocolate agar, brain-heart infusion, and Sabouraud dextrose agar. Eviscerated contents were directly placed on 5% sheep blood agar. All media were incubated at an appropriate temperature and atmospheric conditions.
A positive bacterial growth was considered significant if there were confluent colonies at the site of inoculation. A culture was termed negative if there was no bacterial growth within 7 days. Any growth outside the area of inoculation was considered a contaminant. The bacterial isolate was identified based on Gram staining properties and colony characteristics. In cases where the species of bacteria could not be identified, they were designated as Gram-positive cocci or bacilli or Gram-negative cocci or bacilli. Methicillin-resistant S. aureus (MRSA) was detected using disk diffusion testing with cefoxitin and oxacillin.
Antibiotic susceptibility testing was performed using the Kirby–Bauer disc diffusion method with various antibiotic discs (HiMedia Laboratories Limited, Mumbai, India). Each isolate was labeled sensitive, intermediate, or resistant to a particular antibiotic based on the zone of inhibition as interpreted by the zone size according to the manufacturer’s recommendations using resistance breakpoints according to the guidelines from the Clinical and Laboratory Standards Institute. For this study, an antibiotic was labeled resistant if the zone of inhibition was categorized as intermediate or resistant. The different classes of antibiotics used were aminoglycosides (amikacin, gentamicin, and tobramycin), cephalosporins (cefazolin, ceftazidime, ceftriaxone, cefuroxime, and cefoxitin), fluoroquinolones (ciprofloxacin, ofloxacin, gatifloxacin, and moxifloxacin), carbapenems (imipenem and meropenem), chloramphenicol, vancomycin, colistin, and piperacillin. Gatifloxacin testing in our laboratory was introduced in 2006, moxifloxacin was introduced in 2007, ceftriaxone was introduced in 2008, imipenem and colistin were introduced in 2011, piperacillin was introduced in 2012, meropenem was introduced in 2014, oxacillin and cefoxitin were introduced in 2014, and cefuroxime was introduced in 2017. Ceftazidime, piperacillin, and colistin were not included in the pane of antibiotics for susceptibility testing in Gram-positive bacteria, whereas vancomycin, cefazolin, and cefuroxime were not included in the panel for Gram-negative bacteria. Testing for cefazolin was discontinued in 2017 due to the non-availability of the parenteral preparation in our setting from which a topical ocular preparation was being prepared.
Statistical analysis
All data regarding the resistant isolates are given as the mean of the proportion with a 95% CI. Pearson’s Chi-square test was used to compare the susceptibility and resistance rates between the antibiotics. Pearson’s correlation was used to test the intra-group resistance patterns among the four fluoroquinolones. The trends in antibiotic resistance over time were evaluated using a Cochran-Armitage test for linear trends in proportion.[15] A binary logistic regression test was used with (a) ciprofloxacin (archetype of second-generation fluoroquinolone) as a dependent variable and ofloxacin, gatifloxacin, and moxifloxacin as independent variables and (b) moxifloxacin (archetype of fourth-generation fluoroquinolone) as the dependent variable and the other three fluoroquinolones as independent variables. The odds ratio (OR) and 95% CI were calculated. Statistical analysis was computed using the statistical software SPSS version 23.0 (Statistical Package for Social Sciences, IBM, Chicago, IL). A two-tailed P value less than 0.05 was considered statistically significant.
Results
Source of isolates
During the study period, 5915 clinical samples were received for microbiological culture, of which 1007 samples with bacterial growth and antibiotic susceptibility tests were included in this study. These samples were from 637 patients with microbial keratitis, 210 patients with endophthalmitis, 24 patients with conjunctivitis, and 138 patients with dacryocystitis, lacrimal sac and/or lid abscess, and eviscerated contents. In 16 samples, details of the species of the bacteria were missing. In the remaining 991 samples, Gram-positive bacteria were identified in 725 (73.2%) samples and Gram-negative bacteria were identified in 266 (26.8%) samples. The details of different types of bacteria are provided in Appendix 1. Of the 1007 samples [Appendix 2], ciprofloxacin was tested in 966 (95.9%), ofloxacin was tested in 974 (96.7%), gatifloxacin was tested in 952 (94.5%), and moxifloxacin was tested in 869 (86.3%).
Appendix 1.
Types of bacteria
| Type of bacteria | Number (percent) |
|---|---|
| Gram-positive bacteria | 725 (73.2) |
| Staphylococcus epidermidis | 98 (9.7) |
| Staphylococcus aureus | 147 (14.6) |
| Streptococcus pneumoniae | 156 (15.5) |
| Streptococcus pyogenes | 49 (4.9) |
| Bacillus spp | 28 (2.8) |
| Nocardia | 9 (0.9) |
| Corynebacteria diphtheriae | 6 (0.6) |
| Enterococci | 1 (0.1) |
| Unidentified Gram-positive bacteria | 231 (46.8) |
| Gram-negative bacteria | 266 (26.8) |
| Pseudomonas aeruginosa | 133 (13.2) |
| Gram-negative bacilli (oxidase negative) | 22 (2.2) |
| Moraxella | 4 (0.4) |
| Neisseria | 4 (0.4) |
| Proteus | 3 (0.3) |
| Klebsiella pneumoniae | 3 (0.3) |
| Unidentified Gram-negative bacteria | 97 (36.5) |
Appendix 2.
Frequency of different antibiotics used in antibiotic susceptibility testing from 2005-2020
| Antibiotic | Sensitive | Intermediate | Resistant | Total tested | Not tested | Grand total |
|---|---|---|---|---|---|---|
| Ciprofloxacin | 423 | 150 | 393 | 966 | 41 | 1007 |
| Ofloxacin | 524 | 125 | 325 | 974 | 33 | 1007 |
| Gatifloxacin | 469 | 117 | 366 | 952 | 55 | 1007 |
| Moxifloxacin | 414 | 126 | 329 | 869 | 138 | 1007 |
| Amikacin | 536 | 135 | 334 | 1005 | 2 | 1007 |
| Gentamicin | 520 | 121 | 339 | 980 | 27 | 1007 |
| Tobramycin | 512 | 160 | 316 | 988 | 19 | 1007 |
| Chloramphenicol | 666 | 82 | 240 | 988 | 19 | 1007 |
| Vancomycin | 412 | 106 | 406 | 924 | 83 | 1007 |
| Cefazolin | 400 | 72 | 292 | 764 | 243 | 1007 |
| Cefuroxime | 165 | 5 | 50 | 220 | 787 | 1007 |
| Ceftazidime | 357 | 116 | 510 | 983 | 24 | 1007 |
| Ceftriaxone | 443 | 82 | 221 | 746 | 261 | 1007 |
| Imipenem | 360 | 16 | 56 | 432 | 575 | 1007 |
| Meropenem | 241 | 26 | 62 | 329 | 678 | 1007 |
| Colistin | 270 | 8 | 88 | 366 | 641 | 1007 |
| Piperacillin | 295 | 50 | 226 | 571 | 436 | 1007 |
Cumulative resistance of fluoroquinolones
Of the total bacteria [Table 1], 56.4% (95% CI: 53.3 – 59.6) isolates were resistant to ciprofloxacin, 52.5% (95% CI: 49.1 – 55.9) isolates were resistant to moxifloxacin, 50.9% (95% CI: 47.6 – 54.1) isolates were resistant to gatifloxacin, and 46.2% (95% CI: 43.1 – 49.4) were resistant to ofloxacin (P = 0.001). Ciprofloxacin resistance was highest (P < 0.001) in Gram-positive bacteria (55.9%, 95% CI: 52.2 – 59.6) and moxifloxacin resistance was highest (P = 0.002) in Gram-negative bacteria (74.3%, 95% CI: 68.3 – 80.2).
Table 1.
Pattern of resistance of important ocular pathogenic bacteria to fluoroquinolones
| Ciprofloxacin (resistant isolates) | Ofloxacin (resistant isolates) | Gatifloxacin (resistant isolates) | Moxifloxacin (resistant isolates) | |
|---|---|---|---|---|
| Total bacteria | ||||
| Tests (n=1007) | T: 950; NT: 57 | T: 958; NT: 49 | T: 936; NT: 71 | T: 855; NT: 152 |
| Resistant isolates | 536 (56.4) | 443 (46.2) | 476 (50.9) | 449 (52.5) |
| 95% CI | 53.3-59.6 | 43.1-49.4 | 47.6-54.1 | 49.1-55.9 |
| All Gram-positive bacteria* | ||||
| Tests (n=725) | T: 689; NT: 36 | T: 701; NT: 24 | T: 689; NT: 36 | T: 649; NT: 40 |
| Resistant isolates | 385 (55.9) | 299 (42.7) | 328 (47.6) | 296 (45.6) |
| 95% CI | 52.2-59.6 | 39.0-46.4 | 43.9-51.3 | 41.7-49.5 |
| All Gram-negative bacteria* | ||||
| Tests (n=266) | T: 261;NT: 4 | T: 257; NT: 9 | T: 247; NT: 19 | T: 206; NT: 60 |
| Resistant isolates | 151 (57.9) | 144 (56.0) | 148 (59.9) | 153 (74.3) |
| 95% CI | 51.9-63.9 | 49.7-62.1 | 53.8-66.0 | 68.3-80.2 |
| Coagulase-negative Staphylococci | ||||
| Tests (n=98) | T: 94; NT: 4 | T: 97; NT: 1 | T: 97; NT: 1 | T: 73; NT: 25 |
| Resistant isolates | 54 (57.4) | 33 (34.0) | 31 (32.0) | 30 (41.1) |
| 95% CI | 47.4-67.4 | 28.4-47.8 | 22.7-41.2 | 29.8-52.4 |
| S. aureus | ||||
| Tests (n=102) | T: 92; NT: 10 | T: 94; NT: 8 | T: 101; NT: 1 | T: 96; NT: 6 |
| Resistant isolates | 51 (55.4) | 47 (50.0) | 46 (45.5) | 48 (50.0) |
| 95% CI | 45.3-65.6 | 39.9-60.1 | 35.8-55.3 | 40.0-60.0 |
| Methicillin-resistant S. aureus | ||||
| Tests (n=45) | T: 45; NT: 0 | T: 44; NT: 1 | T: 97; NT: 0 | T: 45; NT: 0 |
| Resistant isolates | 39 (86.7) | 35 (79.5) | 40 (88.9) | 33 (73.3) |
| 95% CI | 76.7-96.6 | 67.6-91.5 | 79.7-98.1 | 60.4-86.3 |
| S. pneumoniae | ||||
| Tests (n=156) | T: 151; NT: 5 | T: 152; NT: 4 | T: 156; NT: 0 | T: 153; NT: 3 |
| Resistant isolates | 68 (45.0) | 39 (25.7) | 62 (39.7) | 42 (27.5) |
| 95% CI | 37.1-53.0 | 18.7-32.6 | 32.0-47.4 | 20.4-34.5 |
| P. aeruginosa | ||||
| Tests (n=144) | T: 129; NT: 15 | T: 128; NT: 16 | T: 122; NT: 22 | T: 102; NT: 42 |
| Resistant isolates | 78 (60.5) | 75 (58.6) | 79 (64.8) | 85 (83.3) |
| 95% CI | 52.0-68.9 | 50.1-67.1 | 56.3-73.2 | 76.1-90.6 |
Number (percentage) denotes resistant bacterial isolates. * As the genus or specie of the bacterial isolates was missing in 16 samples, they were excluded. NT (not tested): Number of isolates against which the antibiotic was not tested for antibiotic susceptibility; T (tested): Number of isolates against which the antibiotic was tested for antibiotic susceptibility
The four fluoroquinolones correlated with each other in their resistance patterns. The correlation of ciprofloxacin to ofloxacin, gatifloxacin, and moxifloxacin resistance was r = 0.582 (P < 0.001), r = 0.597 (P < 0.001), and r = 0.511 (P < 0.001) respectively; ofloxacin to gatifloxacin and moxifloxacin was r = 0.598 (P < 0.001) and r = 0.594 (P < 0.001), respectively, and gatifloxacin to moxifloxacin was r = 0.567 (P < 0.001). Among ciprofloxacin-resistant bacterial isolates, the odds ratios of resistance to ofloxacin, gatifloxacin, and moxifloxacin were OR: 5.21 (95% CI: 3.39 – 8.0, P < 0.001), OR: 5.68 (95% CI: 3.79 – 8.5, P < 0.001), and OR: 2.25 (95% CI: 1.47 – 3.44, P < 0.001), respectively. Similarly, among moxifloxacin-resistant bacterial isolates, the odds of resistance to ciprofloxacin, ofloxacin, and gatifloxacin were OR: 2.23 (95% CI: 1.46 – 3.42, P < 0.001), OR: 5.63 (95% CI: 3.76 – 8.44, P < 0.001), and OR: 4.43 (95% CI: 2.95 – 6.66, P < 0.001), respectively.
A comparison of fluoroquinolone-resistant isolates between intra-ocular (endophthalmitis) and extra-ocular infections (keratitis, conjunctivitis, etc.) revealed statistically significant differences. More ciprofloxacin- (P = 0.088), ofloxacin- (P = 0.001), gatifloxacin- (P = 0.029), and moxifloxacin (P < 0.001)-resistant bacterial isolates were seen in intra-ocular infections [Appendix 3].
Appendix 3.
Difference in fluoroquinolone resistance pattern between extra-ocular (n=797) and intra-ocular infections (n=210)
| Fluoroquinolone | Extra-ocular infection (keratitis, conjunctivitis, etc.) Number (percentage) | Intra-ocular infection (endophthalmitis) Number (percentage) | P |
|---|---|---|---|
| Ciprofloxacin (n=966) | 424 (54.9) | 119 (61.7) | 0.088 |
| Ofloxacin (n=974) | 336 (43.6) | 114 (56.2) | 0.001 |
| Gatifloxacin (n=952) | 371 (48.9) | 112 (57.7) | 0.029 |
| Moxifloxacin (n=869) | 342 (49.4) | 113 (64.2) | <0.001 |
Number and percentage in columns represent resistant isolates
Resistance pattern of fluoroquinolones in different bacterial species
The resistance of the four fluoroquinolones to five important and common ocular bacterial pathogens is given in Table 1. The proportion of coagulase-negative Staphylococci (CoNS) isolates resistant to ciprofloxacin was highest and to gatifloxacin was the least (P = 0.008). The proportion of S. aureus isolates resistant to all fluoroquinolones was high, and although it was lower for gatifloxacin, the difference was not significant (P = 0.596). The proportion of MRSA isolates resistant to all of the four fluoroquinolones was very high but without any significant difference between them (P = 0.203). The proportion of S. pneumoniae isolates resistant to ciprofloxacin was the highest, whereas it was least to ofloxacin (P < 0.0001) and moxifloxacin (P = 0.001). The difference in resistance between ofloxacin and moxifloxacin was not significant (P = 0.723). The proportion of P. aeruginosa isolates resistant to moxifloxacin was the highest (P < 0.001) and was least to ofloxacin and ciprofloxacin (P < 0.001). The difference in resistance between ciprofloxacin and ofloxacin was not significant (P = 0.760).
Trend in antibiotic resistance
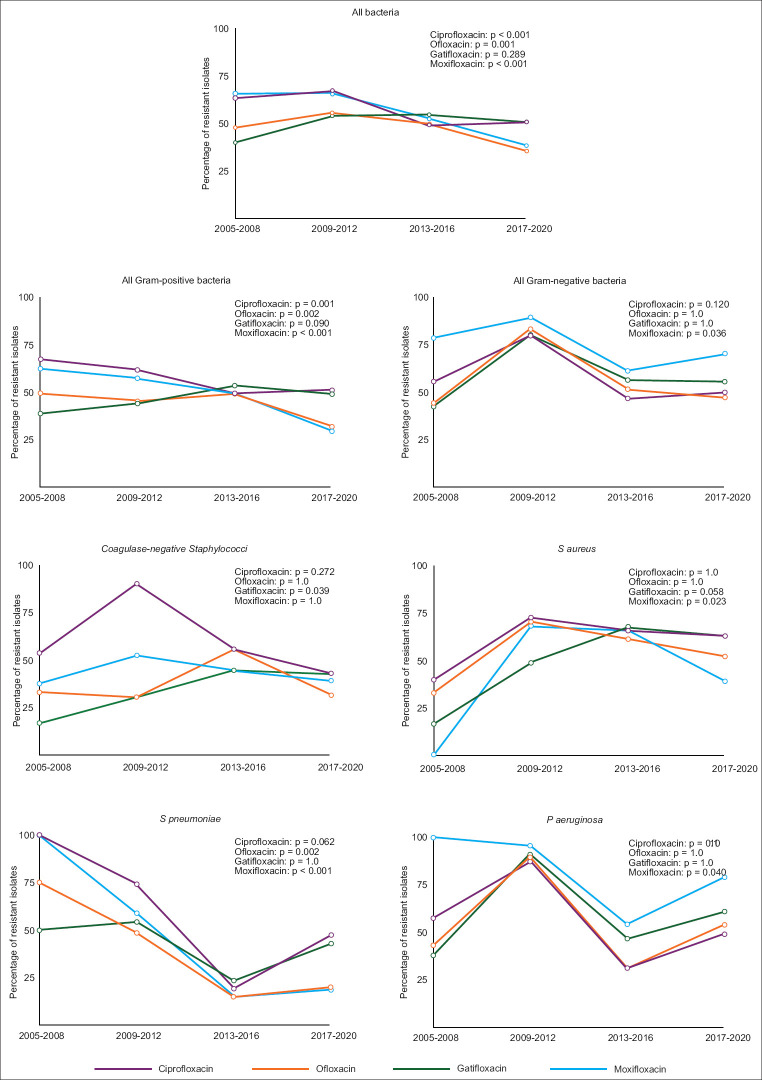
Overall, there was an increasing trend in the resistance rate to all of the fluoroquinolones in most of the bacterial isolates between 2009 and 2012, following which a gradual reduction was observed [Fig. 1 and Appendix 4]. With the exception of gatifloxacin (P = 0.090), there was a statistically significant reduction in the resistance to ciprofloxacin (P = 0.001), ofloxacin (P = 0.002), and moxifloxacin (P < 0.001) within Gram-positive bacteria. A statistically significant reduction in resistance to moxifloxacin was observed in Gram-negative bacteria (P = 0.036) but not toward the other three fluoroquinolones.
Figure 1.
Trends in fluoroquinolone resistance among bacteria. Lines represent the moving average of the proportion of resistant bacteria. P value denotes statistical significance calculated using the Cochran-Armitage test for linear trends in a proportion
Appendix 4.
Trend in bacterial resistance to fluoroquinolones
| Bacteria | Total | 2005-2008 | 2009-2012 | 2013-2016 | 2017-2020 |
|---|---|---|---|---|---|
| Gram-positive | |||||
| Ciprofloxacin | |||||
| Sensitive | 304 (44.1) | 39 (32.5) | 58 (38.2) | 93 (50.5) | 114 (48.9) |
| Resistant | 385 (55.9) | 81 (67.5) | 94 (61.8) | 91 (49.5) | 119 (51.1) |
| Ofloxacin | |||||
| Sensitive | 402 (57.3) | 61 (50.4) | 90 (54.5) | 93 (50.8) | 158 (68.1) |
| Resistant | 299 (42.7) | 60 (49.6) | 75 (45.5) | 90 (49.2) | 74 (31.9) |
| Gatifloxacin | |||||
| Sensitive | 361 (52.4) | 63 (61.2) | 95 (55.9) | 85 (46.2) | 118 (50.9) |
| Resistant | 328 (47.6) | 40 (38.8) | 75 (44.1) | 99 (53.8) | 114 (49.1) |
| Moxifloxacin | |||||
| Sensitive | 353 (54.4) | 21 (37.5) | 75 (42.6) | 93 (50.5) | 164 (70.4) |
| Resistant | 296 (45.6) | 35 (62.5) | 101 (57.4) | 91 (49.5) | 69 (20.6) |
| Gram-negative | |||||
| Ciprofloxacin | |||||
| Sensitive | 110 (42.1) | 28 (44.4) | 13 (20.3) | 33 (53.2) | 36 (50.0) |
| Resistant | 151 (57.9) | 35 (55.6) | 51 (79.7) | 29 (46.8) | 36 (50.0) |
| Ofloxacin | |||||
| Sensitive | 113 (44.0) | 35 (55.6) | 10 (16.7) | 30 (48.4) | 38 (52.8) |
| Resistant | 144 (56.0) | 28 (44.4) | 50 (83.7) | 32 (51.6) | 34 (47.2) |
| Gatifloxacin | |||||
| Sensitive | 99 (40.1) | 27 (57.4) | 13 (19.7) | 27 (43.5) | 32 (44.4) |
| Resistant | 148 (59.9) | 20 (42.6) | 53 (80.3) | 35 (56.5) | 40 (55.6) |
| Moxifloxacin | |||||
| Sensitive | 53 (25.7) | 3 (21.4) | 7 (10.6) | 24 (38.7) | 19 (29.7) |
| Resistant | 153 (74.3) | 11 (78.6) | 59 (89.4) | 38 (61.3) | 45 (70.3) |
| All bacteria | |||||
| Ciprofloxacin | |||||
| Sensitive | 414 (43.6) | 67 (36.6) | 71 (32.9) | 126 (51.2) | 150 (49.2) |
| Resistant | 536 (56.4) | 116 (63.4) | 145 (67.1) | 120 (48.8) | 155 (50.8) |
| Ofloxacin | |||||
| Sensitive | 515 (53.8) | 96 (52.2) | 100 (44.4) | 123 (50.2) | 196 (64.5) |
| Resistant | 443 (46.2) | 88 (47.8) | 125 (55.6) | 122 (49.8) | 108 (35.5) |
| Gatifloxacin | |||||
| Sensitive | 460 (49.1) | 90 (60) | 108 (45.8) | 112 (45.5) | 150 (49.3) |
| Resistant | 476 (50.9) | 60 (40) | 128 (54.2) | 134 (54.5) | 154 (50.7) |
| Moxifloxacin | |||||
| Sensitive | 406 (47.5) | 24 (34.3) | 82 (33.9) | 117 (47.6) | 183 (61.6) |
| Resistant | 449 (52.5) | 46 (65.7) | 160 (66.1) | 129 (52.4) | 114 (38.4) |
Number (percentage) denotes the proportion of resistant bacteria
To gain more clarity, the 16-year period was divided into two equal periods, 2005–2012 and 2013–2020 [Table 2]. Between these two periods, ciprofloxacin resistance decreased significantly (P < 0.05) in CoNS, S. pneumoniae, and P. aeruginosa. Ofloxacin resistance decreased significantly (P < 0.05) in S. pneumoniae and P. aeruginosa. Gatifloxacin resistance increased significantly (P < 0.05) in CoNS and S. aureus but reduced significantly (P = 0.038) in S. pneumoniae with no change in P. aeruginosa (P = 0.105). Moxifloxacin resistance showed a significant reduction (P < 0.05) in S. pneumoniae and P. aeruginosa.
Table 2.
Trend in fluoroquinolone resistance among different bacterial species
| Bacteria and fluoroquinolone | 2005-2012 | 2013-2020 | P |
|---|---|---|---|
| Coagulase-negative Staphylococci | |||
| Ciprofloxacin | 68.0 (53.3-80.1) | 45.5 (30.4-61.2) | 0.027 |
| Ofloxacin | 32.1 (19.9-46.3) | 36.4 (22.4-52.2) | 0.657 |
| Gatifloxacin | 22.6 (12.3-36.2) | 43.2 (28.4-59.0) | 0.031 |
| Moxifloxacin | 48.3 (29.5-67.5) | 36.4 (22.4-52.2) | 0.311 |
| S. aureus | |||
| Ciprofloxacin | 68.4 (51.4-82.5) | 64.7 (54.4-74.0) | 0.677 |
| Ofloxacin | 65.0 (48.3-79.4) | 57.1 (46.8-67.1) | 0.394 |
| Gatifloxacin | 44.7 (30.2-59.9) | 65.7 (55.4-74.9) | 0.016 |
| Moxifloxacin | 66.7 (50.5-80.4) | 53.5 (43.2-63.6) | 0.149 |
| S. pneumoniae | |||
| Ciprofloxacin | 76.5 (58.8-89.3) | 35.9 (27.2-45.3) | <0.001 |
| Ofloxacin | 51.4 (34.0-68.6) | 17.9 (11.5-26.1) | <0.001 |
| Gatifloxacin | 53.8 (37.2-69.9) | 35.0 (26.5-44.4) | 0.038 |
| Moxifloxacin | 61.1 (43.5-76.9) | 17.1 (10.8-25.2) | <0.001 |
| P. aeruginosa | |||
| Ciprofloxacin | 60.8 (46.1-74.2) | 32.1 (21.9-43.6) | 0.001 |
| Ofloxacin | 54.7 (40.5-68.4) | 36.0 (25.2-47.9) | 0.036 |
| Gatifloxacin | 55.8 (39.9-70.9) | 40.5 (29.6-52.2) | 0.105 |
| Moxifloxacin | 88.2 (63.6-98.5) | 47.1 (36.1-58.2) | 0.002 |
Number (95% confidence interval) denotes average percentage of resistant bacteria
Solution to fluoroquinolone resistance: Use of alternative antibiotics
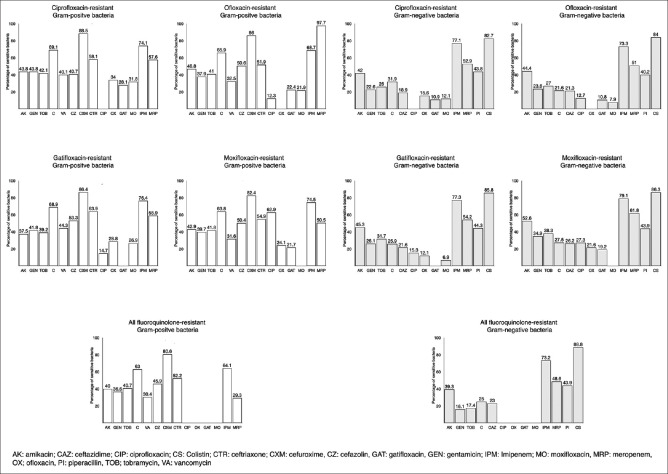
We calculated the sensitivity (susceptibility) of other classes of antibiotics in fluoroquinolone-resistant bacterial isolates [Fig. 2 and Appendix 5]. Among ciprofloxacin-resistant Gram-positive bacteria, cefuroxime (88.5%, 95% CI: 81.8 – 95.2%), imipenem (74.1%, 95% CI: 65.8 – 82.3%), and chloramphenicol (69.1%, 95% CI: 65.1 – 74.3%) had the highest sensitivity. Among ofloxacin-resistant Gram-positive bacteria, meropenem (97.7%, 95% CI: 93.3 – 100%), cefuroxime (86.0%, 95% CI: 77.0 – 95.0%), and imipenem (68.7%, 95% CI: 59.6 – 77.8%) had the highest sensitivity. Among gatifloxacin-resistant Gram-positive bacteria, cefuroxime (86.4%, 95% CI: 79.2 – 93.5%), imipenem (76.4%, 95% CI: 68.9 – 83.9%), and chloramphenicol (68.9%; 95% CI: 63.9 – 73.9%) had the highest sensitivity. Among moxifloxacin-resistant Gram-positive bacteria, cefuroxime (82.4%, 95% CI: 71.9 – 92.8), imipenem (74.5%, 95% CI: 66.2 – 82.8%), and chloramphenicol (63.8%; 95% CI: 58.3 – 69.3%) had the highest sensitivity. Among the different fluoroquinolone-resistant Gram-negative bacteria, the sensitivity rates of colistin and imipenem were highest. In fluoroquinolone-resistant CoNS, cefazolin had the highest sensitivity (79.2 – 85.4%), after imipenem and meropenem. In fluoroquinolone-resistant S. pneumoniae, fluoroquinolone-resistant S. aureus, and fluoroquinolone-resistant MRSA, cefuroxime exhibited the highest sensitivity, which ranged from 95.8 – 100%, 100%, and 70 – 78.6%, respectively. In fluoroquinolone-resistant P. aeruginosa, colistin had the highest sensitivity (93.9 – 97.7%).
Figure 2.
Bar chart showing the susceptibility pattern of fluoroquinolone-resistant bacteria to various antibiotic classes. Numbers denote the proportion of different fluoroquinolone-resistant bacteria susceptible to various antibiotics. For example, 88.5% ciprofloxacin-resistant Gram-positive bacteria were susceptible to cefuroxime (CXM)
Appendix 5.
Antibiotic susceptibility pattern of different antibiotics to bacteria resistant to various fluoroquinolone antibiotics
| 1. Coagulase-negative Staphylococcus | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Fluoroquinolone | Amikacin | Gentamicin | Tobramycin | Chloramphenicol | Vancomycin | Cefazolin | Cefuroxime |
| S | S | S | S | S | S | S | |
| Ciprofloxacin-resistant | 29 (53.7) | 29 (54.7) | 19 (37.3) | 41 (75.9) | 21 (38.9) | 41 (85.4) | 4 (57.1) |
| 95% confidence interval | 39.6-67.4 | 40.4-68.4 | 24.1-51.9 | 62.4-86.5 | 25.9-53.1 | 72.2-93.9 | 21.0-93.8 |
| Ofloxacin-resistant | 21 (63.6) | 12 (37.5) | 13 (40.6) | 23 (69.7) | 12 (38.7) | 24 (88.9) | 4 (66.7) |
| 95% confidence interval | 47.2-80.0 | 20.0-52.8 | 23.6-57.6 | 54.0-85.4 | 21.6-55.9 | 77.0-100 | 29.0-100.0 |
| Gatifloxacin-resistant | 18 (58.1) | 14 (48.3) | 13 (41.9) | 20 (64.5) | 14 (50.0) | 19 (79.2) | 5 (62.5) |
| 95% confidence interval | 39.1-75.4 | 30.1-66.5 | 24.6-59.3 | 47.7-81.4 | 31.5-68.5 | 57.9-92.9 | 24.5-91.5 |
| Moxifloxacin-resistant | 16 (53.3) | 14 (46.7) | 11 (36.7) | 22 (73.3) | 8 (28.6) | 19 (82.6) | 5 (62.5) |
| 95% confidence interval | 34.3-71.7 | 28.3-65.7 | 19.9-56.1 | 54.1-87.7 | 13.2-48.7 | 61.2-95.1 | 28.9-96.1 |
|
| |||||||
| Fluoroquinolone | Ceftriaxone | Ciprofloxacin | Ofloxacin | Gatifloxacin | Moxifloxacin | Imipenem | Meropenem |
|
| |||||||
| S | S | S | S | S | S | S | |
| Ciprofloxacin-resistant | 15 (46.9) | NA | 24 (44.4) | 26 (49.1) | 15 (35.7) | 7 (87.5) | 8 (100) |
| 95% confidence interval | 29.6-65.3 | 31.2-57.7 | 35.6-62.5 | 21.2-50.2 | 47.3-99.7 | 63-1-100.0 | |
| Ofloxacin-resistant | 12 (52.2) | 1 (3.2) | NA | 8 (24.2) | 4 (15.4) | 5 (83.3 | 6 (100) |
| 95% confidence interval | 31.8-72.6 | 0.0-9.8 | 9.6-38.9 | 1.5-29.3 | 53.5-100 | 54.1-100.0 | |
| Gatifloxacin-resistant | 16 (61.5) | 1 (3.6) | 5 (16.7) | NA | 4 (14.8) | 7 (87.5) | 8 (100) |
| 95% confidence interval | 40.6-79.8 | 0.0-18.4 | 3.3-30.0 | 1.4-28.2 | 47.4-99.7 | 63.1-100.0 | |
| Moxifloxacin-resistant | 12 (46.2) | 1 (3.6) | 8 (26.7) | 7 (23.3) | NA | 4 (80.0) | 5 (100) |
| 95% confidence interval | 26.6-66.6 | 0-18.4 | 12.3-45.9 | 8.2-38.5 | 28.4-99.5 | 47.8-100 | |
|
| |||||||
| 2. S. pneumoniae | |||||||
|
| |||||||
| Resistant Fluoroquinolone | Amikacin | Gentamicin | Tobramycin | Chloramphenicol | Vancomycin | Cefazolin | Cefuroxime |
|
| |||||||
| S | S | S | S | S | S | S | |
| Ciprofloxacin-resistant | 13 (19.1) | 28 (41.2) | 22 (32.4) | 51 (75.0) | 36 (52.9) | 30 (68.2) | 23 (95.8) |
| 95% confidence interval | 10.6-30.5 | 29.4-53.8 | 21.5-44.8 | 63.0-84.7 | 40.1-65.2 | 54.4-81.9 | 78.9-99.9 |
| Ofloxacin-resistant | 8 (20.5) | 13 (33.5) | 13 (33.5) | 28 (71.8) | 16 (41.0) | 15 (53.6) | 11 (100) |
| 95% confidence interval | 12.7-47.2 | 19.1-50.2 | 19.1-50.2 | 55.1-88.0 | 25.6-57.9 | 35.1-72.5 | 71.5-100.0 |
| Gatifloxacin-resistant | 7 (11.3) | 24 (39.3) | 13 (21.0) | 49 (79.0) | 40 (65.6) | 28 (71.8) | 23 (100) |
| 95% confidence interval | 4.7-21.9 | 27.1-52.7 | 11.7-33.2 | 66.8-88.3 | 52.3-77.3 | 55.1-85.0 | 85.2-100.0 |
| Moxifloxacin-resistant | 8 (19.0) | 17 (40.5) | 13 (31.0) | 30 (71.4) | 21 (50.0) | 23 (69.7) | 9 (100) |
| 95% confidence interval | 8.6-34.1 | 25.6-56.7 | 17.6-47.1 | 55.4-84.3 | 34.2-65.8 | 51.3-84.4 | 66.4-100.0 |
|
| |||||||
| Resistant Fluoroquinolone | Ceftriaxone | Ciprofloxacin | Ofloxacin | Gatifloxacin | Moxifloxacin | Imipenem | Meropenem |
|
| |||||||
| S | S | S | S | S | S | S | |
| Ciprofloxacin-resistant | 41 (67.2) | NA | 34 (50.0) | 22 (32.4) | 34 (51.5) | 11 (73.3) | 11 (73.3) |
| 95% confidence interval | 54.0-78.7 | 37.6-62.4 | 21.5-44.8 | 38.8-64.0 | 44.9-92.2 | 44.9-92.2 | |
| Ofloxacin-resistant | 20 (57.1) | 4 (10.5) | NA | 12 (30.8) | 14 (36.8) | 6 (60.0) | 6 (60.0) |
| 95% confidence interval | 39.4-73.7 | 2.9-24.8 | 17.2-47.8 | 21.8-54.0 | 26.2-87.8 | 26.2-87.8 | |
| Gatifloxacin-resistant | 42 (72.4) | 13 (22.0) | 32 (54.2) | NA | 29 (48.3) | 12 (80.0) | 12 (75.0) |
| 95% confidence interval | 59.1-83.3 | 12.3-34.7 | 40.8-67.3 | 35.2-61.6 | 51.9-95.7 | 47.6-92.7 | |
| Moxifloxacin-resistant | 24 (64.9) | 7 (17.9) | 16 (40.0) | 11 (26.2) | NA | 6 (75.0) | 6 (75.0) |
| 95% confidence interval | 47.5-79.8 | 7.5-33.5 | 24.9-56.7 | 13.2-40.3 | 34.9-96.8 | 34.9-96.8 | |
|
| |||||||
| 3 .S. aureus | |||||||
|
| |||||||
| Resistant Fluoroquinolone | Amikacin | Gentamicin | Tobramycin | Chloramphenicol | Vancomycin | Cefazolin | Cefuroxime |
|
| |||||||
| S | S | S | S | S | S | S | |
| Ciprofloxacin-resistant | 30 (58.8) | 28 (58.3) | 27 (54.0) | 36 (70.6) | 15 (29.4) | 23 (53.5) | 8 (100) |
| 95% confidence interval | 44.2-72.4 | 43.2-72.4 | 39.3-68.2 | 56.2-82.5 | 17.5-43.8 | 37.7-68.8 | 63.1-100.0 |
| Ofloxacin-resistant | 25 (53.2) | 22 (50) | 21 (45.7) | 32 (68.1) | 10 (21.3) | 18 (45.0) | 6 (100) |
| 95% confidence interval | 38.1-67.9 | 34.6-65.4 | 30.9-61.0 | 52.9-80.9 | 10.7-35.7 | 29.2-61.5 | 54.1-100.0 |
| Gatifloxacin-resistant | 25 (54.3) | 24 (57.1) | 25 (55.6) | 31 (67.4) | 15 (32.6) | 20 (52.6) | 8 (100) |
| 95% confidence interval | 39.0-69.1 | 50.0-72.3 | 40.0-70.4 | 52.0-80.5 | 19.5-48.0 | 35.8-69.0 | 63.1-100.0 |
| Moxifloxacin-resistant | 24 (50.0) | 24 (53.3) | 23 (48.9) | 29 (60.4) | 9 (18.8) | 22 (51.2) | 13 (100.0) |
| 95% confidence interval | 35.8-64.1 | 37.9-68.3 | 34.1-63.9 | 45.3-74.2 | 9.0-32.6 | 35.5-66.7 | 75.3-100.0 |
|
| |||||||
| Resistant Fluoroquinolone | Ceftriaxone | Ciprofloxacin | Ofloxacin | Gatifloxacin | Moxifloxacin | Imipenem | Meropenem |
|
| |||||||
| S | S | S | S | S | S | S | |
| Ciprofloxacin-resistant | 23 (47.9) | NA | 10 (19.6) | 18 (36.0) | 14 (29.2) | 13 (92.1) | 14 (100.0) |
| 95% confidence interval | 33.3-62.8 | 9.8-33.1 | 22.9-50.8 | 17.0-44.1 | 66.1-99.8 | 76.8-100 | |
| Ofloxacin-resistant | 22 (48.9) | 4 (8.9) | NA | 18 (39.1) | 12 (26.7) | 10 (90.1) | 11 (100) |
| 95% confidence interval | 33.7-64.2 | 2.5-21.2 | 25.1-54.6 | 14.6-41.9 | 58.7-99.8 | 71.5-100.0 | |
| Gatifloxacin-resistant | 30 (68.2) | 6 (15.8) | 12 (30.0) | NA | 10 (22.2) | 15 (93.8) | 16 (100) |
| 95% confidence interval | 72.6-96.7 | 12.2-73.8 | 16.6-46.5 | 11.2-37.1 | 69.8-99.8 | 79.4-100.0 | |
| Moxifloxacin-resistant | 28 (58.3) | 5 (12.8) | 7 (17.5) | 12 (25.5) | NA | 13 (92.9) | 14 (100) |
| 95% confidence interval | 43,2-72.4 | 4.3-27.4 | 7.3-32.8 | 14.0-40.4 | 66.1-99.8 | 76.8-100.0 | |
|
| |||||||
| 4. Methicillin-resistant S. aureus | |||||||
|
| |||||||
| Resistant Fluoroquinolone | Amikacin | Gentamicin | Tobramycin | Chloramphenicol | Vancomycin | Cefazolin | Cefuroxime |
|
| |||||||
| S | S | S | S | S | S | S | |
| Ciprofloxacin-resistant | 19 (48.7) | 20 (51.3) | 22 (56.4) | 31 (79.5) | 15 (38.5) | 12 (50) | 12 (75.0) |
| 95% confidence interval | 32.4-65.2 | 34.8-67.6 | 39.6-72.2 | 63.5-90.7 | 23.4-55.4 | 29.1-70.9 | 47.6-92.7 |
| Ofloxacin-resistant | 17 (48.6) | 18 (51.4) | 20 (57.1) | 27 (77.1) | 13 (37.1) | 3 (75.1) | 11 (78.6) |
| 95% confidence interval | 31.4-66.0 | 34.0-68.6 | 39.4-73.7 | 59.9-89.6 | 21.5-55.1 | 19.4-99.4 | 49.2-95.3 |
| Gatifloxacin-resistant | 20 (50.0) | 21 (52.5) | 23 (57.5) | 31 (77.5) | 16 (40.0) | 12 (50.0) | 13 (76.5) |
| 95% confidence interval | 33.8-66.2 | 36.1-68.5 | 41.0-73.0 | 61.6-89.2 | 24.9-56.7 | 29.1-70.9 | 50.1-93.2 |
| Moxifloxacin-resistant | 15 (45.5) | 14 (42.9) | 17 (51.5) | 25 (75.8) | 11 (33.3) | 12 (50.0) | 7 (70.0) |
| 95% confidence interval | 28.1-63.7 | 25.5-60.8 | 33.5-69.2 | 57.7-88.9 | 18.0-51.8 | 29.1-70.9 | 34.8-93.3 |
|
| |||||||
| Resistant Fluoroquinolone | Ceftriaxone | Ciprofloxacin | Ofloxacin | Gatifloxacin | Moxifloxacin | Imipenem | Meropenem |
|
| |||||||
| S | S | S | S | S | S | S | |
| Ciprofloxacin-resistant | 20 (51.3) | NA | 5 (13.2) | 0 | 6 (15.4) | 18 (78.3) | 9 (39.1) |
| 95% confidence interval | 34.8-67.6 | 4.4-28.1 | 0.0 | 5.9-30.5 | 56.3-92.5 | 19.7-61.5 | |
| Ofloxacin-resistant | 18 (51.4) | 2 (5.7) | NA | 1 (2.9) | 6 (17.1) | 15 (71.4) | 8 (38.1) |
| 95% confidence interval | 34.0-68.6 | 0.7-19.1 | 0.0-8.4 | 6.6-33.7 | 47.8-88.7 | 18.1-61.6 | |
| Gatifloxacin-resistant | 21 (52.5) | 1 (2.5) | 5 (12.8) | NA | 7 (17.5) | 18 (78.3) | 9 (39.1) |
| 95% confidence interval | 36.1-68.5 | 0.0-13.2 | 4.3-27.34 | 7.3-32.8 | 56.3-92.5 | 19.7-61.5 | |
| Moxifloxacin-resistant | 16 (48.5) | 0 (0) | 3 (9.4) | 0 (0) | NA | 18 (78.3) | 9 (39.1) |
| 95% confidence interval | 30.8-66.5 | 0.0-10.6 | 2.0-25. | 56.3-92.5 | 19.7-61.5 | ||
|
| |||||||
| 5. P. aeruginosa | |||||||
|
| |||||||
| Resistant Fluoroquinolone | Amikacin | Gentamicin | Tobramycin | Chloramphenicol | Ceftazidime | Ciprofloxacin | Ofloxacin |
|
| |||||||
| S | S | S | S | S | S | S | |
| Ciprofloxacin-resistant | 25 (32.1) | 9 (11.8) | 10 (13.0) | 18 (24.0) | 15 (19.5) | NA | 8 (10.5) |
| 95% confidence interval | 21,9-43.6 | 5.6-21.3 | 6.4-22.6 | 14.9-35.3 | 11.3-30.1 | 4.7-19.7 | |
| Ofloxacin-resistant | 25 (33.3) | 9 (12.3) | 11 (14.9) | 11 (15.3) | 16 (21.6) | 6 (8.1) | NA |
| 95% confidence interval | 22.9-45.2 | 5.8-22.1 | 7.7-25.0 | 7.9-25.7 | 12.9-32.7 | 3.0-16.8 | |
| Gatifloxacin-resistant | 26 (32.9) | 12 (16.0) | 16 (20.3) | 13 (16.9) | 18 (23.4) | 12 (16.0) | 8 (10.8) |
| 95% confidence interval | 22.8-44.4 | 8.6-26.3 | 12.0-30.8 | 9.3-27.1 | 14.5-34.4 | 8.6-26.3 | 4.8-20.2 |
| Moxifloxacin-resistant | 37 (43.5) | 24 (29.6) | 26 (31.0) | 16 (19.3) | 23 (27.4) | 22 (26.8) | 19 (23.5) |
| 95% confidence interval | 32.8-54.7 | 20.0-40.8 | 21.3-42.0 | 11.4-29.4 | 18.2-38.2 | 17.6-37.8 | 14.8-34.2 |
| All fluoroquinolone-resistant | 13 (24.1) | 2 (3.7) | 1 (1.9) | 10 (18.9) | 12 (22.6) | NA | NA |
| 95% confidence interval | 13.5-37.6 | 1.0-12.8 | 0.0-9.9 | 9.4-32.0 | 12.3-36.2 | ||
|
| |||||||
| Resistant Fluoroquinolone | Gatifloxacin | Moxifloxacin | Imipenem | Colistin | Piperacillin | Meropenem | |
|
| |||||||
| S | S | S | S | S | S | ||
| Ciprofloxacin-resistant | 5 (7.4) | 3 (4.8) | 35 (67.3) | 47 (94.0) | 20 (55.6) | 8 (40.0) | |
| 95% confidence interval | 2.4-16.3 | 1.0-13.3 | 52.9-79.7 | 83.5-98.7 | 38.1-72.1 | 19.1-64.0 | |
| Ofloxacin-resistant | 6 (8.3) | 3 (4.6) | 34 (64.2) | 47 (95.9) | 21 (56.8) | 9 (42.9) | |
| 95% confidence interval | 3.1-17.3 | 1.0-12.9 | 49.8-76.9 | 86.2-99.5 | 39.5-72.9 | 21.8-66.0 | |
| Gatifloxacin-resistant | NA | 3 (4.2) | 43 (69.4) | 55 (94.8) | 24 (57.1) | 14 (53.8) | |
| 95% confidence interval | 1.0-11.7 | 56.4-80.4 | 85.6-98.9 | 41.0-72.3 | 33.4-73.4 | ||
| Moxifloxacin-resistant | 16 (18.8) | NA | 50 (71.4) | 62 (93.9) | 30 (58.8) | 23 (65.7) | |
| 95% confidence interval | 11.2-28.8 | 59.4-81.6 | 85.2-98.3 | 44.2-72.4 | 47.8-80.9 | ||
| All fluoroquinolone-resistant | NA | NA | 29 (63.0) | 43 (97.7) | 18 (58.1) | 7 (36.8) | |
| 95% confidence interval | 47.6-76.8 | 88.0-99.9 | 39.1-75.5 | 16.3-61.6 | |||
S: sensitive isolates
NB: To make the tables simple to comprehend the number of isolates which were not tested with any of the alternative antibiotics are not mentioned in this table.
Discussion
Our longitudinal study provides the trend of in vitro susceptibility patterns of four important fluoroquinolone antibiotics among bacterial isolates from ocular infections over a period of nearly two decades. In our study, the overall resistance to the second-generation (ciprofloxacin and ofloxacin) and fourth-generation fluoroquinolones (gatifloxacin and moxifloxacin) were greater than reports from the western hemisphere and were either comparable or greater than the findings from other reports from India [Table 3].[12,13,14,16] Gram-positive bacteria exhibited the highest resistance to ciprofloxacin and the least resistance to ofloxacin, whereas Gram-negative bacteria exhibited the highest resistance to moxifloxacin and the least resistance to ofloxacin.
Table 3.
Comparison of findings of the present study with other studies
| Study, settings, and period | Type of ocular isolates | Resistant isolates |
|---|---|---|
| Asbell et al., 2020[10] United States Multicentric Study period: 2009-2018 | All ocular isolates* | S. aureus |
| Ciprofloxacin: 11.5% | ||
| Ofloxacin: 11.3% | ||
| Levofloxacin: 9.9% | ||
| Moxifloxacin: 10.9% | ||
| Gatifloxacin: 10.8% | ||
| Methicillin-resistant S. aureus | ||
| Ciprofloxacin: 74.3% | ||
| Ofloxacin: 72.5% | ||
| Levofloxacin: 72.2% | ||
| Moxifloxacin: 71.2% | ||
| Gatifloxacin: 71.8% | ||
| CoNS | ||
| Ciprofloxacin: 12.0% | ||
| Ofloxacin: 11.3% | ||
| Levofloxacin: 10.8% | ||
| Moxifloxacin: 10.9% | ||
| Gatifloxacin: 10.8% | ||
| S. pneumoniae | ||
| Ciprofloxacin: NA | ||
| Ofloxacin: 0.8% | ||
| Levofloxacin: 0% | ||
| Moxifloxacin: 0.2% | ||
| Gatifloxacin: 0.2% | ||
| P. aeruginosa | ||
| Ciprofloxacin: 7.1% | ||
| Ofloxacin: 6.9% | ||
| Levofloxacin: 6.1% | ||
| Moxifloxacin: NA | ||
| Gatifloxacin: 5.7% | ||
| Lalitha et al., 2017[12] India Monocentric Study period: 2002-2013 | Cornea* | S. aureus |
| Ciprofloxacin: 55.7% | ||
| Ofloxacin: 42.4% | ||
| Levofloxacin: 47.5% | ||
| Moxifloxacin: 46.9% | ||
| Gatifloxacin: 41.5% | ||
| CoNS | ||
| Ciprofloxacin: 54.3% | ||
| Ofloxacin: 46.7% | ||
| Levofloxacin: 45.7% | ||
| Moxifloxacin: 33.0% | ||
| Gatifloxacin: 29.0% | ||
| S. pneumoniae | ||
| Ciprofloxacin: 24.2% | ||
| Ofloxacin: 4.5% | ||
| Levofloxacin: 2.0% | ||
| Moxifloxacin: 0.6% | ||
| Gatifloxacin: 2.7% | ||
| P. aeruginosa | ||
| Ciprofloxacin: 10.9% | ||
| Ofloxacin: 13.1% | ||
| Levofloxacin: 3.7% | ||
| Gatifloxacin: 8.1% | ||
| Das et al., 2019[13] India Monocentric Study period: 2007-2014 | Cornea† | S. aureus |
| Ciprofloxacin: 46.7% | ||
| Ofloxacin: 29.5% | ||
| Moxifloxacin: 40.8% | ||
| Gatifloxacin: 14.7% | ||
| S. pneumoniae | ||
| Ciprofloxacin: 4.2% | ||
| Ofloxacin: 1.0% | ||
| Moxifloxacin: 1.7% | ||
| Gatifloxacin: 3.8% | ||
| P. aeruginosa | ||
| Ciprofloxacin: 7.4% | ||
| Ofloxacin: 6.4% | ||
| Moxifloxacin: 14.4% | ||
| Gatifloxacin: 5.2% | ||
| Acharya et al., 2019[14] India Monocentric Study period: 2015-2017 | Cornea†† | S. aureus |
| Ciprofloxacin: 31.4% | ||
| Moxifloxacin: 9.4% | ||
| Gatifloxacin: 46.9% | ||
| CoNS | ||
| Ciprofloxacin: 33.6% | ||
| Moxifloxacin: 10.4% | ||
| Gatifloxacin: 45.3% | ||
| P. aeruginosa | ||
| Ciprofloxacin: 43.2% | ||
| Moxifloxacin: 47.2% | ||
| Present study, 2021 India Monocentric Study period: 2005-2020 | All ocular isolates* | S. aureus |
| Ciprofloxacin: 55.4% | ||
| Ofloxacin: 50.0% | ||
| Moxifloxacin: 50.0% | ||
| Gatifloxacin: 45.5% | ||
| Methicillin resistant S. aureus | ||
| Ciprofloxacin: 86.7% | ||
| Ofloxacin: 79.5% | ||
| Moxifloxacin: 73.3% | ||
| Gatifloxacin: 88.9% | ||
| CoNS | ||
| Ciprofloxacin: 57.4% | ||
| Ofloxacin: 34.0% | ||
| Moxifloxacin: 41.1% | ||
| Gatifloxacin: 32.0% | ||
| S. pneumoniae | ||
| Ciprofloxacin: 45.0% | ||
| Ofloxacin: 25.7% | ||
| Moxifloxacin: 27.5% | ||
| Gatifloxacin: 39.7% | ||
| P. aeruginosa | ||
| Ciprofloxacin: 60.5% | ||
| Ofloxacin: 58.6% | ||
| Moxifloxacin: 83.3 | ||
| Gatifloxacin: 64.8% |
CoNS: coagulase-negative Staphylococci; NA: Not available. *Resistant isolates: Intermediate or resistant zones of inhibition. †Resistant isolates: Resistant zone of inhibition. ††Resistant isolates: zones not specified by the authors
In CoNS isolates from our study, resistance to ciprofloxacin was the highest. This corroborated with the findings of other studies from India[12] and elsewhere,[10,11] where CoNS isolates were reported to be more resistant to ciprofloxacin and less resistant to moxifloxacin. In our study, resistance to gatifloxacin, ofloxacin, and moxifloxacin was low. We started identifying MRSA from 2014 and observed it to be highly resistant to all of the four fluoroquinolones. In contrast, in several studies from the USA,[7,8,9,11] Asbell et al. consistently reported low resistance of MRSA to moxifloxacin and besifloxacin. Besifloxacin was reported to have the least minimum inhibitory concentration for MRSA in these studies[7,8,9,10,11] and was recommended for use in MRSA-related ocular infections. However, besifloxacin is not widely used in India nor has its susceptibility pattern been previously reported.[12,13,14,16] However, in the light of our findings of high resistance to gatifloxacin and moxifloxacin, besifloxacin may be an alternative agent against MRSA. Our findings of high resistance of S. pneumoniae to ciprofloxacin, ofloxacin, and gatifloxacin, and low resistance to moxifloxacin are similar to previous studies.[7,8,9,10,11,12,13,14] It is widely reported that P. aeruginosa is highly susceptible to ciprofloxacin and resistant to fourth-generation fluoroquinolones,[7,8,9,10,11,12,13] making it the antibiotic of choice. In our study, although ofloxacin had the least resistance, the difference with ciprofloxacin was statistically not significant.
An optimistic finding in the present study is the declining trend in fluoroquinolone resistance between 2013 and 2020. Most of the bacterial isolates were observed to select for fluoroquinolone resistance in lower numbers [Fig. 1]. This indicates that fluoroquinolones will continue to be useful in the coming years. We found only a few Indian studies to compare our findings. In a study from North India, Acharya et al. reported reduced susceptibility of CoNS, S. aureus, and S. pneumoniae to ciprofloxacin but not moxifloxacin, and reduced susceptibility of P. aeruginosa to both ciprofloxacin and moxifloxacin.[14] Das et al.[13] in their study from eastern India, compared the resistance trends of S. aureus, S. pneumoniae, and P. aeruginosa between 2007 – 2010 and 2011 – 2014. They did not find any change in the resistance to fluoroquinolones in S. aureus or S. pneumoniae but observed an increase in resistance in P. aeruginosa to moxifloxacin. In another study from South India, Lalitha et al. reported that whereas there was no change in the resistance trends to fluoroquinolones in S. pneumoniae or P. aeruginosa, there was a significant increase in resistance to fluoroquinolones in S. aureus between 2002 and 2013.[12] This seems to agree with our findings between 2005 and 2012, where a significant resistance across all bacteria to the fluoroquinolones was observed. It is difficult to ascertain the reason behind this pattern as causes of antibiotic resistance are multi-factorial,[1,6] and the present study was not designed to explore them.
Cross-resistance in fluoroquinolones, either in the same generation or across generations, has been a challenge to physicians over many decades.[6,17] This is evident in our study too. We found a high correlation between the four fluoroquinolone drugs in our study. Moreover, the OR of resistance in ofloxacin and gatifloxacin in ciprofloxacin- and moxifloxacin-resistant isolates was four- to five-fold high. A high proportion of cross-resistance within fluoroquinolones necessitates the search for alternative antibiotics. Based on the sensitivity patterns, cefuroxime, cefazolin, and chloramphenicol are good alternatives against Gram-positive bacteria. However, because chloramphenicol is bacteriostatic, it should not be used as a primary agent. Whereas other studies[12,13,14,16] have found vancomycin to be effective, we observed high vancomycin resistance in our study, raising questions about its usefulness in our setting. We also observed that uncommon ocular antibiotics like imipenem and meropenem had very high sensitivity rates against Gram-positive bacteria. In fluoroquinolone-resistant P. aeruginosa, the only antibiotic with high sensitivity rates was colistin. We had previously reported the effectiveness of treating multi-drug-resistant P. aeruginosa keratitis with colistin,[18] and it continues to remain the agent of choice in this particular type of infection at our center. The emergence of multi-drug-resistant bacterial ocular infections[18,19,20,21,22] necessitates the search for alternative antibiotics, and the findings from our study may be useful in providing future directions in considering the use of non-conventional antibiotics to treat fluoroquinolone-resistant ocular infections. We also observed that fluoroquinolone-resistant bacteria were isolated more from intra-ocular than from extra-ocular infections, which has also been reported from a study in China.[23] This may be explained by our observation that P. aeruginosa was the predominant isolate from intra-ocular infections, and these bacteria are prone to develop resistance to multiple antibiotics[18,19] due to the presence of several mechanisms of antibiotic resistance.
The present study has its limitations. The Kirby–Bauer disc diffusion technique is a qualitative method to study antibiotic susceptibility. It is not a method for studying antibiotic susceptibility patterns, for which the broth dilution technique or automated systems (e.g., Vitek 2, BioMérieux) are more informative and suitable as minimum inhibitory concentrations can be measured. Moreover, the disc diffusion method is based on serum concentrations of the antibiotic, which are often exceeded in the ocular tissues due to frequent dosing and high concentrations of the drug. Nevertheless, the disc diffusion method is a simple test without the requirement for any specialized equipment, is less expensive, standardized, and can easily be interpreted by clinicians.[24]
Based on our findings, fluoroquinolones can continue to be considered as a first-line therapy for prophylaxis and treatment against ocular infections. Ofloxacin can be considered for prophylaxis in intra-ocular surgeries as it had the lowest resistance for both Gram-positive and Gram-negative bacteria. This would reserve the fourth-generation fluoroquinolones for treatment of infections and also reduce the risk of them being selected for resistance. For Gram-positive bacterial infections, moxifloxacin or gatifloxacin because of their superior pharmacokinetic properties can be considered for monotherapy, or fortified preparations of cefuroxime or cefazolin can be good alternatives. For Gram-negative bacterial infections, ciprofloxacin should be considered because it has the lowest minimum inhibitory concentration against Gram-negative bacteria than the other fluoroquinolones, whereas colistin and imipenem can be alternatives in fluoroquinolone-resistant isolates. The lower susceptibility pattern of aminoglycosides in our study questions their usefulness in treating ocular infections.
Conclusion
In this large cohort from central India, we report a high fluoroquinolone resistance in bacteria from ocular infections. However, the gradual decline in resistance in recent years indicates that the usefulness of fluoroquinolones is not over, and they will continue to be frontline ophthalmic antibiotics. In fluoroquinolone-resistant isolates, cefuroxime and cefazolin for Gram-positive bacteria and colistin for Gram-negative bacteria emerged as good alternatives. As the pattern of antibiotic resistance differs geographically, laboratories from different parts of India must be encouraged to share their antibiotic susceptibility in a central nationwide prospective surveillance program like those in existence in other countries. This would aid ophthalmologists working in the community in the selection of antibiotics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Sensitivity pattern of other antibiotics to fluoroquinolone-resistant bacteria
References
- 1.Blondeau JM. Fluoroquinolones:Mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 2004;49(Suppl 2):S73–8. doi: 10.1016/j.survophthal.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein MH, Kowalski RO, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis. Ophthalmology. 1999;106:1313–8. [PubMed] [Google Scholar]
- 3.Garg P, Sharma S, Rao GN. Ciprofloxacin-resistant Pseudomonas keratitis. Ophthalmology. 1999;106:1319–23. doi: 10.1016/S0161-6420(99)00717-4. [DOI] [PubMed] [Google Scholar]
- 4.Kunimoto DY, Sharma S, Garg P, Rao GN. In vitro susceptibility of bacterial keratitis pathogens to ciprofloxacin. Ophthalmology. 1999;106:80–5. doi: 10.1016/S0161-6420(99)90008-8. [DOI] [PubMed] [Google Scholar]
- 5.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497–52. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 6.Acar JF, Goldstein FW. Trends in bacterial resistance to fluoroquinolones. Clin Infect Dis. 1997;24(Suppl 1):S67–73. doi: 10.1093/clinids/24.supplement_1.s67. [DOI] [PubMed] [Google Scholar]
- 7.Asbell PA, Sanfillipo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic resistance among ocular pathogens in the United States five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015;133:1445–54. doi: 10.1001/jamaophthalmol.2015.3888. [DOI] [PubMed] [Google Scholar]
- 8.Asbell PA, Mah FS, Sanfilippo CM, DeCory HH. Antibiotic susceptibility of bacterial pathogens isolated from the aqueous and vitreous humor in the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. J Cataract Refract Surg. 2016;42:1841–3. doi: 10.1016/j.jcrs.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Asbell PA, DeCory HH. Antibiotic resistance among bacterial conjunctival pathogens collected in the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. PLoS One. 2018;13:e0205814. doi: 10.1371/journal.pone.0205814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asbell PA, Sanfillipo CM, Sahm DF, DeCory HH. Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2018. JAMA Ophthalmol. 2020;138:439–50. doi: 10.1001/jamaophthalmol.2020.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas RK, Melton RM, Vollmer PM, Asbell PA. In vitro antibiotic resistance among bacteria from the cornea in the antibiotic resistance monitoring in ocular microorganisms surveillance study. Optom Vis Sci. 2021;98:1113–21. doi: 10.1097/OPX.0000000000001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalitha P, Manoharan G, Karpagam R, Prajna NV, Srinivasan M, Mascarenhas J, et al. Trends in antibiotic resistance in bacterial keratitis isolates from South India. Br J Ophthalmol. 2017;101:108–13. doi: 10.1136/bjophthalmol-2016-308487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S, Samantaray R, Mallick A, Sahu SK, Sharma S. Types of organisms and in-vitro susceptibility of bacterial isolates from patients with microbial keratitis:A trend analysis of 8 years. Indian J Ophthalmol. 2019;67:49–53. doi: 10.4103/ijo.IJO_500_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acharya M, Farooqui JH, Singh A, Gandhi A, Mathur U. Bacterial isolates in microbial keratitis:Three-year trend analysis from north India. Indian J Ophthalmol. 2019;67:1508–9. doi: 10.4103/ijo.IJO_678_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agresti A. Categorical Data Analysis. 2nd ed. New York: Wiley; 2002. pp. 181–2. [Google Scholar]
- 16.Chawla B, Agarwal P, Tandon R, Tityal JS, Sharma N, Agarwal T, et al. In vitro susceptibility of bacterial keratitis isolates to fourth-generation fluoroquinolones. Eur J Ophthalmol. 2010;20:300–5. doi: 10.1177/112067211002000207. [DOI] [PubMed] [Google Scholar]
- 17.Sanders CC. Mechanisms responsible for cross-resistance and dichotomous resistance among the quinolones. Clin Infect Dis. 2001;32(Suppl 1):S1–8. doi: 10.1086/319369. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee S, Agrawal D. Multi-drug resistant Pseudomonas aeruginosa keratitis and its effective treatment with topical colistimethate. Indian J Ophthalmol. 2016;64:153–7. doi: 10.4103/0301-4738.179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020;33:e00181–90. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nithya V, Rathinam S, Karthikeyan RSG, Lalitha P. A ten-year study of prevalence, antimicrobial susceptibility pattern, and genotypic characterization of methicillin resistant Staphylococcus aureus causing ocular infections in a tertiary eye care hospital in south India. Infect Genet Evol. 2019;69:203–10. doi: 10.1016/j.meegid.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Chang VS, Dhaliwal DK, Raju L, Kowalski RP. Antibiotic Resistance in the treatment of staphylococcus aureus keratitis:A 20-year review. Cornea. 2015;34:698–703. doi: 10.1097/ICO.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egrilmez S, Yildrim-Theveny S. Treatment-resistant bacterial keratitis:Challenges and solutions. Clin Ophthalmol. 2020;14:287–97. doi: 10.2147/OPTH.S181997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang N, Yang Q, Tan Y, Lin L, Huang Q, Wu K. Bacterial spectrum and antibiotic resistance patterns of ocular infection:Differences between external and intraocular diseases. J Ophthalmol. 2015;2015:813979. doi: 10.1155/2015/813979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing:A review of general principles and contemporary practices. Clin Infect Dis. 2009;49:1749–55. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity pattern of other antibiotics to fluoroquinolone-resistant bacteria