Abstract
Purpose:
To study the clinical and demographic profile of patients less than 40 years of age presenting to glaucoma services including the reasons for referral.
Methods:
Patients in the age group of 5 to 39 years, visiting the glaucoma clinic, who were either suspected to have glaucoma or who had been newly/previously diagnosed with glaucoma were included in the study. After informed written consent, basic demographic details of the participants including age, gender, education, socioeconomic status, and family history were obtained. A comprehensive ophthalmological evaluation was performed by glaucoma specialists.
Results:
The proportion of glaucoma in the study population (n = 384) was found to be 31.25%, and the incidence of glaucoma among new patients was found to be 11.9%. Among all glaucomas (n = 120), 44.2% of patients had secondary glaucomas, 27.5% had primary glaucomas, and 28.3% had congenital glaucomas. Also, 67.3% of all glaucoma patients were males. Newly diagnosed glaucoma patients presented with a mean intraocular pressure (IOP) of 32.9 mmHg and mild–moderate disc damage with a mean cup-disc ratio of 0.65. Nearly one-third of them had a presenting visual acuity worse than 5/60. The most common reason for referral was raised IOP. Univariate and multivariate analysis revealed that the odds of developing glaucoma were less in females (P = 0.04) and in patients with a higher standard of living index (P < 0.001).
Conclusion:
One-third of the patients had glaucoma and another one-third were suspects. Secondary glaucomas are more common than primary/congenital glaucomas. A comprehensive eye evaluation is a must, especially in those with predisposing factors.
Keywords: Demographic profile, juvenile open-angle glaucoma, reasons for referral, secondary glaucomas, under 40
Glaucoma is the leading cause of irreversible blindness in the world and is largely asymptomatic in the early stages.[1] Aging is an established risk factor, not only for the development but also for the progression of glaucoma.[2] Though considered a disease of aging and more prevalent after 40 years of age, it does occur in the younger age group. A population-based prevalence study in Germany, the Gutenberg health study, found that the prevalence of juvenile glaucoma diagnosed between 2 and 39 years of age was 0.17%, whereas that of adult glaucoma diagnosed over 40 years of age was more than 10 times higher at 2.15%.[3]
Despite the low prevalence, glaucoma in young assumes greater significance because the disease is more aggressive in nature with a higher presenting intraocular pressure (IOP) as compared to primary open-angle glaucoma (POAG).[4] Consequently, the progression of glaucomatous damage is much faster, if left untreated. Moreover, the life expectancy after the diagnosis is considerably longer, hence the disease must be controlled for a longer duration to slow down the progression and reduce the chances of blindness. Severe visual impairment or blindness due to glaucoma in young people reduces their productivity, leaving them vulnerable and dependent on their family or caregivers. Therefore, identifying glaucoma early in young individuals with appropriate intervention is paramount in reducing the magnitude of blindness and the adverse socio-economic impact.
Though primary glaucomas, specifically POAG has been found to be the most common type of glaucoma in adults,[5,6] there is still a paucity of literature regarding the prevalence of various forms of glaucomas, reasons for referral, and disease severity at presentation in the under 40 age group. Most studies done in the younger population are focussed on children below 18 years of age and are retrospective chart reviews.[7,8,9,10,11] Furthermore, studies analyzing glaucoma in young adults primarily included patients with juvenile open-angle glaucoma (JOAG).[12,13,14] We could not find studies specifically analyzing the under 40 years age group considering all clinical types of glaucoma.
Hence, in this prospective, cross-sectional study, we aimed to evaluate the reasons for a referral from general clinics to glaucoma services, the proportion of various types of glaucoma, and the demographic profile of patients less than 40 years of age presenting to the glaucoma services of a tertiary eye hospital. This study will help us to understand the presenting trends of various types of glaucoma in the young population, which may guide in framing protocols to identify the disease early.
Methods
This cross-sectional observational study was conducted at a tertiary eye hospital in south India from October 2018 to September 2020. Patients between 5 and 39 years of age seen in the glaucoma outpatient department including new (referred to glaucoma clinic for the first time from the general outpatient department) and review patients (presenting for follow-up visits) were included in the study. Informed written consent was obtained either from the participants or from the parents of patients below 18 years of age. The study protocol was approved by the Institutional Ethics Committee. This research adhered to the tenets of the Declaration of Helsinki.
Basic demographic details of participants including age, gender, address, education, and occupation were obtained. Socioeconomic status was assessed using the Standard of Living Index (SLI)[15] scale by the study coordinator. It is a scoring system, which includes variables such as household conditions, and accessibility to basic essential services such as water, light, and fuel. For example, the type of housing is assigned a score of 4, 2, and 0 for pucca, semi pucca, and katcha, respectively. A total score of 0–14 was low, 15–24 was medium, and 25–67 was high SLI. A detailed ocular and systemic history were recorded by the principal investigator. Optometrists performed refraction using Snellen’s chart to know the best-corrected visual acuity (BCVA). Central corneal thickness (CCT) was measured using an ultrasound pachymeter (PACSCAN 300P, Sonomed Escalon). Ocular examination was carried out by one of the glaucoma specialists including slit-lamp examination, fundus evaluation using a 90D lens, IOP measurement by Goldmann applanation tonometry (GAT), and anterior chamber angle evaluation using a 2 mirror or 4 mirror gonioscopy (Ocular Instruments Inc., Bellevue, WA, USA). Field analysis using Humphrey field analyzer (HFA II-i, Carl Zeiss Meditec, Dublin, CA) and retinal nerve fiber layer analysis by optical coherence tomography (Cirrus HD-OCT 5000, Carl Zeiss Meditec, Dublin, CA, USA) were done by a trained technician if deemed necessary by the glaucoma consultant either in the same visit or review visit. The final diagnosis for each eye was noted after completing the comprehensive evaluation. Study participants were classified into four main categories: a) Glaucoma: patients who had glaucomatous optic disc and visual field changes with or without raised IOP or patients who were on anti-glaucoma medications; b) glaucoma suspects included i) disc suspects (cup disc ratio [CDR] >0.7, CDR asymmetry of >0.2 between two eyes with IOP below 21 mmHg), ii) primary angle closure suspect [PACS] (At least 270 degrees of irido-trabecular contact, IOP below 21 mmHg without peripheral anterior synechiae and without a glaucomatous optic disc or visual field changes), or iii) ocular hypertension (IOP >21 mmHg, open angles on gonioscopy, without glaucomatous optic disc and visual field changes); c) other conditions predisposing to glaucoma including secondary raised IOP and miscellaneous conditions such as angle recession; d) nil glaucoma who did not have any ocular abnormality predisposing to or suggestive of glaucoma.
Statistical analysis
All the collected data were entered into a REDCap database for ease of analysis. The analysis included the detection of the proportion of glaucoma among the study population along with the proportion of various types of glaucoma, the reasons for referral of patients to glaucoma clinics in the study population with their demographic and clinical characteristics. Mean standard deviation (SD) was given for continuous variables. The categorical variables were given with frequency (percentage). The Chi-square test or Fisher’s exact test was performed to find the association between categorical variables. The relationship among the continuous variables was assessed using Pearson’s correlation method. P values <0.05 were considered statistically significant. Logistic regression analysis was used to predict the risk of glaucoma based on the observed characteristics of the patients, that is, age, gender, education, socioeconomic status, and family history. All the statistical analyses were done using the statistical software STATA statistical software, Version. 14.1 (Statacorp, Texas, USA).
Results
Demographic characteristics
A total of 384 patients were evaluated, including 160 (41.7%) new and 224 (58.3%) follow-up patients. Table 1 shows the baseline demographic characteristics. There were significant differences in the mean age of patients among the various categories studied, with glaucoma suspects being relatively older, whereas patients with other conditions predisposing to glaucoma were relatively younger. Glaucoma was more common in the 31 to 39 years age group (34.2%). There were significant differences in the gender distribution (P < 0.001), with a male predominance among the glaucoma group (65%). Among patients with low and medium SLI, a higher percentage of patients, that is, 63.2% and 46.3%, respectively were diagnosed with glaucoma, whereas among patients with a higher SLI, there were more glaucoma suspects (37.1%). Additionally, with better SLI, the percentage of advanced glaucoma (CDR >0.85) was also found to be low (5.7%, P = 0.002). The educational status, however, did not have any significant association with the distribution across the various categories.
Table 1.
Demographic characteristics of the study participants under 40 years of age visiting glaucoma services based on the glaucoma status
| Parameters | Glaucoma (n=120) | Glaucoma suspect (n=125) | Nil glaucoma (n=80) | Other conditions predisposing to glaucoma* (n=59) | P |
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD) | 25.2 (9.35) | 28.04 (8.80) | 26.63 (9.11) | 22.78 (8.59) | 0.002K |
| Age distribution | 0.115C | ||||
| 5-10 | 11 (9.2) | 7 (5.6) | 4 (5.0) | 5 (8.5) | |
| 11-20 | 30 (25.0) | 22 (17.6) | 19 (23.8) | 20 (33.9) | |
| 21-30 | 38 (31.7) | 37 (29.6) | 25 (31.3) | 21 (35.6) | |
| 31-39 | 41 (34.2) | 59 (47.2) | 32 (40.0) | 13 (22.0) | |
| Gender | |||||
| Male | 78 (65.0) | 62 (49.6) | 32 (40.0) | 44 (74.6) | <0.001C |
| Family history | |||||
| Yes | 19 (15.8) | 22 (17.6) | 34 (42.5) | - | <0.001C |
| SES | |||||
| High SLI | 70 (24.7) | 105 (37.10) | 68 (24.0) | 40 (14.1) | <0.001C |
| Medium SLI | 38 (46.3) | 18 (21.9) | 10 (12.2) | 16 (19.5) | |
| Low SLI | 12 (63.2) | 2 (10.5) | 2 (10.5) | 3 (15.8) | |
| Education | |||||
| Uneducated | 3 (2.5) | 1 (0.8) | 2 (2.5) | 2 (3.4) | 0.244F |
| School | 68 (56.7) | 59 (47.2) | 34 (42.5) | 34 (57.6) | |
| Graduation | 49 (40.8) | 65 (52.0) | 44 (55.0) | 23 (39.0) |
Chi-square test; F-Fisher’s exact test; K-Kruskal Wallis test; SES: Socioeconomic status, SLI: Standard of living index. *Includes secondary raised IOP and miscellaneous conditions such as angle recession and retino-choroidal coloboma
The proportion of glaucoma among the new patients was found to be 11.9% and the overall proportion of glaucoma was 31.2% [Table 2].
Table 2.
Proportion of glaucoma among study participants under 40 years of age visiting glaucoma services
| Categories | New (n=160) (%) | Review (n=224) (%) | Total (n=384) (%) |
|---|---|---|---|
| Glaucoma | 19 (11.9) | 101 (45.1) | 120 (31.2) |
| Glaucoma suspect | 66 (41.2) | 59 (26.3) | 125 (32.6) |
| Nil glaucoma | 59 (36.9) | 21 (9.4) | 80 (20.8) |
| Other conditions predisposing to glaucoma§ | 16 (10.0) | 43 (19.2) | 59 (15.4) |
§include secondary raised IOP and miscellaneous conditions such as angle recession and retino-choroidal coloboma
Diagnoses
Secondary glaucomas (44.2%) were found to be more common than primary glaucomas (27.5%). Among the secondary glaucomas (n = 53), traumatic glaucoma (26.4%) was the most common followed by glaucoma in aphakia (16.9%) and steroid-induced glaucoma (15.1%). The most common causes for secondary raised IOP were steroid response and trauma. Congenital glaucomas constituted 28.3% of all glaucomas. Patients with congenital glaucoma had either primary congenital glaucoma (PCG) or secondary glaucoma, that is, glaucoma associated with ocular or systemic anomalies such as Axenfeld–Reiger’s syndrome, aniridia, nevus of Ota, Peter’s anomaly, nanophthalmos, and microspherophakia [Table 3].
Table 3.
Categorization of ocular diagnoses of patients visiting glaucoma services based on the specific etiologies
| Broad diagnosis | Specific etiology | New n (%) | Review n (%) | Total n (%) |
|---|---|---|---|---|
| Secondary raised IOP | Corticosteroid induced | 6 (3.7) | 15 (6.7) | 21 (5.4) |
| Trauma | 7 (4.4) | 7 (3.1) | 14 (3.6) | |
| Post retinal detachment surgery | 1 (0.6) | 6 (2.6) | 7 (1.8) | |
| Uveitis | 1 (0.6) | 4 (1.7) | 5 (1.3) | |
| Pigment dispersion syndrome | 0 | 1 (0.4) | 1 (0.2) | |
| Post SFIOL implantation | 0 | 1 (0.4) | 1 (0.2) | |
| Post vitreoretinal surgery | 0 | 1 (0.4) | 1 (0.2) | |
| Congenital glaucoma | Axenfeld-Reiger’s syndrome | 1 (0.6) | 4 (1.7) | 5 (1.3) |
| Aniridia | 0 | 5 (2.2) | 5 (1.3) | |
| Nevus of Ota | 1 (0.6) | 1 (0.4) | 2 (0.5) | |
| Peter’s anomaly | 0 | 1 (0.4) | 1 (0.2) | |
| Microspherophakia | 1 (0.6) | 2 (0.8) | 3 (0.8) | |
| Retino-choroidal coloboma | 0 | 2 (0.8) | 2 (0.5) | |
| Nanophthalmos | 1 (0.6) | 1 (0.4) | 2 (0.5) | |
| Primary congenital glaucoma | 0 | 14 (6.2) | 14 (3.6) | |
| Normal tension glaucoma | NTG | 0 | 1 (0.4) | 1 (0.2) |
| Juvenile open angle glaucoma | JOAG | 3 (1.8) | 22 (9.8) | 25 (6.5) |
| Primary angle closure glaucoma | PACG | 1 (0.6) | 4 (1.7) | 5 (1.3) |
| Primary angle closure | PAC | 1 (0.6) | 1 (0.4) | 2 (0.5) |
| Secondary open-angle glaucoma | Pigmentary glaucoma | 1 (0.6) | 1 (0.4) | 2 (0.5) |
| Uveitic glaucoma | 0 | 1 (0.4) | 1 (0.2) | |
| Traumatic glaucoma | 2 (1.2) | 11 (4.9) | 13 (3.3) | |
| Steroid-induced glaucoma | 2 (1.2) | 6 (2.6) | 8 (2.1) | |
| Glaucoma in aphakia | 1 (0.6) | 8 (3.5) | 9 (2.3) | |
| Post penetrating keratoplasty | 0 | 1 (0.4) | 1 (0.2) | |
| Post Retinal detachment surgery | 2 (1.2) | 6 (2.6) | 8 (2.1) | |
| Coats disease | 0 | 1 (0.4) | 1 (0.2) | |
| Ehler-Danlos syndrome | 0 | 1 (0.4) | 1 (0.2) | |
| Secondary angle closure glaucoma | Post retinal detachment | 1 (0.6) | 2 (0.8) | 3 (0.8) |
| Post retinal detachment surgery | 0 | 1 (0.4) | 1 (0.2) | |
| Traumatic glaucoma | 0 | 1 (0.4) | 1 (0.2) | |
| Uveitic glaucoma | 1 | 2 (0.4) | 3 (0.8) | |
| Topiramate induced | 0 | 1 (0.4) | 1 (0.2) | |
| Glaucoma suspect | Disc suspect | 54 (33.7) | 37 (16.5) | 91 (23.6) |
| PACS | 8 (5.0) | 8 (3.5) | 16 (4.1) | |
| Ocular hypertension | 4 (2.5) | 14 (6.2) | 18 (4.6) | |
| Miscellaneous conditions | Angle recession | 1 (0.6) | 7 (3.1) | 8 (2.1) |
| Retino-choroidal coloboma | 0 | 1 (0.4) | 1 (0.2) |
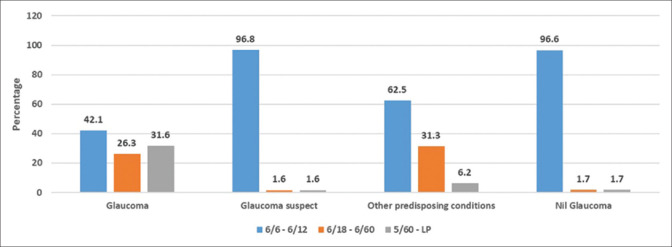
Fig. 1 shows the bar graph depicting the presenting visual acuity in the worse eye among the new patients. There were a significantly higher number of patients with severe visual impairment (visual acuity worse than 6/60 and better than or equal to 3/60) and blindness (visual acuity worse than 3/60) in the glaucoma subset than the other groups (31.6%, P < 0.001).
Figure 1.
Bar graph showing the presenting visual acuity in the worse eye or the study eye among the new patients
The mean IOP in those diagnosed with glaucoma was 32.9 mmHg and the mean CDR was 0.65. High mean presenting IOP (>30 mm Hg) among the new patients was seen in those with secondary raised IOP, JOAG, PACG, and secondary glaucomas. The mean presenting CDR was the highest in PACG (0.9) followed by JOAG (0.83) [Table 1 supplementary material].
Bilaterality was common in congenital glaucomas (79.4%), primary glaucomas (87.9%), and glaucoma suspects (92%), whereas unilaterality was common in secondary glaucomas (73.6%), secondary raised IOP (88%), and miscellaneous conditions (88.9%). Among the secondary congenital glaucomas, all patients with Axenfeld–Reiger’s syndrome, aniridia, microspherophakia, and nanophthalmos had bilateral involvement. All patients with Nevus of Ota and nanophthalmos were females, whereas JOAG (84%), pigmentary glaucoma (100%), steroid-induced glaucoma (87.5%), angle recession (75%), and traumatic glaucoma (76.9%) were more common in males. PACS was noted more in females (81.3%). The most common reason for referral among the new patients was a suspicious-looking optic disc (48.8%), whereas in the review patients it was raised IOP during their initial visit (57.1%) [Table 2 Supplementary Material]. Other reasons for referral included the presence of narrow angles, family history of glaucoma, history of trauma to check for angle recession, high hypermetropia, presence of megalocornea, aniridia, or history of use of anti-glaucoma medications.
Logistic regression analysis was done to find the factors associated with the presence of glaucoma [Table 4]. In both univariate and multivariate analyses, the odds of having glaucoma were less in females (odds ratio [OR] 0.6, 95% confidence interval [CI], 0.37–0.98; P = 0.04) and in patients with higher SLI (OR 0.13, 95% CI, 0.04–0.39; P < 0.001). Parameters such as age, family history, and educational status were not associated with the presence of glaucoma.
Table 4.
Logistic regression analysis to find the factors associated with the diagnosis of glaucoma among patients less than 40 years of age visiting glaucoma services
| Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
|
|
|
|||
| Odds (95% Confidence Interval) | P | Odds (95% Confidence Interval) | P | |
| Age | ||||
| ≤10 | 1.00 | 0.46 | 1.00 | |
| 11-20 | 0.715 (0.295-1.730) | 0.35 | 0.466 (0.164-1.315) | 0.15 |
| 21-30 | 0.665 (0.282-1.571) | 0.20 | 0.438 (0.153-1.250) | 0.12 |
| 31-40 | 0.573 (0.245-1.339) | 0.433 (0.161-1.164) | 0.09 | |
| Gender | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.589 (0.377-0.921) | 0.02 | 0.604 (0.374-0.975) | 0.04 |
| Education | ||||
| Uneducated | 1.00 | 1.00 | ||
| Primary school | 0.923 (0.209-4.062) | 0.92 | 1.494 (0.306-7.305) | 0.62 |
| Secondary school | 0.849 (0.188-3.822) | 0.83 | 2.168 (0.424-11.103) | 0.35 |
| Undergraduate | 0.726 (0.166-3.172) | 0.67 | 2.567 (0.492-13.375) | 0.26 |
| Post-graduate | 0.269 (0.048-1.493) | 0.13 | 1.055 (0.161-6.90) | 0.95 |
| Socio economic status | ||||
| Low | 1.00 | 1.00 | ||
| Medium | 0.504 (0.180-1.408) | 0.19 | 0.393 (0.134-1.154) | 0.09 |
| High | 0.192 (0.073-0.506) | 0.001 | 0.130 (0.043-0.390) | <0.001 |
| Family history of glaucoma | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.698 (0.394-1.238) | 0.22 | 0.941 (0.508-1.743) | 0.85 |
1.00 – Reference category; Boldface indicates statistical significance
Discussion
Our study is the first of its kind in the south Indian population aged 5 to 39 years performed in a prospective fashion. Moreover, this is the largest study to date to analyze the clinical and demographic profiles along with the reasons for referral among these patients visiting glaucoma services at a tertiary eye care hospital. The most common reasons for referral of patients to glaucoma services were found to be raised IOP and suspicious disc. Secondary glaucomas (44.1%) were found to be more common than primary glaucomas (27.5%) or congenital glaucomas (28.3%).
We found that 31% of participants had some form of glaucoma. Our sample is of course a group of patients referred for glaucoma evaluation and is not representative of glaucoma prevalence in the general population. The prevalence of glaucoma in the 18–40 years age group was reported to be 0.16% in the Gutenberg health study done on Caucasian Whites.[3]
It is known that low levels of education are strongly associated with late presentation of glaucoma.[16] In our study, we found that the percentage of glaucoma was higher in patients who had just a school education. However, we did not find any association between educational status and the proportion of advanced disease (P = 0.704). Studies have found that the level of education has an influence on the knowledge and awareness of glaucoma with higher education being associated with greater knowledge of glaucoma and vice versa.[17,18] Though poor education has been linked to adult glaucoma,[16] we did not find such an association as our cohort comprised only people aged below 40. These differences can be attributed to the age group of the cohort studied.
Reports show that glaucoma has also been associated with poor socioeconomic status.[19,20,21] Our study also reflects similar findings. In both univariate and multivariate analysis, high SLI was associated with lesser chances of having glaucoma.
In this age group, we found that secondary glaucomas were more common than primary glaucomas. Steroid response and trauma were the most common causes of secondary raised IOP. Children are more prone to trauma; furthermore, they can have an exaggerated steroid response making them vulnerable to irreversible vision loss if not evaluated in a timely manner.[22] We also found that secondary congenital glaucomas were more common than PCG. Literature search reveals contrasting information with some studies reporting the same,[9] whereas others report PCG to be more common than secondary congenital glaucomas.[7,23] Axenfeld–Reiger’s syndrome was found to be most common among secondary congenital glaucomas, which is in line with the existing literature.[23] Aravind comprehensive eye survey, a population-based prevalence survey in a south Indian population above 40 years of age reported that primary glaucomas were more common than secondary glaucomas.[6] In contrast to adult glaucomas, our study in the under 40 population demonstrates that secondary glaucomas are more common than primary, indicating a change in the trend with increasing age. However, if we look at age-wise distribution, congenital glaucomas were the most common in 5–10 years, secondary glaucomas, especially traumatic and steroid-induced glaucoma in 11–20 and 21–30 years, with primary as well as secondary glaucomas becoming equal in 31–39 years age group indicating the rise in the proportion of primary glaucomas with age.
The proportion of PCG was equal in both genders, whereas PACS was noted more in females in line with the existing literature.[24] A higher number of males were affected with traumatic glaucoma, secondary raised IOP, and angle recession showing a higher incidence of trauma in males, probably due to more outdoor activities. Secondary glaucomas including those following lens removal, retinal detachment surgery, or retinal detachment itself were also more common in males. This is probably a reflection of the greater predisposition to retinal detachment in males.[25,26] As would be expected, primary glaucomas, congenital glaucomas, and glaucoma suspects were bilateral, whereas secondary glaucomas and secondary raised IOP were commonly unilateral.
The presenting BCVA worse than 6/60 was found in 31.6% of glaucoma patients, whereas this proportion was very less in the other categories of patients. The new patients with JOAG, PACG, and secondary glaucomas also presented with larger mean CDR, ranging from 0.75 to glaucomatous optic atrophy indicating advanced disease at presentation. This highlights that if secondary causes are treated appropriately and glaucoma suspects are monitored periodically, progression to glaucoma and vision loss can be lowered. Consequently, the unnecessary economic burden on society can be reduced.
Among the new patients, mean IOP at presentation was found to be above 30 mmHg in JOAG, PACG, secondary glaucomas, and secondary raised IOP. Of note, these are the conditions, which need treatment on an urgent basis to avoid progression. Garzon et al.[27] analyzed the importance of measuring IOP as a part of routine ophthalmic screening in patients aged 18 to 40 years. They found that IOP assessment was quite cost-effective in picking up patients with glaucoma and it cost around 596 Indian rupees (INR) for identifying one such patient.
Among the new patients, a suspicious-looking optic disc was the most common reason for referral. The review group predominantly consisted of patients with glaucoma and the commonest reason for their referral during the initial visit was a raised IOP. Potential causes for the difference in the reasons for a referral might be that those with suspicious discs among the review patients might have undergone investigations during the initial visits and might not have been advised frequent reviews, whereas those with raised IOP need to be watched closely, leading to frequent review visits. Similarly, raised IOP and suspicious disc were reported to be the common reasons for referral in the community and hospital allied network glaucoma evaluation scheme where optometrists with special interest monitored the patients under virtual supervision.[28] Furthermore, routinely measuring the IOP in all young adults who seek care for other reasons has been found to be a very effective method in early diagnosis.[27] Optic disc photography is a proven, cost-effective way of identifying and monitoring glaucomatous optic disc changes. Moreover, it provides a good baseline record to assess progression, offers valuable clinical information to ophthalmologists, and is also easier to interpret than the sophisticated, expensive newer imaging techniques.[29] Our study highlights this information and confirms the importance of a comprehensive evaluation including IOP and disc evaluation even in the under 40 age group.
Family history is associated with a 2–4 times higher risk of developing POAG.[30,31,32] Furthermore, the risk of angle closure in a sibling of a proband with primary angle closure was found to increase by 1.5 folds with every 10 years increase in age.[33] Yet, family history was not associated with an increased risk of glaucoma in our study. Nearly 20% of the entire study population and only 16% of patients with glaucoma had a positive family history. This is possibly due to the younger age of participants. In general, POAG and angle closure develop in older individuals, and these patients with a family history will need a longitudinal follow-up as they can develop glaucoma later in life.[33]
A thorough literature search revealed a scarcity of prospective studies in the under 40 age group proving to be the main advantage of our study. We had included all the patients, irrespective of their new or follow-up status. Moreover, all the evaluations were carried out by glaucoma experts in contrast to other studies where optometrists or a virtual examination was preferred, thus eliminating the chances of examination errors. Our study excluded children below 5 years of age as congenital glaucomas comprise the most common form of glaucoma in that age group. There are previous studies looking at their prevalence, clinical features, and demography and we would not have added much information to the existing literature. Our study is limited by the fact that the presenting visual acuity, IOP, and CDR were analyzed only for the new patients. The period of the study included the period with COVID-19-related lockdown and the following period when restrictions were lifted. However, more than 75% of the recruitment was completed before the pandemic and hence the pandemic is unlikely to impact our observations. We included only the south Indian cohort; hence, the findings might not be generalizable to the rest of the world.
Conclusion
To conclude, secondary glaucomas such as traumatic glaucomas, glaucoma in aphakia, and steroid-induced glaucomas are more common than primary glaucomas in the under 40 age group. Our study along with Garzon et al.[27] stresses the importance of routine comprehensive examination including IOP measurement, gonioscopy, and fundus evaluation without which glaucoma in this age group can be overlooked. More caution is needed, especially when there is either history or findings suggestive of possible secondary glaucoma including trauma, steroid usage, or intraocular surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Table 1 supplementary material.
Range of presenting intraocular pressures and cup to disc ratios among new patients less than 40 years of age visiting glaucoma services based on the diagnosis
| Broad diagnosis | Intra-Ocular Pressure | Cup-to-Disc Ratio | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | Mean (SD) | Min - Max | n | Mean (SD) | Min - Max | |
| Secondary raised IOP | 15 | 31.47 (8.26) | 22-48 | 15 | 0.38 (0.11) | 0.30-0.60 |
| Congenital glaucoma | 4 | 23.50 (11.0) | 14-38 | 4 | 0.62 (0.22) | 0.40-0.90 |
| Juvenile open angle glaucoma | 3 | 30.67 (13.61) | 20-46 | 3 | 0.83 (0.11) | 0.70-0.90 |
| Primary angle closure glaucoma | 1 | 52 (-) | 52-52 | 1 | 0.90 (-) | 0.90-0.90 |
| Primary angle closure | 1 | 26 (-) | 26-26 | 1 | 0.50 (-) | 0.50-0.50 |
| Secondary open-angle glaucoma | 8 | 30.75 (9.68) | 14-44 | 8 | 0.75 (0.14) | 0.60-1.0 |
| Secondary angle closure glaucoma | 2 | 35 (7.07) | 30-40 | 2 | 0.35 (0.07) | 0.30-0.40 |
| Glaucoma suspect | 66 | 16.64 (4.07) | 10-32 | 66 | 0.61 (0.15) | 0.30-0.80 |
| Total | 100 | 21.44 (9.39) | 10-52 | 100 | 0.59 (0.18) | 0.30-1.0 |
Table 2 Supplementary Material.
Reasons for referral of patients under 40 years to glaucoma clinic at their first visit
| Referral reasons at first visit | New Patients n=160(%) | Review patients n=224(%) | Total n=384(%) |
|---|---|---|---|
| Disc suspect | 78 (48.8) | 5 (22.8) | 129 (33.6) |
| Raised IOP | 35 (21.9) | 128 (57.1) | 163 (42.4) |
| Narrow angles | 12 (7.5) | 11 (4.9) | 23 (5.9) |
| Family screening | 23 (14.4) | 5 (2.2) | 28 (7.3) |
| Others¶ | 6 (3.7) | 15 (6.7) | 21 (5.5) |
| Disc suspect + raised IOP | 0 | 1 (0.5) | 1 (0.3) |
| Disc suspect + family screening | 4 (2.5) | 3 (1.3) | 7 (1.8) |
| Raised IOP + family screening | 1 (0.6) | 1 (0.5) | 2 (0.5) |
| Raised IOP + narrow angles | 1 (0.6) | 5 (2.2) | 6 (1.6) |
Others include gonioscopy to rule out angle recession, megalocornea, aniridia, history of use of anti-glaucoma medications, high hypermetropia
Acknowledgments
The authors thank Mrs. Iswarya and Ms. Divya Dharshini, Biostatisticians, Aravind Eye Hospital for the statistical analysis. The authors thank Dr. Lisa Tom, Bascom Palmer Eye Institute, University of Miami, Florida, USA, for her valuable inputs.
References
- 1.GBD 2019 Blindness and Vision Impairment Collaborators;Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020:The Right to Sight:An analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–60. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guedes G, Tsai JC, Loewen NA. Glaucoma and aging. Curr Aging Sci. 2011;4:110–7. doi: 10.2174/1874609811104020110. [DOI] [PubMed] [Google Scholar]
- 3.Marx-Gross S, Laubert-Reh D, Schneider A, Höhn R, Mirshahi A, Münzel T, et al. The prevalence of glaucoma in young people:Findings of the population-based Gutenberg Health Study. Dtsch Arztebl Int. 2017;114:204. doi: 10.3238/arztebl.2017.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonas JB, Gründler A. Optic disc morphology in juvenile primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1996;234:750–4. doi: 10.1007/BF00189356. [DOI] [PubMed] [Google Scholar]
- 5.Vijaya L, Rashima A, Panday M, Choudhari NS, Ramesh SV, Lokapavani V, et al. Predictors for incidence of primary open-angle glaucoma in a South Indian population:The Chennai eye disease incidence study. Ophthalmology. 2014;121:1370–6. doi: 10.1016/j.ophtha.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan R, Nirmalan PK, Krishnadas R, Thulasiraj RD, Tielsch JM, Katz J, et al. Glaucoma in a rural population of southern India:The Aravind comprehensive eye survey. Ophthalmology. 2003;110:1484–90. doi: 10.1016/S0161-6420(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 7.Senthil S, Badakere S, Ganesh J, Krishnamurthy R, Dikshit S, Choudhari N, et al. Profile of childhood glaucoma at a tertiary center in South India. Indian J Ophthalmol. 2019;67:358–65. doi: 10.4103/ijo.IJO_786_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papadopoulos M, Cable N, Rahi J, Khaw PT BIG Eye Study Investigators. The British infantile and childhood glaucoma (BIG) eye study. Invest Ophthalmol Vis Sci. 2007;48:4100–6. doi: 10.1167/iovs.06-1350. [DOI] [PubMed] [Google Scholar]
- 9.Aponte EP, Diehl N, Mohney BG. Incidence and clinical characteristics of childhood glaucoma:A population-based study. Arch Ophthalmol. 2010;128:478–82. doi: 10.1001/archophthalmol.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung DS, Roensch MA, Kooner KS, Cavanagh HD, Whitson JT. Epidemiology and characteristics of childhood glaucoma:Results from the Dallas Glaucoma Registry. Clin Ophthalmol. 2013;7:1739–46. doi: 10.2147/OPTH.S45480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouhenni RA, Ricker I, Hertle RW. Prevalence and clinical characteristics of childhood glaucoma at a tertiary care children's hospital. J Glaucoma. 2019;28:655–9. doi: 10.1097/IJG.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 12.Gupta V, Ganesan VL, Kumar S, Chaurasia AK, Malhotra S, Gupta S. Visual disability among juvenile open-angle glaucoma patients. J Glaucoma. 2018;27:e87–9. doi: 10.1097/IJG.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 13.Moune E, Bella-Hiag A. Le glaucomejuvénile au Cameroun. Bull Soc Belge Ophtalmol. 2007;305:69–77. [PubMed] [Google Scholar]
- 14.Komolafe O, Olawoye O, Fafowora O, Ashaye A, Baiyeroju AM. Demographic and clinical profile of patients with juvenile onset open angle glaucoma in southwestern Nigeria. Niger J Clin Pract. 2011;14:395–9. doi: 10.4103/1119-3077.91742. [DOI] [PubMed] [Google Scholar]
- 15.National Family Health Survey (NFHS-2), 1998-99. India. Mumbai: IIPS; 2000. International Institute of Population Sciences (IIPS) and ORC Macro. [Google Scholar]
- 16.Motlagh BF, Pirbazari TJ. Risk factors for late presentation of chronic glaucoma in an Iranian population. Oman JOphthalmol. 2016;9:97–100. doi: 10.4103/0974-620X.184527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celebi AR. Knowledge and awareness of glaucoma in subjects with glaucoma and their normal first-degree relatives. Med Hypothesis Discov Innov Ophthalmol. 2018;7:40–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Kizor-Akaraiwe NN, Monye HI, Okeke S. Awareness and knowledge about glaucoma and proportion of people with glaucoma in an urban outreach programme in Southeast Nigeria. BMJ Open Ophthalmol. 2017;1:e000018. doi: 10.1136/bmjophth-2016-000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser S, Bunce C, Wormald R. Retrospective analysis of risk factors for late presentation of chronic glaucoma. Br J Ophthalmol. 1999;83:24–8. doi: 10.1136/bjo.83.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravarti T. The Association of socioeconomic status with severity of glaucoma and the impacts of both factors on the costs of glaucoma medications:A cross-sectional study in West Bengal, India. J Ocul Pharmacol Ther. 2018;34:442–51. doi: 10.1089/jop.2017.0135. [DOI] [PubMed] [Google Scholar]
- 21.Eslami Y, Amini H, Zarei R, Fakhraie G, Moghimi S, Mohammadi SF, et al. Socioeconomic factors and disease severity at glaucoma presentation. Iran J Ophthalmol. 2011;23:19–26. [Google Scholar]
- 22.Roberti G, Oddone F, Agnifili L, Katsanos A, Michelessi M, Mastropasqua L, et al. Steroid-induced glaucoma:Epidemiology, pathophysiology, and clinical management. Surv Ophthalmol. 2020;65:458–72. doi: 10.1016/j.survophthal.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Tamçelik N, Atalay E, Bolukbasi S, Çapar O, Ozkok A. Demographic features of subjectswith congenital glaucoma. Indian J Ophthalmol. 2014;62:565. doi: 10.4103/0301-4738.126988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vijaya L, George R, Arvind H, Baskaran M, Paul PG, Ramesh SV, et al. Prevalence of angle-closure disease in a rural southern Indian population. Arch Ophthalmol. 2006;124:403–9. doi: 10.1001/archopht.124.3.403. [DOI] [PubMed] [Google Scholar]
- 25.Mehdizadeh M, Afarid M, Haqiqi MS. Risk factors for giant retinal tears. J Ophthalmic Vis Res. 2010;5:246–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Mitry D, Charteris DG, Yorston D, Siddiqui MR, Campbell H, Murphy AL, et al. The epidemiology and socioeconomic associations of retinal detachment in Scotland:A two-year prospective population-based study. Invest Ophthalmol Vis Sci. 2010;51:4963–8. doi: 10.1167/iovs.10-5400. [DOI] [PubMed] [Google Scholar]
- 27.Garzon C, Odayappan A, Kavitha S, Venkatesh R, Friedman DS. The impact of routinely measuring IOP in younger adults to screen for glaucoma in a large eye hospital. J Glaucoma. 2020;29:362–6. doi: 10.1097/IJG.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 28.Mandalos A, Bourne R, French K, Newsom W, Chang L. Shared care of patients with ocular hypertension in the Community and Hospital Allied Network Glaucoma Evaluation Scheme (CHANGES) Eye. 2012;26:564–7. doi: 10.1038/eye.2011.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaeth GL, Reddy SC. Imaging of the optic disk in caring for patients with glaucoma:Ophthalmoscopy and photography remain the gold standard. Surv Ophthalmol. 2014;59:454–8. doi: 10.1016/j.survophthal.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence of open-angle glaucoma:The visual impairment project. Invest Ophthalmol Vis Sci. 2003;44:3783–9. doi: 10.1167/iovs.03-0077. [DOI] [PubMed] [Google Scholar]
- 31.Green CM, Kearns LS, Wu J, Barbour JM, Wilkinson RM, Ring MA, et al. How significant is a family history of glaucoma? Experience from the Glaucoma Inheritance Study in Tasmania. Clin Exp Ophthalmol. 2007;35:793–9. doi: 10.1111/j.1442-9071.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien JM, Salowe RJ, Fertig R, Salinas J, Pistilli M, Sankar PS, et al. Family history in the primary open-angle African American glaucoma genetics study cohort. Am J Ophthalmol. 2018;192:239–47. doi: 10.1016/j.ajo.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavitha S, Zebardast N, Palaniswamy K, Wojciechowski R, Chan ES, Friedman DS, et al. Family history is a strong risk factor for prevalent angle closure in a South Indian population. Ophthalmology. 2014;121:2091–7. doi: 10.1016/j.ophtha.2014.05.001. [DOI] [PubMed] [Google Scholar]