Abstract
Purpose:
This study was conducted to determine the morphological and functional retinal changes in patients with neovascular age-related macular degeneration (nAMD) treated with intravitreal bevacizumab 1.25 mg.
Methods:
This was a prospective, nonrandomized, interventional study. Eighteen eyes of 18 subjects with nAMD were treated with intravitreal bevacizumab (1.25 mg) injection. Subjects underwent complete ophthalmic evaluation which included visual acuity, slitlamp examination, tonometry, binocular ophthalmoscopy, optical coherence tomography (OCT), and MP1 microperimetry before the intravitreal injection and the follow-up at 1 and 3 months. Test of significance such as Chi-squared test, paired ttest and oneway analysis of variance (ANOVA) linear trend were used to compare the pre- and post-anti-VEGF outcomes. Intraclass correlation was done to assess the intra observer variability.
Results:
Mean retinal sensitivity had increased from 3.77 ± 3.13 dB at baseline to 4.93 ± 2.42 dB at 3 months (P = 0.05). Visual acuity improved from 0.62 ± 0.36 at baseline to 0.52 ± 0.36 at 1 month and 0.48 ± 0.34 at 3-month followup, but overall change was not significant (P = 0.40). There was a significant reduction in central foveal thickness (CFT) from 274.61 ± 117.95 at baseline to 179.83 ± 84.18 at 1 month and 179.00 ± 126.55 at 3-month follow-up (P = 0.013).
Conclusion:
Intravitreal bevacizumab (1.25 mg) injection in nAMD improves retinal function, quantified by retinal sensitivity, scotoma characteristics, fixation stability by MP 1 microperimetry and morphological parameters quantified by CFT in SDOCT. These changes show the effectiveness of treatment with intravitreal bevacizumab in nAMD.
Keywords: Bevacizumab, macular changes, neovascular AMD, retinal function
Wet, exudative, neovascular or disciform age-related macular degeneration (AMD) is characterized by leakage of fluid or hemorrhage from choroidal neovascularization (CNV), which can lead to acute and permanent central vision loss.[1] AMD accounts for 8.7% of world blindness and is the leading cause of irreversible blindness in people >50 years of age in developed countries.[1,2] The previous study determined that the overall prevalence of AMD will increase to 196 million people in 2020 and 288 million by 2040.[2] Although neovascular age-related macular degeneration (nAMD) represents only 10% to 15% of the overall prevalence of AMD, it is responsible for severe vision loss or legal blindness in more than 80% of cases.[1,3,4,5] In the early stages of nAMD, patients complain about the reduction in their quality of life. In progressive stages of the disease, patients develop central dense scotoma (i.e. absolute) due to the subfoveal location of the lesion.[6] The current standard of treatment in nAMD is the use of intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy either alone or in combination with other modalities like photo-dynamic treatment. Previous studies have shown that intravitreal ranibizumab is effective in the treatment of all subtypes of subfoveal CNV.[7,8,9] Despite the licensing of ranibizumab, the most common globally used agent to treat nAMD is off-license bevacizumab. Krebs et al.[10] reported that the bevacizumab was equivalent to ranibizumab for visual acuity at all time-points over 1 year. Also, there was no significant difference of decrease of retinal thickness or number of adverse events.
The aim of the study was to determine the short-term macular morphological and functional changes of the retina in patients with nAMD treated with intravitreal bevacizumab 1.25 mg. Additionally, the depth, size and fixation pattern (stability, location, and preferred retinal locus) of the macular scotoma in patients with central vision loss caused by nAMD has been extensively studied.
Methods
A prospective study was conducted in patients with nAMD who attended the outpatient clinic of a tertiary eye care hospital from May 2010 to April 2011. We included subjects ≥50 years of age with diagnosis of active choroidal neovascular membrane (CNVM) secondary to AMD requiring anti-VEGF treatment, best-corrected visual acuity (BCVA) of more than 20/200, presence of subretinal fluid, cystic maculopathy, or central retinal thickness >250 mm. Only subjects with typical nAMD were included. Exclusion criteria were polypoidal choroidal vasculopathy, subjects with a history of any previous treatment for CNVM, other associated ocular conditions and opaque ocular media that affect the quality OCT and microperimetry. The study was approved by the institutional review board and a written informed consent was obtained from the subjects per the tenets of the Declaration of Helsinki. A comprehensive ophthalmic evaluation was conducted before the intravitreal injection and at follow-up examinations at 1 month and 3 months after the injection. It includes visual acuity measurement, slitlamp examination, tonometry, pupils dilated to ≥6 mm in diameter (using tropicamide 0.5 mg/mL), binocular ophthalmoscopy, fourfield stereoscopic 45° color fundus photography, OCT (SDOCT, Copernicus, Optopol Technologies, Zawierci, Poland). and MP-1 microperimetry (Nidek Technologies, Padova, Italy), all of which were performed before intravitreal injection. Visual acuity measurement, MP1 and OCT evaluations were repeated at 1- and 3-month follow-up.
Intravitreal bevacizumab (1.25 mg) injection was administered to all subjects with the same treatment and re-treatment protocols. On followup, the decision of re-treatment was done based on visual deterioration of >5 letters loss of visual acuity, evidence of persistent subretinal fluid or newly developed macular hemorrhage. Spectral domain OCT (SD-OCT) was performed (Copernicus, Optopol Technologies, Zawierci, Poland) through a dilated pupil. An asterisk scan and 3D scan protocol were chosen. The parameters measured on SDOCT were central foveal thickness (CFT), outer high reflectivity band thickness (OHRBT), and retinal thickness in 9 ETDRS regions. MP-1 microperimetry (Nidek Technologies, Padova, Italy) was performed in a dark room through a dilated pupil. The standard Goldmann III size test spot with stimulus intensity of 16 dB and a grid covering the central 20° area (1°=300 microns, hence 20°=6000 microns) with 33 stimuli points centered on the fovea was used. A 42 double staircase strategy was applied for measuring threshold. A white background with an illumination of 1.27 cd/m2 was used. All subjects were given a pretest stimuli (training) before starting the test to make them aware of the procedure and to minimize the learning curve. The fixation target size was increased until the patient appreciated it. A trigger button was used to respond to the stimulus. False-negative or false-positive results of 5% or higher were included. The retinal sensitivity was measured in central 8°, 12°, and full field central 20°. The fundus tracking software in the instrument measures the location and stability of fixation.
The fixation stability was assessed according to the classification of Fujii et al.[11] The standard central fixation was approximately a 2° diameter (600 microns) centered on the fovea. A predominantly central fixation was with >50% of preferred fixation points located within the central circle, poor central fixation was with >25% but <50%, and predominantly eccentric fixation was with <25%. Eyes with >75% of fixation points located within central 2° were classified as stable fixation. Eyes having <75% fixation points located within 2° but >75% fixation points within the central 4° were labeled as relatively unstable fixation and those with <75% as unstable fixation. Additionally, we also classified the location of PRL as (A) central position, (B) superonasal, (C) superior, (D) superotemporal, (E) inferotemporal, (F) inferior, and (G) Inferonasal to the macula.
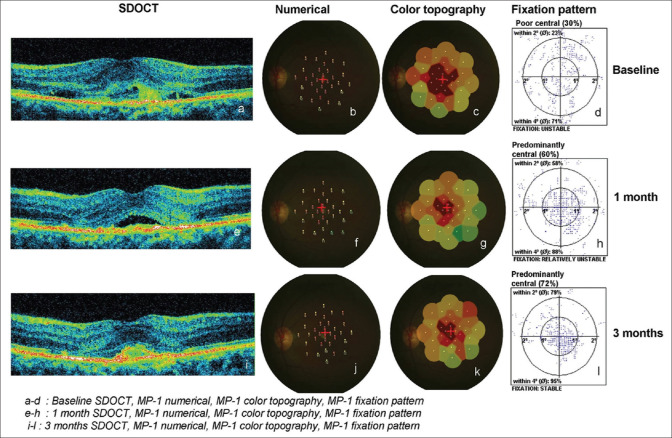
The density and size of the scotoma was categorized as (1) an absolute scotoma when no threshold could be seen (0 dB); (2) a relative scotoma when a threshold value of ≥0 to <10 dB was seen; (3) a normal function when a threshold value of ≥10 to ≤20 dB was noted.[11] Out of 33 test points, the number of absolute scotoma points (<0 dB) was considered as the size of absolute scotoma and the number of relative test points locations was considered as relative scotoma size. The number of test point locations of normal macular function (≥10 dB) was considered as no scotoma area or normal sensitivity area. Fig. 1 shows the SDOCT and microperimetry parameters with a case example.
Figure 1.
Clinical parameters at baseline and follow-up visits (case example)
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) 13 (IBM Corp., Armonk, NY). Data are presented as mean ± standard deviation. Test of significance such as Chi-squared test, paired ttest, and oneway analysis of variance (ANOVA) linear trend were used to compare the pre- and post-anti-VEGF outcomes. Intraclass correlation was done to assess the intra observer variability. A P value of <0.05 was considered statistically significant.
Results
A total of 18 eyes of 18 subjects who met the inclusion and exclusion criteria were recruited for the study. There were 16 males and 2 females. The mean age was 63 ± 8 years (range: 50–81 years). Baseline characteristics of the participants are summarized in Table 1. The mean duration of vision loss was 6.4 ± 6.4 months. At the baseline, the mean BCVA was 0.62 ± 0.36 (logMAR). Subjects received a mean of 2.38 injections (9 patients had 3 injections, 7 had 2 injections and 2 had 1 injection) within a 3-month period. No complications or adverse events happened with intravitreal bevacizumab for any of the participants.
Table 1.
Baseline demographic and clinical parameters
| Characteristics | Baseline |
|---|---|
| Age (years) | 63.0±8 (50-81) |
| *DOV (months) | 6.4±6.4 (1-24) |
| Gender | |
| Male | 16 (89%) |
| Female | 2 (11%) |
| †BCVA (logMAR) | 0.62±0.36 |
| Classification of ‡CNVM | |
| Site of Lesion | |
| Subfoveal | 15 (83%) |
| Juxtafoveal | 3 (17%) |
| Type of Lesion | |
| Classic | 6 (33%) |
| Occult | 12 (67%) |
| OCT Qualitative | |
| Retinal thickening | 14 (78%) |
| §RPE detachment | 13 (72%) |
| Subretinal fluid | 14 (78%) |
*DOV: Duration of decrease of vision; †BCVA: Best-corrected visual acuity; ‡CNVM: Choroidal neovascular membrane; §RPE: Retinal pigment epithelium
The mean BCVA was improved from 0.62 ± 0.36 at baseline to 0.52 ± 0.36 at 1 month (P = 0.174) and to 0.48 ± 0.34 (P = 0.104) at 3 months. The overall change in BCVA was not statistically significant (P = 0.40).
Central foveal thickness (CFT) showed a significant reduction after antiVEGF at both 1-month and 3-month followup visits (274.61 ± 117.95 at baseline to 179 ± 126.55 at 3 months; P = 0.028). The overall change in CFT was statistically significant (P = 0.013). Table 2 summarizes the distribution of retinal thickness parameters in the 9 Early Treatment Diabetic Retinopathy Study (ETDRS) regions. Mean retinal thickness showed a significant reduction in central 1 mm ring from baseline at both 1- and 3-month followup visits (335.28 ± 122.16 to 239.78 ± 114.65 at 1 month; P = 0.008 and to 231.39 ± 114.65 at 3 months; P = 0.013). Similarly, in inner 3 mm, there was a significant reduction in superior and inferior quadrants at both follow-ups. However, in the nasal and temporal quadrant, the thickness showed a significant reduction at 1 month, and moderate but not significant at 3 months after injection. In the outer 6 mm ring, only the inferior quadrant showed a significant reduction from baseline (304.83 ± 49.60 to 273.00 ± 31.96; P = 0.023) 3 months after anti-VEGF therapy.
Table 2.
Comparison of retinal thickness parameters in 9 ETDRS regions at baseline, 1 and 3 months after anti-VEGF
| Region | Baseline (n=18) Mean±SD | 1-month follow-up (n=18) Mean±SD | 3-month follow-up (n=18) Mean±SD | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| ‡ B vs. F | § B vs. S | trend | ||||
| * CFT | 274.61±117.95 | 179.83±84.18 | 179.00±126.55 | 0.012 | 0.028 | 0.013 |
| † OHRBT | 110.94±66.70 | 90.11±66.41 | 96.06±59.51 | 0.233 | 0.396 | 0.49 |
| 9 ETDRS region | ||||||
| Central 1 mm ring | 335.28±122.16 | 239.78±114.65 | 231.39±114.65 | 0.008 | 0.013 | 0.006 |
| Inner 3 mm Ring | ||||||
| Superior | 347.44±64.52 | 300.89±70.09 | 305.56±76.31 | 0.033 | 0.058 | 0.081 |
| Inferior | 358.39±74.33 | 300.78±64.37 | 307.61±82.43 | 0.025 | 0.007 | 0.029 |
| Temporal | 341.50±82.43 | 287.44±64.59 | 288.83±82.73 | 0.032 | 0.071 | 0.045 |
| Nasal | 343.22±85.32 | 288.22±66.00 | 301.94±83.91 | 0.015 | 0.072 | 0.123 |
| Outer 6 mm Ring | ||||||
| Superior | 293.89±39.87 | 287.33±38.10 | 282.00±38.39 | 0.481 | 0.329 | 0.362 |
| Inferior | 304.83±49.60 | 301.28±107.18 | 273.00±31.96 | 0.887 | 0.023 | 0.182 |
| Temporal | 281.72±39.55 | 278.06±29.16 | 285.17±39.33 | 0.768 | 0.82 | 0.777 |
| Nasal | 309.28±55.27 | 284.72±49.58 | 293.44±47.95 | 0.056 | 0.248 | 0.356 |
*CFT: Central foveal thickness; †OHRBT: Outer high reflectivity band thickness; ‡B vs. F: Baseline versus 1-month follow-up; §B vs. S: Baseline versus 3-month follow-up
Mean retinal sensitivity showed a significant improvement in all degrees (8°, 12° and 20°) by microperimetry 1 month after antiVEGF injection (P = 0.033, P = 0.03, P = 0.015, respectively). However, at 3-month followup we found moderate but not significant improvement (P = 0.26, P = 0.05, P = 0.057 for 8°, 12°, and 20°, respectively). Table 3 shows the change in mean retinal sensitivity at 8°, 12°, and 20° macular fields in baseline, 1, and 3 months after antiVEGF therapy. At 1 month, 9 eyes (6 eyes; 2 to <4 dB and 3 eyes; >4 dB) had improved mean retinal sensitivity of ≥2 dB. Three months after anti-VEGF treatment, 6 eyes (3 eyes; 2 to <4 dB and 3 eyes; >4 dB) had improved mean retinal sensitivity of ≥2 dB. We found that at 1 month, 7 eyes improved by 0 to <2 dB, and at 3 months 8 eyes improved by 0 to <2 dB. Whereas 2 eyes at 1 month and 4 eyes at 3 months showed a reduction in mean retinal sensitivity. Fig. 2 shows the change in mean retinal sensitivity 1 and 3 months after anti-VEGF therapy.
Table 3.
Change in mean retinal sensitivity 8°, 12°, and 20° degree macular field at baseline, 1, and 3 months after anti-VEGF
| Mean Retinal Sensitivity (dB) | Baseline n=18 | 1-month follow-up n=18 | 3-month follow-up n=18 | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| *B vs. F | †B vs. S | Trend | ||||
| 8° | 0.73±1.34 | 1.97±2.32 | 1.30±1.86 | 0.033 | 0.26 | 0.367 |
| 12° | 2.23±2.77 | 3.94±2.38 | 3.36±2.49 | 0.03 | 0.057 | 0.191 |
| 20° | 3.77±3.13 | 5.59±2.56 | 4.93±2.42 | 0.015 | 0.057 | 0.205 |
*B vs. F: Baseline versus 1-month follow-up; †B vs. S: Baseline versus 3-month follow-up
Figure 2.
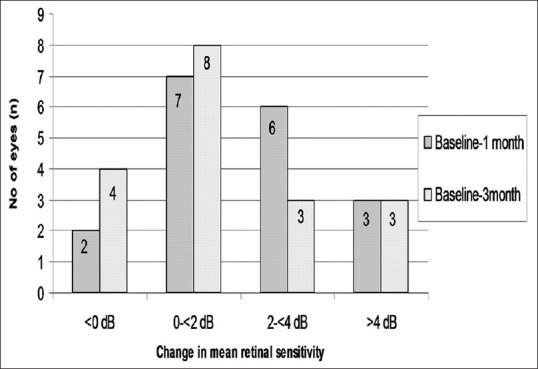
Change in mean retinal sensitivity 1 and 3 months after anti-VEGF treatment
On the whole, the total number of points within the absolute scotoma at each examination decreased significantly (16 points at baseline; 7 points at 1 month; 10 points at 3 months). Overall change was statistically significant (P = 0.030). Fig. 3 shows the change in mean absolute scotoma (0 dB), relative scotoma, and normal (no scotoma) function at test point locations. The mean relative scotoma (0 to <10 dB) in 33 test point location had increased significantly from 10 at baseline to 16 at 1 month (P ≤ 0.0031) then reduced but remained to 15 test point locations at the 3-month follow-up (P = 0.013). Overall change was statistically significant (P = 0.030). The mean normal sensitivity (>10 dB) test point location had increased significantly from 5 points measured at baseline to 7 points (P = 0.009) at 1 month and to 6 points (P = 0.37) at 3 months. The overall change was not statistically significant (P = 0.37).
Figure 3.
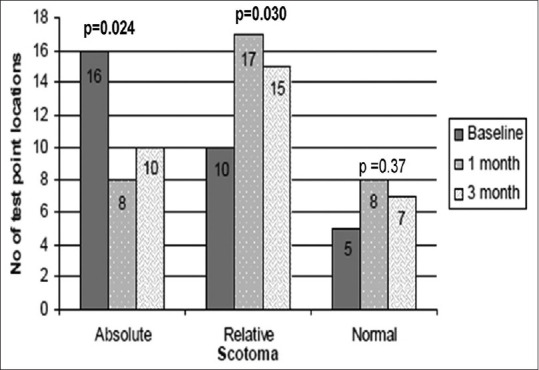
Change in mean absolute scotoma, relative, and normal (no scotoma) size at test point locations
Table 4 shows the fixation pattern at baseline, 1 month, and 3 months after injection. The fixation pattern was improved but not statistically significant (P > 0.05) except the unstable fixation was seen in 7 eyes (38.9%) at baseline and was significantly reduced to 1 eye (5.6%) at 1 month after anti-VEGF.
Table 4.
Changes in microperimetry fixation pattern at baseline, 1, and 3 months after anti-VEGF
| Fixation Pattern | Baseline (n=18) | 1-month follow-up (n=18) | 3-month follow-up (n=18) | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| *B vs. F | †B vs. S | Trend | ||||
| Fixation Stability | ||||||
| Stable | 4 (22.2) | 7 (38.9) | 9 (50.0) | 0.471 | 0.164 | 0.084 |
| Relatively unstable | 9 (50.0) | 9 (50.0) | 7 (38.9) | 1 | 0.738 | 0.742 |
| Unstable | 5 (27.8) | 2 (11.1) | 2 (11.1) | 0.402 | 0.402 | 0.179 |
| Fixation Location | ||||||
| Predominantly central | 7 (38.9) | 11 (61.1) | 12 (66.7) | 0.317 | 0.181 | 0.093 |
| Poor central | 7 (38.9) | 1 (5.6) | 4 (22.2) | 0.041 | 0.47 | 0.229 |
| Predominantly eccentric | 4 (22.2) | 6 (33.3) | 2 (11.1) | 0.711 | 0.658 | 0.423 |
| Preferred Retinal Loci | ||||||
| Central | 9 (50.0) | 8 (44.4) | 8 (44.4) | 1 | 1 | 0.738 |
| Superonasal | 1 (5.6) | 2 (11.1) | 3 (16.7) | 1 | 0.603 | 0.289 |
| Superior | 3 (16.7) | 3 (16.7) | 4 (22.2) | 1 | 1 | 0.668 |
| Superotemporal | 2 (11.1) | 2 (11.1) | 0 (0.0) | 1 | 0.486 | 0.203 |
| Inferotemporal | 1 (5.6) | 2 (11.1) | 2 (11.1) | 1 | 1 | 0.528 |
| Inferior | 1 (5.6) | 0 (0.0) | 0 (0.0) | 1 | 1 | 0.216 |
| Inferonasal | 1 (5.6) | 1 (5.6) | 1 (5.6) | 1 | 1 | 0.999 |
| Temporal | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | NA | NA |
*B vs. F: Baseline versus 1-month follow-up, †B vs. S: Baseline vs 3-month follow-up
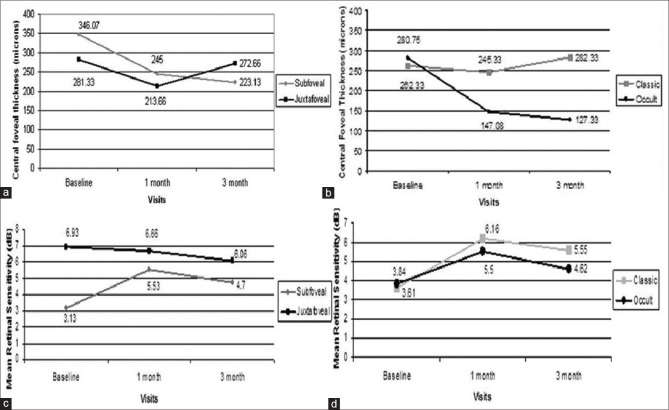
Changes in central retinal thickness and mean retinal sensitivity were observed in CNVM subtype; Fig. 4a shows greater reduction in the mean retinal thickness in those eyes with subfoveal CNVM (15 eyes) (346.07 ± 131.16 at baseline to 245 ± 77.50 at 1 month and to 223.13 ± 91.47 at 3 months) than those with juxtafoveal CNVM (3 eyes) (281.33 ± 34.70 at baseline to 231.66 ± 115.57 at 1 month to 272.66 ± 223.79 at 3 months). Fig. 4b shows that eyes with occult type (12 eyes) (280.75 ± 128.33 at baseline to 147.08 ± 52.57 at 1 month to 127.33 ± 40.39 at 3 months) showed greater reduction than those with classic type (6 eyes) (262.33 ± 10.388 at baseline to 245.33 ± 101.43 at 1 month to 282.33 ± 177.88 at 3 months). Fig. 4c shows greater improvement in the mean retinal sensitivity in those eyes with subfoveal CNVM (15 eyes) (3.13 ± 2.07 at baseline to 5.53 ± 2.35 at 1 month and to 4.70 ± 2.23 at 3 months) than those with juxtafoveal CNVM (3 eyes) (6.93 ± 5.94 at baseline to 6.66 ± 3.05 at 1 month to 6.06 ± 3.56 at 3 months). Fig. 4d shows that eyes with the classic type of CNVM (6 eyes) (3.61 ± 4.65 at baseline to 6.16 ± 2.40 at 1 month to 5.55 ± 2.24 at 3 months) showed greater improvement than those with the occult type of CNVM (12 eyes) (3.84 ± 2.30 at baseline to 5.5 ± 2.30 at 1 month to 4.62 ± 2.54 at 3 months) after bevacizumab injection.
Figure 4.
(a) Change in central foveal thickness in subfoveal Vs juxtafoveal CNVM at baseline, 1, and 3 months after bevacizumab injection. (b) Change in central foveal thickness in occult Vs classic type of CNVM at baseline, 1, and 3 months after bevacizumab injection. (c) Change in mean retinal sensitivity in subfoveal Vs juxtafoveal CNVM at baseline, 1, and 3 months after bevacizumab injection. (d) Change in mean retinal sensitivity in occult Vs classic type of CNVM at baseline, 1, and 3 months after bevacizumab injection
We had seen the relationship between changes in visual acuity, changes in retinal sensitivity, and changes in mean retinal thickness after anti-VEGF treatment. The changes in visual acuity were not significant and they positively correlated to changes in mean retinal thickness (r = 0.425, P = 0.078). However, changes in retinal sensitivity were neither correlated to changes in retinal thickness (r = −0.049, P = 0.846) nor to the changes in visual acuity (r = −0.327, P = 0.185). Intra-observer repeatability was good in the manual measurement of the SDOCT outcomes. The infraclass correlation coefficient (ICC) for CFT and OHRBT was 0.997 and 0.998, respectively.
Discussion
In this study, we found that intravitreal injection bevacizumab (0.5 mg) in nAMD improved retinal function, which might be quantified by retinal sensitivity, scotoma characteristics, fixation stability by MP 1 microperimetry and morphological parameters quantified by central foveal thickness in SDOCT.
Although the CFT changes improved to be the highest at both the 1- and 3-month followup after intravitreal bevacizumab injections, VA improved from 0.62 ± 0.36 at baseline to 0.52 ± 0.36 at 1-month and to 0.48 ± 0.34 at 3-month followup, but the overall change was not statistically significant (P = 0.40), retinal sensitivity shows significant improvement at 1 month followup; however at 3-month followup we found moderate but not statistically significant improvement. This finding can be due to duration of the disease, prolonged intraretinal or subretinal edema that may cause persistent damage to the photoreceptors. Moreover, Sabates et al.[12] found that in healthy macula, there was no relationship between retinal thickness and retinal sensitivity.
We found a statistically significant reduction in CFT (274.61 ± 117.95 at baseline to 179.83 ± 84.18 at 1 month and to 179.00 ± 126.55 at 3 months; P = 0.013) from baseline to 1 and 3 months after anti-VEGF treatment. This finding shows marked reduction in leakage from CNVM and is consistent with the previous studies.[7,9,13,14,15] Following anti-VEGF injection, there was a significant reduction in mean and central 1 mm retinal thickness; however, at 3 mm only vertical (superior and inferior) meridians showed significant reduction and at 6 mm inferior (dependent) meridian showed a significant reduction. Normally, thickness measurements are reported to be higher in vertical meridian (superior and inferior) than horizontal. Thus, in AMD probably the retinal thickness will also show a similar trend. It is also known that a thicker retina shows more reduction in thickness following anti-VEGF; the same was noted in our study in vertical (superior and inferior) meridian. Alternatively, the increased thickness in the inferior quadrant can be exaggerated due to gravity at 6 mm ETDRS circle. Thus, again maximum reduction at 6 mm was seen at the inferior quadrant.
The mean retinal sensitivity within 8°, 12°, and 20° was significantly improved 1 month after anti-VEGF, whereas there was moderate but not significant improvement after 3 months. The initial rapid anatomical restoration 1 month after bevacizumab treatment, however, slowed down after 3 months. Parravano et al.[9] reported improvement in mean retinal sensitivity at ≥2.5 dB in 61.1% (11 of 18) of patients at 24 months of follow-up. Ozdemir et al.[16] showed that within the central 4° area, mean retinal sensitivity had significantly improved 1, 3, and 6 months after bevacizumab injection. Prager et al.[17] reported that bevacizumab injection showed significant improvement in retinal sensitivity at 1-, 3-, and 6-month follow-up. Parravano et al.[9] reported that there was significant improvement in mean retinal sensitivity at 24 weeks after ranibizumab.[9] Cho et al.[7] assessed retinal functional changes after ranibizumab injection at 3-, 6- and 12-month follow-up; retinal sensitivity at the 12-month follow-up improved to the highest level. However, our study assessed the short-term macular functional changes and the retinal sensitivity may improve at longer follow up.
We found a significant reduction in mean absolute scotoma and relative scotoma from baseline to 1 and 3 months after bevacizumab injection. Mean normal test point locations had increased but were not statistically significant. Similar to our study, Ozdemir et al.[16] found that mean absolute scotoma had decreased 1, 3, and 6 months after bevacizumab injection. In previous studies, the result for decrease in absolute scotoma size and increase in relative scotoma size and normal (no scotoma) by bevacizumab injection supported improvement in visual outcomes.[18,19] We found that fixation properties (which include location and stability of fixation) improved after 3 months of bevacizumab injection. Similar to our results, Parravano et al.[9] reported that fixation stability improved at 24 weeks in 33.3% of patients after intravitreal ranibizumab injection, and Ozdemir et al.[16] reported that fixation properties had preserved 3 and 6 months after bevacizumab injection. Parravano et al.[8] reported that fixation stability showed improvement in 61.5% (8 of 13) of patients after 12 months and in 38.5% (5 of 13) of patients after 24 months with three injections of ranibizumab.[9] The improvement in stability of fixation and scotoma characteristics observed in this study resulted in improved visual function.
In wet AMD, the PRL is often developed to the left of the visual field. Guez et al.[20] found that in central scotoma the fixation was on the left or inferior part. Nilsson et al.[21] showed that if a new PRL is located above the retinal lesion it improves the reading speed. Four of our patients (22.2%) showed a shift of PRL superior to the retinal lesion 3 months after bevacizumab injection which is considered favorable for reading and near vision tasks. We found that 50% of our subjects showed significant improvement in fixation stability by ≥2 dB. Squirrell et al.[22] reported that 8 out of 10 (80%) patients showed improvement by ≥2 dB at 1 month of third injection with ranibizumab. These findings also support improvement of visual function.
We found that changes in visual acuity were not significant and positively correlated to changes in mean retinal thickness (r = 0.425, P = 0.078). However, changes in retinal sensitivity neither correlated to changes in retinal thickness (r = −0.049, P = 0.846) nor to the changes in visual acuity (r = −0.327, P = 0.185). Similar to our result, Parravano et al.[9] reported that functional changes expressed as retinal sensitivity and visual acuity were not correlated to central retinal thickness at 6, 12, and 24 months of follow-up. This may be due to duration of the macular disease with prolonged edema that could influence the functional changes after treatment.
This study had a few limitations. The sample size was small and we assessed the short-term macular functional and structural changes with followup period of 3 months. Further studies are required with larger sample sizes and longer followup periods to determine the changes in retinal sensitivity, visual acuity, and CFT after bevacizumab treatment. The treatment does not follow the protocol. We wanted to treat it like three doses of injection followed by pro re nata (PRN). Because of the poor follow-up, the treatment became PRN from the beginning.
Conclusion
In summary, we found that intravitreal injection bevacizumab (0.5 mg) in nAMD improved retinal function, which might be quantified by retinal sensitivity, scotoma characteristics, fixation stability by MP 1 microperimetry, and morphological parameters quantified by central foveal thickness in SD-OCT. These changes showed the effectiveness of the treatment with intravitreal bevacizumab in nAMD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Brucker AJ. Age-related macular degeneration. Retina. 2009;29:S2–4. doi: 10.1097/IAE.0b013e3181ad255f. [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040:A systematic review and meta-analysis. Lancet Glob Health. 2014;2:106–16. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.Spaide RF, Laud K, Fine HF, James M, Klancnik JR, Meyerle CB, Yannuzzi LA, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–90. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 4.Wong TY, Loon SC, Saw SM. The epidemiology of age related eye diseases in Asia. Br J Ophthalmol. 2006;90:506–11. doi: 10.1136/bjo.2005.083733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassell JB, Lamoureux EL, Keeffe J.E Impact of age related macular degeneration on quality of life. Br J Ophthalmol. 2006;90:593–6. doi: 10.1136/bjo.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vingolo EM, Salvatore S, Cavarretta S. Low-vision rehabilitation by means of MP-1 biofeedback examination in patients with different macular diseases:A pilot study. Appl Psychophysiol Biofeedback. 2009;34:127–33. doi: 10.1007/s10484-009-9083-4. [DOI] [PubMed] [Google Scholar]
- 7.Cho HJ, Kim CG, Yoo SJ, Cho SW, Lee DW, Kim JW, et al. Retinal functional changes measured by microperimetry in neovascular age-related macular degeneration treated with ranibizumab. Am J Ophthalmol. 2013;155:118–26. doi: 10.1016/j.ajo.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Parravano M, Oddone F, Tedeschi M, Lomoriello DS, Chiaravalloti A, Ripandelli G, et al. Retinal functional changes measured by microperimetry in neovascular age-related macular degeneration patients treated with ranibizumab. Retina. 2009;29:329–34. doi: 10.1097/IAE.0b013e31819093e6. [DOI] [PubMed] [Google Scholar]
- 9.Parravano M, Oddone F, Tedeschi M, Chiaravalloti A, Perillo L, Boccassini B, et al. Retinal functional changes measured by microperimetry in neovascular age-related macular degeneration treated with ranibizumab:24-month results. Retina. 2010;30:1017–24. doi: 10.1097/IAE.0b013e3181cfd3c6. [DOI] [PubMed] [Google Scholar]
- 10.Krebs I, Schmetterer L, Boltz A, Told R, Vécsei-Marlovits V, Egger S, et al. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97:266–71. doi: 10.1136/bjophthalmol-2012-302391. [DOI] [PubMed] [Google Scholar]
- 11.Fujii GY, de Juan E, Jr, Sunness J, Humayun MS, Pieramici DJ, Chang TS. Patient selection for macular translocation surgery using the scanning laser ophthalmoscope. Ophthalmology. 2002;109:1737–44. doi: 10.1016/s0161-6420(02)01120-x. [DOI] [PubMed] [Google Scholar]
- 12.Sabates FN, Vincent RD, Koulen P, Sabates NR, Gallimore G. Normative data set identifying properties of the macula across age groups:Integration of visual function and retinal structure with microperimetry and spectral-domain optical coherence tomography. Retina. 2011;31:1294–302. doi: 10.1097/IAE.0b013e3182019be2. [DOI] [PubMed] [Google Scholar]
- 13.Fung AE, Rosenfeld PJ, Reichel E. The international intravitreal bevacizumab safety survey:Using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90:1344–9. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser PK, Blodi BA, Shapiro H. Acharya NR;MARINA Study Group Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:1868–75. doi: 10.1016/j.ophtha.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Lalwani GA, Fung AE, Michels S, Dubovy SR, Feuer WJ, Jr, Puliafito CA, et al. An OCT-guided variable-dosing regimen with ranibizumab (Lucentis) in neovascular AMD:Two year results of the PrONTO study. Invest Ophthalmol Vis Sci. 2007;48:1834. [Google Scholar]
- 16.Ozdemir H, Karacorlu M, Senturk F, Karacorlu SA, Uysal O. Microperimetric changes after intravitreal bevacizumab injection for exudative age-related macular degeneration. Acta Ophthalmol. 2012;90:71–5. doi: 10.1111/j.1755-3768.2009.01838.x. [DOI] [PubMed] [Google Scholar]
- 17.Prager F, Michels S, Simader C, Geitzenauer W, Schmidt-Erfurth U. Changes in retinal sensitivity in patients with neovascular age-related macular degeneration after systemic bevacizumab (avastin) therapy. Retina. 2008;28:682–8. doi: 10.1097/IAE.0b013e318161dc70. [DOI] [PubMed] [Google Scholar]
- 18.Algvere PV, Steén B, Seregard S, Kvanta A. A prospective study on intravitreal bevacizumab (Avastin®) for neovascular age-related macular degeneration of different durations. Acta Ophthalmo. 2008;86:482–9. doi: 10.1111/j.1600-0420.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen KB, Sjølie AK, Møller F. Intravitreal bevacizumab (Avastin®) for neovascular age-related macular degeneration in treatment-naive patients. Acta Ophthalmol. 2009;87:714–9. doi: 10.1111/j.1755-3768.2008.01346.x. [DOI] [PubMed] [Google Scholar]
- 20.Guez JE, Le Gargasson JF, Rigaudiere F, O'Regan JK. Is there a systematic location for the pseudo-fovea in patients with central scotoma? Vision Res. 1993;33:1271–9. doi: 10.1016/0042-6989(93)90213-g. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson UL, Frennesson C, Nilsson SE. Patients with AMD and a large absolute central scotoma can be trained successfully to use eccentric viewing, as demonstrated in a scanning laser ophthalmoscope. Vision Res. 2003;43:1777–87. doi: 10.1016/s0042-6989(03)00219-0. [DOI] [PubMed] [Google Scholar]
- 22.Squirrell DM, Mawer NP, Mody CH, Brand CS. Visual outcome after intravitreal ranibizumab for wet age-related macular degeneration:A comparison between best-corrected visual acuity and microperimetry. Retina. 2010;30:436–42. doi: 10.1097/IAE.0b013e3181bd2f29. [DOI] [PubMed] [Google Scholar]