Abstract
Purpose:
Impaired ocular blood flow is an important risk factor in the pathogenesis of open-angle glaucoma (OAG). Studies have reported that dorzolamide 2% may be effective in improving ocular blood flow (OBF) in OAG patients. The objective of this study was to determine the efficacy of dorzolamide 2% (DORZOX, Cipla Ltd.) in improving retrobulbar blood flow in an Indian setting.
Methods:
The study was conducted as an interventional pilot project in 24 healthy subjects and 19 OAG patients. Baseline OBF measurements were done for all glaucoma patients with color Doppler imaging (CDI). Baseline ocular perfusion pressure (OPP) was calculated for all participants. Glaucoma patients were given dorzolamide 2% thrice daily for 12 weeks. The primary efficacy endpoints were mean changes in the CDI parameters of the retrobulbar vessels and OPP posttreatment. The secondary endpoint was mean change in the intraocular pressure (IOP) and adverse events, if any.
Results:
In comparison to healthy subjects, glaucoma patients displayed significantly reduced baseline OPP (P = 0.002). Treatment with dorzolamide 2% for 12 weeks led to a significant increase in OPP (P < 0.001) and a significant increase in end diastolic velocity (EDV) in all major ophthalmic arteries like ophthalmic artery (OA), central retinal artery (CRA), and short posterior ciliary artery (SPCA) (P < 0.001, P = 0.04, and P = 0.0075, respectively). A significant reduction in the intraocular pressure (IOP; P = 0.007) was observed posttreatment, with no adverse events reported.
Conclusion:
Dorzolamide 2% significantly improved parameters such as the EDV and OPP in major ophthalmic arteries. This pilot study shows promising results on using dorzolamide for treating Indian patients with OAG.
Keywords: Dorzolamide, end diastolic velocity, ocular perfusion pressure, open-angle glaucoma
Open-angle glaucoma (OAG) is a multifactorial optic neuropathy characterized by progressive retinal ganglion cell death and visual field loss. The global prevalence of glaucoma is 3.54% for those in the age range of 40–80 years. Prevalence of glaucoma is increasing and is expected to affect 111.8 million people by 2040 throughout the world.[1] In India, the prevalence of OAG is 12.9%.[2] Elevated intraocular pressure (IOP) is the major risk factor for OAG; however, many individuals with elevated IOP do not develop glaucoma, and many individuals with glaucoma progress despite achieving a reasonable level of IOP.[3,4,5]
Growing evidence has shown that ischemia to the optic nerve and dysfunctional vascular regulation are likely responsible for glaucomatous damage in many individuals.[6,7,8,9] In the 2009 World Glaucoma Association report, a consensus was agreed upon that lower ocular perfusion pressure, vascular dysregulation, and lower ocular blood flow (OBF) may be involved in OAG. In large population-based trials, reduced ocular perfusion pressure (OPP) has been linked to both the prevalence and incidence of glaucoma.[10,11,12,13] More recently, color Doppler imaging (CDI) studies have identified that retrobulbar OBF is linked to glaucoma progression and visual field loss.[14,15,16] We have now understood that It is OPP and blood flow play vital role in pathophysiology of glaucoma. Improvement of blood flow could be a part of management in progressive glaucoma after assessing the CDI. Based upon a preponderance of established work, it is hypothesized that dorzolamide will increase the OBF in Indian OAG patients, as assessed in the retrobulbar vasculature by CDI.[17] The objective of this pilot study was to determine the efficacy of dorzolamide 2% (DORZOX, Cipla Ltd.) in improving retrobulbar blood flow in Indian OAG patients.
Methods
We conducted an interventional pilot study over a period of 12 weeks in which 24 healthy subjects and 19 glaucoma patients were included. Baseline IOP and blood pressure measurements were done for healthy subjects as well as glaucoma patients. Baseline OBF measurements were done for all newly diagnosed glaucoma patients with the help of CDI. The study was conducted in South India in accordance with the study protocol, Indian Good Clinical Practice (GCP) standards, and Schedule Y. All procedures were approved by the institutional ethics committee of the hospital. All participating subjects provided their informed consent. Inclusion criteria for the study were treatment-naïve patients and age ≥40 years with mild-to-moderate OAG or normal-tension glaucoma in at least one eye. The severity of glaucoma damage can be categorized as follows: mild: definite optic disk or retinal nerve fiber layer (RNFL) abnormalities consistent with glaucoma and a normal visual field as tested with standard automated perimetry (SAP); moderate: definite optic disk or RNFL abnormalities consistent with glaucoma, and visual field abnormalities in one hemifield that are not within 5° of fixation as tested with SAP; severe: definite optic disk or RNFL abnormalities consistent with glaucoma, and visual field abnormalities in both hemifields and/or loss within 5° of fixation in at least one hemifield as tested with SAP; and indeterminate: definite optic disk or RNFL abnormalities consistent with glaucoma, inability of the patient to perform visual field testing, unreliable/uninterpretable visual field test results, or visual fields not performed yet. Patients willing to follow-up after 12 weeks and adhering to the instructions of the study were included. It was a pilot study since no other studies had been published at the time of initiation of this study on Indian patients, and hence, a shorter duration trial was opted for. Normal healthy subjects participating in the study had no ocular abnormality, had normal optic disks, and IOP <20 mmHg in both eyes.
Exclusion criteria were patients with exfoliation glaucoma, pigmentary glaucoma, history of acute-angle closure or a narrow, occludable anterior chamber angle by gonioscopy, and mean deviation (MD) of visual field testing (Humphrey 30-2 program) >12. The following were also excluded from the study: patients on hypotensives, patients with bradycardia (heart rate <50 beats/min), second- and third-degree heart block, asthma bronchiale, chronic obstructive pulmonary disease, or congestive heart failure; patients who had undergone intraocular surgery or argon laser trabeculoplasty within the last 6 months; patients with a history of chronic or recurrent inflammatory eye diseases (e.g., scleritis, uveitis) or infection, or a history of intraocular trauma; patients with any abnormality preventing reliable applanation tonometry; patients on current use of any ophthalmic or systemic steroid which may interfere with this investigation; patients receiving systemic medications that could affect the IOP or systemic blood pressure, unless the dose had been stable for at least 6 months before the screening visit; patients with severe, unstable, or uncontrolled cardiovascular, renal, or pulmonary disease (creatinine clearance <1.8 l/h); any opacity or patient uncooperativeness that restricted adequate examination of the ocular fundus or anterior chamber in the study eye or poor follow-up; patients with a history of hypersensitivity to one of the study drugs or drugs with similar chemical structure, or a history of non IOP responder to topical beta blockers or topical carbonic anhydrase inhibitors (CAIs); and pregnant patients or nursing mothers. Patients with diabetes were not excluded in our study; however, there was no patient with documented peripheral vascular disease in this study.
For all glaucoma patients (N = 19; 38 eyes), baseline measurements (first visit) were followed by treatment with one drop of dorzolamide 2% (DORZOX) thrice daily (during daytime) for 12 weeks in their study eye/s. Final measurements were done at the end of 12 weeks of the study (final visit). All study visits were conducted approximately at the same time of day by the same research team to eliminate user-initiated variations in the evaluations of OBF.
Parameters evaluated were slit-lamp examination, IOP, brachial artery pressure (systolic and diastolic), radial pulse rate, and CDI of the retrobulbar blood vessels, namely, ophthalmic artery (OA), central retinal artery (CRA), and short posterior ciliary arteries (SPCAs). With the help of CDI in each ophthalmic vessel, peak systolic velocity (PSV) and end diastolic velocity (EDV) were determined, and Pourcelot’s resistive index (RI) was calculated (RI = [PSV – EDV]/PSV). This allowed for a comprehensive assessment of all the blood vessels supplying the retinal ganglion cells and optic nerve during treatment.
We measured systolic blood pressure (SBP), diastolic blood pressure (DBP) and Calculated OPP. Brachial artery blood pressure and pulse were assessed after a 5-min rest period using a calibrated automated sphygmomanometer at the beginning and end of each study visit. The IOP was assessed using Goldmann applanation tonometry. OPPs were calculated using the following equation:
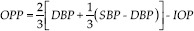
This allows for a comprehensive assessment of the IOP and various measures of OPP during treatment.
Statistical analysis
For analysis of the primary and secondary endpoints, paired t-test was used, and one-way analysis of variance (ANOVA) was used to test the significance between groups (healthy subjects and patients with glaucoma) at baseline and week 12. We used R (Version 3.6.2) software and Excel to analyze the data.
Outcomes
Primary efficacy endpoints at the end of the 12-week study were the mean changes in the CDI parameters of the retrobulbar vessels as well as in the OPP posttreatment. Secondary endpoint at the end of 12-week treatment was the mean change in the IOP. Safety endpoints included noting any adverse events that could occur with the use of dorzolamide 2% (DORZOX) eye drops.
Results
A total of 24 healthy subjects and 19 glaucoma patients who participated in this study underwent baseline OBF evaluation by CDI. The baseline demographics of healthy subjects and glaucoma patients included in the study are presented in Table 1. [Fig. 1] we have presented the age matched comparative data of OBF in normal people’.[18] Patients in the glaucoma and healthy cohorts displayed no differences in the SBP and DBP. The glaucoma patients displayed significantly reduced baseline OPP compared to healthy subjects (P = 0.002) [Table 1, Fig. 2].
Table 1.
Baseline demographics and mean OPP for healthy subjects and glaucoma patients
| Glaucoma patients | Healthy subjects | P | |
|---|---|---|---|
| No. of patients | 19 (38 eyes) | 24 (24 eyes) | N/A |
| Mean IOP (mmHg) | 16.05 | 12.5 | |
| DBP (mmHg) | 80.79 | 81.67 | 0.772 |
| SBP (mmHg) | 125.52 | 125.42 | 0.983 |
| Mean OPP | 46.63 | 55.61 | 0.002* |
DBP=diastolic blood pressure, IOP=intraocular pressure, OPP=ocular perfusion pressure, SBP=systolic blood pressurem, *Denotes that there was significant difference in the ocular perfusion pressure between the healthy subjects and the glaucoma patients
Figure 1.
Normal picture of ocular blood flow with peak and trough
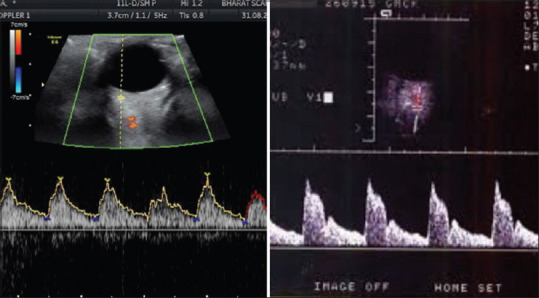
Figure 2.
Ocular blood flow from a glaucoma patient where there is no typical peak and trough
Glaucoma patients were then administered dorzolamide 2% (DORZOX) three times daily for 12 weeks. The mean age of the glaucoma patients was 59.63 years (nine males and 10 females). Table 2 shows a comparison of the clinical parameters between baseline values and values at week 12 for the DORZOX treatment group. Changes in the primary outcome parameters were observed in patients receiving treatment with dorzolamide 2% (DORZOX). After 12 weeks of treatment, dorzolamide 2% (DORZOX) significantly improved OPP (P < 0.001) and significantly increased EDV in all major ophthalmic arteries like OA, CRA, and SPCA (P < 0.001, P = 0.04, and P = 0.0075, respectively). Also, there was a significant reduction in the IOP (P = 0.007) after dorzolamide 2% (DORZOX) treatment at the end of 12 weeks [Table 2].
Table 2.
Comparison of values of clinical parameters at baseline and at week 12 in the DORZOX treatment group
| Variable (n=38) | Baseline (Day 1) | After 12 weeks of treatment with DORZOX | Difference n (%) | P |
|---|---|---|---|---|
| OPP | 46.63 | 48.71 | 2.06 (4.45) | 0.000587* |
| IOP | 16.05 | 14.53 | −1.53 (−9.51) | 0.007236* |
| OA | ||||
| PSV | 29.81 | 31.78 | 1.97 (6.59) | 0.051379 |
| EDV | 5.21 | 6.44 | 1.23 (23.50) | 0.000291* |
| RI | 0.78 | 0.76 | −0.02 (−2.91) | 0.129192 |
| CRA | ||||
| PSV | 13.07 | 12.09 | −0.97 (−7.44) | 0.094296 |
| EDV | 3.02 | 3.60 | 0.59 (19.40) | 0.044408* |
| RI | 0.74 | 0.68 | −0.06 (−7.92) | 0.051113 |
| SPCA | ||||
| PSV | 12.75 | 14.23 | 1.48 (11.63) | 0.115915 |
| EDV | 3.13 | 4.41 | 1.28 (40.97) | 0.00755* |
| RI | 0.70 | 0.69 | –0.01 (–1.06) | 0.395842 |
CRA=central retinal artery, EDV=end diastolic velocity, IOP=intraocular pressure, OA=ophthalmic artery, OPP=ocular perfusion pressure, PSV=peak systolic velocity, RI=resistive index, SPCA=short posterior ciliary artery. *Denotes that there was significant difference in the ocular perfusion pressure between the healthy subjects and the glaucoma patients
No adverse events were reported due to dorzolamide 2% (DORZOX) eye drops or the study procedure during the study.
Discussion
Worldwide, 60 million people suffer from glaucoma, and it is expected that, by 2040, around 111 million people will be affected by glaucoma.[3] Traditionally, IOP is considered to be the only modifiable risk factor for glaucoma management. However, many individuals with elevated IOP do not develop glaucoma and many individuals with glaucoma progress despite achieving a reasonable level of IOP.[4,5,6] There is ample evidence available which has shown that the prevalence and progression of OAG is often related to low OPP.[13,19,20,21] OAG seems to be associated with decreased mean blood flow velocity and increased mean RI in the CRA and the SPCAs. Reduced EDV and increased RI in the OA and reduced PSV in the CRA and the SPCAs have also been observed in eyes with POAG when compared to normal control eyes.[15]
Much controversy exists regarding POAG therapies and their potential influence on the OBF. It is of interest to know the effects of antiglaucoma drugs on ocular hemodynamic parameters, since they may affect glaucoma progression. To significantly impact OBF, topical glaucoma medications must penetrate the anterior surface of the eyes, reach critical concentrations, and exert a physiological effect on the vascular tissue. The vascular effect of therapies have role in IOP reduction (by increasing OPP) and by reducing their production. Topical CAIs have been proven to increase ocular hemodynamic parameters in various studies; however, their effect on Indian glaucoma patients is yet to be evaluated.[17]
In this pilot study, we found that healthy subjects have significantly reduced OPP compared to glaucoma patients. When these OAG glaucoma patients were treated with dorzolamide 2% (DORZOX) for 12 weeks, three times daily, we found that there was a significant increase in the OPP and other retrobulbar blood flow parameters such as EDV (in the OA, CRA, and SPCA) and a significant decrease in IOP. Also, there was a decrease in RI in all ophthalmic arteries (OA, CRA, and SPCA) and an increase in PSV in OA and SPCA; however, the difference was not statistically significant.
In our study, increased OBF in the retrobulbar vessels after dorzolamide 2% treatment could be an IOP-independent effect. It may be due to the direct metabolic effects of dorzolamide 2% on the blood vessels, that is, a CO2-mediated vasorelaxing effect due to blockade of the carbonic anhydrase enzyme in the local tissues, increased OPP (via IOP reduction), or a combination of IOP reduction and direct vascular interactions.[15] Our study results are supported by various published studies investigating dorzolamide 2%, which have found it to increase ocular hemodynamic parameters in glaucoma patients.[17]
Conclusion
In conclusion, our study on Indian glaucoma patients shows that glaucoma patients have significantly lower OPP compared to healthy subjects. To our knowledge, this is the first trial in Indian patients to evaluate the effects of dorzolamide 2% (DORZOX) on OBF in glaucoma patients, wherein we found that dorzolamide 2% (DORZOX) caused significant hemodynamic changes (improved OPP and EDV) in the major ophthalmic vessels. Although this study showed promising results regarding the use of dorzolamide in improving OBF and decreasing IOP in glaucoma patients, more studies with larger sample sizes and across multiple centers need to be conducted to validate the findings of this pilot study. Also, it remains to be established whether the above-mentioned effects on OBF can help to reduce visual field loss in Indian patients with glaucoma.
Financial support and sponsorship
Research grant was obtained from Cipla Ltd.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kreft D, Doblhammer G, Guthoff RF, Frech S. Prevalence, incidence, and risk factors of primary open-angle glaucoma-a cohort study based on longitudinal data from a German public health insurance. BMC Public Health. 2019;19:851. doi: 10.1186/s12889-019-6935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandelwal RR, Raje D, Khandelwal RR. Original Article clinical profile and burden of primary glaucoma in rural camp patients attending a tertiary care center in India. J Clin Ophthalmol Res. 2019;7:55–60. [Google Scholar]
- 3.Hollowst FC, Graham PA. Intra-ocular pressure, glaucoma, and glaucoma suspects in a defined population. Br J Ophthalmol. 1966;50:570–86. doi: 10.1136/bjo.50.10.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression:Results from the Early Manifest glaucoma trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DR. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14:86–90. doi: 10.1097/00055735-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Moore D, Harris A, Wudunn D, Kheradiya N, Siesky B. Dysfunctional regulation of ocular blood flow:A risk factor for glaucoma? Clin Ophthalmol (Auckland, NZ) 2008;2:849–61. doi: 10.2147/opth.s2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinreb RN. Ocular blood flow in glaucoma. Can J Ophthalmol. 2008;43:281–3. doi: 10.3129/i08-058. [DOI] [PubMed] [Google Scholar]
- 8.Flammer J, Org S, Costa VP, Orzalesi N, Metzner L, Renard J, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–93. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 9.Harris A, Kagemann L, Ehrlich R, Rospigliosi C, Moore D, Siesky B. Measuring and interpreting ocular blood flow and metabolism in glaucoma. Can J Ophthalmol. 2008;43:328–36. doi: 10.3129/i08-051. [DOI] [PubMed] [Google Scholar]
- 10.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol. 1995;113:216–21. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 11.Friedman DS, Wolfs RC, O'Colmain BJ, Klein BE, Taylor HR, West S, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–8. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma:The Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–93. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 13.Leske MC, Wu S-Y, Nemesure B, Hennis A, Group BES. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol. 2002;120:954–9. doi: 10.1001/archopht.120.7.954. [DOI] [PubMed] [Google Scholar]
- 14.Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M. Ocular hemodynamics and glaucoma prognosis:A color Doppler imaging study. Arch Ophthalmol. 2003;121:1711–5. doi: 10.1001/archopht.121.12.1711. [DOI] [PubMed] [Google Scholar]
- 15.Martinez A, Gonzalez F, Capeans C, Perez R, Sanchez-Salorio M. Dorzolamide effect on ocular blood flow. Invest Ophthalmol Vis Sci. 1999;40:1270–5. [PubMed] [Google Scholar]
- 16.Tobe LA, Harris A, Hussain RM, Eckert G, Huck A, Park J, et al. The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fibre layer structure in patients with open-angle glaucoma over an 18-month period. Br J Ophthalmol. 2015;99:609–12. doi: 10.1136/bjophthalmol-2014-305780. [DOI] [PubMed] [Google Scholar]
- 17.Siesky B, Harris A, Brizendine E, Marques C, Loh J, Mackey J, et al. Literature review and meta-analysis of topical carbonic anhydrase inhibitors and ocular blood flow. Surv Ophthalmol. 2009;54:33–46. doi: 10.1016/j.survophthal.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Nivean PD, Ariga M, Nivean M, Palani M. Ocular blood flow study using color doppler imaging:Normative value and its relevance in glaucoma. TNOA J Ophthalmic Sci Res. 2021;59:10–2. [Google Scholar]
- 19.Rojanapongpun P, Drance SM, Morrison BJ. Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br J Ophthalmol. 1993;77:25–9. doi: 10.1136/bjo.77.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt Z, Mckillop G, O'Brien C, Allan P, Aspinall P. Measurement of ocular blood flow velocity using colour doppler imaging in low tension glaucoma. Eye (Lond) 1995;9:29–33. doi: 10.1038/eye.1995.4. [DOI] [PubMed] [Google Scholar]
- 21.Galassi F, Sodi A, Ucci F, Harris A, Chung HS. Ocular haemodynamics in glaucoma associated with high myopia. Int Ophthalmol. 1998;22:299–305. doi: 10.1023/a:1006347509491. [DOI] [PubMed] [Google Scholar]


