Abstract
Pathologic chromosome breaks occur in human dividing cells ~10 times per day, and physiologic breaks occur in each lymphoid cell many additional times per day. Nonhomologous DNA end joining (NHEJ) is the major pathway for the repair of all of these double-strand breaks (DSBs) during most of the cell cycle. Nearly all broken DNA ends require trimming before they can be suitable for joining by ligation. Artemis is the major nuclease for this purpose. Artemis is tightly regulated by one of the largest protein kinases, which tethers Artemis to its surface. This kinase is called DNA-dependent protein kinase catalytic subunit (or DNA-PKcs) because it is only active when it encounters a broken DNA end. With this activation, DNA-PKcs permits the Artemis catalytic domain to enter a large cavity in the center of DNA-PKcs. Given this remarkably tight supervision of Artemis by DNA-PKcs, it is an appropriate time to ask what we know about the Artemis:DNA-PKcs complex, as we integrate recent structural information with the biochemistry of the complex and how this relates to other NHEJ proteins and to V(D)J recombination in the immune system.
Keywords: DNA repair, nonhomologous DNA end joining (NHEJ), V(D)J recombination, immunoglobulin class switch recombination, chromosomal translocations, severe combined immune deficiency, DNA-PKcs, Artemis, cryo-EM
INTRODUCTION
When a chromosome break arises, it is nearly always the site of a double-strand DNA break (DSB). The cell relies on the nonhomologous DNA end joining (NHEJ) pathway to repair and join the two DNA ends together 1,2. The primary NHEJ proteins are Ku70/80 [consisting of Ku70 and Ku80], DNA-PKcs, Artemis, XLF, XRCC4 and DNA ligase IV 3,4. Ku70/80 is the first protein to bind, and it recruits the Artemis:DNA-PKcs complex to trim off any damage DNA. Polymerases of the POL X family (including pol mu and pol lambda) can add or fill-in nucleotides, if necessary. The synapsis and ligation of the ends involves Ku70/80 interacting with the ligase complex, which consists of XLF, XRCC4 and DNA ligase IV 5–7 (Figure 1A).
Figure 1.

NHEJ mechanism and its role in V(D)J recombination.
(A) NHEJ mechanism. The general steps of nonhomologous DNA end joining (NHEJ) are shown. When double-strand DNA breaks (DSBs) occur by ionizing radiation, reactive oxygen species, nicks at replication forks or enzymatic causes of a DSB (including RAG complex and topoisomerases), NHEJ begins with Ku70/80 binding to the dsDNA ends at DSB ends. Then, the nuclease complex (Artemis:DNA-PKcs complex), the DNA polymerases (μ, λ and TdT), and the ligase complex (XFL:XRCC4:DNA ligase IV and PAXX) can bind act. These proteins work on either of the two DNA ends independently, iteratively (loading repeatedly) and in any order until both strands are ligated. In addition to this mechanistic flexibility, each component exhibits enzymatic flexibility.
(B) The role of the Artemis:DNA-PKcs complex in V(D)J recombination during antigen receptor development. The basal state of the Artemis:DNA-PKcs complex, where the catalytic region of Artemis is external to DNA-PKcs, binds to the hairpin ends generated by the RAG complex. Next, the activated Artemis:DNA-PKcs complex, where the catalytic region of Artemis is now inside of the DNA-PKcs HEAT cradle, opens the hairpins. After modification by the nuclease complex and the NHEJ polymerases, the XRCC4:DNA ligase IV complex can join the coding ends. Hairpin opening is essential for completion of V(D)J recombination. Patients with failure to this hairpin opening leads to radiosensitive T−B− severe combined immunodeficiency (RS-SCID).
Recent structural and biochemical progress on Artemis and DNA-PKcs as well as the complex of the two proteins, has set the stage for the next generation of insights on this dynamic pair of proteins 8–10. The dynamics of this complex appears to be the most highly regulated phase of NHEJ since the broken DNA ends must activate DNA-PKcs, and this activation permits Artemis to function as an endonuclease in all human cells and responsible for the hairpin opening step during V(D)J recombination in early lymphoid cells 11,12 (Figure 1B). Here, we examine the following set of key questions that integrate the earlier biochemical data with the recent structural data.
First, two key steps of the NHEJ repair process – DNA end trimming by the Artemis nuclease and ligation by the XRCC4:DNA ligase IV complex – involve some degree of coordination via their interaction with a key cleft denoted as “Art-X4 cleft” on the surface of DNA-PKcs 8. What does this interaction tell us about the NHEJ process?
Second, the roles and functions of the long Artemis C-terminal tail (330 aa out of the total of 692 aa) are not fully understood. This region is an unstructured dynamic region, which makes it difficult to study. However, recent cryo-EM structures along with previous biochemical studies allow us to propose testable possibilities 8,9,13–15.
Third, in addition to the Artemis tail, the regulation of the Artemis catalytic region when in complex with DNA-PKcs is still unknown. How does such dynamic catalytic region enter the cavity of DNA-PKcs through a tight opening? How exactly is the self-inhibitory mechanism of Artemis blocking the active site and regulating its 5’-exonuclease and its endonuclease activities?
Fourth, we discuss the Artemis active site metal ions 9,16–18. The number and position of the divalent cations in the active site of Artemis are not fully defined. We propose testable possibilities for the active site metals ions.
Lastly, during the evolution of V(D)J recombination in vertebrates, Artemis evolved to open the hairpins formed by the RAG complex 19–23. The only structure published indicates that this position is one nt 3’ of the hairpin tip 9. Yet, the hairpin opening of all tested hairpin DNA ends is predominantly at a location that is two nucleotides 3’ of the hairpin tip 24. There must be some flexibility within the Artemis active site to permit hairpin opening at more than one position for the same sequence, and this would contribute to immune diversity. How does such a rigid active site permit this flexibility?
THE ROLE OF THE STRUCTURAL CLEFT ON DNA-PKcs FOR INTERACTION WITH ARTEMIS
Recently, we defined an Art-X4 cleft (~2966-~3020 aa) on the FAT domain of DNA-PKcs where the Artemis C-terminal tail (401-407 aa) and the XRCC4 C-terminal tail (267-278 aa) may bind mutually exclusively 8. In addition to our basal state of Artemis:DNA-PKcs complex, a very similar interaction between the Artemis tail and DNA-PKcs in the activated state was described in Liu et al9. The positioning of amino acids of the Artemis tail on the FAT domain in both states still holds uncertainty. Computer simulation, such as protein dynamics, should be used to model the transition from the basal state to the activated state, and also within each state to see if one can identify various motion-based interactions.
We do not know yet whether any other proteins may also interact with this highly negatively charged cleft. This cleft may have other roles in the NHEJ mechanism. We examined three contact points (which include the Art-X4 cleft) for the Artemis tail interacting regions on the FAT domain of DNA-PKcs. Considering the length of the unstructured Artemis tail, it is possible that additional interacting regions exist between the Artemis tail and DNA-PKcs, or between the XRCC4 tail and DNA-PKcs. Our competition assays show that addition of an XRCC4 C-terminal fragment (X4 peptide) to the Artemis:DNA-PKcs complex disrupts the complex formation 8. Now, one can design disruptors based on this structural information. The gel assays also indicate that the transition from the Artemis:DNA-PKcs complex to DNA-PKcs:XRCC4:LigaseIV may be favored during NHEJ processing. Therefore, this Art-X4 cleft may be responsible for this transition since it is still unclear how the Artemis:DNA-PKcs complex dissociates from the processed DNA bound by Ku70/80 prior to the ligation step by XRCC4:DNA ligase IV.
THE ROLE OF THE ARTEMIS TAIL IN REGULATING NUCLEASE ACTION DURING NHEJ
The C-terminal half of Artemis is an unstructured tail (~330 aa). As we collect more information of direct interactions between Artemis and other proteins such as DNA-PKcs and DNA ligase IV, the role of this tail is clearly involved in key protein-protein interaction steps. Interestingly, a double mutation of L401G+R402N within Artemis abolishes the interaction with DNA-PKcs 25. However, major aspects of the functions of this C-terminal regulatory region of Artemis are unclear 26. For example, we still do not know the role of phosphorylation of this tail by DNA-PKcs 15. One might think that phosphorylation of the Artemis tail helps dissociate the self-inhibitory region of the tail from its active site 15. However, the activation of Artemis does not seem to require phosphorylation 27. Based on how the tail interacts with DNA-PKcs 8,9, the tail seems to increase the affinity of Artemis for DNA-PKcs, perhaps by reducing koff.
Cryo-EM studies of the basal state of Artemis:DNA-PKcs revealed that the Artemis catalytic domain is continuously in motion external to the DNA-PKcs N-HEAT region 8 (Figure 2). This continuous motion of the Artemis catalytic domain might be facilitated by the Artemis tail. One possibility is that the high degree of freedom of the tail might make the Artemis catalytic domain more dynamic. If the Artemis tail extending from the Art-X4 cleft is contributing to the dynamic positioning of the Artemis catalytic domain, this can be tested by using a truncated Artemis (e.g., 1~455 aa), which does not contain the autoinhibitory amino acids (456~458 aa) and the rest of the tail. If this complex (Artemis1-455:DNA-PKcs) becomes more stable, it indicates that the C-terminal portion of the tail makes the position of the catalytic domain dynamic. Alternatively, the Artemis tail may also help the Artemis catalytic domain to position on the N-HEAT external to DNA-PKcs so that Artemis can enter the DNA-PKcs cavity more easily 9.
Figure 2.
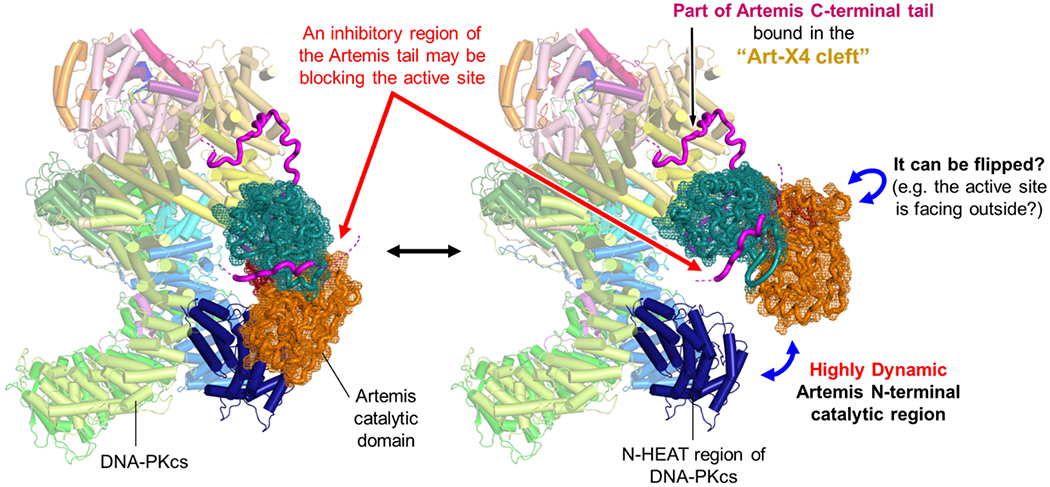
Dynamic nature of the Artemis catalytic domain in the complex with DNA-PKcs.
The basal state of Artemis:DNA-PKcs is shown (PDB: 7TYR) along with the Artemis catalytic domain (PDB: 7AF1), which exhibits highly dynamic positioning external to DNA-PKcs based on our previous cryo-EM work 8. It is still unclear whether a self-inhibitory tail (magenta), pointed by red arrows, is blocking the active site of Artemis in this state.
The self-inhibitory region of the tail maintains Artemis endonucleolytically inactive, even though the 5’ exonuclease activity of Artemis is always active 14. In order for Artemis to gain its endonuclease activity, DNA-PKcs and ATP/Mg2+ and dsDNA are required so that DNA-PKcs autophosphorylates to activate itself first. Perhaps the ABCDE phosphorylation helps the Artemis catalytic region to enter the cradle cavity where the dsDNA end is located 9. It is still unknown if the Artemis tail extending from the Art-X4 cleft interacts with the Artemis catalytic region in the basal state, keeping it from being endonucleolytically active (Figure 2). Importantly, even without DNA-PKcs, Artemis interacts with DNA ligase IV through the Artemis’ tail (485-495 aa) 28 and can process 3’-overhang DNA ends containing a terminal 3’-hydroxyl group 29.
Therefore, the Artemis tail not only helps form a complex with DNA-PKcs, but also it regulates Artemis activity and permits mechanistic flexibility by intramolecularly interacting with its catalytic region and by intermolecularly interacting with DNA ligase IV.
POSITIONING THE ARTEMIS CATALYTIC DOMAIN AND EXONUCLEASE VERSUS ENDONUCLEASE ACTIVITIES
Cryo-EM studies have shown how dynamic DNA-PKcs itself is, especially the N-HEAT and M-HEAT regions which account for ~68% of the entire protein 8,30. These HEAT regions create a large open space, whose primary purpose could be to provide a nuclease workshop for Artemis. This seems quite likely, as the other DNA end processing enzymes (e.g. polymerases, PNK, and glycosylases) do not appear to enter into the center of DNA-PKcs as Artemis does. One of many unsolved questions is how and when exactly large rearrangements of the N-HEAT, which Ku70/80 is attached to, occur and how the Artemis catalytic region enters the cradle cavity of DNA-PKcs.
Our cryo-EM analysis on the Artemis:DNA-PKcs:DNA complex shows that the Artemis catalytic region stays external to DNA-PKcs even after the complex binds DNA 8. This indicates that it still requires either 1) the DNA-PKcs ABCDE autophosphorylation (or other sites) followed by large conformational change, 2) phosphorylation of the C-terminal tail of Artemis, or 3) both events, in order for the Artemis catalytic region to enter the cavity. Our preliminary data of Artemis:DNA-PKcs:Ku70/80:DNA in the absence of ATP show that the Artemis catalytic region is not observed at the same position as we observed in the Artemis:DNA-PKcs complex (unpublished data). It is possible that the Artemis catalytic region becomes more dynamic as the position of the Artemis catalytic region observed in the basal state complex is occupied by the Ku70 portion of Ku70/80, and this indicates that phosphorylation events followed by large conformational change are key. The proposed transient interactions between the N-HEAT region of DNA-PKcs and the catalytic region of Artemis are still speculative 8. We do not know how strong they are or how long Artemis can stay there once it binds on the N-HEAT region. The phosphorylation of the N-site serines might have an effect on the local environment; hence, it may interfere the interaction with Artemis 31.
In our recent paper, four possible orientations of the Artemis catalytic region were discussed 8. However, the Artemis catalytic region is so dynamic, it could orient position in many ways (Figure 2), such that the active site of Artemis is more exposed 8. Given this highly flexible nature of the Artemis catalytic domain, it is possible that DNA ends can have access to the active site. In the presence of the self-inhibitory region of the Artemis tail, which is blocking the active site, it is likely that a dsDNA end is not be able to reach the catalytic residues for endonucleolytic action. However, a ssDNA or 5’-terminus of a duplex DNA end might be able to reach the active site, even though more internal portions of the duplex may not. This view agrees with our previous work, showing that a 5’-overhang can be trimmed by the exonuclease activity of Artemis:DNA-PKcs complex in the absence of ATP 18. We have also previously shown that the 5’ exonuclease action can extend inward along the ssDNA portion 19. Therefore, the ‘endonucleolytically autoinhibited’ state of the Artemis:DNA-PKcs complex or freely diffusing Artemis alone is always in a state that has sufficient space for the 5’ terminus to be subject to the 5’ exonucleolytic activity. Although the recent AlphaFold2 analysis has provided some insight, how the active site is covered up by the Artemis tail to inhibit the endonuclease activity but not the 5’ exonuclease activity is unknown 8,32–34.
Baddock et al., reported that a 5’ phosphate binding pocket identified near the active site of SNMIB/Apollo nuclease has a key role in determining endo- versus exonuclease activity across the SNM1 family as the 5’-phosphate is required for the exonucleolytic activity of SNM1B and SNM1A 35. Interestingly, the residues in this region are conserved between them, but not Artemis. The sidechain of F318 of Artemis partially occludes this phosphate binding pocket; however, it is not clear how this aromatic ring affects the binding of various DNA substrates for Artemis or whether it has the role in determining Artemis’ endo- versus exonuclease activity.
Given that activated Artemis can endonucleolytically cut long 3’ or 5’ overhangs, it is worth considering how deep into a DNA end that resection can occur 36. When DNA ligase IV or XRCC4 is missing, then resection might extend deeply into each end of a DSB. This may contribute to the embryonic lethality of the DNA Ligase IV and XRCC4 null mice. Such an explanation would seem unlikely except for the fact that the lethality of the DNA ligase IV and XRCC4 mice is reversed if one also deletes Ku70/80 37. XRCC4 lethality is also reversed when DNA-PKcs is also knocked out (personal comm. F. W. Alt). In light of the viability of the Ku70/80 and DNA ligase IV double null mice and XRCC4 and DNA-PKcs null mice, we wonder if unchecked resection occurs when the XRCC4:DNA ligase IV complex is missing. But if one knocks out Ku70/80, then recruitment of the Artemis:DNA-PKcs complex is made sufficiently inefficient that sites of DSB resection no longer occur prior to DNA ligase I or DNA ligase III ligation of the DSB (DNA ligase I and DNA ligase III can substitute slowly and inefficiently, despite deep resection).
IDENTITY OF THE CATALYTIC DIVALENTS WITHIN ARTEMIS
The identification and the role of catalytic cations in Artemis is still currently unclear. It has been proposed that there are two metal binding sites at the catalytic site in Artemis, potentially with two Zn2+ ions, hence with a two-metal-ion-dependent mechanism 38–40. The first one, which is surrounded by H33, H35, H115 and D136, should be a Zn2+ ion, and this is supported by the recent crystal structures 16,17 (Figure 3A and B). The interactions between this Zn2+ and these residues are strong, and the Zn2+ seems tightly bound. With the known physiological concentration of Mg2+ (e.g. ~1 mM free within cells), it is unlikely that Mg2+ will replace it. However, interestingly Yosaatmadja et al. showed that Artemis, when purified with Ni-NTA column, can also replace the Zn1 with a Ni2+ ion while another Zn2+ ion in the metal 2 position exists (PDB: 6TT5) 17. They also showed a structure with a Ni2+ ion in the metal 1 position with a ceftriaxone close to the Ni2+ ion (PDB: 7APV), but without the second metal cation in the active site 17, along with many other structures derived from the PanDDA analysis.
Figure 3.
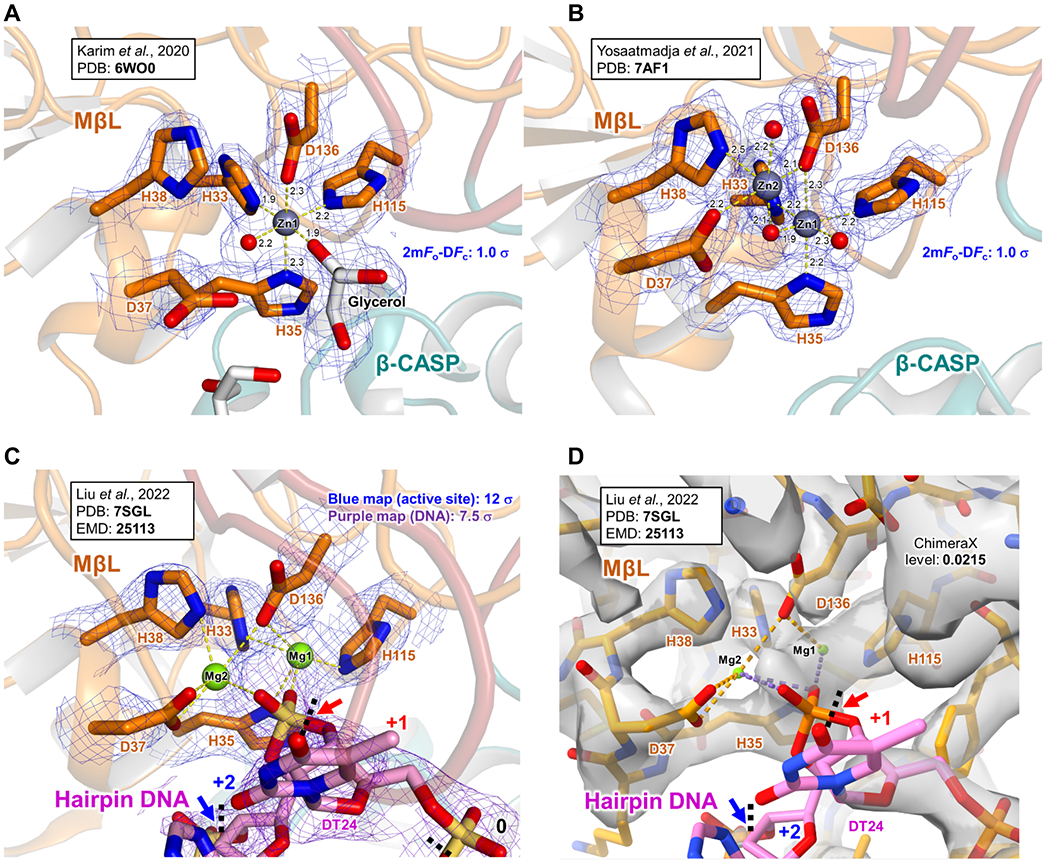
Comparison of the Artemis active site focusing on the catalytic metals.
(A) A crystal structure of Artemis 3-361 is shown (PDB: 6WO0) 16. Only one Zn2+ (grey sphere) is observed in the density. A red sphere is the oxygen of a water molecule. A density of the 2mFo-DFc map for the select residues is shown in blue (1.0 σ).
(B) A crystal structure of Artemis 3-361 is shown (PDB: 7AF1) 17. Two Zn2+ ions (grey spheres) are observed in the density. Red spheres are the oxygens of water molecules. A density of the 2mFo-DFc map for the select residues is shown in blue (1.0 σ).
(C) A cryo-EM structure of Artemis:DNA-PKcs:Ku70/80:DNA (PDB: 7SGL) 9. This is a zoomed view of the Figure 4B. Two Mg2+ ions (green spheres) were assigned in the active site. The cryo-EM map (EMD-25113) is shown for protein residues and the hairpin DNA, in blue (12 σ) and in purple (7.5 σ), respectively. The red arrow points the phosphate of the +2 nucleotide (DA25, not labeled here), indicating the +1 cutting site. The blue arrow points the phosphate of the +3 nucleotide (DT26, not labeled here), indicating the +2 cutting site.
(D) The same as (C), but shown in ChimeraX. The map level is 0.0215.
As for the second metal cation at the active site, perhaps Zn2+, could occupy this position as the interaction with surrounding residues (D37, H38 and D136) seems very weak and the metal is loosely bound 17. In the presence of Mg2+, it is likely that Mg2+ occupies this site and coordinates a water molecule for a nucleophilic reaction to cleave the DNA backbone. Therefore, in the presence of DNA-PKcs and ATP/Mg2+, it is likely that after autophosphorylation of the ABCDE cluster of DNA-PKcs, the catalytic region of Artemis moves to the DNA end. Then, a Mg2+ ion, replacing the second Zn2+, if present, may act as the catalytic metal with the first Zn2+ remaining bound at the catalytic site. Some groups proposed that two Mg ions may be at the active site 9,41. Hognon and Monari’s study provides interesting insight from their Artemis:DNA model using a published crystal structure of the Artemis catalytic domain (PDB: 6WO0), an ideal B-DNA double strand and the additional second Mg2+, using classical MD simulations. They also analyzed the hydrolysis reaction with two Mg2+ ions in the active site using a hybrid QM/MM 41. However, we must point out that it does not factor in the key feature of Artemis as a structure-specific nuclease in which it focuses on dsDNA/ssDNA overhang configurations 36. (Moreover, the amino acids designations discussed do not match the published structures.) Future work using this approach would benefit from considering the critical structure-specific nuclease feature that distinguishes Artemis from other structure-specific endonucleases. Interestingly, Liu et al. reported two Mg2+ ions in this region 9 (Figure 3C and D). However, the second Mg2+ (denoted as “Mg2” in this review) does not have clear density. Moreover, the coordinates of these Mg2+ ions (e.g., angles and distances) still hold questions as, often, it is difficult to identify metals with confidence. In addition, when or if the metal replacement from Zn2+ to Mg2+ occurs is not known.
In general, two metal divalent cations in the active site of nucleases function to neutralize the developing negative charge at the active site area due to ASP residues of proteins and a phosphodiester backbone of DNA38,39. Then, a nucleophilic water molecule, probably coordinated by metal 1 (e.g., Zn2+), performs the nucleophilic attack on the scissile phosphate. The metal 2 (e.g., Mg2+) likely stabilizes the 3’-leaving group and also reduces the energy barrier between the pre-cut and post-cut states.
In this context, the effect of Mn2+ on Artemis deserves mention. We know that full-length Artemis is active as an endonuclease when activated with DNA-PKcs in the presence of ATP/Mg2+. Without DNA-PKcs, full-length Artemis is not endonucleolytically active. However, when Mn2+ is supplemented to the nuclease reaction, full-length Artemis gains endonuclease activity without DNA-PKcs 18,42. One possibility is that Mn2+ ion(s) might disrupt the interaction between the catalytic domain of Artemis and the autoinhibitory region of the C-terminal tail or other potential intra-interaction sites; then, the autoinhibitory region may move away from the catalytic site of Artemis such that Artemis becomes endonucleolytically active. Additionally, Mn2+ can act as the metal 2 as described above. However, we still do not know exactly how Mn2+ works or whether it acts as the catalytic cation. This is indeed an important aspect.
THE POSITION OF HAIRPIN OPENING BY ARTEMIS:DNA-PKCS DURING V(D)J RECOMBINATION
A key issue important for the generation of immunologic antigen receptor diversity is the position of the DNA hairpin opening by Artemis, when in complex with DNA-PKcs (Figure 1B). Our lab previously examined the diversity of hairpin opening locations in all of the human VH coding ends in a biochemical system using purified Ku70/80, Artemis and DNA-PKcs. Regardless of the DNA coding end sequence, we found that Artemis nicking predominantly occurred at a location two nts on the 3’ side of the hairpin tip for each of the 39 VH coding ends without exception 24. We designated this as the “+2 position” along the hairpin tip structure. Nicking of the hairpin tip at the +1, +3, or +4 positions occurred, but was substantially less frequent. Moreover, all of the cellular and in vivo experimental studies on the coding end state after cutting indicate nicking at two nts past the hairpin tip to generate a 4 nt 3’-overhang 43 (Figure 4A).
Figure 4.
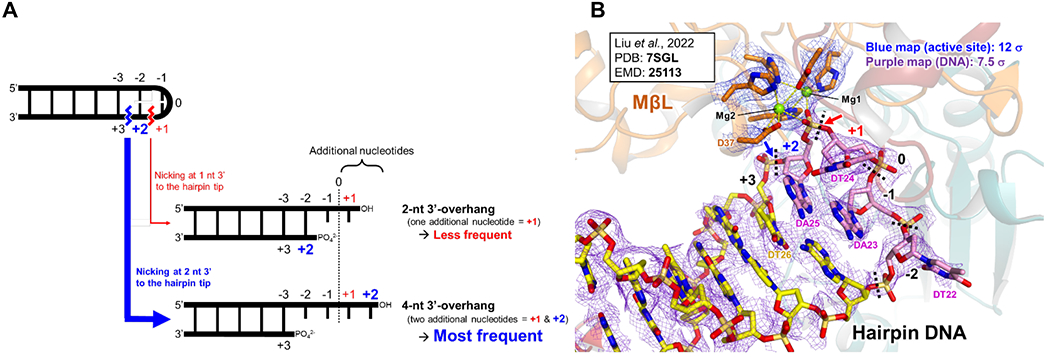
The position of hairpin opening by Artemis:DNA-PKcs during V(D)J recombination.
(A) Hairpin nicking sites by Artemis endonuclease. Artemis activated by DNA-PKcs nicks primarily 2 nt 3’ of the hairpin tip, which generates a 4 nt 3’-overhang as the major product (>70% of the product in nearly all sequences studied)24. Importantly, nicking at the +1, +3 and +4 positions also occurs but less frequently, generating 3’ overhangs of length 2, 6 and 8 nts 24. Thus, the nicking position is a distribution among the +1 to + 4 positions, but very predominantly at the +2 position.
(B) Liu et al.’s cryo-EM structure of Artemis:DNA-pKcs:Ku70/80:DNA structure 9. This model (PDB: 7SGL) implies that a water molecule would attack the phosphate of the DA25 nucleotide, creating a nick at the +1 position (red arrow) and generating a 2 nt 3’-overhang. However, for most DNA hairpin sequences, Artemis nicking at this position is known to be less frequent (see text). The cryo-EM map (EMD-25113) corresponding to the metal coordinating residues in the MβL domain (orange) is shown in blue (12 σ). The map corresponding to the hairpin DNA is shown in purple (7.5 σ) with the carbon atoms of the −2, −1, +1 and +2 residues in magenta; and those of the rest of the residues are in yellow. The zoomed view is in Figure 3C and D.
In contrast, Liu et al.’s recent cryo-EM structure (PDB: 7SGL) described predominant nicking one nt beyond the hairpin tip, which would leave a 2 nt 3’ overhang 9. Since Artemis primarily cuts most DNA hairpin sequences at +2, why is Artemis in the activated state cutting at +1? We have wondered about the possible explanation for this, because this one nt difference is important when one considers antigen receptor junctional diversity (Figure 4A and B).
One possible explanation is that there is only one divalent cation, probably Zn2+ (see below), in the catalytic center. This might permit more space for water to act as a nucleophile in opening the hairpin. Such flexibility might explain why more than one position is a site of potential nicking, even though only one site is nicked for each individual hairpin opening event. Though the +2 position is the predominant site of nicking for all physiologically-relevant hairpin sequences, the +1, +3, and +4 positions are the site of hairpin opening much less frequently 24. Such flexibility may indicate more space for a water nucleophile to position for hairpin opening. A second possible explanation is that the position of the DNA hairpin tip may be more flexible than is seen in the one case structurally analyzed thus far 9. Intriguingly, the bases of the hairpin tip (e.g., DT22, DT24 and DA25) are fairly distorted. This distortion may change the positioning of the hairpin tip in the active site of Artemis. In fact, the cryo-EM density of the +2 and +3 nucleotides (DA25 and DT26, respectively) is less clear than the stem part of dsDNA. Also, the position of the D37 residue and the second Mg2+ ion (= Mg2 in our Figures 3C, D and 4B or “MG801” in 7SGL) can be ambiguous (Figures 3C, D and 4B). Further studies of the structure will help clarify the explanation for the differences between the cryo-EM structure and the biochemical studies of the hairpin opening location.
CONCLUDING REMARKS
We have discussed a subset of the many interesting aspects of the recently described Artemis:DNA-PKcs structures. Further studies are required to gain a comprehensive understanding of the mechanism and regulation of NHEJ and the role of the Artemis:DNA-PKcs complex in antigen receptor diversity.
Acknowledgements:
This work was supported by NIH CA100504 and NIH GM118009 to MRL. The coordinate file (PDB: 7TYR) available in the PDB still has incorrect secondary structure annotations, which were, according to the PDB, automatically recalculated and replaced during the PDB deposition process using the PROMOTIF algorithm. The request for fixing these still has not resolved the issue as of this writing. If readers would like to discuss the correct secondary structure annotations that the authors originally deposited to the PDB, please contact gwatanab@usc.edu.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Zhao B, Rothenberg E, Ramsden DA & Lieber MR The molecular basis and disease relevance of non-homologous DNA end joining. Nat Rev Mol Cell Biol 21, 765–781 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pannunzio NR, Watanabe G & Lieber MR Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J Biol Chem 293, 10512–10523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang S et al. Stages, scaffolds and strings in the spatial organisation of non-homologous end joining: Insights from X-ray diffraction and Cryo-EM. Prog Biophys Mol Biol 163, 60–73 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Lees-Miller JP, He Y & Lees-Miller SP Structural insights into the role of DNA-PK as a master regulator in NHEJ. Genome Instab Dis 2, 195–210 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao B et al. The essential elements for the noncovalent association of two DNA ends during NHEJ synapsis. Nat Commun 10, 3588 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S et al. Structural basis of long-range to short-range synaptic transition in NHEJ. Nature 593, 294–298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaplin AK et al. Cryo-EM of NHEJ supercomplexes provides insights into DNA repair. Mol Cell 81, 3400–3409 e3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe G, Lieber MR & Williams DR Structural analysis of the basal state of the Artemis:DNA-PKcs complex. Nucleic Acids Res 50, 7697–7720 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L et al. Autophosphorylation transforms DNA-PK from protecting to processing DNA ends. Mol Cell 82, 177–189 e4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang S et al. Structural insights into inhibitor regulation of the DNA repair protein DNA-PKcs. Nature 601, 643–648 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moshous D et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell 105, 177–86 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Felgentreff K et al. Functional analysis of naturally occurring DCLRE1C mutations and correlation with the clinical phenotype of ARTEMIS deficiency. J Allergy Clin Immunol 136, 140–150 e7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niewolik D & Schwarz K Physical ARTEMIS:dNa-PKcs interaction is necessary for V(D)J recombination. Nucleic Acids Res (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niewolik D, Peter I, Butscher C & Schwarz K Autoinhibition of the Nuclease ARTEMIS Is Mediated by a Physical Interaction between Its Catalytic and C-terminal Domains. J Biol Chem 292, 3351–3365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y et al. The DNA-dependent protein kinase catalytic subunit phosphorylation sites in human Artemis. J Biol Chem 280, 33839–46 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Karim MF et al. Structural analysis of the catalytic domain of Artemis endonuclease/SNM1C reveals distinct structural features. J Biol Chem 295, 12368–12377 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yosaatmadja Y et al. Structural and mechanistic insights into the Artemis endonuclease and strategies for its inhibition. Nucleic Acids Res 49, 9310–9326 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu J et al. DNA-PKcs regulates a single-stranded DNA endonuclease activity of Artemis. DNA Repair (Amst) 9, 429–37 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Pannicke U, Schwarz K & Lieber MR Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell 108, 781–94 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Teng G & Schatz DG Regulation and Evolution of the RAG Recombinase. Adv Immunol 128, 1–39 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Kim MS et al. Cracking the DNA Code for V(D)J Recombination. Mol Cell 70, 358–370 e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X et al. Cutting antiparallel DNA strands in a single active site. Nat Struct Mol Biol 27, 119–126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Gellert M & Yang W Inner workings of RAG recombinase and its specialization for adaptive immunity. Curr Opin Struct Biol 71, 79–86 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu H, Schwarz K & Lieber MR Extent to which hairpin opening by the Artemis:DNA-PKcs complex can contribute to junctional diversity in V(D)J recombination. Nucleic Acids Res 35, 6917–23 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soubeyrand S et al. Artemis phosphorylated by DNA-dependent protein kinase associates preferentially with discrete regions of chromatin. J Mol Biol 358, 1200–11 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Pannicke U et al. Functional and biochemical dissection of the structure-specific nuclease ARTEMIS. EMBO J 23, 1987–97 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodarzi AA et al. DNA-PK autophosphorylation facilitates Artemis endonuclease activity. EMBO J 25, 3880–9 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malu S et al. Artemis C-terminal region facilitates V(D)J recombination through its interactions with DNA Ligase IV and DNA-PKcs. J Exp Med 209, 955–63 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerodimos CA, Chang HHY, Watanabe G & Lieber MR Effects of DNA end configuration on XRCC4-DNA ligase IV and its stimulation of Artemis activity. J Biol Chem 292, 13914–13924 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaplin AK et al. Dimers of DNA-PK create a stage for DNA double-strand break repair. Nat Struct Mol Biol 28, 13–19 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Neal JA et al. Unraveling the complexities of DNA-dependent protein kinase autophosphorylation. Mol Cell Biol 34, 2162–75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu HY et al. Structure and Function of SNM1 Family Nucleases. Adv Exp Med Biol (2022). [DOI] [PubMed] [Google Scholar]
- 33.Jumper J et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tunyasuvunakool K et al. Highly accurate protein structure prediction for the human proteome. Nature 596, 590–596 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baddock HT et al. A phosphate binding pocket is a key determinant of exo-versus endonucleolytic activity in the SNM1 nuclease family. Nucleic Acids Res 49, 9294–9309 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang HH & Lieber MR Structure-Specific nuclease activities of Artemis and the Artemis: DNA-PKcs complex. Nucleic Acids Res 44, 4991–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karanjawala ZE et al. The embryonic lethality in DNA ligase IV-deficient mice is rescued by deletion of Ku: implications for unifying the heterogeneous phenotypes of NHEJ mutants. DNA Repair (Amst) 1, 1017–26 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Yang W Nucleases: diversity of structure, function and mechanism. Q Rev Biophys 44, 1–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pettinati I, Brem J, Lee SY, McHugh PJ & Schofield CJ The Chemical Biology of Human Metallo-beta-Lactamase Fold Proteins. Trends Biochem Sci 41, 338–355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poinsignon C et al. The metallo-beta-lactamase/beta-CASP domain of Artemis constitutes the catalytic core for V(D)J recombination. J Exp Med 199, 315–21 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hognon C & Monari A Staring at the Naked Goddess: Unraveling the Structure and Reactivity of Artemis Endonuclease Interacting with a DNA Double Strand. Molecules 26(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y et al. Impact of a hypomorphic Artemis disease allele on lymphocyte development, DNA end processing, and genome stability. J Exp Med 206, 893–908 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlissel MS Structure of nonhairpin coding-end DNA breaks in cells undergoing V(D)J recombination. Mol Cell Biol 18, 2029–37 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]


