Abstract
Background & Aims:
GS-9620, an oral agonist of toll-like receptor 7 (TLR7), is in clinical development for the treatment of chronic hepatitis B (CHB). GS-9620 was previously shown to induced prolonged suppression of serum viral DNA and antigens in the chimpanzee and woodchuck models of CHB. Here we investigated the immunomodulatory mechanisms underlying these antiviral effects.
Methods:
Archived liver biopsies and paired PBMC samples from a previous chimpanzee study were analyzed by RNA-Seq, qRT-PCR, immunohistochemistry (IHC) and in situ hybridization (ISH).
Results:
GS-9620 treatment of CHB chimpanzees induced an intrahepatic transcriptional profile significantly enriched with genes associated with hepatitis B virus (HBV) clearance in acutely infected chimpanzees. Type I and II interferon, CD8+ T cell and B cell transcriptional signatures were associated with treatment response, together with evidence of hepatocyte death and liver regeneration. IHC and ISH confirmed an increase in intrahepatic CD8+ T cell and B cell numbers during treatment, and revealed that GS-9620 transiently induced aggregates predominantly comprised of CD8+ T cells and B cells in portal regions. There were no follicular dendritic cells (FDCs) or IgG-positive cells in these lymphoid aggregates and very few CD11b+ myeloid cells. There was no change in intrahepatic NK cell number during GS-9620 treatment.
Conclusion:
The antiviral response to GS-9620 treatment in CHB chimpanzees was associated with an intrahepatic interferon response and formation of lymphoid aggregates in the liver. Our data indicate these intrahepatic structures are not fully differentiated follicles containing germinal center reactions. However, the temporal correlation between development of these T and B cell aggregates and the antiviral response to treatment suggests they play a role in promoting an effective immune response against HBV.
Keywords: TLR7, Hepatitis B virus, immunomodulation, chimpanzee animal model, CD8 T cell, B cell, NK cell, interferon-alfa, interferon-stimulated gene, lymphocyte aggregate, tertiary lymphoid structure
Graphical Abstract
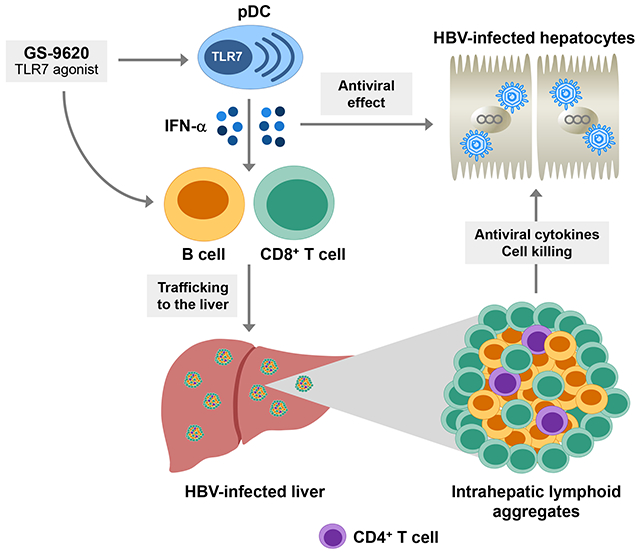
Lay summary:
New therapies to treat chronic hepatitis B (CHB) are urgently needed. In this study we performed a retrospective analysis of liver and blood samples from a chimpanzee model of CHB to help understand how GS-9620, a drug in clinical trials, suppressed hepatitis B virus (HBV). We found that the antiviral response to GS-9620 was associated with accumulation of immune cells in the liver that can either kill cells infected with HBV or can produce antibodies that may prevent HBV from infecting new liver cells. These findings have important implications for how GS-9620 may be used in patients and may also help guide the development of new therapies to treat chronic HBV infection.
INTRODUCTION
Approximately 240 million individuals live with chronic hepatitis B (CHB), and over half a million people are estimated to die each year due to CHB-associated liver diseases such as cirrhosis and hepatocellular carcinoma. Various nucleos(t)ides and interferon-α (IFN-α) are currently licensed for the treatment of CHB, but while these therapies reduce viremia and improve long-term outcome, they rarely lead to functional cure. The rarity of complete treatment response, together with the difficulty in obtaining liver biopsy specimens from CHB patients, has hindered characterization of the immune determinants of viral control. Accordingly, animal models have been extensively used to identify potential mechanisms of HBV clearance. Most notably, characterization of acute hepadnavirus infection in woodchucks and chimpanzees, together with studies in transgenic and hydrodynamic mouse models have identified important determinants of HBV clearance [1–4]. However, these studies were performed in models of self-limiting natural infection and/or in models that are absent viral spread and the authentic HBV genome, and therefore it remains to be determined whether the identified mechanisms can be therapeutically induced to achieve functional cure of CHB in patients. Fortunately, the development of novel therapies provides new possibilities for studying the immune determinants of viral clearance during chronic infection.
GS-9620 is a potent, orally active small molecule agonist of toll-like receptor 7 (TLR7) in clinical development for the treatment of CHB [5]. In humans, TLR7 is expressed predominantly by plasmacytoid dendritic cells (pDCs) and B cells. Activation of pDCs by TLR7 agonists induces IFN-α and various other cytokines. IFN-α is a pleiotropic cytokine that has both direct antiviral and immunomodulatory functions, including activation of natural killer (NK) cells, B cells and cytotoxic CD8+ T cells (CTLs) [6]. Direct activation of TLR7 in B cells can also contribute to the development of effective antiviral antibody responses [7]. GS-9620 was previously shown to induce prolonged suppression of serum viral DNA and antigens in the woodchuck and chimpanzee models of CHB [8, 9]. However, these preclinical studies did not comprehensively characterize the intrahepatic cellular and molecular characteristics of the antiviral response to GS-9620. Defining the immunological basis for response is an important goal since mechanistic understanding of GS-9620 activity could drive rational design of novel immunotherapeutic strategies.
The chimpanzee is the only immunocompetent model of natural HBV infection. Chimpanzees also share high genetic similarity with humans, and have a comparable immune system. In addition, the cross-reactivity of human-specific reagents with the chimpanzee permits more extensive immune characterization than is possible in the woodchuck model. This is an important distinction because a number of studies have illustrated the challenge in determining the relative contribution of intrahepatic NK and CD8+ T cells to antiviral response in the woodchuck model [8, 10]. Comprehensive characterization of the immune determinants of antiviral response to GS-9620 treatment in CHB chimpanzees is therefore of translational value and is also practicable.
In the current study, we performed transcriptome, immunohistochemistry (IHC) and in situ hybridization (ISH) analysis of archived liver biopsy samples from previous chimpanzee studies to characterize the immune environment in the liver of chimpanzees chronically infected with HBV, and to identify the intrahepatic immune correlates of GS-9620 treatment response in these animals.
MATERIALS AND METHODS
Animals and treatment
Chimpanzee liver biopsy and PBMC samples were archived from previous studies (Table 1) and were collected prior to December 15, 2011. The GS-9620 study has been previously described [9]. Additional details are provided in the Supplementary Materials
Table 1.
Summary of chimpanzee characteristics.
| Animal numbera | Sex | Infection | Duration of infection (years) | Age at time of biopsy | Serum HBV DNA (IU/mL) | Serum HBsAgb (μg/mL) | Serum HBeAgb (ng/mL) | Serum HCV RNA (copies/mL) | ALTb,c (U/mL) | ASTb,c (U/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 4x0139 | F | HBV | 30 | 32 | 1.1 x 107 | 43.9 | 43.8 | N/A | 31 | 21 |
| 4x0328 | M | HBV | >24 | 32 | 2.0 x 104 | 3.0 | 2.4 | N/A | 36 | 32 |
| 4x0506 | F | HBV | >27 | 30 | 2.4 x 103 | 7.4 | Anti-e positive | N/A | 37 | 14 |
| 4x0497 | M | HCV | 22 | 27 | n/a | n/a | n/a | 3.9 x 106 | 102* | 62* |
| 4x0499 | M | HCV | 20 | 22 | n/a | n/a | n/a | 2.9 x 106 | 67* | 38 |
| 4x0500 | F | HCV | 20 | 21 | n/a | n/a | n/a | 8.4 x 106 | 59* | 20 |
| 4x0548 | M | HCV | >1 | 29 | n/a | n/a | n/a | 1.3 x 107 | 39 | 35 |
| 4x0364 | M | Naïve | N/A | 18 | n/a | n/a | n/a | n/a | 24 | 17 |
| 4x0427 | F | Naïve | N/A | 14 | n/a | n/a | n/a | n/a | 20 | 15 |
| 4x0428 | F | Naïve | N/A | 14 | n/a | n/a | n/a | n/a | 31 | 17 |
| 4x0437 | M | Naïve | N/A | 14 | n/a | n/a | n/a | n/a | 34 | 34 |
HBV-infected animal information is adapted from Lanford et al. [9], with permission.
Mean of at least two historical bleeds.
The normal ranges for liver enzymes in chimpanzees are: ALT, male: 25-53 U/L; ALT, female: 21-55 U/L; AST, male: 2-48 U/L; AST, female: 11-25 U/L. Asterisks denote values higher than the normal range.
n/a: not applicable.
Additional materials and methods
Reagent details are provided in the Supplementary Materials and the Supplementary CTAT Table. Total cellular RNA was analyzed by RNA-Seq and real-time quantitative RT-PCR (qRT-PCR) as described in the Supplementary Materials. Immunohistochemistry (IHC) and in situ hybridization (ISH) procedures are also provided in the Supplementary Materials. Validation of IHC antibodies (Supplementary Table 1) and ISH probes is presented in Supplementary Figs. 1 and 2, respectively.
Statistical analysis
Data is expressed as mean ± standard error of the mean (SEM). Statistical significance was tested by one-way ANOVA with multiple comparison correction. A value of p<0.05 was considered significant.
RESULTS
GS-9620 treatment dramatically altered the liver and PBMC transcriptomes of chimpanzees chronically infected with HBV
To examine the host immune response to chronic HBV infection prior to treatment with GS-9620, we performed whole transcriptome profiling and IHC of liver biopsies taken from chimpanzees chronically infected with either HBV or HCV, as well as from uninfected (naïve) animals (Table 1). These data (Supplementary Figs. 3–5, Supplementary Tables 2 and 3) are described in the Supplementary Materials. We next performed a series of studies to identify the immune correlates of antiviral response to GS-9620 treatment in a previous study in CHB chimpanzees [9]. The design of the GS-9620 study is summarized in Fig. 1A and additional details are provided in the Supplementary Materials. There was a modest reduction in serum HBV DNA in the first treatment cycle (1 mg/kg GS-9620) without ALT or AST elevation (Fig. 1B). In contrast, there was a rapid decline in serum HBV DNA during the second treatment cycle (2 mg/kg GS-9620) that was accompanied by transient increase in both ALT and AST (Fig. 1B). Changes in serum HBsAg paralleled HBV DNA, although the degree of reduction was more modest [9].
Fig. 1. GS-9620 study in CHB chimpanzees.
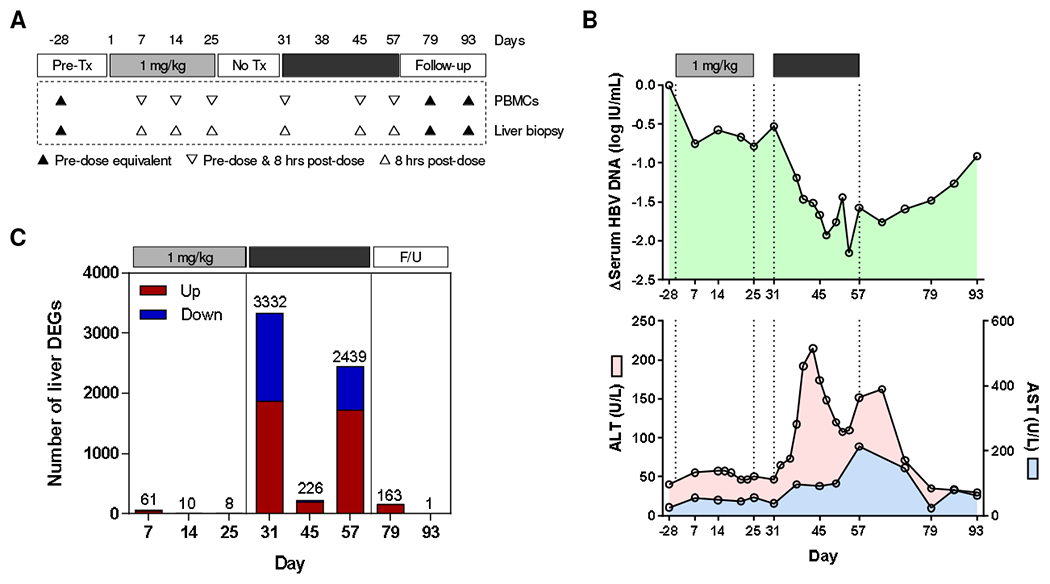
(A) Design of the GS-9620 study in CHB chimpanzees (n=3). The timing of liver biopsies and PBMC sampling are indicated. Tx: treatment. (B) Mean change in serum HBV DNA (top), ALT (pink, bottom) and AST (blue, bottom). Data is adapted from Lanford et al. [9], with permission. (C) DEG count relative to pre-treatment (day -28) for each time point (red: over-expressed; blue: under-expressed). F/U: follow-up. Note: animal 4x0139 was excluded from analysis at day 45 because GS-9620 was not administered to this animal on days 43, 45 and 47.
In the current study we performed retrospective analysis of archived liver biopsies and paired PBMC samples collected before treatment, during both treatment cycles and after treatment (Fig. 1A). RNA-Seq analysis of the liver biopsies demonstrated that 1 mg/kg GS-9620 had little impact on intrahepatic gene expression, with less than 65 of the 21,787 genes analyzed being differentially expressed at any one time-point (Fig. 1C). In contrast, 2 mg/kg GS-9620 strongly modulated the intrahepatic transcriptome of these animals, with >2000 DEGs after the first (day 31) and last dose (day 57) of the second treatment cycle (Fig. 1C). There was a very similar trend for PBMC DEGs (Supplementary Fig. 6). However, there were markedly fewer liver and PBMC DEGs after the 7th dose (day 45) of 2 mg/kg GS-9620. Lower compound exposure after this dose may account for the weaker pharmacodynamic response [9]. Alternatively, frequent administration of GS-9620 may have induced tachyphylaxis (TLR tolerance), as has been described for other TLR7 agonists [11]. The complete liver and PBMC DEG lists are provided in Supplementary Tables 9 and 10.
Intrahepatic IFN, CD8+ T cell and B cell transcriptional signatures correlate with the antiviral response to GS-9620 treatment
Module analysis determined that 1 mg/kg GS-9620 had only a modest effect on the transcriptional profiles of liver and PBMCs, with only the IFN gene signature (Module, M3.1) being induced (Fig. 2A, Supplementary Fig. 7). This is consistent with IFN-α being the most strongly induced serum cytokine during the first treatment cycle [9]. There was also pronounced induction of the IFN module (M3.1) during the second treatment cycle in both compartments (Fig. 2A, Supplementary Fig. 7). Interestingly, there were different patterns of intrahepatic interferon-stimulated gene (ISG) induction during GS-9620 treatment. For example, Viperin (RSAD2) and OASL, which are elevated in the liver during resolution of acute WHV infection of woodchucks [4], were significantly induced during both treatment cycles (Supplementary Table 4). ISG15 and PD-L1 (CD274) displayed a similar pattern, albeit induction of these ISGs was only statistically significant during the second treatment cycle (Fig. 2B). In contrast, the type II IFN-biased ISG CXCL9 was only significantly induced during the later stages of second treatment cycle (Fig. 2B), suggesting a differential dose- and time-dependence for IFN-α vs. IFN-γ induction by GS-9620.
Fig. 2. Intrahepatic transcriptional signature of CHB chimpanzees treated with GS-9620.
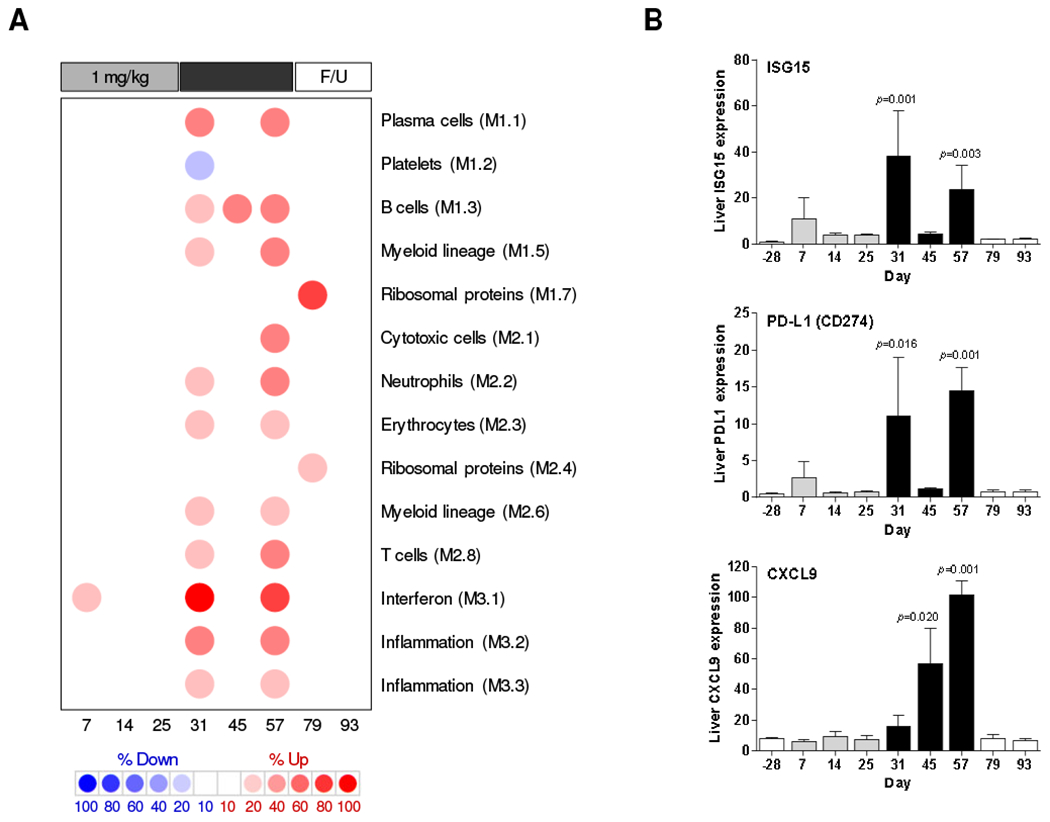
(A) Modular analysis of intrahepatic transcriptional signatures relative to pre-treatment (day -28). Columns represents the mean of n=3 animals (except day 45, n=2) on different study days. Spot intensity (red: over-expressed; blue: under-expressed) denotes the percentage of transcripts significantly changed in each module and is defined by the scale bar. The functional interpretation of each module is displayed on the right. Only modules with a functional annotation and enrichment greater than 10% at one or more time-point are displayed. F/U: follow-up. (B) qRT-PCR data expressed as fold-change relative to naïve animals. The bar height indicates the mean and the errors bars represent the SEM. Column color matches the study phase as described in Fig. 1A. Statistical significance relative to pre-treatment (day −28) was calculated with log-transformed values by one-way ANOVA with Dunnett’s multiple comparison correction. Only p values <0.05 are labeled.
In contrast to the lower dose, 2 mg/kg GS-9620 induced a broad intrahepatic immune response, with cytotoxic cell (NK cell/CD8+ T cell) (M2.1), T cell (M2.8), B cell (M1.3) and plasma cell (M1.1) gene signatures prominently up-regulated (Fig. 2A). Notably, T cell (M2.8) and B cell (M1.3) gene signatures in the liver were progressively induced during the second treatment cycle, and most strongly up-regulated at the peak of antiviral response (day 54-64) (Fig. 2A). The cytotoxic cell module (M2.1) was only induced at the peak of antiviral response. The progressive change in intrahepatic signatures during the second treatment cycle was not clearly reflected in module analysis of paired PBMC samples (Supplementary Fig. 7).
The transcriptional signature induced by the second treatment cycle was significantly enriched with genes induced during HBV clearance in acutely infected chimpanzees [2] (Fig. 3A). Strikingly, this HBV clearance signature, which is predominantly comprised of genes associated with T cell response, was temporally associated with the peak antiviral response to GS-960 treatment. The induction of CD3D and CD8A but not CD4, suggests that the number of CTLs (but not T helper cells) was increased in the liver during the second treatment cycle (Fig. 3B, Supplementary Table 4). The over-expression of genes associated with T cell activation (ICOS), inhibition (PD-1, CTLA4) and survival (TNFSF18, GITR ligand) is also consistent with increased intrahepatic T cell frequency during 2 mg/kg GS-9620 treatment (Fig. 3B, Supplementary Table 4). Interestingly, the second treatment cycle also upregulated a large number of genes associated with MHC class I antigen processing and presentation (Supplementary Table 5), suggesting that GS-9620 may enhance recognition of infected hepatocytes by CD8+ T cells. The induction of CD19 and CD79a suggest that B cell numbers in the liver were also increased by the second cycle of GS-9620 treatment (Fig. 3B, Supplementary Table 4). The strong intrahepatic upregulation of various CC and CXC chemokine mRNAs (Supplementary Table 4), is consistent with elevated serum chemokine levels [9] and suggests that the apparent increase in cell number is due, at least in part, to migration of CTLs and B cells into the liver during treatment. In contrast to T and B cell response genes, intrahepatic NK, NKT and MAIT cell associated genes such as CD56 (NCAM1), CD16 (FCGR3A), CD161 (KLRB1), NCR1 (NKp46), NKG2A (KLRC1) and NKG2D (KLRK1) were not significantly modulated by GS-9620 treatment (Fig 3B, Supplementary Table 4). Collectively, these data indicate that IFN signaling, together with migration (and/or expansion) of CD8+ T cells and B cells in the liver of CHB chimpanzees, is associated with the antiviral response to GS-9620 treatment.
Fig. 3. Intrahepatic expression of genes associated with HBV clearance in CHB chimpanzees treated with GS-9620.
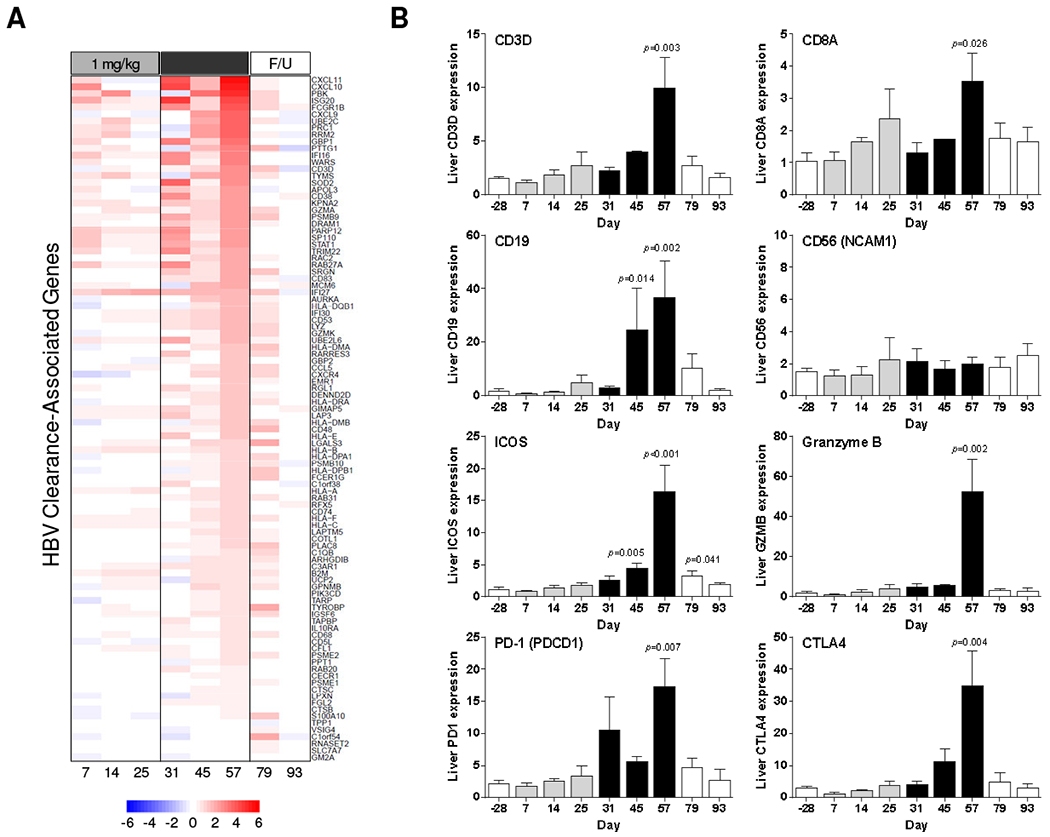
(A) Expression of HBV clearance-associated genes [2] relative to pre-treatment (day −28) plotted by mean log2 fold-change. Heatmap columns represents the mean of n=3 animals (except day 45, n=2) on different study days. Rows represent individual genes; over-expression (red) and under-expression (blue) indicated by the scale bar for log2 fold-change values. F/U: follow-up. (B) qRT-PCR data expressed as fold-change relative to naïve animals as described in Fig. 2.
Intrahepatic CD8+ T cell response to GS-9620 is associated with cytolytic and non-cytolytic transcriptional signatures
CD8+ T cells can inhibit HBV infection by non-cytolytic mechanisms mediated by TNF-α and IFN-γ, as well as by killing infected hepatocytes via cytotoxic effector molecules. As mentioned previously, the IFN-γ responsive gene CXCL9 was highly induced during the later stages of the second GS-9620 treatment cycle. CXCL9 expression correlated closely with the intrahepatic T cell signature, whereas expression of the type I IFN-biased ISG15 did not (compare Fig. 2B and Fig. 3B). Since intrahepatic levels of NK, NKT and MAIT cell-associated genes were not modulated, these data suggest that intrahepatic CTLs are the predominant source of IFN-γ during GS-9620 treatment. This interpretation is consistent with in vitro studies in human PBMCs demonstrating that GS-9620 induces a weak IFN-γ response in NK and MAIT cells (data not shown).
It was previously demonstrated that the antiviral response to GS-9620 treatment and elevation of liver injury biomarkers was temporally correlated (Fig. 1B, Supplementary Fig. 12) [9]. This biochemical evidence of liver damage is consistent with intrahepatic induction of the cytotoxic effector genes granzyme B (GZMB) and perforin 1 (PRF1), as well as the receptor-mediated cell death gene Fas ligand (FASLG/CD95L) during the second treatment cycle (Fig. 3B, Supplementary Table 4). In contrast, TRAIL (TNFSF10) was not significantly induced during either treatment cycle (Supplementary Table 4). The induction of transcriptional signatures indicative of hepatocyte apoptosis and proliferation are consistent with the upregulation of cytotoxic effector genes (Supplementary Table 6). These expression data are also in line with a previous analysis, which demonstrated there are increased numbers of hepatocytes expressing activated caspase 3 (apoptosis marker) and Ki67 (proliferation marker), as well as a substantially lower number of HBV core-positive cells, at the end of the second treatment cycle [9].
GS-9620 treatment induced lymphoid aggregates in the liver of chimpanzees chronically infected with HBV
The histological features of liver biopsies from CHB chimpanzees treated with GS-9620 have previously been described [9]. H&E staining confirmed pronounced infiltration of mononuclear cells, primarily in the portal areas of the liver, during the second treatment cycle (Supplementary Fig. 8). To better characterize this immune infiltrate we performed IHC with markers for total T cells (CD3), CTLs (CD8), B cells (CD20) and NK cells (CD56). This revealed that CD3+ T cells, CD8+ T cells and B cells formed lymphoid aggregates in the portal regions of the liver during the second GS-9620 treatment cycle (Fig. 4, Supplementary Figs. 9 and 10). These lymphoid aggregates had formed at most portal regions at the peak of antiviral response (Supplementary Fig. 11). Morphometric analysis demonstrated that a significant increase in intrahepatic T cell, CTL and B cell numbers in these aggregates temporally correlated with the peak of antiviral response (Fig. 5, Supplementary Fig. 12). Interestingly, these immune cells were also increased in the adjacent liver parenchyma (or lobule), but did not form aggregates there (Supplementary Fig. 13). Intrahepatic T and B cell numbers returned to almost pre-treatment levels during the follow-up period (Fig. 5, Supplementary Fig. 13). Staining of serial sections suggested that majority of CD3+ cells in the aggregates (although not all) were CD8+, indicating that CTLs were the prominent T cell population in these structures. Consistent with the transcriptional analysis (Fig. 3B), there was moderate PD-1 staining throughout the lymphoid aggregates as well as in a few cells within the hepatic sinusoids (Fig. 6). CD20+ cells comprised the bulk of the remaining cells in the aggregates. In contrast to T and B cells, very few CD56+ cells were detected and there was not a significant change in intrahepatic NK cell number during GS-9620 treatment (Fig. 5, Supplementary Fig. 13). In line with the transcriptome analysis, these data indicate that an increase in the numbers of intrahepatic CTLs and B cells (but not NK cells) is temporally associated with the antiviral response to GS-9620 in CHB chimpanzees.
Fig. 4. Lymphoid aggregates of CD8+ T cells and B cells in CHB chimpanzees treated with GS-9620.
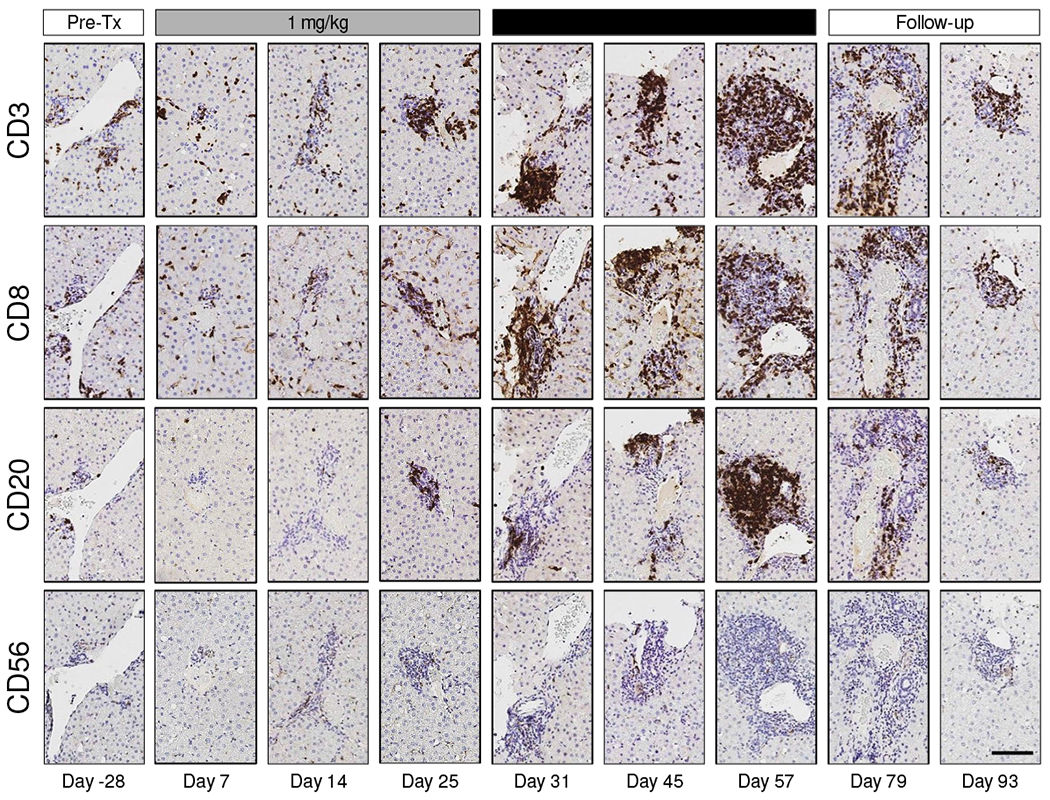
IHC labeling of serial liver biopsy sections for CD3 (T cell), CD8 (CTL), CD20 (B cell) and CD56 (NK cell) immunoreactivity on different study days for a single representative animal (4x0139). All immunolabels are stained dark brown. The maximum viral load reduction in this animal occurred on study day 54. Image magnification is 10X. The scale bar (bottom right image) represents 100 μm. Tx: treatment.
Fig. 5. Quantitation of intrahepatic immune cells in CHB chimpanzees treated with GS-9620.
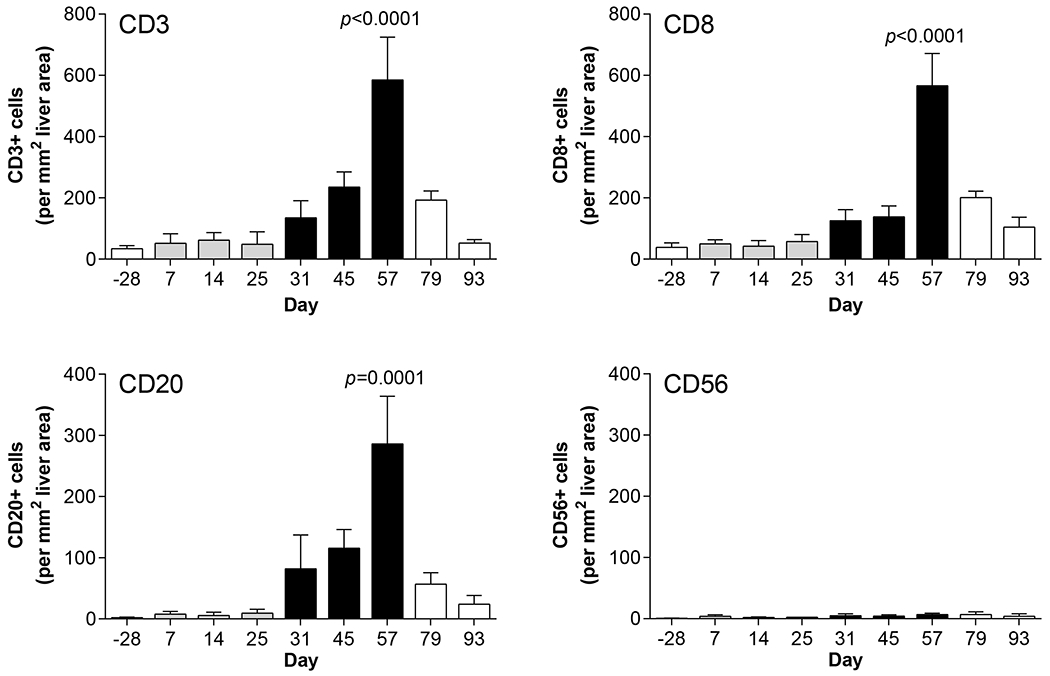
Number of lymphocytes in areas of immune cell aggregation on different study days as determined by quantitative image analysis. Bar height indicates the mean and error bars represent the SEM for all animals for which IHC slides were evaluable (n=2-3, except for n=1 on day 25 for CD56 labeling). Column color matches the study phase as described in Fig. 1A. Statistical significance relative to pre-treatment (day −28) was calculated by one-way ANOVA with Dunnett’s multiple comparison correction. Only p values <0.05 are labeled.
Fig. 6. Intrahepatic aggregates in CHB chimpanzees treated with GS-9620 contain PD-1+ lymphocytes but not follicular dendritic cells or IgG+ plasma cells.
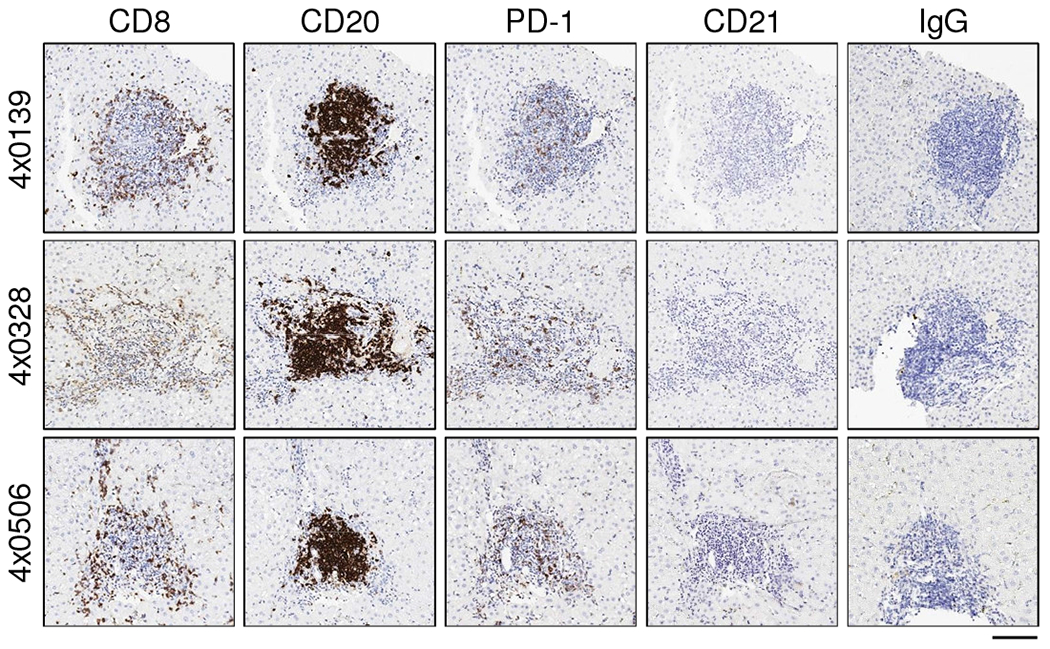
IHC labeling of serial liver biopsy sections for CD8 (CTL), CD20 (B cell), PD-1 (marker of T cell activation/exhaustion), CD21 (follicular dendritic cell) and IgG (subset of plasma cells) immunoreactivity on study day 57. All immunolabels are stained dark brown. Image magnification is 10X. The scale bar (bottom right) represents 100 μm.
TLR agonists have previously been shown to induce intrahepatic aggregates of CD11b+ myeloid cells that facilitate expansion of CTLs in the liver of mice [12]. These structures (“iMATEs”) induced intrahepatic expansion of HBV-specific CTLs generated by vaccination, and led to HBV clearance in the adenovirus mouse model [12]. In contrast to iMATES, the intrahepatic aggregates induced by GS-9620 treatment were comprised of a B cell “core” surrounded and admixed with T cells (Fig. 4, Supplementary Figs. 9 and 10), and lacked CD11b+ myeloid cells (Supplementary Fig. 14). Therefore, the lymphoid aggregates in the liver of HBV-infected chimpanzees temporally associated with GS-9620 treatment response were structurally and phenotypically distinct from iMATEs described in mice.
Intrahepatic lymphoid aggregates induced by GS-9620 treatment are not fully differentiated tertiary lymphoid structures
In contrast to iMATEs, the lymphoid aggregates induced by GS-9620 treatment in the liver of HBV-infected chimpanzees are reminiscent of intrahepatic lymphoid aggregates commonly observed in portal areas of individuals with chronic hepatitis C virus (HCV) infection [13]. A similar lymphoid structure was identified in the portal region of an untreated HCV-infected chimpanzee (Supplementary Fig. 15). The majority of intrahepatic lymphoid aggregates/follicles in HCV patients comprise a core of B cells mixed with CD4+ T cells, with an outer ring formed predominantly of CD8+ T cells [13]. A small percentage of these follicles have been reported to contain a functional germinal center (GC) and stain positive for both follicular dendritic cells (FDCs) and CD4+ T follicular helper cells (TFH) [14–16]. The presence of organized tertiary lymphoid structures (TLS) in HBV-infected chimpanzees treated with GS-9620 was suggested by the temporal association between intrahepatic induction of CXCL13 (a major organizational chemokine for B cell follicles and GCs), CXCR5 (the CXCL13 receptor, expressed by both B cells and TFH in GCs) and ICOS (highly expressed by TFH) with T and B cell infiltration into the liver (Fig. 3B, Supplementary Table 4). Consistent with TLS formation, B cells and potentially admixed CD3+CD8− T cells (e.g. TFH cells) –but likely not CD8+ T cells– expressed CXCR5 in the lymphoid aggregates (Fig. 7). An increase in the intrahepatic expression of CCR7 and its ligands CCL19 and CCL21 also suggested that organized lymphoid structures were induced by GS-9620 treatment (Supplementary Table 4). However, the absence of positive CD21 and IgG staining (markers of FDCs and a subset of plasma cells, respectively) indicated that these aggregates do not represent fully differentiated follicles that support germinal center reactions (Fig. 6). Moreover, intrahepatic expression of AICDA (activation-induced cytidine deaminase, AID) was not induced by GS-9620 treatment (Supplementary Table 4), suggesting that immunoglobulin class switching and somatic hypermutation did not occur in these structures. Collectively these data indicate that the intrahepatic lymphocyte aggregates induced by GS-9620 treatment lack key features of fully differentiated TLS.
Fig. 7. Lymphoid aggregates in CHB chimpanzees treated with GS-9620 contain CXCR5+ B cells.
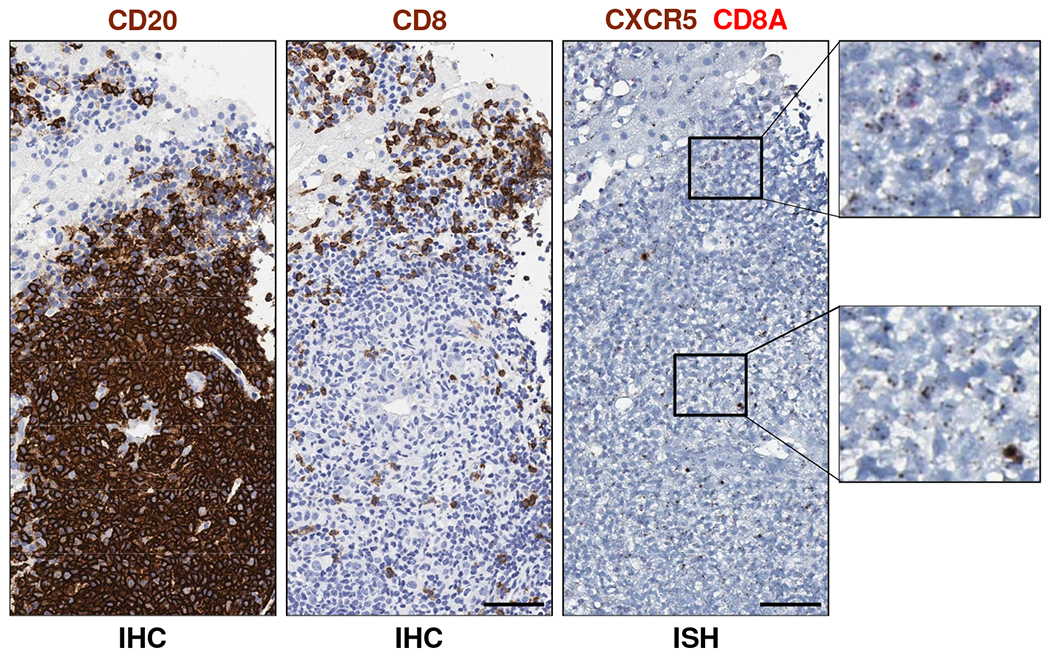
IHC and ISH staining of serial liver biopsy sections from animal 4x0139 for CD20 (left image, dark brown), CD8 (middle image, dark brown), CD8A RNA (right image, red) and CXCR5 RNA (right image, dark brown) on study day 57. Image magnification is 40X. The scale bars (bottom right of images) represent 50 μm. Higher magnification views of ISH staining are provided on the right. Cells that stained positive for both CD8A RNA and CXCR5 RNA were not detected. Note that biopsies from animals 4x0328 and 4x0506 were not evaluable by ISH (see Supplementary Materials).
DISCUSSION
In this study we performed a retrospective analysis of archived samples to characterize the immune environment in the liver of chimpanzees chronically infected with HBV. Transcriptome and immunohistochemical analyses revealed important parallels with the intrahepatic immune environment of CHB in man. The detection of intrahepatic CD8+ T cells together with a T cell exhaustion transcriptional signature in HBV-infected chimpanzees is consistent with the presence of exhausted HBV-specific CD8+ T cells in the liver of CHB patients [17]. Although it was not possible to determine the antigen-specificity of the intrahepatic CD8+ T cells in HBV-infected chimpanzees (see below), there was no biochemical evidence of hepatocyte injury in these animals (Table 1). This is notable because non virus-specific CD8+ T cells, rather than HBV-specific CD8+ T cells, are associated with liver damage in untreated CHB patients [18]. These data therefore suggest that CD8+ T cell dysfunction may play a role in HBV persistence in chimpanzees.
The absence of an intrahepatic type I IFN transcriptional signature in HBV-infected chimpanzees is consistent with various studies in woodchucks, chimpanzees and humans [2, 4, 19–21]. However additional comparisons between the intrahepatic immune environment associated with chronic HBV infection in chimpanzees and humans is challenging because the latter is poorly characterized. In contrast, the transcriptional response of woodchucks to chronic hepadnavirus infection has recently been described [20]. We identified important parallels between the intrahepatic transcriptome profiles of the chimpanzee and woodchuck models of CHB (Supplementary Table 7). However, the absence of a neutrophil transcriptional signature in chronically infected chimpanzees may represent an important difference between these animal models, and raises the possibility that they might reflect different stages of HBV natural history in humans. This difference in intrahepatic immune environment may also have contributed to the difference in degree of antiviral response to GS-9620 in the woodchuck and chimpanzee models [8, 9].
We also performed a retrospective analysis of archived samples to characterize the intrahepatic immune correlates of antiviral response to the oral TLR7 agonist GS-9620 in CHB chimpanzees. An important finding from this study is that the disparate antiviral response to low (1 mg/kg) and high dose (2 mg/kg) GS-9620 appears to reflect differential dose requirements for induction of intrahepatic innate and adaptive immune responses. In the first treatment cycle, the modest antiviral response to GS-9620 was associated with intrahepatic ISG induction, but not a significant increase in B cell or CTL number, or evidence of liver injury. Although transcriptional analysis of whole biopsy samples cannot determine whether these ISGs are expressed in hepatocytes and/or non-parenchymal cells, it was previously demonstrated that hepatocyte ISG15 protein levels are elevated following GS-9620 treatment [9]. In addition, the accompanying study demonstrates that type I IFN induced by GS-9620 in human PBMCs stimulates ISG expression in HBV-infected primary human hepatocytes (PHH) in vitro [22]. These data therefore indicate that HBV was primarily controlled by type I IFN-mediated, non-cytolytic mechanisms during low dose GS-9620 treatment. Based on comprehensive kinetic studies of self-limiting HBV infection in chimpanzees, it was suggested that immune-mediated non-cytolytic mechanisms can control cccDNA levels by inhibiting viral replication (thereby preventing viral spread) and, at least theoretically, by destabilizing cccDNA [23]. Although intrahepatic cccDNA content was not monitored during the GS-9620 chimpanzee study, GS-9620-induced type I IFN reduced viral RNA, DNA and antigens in HBV-infected PHH, but did not alter cccDNA levels [22]. Taken together, these data suggest that the first GS-9620 treatment cycle induced non-cytolytic control of HBV by suppressing viral replication.
In contrast to the first treatment cycle, the higher GS-9620 dose induced accumulation of CTLs and B cells (but not NK cells) in the liver. This intrahepatic response is consistent with activation of CD8+ T cells in the periphery being limited to the second GS-9620 treatment cycle [9]. The higher GS-9620 dose also increased intrahepatic expression of genes associated with MHC class I antigen processing and presentation, suggesting the second cycle of treatment may have promoted antigen recognition by HBV-specific CD8+ T cells. Consistent with this notion, GS-9620-induced cytokines increased expression of a similar set of genes and enhanced presentation of an immunodominant viral peptide by HBV-infected PHH [22]. Elevation of intrahepatic CTL numbers during GS-9620 treatment was temporally associated with the peak of antiviral response and was accompanied by biochemical, immunohistochemical and transcriptional evidence of hepatocyte killing and liver regeneration [9]. Collectively, these data indicate that the antiviral response induced by higher dose GS-9620 was mediated, at least in part, by killing of HBV-infected hepatocytes by CD8+ T cells. The intrahepatic expression of IFN-γ responsive genes during the second treatment cycle suggests that non-cytolytic CD8+ T cell effector mechanisms may also have contributed to the antiviral response. Comparable immune mechanisms are associated with viral clearance during self-limiting hepadnavirus infection [2–4, 23], indicating there are important parallels between the immune pathways that mediate natural resolution of infection and those induced by GS-9620 treatment.
The transcriptomic analysis of paired liver biopsy and PBMC samples in CHB chimpanzees treated with GS-9620 provides a rare opportunity to directly compare immune parameters in the liver and periphery. This revealed that ISGs were strongly induced in both compartments, but the T and B cell signatures that closely correlated with antiviral response were only significantly induced in the liver. The pharmacokinetic properties of GS-9620 likely explain these anatomic differences in immune response, with oral administration expected to provide high gut and liver exposure but minimal systemic exposure [24]. Activation of specialized immune cells enriched or compartmentalized in the liver may also have contributed to the disparity between the intrahepatic and peripheral immune responses. The localized intrahepatic response to GS-9620 underscores the need to include immune monitoring of the liver in future immunotherapeutic studies.
Interestingly, the increase in immune cell numbers in the liver during GS-9620 treatment was predominantly due to the formation of lymphoid aggregates in portal regions. These aggregates contained large numbers of CD8+ T cells and CD20+ B cells, but very few CD11b+ myeloid lineage cells, and so were distinct from iMATEs induced by TLR agonists in the liver of rodents [12]. FDCs and IgG-positive cells were not detected in the aggregates, suggesting that they are not fully differentiated follicles containing a GC reaction. Instead, the aggregates resemble the FDC-negative intrahepatic lymphoid follicles regularly observed in HCV patients (and infrequently in CHB patients) [13, 15], and which we also identified in an HCV-infected chimpanzee. The temporal association between the development of these structures and the antiviral response to GS-9620 treatment raises the possibility that they support development of an effective immune response to HBV. In accordance with this hypothesis, various studies have suggested that similar structures promote antiviral, antibacterial and antitumor immunity [25]. However, our data does not establish a causal relationship between the intrahepatic aggregates and antiviral response. Additional studies are therefore required to confirm these structures play a role in initiation, differentiation, maturation or expansion of HBV-specific CD8+ T cells and/or B cells. It is also important to identify the molecular and cellular pathways necessary for the formation and function of the aggregates. In particular, given the close proximity of T and B cells, it will be interesting to determine whether these structures support T-B cooperation, e.g. T cell help to B cells or antigen presentation by B cells to T cells.
Antiviral CD8+ T cell responses can be limited by a variety of mechanisms, including NK cell-mediated deletion and T cell exhaustion [17, 26]. GS-9620 substantially increased intrahepatic CD8+ T cell numbers without altering NK cell frequency, and did not induce intrahepatic TRAIL expression. Our data therefore do not suggest that GS-9620 treatment stimulated NK cell-mediated elimination of antiviral CD8+ T cells in the liver of HBV-infected chimpanzees. In contrast, GS-9620 strongly induced intrahepatic expression of the inhibitory T cell receptor CTLA4, as well as PD-1 and its ligand PD-L1. This raises the possibility that combination with checkpoint blockade may improve the antiviral response to GS-9620 treatment.
One limitation of the current study is the small number of animals that were evaluated. Nevertheless, the intrahepatic IFN and T cell transcriptional signatures are a common feature of antiviral response to GS-9620 treatment in both chimpanzee and woodchuck models (Supplementary Fig. 16). Another limitation is that the original chimpanzee efficacy study did not characterize the antigen specificity of the intrahepatic CD8+ T cells during GS-9620 treatment. Unfortunately, it is now extremely challenging to do so with the remaining FFPE liver samples. While it is possible that virus non-specific cells induced liver injury during GS-9620 treatment and contributed to antiviral response, this is considered unlikely for several reasons. Firstly, in the woodchuck study substantial ALT elevations (mean >4-fold over baseline at end-of-treatment) were observed in chronically infected but not uninfected animals dosed with the GS-9620 [8]. Secondly, in the chimpanzee study the largest ALT elevation occurred in the animal with the highest percentage of HBV core-positive hepatocytes [9]. Importantly, the relative increase in intrahepatic CD8+ cells at the peak of antiviral response was similar in all chimpanzees. Thirdly, the lack of viral rebound after cessation of treatment (both models) together with the induction of an anti-surface antigen antibody response (woodchuck study) suggests induction of an effective antiviral memory T and/or B cell response [8, 9].
In summary, by characterizing the intrahepatic immune correlates of antiviral response to GS-9620 in the chimpanzee model of CHB, this study provides new insights into the immune mechanisms that can suppress chronic HBV infection. Encouragingly, our data suggest that administration of an immunomodulatory agent can induce an effective antiviral CD8+ T cell response despite HBV infection persisting for more than two decades. Ongoing clinical studies with a variety of immunotherapies are evaluating whether it is possible to achieve the same goal in CHB patients.
Supplementary Material
GS-9620 treatment induced the expression of genes associated with HBV clearance
GS-9620 treatment transiently induced intrahepatic lymphoid aggregates
The aggregates were not fully differentiated tertiary lymphoid structures
Intrahepatic NK cell number did not change during GS-9620 treatment
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Deborah Chavez for organizing the chimpanzee studies; Kathleen Brasky for veterinary expertise; Allyson Shauf and David Newstrom for supporting the IHC and ISH analyses; Peng Yue, Tomas Cihlar, Anuj Gaggar, Kathryn Bowenkamp, Daniel Tumas and Frank Chisari for discussions and support. Finally, we thank the many colleagues whose work we were unable to describe because of space limitations.
Financial support:
This study was sponsored by Gilead Sciences, Inc. The Southwest National Primate Research Center resources are supported by NIH grant P51-OD011133 from the Office of Research Infrastructure Programs/Office of the Director.
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CHB
chronic hepatitis B
- CTL
cytotoxic T lymphocyte
- DEG
differentially expressed gene
- FDC
follicular dendritic cell
- FDR
false discovery rate
- GC
germinal center
- GSEA
gene set enrichment analysis
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- IFN-α
interferon-alpha
- iMATEs
intrahepatic myeloid-cell aggregates for T cell population expansion
- IPA
Ingenuity Pathway Analysis
- ISG
interferon-stimulated gene
- M
module
- pDC
plasmacytoid dendritic cell
- PHH
primary human hepatocytes
- TFH
T follicular helper cell
- TLR
toll-like receptor
- TLS
tertiary lymphoid structure
- WHV
woodchuck hepatitis virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: L. Li, V. Barry, S. Daffis, C. Niu, E. Huntzicker, D. French, I. Mikaelian, W. Delaney and S. Fletcher are employees of Gilead Sciences, Inc.
REFERENCES
- [1].Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003;77:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A 2004;101:6669–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M, et al. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci U S A 2010;107:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fletcher SP, Chin DJ, Cheng DT, Ravindran P, Bitter H, Gruenbaum L, et al. Identification of an intrahepatic transcriptional signature associated with self-limiting infection in the woodchuck model of hepatitis B. Hepatology 2013;57:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Roethle PA, McFadden RM, Yang H, Hrvatin P, Hui H, Graupe M, et al. Identification and optimization of pteridinone Toll-like receptor 7 (TLR7) agonists for the oral treatment of viral hepatitis. J Med Chem 2013;56:7324–7333. [DOI] [PubMed] [Google Scholar]
- [6].Swiecki M, Colonna M. Type I interferons: diversity of sources, production pathways and effects on immune responses. Curr Opin Virol 2011;1:463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A 2013;110:E3216–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the woodchuck model of chronic hepatitis B. J Hepatol 2015;62:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013;144:1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fletcher SP, Chin DJ, Gruenbaum L, Bitter H, Rasmussen E, Ravindran P, et al. Intrahepatic Transcriptional Signature Associated with Response to Interferon-alpha Treatment in the Woodchuck Model of Chronic Hepatitis B. PLoS Pathog 2015;11:e1005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Clarke S, Laxton C, Horscroft N, Richard V, Thomas A, Parkinson T. Comparison of rat and human responses to toll-like receptor 7 activation. J Interferon Cytokine Res 2009;29:113–126. [DOI] [PubMed] [Google Scholar]
- [12].Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol 2013;14:574–583. [DOI] [PubMed] [Google Scholar]
- [13].Freni MA, Artuso D, Gerken G, Spanti C, Marafioti T, Alessi N, et al. Focal lymphocytic aggregates in chronic hepatitis C: occurrence, immunohistochemical characterization, and relation to markers of autoimmunity. Hepatology 1995;22:389–394. [PubMed] [Google Scholar]
- [14].Mosnier JF, Degott C, Marcellin P, Henin D, Erlinger S, Benhamou JP. The intraportal lymphoid nodule and its environment in chronic active hepatitis C: an immunohistochemical study. Hepatology 1993;17:366–371. [PubMed] [Google Scholar]
- [15].de Ruiter PE, van der Laan LJ. Evidence of B-cell follicles with germinal centers in chronic hepatitis C patients. Eur J Immunol 2015;45:1570–1571. [DOI] [PubMed] [Google Scholar]
- [16].Spaan M, Kreefft K, de Graav GN, Brouwer WP, de Knegt RJ, ten Kate FJ, et al. CD4+ CXCR5+ T cells in chronic HCV infection produce less IL-21, yet are efficient at supporting B cell responses. J Hepatol 2015;62:303–310. [DOI] [PubMed] [Google Scholar]
- [17].Fisicaro P, Valdatta C, Massari M, Loggi E, Ravanetti L, Urbani S, et al. Combined blockade of programmed death-1 and activation of CD137 increase responses of human liver T cells against HBV, but not HCV. Gastroenterology 2012;143:1576–1585. [DOI] [PubMed] [Google Scholar]
- [18].Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000;191:1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology 2009;137:1289–1300. [DOI] [PubMed] [Google Scholar]
- [20].Fletcher SP, Chin DJ, Ji Y, Iniguez AL, Taillon B, Swinney DC, et al. Transcriptomic analysis of the woodchuck model of chronic hepatitis B. Hepatology 2012;56:820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lebosse F, Testoni B, Fresquet J, Facchetti F, Galmozzi E, Fournier M, et al. Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B. J Hepatol 2017;66:897–909. [DOI] [PubMed] [Google Scholar]
- [22].Niu C, Li L, Daffis S, Lucifora J, Bonnin M, Maadadi S, et al. Toll-like receptor 7 agonist GS-9620 induces prolonged inhibition of HBV via a type-I interferon-dependent mechanism. Submitted. [DOI] [PubMed]
- [23].Wieland SF, Spangenberg HC, Thimme R, Purcell RH, Chisari FV. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc Natl Acad Sci U S A 2004;101:2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fosdick A, Zheng J, Pflanz S, Frey CR, Hesselgesser J, Halcomb RL, et al. Pharmacokinetic and pharmacodynamic properties of GS-9620, a novel Toll-like receptor 7 agonist, demonstrate interferon-stimulated gene induction without detectable serum interferon at low oral doses. J Pharmacol Exp Ther 2014;348:96–105. [DOI] [PubMed] [Google Scholar]
- [25].Jones GW, Jones SA. Ectopic lymphoid follicles: inducible centres for generating antigen-specific immune responses within tissues. Immunology 2016;147:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peppa D, Gill US, Reynolds G, Easom NJ, Pallett LJ, Schurich A, et al. Upregulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med 2013;210:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


