Abstract
Background:
There are limited data on the prognostic role of hepatorenal function indices in ambulatory patients with congenital heart disease (CHD). The purpose of this study was to determine the prevalence, risk factors, and prognostic implications of hepatorenal dysfunction, as measured by model for end-stage liver disease excluding international normalized ratio (MELD-XI) score, in adults with CHD.
Methods:
Retrospective study of CHD patients with comprehensive metabolic panel (2003–2019). Mild/moderate and severe hepatorenal dysfunction were defined as MELD-XI 11–15 and >15 respectively.
Results:
Of 4,977 patients, 1,376 (28%) had hepatorenal dysfunction (mild/moderate n=935[19%]; severe n=441[9%]). Hepatorenal dysfunction was most common in Fontan/unrepaired single ventricle (46%) and right heart disease (31%). Baseline MELD-XI was associated with all-cause mortality (HR 1.27, 1.21–1.33, p<0.001) after adjustment for age, sex, and congenital heart lesion.
In 3,864 patients with serial MELD-XI data, there was a temporal increase in MELD-XI, and this was associated with an increased risk of mortality (HR 1.24, 1.15–1.36 per unit increase in MELD-XI, p=0.004), independent of baseline MELD-XI score. In the subset of 1,856 patients that underwent surgical/transcatheter interventions, there was a postoperative reduction in MELD-XI, and this was associated with a lower risk of mortality (HR 0.94, 0.90–0.98 per unit decrease in MELD-XI, p=0.008), independent of the baseline MELD-XI score.
Conclusions:
Hepatorenal dysfunction was common in adults with CHD. Both baseline MELD-XI score and temporal changes in MELD-XI score were associated with clinical outcomes, and hence could be used to monitor for deterioration in clinical status, and therapeutic response to interventions.
Keywords: Congenital heart disease, Hepatorenal dysfunction, Prognostication, Mortality
BRIEF SUMMARY
Of 4,977 patients, 1,376 (28%) had hepatorenal dysfunction (mild/moderate n=935[19%]; severe n=441[9%]). Hepatorenal dysfunction was most common in Fontan/unrepaired single ventricle (46%) and right heart disease (31%). Both baseline MELD-XI score and temporal changes in MELD-XI score were associated with clinical outcomes, and hence could be used to monitor for deterioration in clinical status, and therapeutic response to interventions.
INTRODUCTION
There is a complex interaction between the heart, kidney, and liver, and as a result, structural and functional changes in one organ-system can lead to pathologic changes in the other organ-systems.1, 2 Hence, patients with heart failure often develop structural remodeling and dysfunction of the liver and kidney (hepatorenal dysfunction). 1, 2 The model for end-stage liver disease (MELD) score (and other modifications of this score such as the model for end-stage liver disease excluding international normalized ratio [MELD-XI]) provides an assessment of hepatorenal function, and it is used for prognostication in patients with heart failure, and in patients undergoing cardiac surgery or awaiting organ transplantation.3–8 In the adult congenital heart disease (CHD) population, MELD-XI score has been used for prognostication in patients with Fontan palliation, and patients undergoing CHD cardiac surgery.9–11 However, there are limited data about the role of MELD-XI score for risk stratification in ambulatory adults with CHD.11
We recently described the prevalence and prognostic implications of hepatorenal dysfunction in adults with Ebstein anomaly, and the risk factors for hepatorenal dysfunction in this population were right atrial (RA) dysfunction and RA hypertension (systemic venous congestion), and low cardiac output (hypoperfusion).12 However, these hemodynamic factors are not unique to Ebstein anomaly, but are also present in other congenital heart lesions. Therefore, it is logical to expect that hepatorenal dysfunction will also be present in other congenital heart lesions, but such data are currently lacking. The purpose of the study was to determine the prevalence, risk factors, and prognostic implications of hepatorenal dysfunction in adults with CHD.
METHODS
Study Population
This is a retrospective cohort study of adults (≥18 years of age) with CHD that had at least one comprehensive metabolic panel performed in the outpatient clinic from January 1, 2003 to December 31, 2019. The patients were identified through the Mayo Adult Congenital Heart Disease (MACHD) Registry, and the Rationale and Methods for the creation the MACHD Registry are described in the Supplementary Appendix S1. From this cohort, we excluded patients that were hospitalized within 90 days prior to comprehensive metabolic panel. Data from a subset of this cohort have been reported in a previous study.12 The Mayo Clinic Institutional Review Board approved the study.
Congenital heart lesions were classified as severe CHD (tetralogy of Fallot, truncus arteriosus, transposition of great arteries, univentricular heart, and atrioventricular canal defect) vs non-severe CHD based on the modified CHD severity classification scheme proposed by Marelli et al.13 In order to assess the prevalence of hepatorenal dysfunction across different cardiovascular physiologic states/hemodynamics, we classified the congenital heart lesions into the following anatomic/physiologic subgroups: (1) right heart disease (tetralogy of Fallot, Ebstein anomaly, pulmonic stenosis, pulmonary atresia with intact ventricular septum, double chambered right ventricle and truncus arteriosus); (2) left heart disease (coarctation aorta, aortic stenosis, subaortic stenosis, and Shone’s complex); (3) systemic right ventricle; (4) Fontan palliation; (4)unrepaired single ventricle; (5) others (Supplementary Appendix S1).
Study Objectives
The primary objective was to determine the prevalence, risk factors, and prognostic implications of hepatorenal dysfunction. For this analysis, we used the first comprehensive metabolic panel performed during the first clinical encounter in the outpatient CHD clinic to determine hepatorenal function, (baseline test), and then assessed the relationship between hepatorenal function at baseline and all-cause mortality during follow-up.
The secondary objective was to assess the relationship between temporal changes in hepatorenal function and all-cause mortality. We used 2 different subgroups of patients for this analysis. The first subgroup comprised of patients that had ≥2 comprehensive metabolic panels ≥24 months apart without any cardiac interventions between the tests (n=3,864). The second subgroup comprised of patients that had preprocedural comprehensive metabolic panel (within 3 months prior to cardiac surgical or transcatheter intervention) and postprocedural comprehensive metabolic panel (≥12 months after intervention), (n=1,856). Both the preprocedural and postprocedural comprehensive metabolic panels were based on tests performed in the outpatient clinic.
The exploratory objective to determine whether MELD-XI score had additional prognostic value beyond that of total bilirubin (hepatic function) and serum creatinine (renal function) when analyzed as individual variables.
Assessment of Hepatorenal Function
Hepatorenal function was assessed using the MELD-XI score since it can used in all patients including those on vitamin K antagonist. MELD-XI score was calculated as: 5.11× ln (serum total bilirubin in mg/dL) + 11.76 × ln (serum creatinine in mg/dL) + 9.44.3, 12 To avoid negative scores, a lower limit of total bilirubin and creatinine was set at 1.0 mg/dl. To convert from millimoles per liter to mg/dL, multiply the value in millimoles per liter by 18, prior to calculating MELD-XI score. Based on data from previous studies, we defined hepatorenal dysfunction as MELD-XI score ≥11, and we further classified the severity of hepatorenal dysfunction as mild/moderate dysfunction (MELD-XI score 11–15) and severe hepatorenal dysfunction (MELD-XI score >15).6, 7, 12
Imaging and Clinical Variables
The medical records were reviewed, and demographic, surgical, and echocardiographic data were retrieved. All-cause mortality was ascertained through a detailed review of the medical records and Accurint database, which is an institutional database for verification of mortality data.
All patients underwent comprehensive 2-dimensional, Doppler, and speckle tracking echocardiography, and offline image analyses and measurements were performed in all patients. Chamber function was assessed using strain imaging while valve function was assessed using qualitative and quantitative Doppler techniques.12, 14 RA pressure and right ventricular (RV) systolic pressure were estimated using standard techniques.15 We excluded patients with Fontan palliation from the analysis for the hemodynamic determinants of hepatorenal dysfunction, because of the lack of a standardized method for the assessment of systemic venous pressure and hemodynamics by echocardiography in this subgroup of patients.
Statistical Analysis
Between-group comparisons were performed using unpaired t-test, Wilcoxon rank sum test, analysis of variance, and chi-squared test as appropriate. Temporal changes in hepatorenal function were assessed using paired t-test. The relationship between hemodynamic variables and hepatorenal function indices was assessed by univariable linear regression analysis, and the variables with p<0.1 on univariate analysis were used to create a multivariable regression model. Time-to-event analyses were performed using the Kaplan Meier method and Cox regression. All models were adjusted for age, sex, type of congenital heart lesion, and other indices with known association with clinical outcomes in this population.16 The congenital heart lesions were modeled as categorical variables using the most common lesion (coarctation of aorta) as the reference group. The relative prognostic performance of MELD-XI score as compared to other indices (total bilirubin, creatinine, and total bilirubin plus creatinine modeled as individual indices) was assessed by comparing the area under the curve (AUC). The single conditional imputation method was used to correct for missing data.17 A p<0.05 was considered statistically significant. All statistical analyses were performed with JMP software (version 14.1.0; SAS Institute Inc, Cary NC) and GraphPad software (version 9.0.1; San Diego, CA).
RESULTS
Baseline Characteristics
Of 5,321 patients in the Mayo Adult Congenital Heart Disease Registry, 4,977 (94%) had at least one comprehensive metabolic panel that met the study inclusion criteria. Table 1 shows the baseline characteristics of the 4,977 patients included in the study. The median age was 35 (24–47) years and 2,589 (52%) were males. The 3 most common congenital heart lesions were coarctation of aorta (n=805, 16%), tetralogy of Fallot (n=744, 15%), and Ebstein anomaly (n=683, 14%), Table 1. Excluding the patients with Fontan palliation, the mean estimated RA pressure and Doppler cardiac index among the remaining 4,610 patients was 8±4 mmHg and 3.2±0.7 L/min/m2, respectively (Table 1).
Table 1:
Baseline Clinical Indices (n=4,977)
| Age, years | 35 (24–47) |
| Male sex | 2,589 (52%) |
| Severe CHD | 2,080 (42%) |
| Non-severe CHD | 2,897 (58%) |
| Congenital heart lesions | |
| *Coarctation of aorta | 805 (16%) |
| **Tetralogy of Fallot | 744 (15%) |
| Ebstein anomaly | 683 (14%) |
| ***Congenital aortic stenosis (isolated) | 639 (13%) |
| Fontan palliation | 367 (7%) |
| Atrioventricular canal defect | 304 (6%) |
| ASD/PAPVR (repaired) | 281 (6%) |
| Valvular pulmonic stenosis | 240 (5%) |
| Congenitally corrected TGA | 205 (4%) |
| TGA status post atrial switch operation | 179 (4%) |
| TGA status post arterial switch operation | 166 (3%) |
| Subaortic stenosis (isolated) | 130 (3%) |
| Eisenmenger syndrome/unrepaired single ventricle | 75 (2%) |
| Pulmonary atresia-intact ventricular septum | 43 (0.9%) |
| Truncus arteriosus | 40 (0.8%) |
| Double chambered RV | 33 (0.6%) |
| ASD/APVR (unrepaired) | 24 (0.4%) |
| Others | 19 (0.9%) |
| Comorbidities | |
| Hypertension | 1,216 (24%) |
| Hyperlipidemia | 1,027 (21%) |
| Diabetes | 324 (6%) |
| Atrial fibrillation | 738 (15%) |
| Atrial flutter/tachycardia | 582 (12%) |
| Hepatitis B/C | 6 (0.1%) |
| Medications | |
| Beta blockers | 1,303 (26%) |
| ACEI/ARB | 1,280 (26%) |
| Spironolactone | 270 (6%) |
| Loop diuretics | 930 (19%) |
| Warfarin | 849 (17%) |
| Direct oral anticoagulant | 84 (2%) |
| Antiplatelet therapy | 1,136 (23%) |
| ¶ Echocardiography (n=4,610) | |
| RA reservoir strain, % | 36±11 |
| RV (non-systemic) global longitudinal strain | −21±5 |
| Estimated RA pressure, mmHg | 8±4 |
| ≥Moderate TR severity | 926 (20%) |
| Estimated RVSP, mmHg | 34 (28–46) |
| LA reservoir strain, % | 36±13 |
| LV cardiac index, L/min/m2 | 3.2 ±0.7 |
| LV ejection fraction, % | 61±8 |
| LV (systemic) global longitudinal strain, % | −21±3 |
TGA: Transposition of great arteries; ASD/PAPVR: atrial septal defect/partial anomalous pulmonary venous return; ACEI/ARB: angiotensin converting enzyme inhibitor/angiotensin-II receptor blocker; RA: Right atrium; RV: Right ventricle; RVSP: right ventricular systolic pressure; LV: Left ventricle; CHD: congenital heart disease
Coarctation of aorta included patients with isolated coarctation and those with associated left ventricular inflow and outflow disease
Tetralogy of Fallot includes the entire disease spectrum including pulmonary atresia and ventricular septal defect and absent pulmonary valve syndrome
Congenital aortic stenosis defined as having >moderate aortic stenosis prior to 18 years of age regardless of aortic valve morphology
Echocardiographic data presented in the tables were derive from all patients except those with Fontan palliation
Prevalence and Determinants of Hepatorenal Dysfunction
The median total bilirubin (hepatic function) and serum creatinine (renal function) were 0.7 (0.4–1.2) mg/dl and 1.0 (0.8–1.1) mg/dl, respectively (Supplementary Table S1). The median MELD-XI score (hepatorenal function) was 9.5 (9.4–12.6), and there was significant variability in MELD-XI score between the different congenital heart lesions (Table 2).
Table 2:
MELD-XI Score and Hepatorenal Dysfunction
| MELD-XI | HRD (MELD-XI ≥11) | Mild/mod HRD (MELD-XI 11–15) | Severe HRD (MELD-XI >15) | |
|---|---|---|---|---|
|
| ||||
| All patients | 9.5 (9.4–12.6) | 1,376 (28%) | 935 (19%) | 441 (9%) |
| Congenital heart lesions | ||||
| Coarctation of aorta | 9.4 (9.4–11.2) | 209 (26%) | 175 (22%) | 34 (4%) |
| Tetralogy of Fallot | 10.9 (9.8–13.6) | 253 (34%) | 180 (24%) | 73 (10%) |
| Ebstein anomaly | 10.6 (9.5–12.8) | 212 (31%) | 151 (22%) | 61 (9%) |
| Congenital aortic stenosis (isolated) | 9.4 (9.4–10.9) | 125 (19%) | 82 (12%) | 43 (7%) |
| Fontan palliation | 11.2 (10.1–14.6) | 169 (46%) | 100 (27%) | 69 (19%) |
| Atrioventricular canal defect | 9.5 (9.4–11.3) | 85 (28%) | 54 (18%) | 31 (10%) |
| ASD/PAPVR (repaired) | 9.4 (9.4–10.7) | 67 (24%) | 46 (17%) | 21 (7%) |
| Valvular pulmonic stenosis | 9.4 (9.4–10.3) | 41 (17%) | 23 (10%) | 18 (8%) |
| Congenitally corrected TGA | 9.6 (9.4–12.6) | 47 (23%) | 28 (14%) | 19 (9%) |
| TGA status post atrial switch operation | 9.4 (9.4–11.2) | 34 (17%) | 18 (10%) | 16 (9%) |
| TGA status post arterial switch operation | 9.4 (9.4–9.9) | 30 (18%) | 17 (10%) | 13 (8%) |
| Subaortic stenosis (isolated) | 9.4 (9.4–10.5) | 25 (19%) | 16 (12%) | 9 (7%) |
| Eisenmenger /unrepaired single ventricle | 11.4 (10.9–13.7) | 37 (49%) | 21 (28%) | 16 (21%) |
| Pulmonary atresia-intact ventricular septum | 11.1 (10.4–14.3) | 21 (48%) | 11 (25%) | 10 (23%) |
| Truncus arteriosus | 9.9 (9.5–12.8) | 10 (26%) | 5 (14%) | 5 (13%) |
| Double chambered RV | 9.4 (9.4–10.6) | 6 (19%) | 4 (13%) | 2 (6%) |
| ASD/APVR (unrepaired) | 9.5 (9.4–10.8) | 5 (21%) | 4 (17%) | 1 (4%) |
TGA: Transposition of great arteries; ASD/PAPVR: atrial septal defect/partial anomalous pulmonary venous return; MELD-XI: model for end-stage liver disease excluding international normalized ratio; HRD: hepatorenal dysfunction
Note: (%) denotes the prevalence of hepatorenal dysfunction within each congenital heart lesion
Of the 4,977 patients, 1,376 (28%) had hepatorenal dysfunction (MELD-XI ≥11), of which 935 (19%) and 441 (9%) were mild/moderate and severe hepatorenal dysfunction respectively. The prevalence of hepatorenal dysfunction was higher in patients with severe CHD (as compared to non-severe CHD), and in patients with Fontan palliation and right heart disease (as compared to other CHD subgroups) (Figure 1). With regards to specific congenital heart lesions, the highest prevalence of hepatorenal dysfunction occurred patients with Fontan palliation (n=169, 46%), Eisenmenger syndrome (n=37, 49%) and pulmonary atresia with intact ventricular septum (n=21, 48%), and there was no significant between-group differences in the prevalence of hepatorenal dysfunction among these 3 congenital heart lesions (p=0.6). Among the patients with left heart disease, there was correlation between MELD-XI score and left atrial reservoir strain (r=−0.31, p=0.03), RA reservoir strain (r=−0.44, p=0.008), RA pressure (r=0.42, p=0.01), and RV systolic pressure (r=0.49, p<0.001).
Figure 1.
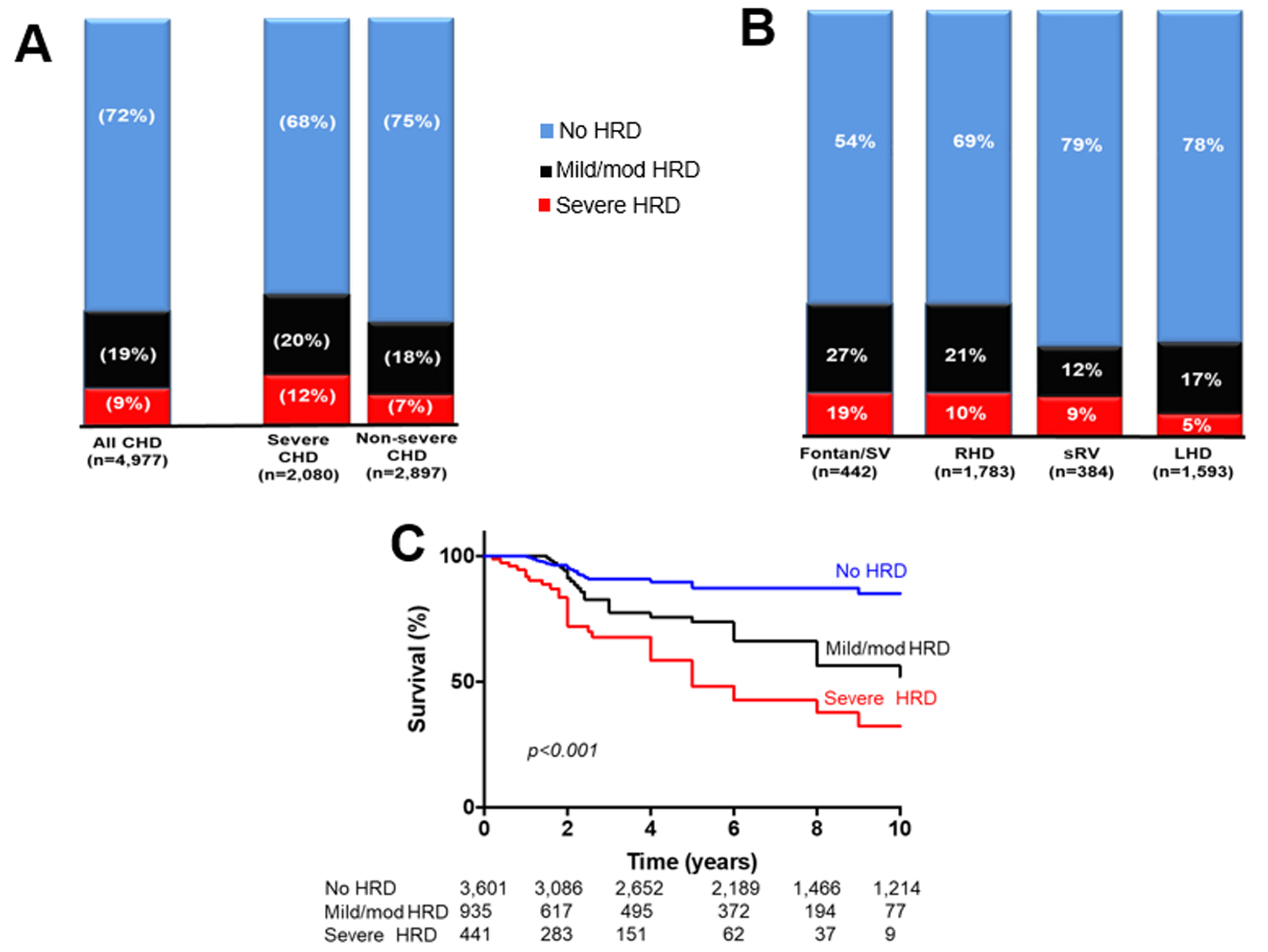
(A) Bar chart comparing the prevalence of hepatorenal disease (HRD) in patients with severe and non-severe congenital heart disease (CHD). Compared to patients with non-severe CHD, those with severe CHD had a higher prevalence of severe HRD (p=0.002) but similar prevalence of mild/moderate HRD (p=0.1).
(B) Bar chart comparing the prevalence of HRD across different CHD subgroups. There were significant between-group differences in the prevalence of mild/moderate HRD (p<0.001) and severe HRD (p<0.001).
(C) Kaplan-Meier curves comparing survival across different HRD severity subgroups.
SV: single ventricle; RHD: right heart disease, LHD: left heart disease, sRV: systemic right ventricle
Of the different hemodynamic indices analyzed (Supplementary Table S2), RA reservoir strain, estimated RA pressure, and estimated RV (subpulmonary ventricle) systolic pressure were independently associated with hepatorenal function after adjustment for age, sex, and type of congenital heart lesions (Table 3).
Table 3:
Multivariable Linear Regression Model Showing the Relationship Between Hemodynamic Indices and Hepatorenal Function (MELD-XI score)
| β±SE | p | |
|---|---|---|
|
| ||
| Age, years | 0.06±0.06 | 0.08 |
| Male sex | 0.09±0.17 | 0.3 |
| Echocardiography | ||
| RA reservoir strain, % | −0.14±0.05 | <0.001 |
| *RV global longitudinal strain | 0.10±0.12 | 0.2 |
| Estimated RA pressure, mmHg | 0.19±0.11 | <0.001 |
| Estimated RVSP, mmHg | 0.13±0.08 | 0.004 |
| **LV cardiac index, L/min/m2 | −0.04±0.14 | 0.7 |
RA: Right atrium; RV: Right ventricle; LV: Left ventricle; Note that
RV denotes non-systemic ventricle while
LV denotes systemic ventricle. Note that patients with Fontan palliation were excluded from this analysis
Model was adjusted for specific congenital heart disease diagnosis by modeling the different congenital heart lesions as categorical variables using coarctation of aorta as the reference group.
Prognostic Implications of Baseline Hepatorenal Function
The median follow-up for the 4,977 patients was 74 (39–122) months, and during this period 549 (13%) died. The cause of death was end-stage heart failure (n=187), arrhythmia/sudden cardiac death (n=62), death after cardiac surgery (n=51), stroke/intracranial hemorrhage (n=53), malignancy (n=21), sepsis/multi-organ failure (n=102), and mixed etiology (n=32). The cause of death was unknown in 41 patients. Baseline MELD-XI score was associated with all-cause mortality on univariable analysis (hazard ratio [HR] 1.31, 95% confidence interval [95% CI] 1.24–1.39, p<0.001), and after adjustment for age, sex, and type of congenital heart lesion (HR 1.27, 95%CI 1.21–1.33, p<0.001), Table 4. On subgroup analysis, MELD-XI score was also associated with all-cause mortality in patients with right heart disease (HR 1.43, 95% CI 1.26–1.81, p<0.001), Fontan palliation/unrepaired single ventricle (HR 1.39, 95% CI 1.19–1.76, p<0.001), systemic right ventricle (HR 1.19, 95%CI 1.07–1.31, p=0.007), and left heart disease (HR 1.11, 95%CI 1.01–1.24, p=0.003).
Table 4:
Multivariable Cox Model Showing the Relationship Between Hepatorenal Function (MELD-XI score) and All-cause Mortality
| Model 1 | HR (95%CI) | p |
|
| ||
| Baseline MELD-XI score, per unit | 1.27 (1.21–1.33) | <0.001 |
| Age, per year | 1.07 (1.03–1.12) | <0.001 |
| Male sex | --- | --- |
| NYHA III/IV | 1.66 (1.47–1.93) | 0.004 |
| Cyanosis | 1.36 (1.08–1.52) | 0.03 |
| Prior palliative surgery | -- | --- |
| Number prior cardiac surgeries | --- | --- |
| *Systemic ventricular dysfunction | 2.84 (1.45–4.11) | <0.001 |
| ≥Moderate systemic AVV regurgitation | --- | --- |
| Diuretics | 1.14 (0.98–1.31) | 0.09 |
| RAAS antagonist | --- | --- |
| Atrial fibrillation | 1.99 (1.17–3.38) | 0.002 |
| Hypertension | --- | --- |
|
| ||
| Model 2 | HR (95%CI) | p |
|
| ||
| Baseline MELD-XI score, per unit | 1.18 (1.06–1.31) | 0.01 |
| Temporal change in MELD-XI, per unit ↑ | 1.24 (1.15–1.36) | 0.004 |
| Age, per year | 1.04 (1.01–1.07) | 0.006 |
| Male sex | --- | --- |
| NYHA III/IV | 1.45 (1.21–1.64) | 0.02 |
| Cyanosis | --- | --- |
| Prior palliative surgery | -- | --- |
| Number prior cardiac surgeries | --- | --- |
| *Systemic ventricular dysfunction | 1.78 (1.19–2.66) | 0.008 |
| ≥Moderate systemic AVV regurgitation | --- | --- |
| Diuretics | --- | --- |
| RAAS antagonist | --- | --- |
| Atrial fibrillation | 2.16 (1.23–3.09) | 0.001 |
| Hypertension | --- | --- |
|
| ||
| Model 3 | HR (95%CI) | p |
|
| ||
| Baseline MELD-XI score, per unit | 1.21 (1.02–1.39) | 0.02 |
| Postop change in MELD-XI, per unit ↓ | 0.94 (0.90–0.98) | 0.008 |
| Age, per year | 1.08 (1.02–1.114) | 0.009 |
| Male sex | --- | --- |
| NYHA III/IV | 1.31 (0.97–2.63) | 0.08 |
| Cyanosis | --- | --- |
| Prior palliative surgery | -- | --- |
| Number prior cardiac surgeries | --- | --- |
| *Systemic ventricular dysfunction | 1.48 (1.15–2.12) | 0.007 |
| ≥Moderate systemic AVV regurgitation | --- | --- |
| Diuretics | --- | --- |
| RAAS antagonist | --- | --- |
| Atrial fibrillation | 1.46 (1.09–2.92) | 0.01 |
| Hypertension | --- | --- |
Model 1 shows the relationship between baseline MELD-XI score and all-cause mortality, and this model was based on the all patients in the study (n=4, 977).
Model 2 shows the relationship between baseline MELD-XI score, temporal change in MELD-XI score and all-cause mortality, and this model was based on the subgroup of patients with serial MELD-XI data (n=3,864).
Model 3 shows the relationship between baseline MELD-XI score, postoperative change in MELD-XI score and all-cause mortality, and this model was based on the subgroup of patients with preoperative and postoperative MELD-XI data (n=1,856).
Note that all models were adjusted for specific congenital heart disease diagnosis by modeling the different congenital heart lesions as categorical variables using coarctation of aorta as the reference group. Systemic ventricular dysfunction was defined global longitudinal strain less negative that −18%
MELD: Model for end-stage liver disease; HR: Hazard ratio; CI: Confidence interval; AVV: atrioventricular valve; RAAS: renin angiotensin aldosterone system; NYH: New York Heart Association
Compared to patients without hepatorenal dysfunction (MELD-XI score <11), those with mild/moderate hepatorenal dysfunction and severe hepatorenal dysfunction had significantly lower 10-year survival (83% vs 57% vs 38%, p<0.001), Figure 1. Mild/moderate hepatorenal dysfunction (HR 1.61, 1.27–3.11, p=0.007) and severe hepatorenal dysfunction (HR 3.93, 2.81–6.67, p<0.001) were associated with all-cause mortality after adjustment for age, sex, and type of congenital heart lesion.
For the exploratory analysis, we compared the ability of MELD-XI to discriminate between patients with different outcomes (dead vs alive) to that of total bilirubin and/or creatinine. MELD-XI (AUC 0.773), total bilirubin (AUC 0.712), creatinine (AUC 0.691) and total bilirubin plus creatinine (AUC 0.728) were associated with all-cause mortality after adjustment for age, sex, and congenital heart lesion. However, MELD-XI provided a more robust prognostic performance as compared to total bilirubin (AUC difference 0.061, 95%CI 0.045–0.076, p<0.001), creatinine (AUC difference 0.082, 95%CI 0.066–0.094, p<0.001), and total bilirubin plus creatinine (AUC difference 0.044, 95%CI 0.018–0.069, p=0.007).
Prognostic Implications of Temporal Change in Hepatorenal Function
Of the 4,977 patients, 3,864 (78%) had ≥2 comprehensive metabolic panels ≥24 months apart, and the median interval between tests was 29 (26–34) months. The MELD-XI scores at baseline and follow-up assessments were 9.5 (9.4–11.7) and 9.8 (9.5–12.7), and the Δ MELD-XI score was +0.3 (95%CI +0.1 - +0.5). The average annual change in MELD-XI score (Δ MELD-XI score/year) was +0.14/year (95%CI +0.08 - +0.19). RA reservoir strain (β±SE −0.09±0.04, p=0.006), estimated RA pressure (β±SE −0.11±0.09, p<0.001), and estimated RV (subpulmonary ventricle) systolic pressure (β±SE 0.06±0.03, p=0.02) at baseline were associated with temporal decline in MELD-XI score during follow-up. Of the 3,864 patients with serial MELD-XI scores, 327 (9%) died during follow-up. Temporal increase in MELD-XI score was associated with an increased risk of all-cause mortality (HR 1.24, 95%CI 1.15–1.36 per unit increase in MELD-XI score, p=0.004), and this relationship was independent of baseline MELD-XI score (Table 4).
Of the 4,977 patients, 1,856 (37%) had comprehensive metabolic panels pre- and post- surgical or transcatheter interventions, and the list of cardiac interventions are shown in Supplementary Table S3. The MELD-XI scores at baseline and follow-up assessments were 10.7 (9.8–12.9) and 9.6 (9.4–11.5), and the Δ MELD-XI score was −1.1 (95%CI −1.6 - −0.7). The magnitude of reduction in MELD-XI scores (Δ MELD-XI score) was inversely correlated with interval between cardiac intervention and comprehensive metabolic panel assessment (r=0.37, p=0.01), Figure 2.
Figure 2.
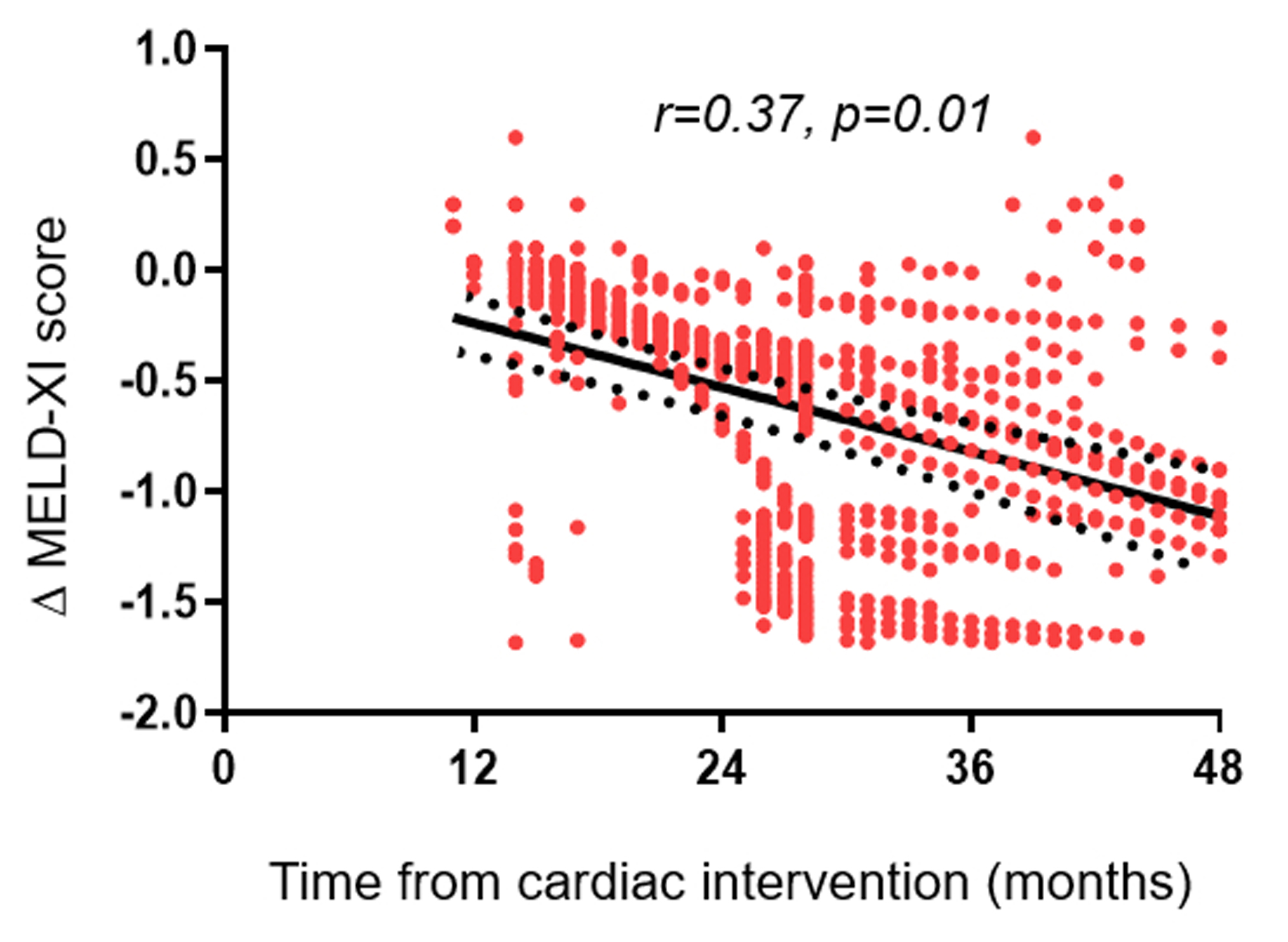
Pearson correlation showing the relationship between temporal change in MELD-XI (Δ MELD-XI) score after a cardiac procedure and the interval between the cardiac procedure and the comprehensive metabolic panel test.
Of the 5 most common procedures, the temporal change in MELD-XI score was highest in patients that underwent non-systemic atrioventricular valve repair/replacement (n=471, Δ MELD-XI score was −1.6 [95%CI −1.9 - −1.2]). Using the non-systemic atrioventricular valve repair/replacement as the reference group, the temporal change in MELD-XI score was comparable after pulmonary valve replacement (n=514, Δ MELD-XI score was −1.3 [95%CI −1.7 - −0.9], p=0.2), but lower in patients that underwent systemic atrioventricular valve repair/replacement (n=139, Δ MELD-XI score was −1.1 [95%CI −1.5 - −0.7], p=0.03), aortic valve replacement (n=364, Δ MELD-XI score was −0.9 [95%CI −1.2 - −0.6], p<0.001), and coarctation of aorta repair (n=141, Δ MELD-XI score was −0.8 [95%CI −1.3 - −02], p<0.001).
Of the 1,856 patients, 259 (14%) died during follow-up. Postoperative decrease in MELD-XI score was associated with a lower risk of all-cause mortality (HR 0.94, 95%CI 0.90–0.98 per unit decrease in MELD-XI score, p=0.008), and this relationship was independent of baseline MELD-XI score (Table 4).
DISCUSSION
In this study, we assessed the prevalence, risk factors and prognostic implications of hepatorenal dysfunction in adults with CHD, and these are the main findings. (1) Hepatorenal dysfunction, as defined by MELD-XI score, was present in 28% of the patients, and hepatorenal dysfunction was associated with hemodynamic indices of right heart dysfunction (RA reservoir strain, RA pressure and RV [subpulmonary ventricle] systolic pressure); (2) The baseline hepatorenal function and temporal changes in hepatorenal function were independently associated with all-cause mortality; (3) The MELD-XI score improved prognostication beyond that of hepatic and renal function indices (total bilirubin and creatinine), suggesting this to be the preferred risk stratification tool in this population.
MELD-XI score has been shown to be prognostic in subsets of patients with CHD such as Ebstein anomaly, Fontan palliation, and CHD patients undergoing heart transplant for end-stage heart failure.9, 10, 12, 18 However, there are limited data regarding the role of MELD-XI for risk stratification in ambulatory CHD patients.11 In a retrospective study of 637 adults with CHD, Konno et al11 reported a median MELD-XI score of 9.5, and that 18% of their cohort had hepatorenal dysfunction (defined as a MELD-XI >10.4). The median MELD-XI score and the definition of hepatorenal dysfunction used in the Konno et al study was similar to that of the current study. However, the higher prevalence of hepatorenal dysfunction in the current study (28% vs 18%) may be related to a higher proportion of patients with complex/severe CHD in the current study (42% vs 22%), and the higher proportion of complex/severe CHD in the current study is likely due to the inclusion criteria used for creating the MACHD Registry (Supplementary Online Data). Furthermore, the larger sample used in the current study allowed for more robust statistical analysis to determine the hemodynamic factors associated with hepatorenal dysfunction, and all these factors were markers of right heart dysfunction. This suggests that systemic venous congestion is a more important determinant of hepatorenal dysfunction (as compared to hypoperfusion) in this population. An important and novel observation from this study was that patients with Eisenmenger syndrome and those with pulmonary atresia with intact ventricular septum had the highest prevalence of hepatorenal dysfunction (comparable only to patients with Fontan palliation). The risk and clinical relevance of liver disease in patients with Fontan palliation are well described, and as a result, imaging and laboratory screening for liver disease are now part of routine clinical evaluation in this population.19–22 The current results suggest that the risk of liver disease (or hepatorenal dysfunction) is not limited to the Fontan physiology but rather can affect any CHD patient with significant right heart dysfunction. This has important clinical implications with regards to clinical surveillance since more than one-third of the adult CHD population can be classified as having predominantly right heart disease.13, 23, 24 An unexpected finding in this study was the occurrence of hepatorenal dysfunction in 22% of patients with left heart disease (Figure 1). We postulate that this may be related to pulmonary hypertension and right heart dysfunction resulting from left atrial and pulmonary vascular remodeling as evidenced by the correlation between MELD-XI score and indices of left atrial and right heart dysfunction. Pulmonary hypertension in the setting of left heart is a well-recognized entity in adults with CHD and has been reported in more than 20% of patient with coarctation of aorta and other left-sided cardiac pathologies. 25, 26
A consistent finding across the different studies conducted in adults with CHD is that a high MELD-XI score at the time of baseline assessment was associated with adverse events during follow-up.9–12, 18 However, previous studies have not explored whether MELD-XI score changed over time, or the prognostic implications of such temporal changes. In the current study, we showed that a temporal increase in MELD-XI score can occur in some patients, and this was associated with a higher risk of mortality independent of the baseline MELD-XI score. Similarly, some patients showed a postoperative improvement in MELD-XI score after surgical or transcatheter intervention, and this was associated with a lower mortality during follow-up. Of note, the magnitude of reduction in MELD-XI score after a cardiac procedure was most robust after right sided (non-systemic) valve intervention as compared other procedures suggesting that improvement in systemic venous congestion resulting from unloading of the right heart may be the underlying mechanism for improvement.
Clinical Implications and Future Directions
Risk stratification in adults with CHD is challenging because of significant heterogeneity resulting from differences in anatomy and physiology of the different congenital lesions. The consistent prognostic performance of MELD-XI score across the different congenital heart lesions observed in the current study suggests that MELD-XI score can be used for risk stratification in this population regardless of the underlying congenital heart lesion. Additionally, serial MELD-XI score can be used to monitor clinical deterioration during follow-up or clinical improvement after cardiovascular procedures.
Both total bilirubin and serum creatinine are part of a standard comprehensive metabolic panel and are often used to assess hepatic and renal function respectively. Although MELD-XI score is derived from total bilirubin and serum creatinine, our results show that the MELD-XI score improved risk stratification beyond that of total bilirubin and/or serum creatinine, and perhaps should be the preferred risk stratification tool.
Limitations
This is a retrospective single center study, and as a result, it is prone to selection and ascertainment bias. We did not have complementary imaging data to assess structural changes in the liver and kidney, and hence we are unable to provide more in-depth mechanistic insight about the pathophysiology of hepatorenal dysfunction in this population. Finally, we could not control for changes in the intensity of heart failure therapy (diuretics, beta blockers and renin angiotensin aldosterone system antagonists), and such therapies can potentially confound our results because of their effect on volume status and neurohormonal activation.
Conclusions
The superior prognostic performance of MELD-XI score (as compared to total bilirubin and/or creatinine) suggests that MELD-XI should be the preferred clinical tool for the assessment of hepatorenal function in the adult CHD population. The clinical applications of these findings would be seamless and inexpensive since MELD-XI score can easily be calculated using online calculators and will not require any additional tests or specialized skillset. Further studies are required to determine whether the integration of MELD-XI score into the clinical decision process with regards to the timing of intervention will improve clinical outcomes in this population.
Supplementary Material
Acknowledgement:
James Welper and Katrina Tollefsrud performed offline image analysis for this study.
Funding:
Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grants (R01 HL158517 and K23 HL141448). The MACHD Registry is supported by the Al-Bahar Research grant.
Abbreviations:
- CHD
congenital heart disease
- RA
right atrium
- RV
right ventricle
- MELD
XI model for end-stage liver disease excluding international normalized ratio
- AUC
area under the curve
- HR
hazard ratio
- CI
confidence interval
Footnotes
Conflict of Interest: none
Disclosures: none
REFERENCES
- 1.Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG and Hernandez AF. Cardiohepatic interactions in heart failure: an overview and clinical implications. Journal of the American College of Cardiology. 2013;61:2397–2405. [DOI] [PubMed] [Google Scholar]
- 2.Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, Ronco C, Tang WHW, McCullough PA, American Heart Association Council on the Kidney in Cardiovascular D and Council on Clinical C. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e840–e878. [DOI] [PubMed] [Google Scholar]
- 3.Heuman DM, Mihas AA, Habib A, Gilles HS, Stravitz RT, Sanyal AJ and Fisher RA. MELD-XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 2007;13:30–7. [DOI] [PubMed] [Google Scholar]
- 4.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER and Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. [DOI] [PubMed] [Google Scholar]
- 5.Kim MS, Kato TS, Farr M, Wu C, Givens RC, Collado E, Mancini DM and Schulze PC. Hepatic dysfunction in ambulatory patients with heart failure: application of the MELD scoring system for outcome prediction. Journal of the American College of Cardiology. 2013;61:2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Liu YX, Seto WK, Wu MZ, Yu YJ, Lam YM, Au WK, Chan D, Sit KY, Ho LM, Tse HF and Yiu KH. Prognostic Value of Hepatorenal Function By Modified Model for End-stage Liver Disease (MELD) Score in Patients Undergoing Tricuspid Annuloplasty. J Am Heart Assoc. 2018;7 (14):e009020. doi: 10.1161/JAHA.118.009020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins RB, Young BAC, Mehaffey JH, Speir AM, Quader MA, Rich JB, Ailawadi G and Investigators for the Virginia Cardiac Services Quality I. Model for End-Stage Liver Disease Score Independently Predicts Mortality in Cardiac Surgery. The Annals of thoracic surgery. 2019;107:1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inohara T, Kohsaka S, Shiraishi Y, Goda A, Sawano M, Yagawa M, Mahara K, Fukuda K, Yoshikawa T and West Tokyo Heart Failure Registry I. Prognostic impact of renal and hepatic dysfunction based on the MELD-XI score in patients with acute heart failure. International journal of cardiology. 2014;176:571–3. [DOI] [PubMed] [Google Scholar]
- 9.Adams ED, Jackson NJ, Young T, DePasquale EC and Reardon LC. Prognostic utility of MELD-XI in adult congenital heart disease patients undergoing cardiac transplantation. Clin Transplant. 2018;32:e13257. [DOI] [PubMed] [Google Scholar]
- 10.Assenza GE, Graham DA, Landzberg MJ, Valente AM, Singh MN, Bashir A, Fernandes S, Mortele KJ, Ukomadu C, Volpe M and Wu F. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. 2013;99:491–6. [DOI] [PubMed] [Google Scholar]
- 11.Konno R, Tatebe S, Sugimura K, Satoh K, Aoki T, Miura M, Suzuki H, Yamamoto S, Sato H, Terui Y, Miyata S, Adachi O, Kimura M, Saiki Y and Shimokawa H. Prognostic value of the model for end-stage liver disease excluding INR score (MELD-XI) in patients with adult congenital heart disease. PloS one. 2019;14:e0225403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egbe AC, Miranda WR, Dearani J, Kamath PS and Connolly HM. Prognostic Role of Hepatorenal Function Indexes in Patients With Ebstein Anomaly. Journal of the American College of Cardiology. 2020;76:2968–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E and Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–72. [DOI] [PubMed] [Google Scholar]
- 14.Egbe A, Miranda W, Connolly H and Dearani J. Haemodynamic determinants of improved aerobic capacity after tricuspid valve surgery in Ebstein anomaly. Heart. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010;23:685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 16.Dimopoulos K, Diller GP, Koltsida E, Pijuan-Domenech A, Papadopoulou SA, Babu-Narayan SV, Salukhe TV, Piepoli MF, Poole-Wilson PA, Best N, Francis DP and Gatzoulis MA. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320–8. [DOI] [PubMed] [Google Scholar]
- 17.Ali AM, Dawson SJ, Blows FM, Provenzano E, Ellis IO, Baglietto L, Huntsman D, Caldas C and Pharoah PD. Comparison of methods for handling missing data on immunohistochemical markers in survival analysis of breast cancer. British journal of cancer. 2011;104:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm JC, Magruder JT, Do N, Spinner JA, Dungan SP, Kilic A, Patel N, Nelson KL, Jacobs ML, Cameron DE and Vricella LA. Modified Model for End-Stage Liver Disease eXcluding INR (MELD-XI) Score Predicts Early Death After Pediatric Heart Transplantation. The Annals of thoracic surgery. 2016;101:730–5. [DOI] [PubMed] [Google Scholar]
- 19.Egbe A, Miranda WR, Connolly HM, Khan AR, Al-Otaibi M, Venkatesh SK, Simonetto D, Kamath P and Warnes C. Temporal changes in liver stiffness after Fontan operation: Results of serial magnetic resonance elastography. International journal of cardiology. 2018;258:299–304. [DOI] [PubMed] [Google Scholar]
- 20.Egbe AC, Miranda WR, Veldtman GR, Graham RP and Kamath PS. Hepatic Venous Pressure Gradient in Fontan Physiology Has Limited Diagnostic and Prognostic Significance. CJC Open. 2020;2:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Possner M, Gordon-Walker T, Egbe AC, Poterucha JT, Warnes CA, Connolly HM, Ginde S, Clift P, Kogon B, Book WM, Walker N, Wagenaar LJ, Moe T, Oechslin E, Kay WA, Norris M, Dillman JR, Trout AT, Anwar N, Hoskoppal A, Broering DC, Bzeizi K and Veldtman G. Hepatocellular carcinoma and the Fontan circulation: Clinical presentation and outcomes. International journal of cardiology. 2021;322:142–148. [DOI] [PubMed] [Google Scholar]
- 22.Wu FM, Kogon B, Earing MG, Aboulhosn JA, Broberg CS, John AS, Harmon A, Sainani NI, Hill AJ, Odze RD, Johncilla ME, Ukomadu C, Gauvreau K, Valente AM, Landzberg MJ and Alliance for Adult Research in Congenital Cardiology I. Liver health in adults with Fontan circulation: A multicenter cross-sectional study. The Journal of thoracic and cardiovascular surgery. 2017;153:656–664. [DOI] [PubMed] [Google Scholar]
- 23.Zomer AC, Vaartjes I, van der Velde ET, de Jong HM, Konings TC, Wagenaar LJ, Heesen WF, Eerens F, Baur LH, Grobbee DE and Mulder BJ. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. International journal of cardiology. 2013;168:2487–93. [DOI] [PubMed] [Google Scholar]
- 24.Diller GP, Kempny A, Alonso-Gonzalez R, Swan L, Uebing A, Li W, Babu-Narayan S, Wort SJ, Dimopoulos K and Gatzoulis MA. Survival Prospects and Circumstances of Death in Contemporary Adult Congenital Heart Disease Patients Under Follow-Up at a Large Tertiary Centre. Circulation. 2015;132:2118–25. [DOI] [PubMed] [Google Scholar]
- 25.Oliver JM, Gallego P, Gonzalez AE, Sanchez-Recalde A, Bret M and Aroca A. Pulmonary hypertension in young adults with repaired coarctation of the aorta: an unrecognised factor associated with premature mortality and heart failure. International journal of cardiology. 2014;174:324–9. [DOI] [PubMed] [Google Scholar]
- 26.Jain CC, Warnes CA, Egbe AC, Cetta F, DuBrock HM, Connolly HM and Miranda WR. Hemodynamics in Adults With the Shone Complex. The American journal of cardiology. 2020;130:137–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


