Abstract
Abstract
Purpose
This study aimed at the population receiving thrombolytic therapy and to explore the optimal time point for neutrophil-to-lymphocyte ratio (NLR) in predicting stroke-associated pneumonia (SAP).
Methods
We assessed patients undergoing intravenous thrombolysis (IVT) for acute ischemic stroke. Blood parameters were sampled before thrombolysis (within 30 min after admission) and within 24–36 h after thrombolysis, respectively. The primary outcome measure was the occurrence of SAP. Multivariate logistic regression analysis was performed to analyze the association between admission blood parameters and the event of SAP. We also used receiver operating characteristic (ROC) curve analysis to assess the discriminative ability of blood parameters measured at different times in predicting SAP.
Results
Among the 388 patients, SAP occurred in 60 (15%) patients. Multivariate logistic regression analysis showed that NLR was significantly associated with SAP (NLR before IVT: aOR = 1.288; 95%CI = 1.123–1.476; p < 0.001; NLR after IVT: (aOR = 1.127, 95%CI = 1.017–1.249; p = 0.023). The ROC curve showed that the predictive ability of NLR after IVT was better than NLR before IVT, not only in predicting the occurrence of SAP but also in predicting short-term and long-term functional outcomes, hemorrhagic transformation, and 1-year mortality.
Conclusion
Increased NLR measured within 24–36 h after IVT has a significant predictive effect on the occurrence of SAP and can be used to predict short-term and long-term poor functional outcomes, hemorrhagic transformation, and 1-year mortality.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10072-023-06654-7.
Keywords: Stroke, Thrombolysis, Pneumonia, Prognosis, Neutrophil, Lymphocyte
Introduction
Acute ischemic stroke (AIS) occurs when blood flow in the brain region is interrupted due to cerebrovascular obstruction. Although the direct cause of brain injury is insufficient blood supply, the deterioration of neurological deficit is closely related to the inflammatory process after AIS. It has been proved that stroke could destroy the homeostasis of the regular and strictly regulated immune system, making patients vulnerable to complications such as immunosuppression and infection [1–3]. Stroke-associated pneumonia (SAP) is one of the significant clinical complications encountered by elderly stroke survivors, and its etiology is mainly due to dysphagia leading to oropharyngeal aspiration and the disruption of immune homeostasis after stroke [2, 4]. SAP has been known to associate with delayed recovery, poorer functional outcomes, longer hospital stays, and higher medical costs [5–7]. However, the diagnosis of SAP is challenging because the hallmark symptoms of clinical infection, such as fever, leucocytosis, and elevated C reactive protein (CRP) can be caused by the destruction of thermoregulation and non-infectious inflammation or infections elsewhere [8]. In addition, the administration of some drugs, such as aspirin and paracetamol can mask fever. Even chest X-ray, as a core component of diagnosis, has limited utility because there may be no infiltration in the early stage and may only be helpful in the late stage of infection [9]. However, late treatment may reduce the rehabilitation rate and worsen the functional prognosis of patients [10]. Therefore, pragmatic markers are needed to predict the occurrence of SAP more accurately.
Recently, a growing number of studies have shown that NLR, which can be easily calculated through blood cell counts, could adequately predict the severity of systemic inflammation and infection, for example, in sepsis, solid tumors, and even COVID-19 [11–13]. Previous studies have shown that the neutrophil-to-lymphocyte ratio (NLR) outperformed conventional blood inflammatory markers in predicting stroke-related pneumonia (SAP) [14–16]. However, these studies only focused on the general stroke population and did not consider the impact of thrombolytic therapy. It is well known that thrombolytic therapy can change the early inflammatory stage of stroke patients by protecting the ischemic penumbra. Therefore, in this research, we observed the potential, undetected but existing inflammatory process in the different stages of thrombolytic therapy, thus predicting the onset of SAP before clinical manifestations. Furthermore, we planned to explore its utility in predicting short-term and long-term functional prognosis and mortality.
Methods
Study design
This is a single-center cohort study evaluating patients undergoing IVT for acute ischemic stroke between 2018 and 2020 in the comprehensive stroke center. Data were collected retrospectively for treated patients. All patients included in this study signed informed consent in follow-up visits. The data supporting the results of this study are available from the corresponding author upon reasonable request from any qualified investigator.
Population
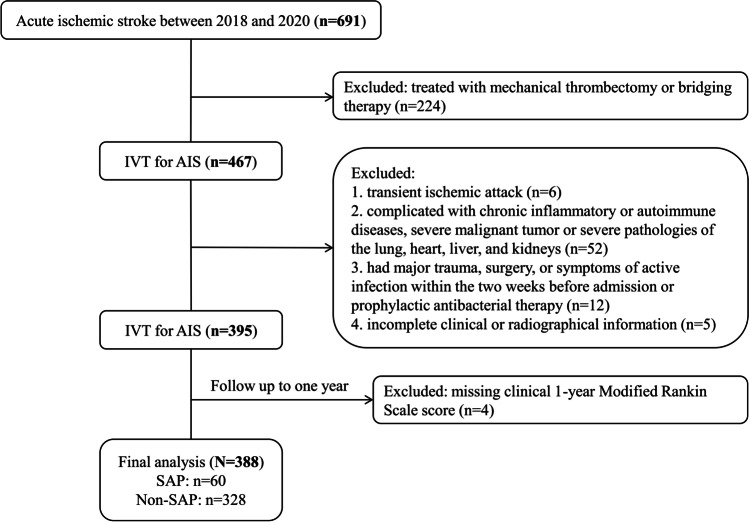
The inclusion criteria were patients with clinically and radiographically diagnosed acute ischemic stroke. Eligible patients were treated with intravenous thrombolysis (IVT) [by recombinant human tissue plasminogen activator (rtPA) at the dose of 0.9 mg/kg] according to national clinical guidelines during the study period. Considering that factors such as time window, operation, and anesthesia methods of endovascular therapy (EVT) patients may indirectly affect the inflammatory process, only patients with simple thrombolysis were included in this study. Exclusion criteria were as follows: (1) transient ischemic attack (n = 6), (2) complicated with chronic inflammatory or autoimmune diseases, severe malignant tumor or severe pathologies of the lung, heart, liver, and kidneys (n = 52), (3) had a major trauma, surgery, or symptoms of active infection within the 2 weeks before admission or prophylactic antibacterial therapy (n = 12), (4) incomplete clinical or radiographical information (n = 5), or missing clinical 1-year Modified Rankin Scale (mRS) score (n = 4), and (5) patients were treated with mechanical thrombectomy or bridging therapy (n = 224). Among the 467 patients treated with IVT, 79 were subsequently excluded, leaving 388 subjects for the final analyses (Fig. 1).
Fig. 1.
Flow chart
At this comprehensive stroke center, all patients were routinely examined by chest computerized tomography (CT) on admission to confirm the presence of serious lung or heart disease. All pneumonia cases were confirmed by chest CT and were re-examined according to their condition.
Data collection and assessment
Information, such as demographic characteristics (age, sex), vascular risk factors (hypertension, diabetes mellitus, ischemic heart disease, hyperlipidemia, atrial fibrillation, smoking, drinking, previous stroke), clinical characteristics (stroke severity based on the infarct volume and the National Institutes of Health Stroke Scale [NIHSS] score, dysphagia, and stroke etiology), treatment parameters (OTT, DNT, and hospital stays), and blood parameters, was collected (see Table 1 for details). OTT time refers to the time from symptom onset to treatment with IVT, and DNT time refers to door-to-needle time. Blood parameters in the current analysis were obtained before IVT (within 30 min after arriving at the emergency department) and within 24–36 h after IVT, respectively. The blood parameters included leukocytes, neutrophils, lymphocytes, monocytes, NLR, and C-reactive protein (CRP). The NLR was calculated after dividing absolute neutrophil counts by absolute lymphocyte counts. A trained speech therapist performed a dysphagia assessment in the first 24 h after admission by the modified water swallowing test, a Japanese bedside dysphagia assessment [17]. Patients were evaluated on swallowing reflex, wet hoarseness, and cough after drinking 30 ml of water and were grouped according to the Functional Oral Intake Scale (FOIS) score (1–3: use of an alternative route for feeding; 4–5: oral feeding with a necessity for the modification of food consistency; and 6–7: no changes in food consistency) [18, 19]. At least two professional radiologists reported computed tomography or magnetic resonance. The large infarct volume was defined as the main blood supply area of large vessels with an infarct diameter greater than 3 cm and involving more than two anatomical sites. The A2DS2 score is a well-established tool for predicting SAP, which includes age (1 point for ≥75 years), atrial fibrillation (1 point), dysphagia (2 points), sex (1 point for male sex), and initial NIHSS score (0 point for NIHSS score 0–4, 3 points for NIHSS score 5–15, and 5 points for NIHSS score ≥16). The A2DS2 score ≥5 can be used for preliminary clinical screening of patients with SAP [20–22].
Table 1.
Baseline characteristics of patients with SAP and non-SAP with acute ischemic stroke
| Total (n = 388) | Non-SAP (n = 328) | SAP (n = 60) | P-value | |
|---|---|---|---|---|
| Age, years | 69±12 | 67±12 | 75±12 | <0.001* |
| Sex, male (%) | 255(66) | 213(65) | 42(71) | 0.337 |
| Drinking, n (%) | 155(40) | 136(41) | 19(32) | 0.187 |
| Smoking, n (%) | 163(42) | 136(41) | 27(46) | 0.526 |
| Previous stroke, n (%) | 52(13) | 40(12) | 12(20) | 0.089 |
| Atrial fibrillation, n (%) | 94(24) | 71(22) | 23(38) | 0.006* |
| Hypertension, n (%) | 273(70) | 226(69) | 47(80) | 0.055 |
| Ischemic heart disease, n (%) | 27(7) | 22(7) | 5(8) | 0.619 |
| Diabetes mellitus, n (%) | 182(47) | 152(46) | 30(51) | 0.510 |
| Hyperlipidemia, n (%) | 229(59) | 199(60) | 30(51) | 0.285 |
| Initial NIHSS score, median (IQR) | 5(3–10) | 4(3–8) | 13(8–17) | <0.001* |
| Dysphagia, n (%) | 47(12) | 13(4) | 34(58) | <0.001* |
| FOIS score, n (%) | <0.001* | |||
| 1–3 | 41(11) | 14(4) | 27(51) | |
| 4–5 | 198(52) | 176(53) | 22(42) | |
| 6–7 | 145(38) | 141(43) | 4(8) | |
| A2DS2 score | 3(1–5) | 3(1–4) | 5(4–7) | <0.001* |
| Large infarct volume, n (%) | 51(13) | 28(9) | 23(38) | <0.001* |
| Stroke etiology, n (%) | 0.088 | |||
| Large vessel disease | 202(52) | 166(50) | 36(61) | |
| Cardioembolism | 72(19) | 60(18) | 12(20) | |
| Small vessel occlusion | 81(21) | 76(23) | 5(8) | |
| Undetermined or other etiology | 33(9) | 27(8) | 6(10) | |
| Blood parameters measured within 30 min after admission | ||||
| Leukocytes (×109/L), median (IQR) | 7.17(5.92–8.95) | 7.07(5.72–8.60) | 8.48(6.66–10.58) | 0.004* |
| Neutrophils (×109/L), median (IQR) | 4.59(3.47–6.24) | 4.45(3.36–5.85) | 6.29(4.05–8.92) | <0.001* |
| Monocyte (×109/L), median (IQR) | 0.47(0.37–0.61) | 0.46(0.36–0.61) | 0.52(0.38–0.70) | 0.035* |
| Lymphocyte (×109 /L), median (IQR) | 1.68(1.28–2.21) | 1.74(1.34–2.26) | 1.42(1.05–1.75) | 0.002* |
| NLR, median (IQR) | 2.59(1.76–4.24) | 2.47(1.67–3.94) | 4.44(2.70–7.59) | <0.001* |
| CRP (mg/L), median (IQR) | 3.42(3.00–9.85) | 3.26(3.00–8.46) | 8.95(4.05–16.80) | <0.001* |
| Blood parameters measured within 24–36 h after IVT | ||||
| Leukocytes (×109/L), median (IQR) | 7.66(6.42–9.59) | 7.44(6.24–9.06) | 9.28(7.41–12.13) | <0.001* |
| Neutrophils (×109/L), median (IQR) | 5.37(4.09–7.11) | 5.02(3.84–6.71) | 7.51(5.62–9.87) | <0.001* |
| Monocyte (×109/L), median (IQR) | 0.51(0.39–0.66) | 0.50(0.39–0.65) | 0.57(0.38–0.87) | 0.060 |
| Lymphocyte (×109/L), median (IQR) | 1.47(1.12–1.88) | 1.51(1.19–1.91) | 1.20(0.83–1.55) | <0.001* |
| NLR, median (IQR) | 3.42(2.32–5.59) | 3.13(2.23–4.93) | 6.43(4.75–9.02) | <0.001* |
| CRP (mg/L), median (IQR) | 3.32(3.00–9.87) | 3.29(3.00–8.47) | 9.10(3.78–22.40) | <0.001* |
| DNT (min), median (IQR) | 47(35–59) | 46(34–59) | 50(39–62) | 0.109 |
| OTT, n (%) | 0.889 | |||
| ≤ 90 min | 32(8) | 27(8) | 5(8) | |
| 90–180 min | 184(47) | 154(47) | 30(50) | |
| ≥ 180 min | 172(44) | 147(45) | 25(42) | |
| Hospital stays (days), median (IQR) | 9(7–13) | 9(7–12) | 14(10–20) | <0.001* |
| Early neurological outcomes, n (%) | 0.024* | |||
| Neither END nor ENI | 176(45) | 149(45) | 27(45) | |
| ENI | 182(47) | 159(48) | 23(38) | |
| END | 30(8) | 20(6) | 10(17) | |
| Hemorrhagic transformation, n (%) | 43(11) | 29(9) | 14(24) | <0.001* |
| mRS 0-2 at discharge, n (%) | 263(68) | 250(76) | 13(22) | <0.001* |
| mRS 0-2 at 1 year, n (%) | 265(68) | 247(75) | 18(31) | <0.001* |
| Mortality, n (%) | 43(11) | 21(6) | 22(37) | <0.001* |
FOIS the Functional Oral Intake Scale, CRP C-reactive protein, NIHSS National Institutes of Health Stroke Scale, NLR neutrophil-to-lymphocyte ratio, OTT onset-to-treatment time, DNT door-to-needle time, SAP stroke-associated pneumonia, ENI early neurological improvement, END early neurological deterioration, mRS Modified Rankin Scale; IQR, interquartile range
*p < 0.05
Outcome
The primary outcome measure was the morbidity of SAP, defined as pneumonia diagnosed within the first 7 days after stroke onset [23]. Over two neurologists diagnosed pneumonia during the hospital stay based on signs, symptoms, laboratory measurements suggestive of acute lower respiratory tract infection, and confirmation via chest CT, according to the modified Center for Disease Control and Prevention criteria [23].
We additionally noted post-thrombolysis early neurological outcomes (early neurological improvement [ENI], early neurological deterioration [END]), hemorrhagic transformation, mRS score at discharge and 1 year from AIS onset, and mortality. END was defined as an increase of ≥ 4 points in the NIHSS score within 24 h after IVT, and ENI was defined as a decrease of ≥ 4 points in the NIHSS score or a complete resolution of neurological deficits within 24 h after IVT. The favorable outcome was defined as an mRS score of 0–2, and functional dependence was defined as an mRS score of 3–5. The follow-up assessments were conducted in face-to-face outpatient clinics or by telephone with patients or relatives. The physicians who assessed the NIHSS and the mRS score were specially trained and blind to the diagnosis of SAP. Mortality was defined as the percentage of deaths from treatment to 1-year follow-up. Hemorrhagic transformation was defined as any hemorrhage with neurologic deterioration and confirmed radiographically.
Statistical analysis
The continuous variables were presented as mean ± standard deviation or medians (interquartile range), and counts and percentages were shown in the case of categorical data. Continuous variables were tested for normality using the Kolmogorov-Smirnov test and then compared, as appropriate, by a Student’s t-test or the Mann-Whitney U test. Pearson’s χ2 test or Fisher’s exact test was used to compare categorical variables. We referred to the NLR measured before IVT (within 30 min after admission) as Model 1 and the NLR measured within 24–36 h after IVT as Model 2. The multivariable logistic regression analyses were used to identify significant predictors of SAP and to analyze outcomes of the high NLR group, which were adjusted for variables selected from univariate analysis with p < 0.1 and previous studies. The predictive power of NLR was compared with other peripheral inflammatory cell counts using the area under the receiver operating characteristic curve (AUROC), and the optimal cut-off point was calculated based on Youden’s index. Meanwhile, in order to better define the added value of NLR to the established clinical model for the diagnosis of SAP, we combined the NLR after IVT with the A2DS2 score to establish the modified A2DS2 score model and included it in the ROC analysis. In addition, the relationship between NLR and relevant baseline variables was performed using Pearson correlation. Multiple linear regression further analyzed variables with p < 0.05 in Pearson correlation analysis. All statistical analysis was performed using SPSS version 26 (IBM SPSS, Chicago, IL, USA). The receiver operating characteristic curve (ROC) was performed using GraphPad Prism 8. A two-tailed value of p < 0.05 was considered significant.
Results
Participants characteristics
Table 1 displays the characteristics of included patients according to dichotomized SAP. The prevalence of SAP was 15% (n = 60). Across the entire study population, the mean age was 69 years, and 66% of patients were male. The median initial NIHSS score and hospital stays were 5(3–10) and 9(7–13), respectively. 263 subjects (68%) achieved favorable outcome at discharge.
As shown in Table 1, compared to non-SAP, patients with SAP were older (mean 75 versus 67 years, p < 0.001), more likely to combine with dysphagia (58% vs 4%, p < 0.001), atrial fibrillation (38% vs 22%, p = 0.006), and hemorrhagic transformation (24% vs 9%, p < 0.001), and had higher median initial NIHSS score (13 vs 4, p < 0.001), A2DS2 score (5 vs 3, p < 0.001), longer hospital stays (14 vs 9 days, p < 0.001), higher rate of FOIS score of 1–3 (51% vs 4%, p < 0.001), higher rate of large infarct volume (38% vs 9%, p < 0.001), and a higher rate of END (17% vs 6%, p = 0.024). The favorable outcome at discharge (22% vs 76%, p < 0.001) and 1 year (31% vs 75%, p < 0.001) were less commonly found in patients with SAP, but mortality (37% vs 6%, p < 0.001) was higher in patients with SAP. Compared with non-SAP patients, SAP patients also had higher leukocyte, neutrophil, monocyte counts, and CRP levels, but lower lymphocyte levels both at admission and 24–36 h after IVT. In the total group, OTT time of fewer than 90 min accounts for 8%, and the median DNT time is 47 min, both of which have no significant correlation with the occurrence of SAP. In this study, no significant difference in stroke etiology, drinking, smoking, history of previous stroke, hyperlipidemia, hypertension, or diabetes mellitus was shown between the two groups.
Association between NLR and outcomes
It can be seen from the data in Table 1 that the SAP group reported significantly higher NLR than the non-SAP group (4.44–6.43 vs. 2.47–3.13, p < 0.001). When we divided the NLR respectively into two groups bounded by 4, patients in the high NLR groups had more pneumonia events (24–27% vs. 3–7%, p < 0.001) compared with patients in the low NLR groups (Table 2). In the multivariable logistic regression analysis, the NLR before IVT (adjusted odds ratio [aOR] = 1.288; 95% confidence interval [CI] = 1.123–1.476; p < 0.001), as well as the NLR after IVT (aOR = 1.127, 95%CI = 1.017–1.249; p = 0.023), remained an independent predictor of SAP after adjusting for confounders (Table 3). Initial NIHSS score, atrial fibrillation, dysphagia, and A2DS2 score were also significant predictors of SAP in Model 1 and Model 2 (p < 0.05, Table 3).
Table 2.
Baseline characteristics of patients with low and high NLRs
| Variables | aModel 1 | bModel 2 | ||||
|---|---|---|---|---|---|---|
| Low NLR (NLR≤4, n = 228) |
High NLR (NLR>4, n = 160) |
P-value | Low NLR (NLR≤4, n = 158) |
High NLR (NLR>4, n = 230) |
P-value | |
| Pneumonia events, n (%) | 16(7) | 44(27) | <0.001* | 4(3) | 56(24) | <0.001* |
| Age (years), median (IQR) | 67±12 | 70±12 | 0.140 | 67±12 | 70±13 | 0.002* |
| Sex, male, n (%) | 151(66) | 104(65) | 0.802 | 107(68) | 148(64) | 0.492 |
| Atrial fibrillation, n (%) | 44(19) | 43(27) | 0.078 | 33(21) | 54(23) | 0.548 |
| Previous stroke, n (%) | 28(12) | 24(15) | 0.439 | 21(13) | 31(13) | 0.958 |
| Hyperlipidemia, n (%) | 148(65) | 81(51) | 0.005* | 102(65) | 127(55) | 0.066 |
| Diabetes mellitus, n (%) | 106(46) | 76(48) | 0.845 | 73(46) | 109(47) | 0.818 |
| Hypertension, n (%) | 160(70) | 113(71) | 0.924 | 103(65) | 170(74) | 0.065 |
| Ischemic heart disease, n (%) | 15(7) | 12(8) | 0.726 | 11(7) | 16(7) | 0.998 |
| Initial NIHSS, median (IQR) | 4(2–8) | 7(3–10) | <0.001* | 3(2–6) | 7(3–12) | <0.001* |
| A2DS2 score, median (IQR) | 2(1–4) | 4(2–5) | <0.001* | 2(1–4) | 4(1–5) | <0.001* |
| Dysphagia, n (%) | 16(7) | 31(19) | <0.001* | 5(3) | 42(18) | <0.001* |
| Large infarct volume, n (%) | 20(9) | 31(19) | 0.003* | 7(4) | 44(19) | <0.001* |
| Stroke etiology, n (%) | ||||||
| Large vessel disease | 115(50.4) | 88(55) | 0.667 | 75(48) | 128 (56) | 0.014* |
| Cardioembolism | 44(19.3) | 30(18.8) | 30(19) | 44 (19) | ||
| Small vessel occlusion | 51(22.4) | 28(17.5) | 44(28) | 35(15) | ||
| Undetermined or other etiology | 18(7.9) | 14(8.8) | 9(6) | 23(10) | ||
| DNT (h), median (IQR) | 44(34–59) | 49(36–59) | 0.217 | 43(34–58) | 48(36–60) | 0.093 |
| OTT, n (%) | ||||||
| ≤90 min | 23(10) | 9(6) | 0.013* | 8(5) | 10(4) | 0.378 |
| 90–180 min | 117(52) | 67(42) | 69(44) | 117(51) | ||
| ≥180 min | 87(38) | 85(53) | 81(51) | 103(45) | ||
| Hospital stays (days), median (IQR) | 9(7–12) | 10(8–15) | <0.001* | 9(7–11) | 10(7–14) | 0.005* |
| Early neurological outcomes, n (%) | ||||||
| Neither END nor ENI | 97(43) | 79(49) | 0.002* | 70(44) | 106(46) | 0.086 |
| ENI | 120(53) | 62(39) | 81(51) | 101(44) | ||
| END | 10(4) | 20(12) | 7(4) | 23(10) | ||
| Hemorrhagic transformation, n (%) | 14(6) | 29(18) | <0.001* | 6(4) | 37(16) | <0.001* |
| mRS 0-2 at discharge, n (%) | 180(79) | 83(52) | <0.001* | 137(87) | 126(55) | <0.001* |
| mRS 0-2 at 1 year, n (%) | 176(77) | 89(56) | <0.001* | 133(84) | 132(57) | <0.001* |
| Mortality, n (%) | 13(6) | 30(19) | <0.001* | 3(2) | 40(17) | <0.001* |
NIHSS National Institutes of Health Stroke Scale, NLR neutrophil-to-lymphocyte ratio, OTT onset-to-treatment time, DNT door-to-needle time, ENI early neurological improvement, END early neurological deterioration, mRS Modified Rankin Scale, IQR interquartile range
aModel 1:NLR measured before IVT (within 30 min after admission)
bModel 2:NLR measured within 24–36 h after IVT
*p < 0.05
Table 3.
Multivariate logistic regression analysis of possible predicts of SAP
| Variables | aModel 1 | bModel 2 | ||
|---|---|---|---|---|
| Adjusted OR | P-value | Adjusted OR | P-value | |
| Age | 1.027(0.990–1.066) | 0.150 | 1.02(0.984–1.057) | 0.290 |
| NLR | 1.288(1.123–1.476) | <0.001* | 1.127(1.017–1.249) | 0.023* |
| Initial NIHSS score | 1.118(1.036–1.205) | 0.004* | 1.112(1.031–1.199) | 0.006* |
| Dysphagia | 18.859(7.282–48.839) | <0.001* | 14.576(5.708–37.218) | <0.001* |
| A2DS2 score | 1.656(1.4–1.959) | <0.001* | 1.574(1.331–1.863) | <0.001* |
| Atrial fibrillation | 4.387(1.110–17.341) | 0.035* | 6.523(1.826–3.298) | 0.004* |
| Hyperlipidemia | 1.087(0.479–2.469) | 0.842 | 0.974(0.44–2.156) | 0.948 |
| Diabetes mellitus | 1.543(0.694–3.431) | 0.288 | 1.958(0.883–4.338) | 0.098 |
| Previous stroke | 0.936(0.3–2.922) | 0.910 | 1.308(0.439–3.895) | 0.630 |
| Hypertension | 1.135(0.435–2.96) | 0.796 | 1.101(0.436–2.782) | 0.838 |
| Ischemic heart disease | 0.825(0.217–3.134) | 0.778 | 0.97(0.260–3.615) | 0.964 |
| Drinking | 0.720(0.276–1.881) | 0.503 | 0.588(0.233–1.486) | 0.262 |
| Smoking | 2.842(0.978–8.257) | 0.055 | 2.761(0.977–7.806) | 0.055 |
NIHSS National Institutes of Health Stroke Scale, NLR neutrophil-to-lymphocyte ratio, OR odds ratio, SAP stroke-associated pneumonia
aModel 1:NLR measured before IVT (within 30 min after admission)
bModel 2:NLR measured within 24–36 h after IVT
Model 1 and Model 2 were both adjusted with P < 0.1 in univariate analysis and diabetes mellitus, smoking, drinking, ischemic heart disease, hyperlipidemia, and stroke etiology
*p < 0.05
As shown in Table 4, the results of multiple logistic regression analysis after adjustment for confounding factors showed that a high NLR level was also an independent risk factor for SAP, poor short-term and long-term functional outcomes, and mortality (p < 0.05, Table 4).
Table 4.
Multivariate logistic regression analysis for outcomes of high NLR group (NLR>4)
| Variables | aModel 1 | bModel 2 | ||
|---|---|---|---|---|
| Adjusted OR | P-value | Adjusted OR | P-value | |
| mRS 0-2 at discharge | 0.350 (0.186–0.659) | 0.001* | 0.379 (0.203–0.708) | 0.002* |
| mRS 0-2 at 1 year | 0.488 (0.274–0.869) | 0.015* | 0.542 (0.303–0.969) | 0.039* |
| Mortality | 4.615 (1.243–17.142) | 0.022* | 3.749 (1.018–13.804) | 0.047* |
| SAP | 5.198 (1.607–16.814) | 0.006* | 4.996 (1.543–16.179) | 0.007* |
NLR neutrophil-to-lymphocyte ratio, mRS Modified Rankin Scale, SAP stroke-associated pneumonia, OR odds ratio
aModel 1:NLR measured before IVT (within 30 min after admission)
bModel 2:NLR measured within 24–36 h after IVT
Model 1 and Model 2 were both adjusted with P < 0.1 in univariate analysis
*p < 0.05
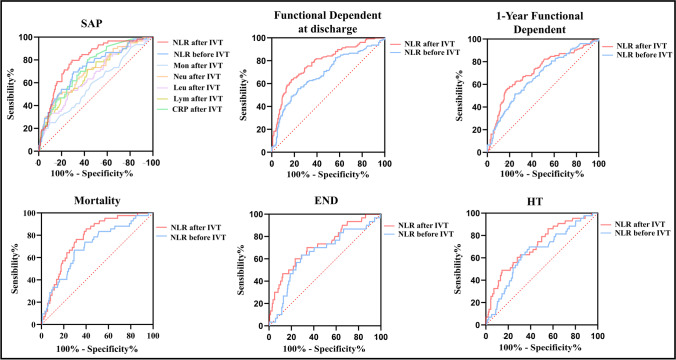
ROC analysis for SAP showed that the area under the curve of NLR after IVT (0.80, 95%CI = 0.74–0.86; p < 0.001) was higher than NLR before IVT (0.72, 95%CI = 0.66–0.80; p < 0.001) and conventional inflammatory markers, such as CRP (0.73, 95%CI = 0.67–0.80; p < 0.001), lymphocyte counts (0.68, 95%CI = 0.61–0.75; p < 0.001), neutrophils (0.70, 95%CI = 0.63–0.77; p < 0.001), monocyte (0.59, 95%CI = 0.51–0.67; p = 0.034) and leukocyte counts (0.66, 95%CI = 0.58–0.73; p < 0.001) (Fig. 2). The optimal point of NLR after IVT identified by the Youden index was 4.4 with 80% sensitivity, and 70% specificity (Youden index = 0.507). In addition, the area under the curve of NLR after IVT was also higher than NLR before IVT in the ROC analysis for functional dependence at discharge (after IVT: 0.78, 95%CI = 0.73–0.83, p < 0.001; before IVT: 0.68, 95%CI = 0.63–0.74, p < 0.001), functional dependence at 1 year (after IVT: 0.71, 95%CI = 0.65–0.77, p < 0.001; before IVT: 0.66, 95%CI = 0.61–0.72; p < 0.001), mortality (after IVT: 0.77, 95%CI = 0.70–0.83, p < 0.001; before IVT: 0.70, 95%CI = 0.61–0.78, p < 0.001), END (after IVT: 0.72, 95%CI = 0.62–0.82, p < 0.001; before IVT: 0.64, 95%CI = 0.54–0.75, p = 0.007), and HT (after IVT: 0.71, 95%CI = 0.63–0.80, p < 0.001; before IVT: 0.64, 95%CI = 0.56–0.73, p = 0.002) (Fig. 2). Surprisingly, NLR after IVT was similar in AUROC to the widely accepted A2DS2 score (0.80, 95%CI = 0.74–0.86; p < 0.001), and the best AUROC (0.84, 95%CI = 0.0.79–0.90; p < 0.001) was achieved in the modified A2DS2 score (NLR after IVT combined with the A2DS2 score) (Fig. 3).
Fig. 2.
Receiver operator curves generated for SAP, functional dependent at discharge (modified Rankin Scale score 3–5), 1-year functional dependent, mortality, END, and HT. Abbreviation: neutrophils (Neu), lymphocytes (Lym), Leukocytes (Leu), Monocyte (Mon), C-reactive protein (CRP), Early neurological deterioration (END), hemorrhagic transformation (HT)
Fig. 3.
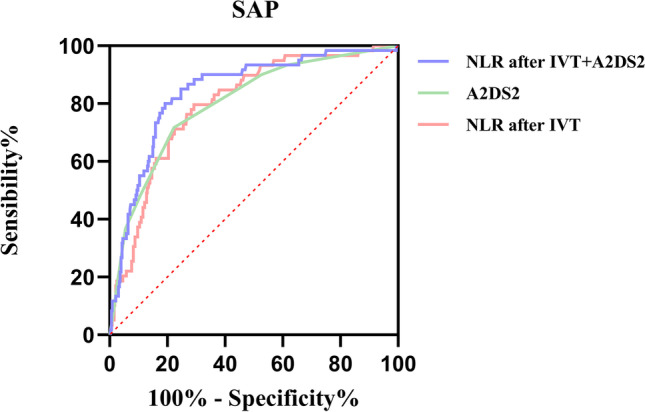
Comparison of area under the receiver operating characteristic curve (AUROC) values among A2DS2 score and modified A2DS2 score (NLR combined with A2DS2 score)
Association between NLR and relevant baseline variables
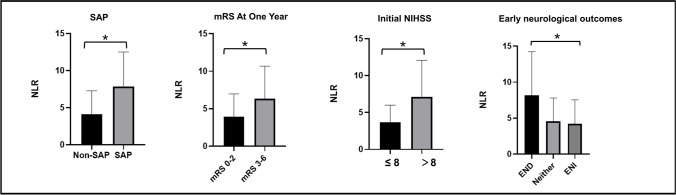
As shown in Table 2, compared with patients in the low NLR group of Model 1, patients in the high NLR group had higher median initial NIHSS score (7 vs 4, p < 0.001) and A2DS2 score (4 vs 2, p < 0.001), higher rate of END (12% vs 4%, p = 0.002), higher rate of OTT time ≥180 min (53% vs 38%, p = 0.013), higher rate of large infarct volume (19% vs 9%, p = 0.003), longer hospital stays (10 vs 9, p < 0.001), higher mortality (19% vs 6%, p < 0.001), and less favorable outcomes both at discharge (52% vs 79%, p < 0.001) and 1 year (56% vs 77%, p < 0.001), and more likely to combine with dysphagia (19% vs 7%, p < 0.001), hemorrhagic transformation (18% vs 6%, p < 0.001). In Model 2, the A2DS2 score, initial NIHSS score, dysphagia, hemorrhagic transformation, hospital stays, favorable outcomes, and mortality were also associated with NLR (p < 0.05, Table 2 and Fig. 4).
Fig. 4.
Comparison of different groups divided by NLR measured within 24–36 h (p < 0.05)
In Pearson correlation analysis (see Supplementary Table 1), NLR before IVT was significantly correlated with the initial NIHSS score (p < 0.001) and OTT (p < 0.001), while NLR after IVT was significantly associated with the initial NIHSS score (p < 0.001) and early NIHSS changes (p = 0.004). Some of these associations remained statistically significant (NLR measured before IVT: Initial NIHSS: β = 0.124, p < 0.001; OTT: β = 0.009, p < 0.001; NLR measured within 24–36 h after IVT: early NIHSS changes: β = −0.249, p < 0.001; Initial NIHSS: β = 0.360, p < 0.001) in a multivariate linear regression model where we corrected for parameters that have a known influence on peripheral leukocyte counts (diabetes mellitus, hyperlipidemia, smoking, drinking, ischemic heart disease, Supplementary Table 2). Therefore, OTT was only significantly associated with NLR before IVT, while early neurological outcomes were only significantly correlated with NLR after IVT. The initial NIHSS score was significantly correlated with the NLR at both time points.
Discussion
First of all, this current study supported that a high NLR level was an independent predictor of SAP in stroke patients and had better predictive validity than established biomarkers like leukocyte counts, which was in agreement with previous research findings [14–16, 24–27]. Furthermore, a high NLR level is an independent risk factor for poor short-term and long-term outcomes and mortality, further underscoring the usefulness of this readily available ratio in everyday practice.
Our research showed that NLR measured before IVT and within 24–36 h after IVT can both predict the onset of SAP, functional dependence, and mortality after stroke. However, NLR measured within 24–36 h after IVT had a higher predictive value. As indicated in this study, the area under the ROC curve of NLR measured within 24–36 h after IVT was larger than before thrombolytic treatment. Therefore, the calculated cut-off showed adequate sensitivity and specificity (sens.: 80%, spec.: 70%). Intriguingly, the combination of NLR after IVT and A2DS2 scores greatly improved the ability to predict SAP in our cohort (AUROC = 0.84, p < 0.001). Since stroke patients undergoing IVT are often admitted as an emergency and may lack a clear history, monitoring NLR is a more effective and simple method compared to the A2DS2 score in predicting SAP.
A possible explanation for the above results might be that the dynamic development of neuroinflammation after a stroke takes several hours, and the immune activity changes after taking thrombolytic treatment. Ischemic stroke causes the death of brain cells by cutting off the blood supply, activating pro-inflammatory factors, resulting in a hierarchical biological effect of systemic inflammation as shown by an increase in peripheral blood neutrophil counts. Subsequently, increased activity of the adrenal medulla and hypothalamic axis leads to stroke-induced immunosuppression syndrome, which can cause lymphocyte apoptosis and T-cell inactivation. Thus, the immune system is suppressed as compensation for brain injury, but this results in increased susceptibility to infection for the host. Interestingly, the number of circulating lymphocytes in uninfected patients recovered on the day after AIS, while infected patients showed a continuous decrease in lymphocytes [28]. In a mouse experiment [29] and a human observational study [30], it was found that in patients with post-stroke infection, the increase of neutrophils might occur in the early stage of AIS, but the decrease of lymphocytes occurred within 6 h after stroke and lasted for at least 6 days. Therefore, a sustained decline in lymphocytes may also be a predictor of post-stroke infection. Enhancing lymphocyte levels may be a viable alternative therapeutic option for reversing immune impairment after stroke in the future.
In addition, for the elderly with poor foundations, the restoration of cerebral blood flow after reperfusion therapy may further induce the production of reactive oxygen species (ROS) and the destruction of the blood-brain barrier, making it easier for immune cells in the peripheral circulation to penetrate the brain, promoting brain swelling, and exacerbating tissue damage at the injury site and the ischemic penumbra [31, 32]. Therefore, reperfusion injury caused by thrombolytic therapy should be given special consideration in inflammatory events after AIS.
Previous studies have proved that early thrombolytic therapy can save the penumbra and reduce the development of inflammation [33, 34]. Therefore, if inflammation indicators such as NLR are still high after thrombolytic therapy, it may be an indicator of irreversible injury in the edema penumbra. In addition, in our cohort, the low NLR group had a better effect on IVT, as shown by a lower rate of END and lower incidence of hemorrhagic transformation. This is consistent with a previous study that has shown that higher NLR is associated with reperfusion failure after endovascular treatment [14]. Therefore, although NLR can effectively predict the occurrence of SAP and adverse outcomes, the optimal time to detect NLR must be considered. According to the results of this study, NLR measured 24–36 h after IVT may be a better choice.
For the relevant baseline variables, we found that NLR measured after IVT was closely related to the severity of the stroke and early neurological outcomes, as indicated by having significant correlations with initial NIHSS scores and early NIHSS changes in a multivariate linear regression model. Previous studies have also shown that the severity of stroke is a significant risk factor for SAP, and NLR is closely related to infarct volume and may be able to predict post-thrombolysis END [35, 36]. Another finding in this study was that the OTT time was only significantly associated with NLR before IVT. It may suggest that with the increase in OTT time, the potential inflammatory response in patients of AIS before IVT may be more intense. On the other hand, our study also showed that OTT time had no significant effect on NLR measured within 24–36 h after IVT when thrombolytic therapy was actively administered within the time range of 4.5 h. Therefore, NLR measured within 24–36 h after IVT may be a relatively stable marker for predicting SAP and functional outcome of patients with AIS because it is not affected by OTT time. However, several studies have shown that ultra-early thrombolysis contributes to active recovery and lower mortality after cerebral infarction [37–39]. Indeed, it should be noted that, in our whole group, the median OTT was 2.93 h, and ultra-early thrombolysis of fewer than 90 min accounted for only 8% of the total number, which is a relatively small number and may cause deviation. Meanwhile, it is worth noting that the OTT time has a confounding bias with the severity of stroke. Patients with more severe strokes are often found earlier, thus shortening the OTT time. In this study, there was no significant association between DNT and SAP, as well as NLR, so the extension of OTT was mainly related to the time from onset to arrival at the hospital.
Once the infection is diagnosed, antibiotics are the key strategy and “gold-standard” treatment. Broad-spectrum antibiotics were once advocated by some scholars in order to avoid post-stroke infections. However, most of the subsequent studies have indicated that prophylactic antibiotics could not be effective for SAP [40–44]. To develop a preventive therapy for SAP, future research may consider to explore improving immune regulation.
The study has some potential limitations. First, this is a retrospective single-center study with a relatively small sample size. Future studies need larger sample sizes and prospective multicenter studies to ensure more reliable results. Second, our study included patients who underwent IVT alone and did not include patients who did not receive thrombolysis, or who received mechanical thrombectomy or bridging therapy. So, future studies should further discuss the impact of different treatments on NLR. Third, other variables that may affect the risk of SAP and NLR values were not included, such as congestive heart failure, chronic obstructive pulmonary disease, lesion site of stroke, and type of vessel occlusion. Fourth, even if we have formulated strict exclusion criteria, we cannot guarantee that patients with latent infections before admission can be completely excluded. However, in this study, the incidence rate of SAP was 15%, which is congruous with the rates reported in previous research [45–47]. Thus, this bias may be within acceptable ranges. In addition, it is also a pity that we did not record the specific time of onset of pneumonia, nor did we record its severity. Finally, the results of this study cohort were not re-examined and confirmed in a validation cohort. Future research should focus on solving these problems.
Conclusion
In conclusion, our analysis considered the impact of NLR at different time points before and after IVT on SAP prediction, indicating that increased systemic inflammation after IVT, expressed by increased NLR measured within 24–36 h after IVT, is tightly associated with the occurrence of SAP and the poor prognosis. Our results complement the role of NLR in acute ischemic cerebrovascular disease and provide solid evidence for clinical practice.
Supplementary information
Acknowledgements
We are very grateful to the stroke patients and other researchers who participated in this study for their contributions.
Abbreviations
- AIS
Acute ischemic stroke
- SAP
Stroke-associated pneumonia
- IVT
Intravenous thrombolysis
- NLR
Neutrophil to lymphocyte ratio
- CRP
C-reactive protein
- OTT
Onset to treatment time
- DNT
to door-to-needle time
- FOIS
the Functional Oral Intake Scale
- ENI
Early neurological improvement
- END
Early neurological deterioration
- mRS
Modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- IQR
Interquartile range
- OR
Odds ratio
- 95% CI
95% Confidence interval
- AUROC
Area under the receiver operating characteristic curve.
Authors' contributions
Liu-Zhu Chen and Xiao-Qian Luan conceived and designed the study. Liu-Zhu Chen analyzed and interpreted the data. Liu-Zhu Chen drafted the article and all authors revised it. Hu-Wei Xia, Shu-Zhen Wu, Lu-Qian Zhan, and Yi-Si Lin screened the subjects, collected the basic data, and checked the medical records. Jin-Cai He supervised the study and made critical comments on the article. All authors contributed to the article and approved the publishing of the final manuscript.
Funding
This research was supported by a grant from the Projects of the National Natural Science Foundation of China (No. 81873799) and Wenzhou Municipal Sci-Tech Bureau Program (Y2020421).
Data availability
All datasets presented in this study are included in the article material. The data supporting the results of this study are available from the corresponding author upon reasonable request from any qualified investigator.
Declarations
Ethics approval and consent to participate
Our study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and was conducted by the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Consent for publication
All authors approved the publishing of the final manuscript.
Competing interests
The authors declare no conflicts of interest in this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Liu-Zhu Chen and Xiao-Qian Luan contributed equally to this work.
Contributor Information
Liu-Zhu Chen, Email: liuzhu.chen7@gmail.com.
Xiao-Qian Luan, Email: 15058753868@163.com.
Shu-Zhen Wu, Email: wushuzhen0412@126.com.
Hu-Wei Xia, Email: 920767497@qq.com.
Yi-Si Lin, Email: linyisi2020@163.com.
Lu-Qian Zhan, Email: 429744733@qq.com.
Jin-Cai He, Email: hjc@wmu.edu.cn.
References
- 1.Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab. 2014;34(10):1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38(3):1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 3.Liu Q, Jin WN, Liu Y, Shi K, Sun H, Zhang F, et al. Brain ischemia suppresses immunity in the periphery and brain via different neurogenic innervations. Immunity. 2017;46(3):474–487. doi: 10.1016/j.immuni.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6(10):775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 5.Indredavik B, Rohweder G, Naalsund E, Lydersen S. Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke. 2008;39(2):414–420. doi: 10.1161/strokeaha.107.489294. [DOI] [PubMed] [Google Scholar]
- 6.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G, et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77(14):1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9(1):105–118. doi: 10.1016/s1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 8.Wong AA, Davis JP, Schluter PJ, Henderson RD, O'Sullivan JD, Read SJ. The time course and determinants of temperature within the first 48 h after ischaemic stroke. Cerebrovasc Dis. 2007;24(1):104–110. doi: 10.1159/000103124. [DOI] [PubMed] [Google Scholar]
- 9.Solbiati M, Dipaola F, Ceriani E, Casazza G. Diagnostic value of chest radiographs in bedridden patients with suspected pneumonia. Am J Med. 2010;123(8):e13. doi: 10.1016/j.amjmed.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Hetze S, Engel O, Römer C, Mueller S, Dirnagl U, Meisel C, et al. Superiority of preventive antibiotic treatment compared with standard treatment of poststroke pneumonia in experimental stroke: a bed to bench approach. J Cereb Blood Flow Metab. 2013;33(6):846–854. doi: 10.1038/jcbfm.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38(3):641–647. doi: 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Jang MU, Kim Y, Park SY, Kim C, Kim YJ, et al. The neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict reperfusion and prognosis after endovascular treatment of acute ischemic stroke. J Pers Med. 2021;11(8):696. doi: 10.3390/jpm11080696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocaturk O, Besli F, Gungoren F, Kocaturk M, Tanriverdi Z. The relationship among neutrophil to lymphocyte ratio, stroke territory, and 3-month mortality in patients with acute ischemic stroke. Neurol Sci. 2019;40(1):139–146. doi: 10.1007/s10072-018-3604-y. [DOI] [PubMed] [Google Scholar]
- 16.Qun S, Tang Y, Sun J, Liu Z, Wu J, Zhang J, et al. Neutrophil-to-lymphocyte ratio predicts 3-month outcome of acute ischemic stroke. Neurotox Res. 2017;31(3):444–452. doi: 10.1007/s12640-017-9707-z. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky MB, Suiter DM, Gonzalez-Fernandez M, Michtalik HJ, Frymark TB, Venediktov R, et al. Screening accuracy for aspiration using bedside water swallow tests: a systematic review and meta-analysis. Chest. 2016;150(1):148–163. doi: 10.1016/j.chest.2016.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86(8):1516–1520. doi: 10.1016/j.apmr.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 19.Souza JT, Ribeiro PW, de Paiva SAR, Tanni SE, Minicucci MF, Zornoff LAM, et al. Dysphagia and tube feeding after stroke are associated with poorer functional and mortality outcomes. Clin Nutr. 2020;39(9):2786–2792. doi: 10.1016/j.clnu.2019.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann S, Malzahn U, Harms H, Koennecke HC, Berger K, Kalic M, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. 2012;43(10):2617–2623. doi: 10.1161/STROKEAHA.112.653055. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Yu S, Wei L, Ye R, Lin M, Li X, et al. The A2DS2 Score as a predictor of pneumonia and in-hospital death after acute ischemic stroke in Chinese populations. PLoS One. 2016;11(3):e0150298. doi: 10.1371/journal.pone.0150298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zapata-Arriaza E, Moniche F, Blanca PG, Bustamante A, Escudero-Martínez I, Uclés O, et al. External validation of the ISAN, A2DS2, and AIS-APS scores for predicting stroke-associated pneumonia. J Stroke Cerebrovasc Dis. 2018;27(3):673–676. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 23.Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. 2015;46(8):2335–2340. doi: 10.1161/STROKEAHA.115.009617. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Liu Y, Han L, He F, Cai N, Zhang Q, et al. Risk factors for acute stroke-associated pneumonia and prediction of neutrophil-to-lymphocyte ratios. Am J Emerg Med. 2021;41:55–59. doi: 10.1016/j.ajem.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Derbisz J, Nowak K, Wnuk M, Pulyk R, Jagiella J, Slowik J, et al. Prognostic significance of stroke-associated infection and other readily available parameters in acute ischemic stroke treated by intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2021;30(2):105525. doi: 10.1016/j.jstrokecerebrovasdis.2020.105525. [DOI] [PubMed] [Google Scholar]
- 26.Lan Y, Sun W, Chen Y, Miao J, Li G, Qiu X, et al. Nomogram including neutrophil-to-lymphocyte ratio for the prediction of stroke-associated infections. Front Neurol. 2020;11:574280. doi: 10.3389/fneur.2020.574280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gens R, Ourtani A, De Vos A, De Keyser J, De Raedt S. Usefulness of the neutrophil-to-lymphocyte ratio as a predictor of pneumonia and urinary tract infection within the first week after acute ischemic stroke. Front Neurol. 2021;12:671739. doi: 10.3389/fneur.2021.671739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogelgesang A, Grunwald U, Langner S, Jack R, Bröker BM, Kessler C, et al. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke. 2008;39(1):237–241. doi: 10.1161/strokeaha.107.493635. [DOI] [PubMed] [Google Scholar]
- 29.Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198(5):725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haeusler KG, Schmidt WU, Föhring F, Meisel C, Helms T, Jungehulsing GJ, et al. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc Dis. 2008;25(1-2):50–58. doi: 10.1159/000111499. [DOI] [PubMed] [Google Scholar]
- 31.Shim R, Wong CH (2016) Ischemia, Immunosuppression and infection--tackling the predicaments of post-stroke complications. Int J Mol Sci. 17(1). 10.3390/ijms17010064 [DOI] [PMC free article] [PubMed]
- 32.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27(5):1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Audebert HJ, Rott MM, Eck T, Haberl RL. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke. 2004;35(9):2128–2133. doi: 10.1161/01.STR.0000137607.61697.77. [DOI] [PubMed] [Google Scholar]
- 34.Ye L, Cai R, Yang M, Qian J, Hong Z. Reduction of the systemic inflammatory induced by acute cerebral infarction through ultra-early thrombolytic therapy. Exp Ther Med. 2015;10(4):1493–1498. doi: 10.3892/etm.2015.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokgoz S, Keskin S, Kayrak M, Seyithanoglu A, Ogmegul A. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J Stroke Cerebrovasc Dis. 2014;23(8):2163–2168. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18(1):51. doi: 10.1186/s12974-021-02090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strbian D, Soinne L, Sairanen T, Happola O, Lindsberg PJ, Tatlisumak T, et al. Ultraearly thrombolysis in acute ischemic stroke is associated with better outcome and lower mortality. Stroke. 2010;41(4):712–716. doi: 10.1161/STROKEAHA.109.571976. [DOI] [PubMed] [Google Scholar]
- 38.Grotta J. Timing of thrombolysis for acute ischemic stroke: "timing is everything" or "everyone is different". Ann N Y Acad Sci. 2012;1268:141–144. doi: 10.1111/j.1749-6632.2012.06690.x. [DOI] [PubMed] [Google Scholar]
- 39.Yafasova A, Fosbol EL, Johnsen SP, Kruuse C, Petersen JK, Alhakak A, et al. Time to thrombolysis and long-term outcomes in patients with acute ischemic stroke: a nationwide study. Stroke. 2021;52(5):1724–1732. doi: 10.1161/STROKEAHA.120.032837. [DOI] [PubMed] [Google Scholar]
- 40.Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holasou M, Rezaei Y. An open-label evaluator-blinded clinical study of minocycline neuroprotection in ischemic stroke: gender-dependent effect. Acta Neurol Scand. 2015;131(1):45–50. doi: 10.1111/ane.12296. [DOI] [PubMed] [Google Scholar]
- 41.Westendorp WF, Vermeij JD, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJ, et al. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet. 2015;385(9977):1519–1526. doi: 10.1016/s0140-6736(14)62456-9. [DOI] [PubMed] [Google Scholar]
- 42.Yan L, Qing Y, Xingyi J, Hongbo Q. Etiologic diagnosis and clinical treatment of multiple drug-resistant bacteria infection in elderly patients with stroke-associated pneumonia after neurosurgery. Cell Biochem Biophys. 2015;71(2):731–734. doi: 10.1007/s12013-014-0256-2. [DOI] [PubMed] [Google Scholar]
- 43.Harms H, Prass K, Meisel C, Klehmet J, Rogge W, Drenckhahn C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS One. 2008;3(5):e2158. doi: 10.1371/journal.pone.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalra L, Irshad S, Hodsoll J, Simpson M, Gulliford M, Smithard D, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet. 2015;386(10006):1835–1844. doi: 10.1016/s0140-6736(15)00126-9. [DOI] [PubMed] [Google Scholar]
- 45.Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: a multicenter study. Stroke. 2000;31(6):1223–1229. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- 46.Badve MS, Zhou Z, van de Beek D, Anderson CS, Hackett ML. Frequency of post-stroke pneumonia: systematic review and meta-analysis of observational studies. Int J Stroke. 2019;14(2):125–136. doi: 10.1177/1747493018806196. [DOI] [PubMed] [Google Scholar]
- 47.Vernino S, Brown RD, Jr, Sejvar JJ, Sicks JD, Petty GW, O'Fallon WM. Cause-specific mortality after first cerebral infarction: a population-based study. Stroke. 2003;34(8):1828–1832. doi: 10.1161/01.Str.0000080534.98416.A0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets presented in this study are included in the article material. The data supporting the results of this study are available from the corresponding author upon reasonable request from any qualified investigator.