Abstract
Background
Incidence of colorectal cancer (CRC) in younger adults is increasing in many countries. Smoking is an established risk factor of CRC risk, but evidence on its impact on early-onset CRC (EOCRC) risk is limited. We aimed to evaluate the association of smoking exposure with EOCRC and compare it with late-onset CRC (LOCRC).
Methods
Smoking history and other known or suspected CRC risk factors were ascertained in detail in personal interviews among 6264 CRC patients and 6866 controls (frequency matched for age, sex, and county of residence) who were recruited in 2003-2020 in the DACHS study (Darmkrebs: Chancen der Verhütung durch Screening [German]; Colorectal Cancer: Chances for Prevention Through Screening [English]), a population-based case-control study from Germany. Associations of smoking with EOCRC (<55 years, 724 cases, 787 controls) and LOCRC (≥55years, 5540 cases, 6079 controls) were estimated using multiple logistic regression.
Results
Smoking exposure was much higher among EOCRC cases than among controls, and strong associations of smoking were observed for both EOCRC and LOCR. Adjusted odds ratios for EOCRC and LOCRC were as follows: current smoking: 1.57 (95% confidence interval [CI] = 1.20 to 2.04, P < .001) and 1.46 (95% CI = 1.28 to 1.67, P < .001); former smoking: 1.39 (95% CI = 1.07 to 1.81, P = .01) and 1.24 (95% CI = 1.13 to 1.36, P < .001); per 10 pack-years: 1.15 (95% CI = 1.05 to 1.27, P < .001) and 1.05 (95% CI = 1.03 to 1.08, P < .001). These patterns were similar for colon and rectum cancer and for early- and late-stage CRC.
Conclusion
Smoking is a strong risk factor for both EOCRC and LOCRC.
Colorectal cancer (CRC) is the third-most common cancer and the second-most common cause of death due to cancer, with approximately 1.9 million incident cases and 0.9 million deaths estimated globally in 2020 (1). Furthermore, the incidence of CRC among younger persons (those younger than the screening age) is increasing in many countries, including those that have previously shown a decrease in the incidence of CRC in older adults (eg, Australia, Canada, Denmark, New Zealand, the United Kingdom, and the United States) (2-8). As a consequence of these trends, the US Preventive Services Task Force recently lowered the recommended age of initiating screening in the average-risk population from 50 years to 45 years (9). Identification of the specific role of key risk factors for early-onset CRC (EOCRC) might be crucial for even more effective, targeted primary and secondary prevention.
Numerous epidemiological studies and several meta-analyses have established smoking as a risk factor for CRC (10-12). However, the vast majority of CRC cases occur at older ages, and previous evidence therefore mostly reflects the role of smoking for late-onset CRC (LOCRC). Only a few recent studies from the United States have specifically addressed the role of smoking for EOCRC, and results were inconsistent, from positive to null (13-16). In this large, population-based, case-control study from Germany, we aimed to provide a detailed assessment and comparison of the associations between smoking and risk of EOCRC and LOCRC, paying particular attention to the amount of smoking exposure and specific associations by cancer site and CRC stage.
Methods and Materials
Study design and study population
The DACHS study (Darmkrebs: Chancen der Verhütung durch Screening [German]; Colorectal Cancer: Chances for Prevention Through Screening [English]) is an ongoing, population-based, case-control study conducted in the Rhine-Neckar region in southwestern Germany since 2003. All of the >20 clinics providing CRC surgery in the catchment area of approximately 2 million people contributed to recruitment. Details of the DACHS study have been reported elsewhere (17,18). Briefly, patients with a histologically confirmed first diagnosis of CRC (International Classification of Diseases, 10th Revision codes C18-C20) are eligible if they are at least 30 years of age, can speak German, and are physically and mentally able to participate in an interview of approximately 1 hour. Community-based controls are randomly selected from population-based registries using frequency matching with respect to age (5-year groups), sex, and county of residence. Controls with a history of CRC are excluded; otherwise inclusion and exclusion criteria are the same as in cases. The study was approved by the ethics committees of the Heidelberg Medical Faculty of Heidelberg University and of the state Medical Boards of Baden-Württemberg and Rhineland-Palatinate. Written informed consent is obtained from each participant. The current analysis is based on cases and controls recruited between 2003 and 2020. This observational study has been registered in the German Clinical Trials Register (ID DRKS00011793), which is a primary registry in the World Health Organization (WHO) Registry Network.
Data collection
Patients were informed about the study by their physicians, usually during or shortly after their hospital stay for CRC surgery. In addition, patients who could not be recruited during their hospital stay were contacted by mail shortly after discharge by clinicians or through cancer registries. According to estimates based on cancer registries, approximately 50% of eligible cases in the study area could be recruited. Controls were randomly selected and frequency matched by sex, 5-year age groups, and county of residence from population registries and contacted by the study center through mail and follow-up calls (participation rate was 51%).
Personal interviews by trained interviewers were conducted with both cases and controls using a standardized questionnaire and included detailed information on sociodemographic, medical, and lifestyle history. Interviews with cases were conducted during their hospital stay or shortly after hospital discharge at their homes. Interviews with controls were scheduled at their homes. A minority of control participants not willing to participate in a personal interview provided some key information in a self-administered questionnaire that also addressed sociodemographic, medical, and lifestyle factors, including smoking.
Assessment of smoking behavior
Information on current as well as prior smoking behavior was ascertained in great detail. We classified participants as current smokers if they were still smoking at the time of diagnosis (cases) or interview (controls) or reported stopping smoking in the year before; as former smokers if they had stopped more than 1 year before the diagnosis or interview; and as never smokers if they had never smoked regularly. As a measure of cumulative smoking exposure, pack-years of active smoking were calculated for both current and former smokers from the average number of cigarettes smoked daily multiplied by the duration of smoking in years divided by 20 (eg, smoking 20 cigarettes per day for 1 year corresponds to 1 pack-year).
Statistical analysis
There are no uniform definitions of EOCRC. The majority of studies have used 50 years as the threshold age for defining EOCRC or LOCRC, which is the starting age for CRC screening in the average risk population recommended in most countries’ guidelines (19). We used 55 years as the cutoff age in our main analysis because screening colonoscopy, which was added in Germany in 2002, has been offered for CRC screening from age 55 years on throughout most of the study period in Germany (for men, it was lowered to 50 years in 2019 only).
The distribution of demographic and lifestyle characteristics among cases and controls was compared in both younger and older participants (<55 or ≥55 years). Differences were tested for statistical significance using the Pearson χ2 test for categorical data. Multiple logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the associations of smoking status and pack-years (entered as continuous variable or as categorical variable, with categories 0, ≤15, and >15 to ensure reasonable sample size in each category) with risk of both EOCRC (<55 years) and LOCRC (≥55 years). Two adjustment levels were applied. Model A adjusted for age and sex. Model B additionally adjusted for education, family history of CRC in the first-degree relative, previous large bowel endoscopy, body mass index (BMI) approximately 10 years before diagnosis or interview, alcohol consumption, use of nonsteroidal antiinflammatory drugs (NSAIDs, including aspirin), physical activity, and diabetes. Results from model B are reported as main results. Subsite-specific and stage-specific analyses were also performed for colon and rectal cancer and for early- (stages I and II) and late- (stages III and IV) stage cancer, respectively, using model B. Potential interaction of smoking exposure with age was tested for statistical significance by additionally including a cross-product term of smoking status or pack-years of smoking with age (<55 or ≥55 years) as categorical variable in the comprehensively adjusted models (model B). All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). Statistical tests were 2-sided, with an alpha level of .05.
Results
Characteristics of the study participants
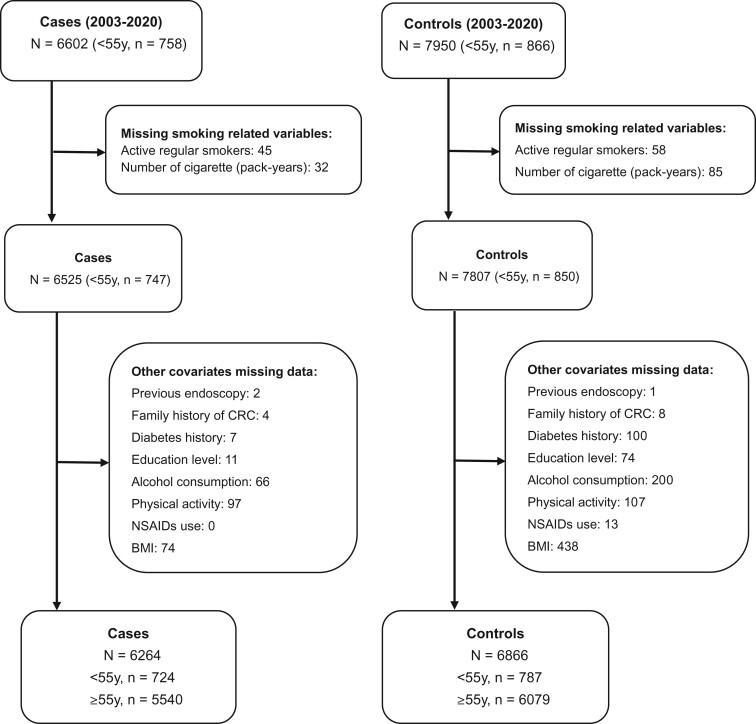
Figure 1 shows the selection of the study population. After excluding participants with missing data on relevant covariates, 724 EOCRC cases (mean age at diagnosis: 48.4 years) and 787 controls younger than 55 years were included. The corresponding numbers for LOCRC were 5540 cases (mean age at diagnosis: 71.2 years) and 6079 controls aged at least 55 years. Characteristics of the participants are shown in Table 1. In both age groups, statistically significant higher proportions of cases than of controls had a lower level of education, a family history of CRC, and a higher BMI. A previous large bowel colonoscopy was less often reported by cases than by controls. There were also differences between cases and controls regarding alcohol consumption, diabetes, and NSAIDs use, but these differences reached statistical significance in the older, much larger age group only.
Figure 1.
Flow chart showing selection of the study population. BMI = body mass index; CRC = colorectal cancer; NSAIDs = nonsteroidal antiinflammatory drugs.
Table 1.
Characteristics of participants aged younger than 55 years old and 55 years and older
| <55 years |
≥55 years |
|||||
|---|---|---|---|---|---|---|
| Cases (724) | Controls (787) | P | Cases (5540) | Controls (6079) | P | |
| Variables | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Sex | ||||||
| Female | 312 (43) | 350 (44) | 2202 (40) | 2393 (40) | ||
| Male | 412 (57) | 437 (56) | 3338 (60) | 3686 (60) | ||
| Age, y | ||||||
| 30-39/55-64 | 58 (8) | 62 (8) | 1375 (25) | 1520 (25) | ||
| 40-49/65-74 | 271 (37) | 291 (37) | 2135 (39) | 2360 (39) | ||
| 50-54/≥75 | 395 (55) | 434 (55) | 2030 (36) | 2199 (36) | ||
| Education, y | <.001 | <.001 | ||||
| ≤9 | 307 (42) | 194 (25) | 3695 (67) | 3326 (55) | ||
| 10-11 | 1939 (27) | 273 (35) | 925 (17) | 1282 (21) | ||
| 12-13 | 224 (31) | 320 (40) | 920 (16) | 1471 (24) | ||
| Alcohol consumptiona | .17 | <.001 | ||||
| Light drinker | 577 (80) | 650 (83) | 4148 (75) | 4699 (77) | ||
| Moderate drinker | 102 (14) | 104 (13) | 961 (17) | 1045 (17) | ||
| Heavy drinker | 45 (6) | 33 (4) | 431 (8) | 335 (6) | ||
| Physical activity (MET-h/wk)b | .09 | .01 | ||||
| <115.1/<78.3 | 203 (28) | 263 (33.5) | 1780 (32) | 2015 (33) | ||
| 115.1-188.8/78.3-139.5 | 254 (35) | 261 (33) | 1997 (36) | 2032 (33.5) | ||
| >188.8/>139.5 | 267 (37) | 263 (33.5) | 1763 (32) | 2032 (33.5) | ||
| First-degree family history of CRC | <.001 | <.001 | ||||
| No | 617 (85) | 727 (92) | 4732 (85) | 5385 (89) | ||
| Yes | 107 (15) | 60 (8) | 808 (15) | 694 (11) | ||
| Previous large bowel endoscopy | <.001 | <.001 | ||||
| No | 609 (84) | 552 (70) | 3913 (71) | 2161 (36) | ||
| Yes | 115 (16) | 235 (30) | 1627 (29) | 3918 (64) | ||
| Diabetes | .11 | <.001 | ||||
| No | 684 (94) | 757 (96) | 4393 (79) | 5182 (85) | ||
| Yes | 40 (6) | 30 (4) | 1147 (21) | 897 (15) | ||
| NSAIDs usec | .34 | <.001 | ||||
| No | 666 (92) | 713 (91) | 4052 (73) | 4142 (68) | ||
| Yes | 58 (8) | 74 (9) | 1488 (27) | 1937 (32) | ||
| BMI approx. 10 years before diagnosis/interview (kg/m2) | <.001 | <.001 | ||||
| <25 | 351 (48) | 476 (61) | 1578 (28) | 2203 (36) | ||
| 25 to <30 | 252 (35) | 232 (29) | 2600 (47) | 2812 (46) | ||
| ≥30 | 121 (17) | 79 (10) | 1362 (25) | 1064 (18) | ||
Average alcohol consumption in past 10 years, using sex-specific cutoffs: light drinkers: 0-12 g/d (women) and 0-24 g/d (men); moderate drinkers: more than 12-25 g/d (women) and more than 24-50 g/d (men); and heavy drinkers: more than 25 g/d (women) and more than 50 g/d (men). BMI = body mass index; CRC = colorectal cancer; MET = metabolic equivalent of task; NSAIDs = nonsteroidal antiinflammatory drugs.
Average physical activity in past 10 years, classified according to tertiles among controls.
NSAID (including aspirin) use at least 2 times per week for at least 1 year.
Associations of smoking exposure with EOCRC and LOCRC
Table 2 shows the associations of smoking exposure (smoking status and pack-years of active smoking) with EOCRC and LOCRC. Former and current smoking were strongly associated with a higher risk of both EOCRC and LOCRC, and strong associations persisted after comprehensive adjustment for multiple potential confounders. Former and current smoking were associated with a 1.39-fold (95% CI = 1.07 to 1.81, P = .01) and 1.57-fold (95% CI = 1.20 to 2.04, P < .001) increased risk of EOCRC, respectively, and a 1.24-fold (95% CI = 1.13 to 1.36, P < .001) and 1.46-fold (95% CI = 1.28 to 1.67, P < .001) increased risk of LOCRC, respectively, compared with never smokers. Cumulative smoking exposure showed a dose-response relationship with both EOCRC and LOCRC. The odds ratios per 10 pack-years increment were 1.15 (95% CI = 1.05 to 1.27, P < .001) and 1.05 (95% CI = 1.03 to 1.08, P < .001) for EOCRC and LOCRC, respectively. Although all the associations with smoking exposure were slightly stronger for EOCRC than for LOCRC, the interactions between smoking exposure and age (<55 or ≥55 years) did not reach statistical significance.
Table 2.
Associations of smoking with early- and late-CRC risk
| Smoking exposure | <55 years |
≥55 years |
P (interaction) c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | ORa (95% CI) | ORb (95% CI) | Cases | Controls | ORa (95% CI) | ORb (95% CI) | ||
| No. (%) | No. (%) | No. (%) | No. (%) | ||||||
| Smoking status | .40 | ||||||||
| Never | 277 (38) | 391 (50) | 1 (Ref) | 1 (Ref) | 2527 (46) | 3141 (52) | 1 (Ref) | 1 (Ref) | |
| Former | 216 (30) | 208 (26) | 1.47 (1.15 to 1.88) | 1.39 (1.07 to 1.81) | 2267 (41) | 2333 (38) | 1.25 (1.15 to 1.35) | 1.24 (1.13 to 1.36) | |
| Current | 231 (32) | 188 (24) | 1.74 (1.36 to 2.23) | 1.57 (1.20 to 2.04) | 746 (13) | 605 (10) | 1.60 (1.41 to 1.81) | 1.46 (1.28 to 1.67) | |
| Pack-years | .20 | ||||||||
| 0 | 277 (38) | 391 (50) | 1 (Ref) | 1 (Ref) | 2527 (46) | 3141 (52) | 1 (Ref) | 1 (Ref) | |
| 1-15 | 242 (33) | 237 (30) | 1.44 (1.14 to 1.83) | 1.39 (1.08 to 1.79) | 1444 (26) | 1514 (25) | 1.22 (1.11 to 1.34) | 1.25 (1.13 to 1.38) | |
| ≥16 | 205 (29) | 159 (20) | 1.85 (1.42 to 2.41) | 1.61 (1.21 to 2.14) | 1569 (28) | 1424 (23) | 1.43 (1.31 to 1.57) | 1.33 (1.20 to 1.48) | |
| Per 10 pack-years | 1.22 (1.11 to 1.33) | 1.15 (1.05 to 1.27) | 1.07 (1.05 to 1.10) | 1.05 (1.03 to 1.08) | |||||
Adjusted for age and sex. CI = confidence interval; NSAIDs = nonsteroidal antiinflammatory drugs; OR = odds ratio; Ref = reference.
Additionally adjusted for previous endoscopy, family history of CRC, education, BMI approximately 10 years before diagnosis (cases) or interview (controls), alcohol consumption, NSAID use (including aspirin), physical activity, and diabetes.
P interaction between age (<55 or ≥55 years) and smoking exposure in the comprehensively adjusted model (model B).
Associations of smoking exposure with EOCRC or LOCRC by cancer site and stage
Tables 3 and 4 show the associations between smoking exposure and EOCRC and LOCRC by cancer site and cancer stage, respectively. Overall, very similar patterns were seen for colon and rectal cancer (Table 3) and for early- and late-stage cancer (Table 4), even though not all of the smoking category-specific associations in the younger age group reached statistical significance, given the much lower numbers of cases and controls in this group.
Table 3.
Associations of smoking with early- and late-CRC risk by subsite
| Cancer site | <55 years |
≥55 years |
P interaction b | ||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | ORa (95% CI) | Cases | Controls | ORa (95% CI) | ||
| No. (%) | No. (%) | No. (%) | No. (%) | ||||
| Colon | |||||||
| Smoking status | .78 | ||||||
| Never | 149 (40) | 391 (50) | 1 (Ref) | 1643 (47) | 3141 (52) | 1 (Ref) | |
| Former | 102 (28) | 208 (26) | 1.26 (0.91 to 1.75) | 1388 (40) | 2333 (38) | 1.21 (1.10 to 1.34) | |
| Current | 116 (32) | 188 (24) | 1.54 (1.12 to 2.12) | 449 (13) | 605 (10) | 1.49 (1.28 to 1.73) | |
| Pack-years | |||||||
| 0 | 149 (41) | 391 (50) | 1 (Ref) | 1643 (47) | 3141 (52) | 1 (Ref) | .60 |
| 1-15 | 120 (33) | 237 (30) | 1.33 (0.98 to 1.81) | 895 (26) | 1514 (25) | 1.23 (1.10 to 1.38) | |
| ≥16 | 98 (26) | 159 (20) | 1.50 (1.06 to 2.13) | 942 (27) | 1424 (23) | 1.31 (1.17 to 1.47) | |
| Per 10 pack-years | 1.14 (1.02 to 1.28) | 1.05 (1.02 to 1.08) | |||||
| Rectum | |||||||
| Smoking status | .47 | ||||||
| Never | 128 (36) | 391 (50) | 1 (Ref) | 884 (43) | 3141 (52) | 1 (Ref) | |
| Formerc | 114 (32) | 208 (26) | 1.52 (1.10 to 2.11) | 879 (43) | 2333 (38) | 1.30 (1.14 to 1.47) | |
| Current | 115 (32) | 188 (24) | 1.58 (1.13 to 2.20) | 297 (14) | 605 (10) | 1.47 (1.22 to 1.76) | |
| Pack-years | |||||||
| 0 | 128 (36) | 391 (50) | 1 (Ref) | 884 (43) | 3141 (52) | 1 (Ref) | .39 |
| 1-15 | 122 (34) | 237 (30) | 1.46 (1.06 to 2.00) | 549 (27) | 1514 (25) | 1.29 (1.12 to 1.49) | |
| ≥16 | 107 (30) | 159 (20) | 1.70 (1.20 to 2.41) | 627 (30) | 1424 (23) | 1.38 (1.20 to 1.59) | |
| Per 10 pack-years | 1.15 (1.02 to 1.29) | 1.05 (1.02 to 1.08) | |||||
Adjusted for age, sex, previous endoscopy, family history of CRC, education, BMI approximately 10 years before diagnosis (cases) or interview (controls), alcohol consumption, NSAID use (including aspirin), physical activity, and diabetes. BMI = body mass index; CI = confidence interval; CRC = colorectal cancer; OR = odds ratio; Ref = reference.
P interaction between age (<55 or ≥55 years) and smoking exposure.
Table 4.
Associations of smoking with early- and late-CRC risk by stage
| CRC stage | <55 years |
≥55 years |
P interaction b | ||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | ORa (95% CI) | Cases | Controls | ORa (95% CI) | ||
| No. (%) | No. (%) | No. (%) | No. (%) | ||||
| Early-stage CRC (stages I or II) | .22 | ||||||
| Smoking status | |||||||
| Never | 107 (36) | 391 (50) | 1 (Ref) | 1306 (45) | 3141 (52) | 1 (Ref) | |
| Former | 104 (35) | 208 (26) | 1.61 (1.15 to 2.26) | 1215 (42) | 2333 (38) | 1.29 (1.16 to 1.44) | |
| Current | 86 (29) | 188 (24) | 1.58 (1.11 to 2.24) | 376 (13) | 605 (10) | 1.52 (1.30 to 1.79) | |
| Pack-years | .27 | ||||||
| 0 | 107 (36) | 391 (50) | 1 (Ref) | 1306 (45) | 3141 (52) | 1 (Ref) | |
| 1-15 | 102 (34) | 237 (30) | 1.52 (1.09 to 2.12) | 784 (27) | 1514 (25) | 1.32 (1.17 to 1.49) | |
| ≥16 | 88 (30) | 159 (20) | 1.70 (1.18 to 2.46) | 807 (28) | 1424 (23) | 1.36 (1.20 to 1.53) | |
| Per 10 pack-years | 1.18 (1.05 to 1.33) | 1.05 (1.02 to 1.08) | |||||
| Late-stage CRC (stages III or IV) | |||||||
| Smoking status | |||||||
| Never | 155 (40) | 391 (50) | 1 (Ref) | 1158 (47) | 3141 (52) | 1 (Ref) | .57 |
| Former | 100 (26) | 208 (26) | 1.22 (0.88 to 1.70) | 969 (39) | 2333 (38) | 1.16 (1.03 to 1.30) | |
| Current | 135 (34) | 188 (24) | 1.65 (1.20 to 2.26) | 343 (14) | 605 (10) | 1.43 (1.21 to 1.69) | |
| Pack-years | |||||||
| 0 | 155 (40) | 391 (50) | 1 (Ref) | 1158 (47) | 3141 (52) | 1 (Ref) | .31 |
| 1-15 | 124 (32) | 237 (30) | 1.30 (0.95 to 1.76) | 610 (25) | 1514 (25) | 1.16 (1.02 to 1.32) | |
| ≥16 | 111 (28) | 159 (20) | 1.67 (1.18 to 2.36) | 702 (28) | 1424 (23) | 1.28 (1.12 to 1.46) | |
| Per 10 pack-years | 1.15 (1.03 to 1.30) | 1.04 (1.01 to 1.07) | |||||
Adjusted for age, sex, previous endoscopy, family history of CRC, education, BMI approximately 10 years before diagnosis (cases) or interview (controls), alcohol consumption, NSAID use (including aspirin), physical activity, and diabetes. BMI = body mass index; CI = confidence interval; CRC = colorectal cancer; OR = odds ratio; Ref = reference.
P interaction between age (<55 or ≥55 years) and smoking exposure.
Discussion
In this large, population-based, case-control study from Germany, smoking was strongly associated with both EOCRC and LOCRC risk in a dose-response manner. The associations persisted after comprehensive confounder adjustment and were of similar size for colon and rectum cancer, and for early- and late-stage cancer.
Although the association of smoking with CRC risk has long been established (10-12), only a few recent studies from the United States have specifically addressed the role of smoking for EOCRC. Inconsistent results were reported from 3 smaller studies (between 239 and 651 EOCRC cases). In a single-institution electronic health records study, no association was found between smoking and EOCRC (269 cases) (14). In a retrospective, registry-based case-control study among US veterans, the association of current smoking with EOCRC risk (651 cases) likewise did not reach statistical significance (OR = 1.10, 95% CI = 0.89 to 1.35), but smoking information was missing for a large proportion (36.6%) of EOCRC cases (16). In an analysis of the US National Interview Survey, former or current smoking was associated with ever having had a diagnosis of CRC among those aged 18-49 years (239 cases, OR = 1.51, 95% CI = 1.10 to 2.08) (15). In a very large study (5710 EOCRC cases) using Explorys, a US national database, “tobacco use” (with no further distinction between current or former smoking, or by amount of exposure) was strongly associated with increased risk of EOCRC (OR = 2.46, 95% CI = 2.33 to 2.59, P < .001) (13). The apparent inconsistency of previous results may have been partly due to small sample size in some of the studies as well as large variations in study design and details of exposure and relevant covariate information. Our results are based on a large, prospective, population-based case-control study from Germany, which has been specifically designed to assess risk factors of CRC and thereby substantially strengthens the evidence for the role of smoking in increasing EOCRC risk.
The biological mechanisms underlying the association between smoking and CRC have not yet been fully revealed. Smoking has been found to be even more strongly associated with both advanced adenomas and serrated polyps (20), the precursors of CRC, than with CRC, which often develop already at “EOCRC ages.”
Cigarette smoke carcinogens, such as nitrosamines, benzene, heterocyclic amines, and polycyclic aromatic hydrocarbons, may reach the colorectal mucosa by direct ingestion or the circulation and may have a direct carcinogenic impact on both the colon and the rectum (21). There is increasing evidence that smoking is particularly strongly associated with the microsatellite instability-high, CpG island methylator phenotype-positive, and B-Raf protein encoding gene (BRAF) mutation-positive subtypes of CRC (22-25), implying that epigenetic modification may be functionally involved in smoking-related colorectal carcinogenesis. There is also accumulating evidence that the composition of the gut microbiome, which is altered by smoking exposure, plays an important role in colorectal carcinogenesis (26). A recent animal study (27) showed that cigarette smoke–induced dysbiosis of the gut microbiota exerts a protumorigenic function in CRC. Smoke-induced gut microbiota dysbiosis changed gut metabolites and compromised gut barrier function, potentially activating oncogenic MAPK/ERK (mitogen-activated protein kinases/extracellular signal-regulated kinases) signaling in colonic epithelial cells. It appears plausible that such mechanisms may affect both EOCRC and LOCRC, which is supported by the consistent associations of smoking with both outcomes found in our study.
Our study has a number of specific strengths, including its design as a population-based case-control study with prospectively designed detailed collection of information on smoking exposure and relevant potential confounders and its large overall sample size. However, several limitations also require careful consideration. Firstly, despite the overall large sample size, the number of EOCRC cases (n = 724) and controls (n = 787) was still quite limited, which limited the power and precision of analyses of EOCRC-specific associations, in particular for site stage-specific analyses. In particular, power was insufficient to assess interactions between smoking and age, and it is unclear to what extent the apparently stronger associations seen between smoking and EOCRC than between smoking and LOCRC may be due to chance rather than a true difference in smoking effects. Secondly, with recruitment rates of approximately 50% of cases and slightly more than 50% of controls, we cannot rule out some selection bias. The main barrier to complete recruitment of cases in this large population-based study was overload of physicians in the more than 20 clinics involved in the recruitment, which is unlikely to be related to smoking status exposure of the cases. Also, recruitment rates of both cases and controls were by far the lowest in the oldest age groups in this study, which, in contrast to most previous studies, did not set an upper age limit. Although this has led to lower overall participation rates, it should likewise not be a major source of selection bias. Nevertheless, it is conceivable that more health-conscious people may have been more likely to be willing to participate as controls in this study, which may have led to some overestimation of smoking effects. Thirdly, all smoking information was based on self-reports. Therefore, despite most detailed ascertainment of current and former smoking habits in personal interviews, some misclassification of smoking exposure cannot be excluded, which could have led to some underestimation of smoking effects.
Despite its limitations, our study adds to the evidence that smoking is no less (and possibly even stronger) related to EOCRC than it is related to LOCRC. Further efforts to reduce smoking exposure, which is related to a large variety of adverse health effects beyond CRC, should be a public health priority and should also help to reduce the burden of both EOCRC and LOCRC in the long term. Some progress in that regard, especially among younger generations, has been made in several high-income countries in the past decades, including countries for which an increase in EOCRC rates has been observed (28). In Germany, based on data from 1998 through 2014, for all birth cohorts (range from 1910-1929 to 1990-1996), smoking prevalence decreased with age. The share of smokers increased consistently up to 45 or 60 years and then decreased. Also, except for the 1950-1959 cohort, younger cohorts had a lower smoking prevalence than older ones in comparable age groups. In 1998, smoking rates were highest between ages 26 and 45 years. Sixteen years later, in 2014, smoking rates were highest between age 46 and 60 years. The high former smoking rates of the 55 years of age and older group can be explained by younger age groups with high shares of smokers in the past getting older (29). Hence, although smoking most likely increases the risk of EOCRC, trends in smoking are unlikely to explain the rise in EOCRC incidence rates in these countries (28). Such trends may well be explained, however, by other major risk factors of CRC (including EOCRC), such as overweight and obesity, the prevalence of which keeps increasing almost globally (30-32). However, stable or increasing smoking prevalence among younger generations, along with other adverse risk factor trends, may lead to substantial increases in EOCRC in the future in many other countries, especially low-income countries (28). Comprehensive efforts to promote healthy lifestyles that can most substantially reduce the risk of CRC and many other adverse health outcomes (33-35) would have enormous public health potential.
Contributor Information
Hengjing Li, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, Germany; Medical Faculty Heidelberg, Heidelberg University, Heidelberg, Germany.
Xuechen Chen, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, Germany; Medical Faculty Heidelberg, Heidelberg University, Heidelberg, Germany.
Michael Hoffmeister, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Hermann Brenner, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, Germany; Division of Preventive Oncology, German Cancer Research Center (DKFZ), National Center for Tumor Diseases (NCT), Heidelberg, Germany; German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Funding
This work was supported by grants from the German Research Council (BR 1704/6-1, BR1704/6-3, BR 1704/6-4, BR 1704/6-6, CH 117/1-1, BR 1704/17-1, and HO 5117/2-1) and the German Federal Ministry of Education and Research (01KH0404, 01ER0814, 01ER0815, and 01GL1712). The sponsors had no role in the study design and in the collection, analysis, and interpretation of data. The first author (H.L.) was supported by a grant from the China Scholarship Council (CSC).
Notes
Role of the funder: The Funder had no role in the design of the study, the collection, analysis, and interpretation of the data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Disclosures: The authors have no disclosures.
Author contributions: Conceptualization: HJL and HB. Formal Analysis: HJL and HB. Methodology: HJL and HB. Writing—original draft: HJL and HB. Funding acquisition: HB and MH. Project administration: HB and MH. Supervision: HB and MH. Writing-review & editing: HJL, MH, XCC, and HB.
Acknowledgements: We would like to thank Ute Handte-Daub and Ansgar Brandhorst for their excellent technical assistance. We are particularly grateful to the study participants, as well as the interviewers who assisted in the data collection. We also gratefully appreciate the cooperation of the below-listed clinics and institutions: Chirurgische Universitätsklinik Heidelberg, Klinik am Gesundbrunnen Heilbronn, St. Vincentiuskrankenhaus Speyer, St. Josefskrankenhaus Heidelberg, Chirurgische Universitätsklinik Mannheim, Diakonissenkrankenhaus Speyer, Krankenhaus Salem Heidelberg, Kreiskrankenhaus Schwetzingen, St. Marienkrankenhaus Ludwigshafen, Klinikum Ludwigshafen, Stadtklinik Frankenthal, Diakoniekrankenhaus Mannheim, Kreiskrankenhaus Sinsheim, Klinikum am Plattenwald Bad Friedrichshall, Kreiskrankenhaus Weinheim, Kreiskrankenhaus Eberbach, Kreiskrankenhaus Buchen, Kreiskrankenhaus Mosbach, Enddarmzentrum Mannheim, Kreiskrankenhaus Brackenheim, and Cancer Registry of Rhineland-Palatinate, Mainz.
Data availability
The data underlying this article can only be shared upon request for purposes defined in participants' informed consent based on a legally binding contract that ensures the privacy of individuals that participated in the study.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2. Troeung L, Sodhi-Berry N, Martini A, et al. Increasing incidence of colorectal cancer in adolescents and young adults aged 15-39 years in Western Australia 1982-2007: examination of colonoscopy history. Front Public Health. 2017;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy CC, Lund JL, Sandler RS.. Young-onset colorectal cancer: earlier diagnoses or increasing disease burden? Gastroenterology. 2017;152(8):1809-1812.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang W, Chen W, Lin J, et al. Incidence and characteristics of young-onset colorectal cancer in the United States: an analysis of SEER data collected from 1988 to 2013. Clin Res Hepatol Gastroenterol. 2019;43(2):208-215. [DOI] [PubMed] [Google Scholar]
- 5. Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68(10):1820-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lui RN, Tsoi KKF, Ho JMW, et al. Global increasing incidence of young-onset colorectal cancer across 5 continents: a joinpoint regression analysis of 1,922,167 cases. Cancer Epidemiol Biomarkers Prev. 2019;28(8):1275-1282. [DOI] [PubMed] [Google Scholar]
- 7. Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179-2185. [DOI] [PubMed] [Google Scholar]
- 8. Araghi M, Soerjomataram I, Bardot A, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019;4(7):511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. [DOI] [PubMed] [Google Scholar]
- 10. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778. [DOI] [PubMed] [Google Scholar]
- 11. Liang PS, Chen TY, Giovannucci E.. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124(10):2406-2415. [DOI] [PubMed] [Google Scholar]
- 12. Cheng J, Chen Y, Wang X, et al. Meta-analysis of prospective cohort studies of cigarette smoking and the incidence of colon and rectal cancers. Eur J Cancer Prev. 2015;24(1):6-15. [DOI] [PubMed] [Google Scholar]
- 13. Syed AR, Thakkar P, Horne ZD, et al. Old vs new: risk factors predicting early onset colorectal cancer. World J Gastrointest Oncol. 2019;11(11):1011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gausman V, Dornblaser D, Anand S, et al. Risk factors associated with early-onset colorectal cancer. Clin Gastroenterol Hepatol. 2020;18(12):2752-2759.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanford NN, Giovannucci EL, Ahn C, et al. Obesity and younger versus older onset colorectal cancer in the United States, 1998-2017. J Gastrointest Oncol. 2020;11(1):121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Low EE, Demb J, Liu L, et al. Risk factors for early-onset colorectal cancer. Gastroenterology. 2020;159(2):492-501.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22-30. [DOI] [PubMed] [Google Scholar]
- 18. Brenner H, Chang-Claude J, Rickert A, et al. Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: population-based case-control study. J Clin Oncol. 2012;30(24):2969-2976. [DOI] [PubMed] [Google Scholar]
- 19. Ebell MH, Thai TN, Royalty KJ.. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev. 2018;39:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson JC, Calderwood AH, Christensen BC, et al. Smoking and other risk factors in individuals with synchronous conventional high-risk adenomas and clinically significant serrated polyps. Am J Gastroenterol. 2018;113(12):1828-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng J, Chen Y, Wang X, et al. Meta-analysis of prospective cohort studies of cigarette smoking and the incidence of colon and rectal cancers. Eur J Cancer Prev. 2015;24(1):6-15. [DOI] [PubMed] [Google Scholar]
- 22. Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102(14):1012-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen K, Xia G, Zhang C, et al. Correlation between smoking history and molecular pathways in sporadic colorectal cancer: a meta-analysis. Int J Clin Exp Med. 2015;8(3):3241-3257. eCollection 2015. [PMC free article] [PubMed] [Google Scholar]
- 24. Amitay EL, Carr PR, Jansen L, et al. Smoking, alcohol consumption and colorectal cancer risk by molecular pathological subtypes and pathways. Br J Cancer. 2020;122(11):1604-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Amitay E, Harrison TA, et al. Association between smoking and molecular subtypes of colorectal cancer. JNCI Cancer Spectr. 2021;14;5(4):pkab056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song M, Chan AT.. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol. 2019;17(2):275-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bai X, Wei H, Liu W, et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut. 2022;71(12):2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dai X, Gakidou E, Lopez AD.. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob Control. 2022;31(2):129-137. [DOI] [PubMed] [Google Scholar]
- 29. Heilert D, Kaul A. The German Socio-Economic Panel Study. Smoking behaviour in Germany–evidence from the SOEP. SOEP papers 920,103S. 2017. https://www.diw.de/documents/publikationen/73/diw_01.c.563343.de/diw_sp0920.pdf. Accessed October 30, 2022.
- 30. Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5(1):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li H, Boakye D, Chen X, et al. Association of body mass index with risk of early-onset colorectal cancer: systematic review and meta-analysis. Am J Gastroenterol. 2021;116(11):2173-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Boakye D, Chen X, et al. Associations of body mass index at different ages with early-onset colorectal cancer. Gastroenterology. 2022;162(4):1088-1097.e3. [DOI] [PubMed] [Google Scholar]
- 33. Aleksandrova K, Pischon T, Jenab M, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med. 2014;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carr PR, Weigl K, Jansen L, et al. Healthy lifestyle factors associated with lower risk of colorectal cancer irrespective of genetic risk. Gastroenterology. 2018;155(6):1805-1815.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Erben V, Carr PR, Holleczek B, et al. Strong associations of a healthy lifestyle with all stages of colorectal carcinogenesis: results from a large cohort of participants of screening colonoscopy. Int J Cancer. 2019;144(9):2135-2143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article can only be shared upon request for purposes defined in participants' informed consent based on a legally binding contract that ensures the privacy of individuals that participated in the study.