Abstract
Introduction. Although lung damages are among the leading causes of death from Rheumatoid Arthritis (RA), few studies have assessed the spirometric and plethysmographic data and profile of patients with RA, particularly those with Anti-Citrullinated Peptides Antibodies Positive (ACPA+).
Aim. To compare the spirometric and plethysmographic data and profile of RA patients ACPA+ and ACPA-.
Methods. This comparative pilot study was performed over a two-year period (2018-2019) in Algiers (Algeria). The study included two groups of RA non-smoker patients: 26 ACPA+ and 33 ACPA-. RA was diagnosed according to the ACR/EULAR 2010 RA classification criteria. Spirometry and plethysmography were performed. The following definitions were applied: Obstructive Ventilatory Impairment (OVI): FEV1/FVC z-score < -1.645; Restrictive Ventilatory Impairment (RVI): Total Lung Capacity (TLC) z-score < -1.645; Mixed Ventilatory Impairment (MVI): FEV1/FVC z-score < -1.645 and TLC z-score < -1.645; lung- hyperinflation: residual volume z-score > +1.645; Nonspecific Ventilatory Impairment (NSVI): FEV1 z-score < -1.645, FVC z-score < -1.645, FEV1/FVC z-score ≥ -1.645, and TLC z-score ≥ -1.645.
Results. The ACPA- group was older than the ACPA+ one by ∼ 10 years (63±13 vs. 53±12 years, p=0.0025; respectively). The ACPA+ and ACPA- groups included comparative percentages of patients having RVI, MVI, and NSVI (23.1 vs. 45.5%, p=0.0745; 3.8 vs. 3.0%, p=0.8654; and 7.7 vs. 6.1%, p=0.8086; respectively). Compared to the ACPA- group, the ACPA+ group included a higher percentage of patients having OVI and lung-hyperinflation (9.1 vs. 38.5%, p=0.0069; 9.1 vs. 42.3%, p=0.0029; respectively).
Conclusion. Compared to the ACPA- group, the ACPA+ one had more lung-hyperinflation and OVI, and comparative percentages of RVI, MVI, and NSVI.
Keywords: Airway obstruction, Algeria, Antibodies, Lung function tests, Tomography, X-Ray Computed.
Résumé
Introduction. Bien que les lésions pulmonaires soient parmi les principales causes de décès par Polyarthrite Rhumatoïde (PR), peu d'études ont évalué les données spirométriques et pléthysmographiques et le profil des patients atteints de PR, en particulier ceux avec des Anticorps Anti-Peptides Citrullinés Positifs (AAPC+).
Objectif. Comparer les données spirométriques et pléthysmographiques et le profil des patients PR AAPC+ et AAPC-.
Méthodes. Cette étude pilote comparative a été réalisée sur une période de deux ans (2018-2019) à Alger (Algérie). Elle a inclus deux groupes de patients non-fumeurs ayant une PR: 26 AAPC+ et 33 AAPC-. La PR a été diagnostiquée selon les critères de classification internationale (ACR/EULAR-2010). Une spirométrie et une pléthysmographie ont été réalisées. Les définitions suivantes ont été appliquées: Déficit Ventilatoire Obstructif (DVO): z-score du VEMS/CVF <-1,645; Déficit Ventilatoire Restrictif (DVR): z-score de la Capacité Pulmonaire Totale (CPT) <-1,645; Déficit Ventilatoire Mixte (DVM): z-score du VEMS/CVF <-1,645 et z-score de la CPT <-1,645; distension pulmonaire: z-score du volume résiduel >+1,645; Déficit Ventilatoire Non-spécifique (DVNS): z-score du VEMS <-1,645, z- score de la CVF <-1,645, z-score du VEMS/CVF ≥-1,645 et z-score de la CPT ≥-1,645.
Résultats. Le groupe AAPC- était plus âgé que le groupe AAPC+ d'environ 10 ans (63±13 contre 53±12 ans, p=0,0025 ; respectivement). Les groupes AAPC+ et AAPC- incluaient des pourcentages similaires de patients ayant un DVR, un DVM, et un DVNS (23,1 contre 45,5%, p=0,0745; 3,8 contre 3,0%, p=0,8654; et 7,7 contre 6,1%, p=0,8086;
respectivement). Comparé au groupe AAPC-, le groupe AAPC+ incluait un pourcentage plus élevé de patients ayant une DVO et une distension pulmonaire (9,1 contre 38,5%, p=0,0069; 9,1 contre 42,3%, p=0,0029; respectivement).
Conclusion. Comparé au groupe AAPC-, le groupe AAPC+ incluait plus patients ayant une distension pulmonaire et/ou un DVO, et des pourcentages similaires de DVR, DVM et DVNS.
Mots clés: Obstruction des voies aériennes, Algérie, Anticorps, Exploration fonctionnelle respiratoire, Tomographie, Radiographie du thorax.
INTRODUCTION
Rheumatoid Arthritis (RA) is the most common inflammatory rheumatic disease, with a prevalence of 0.1 to 2.5% in the African continent (1 ). Females are twice to five times more susceptible to RA than males, and the disease is more common at the age of 40-50 years (2 ). Pulmonary involvement is one of the most frequent extra-articular manifestations of RA, and it is one of the leading causes of RA mortality (3 ). Several studies have evaluated the respiratory function and the bronchial inflammatory state in patients with RA (3, 4, 5, 6, 7, 8, 9, 10). It appears that RA is associated with increased odds of Restrictive and Obstructive Ventilatory Impairments (RVI, OVI, respectively) (7 ). Moreover, the existing body of evidence suggests that the lung is an organ of immune defense and has a role in the pathogenesis of the autoimmune disease Anti-Citrullinated Protein Antibody positive (ACPA+) RA (11 ). ACPAs are highly specific for RA (12 ), and ACPA+ represents a subclass of RA that differs in terms of pathogenesis, disease course, and response to therapy when compared with ACPA negative (ACPA-) (13, 14, 15). However, to the best of the authors’ knowledge, only a few studies have evaluated the relationship between lung impairments and immunological markers, such as the ACPA (3, 4, 5, 6).
ACPAs are considered as both sensitive and specific markers for rheumatoid disease ( 16, 17). They bind to proteins or peptides containing citrulline, and are detected in joints as well as in other tissues and organs, such as the lung (16 ). Although recent studies have suggested that the lung is the initial site for ACPA production, and that specific citrullination in the lung may precede the onset of joint involvement in RA (11, 13), the mechanism explaining the association of ACPA with pulmonary impairments has not been elucidated yet. Furthermore, there is not enough data explaining the different types of pulmonary involvement according to the serologic status of RA, whether ACPA+ or ACPA-. Indeed, the question whether ACPA levels could influence the clinical, Computed-Tomography (CT) and respiratory functional expressions of the rheumatoid lung is an issue worth raising (18 ).
According to Daha and Toes (18 ) “contrasting genetic backgrounds in ACPA+ and ACPA- support the notion that these are two distinct disease subsets, with different underlying pathogenesis”. Moreover, another study concluded that ACPA+ is a distinct entity from ACPA−, and that ACPA+ is independently associated with more severe erosive disease (19 ). Since the lung is a potential primary initiation site for ACPA+ (11 ), it is possible that pulmonary manifestations of ACPA+ and ACPA- are distinct on spirometry and plethysmography. Thus, it seems to be relevant to evaluate the spirometric and plethysmographic data and profiles of RA patients, and to determine their variabilities based on the patients’ ACPA status.
The objective of the present comparative pilot study, which was performed during the
2018-2019 period in Algiers, Algeria, was to compare the spirometric and plethysmographic data and profiles of RA non-smoker patients depending in the presence/absence of ACPA (ACPA+ vs. ACPA-). The primary outcome was the frequency of RVI, and the second outcomes were the frequencies of the following impairments: OVI; Mixed Ventilatory Impairment (MVI); Nonspecific Ventilatory Impairment (NSVI), and lung-hyperinflation.
METHODS
Study design
This was a cross-sectional comparative pilot study. The study was performed over a two-year period (2018-2019) in the Department of Pneumology, Phthisiology, and Allergology (Rouiba Hospital, Algiers, Algeria). The medical council of Rouiba hospital was consulted in reviewing and approving this manuscript for its design, conduct, reporting, and dissemination of this research work. The above-cited medical council approved this study (approval number 11/2018). As the spirometry and plethysmography are routine tests used for diagnostic activity, and after a discussion with the medical council of Rouiba hospital, the patients’ participation or individual ‘written’ consent from the study participants was not required.
Population
Only patients diagnosed with RA were included. Patients were recruited20, 21
the age- and sex- adjusted prevalence of asthma was 3.45% (22 ).
The following non-inclusion criteria were applied: malignant tumor, history of pulmonary surgery, active infection, human immunodeficiency virus/acquired immune
deficiency syndrome, heart failure, chronic respiratory conditions such as COPD or asthma, active smoking, previous treatment by methotrexate (because of its possible impacts on lung interstitium and air trapping (23 )), destruction or fibrosis of the lung parenchyma due to previous infection or having pulmonary pathologies that can cause sequelae lung lesionssuch asandplethysmographymeasurements.PatientshavingInterstitialLungDiseases(ILDs)werenot excluded from the study. Files of RA patients with missing data were excluded from thefinal statistical analysis.presentsthestudyflowchart.
Positive diagnosis of RA
The positive diagnosis of RA was based on a body of clinical, biological, and radiological arguments, and in particular on the absence of clinical and biological arguments for another cause of inflammatory rheumatism (24 ). The criteria useful for diagnosis were those of the American college of rheumatology and European League of associations for rheumatology 2010 (25 ). However, in the absence of these clinico-biological criteria and the presence of erosions typical of RA identified at the standard x-rays of the hands-wrists and frontal forefoot, the disease is also classified as RA (25 ). Indeed, the concept of "typical RA erosion" has recently been defined as having at least three erosive joints among the metacarpophalangeal, proximal interphalangeal, wrist, and metatarsophalangeal bone (26 ).
Collected data
Clinical data were collected using a standard non-validated French medical questionnaire. The latter is part of normal clinical care at our department, and the questionnaire is used in the daily activity at the outpatient consultation Department. The questions, which were asked in Arabic language dialect, were essentially closed-ended and
most often dichotomous. Frequent comorbidities like arterial-hypertension, diabetes- mellitus, and heart diseases were noted. The following RA characteristics were noted from the patients’ medical records: length of time, age of symptoms onset, time of diagnosis, extra-articular symptomatology (Raynaud's phenomenon, morning stiffness), and Disease Activity Score (DAS) (27 ). The DAS was calculated at 28 joints using the level of C-Reactive Protein (CRP, DAS28-CRP) (28 ). The DAS28-CRP takes into account the number of painful and swollen joints, the overall evaluation of the disease by the patient, and CRP (28 ). The DAS28-CRP was considered severe, moderate, weak, or in remission if it was > 5.1, 3.2 to 5.1, 2.6 to 3.2, or ≤ 2.6 (29 ), respectively.
Cough and dyspnea were noted. Dyspnea was classified according to the modified medical research council scale [from grade 0 (breathless with strenuous exercise) to grade
4 (breathless to leave the house or when dressing)] (30 ). The smoking status [passive smoker, active smoker (smoking history > one pack-year)] was determined. The durations of the respiratory and extra-respiratory signs (in months) before RA diagnosis were noted.
Sex and some anthropometric data were determined. Age was calculated from the date of birth to the date of measurement. Standing height (m) and weight (kg) were recorded (seca medical scales and measuring systems, Germany). Body Mass Index (BMI, kg/m2) was calculated, and the following corpulence status were determined: underweight (BMI <
18.5 kg/m2), normal weight (BMI: 18.5-24.9 kg/m2), overweight (BMI: 25.0-29.9 kg/m2), and obesity (BMI ≥ 30.0 kg/m2) (31 ).
Some biological data such as Erythrocyte Sedimentation Rate (ESR, first hour, mm), CRP (mg/L), hemoglobin (g/L), white blood cells (/mm3), polynuclear neutrophils or eosinophils (/mm3), lymphocytes (/mm3), monocytes (/mm3)] were determined using standard hospital methods. Biological data were determined during the same week in which spirometry and plethysmography were performed. In patients aged < 50 years, ESR was considered elevated if it was > 15 in males or > 20 in females (32 ). In patients aged ≥ 50 years, ESR was considered elevated if it was > 20 in males or > 30 in females (32 ). CRP was considered elevated if its level was > 12 mg/L (33 ). Biological inflammatory syndrome was retained in the presence of an increase in CRP and/or ESR. Anemia was defined as hemoglobinemia < 12 g/L in females and <13 g/L in males (34 ). A number of white blood cells being > 11000/mm3 defined leukocytosis (35 ).
The following immunological data were determined: Rheumatoid Factor (RF, IU/ml), Anti-Nuclear Antibody (ANA, titer), and ACPA (U/ml). Immunoglobulin (Ig)M-RF, IgG-RF, and IgA-RF were detected by immunoturbidimetry (OCD VITROS, USA). According to the RF values, five RF levels were determined: negative (FR < 10 IU/mL), light (FR: 10 to 40), moderate (FR: 40 to 100), high (FR: 100 to 300), and very high (FR > 300) (36 ). Two groups of patients were identified: FR negative (FR-) and FR positive (FR+). A positive ANA test is
usually reported as a ratio (called a titer), and looked for using the indirect Hep-2 cell immunofluorescence method (Medical Biological Lab, Tokyo, Japan) with an initial dilution of 1:20, following a standardized protocol (37 ). Positive reactions were titrated by double dilution to the end-point. Serum reactivity at a dilution of at least 1:160 was considered a positive result for ANA in the present study (38 ). ACPAs were measured using an electro- chemiluminescence test on Cobas 6000 (ROCHE, Switzerland). RA patients were divided into two groups, ACPA+ (ACPA ³ 5 U/ml) and ACPA- (ACPA < 5 U/ml) (39 ). The ACPA+ and ACPA- groups were divided into subgroups according to the FR status: ACPA+-FR+, ACPA+- FR-, ACPA--FR+, and ACPA--FR- (Figure 1 ).
Figure 1. Study flowchart (Algiers (Algeria): 2018-2019).
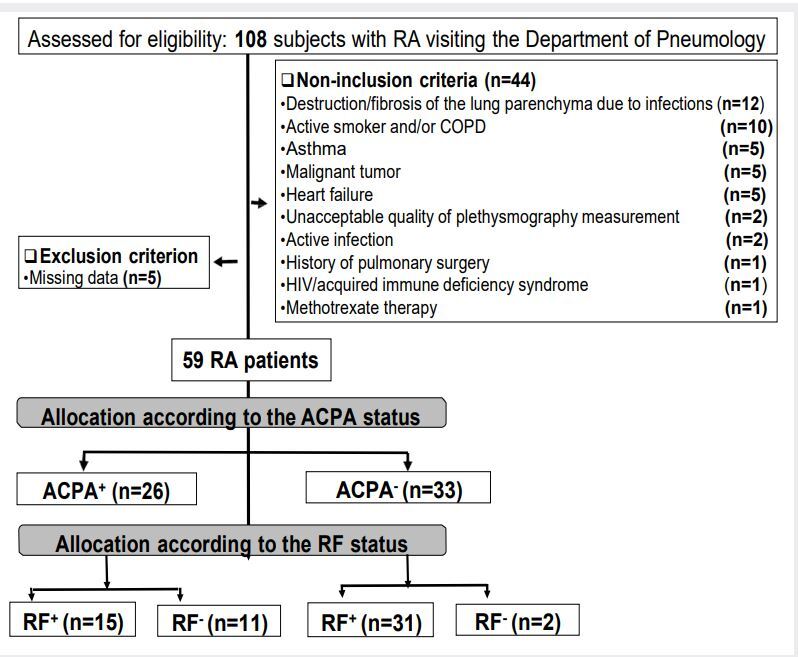
usually reported as a ratio (called a titer), and looked for using the indirect Hep-2 cell immunofluorescence method (Medical Biological Lab, Tokyo, Japan) with an initial dilution of 1:20, following a standardized protocol (37 ). Positive reactions were titrated by double dilution to the end-point. Serum reactivity at a dilution of at least 1:160 was considered a positive result for ANA in the present study (38 ). ACPAs were measured using an electro- chemiluminescence test on Cobas 6000 (ROCHE, Switzerland). RA patients were divided into two groups, ACPA+ (ACPA ³ 5 U/ml) and ACPA- (ACPA < 5 U/ml) (39 ). The ACPA+ and ACPA- groups were divided into subgroups according to the FR status: ACPA+-FR+, ACPA+- FR-, ACPA--FR+, and ACPA--FR- (Figure 1 ).
Radiological data were determined using standard hospital methods [24 multi-bar 16- slice scanner (Optima 540 general electrique USA)]. No patient received intravenous contrast media. An experienced radiologist with high experience in thoracic CT and ILDs performed a semi-quantitative visual evaluation based on the lobe of the thoracic scanner, and a quantitative evaluation with computer-assisted calculation of emphysema and lung volume computed-assisted reading for pulmonary nodules. Inspiratory CT images were first evaluated, followed by expiratory CT images, with no time limit for visual evaluation. The radiologist was not informed about the patients’ clinical data, their biological, immunological, and spirometric/plethysmographic results. The radiologist was asked to apply the recommendations of the Fleischner society (40 ), and to determine the presence or not of an ILD, and if yes to determine its type, such as usual interstitial pneumonia, nonspecific ILD,
large airway abnormalities such as thickening of the bronchial wall, and bronchiectasis, or
small airway abnormalities like centrilobular nodules, and entrapment of expiratory air.
Lung Function Data (LFD) were measured by a plethysmograph (Body-box 5500, MediSoft, Belgium) between 9:00 a.m. and 3:00 p.m. Only one qualified person (KA in the authors’ list) performed the lung function measurements. The Lilly heated pneumotachograph (MICRO 5000 spirometer) combined to the plethysmograph, was used
to measure LFD. Calibration of the expiratory flow rates (daily calibration with a 3-liter syringe) and plethysmograph (daily calibration according to the manufactory guideline) were performed according to the international recommendations ( 41, 42 ). LFD were measured/calculated according to the international recommendations for spirometry and plethysmography (41, 42 ).
Spirometry was carried out in the sitting position. The following spirometric data were measured: Forced Vital Capacity (FVC, L), Forced Expiratory Volume in one second (FEV1, L), and Maximal Mid-Expiratory Flow (MMEF, L/s). The FEV1/FVC ratio (absolute value) was calculated. The spirometric data were expressed at ‘‘body temperature, barometric pressure saturated with water vapor’’ (41 ). The FVC maneuver had three distinct phases: maximal inspiration, a blast of exhalation, and continued complete exhalation to the end of testing. Patients were verbally encouraged to continue exhaling air at the end of the maneuver to obtain optimal effort. The criterion for end of testing was a volume-time curve showing no change in volume (25 mL) for one second, despite the patient’s effort to exhale for at least six seconds. The FVC maneuver repeatability was considered acceptable when the differences between the largest and the next largest FVC or between the largest and the next largest FEV1 were < 0.150 L (if FVC > 1 L) or < 0.100 L (if FVC ≤ 1 L) (41 ).
The steps for performing plethysmography were previously described ( 43, 44 ). Slow Vital Capacity (L), expiratory reserve volume (L), and Functional Residual Capacity (FRC, L) were measured, and the following static lung volumes were calculated (43, 44 ): Residual Volume (RV, L), Total Lung Capacity (TLC, L), and inspiratory capacity (L). The RV/TLC ratio (absolute value) was calculated.
LFD were expressed in three ways: i) absolute values, ii) percentages of predicted values from the global lung initiative norms for spirometry and static lung volumes (44, 45, 46, 47 ), and iii) z-scores (44, 45, 46, 47 ). Any LFD’ z-score below the Lower Limit of Normal (LLN = -1.645) or above the Upper Limit of Normal (ULN = +1.645) was considered abnormal (48 ).
The following definitions of ventilatory impairments were applied (48, 49, 50, 51):
• OVI: FEV1/FVC z-score < LLN;
• RVI: TLC z-score < LLN;
• MVI: FEV1/FVC z-score < LLN and TLC z-score < LLN;
• NSVI: FVC z-score < LLN and z-score FEV1 < LLN and FEV1/FVC z-score ≥ LLN and TLC z-score ≥ LLN;
• Lung-hyperinflation: RV z-score > ULN
The following three-level system assessing the severity of lung function impairments (ie; OVI and RVI) using FEV1 z-score values was used (50): i) mild: z-score between -1.65 and -2.50; ii) moderate: z-score between -2.50 and -4.00; and iii) severe: z-score < - 4.00.
Sample size calculation and statistical analyses
The sample size was calculated using the following formula (52 ): N= [(Zα/2)2 x P x (1- P) x D]/E2; where “ P ” was the frequency of the main event of interest (ie; frequency of RVI in RA patients), “ E ” was the margin of error (= 6.5%), “ Z α/2 ” was the normal deviate for two- tailed alternative hypotheses at a level of significance (Z α/2 = 1.96 for a 95%CI) and “ D ” was the design (= 1 for simple random sampling). Given the pioneer character of this pilot study, “ P ” was determined from a previous North-African study (5 ), where the frequency of RVI was 7% (p = 0.07). The total sample size was 59 consecutive RA patients. Assumption of 45% for the non-inclusion- and exclusion- criteria gave a corrected total sample of 108 patients [108 = 59/ (1-0.45)].
To assess the normal distribution of quantitative data, the Kolmogorov-Smirnov normality test was used. Quantitative data were expressed as mean±standard-deviation (and 95% CIfor ACPA values) if they had a normal distribution, and they were then compared using the Student’s T test for independent samples. If not, quantitative data were expressed as median [interquartile], and they were then compared using the Mann Whitney U Test. Categorical data were expressed as number (%). The two-sided Chi-square test was used
to compare the categorical data of two groups (ACPA+ vs. ACPA-) or subgroups (ACPA+- FR+ vs. ACPA+-FR-). No comparison was made between the ACPA--FR+ and ACPA--FR- subgroups since the ACPA--FR- subgroup included only two patients (Figure 1). Hedge value of TLC z-score was used for measuring the effect size (53 ). The latter was described as small, medium, large, and very large if it was ≤ 0.2, around 0.5, around 0.8, and more than 1.30, respectively (53 ). All mathematical computations and statistical procedures were performed using statistical software (Statistica, version 12). Significance level was set at 0.05.
RESULTS
An initial sample of 108 RA patients was included. After application of the non- inclusion- and exclusion- criteria, only 59 (8 males) were included in the final sample (Figure 1): 26 (4 males) in the ACPA+ group, and 33 (4 males) in the ACPA- group.
Table 1 presents some characteristics of the RA patients. Compared to the ACPA- group, the ACPA+ group was ∼ 10-years younger, and included a significantly higher percentage of patients with dyspnea grade 0.
Table 1. Characteristicsofpatientswithrheumatoid arthritis(RA):Algiers(Algeria):2018-2019.
|
Data |
Outcome |
Unit/Category |
Total sample (n=59) |
ACPA - (n=33) |
ACPA + (n=26) |
p-value |
|
Sex |
(male) |
8 (13.6) |
4 (12.1) |
4 (15.4) |
0.7219 |
|
|
Age |
(year) |
59±14 |
63±13 |
53±12 |
0.0025 * |
|
|
Sex and anthropometric data |
Weight |
(kg) |
69±15 |
67±14 |
71±15 |
0.3244 |
|
Height |
(m) |
156±7 |
155±8 |
158±6 |
0.0897 |
|
|
Body mass index |
(kg/m2) |
28.1±5.7 |
27.9±6.0 |
28.2±5.5 |
0.8507 |
|
|
(underweight) |
5 (8.5) |
3 (9.1) |
2 (7.7) |
0.8480 |
||
|
(normal weight) |
9 (15.3) |
5 (15.2) |
4 (15.4) |
0.9831 |
||
|
Corpulence status |
(overweight) |
22 (37.3) |
10 (30.3) |
12 (46.2) |
0.2099 |
|
|
(obesity) |
23 (39.0) |
15 (45.5) |
8 (30.8) |
0.2505 |
||
|
Comorbidities and passive smoking |
Arterial-hypertension |
(yes) |
14 (23.7) |
8 (24.2) |
6 (23.1) |
0.9214 |
|
Mellitus-diabetes |
(yes) |
10 (16.9) |
5 (15.2) |
5 (19.2) |
0.6844 |
|
|
Heart diseases |
(yes) |
8 (13.6) |
5 (15.2) |
3 (11.5) |
0.6803 |
|
|
Passive smoking |
(yes) |
11 (18.6) |
7 (21.2) |
4 (15.4) |
0.5701 |
|
|
Cough |
(yes) |
49 (83.1) |
28 (87.9) |
21 (80.8) |
0.4511 |
|
|
(grade 0) |
4 (6.8) |
0 (0.0) |
4 (15.4) |
0.0195 * |
||
|
(grade 1) |
22 (37.3) |
12 (36.4) |
10 (38.5) |
0.8685 |
||
|
Respiratory signs |
Dyspnea (modified medical research council) |
(grade 2) |
18 (30.5) |
13 (39.4) |
5 (19.2) |
0.0943 |
|
(grade 3) |
14 (23.7) |
8 (24.2) |
6 (23.1) |
0.9214 |
||
|
(grade 4) |
1 (1.7) |
0 (0.0) |
1 (3.8) |
0.2588 |
||
|
(score) |
1.8±1.0 |
1.9±0.8 |
1.6±1.1 |
0.2959 |
||
|
Duration of signs before RA diagnosis |
(months) |
137±173 |
106±160 |
176±183 |
0.1261 |
|
|
Extra-respiratory signs and symptoms |
Raynaud's phenomenon |
(yes) |
29 (49.2) |
15 (45.5) |
14 (53.8) |
0.5267 |
|
Morning stiffness |
(yes) |
51 (86.4) |
29 (87.9) |
22 (84.6) |
0.4511 |
|
|
Duration of signs before RA diagnosis |
(months) |
118±144 |
90±145 |
154±138 |
0.0944 |
|
|
RA activity |
DAS28-CRP |
(absolute value) |
6.00±1.02 |
6.04±0.86 |
5.94±1.21 |
0.7279 |
|
Disease activity status |
(severe) |
48 (81.4) |
28 (84.8) |
20 (76.9) |
0.4396 |
|
|
(moderate) |
11 (18.6) |
5 (15.2) |
6 (23.1) |
0.4396 |
ACPA: Anti-Citrullinated Peptides Antibodies. CRP: C-Reactive Protein. DAS28: Disease Activity Score 28. Quantitative and categorical data were mean±SD and number (%). *p-value < .05: 2 sided Chi-square test (comparison of categorical data between the 2 groups). Student's T test (comparison of quantitative data between the 2 groups).
Table 2 presents the immunological, biological, hematological, and radiological data of RA patients. The ACPA+ and ACPA- groups had comparative RF, ANA, biological, and hematological data, and comparative biological and hematological data/profiles. Compared to the ACPA- group, the ACPA+ group had a significantly higher value of ACPA [mean±standard-deviation (95%CI): 1.4±0.7 (1.2 to 1.7) vs. 133.1±247.7 (33.1 to 233.2), p=0.003, respectively], included lower percentages of patients RF+, or with a light RF level. The two groups had comparative radiological data. No patient has radiological signs of emphysema.
Table2.Immunological,biological,hematological,andradiologicaldataandprofileofpatientswithrheumatoidarthritis(RA):Algiers(Algeria):2018-2019.
|
Data |
Outcome |
Unit/Category |
Total sample (n=59) |
ACPA - (n=33) |
ACPA + (n=26) |
p-value |
|
Immunological data and profile |
ACPA |
(U/ml) |
59±175 |
1±1 |
133±248 |
0.0034 * |
|
ANA cut-off (titer) |
≥1/160(%) |
161±238 |
128±161 |
202±308 |
0.2453 |
|
|
Rheumatoid factor (RF) |
(IU/ml) |
85±124 |
62±91 |
114±154 |
0.1122 |
|
|
RF subgroups |
Negative |
13 (22.0) |
2 (6.1) |
11 (42.3) |
0.009 * |
|
|
Positive |
46 (78.0) |
31 (93.9) |
15 (57.7) |
0.009 * |
||
|
RF levels |
Light |
22 (37.3) |
18 (54.5) |
4 (15.4) |
0.0020 * |
|
|
Moderate |
11 (18.6) |
9 (27.3) |
2 (7.7) |
0.0551 |
||
|
High |
7 (11.9) |
2 (6.1) |
5 (19.2) |
0.1225 |
||
|
Very high |
6 (10.2) |
2 (6.1) |
4 (15.4) |
0.2412 |
||
|
Biological data and profile |
ESR (1st hour) |
(mm) |
52±33 |
51±30 |
54±37 |
0.6831 |
|
C-reactive protein |
(mg/L) |
28±43 |
21±33 |
36±51 |
0.1640 |
|
|
High ESR |
(yes) |
15 (25.4) |
8 (24.2) |
7 (26.9) |
0.8130 |
|
|
High C-reactive protein |
(yes) |
28 (47.5) |
13 (39.4) |
15 (57.7) |
0.1623 |
|
|
Biological inflammatory syndrome |
(yes) |
48 (81.4) |
25 (75.8) |
23 (88.5) |
0.2204 |
|
|
Hemoglobin |
(g/L) |
12±2 |
12±2 |
12±2 |
0.9362 |
|
|
Hematological data and profile |
White blood cells |
(/mm3) |
7810±2682 |
7953±2551 |
7627±2881 |
0.6469 |
|
Polynuclear neutrophils |
(/mm3) |
4657±1916 |
4796±1939 |
4481±1910 |
0.5352 |
|
|
Polynuclear eosinophils |
(/mm3) |
258±543 |
189±139 |
346±804 |
0.2746 |
|
|
Lymphocytes |
(/mm3) |
2272±948 |
2304±846 |
2231±1080 |
0.7733 |
|
|
Monocytes |
(/mm3) |
623±244 |
665±257 |
570±219 |
0.1373 |
|
|
Anemia |
(yes) |
22 (37.3) |
13 (39.4) |
9 (34.6) |
0.7050 |
|
|
Leukocytosis |
(yes) |
8 (13.6) |
5 (15.2) |
3 (11.5) |
0.6803 |
|
|
Bronchiectasis |
(yes) |
16 (27.1) |
7 (21.2) |
9 (34.6) |
0.2503 |
|
|
Radiological data |
Bronchial wall thickening |
(yes) |
2 (3.4) |
0 (0.0) |
2 (7.7) |
0.1048 |
|
Thickened septal/non-septal lines |
(yes) |
45 (76.3) |
23 (69.7) |
12 (46.2) |
0.0681 |
|
|
Interstitial lung disease |
(yes) |
43 (72.9) |
27 (81.8) |
16 (61.5) |
0.0817 |
|
|
Nonspecific interstitial pneumonia |
(yes) |
32 (54.2) |
19 (57.6) |
13 (50.0) |
0.5607 |
|
|
Bronchiolitis |
(yes) |
18 (30.5) |
8 (24.2) |
10 (38.5) |
0.2362 |
|
|
Ground glass attenuation |
(yes) |
34 (57.6) |
20 (60.6) |
14 (53.8) |
0.5998 |
|
|
Usual interstitial pneumonia |
(yes) |
9 (15.3) |
6 (18.2) |
3 (11.5) |
0.4772 |
|
|
Micro nodule |
(yes) |
35 (59.3) |
19 (57.6) |
16 (61.5) |
0.7621 |
|
|
Air space consolidation |
(yes) |
32 (54.2) |
19 (57.6) |
13 (50.0) |
0.5607 |
ACPA: Anti-Citrullinated Peptides Antibodies. ANA: Anti-Nuclear Antibody. ESR: Erythrocyte Sedimentation Rate. Quantitative and categorical data were mean±SD and number (%), respectively. *p-value < 0.05: 2 sided Chi-square test (comparison of categorical data between the 2 groups) Student's T test (comparison of quantitative data between the 2 groups).
Table 3 presents the LFD of RA patients. When LFD were expressed as z-scores, compared to the ACPA- group, the ACPA+ one had significantly lower MMEF and FEV1/FVC ratio, and significantly higher values of FRC, RV, TLC, and RV/TLC. When LFD were expressed as percentage of predicted values, compared to the ACPA- group, the ACPA+ one had significantly higher values of FRC, RV, TLC, and RV/TLC, and a significantly lower FEV1/FVC ratio. The TLC z-score effect size was medium (Hedges’ unbiased d = -0.442).
Table3.Lungfunctiondataofpatientswithrheumatoidarthritis(RA):Algiers(Algeria):2018-2019.
|
Data |
Outcome |
Unit |
Total sample (n=59) |
ACPA - (n=33) |
ACPA + (n=26) |
p-value |
|
FVC |
L |
2.20±0.80 |
1.99±0.74 |
2.46±0.81 |
0.0260 * |
|
|
Spirometric data |
% |
73±23 |
71±23 |
76±23 |
0.3837 |
|
|
z-score |
-1.91±1.61 |
-1.95±1.53 |
-1.86±1.73 |
0.8386 |
||
|
FEV1 |
L |
1.70±0.69 |
1.63±0.63 |
1.80±0.76 |
0.3470 |
|
|
% |
72±26 |
73±24 |
70±30 |
0.7308 |
||
|
z-score |
-1.92±1.80 |
-1.72±1.48 |
-2.17±2.14 |
0.3419 |
||
|
MMEF |
L/s |
2.39±1.13 |
2.44±1.04 |
2.32±1.26 |
0.7044 |
|
|
% |
109±51 |
118±43 |
98±59 |
0.1396 |
||
|
z-score |
-0.06±1.61 |
0.33±1.13 |
-0.56±1.98 |
0.0349 * |
||
|
SVC |
L |
2.30±0.85 |
2.07±0.74 |
2.58±0.89 |
0.0200 * |
|
|
% |
71±22 |
69±21 |
74±22 |
0.3723 |
||
|
z-score |
-2.25±1.68 |
-2.40±1.64 |
-2.06±1.75 |
0.4432 |
||
|
Static lung volumes |
FRC |
L |
3.03±1.22 |
2.60±0.73 |
3.57±1.50 |
0.0018 * |
|
% |
123±51 |
108±39 |
141±59 |
0.0114 * |
||
|
z-score |
0.81±1.87 |
0.25±1.47 |
1.52±2.10 |
0.0083 * |
||
|
RV |
L |
2.24±1.15 |
1.86±0.73 |
2.73±1.40 |
0.0032 * |
|
|
% |
150±96 |
117±51 |
192±121 |
0.0021 * |
||
|
z-score |
1.18±2.03 |
0.43±1.47 |
2.13±2.27 |
0.0010 * |
||
|
TLC |
L |
4.51±1.46 |
3.90±0.91 |
5.27±1.67 |
0.0002 * |
|
|
% |
94±28 |
85±24 |
106±30 |
0.0048 * |
||
|
z-score |
-0.56±2.21 |
-1.26±1.81 |
0.33±2.37 |
0.0052 * |
||
|
IC |
L |
1.53±0.54 |
1.38±0.53 |
1.72±0.50 |
0.0146 * |
|
|
% |
67±23 |
65±25 |
71±19 |
0.3152 |
||
|
z-score |
-1.69±1.17 |
-1.80±1.28 |
-1.54±1.01 |
0.4081 |
||
|
FEV1/FVC |
AV |
0.77±0.13 |
0.81±0.10 |
0.72±0.14 |
0.0055 * |
|
|
Ratios |
% |
97±17 |
103±13 |
90±18 |
0.0032 * |
|
|
z-score |
-0.23±1.67 |
0.35±1.30 |
-0.97±1.83 |
0.0019 * |
||
|
RV/TLC |
AV |
48.89±13.00 |
47.82±13.14 |
50.25±12.94 |
0.4810 |
|
|
% |
153±51 |
137±34 |
174±61 |
0.0045 * |
||
|
z-score |
2.22±1.90 |
1.67±1.56 |
2.92±2.09 |
0.0114 * |
ACPA: Anti-Citrullinated Peptides Antibodies. AV: Absolute Value. FEV 1: Forced Expiratory Volume in one second. FRC: Functional Residual Capacity. FVC: Forced Vital Capacity. IC: Inspiratory Capacity. MMEF: Maximal-Mid Expiratory Flow. RV: Residual Volume. SVC: Slow Vital Capacity. TLC: Total Lung Capacity. %: percent of predicted values. Data were mean±SD. * p-value < 0.05: Student's T test (comparison between the 2 groups).
Table 4 presents the patients’ spirometric/plethysmographic profiles of RA patients. Compared to the ACPA- group, the ACPA+ group included higher percentages of patients with i) low MMEF (3.0 vs. 30.8%, respectively), ii) high FRC (9.1 vs. 38.5%, respectively),
Table4.Spirometricand plethysmographicprofilesofpatientswithrheumatoidarthritis(RA):Algiers(Algeria):2018-2019.
|
Data |
Outcome |
Total sample(n=59) |
ACPA - (n=33) |
ACPA + (n=26) |
p-value |
|
FVC |
33 (55.9) |
18 (54.5) |
15 (57.7) |
.8058 |
|
|
FEV1 |
32 (54.2) |
16 (48.5) |
16 (61.5) |
.3197 |
|
|
FEV1/FVC (OVI) |
13 (22.0) |
3 (9.1) |
10 (38.5) |
.0069 * |
|
|
z-score < -1.645 |
MMEF |
9 (15.3) |
1 (3.0) |
8 (30.8) |
.032 * |
|
SVC |
38 (64.4) |
21 (63.6) |
17 (65.4) |
.8860 |
|
|
FRC |
2 (3.4) |
2 (6.1) |
0 (0.0) |
.2000 |
|
|
RV |
2 (3.4) |
2 (6.1) |
0 (0.0) |
.2000 |
|
|
IC |
30 (50.8) |
18 (54.5) |
12 (46.2) |
.5267 |
|
|
TLC (RVI) |
21 (35.6) |
15 (45.5) |
6 (23.1) |
.0745 |
|
|
RV (lung-hyperinflation) |
14 (23.7) |
3 (9.1) |
11 (42.3) |
.0029 * |
|
|
TLC |
9 (15.3) |
3 (9.1) |
6 (23.1) |
.1377 |
|
|
z-score > +1.645 |
FRC |
13 (22.0) |
3 (9.1) |
10 (38.5) |
.0069 * |
|
IC |
0 (0.0) |
0 (0.0) |
0 (0.0) |
- |
|
|
RV/TLC |
33 (55.9) |
17 (51.5) |
16 (61.5) |
.4424 |
|
|
Ventilatory impairments |
Mixed ventilatory impairment |
2 (3.4) |
1 (3.0) |
1 (3.8) |
.8654 |
|
Nonspecific ventilatory impairment |
4 (6.8) |
2 (6.1) |
2 (7.7) |
.8086 |
|
|
Classifications of OVI |
Moderate |
8 (13.6) |
2 (6.1) |
6 (23.1) |
0.0585 |
|
Severe |
5 (8.5) |
1 (3.0) |
4 (15.4) |
0.0894 |
|
|
Classifications of RVI |
Mild |
8 (13.6) |
7 (21.2) |
1 (3.8) |
0.0524 |
|
Moderate |
13 (22.0) |
8 (24.2) |
5 (19.2) |
0.3226 |
|
OVI: Obstructive Ventilatory Impairment. RVI: Restrictive Ventilatory Impairment. For the remaining abbreviations, see table 3. Data were number (%). * p-value < 0.05: 2 sided Chi-square test: comparison between the 2 groups. Notes: 1. ACPA - group: among the 3 subjects with an OVI, 1 had lung-hyperinflation, and among the 3 subjects with lung-hyperinflation, 1 had an OVI. 2. ACPA + group: among the 10 subjects with an OVI, 9 had lung-hyperinflation, and among the 11 subjects with lung-hyperinflation, 9 had an OVI
iii) OVI (9.1 vs. 38.5%, respectively), and iv) lung-hyperinflation (9.1 vs. 42.3%, respectively). The two groups included comparative percentages of patients i) with low FVC,
FEV1, SVC, FRC, RV, and IC; ii) with high TLC, IC and RV/TLC; iii) with RVI, MVI, and NSVI; and iv) divided according to the severity of OVI and RVI.
Table 5 llustrates the lung function and radiological data of the ACPA+-FR+ and ACPA+-FR- subgroups. Whatever the expression mode of the LFD (ie; L, %, z-score), the two groups had comparative FVC, FEV1, MMEF, SVC, IC, and RV/TLC. Compared to the ACPA+-FR- subgroup, the ACPA+-FR+ subgroup had significantly higher FRC (z-score and
Table 5.Lung function and radiological data of patients with rheumatoid arthritis ACPA+(n=26)divided according to the rheumatoid factor (RF) status): Algiers (Algeria):2018-2019.
|
Data |
Outcome |
Unit/Category |
RF - (n=11) |
RF + (n=15) |
p-value |
|
FVC |
L |
2.35±0.80 |
2.53±0.84 |
.5760 |
|
|
% |
75±23 |
77±23 |
.8298 |
||
|
z-score |
-1.89±1.68 |
-1.85±1.82 |
.9525 |
||
|
FEV1 |
L |
1.83±0.69 |
1.78±0.83 |
.8694 |
|
|
% |
75±30 |
67±30 |
.4998 |
||
|
z-score |
-1.76±2.07 |
-2.47±2.20 |
.4166 |
||
|
MMEF |
L/s |
2.55±1.32 |
2.15±1.22 |
.4309 |
|
|
% |
116±66 |
84±51 |
.1750 |
||
|
z-score |
0.09±1.69 |
-1.03±2.10 |
.1571 |
||
|
SVC |
L |
2.46±0.93 |
2.67±0.88 |
.5715 |
|
|
% |
73±25 |
75±21 |
.7860 |
||
|
Lung function data |
z-score |
-2.16±1.91 |
-1.99±1.69 |
.8108 |
|
|
FRC |
L |
2.99±1.52 |
4.00±1.37 |
.0888 |
|
|
% |
115±47 |
161±60 |
.0443 * |
||
|
z-score |
0.44±1.82 |
2.30±1.98 |
.0218 * |
||
|
RV |
L |
2.23±1.27 |
3.09±1.41 |
.1220 |
|
|
% |
137±62 |
233±139 |
.0423 * |
||
|
z-score |
1.12±1.88 |
2.88±2.30 |
.0490 * |
||
|
TLC |
L |
4.62±1.86 |
5.74±1.39 |
.0916 |
|
|
% |
91±27 |
117±27 |
.0271 * |
||
|
z-score |
-0.88±2.29 |
1.22±2.08 |
.0224 * |
||
|
IC |
L |
1.67±0.54 |
1.76±0.49 |
.6746 |
|
|
% |
69±22 |
72±18 |
.7144 |
||
|
z-score |
-1.63±1.13 |
-1.48±0.94 |
.7048 |
||
|
FEV1/FVC |
AV |
0.79±0.13 |
0.68±0.13 |
.0424 * |
|
|
% |
99±17 |
83±17 |
.0292 * |
||
|
z-score |
-0.00±1.74 |
-1.68±1.59 |
.0178 * |
||
|
RV/TLC |
AV |
47.09±8.54 |
52.56±15.28 |
.2960 |
|
|
% |
151±37 |
191±70 |
.1029 |
||
|
z-score |
2.25±1.63 |
3.40±2.30 |
.1696 |
||
|
Bronchiectasis |
(yes) |
3 (27.3) |
6 (40.0) |
.5013 |
|
|
Bronchial wall thickening |
(yes) |
1 (9.1) |
1 (6.7) |
.8207 |
|
|
Thickened septal/non- septal lines |
(yes) |
6 (54.5) |
6 (40.0) |
.4637 |
|
|
Radiological data |
|||||
|
Interstitial lung disease |
(yes) |
9 (81.8) |
7 (46.7) |
.0691 |
|
|
Nonspecific interstitial pneumonia |
(yes) |
8 (72.7) |
5 (33.3) |
.0471 * |
|
|
Bronchiolitis |
(yes) |
6 (54.5) |
4 (26.7) |
.1500 |
|
|
Ground glass attenuation |
(yes) |
7 (63.6) |
7 (46.7) |
.3931 |
|
|
Usual interstitial pneumonia |
(yes) |
2 (18.2) |
1 (6.7) |
.3650 |
|
|
Micro nodule |
(yes) |
7 (63.6) |
9 (60.0) |
.8521 |
|
|
Air space consolidation |
(yes) |
6 (54.5) |
7 (46.7) |
.6943 |
ACPA: Anti-Citrullinated Peptides Antibodies. AV: Absolute Value. FEV 1: Forced Expiratory Volume in one second. FRC: Functional Residual Capacity. FVC: Forced Vital Capacity. IC: Inspiratory Capacity. MMEF: Maximal-Mid Expiratory Flow. RV: Residual Volume. SVC: Slow Vital Capacity. TLC: Total Lung Capacity. %: percent of predicted values. Quantitative and categorical data were mean±SD and number (%), respectively. * p-value < 0.05: 2 sided Chi-square test (comparison of categorical data between the 2 groups) Student's T test (comparison of quantitative data between the 2 groups).
%), RV (z-score and %), and TLC (z-score and %), and a significantly lower FEV1/FVC (absolute value, z-score, %). Compared to the ACPA+-FR- subgroup, the ACPA+-FR+ subgroup included lower percentages of patients with nonspecific interstitial pneumonia (72.7 vs. 33.3%, respectively).
DISCUSSION
The main results of the present comparative North-African pilot study were that compared to the ACPA- group (n=33), the ACPA+ group (n=26) included i) higher percentages of patients with OVI (9.1 vs. 38.5%, respectively) or lung-hyperinflation (9.1 vs. 42.3%, respectively), and ii) comparative percentages of patients with RVI, MVI, and NSVI. RA is a heterogeneous condition (11 ). The subdivision of RA by autoantibody status, conservatively RF and recently ACPA, has facilitated better understanding of possible pathogenic mechanisms in the genesis of RA (11 ). This is especially the case for ACPA+ RA, where RA initiating locations distant from the joints have been proposed within the gastrointestinal and respiratory systems (11 ). ACPAs are an interesting biomarker in RA (11 ). ACPA+ patients differ from ACPA- ones in terms of pathogenesis, disease course, and response to treatment (11, 12, 13 ). ACPA can develop before the onset of clinically detectable RA, and the lung can be an early site of autoimmune damage and ACPA production in the nonexistence of any clinical signs of the joint (17 ). Higher ACPA levels in bronchial alveolar lavage fluid have been reported compared with serum from ACPA+ patients, demonstrating that the lung is a local site of ACPA production (54 ). In addition to its higher prevalence in smokers (55 ), ACPA higher prevalence is also recognized in RA-related lung diseases, including patients with RA and bronchiectasis, a condition with a high prevalence of never smokers (56 ). On high-resolution CT, three commonly reported changes identified of RA populations are RA-bronchiectasis, RA-ILD, and RA-obstructive disease of smaller airways (57 ). To the best of the authors’ knowledge, this is the first study comparing the spirometric/plethysmographic data/profiles of RA patients divided according to their ACPA
status.
Spirometric and plethysmographic profiles of RA patients
Extra-articular manifestations of RA are frequent and dominated by pulmonary involvement (3 ). In this study including non-smokers RA patients free from asthma and
COPD, the most frequent spirometric/plethysmographic impairments were RVI (35.6%), lung-hyperinflation (23.7%), OVI (22.0%), NSVI (6.8%), and MVI (3.4%) (Table 4). According to the literature, the frequencies of spirometric/plethysmographic impairments vary largely from one study to another ( 5, 9, 10): RVI [from 5.8% to 22%], lung-hyperinflation
[3.4%], OVI [from 9% to 23.1%], NSVI [2.8%], and MVI [from 1% to 26.9%]. The divergence in the reported frequencies of spirometric/plethysmographic impairments can be explained
by the divergence of the patients’ profiles in terms of age, sex, smoking status, and corpulence status, presence of comorbidities such as asthma and/or COPD, RA
characteristics in terms of duration, stage, whether receiving treatment or not, different RF status (RF+ vs. RF-), patients’ recruitment mode (population-based setting (54 ) vs. rheumatology clinic), and mainly the applied criteria to retain the spirometric
plethysmographic impairments [for example LFD < 80% (58 ) vs. < LLN (present study)]. In the context of the hypothesis that RA-related autoimmunity is initiated at an extra-articular site (59 ), the high frequencies of some spirometric/plethysmographic impairments may strengthen the hypothesis that RA-related autoimmunity RA is initially generated in the lungs (17 ).
Spirometric and plethysmographic profiles of ACPA+ and ACPA- patients
In this study, the ACPA+ and ACPA- groups have comparative percentages of patients with RVI (main outcome), MVI and NSVI (secondary outcomes), and lung-hyperinflation and OVI (secondary outcomes) occur more frequently in patients with ACPA+ (Table 4). On the one hand, our results are “inconsistent” with those reported in a population-based study reporting no differences in the distribution of airway impairments between ACPA+ and ACPA- patients (54 ). On the other hand, our findings are “closer” to those reported by some authors (17, 60, 61 ). First, Huang et al. (60 ) investigated whether RA-related autoantibodies are associated with lung function impairments. RA serostatus was assessed by research assays for Cyclic Citrullinated Peptide (CCP) and RF (60 ). Among the 1272 analyzed patients
(mean age: 56.3±14.1 years, 17.8% were males, 69.5% were seropositive), 100 patients (7.9%) present lung function impairment (60 ). Compared with seronegativity, seropositivity is associated with increased odds of any lung function impairment (odds-ratio: 2.29, 95% CI: 1.30-4.03). When analyzing the type of lung function impairment, seropositivity was also associated with RVI (odds-ratio: 2.48; 95%CI: 1.26-4.87), OVI (odds-ratio: 3.12; 95%CI: 1.28-7.61), and diffusion impairment (odds-ratio: 2.30; 95%CI: 1.09-4.83) (60 ). When analyzing by CCP status, the associations were stronger for CCP+ (any lung function impairment odds-ratio: 1.67, 95%CI 1.03-2.69 for CCP+ vs. CCP−) (60 ). Huang et al. (60 ) concluded that CCP+ patients have a two-fold increased risk for impairments on lung function tests compared with CCP- patients. Second, Demoruelle et al. (17 ) reported the presence of subclinical lung impairments at high-resolution CT in more than 70% of ACPA+ patients without evidence of arthritis. The most frequent impairments are airways alterations, such as bronchial wall thickening and air trapping, with a minority of parenchymal alterations (17 ). Third, Fischer et al. (61 ) reported a prevalence of 54% of airways impairments, 14% of ILD, and 26% of a combination of both in ACPA+ patients without arthritis and with respiratory complaints.
In our study, the ACPA- and ACPA+ groups included comparable percentages of patients with the main outcome (frequency of a RVI: 45.5 vs. 23.1%, p = 0.0745) (Table 4). This result is “expected” since the ACPA- and ACPA+ groups included comparable percentage of patients with ILD (81.8 vs. 61.5%, p = 0.0817). However, the latter radiological finding is “unexpected”. In patients with RA-ILD, while one small study reported no association with ACPA+ (62 ), another study identified a strong relationship with ACPA+ and RF+ in 94% and 89% of patients (63 ). Moreover, a cohort including patients with early-RA and patients with longstanding-RA reported a higher frequency of both interstitial and airway diseases, with the latter being more noticeable in longstanding-RA (64 ). Finally, a study including 60 patients with early-RA concluded that the RF and ILD are linked (65 ).
In our study, compared to the ACPA- group, the ACPA+ group included a higher percentage of patients with lung-hyperinflation (9.1 vs. 42.3%, p=0.009, respectively) (Table 4). Lung-hyperinflation is a chief concern in the management of some chronic respiratory conditions (66 ). It leads to a rise in the relaxation volume due to the reduction of the lung’ elastic retraction forces (67 ). Lung-hyperinflation has deleterious clinical, functional, and radiological consequences that make it a major source of impaired quality of life ( 67, 68). In our ACPA+ group, the presence of lung-hyperinflation can explain the higher percentage of patients with dyspnea grade 0 (breathless with strenuous exercise) (68 ). Lung- hyperinflation can be understood as an aging index of the ventilatory mechanics, or an indirect sign towards an OVI and/or an expiratory muscle weakness (66 ). Lung- hyperinflation can also indirectly reflects damage to the distal airways (69 ). In the present, the last hypothesis is plausible since compared to the ACPA- group, the ACPA+ group included a higher percentage of patients with low MMEF (3.0 vs. 30.8%, respectively) (Table 4). MMEF is considered more sensitive (but nonspecific) in detecting early airway obstruction which tends to take place at lower lung volumes (70 ). Since lung-hyperinflation (eg, higher RV) can be observed in patients with expiratory muscle weakness, it was better to evaluate the maximal expiratory pressure (71 ).
Compared to the ACPA- group, the ACPA+ group included a higher percentage of patients with OVI (9.1 vs. 38.5%, p=0.009, respectively). This result is “unexpected” and some remarks should be noted. First, since the two groups had comparative inflammatory data/profile (Table 2), therefore the hypothetic relationship between inflammation and airway obstruction cannot be advanced in our study (72 ). Second, since the two groups of patients were no smokers, and included comparative percentages of passive smokers (Table 1), the role of smoking in the pathogenesis of OVI in the ACPA+ group cannot be forwarded. Concerning smoking, there is wide literature concerning its potential role in the pathogenesis of ACPA+ (11 ). Because of the robust relationship between smoking and
ACPA’ pathogenesis, the lung has become the attention of investigation to decide whether processes within the lung are connected to the generation of ACPA (11 ). Smoking was identified as an environmental factor that in the context of human leukocyte antigen-D related isotype shared epitope genes may trigger RA-specific immune reactions to citrullinated proteins (13 ). In one animal study (73 ), ACPA levels were lower in mice exposed to cigarette smoke. In another study (74 ), no patient with COPD has a carrier of ACPA, suggesting that smoking is not directly responsible for triggering ACPA production. Third, since no patient from the two groups were asthmatic and/or COPD, the presence of OVI in the ACPA+ group cannot be linked to these two obstructive lung diseases. However, the high frequency of OVI observed in the ACPA+ group compared to the ACPA- group is “similar” to the high frequency of COPD or asthma in ACPA+ (8, 75 ). Zaccardelli et al. (8 ) reported that i) 17.7% of pre-ACPA+ cases and 6.3% of matched controls reported physician-diagnosed asthma, ii) After adjusting for matching factors, smoking pack-years, passive smoking, and BMI, asthma remains significantly associated with pre-ACPA+ [odds- ratio: 3.57, 95%CI: 1.58-8.04], and iii) Asthma is significantly associated with seropositive RA (odds-ratio: 1.79, 95%CI: 1.01-3.18). Moreover, Zaccardelli et al. (75 ) analyzed 283 females with pre-RA (before RA diagnosis) and 842 controls, and reported that i) Pre-ACPA+ is associated with increased COPD risk [hazard-ratio: 3.04, 95%CI: 1.33-7.00] after adjusting for covariates, including smoking pack-years, and ii) Pre-ACPA+ has a hazard- ratio for asthma of 1.74 (95%CI: 0.72-4.24). The authors concluded that “females with elevated ACPA before RA diagnosis have increased risk for developing COPD compared to controls, and that females who later develop RA are more likely to develop asthma, regardless of pre-ACPA status” (75 ). Another study identified an association between ACPA and bronchial wall thickening on chest CT, suggesting that structural airway abnormalities may be present early in the course of RA, even though the patients are asymptomatic or have few respiratory symptoms (76 ). Finally, in 60 patients with early RA, the presence of
ACPAs is associated with bronchial wall thickening (65 ). One possible explanation of the OVI observed in the ACPA+ group could be the presence of an oxidative stress (77 ). One study reported that autoreactivity to carbamylated and acetylated epitopes is more commonly found within RA patients with citrulline-reactivity (77 ). NSVI is an old ventilatory impairment described in 1972 in a study involving asymptomatic asthmatic patients (78 ). The NSVI can reflect reduced effort, a RVI, or be an early consequence of small airway condition with emphysema and/or air trapping (79 ). The NSVI has been titled “preserved ratio-impaired spirometry” which, in follow-up has been shown to be associated with both more typical RVI or OVI (80, 81, 82 ). The NSVI occurs in 9.5%90 to 15% (83 ) of patients with complete lung function tests. The two frequent etiologies of NSVI are airway hyperreactivity (68%) and/or obesity (50%), and RA is identified only in 1% of patients (84 ). Since the two groups of ACPA+ and ACPA- have comparative corpulence status (Table 1), obesity cannot be advanced to explain the presence of NSVI. The latter may be secondary to changes, such as bronchial inflammation, bronchoconstriction, or mucus accumulation.
Compared to the ACPA+-FR- subgroup, the ACPA+-FR+ subgroup included lower
percentages of patients with nonspecific interstitial pneumonia and comparable frequencies of patients with ILD or bronchiectasis (Table 5). Our finding is opposite to the evidence suggesting an association between ACPA+, RF+ and RA-ILD (11 ). Perry et al. (11 ) identified that 94% and 97% of RA-bronchiectasis patients were ACPA+ and RF+, respectively. The aforementioned frequencies were significantly higher than would normally be seen in patients with RA alone (56).
Study limitations
This study has some limitations. First, our sample size (n=59) “appears” small. However, the sample size was calculated according to a predictive equation (52 ). Calculation of an optimal size is a crucial point since it helps avoid an inadequate power to detect statistical effects (85 ). On the one hand, using few participants in a study may lead
to lower "precision" in findings. On the other hand, a large sample size is, however, expensive and exposes more participants to procedures (85 ). Second, as the sample size was calculated only for the primary outcome measure (frequency of a RVI), our statements that there was difference or no difference between ACPA- and ACPA+ groups for the secondary outcomes (frequencies of lung hyperinflation, OVI, MVI, or NSVI) should be interpreted with a caution, as this could be a consequence of the low power of the study. Third, it was better to include two groups matched for age, since age is a predictor factor of adults’ LFD (86 ). To counterbalance this limitation, LFD were expressed as z-scores ( 45, 46). z-scores are free from bias due to age, height, sex and ethnic group ( 45, 46 ). Fourth, it could have been better to explore the alveolar-capillary diffusion capacity (eg; diffusing capacity for carbon monoxide), the submaximal aerobic capacity via the 6-minute walk test for example, and the oxidant/antioxidant status of the RA patients. Fifth, our study’ one- center character did not allow us to generalize our findings. Moreover, since our patients were selected from those referred to a pulmonology service, this will confound the results, as the patients studied will not be representative of all RA patients. Sixth, it could have been better if a bronchodilator test was performed. The latter would made possible to distinguish between true MVI (or NSVI) and RVI due to the eventual reduction of static lung volumes in terms of air trapping (48 ). Seventh, we have compared the subgroups according to RF serology status, but the study was not powered for these comparisons. Therefore, conclusion related to the RF status must be considered with caution. Finally, it was better to
evaluate if the ventilatory impairments reported in our study have a clinically meaningful, for
example; do they influence patients’ symptoms or walk distance or quality of life? For future studies, it will be interesting to evaluate the correlation between LFD and quality of life, evaluated via an Arabic validated short questionnaire such as the VQ11 (87 ), of ACPA+ and ACPA- patients (24 ).
CONCLUSION
Our study revealed significantly higher risks of occurrence of lung-hyperinflation and OVI in patients with ACPA+, but comparable risk of occurrence of RVI, MVI, and NSVI in both ACPA- and ACPA+ groups. Our study “confirms” the benefit of early screening for lung- hyperinflation and OVI in patients with ACPA+, and testing for ACPA in non-smokers patients with OVI and/or lung-hyperinflation, particularly in those with clinical signs suggestive of RA. This attitude could improve the management of RA patients and some patients with lung- hyperinflation and/or OVI. There is a significant clinical implication of our findings, since physicians would treat isolated lung-hyperinflation seen on lung function tests especially in dyspneic patients. Lung-hyperinflation is a “natural” target for bronchodilators, and it is by decreasing it that bronchodilators can reduce the dyspnea (66 ).
References
- Usenbo Anthony, Kramer Veronika, Young Taryn, Musekiwa Alfred. PLOS ONE. 8. Vol. 10. Public Library of Science (PLoS); 2015. Prevalence of Arthritis in Africa: A Systematic Review and Meta-Analysis; pp. e0133858–e0133858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvien T K, Uhlig T, Ødegård S, Heiberg M S. Annals of the New York Academy of Sciences. 1. Vol. 1069. Wiley; 2006. Epidemiological Aspects of Rheumatoid Arthritis: The Sex Ratio; pp. 212–222. [DOI] [PubMed] [Google Scholar]
- Bongartz Tim, Nannini Carlotta, Medina-Velasquez Yimy F, Achenbach Sara J, Crowson Cynthia S, Ryu Jay H, Vassallo Robert, Gabriel Sherine E, Matteson Eric L. Arthritis & Rheumatism. 6. Vol. 62. Wiley; 2010. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: A population-based study; pp. 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Yoshitaka, Ito Satoru, Matsuda Toshiaki, Iwano Shingo, Uchida Akemi, Takahashi Nobunori, Kojima Toshihisa, Wakahara Keiko, Yamaguchi Etsuro, Hasegawa Yoshinori. Respiratory Physiology & Neurobiology. Vol. 261. Elsevier BV; 2019. Longitudinal changes in pulmonary function and respiratory impedance of rheumatoid arthritis; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- Ben Fredj H, Saad Ben, Mhaouech H, Bouajina N, Tabka I, Rouatbi Z, S. Pulmonary function in rheumatoid arthritis in a Tunisian population. Tunis Med. 2013;91(4):248–253. [PubMed] [Google Scholar]
- Agrawal Abhinav, Thyagarajan Braghadheeswar, Ceniza Sidney, Hasan Yusuf Syed. Case Reports in Pulmonology. Vol. 2015. Hindawi Limited; 2015. Interstitial Lung Disease of the UIP Variant as the Only Presenting Symptom of Rheumatoid Arthritis; pp. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisco Lauren, Moll Matthew, Wang Jiaqi, Hobbs Brian D, Huang Weixing, Martin Lily W, Kronzer Vanessa L, Huang Sicong, Silverman Edwin K, Doyle Tracy J, Cho Michael H, Sparks Jeffrey A. Arthritis & Rheumatology. 11. Vol. 73. Wiley; 2021. Relationship Between Rheumatoid Arthritis and Pulmonary Function Measures on Spirometry in the UK Biobank; pp. 1994–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccardelli Alessandra, Liu Xinyi, Ford Julia A, Cui Jing, Lu Bing, Chu Su H, Schur Peter H, Speyer Cameron B, Costenbader Karen H, Robinson William H, Sokolove Jeremy, Karlson Elizabeth W, Camargo Carlos A, Sparks Jeffrey A. Arthritis Research & Therapy. 1. Vol. 21. Springer Science and Business Media LLC; 2019. Asthma and elevation of anti-citrullinated protein antibodies prior to the onset of rheumatoid arthritis; pp. 246–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortet B, Perez T, Roux N, Flipo R-M M, Duquesnoy B, Delcambre B, Remy-Jardin M. Annals of the Rheumatic Diseases. 10. Vol. 56. BMJ; 1997. Pulmonary function tests and high resolution computed tomography of the lungs in patients with rheumatoid arthritis; pp. 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnenegre A, Pugnere N, Antonini MT, Arnaud M, Melloni B, Treves R, Bonnaud F. European Respiratory Journal. 5. Vol. 10. Eur Respiratory Soc; 1997. Airway obstruction and rheumatoid arthritis; pp. 1072–1078. [DOI] [PubMed] [Google Scholar]
- Perry E, Kelly C, Eggleton P, De Soyza A, Hutchinson D. Rheumatology. 11. Vol. 53. Oxford University Press (OUP); 2014. The lung in ACPA-positive rheumatoid arthritis: an initiating site of injury? pp. 1940–1950. [DOI] [PubMed] [Google Scholar]
- Avouac J, Gossec L, Dougados M. Annals of the Rheumatic Diseases. 7. Vol. 65. BMJ; 2006. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review; pp. 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog Lars, Stolt Patrik, Lundberg Karin, Källberg Henrik, Bengtsson Camilla, Grunewald Johan, Rönnelid Johan, Erlandsson Harris Helena, Ulfgren Ann-Kristin, Rantapää-Dahlqvist Solbritt, Eklund Anders, Padyukov Leonid, Alfredsson Lars. Arthritis & Rheumatism. 1. Vol. 54. Wiley; 2006. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination; pp. 38–46. [DOI] [PubMed] [Google Scholar]
- De Vries-Bouwstra J K, Goekoop-Ruiterman Y P M, Verpoort K N, Schreuder G M T, Ewals J A P M, Terwiel J P, Ronday H K, Kerstens P J S M, Toes R E M, De Vries R R P, Breedveld F C, Dijkmans B A C, Huizinga T W J, Allaart C F. Arthritis & Rheumatism. 5. Vol. 58. Wiley; 2008. Progression of joint damage in early rheumatoid arthritis: Association with HLA–DRB1, rheumatoid factor, and anti–citrullinated protein antibodies in relation to different treatment strategies; pp. 1293–1298. [DOI] [PubMed] [Google Scholar]
- Thurlings R M, Vos K, Wijbrandts C A, Zwinderman A H, Gerlag D M, Tak P P. Annals of the Rheumatic Diseases. 7. Vol. 67. BMJ; 2008. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response; pp. 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woude Diane van der. Leiden University; 2012. Anti-citrullinated protein antibodies (ACPA) in rheumatoid arthritis: linking genetic predisposition to clinical outcome. [Google Scholar]
- Demoruelle M Kristen, Weisman Michael H, Simonian Philip L, Lynch David A, Sachs Peter B, Pedraza Isabel F, Harrington Annie R, Kolfenbach Jason R, Striebich Christopher C, Pham Quyen N, Strickland Colin D, Petersen Brian D, Parish Mark C, Derber Lezlie A, Norris Jill M, Holers V Michael, Deane Kevin D. Arthritis & Rheumatism. 6. Vol. 64. Wiley; 2012. Brief Report: Airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: Early injury or initiating site of autoimmunity? pp. 1756–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daha Nina A, Toes Rene EM. Nature Reviews Rheumatology. 4. Vol. 7. Nature Publishing Group; 2011. Are ACPA-positive and ACPA-negative RA the same disease? pp. 202–203. [DOI] [PubMed] [Google Scholar]
- Grosse Julien, Allado Edem, Roux Camille, Pierreisnard Audrey, Couderc Marion, Clerc-Urmes Isabelle, Remen Thomas, Albuisson Éliane, De Carvalho-Bittencourt Marcelo, Chary-Valckenaere Isabelle, Loeuille Damien. Rheumatology International. 4. Vol. 40. Springer Science and Business Media LLC; 2020. ACPA-positive versus ACPA-negative rheumatoid arthritis: two distinct erosive disease entities on radiography and ultrasonography; pp. 615–624. [DOI] [PubMed] [Google Scholar]
- Slimani S, Ladjouze-Rezig A. Rheumatology. 3. Vol. 53. Oxford University Press (OUP); 2014. Prevalence of rheumatoid arthritis in an urban population of Algeria: a prospective study; pp. 571–573. [DOI] [PubMed] [Google Scholar]
- Mosrane Y, Bougrida M, Alloui A S, Martani M, Rouabah L, Bourahli M K. Systemic inflammatory profile of smokers with and without COPD. Rev Pneumol Clin. 2017;73(4):188–198. doi: 10.1016/j.pneumo.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Nafti Salim, Taright Samya, El Ftouh Mustapha, Yassine Najiba, Benkheder Ali, Bouacha Hend, Fakhfakh Hachemi, Ali-Khoudja Moufida, Texier Nathalie, El Hasnaoui Abdelkader. Respiratory Medicine. 2. Vol. 103. Elsevier BV; 2009. Prevalence of asthma in North Africa: the Asthma Insights and Reality in the Maghreb (AIRMAG) study; pp. S2–S11. [DOI] [PubMed] [Google Scholar]
- Dayton C S, Schwartz D A, Sprince N L, Yagla S J, Davis C S, Koehnke R K, Furst D E, Hunninghake G W. American Journal of Respiratory and Critical Care Medicine. 4. Vol. 151. American Thoracic Society; 1995. Low-dose methotrexate may cause air trapping in patients with rheumatoid arthritis. pp. 1189–1193. [DOI] [PubMed] [Google Scholar]
- Lataoui S, Belghali S, Zeglaoui H, Bouajina E, Saad Ben, H. Sub-maximal aerobic capacity and quality of life of patients with rheumatoid arthritis. Rev Mal Respir. 2017;34(1):74–85. doi: 10.1016/j.rmr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Neogi Tuhina, Aletaha Daniel, Silman Alan J, Naden Raymond L, Felson David T, Aggarwal Rohit, Bingham III Clifton O, Birnbaum Neal S, Burmester Gerd R, Bykerk Vivian P. Arthritis & Rheumatism. 9. Vol. 62. Wiley Online Library; 2010. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: phase 2 methodological report; pp. 2582–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Heijde Désirée, Van Der Helm-Van Mil Annette H M, Aletaha Daniel, Bingham Clifton O, Burmester Gerd R, Dougados Maxime, Emery Paul, Felson David, Knevel Rachel, Kvien Tore K, Landewé Robert B M, Lukas Cédric, Mcinnes Iain, Silman Alan J, Smolen Josef S, Stanislawska-Biernat Ewa, Zink Angela, Combe Bernard. Annals of the Rheumatic Diseases. 4. Vol. 72. BMJ; 2013. EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria; pp. 479–481. [DOI] [PubMed] [Google Scholar]
- Prevoo M L L, Van't Hof M A, Kuper H H, Van Leeuwen M A, Van De Putte L B A, Van Riel P L C M. Arthritis & Rheumatism. 1. Vol. 38. Wiley; 1995. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis; pp. 44–48. [DOI] [PubMed] [Google Scholar]
- Wells G, Becker J-C C, Teng J, Dougados M, Schiff M, Smolen J, Aletaha D, Van Riel P L C M. Annals of the Rheumatic Diseases. 6. Vol. 68. BMJ; 2009. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate; pp. 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Annals of the Rheumatic Diseases. 3. Vol. 66. BMJ; 2007. Comparison of Disease Activity Score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values; pp. 407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher C M, Elmes P C, Fairbairn A S, Wood C H. BMJ. 5147. Vol. 2. BMJ; 1959. Significance of Respiratory Symptoms and the Diagnosis of Chronic Bronchitis in a Working Population; pp. 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai A G, Wadden T A. In the clinic: obesity. Ann Intern Med. 2013;159(5):3–16. doi: 10.7326/0003-4819-159-5-201309030-01003. [DOI] [PubMed] [Google Scholar]
- Bottiger L E, Svedberg C A. BMJ. 5544. Vol. 2. BMJ; 1967. Normal erythrocyte sedimentation rate and age. pp. 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombet Isabelle, Pouchot Jacques, Kronz Vladimir, Hanras Xavier, Capron Loïc, Durieux Pierre, Wyplosz Benjamin. The American Journal of Medicine. 9. Vol. 123. Elsevier BV; 2010. Agreement between Erythrocyte Sedimentation Rate and C-Reactive Protein in Hospital Practice; pp. 863.e7–863.e13. [DOI] [PubMed] [Google Scholar]
- Villar Emmanuel, Lièvre Michel, Kessler Michèle, Lemaître Vincent, Alamartine Eric, Rodier Michel, François Maud, Zaoui Philippe, Moranne Olivier, Choukroun Gabriel, Guerraoui Abdallah, Jolivot Anne, Janin Gérard, Branger Bernard, Heng Anne-Elisabeth, Boudray Catherine, Bissery Alvine, Rabilloud Muriel, Pouteil-Noble Claire. Journal of Diabetes and its Complications. 4. Vol. 25. Elsevier BV; 2011. Anemia normalization in patients with type 2 diabetes and chronic kidney disease: results of the NEPHRODIAB2 randomized trial; pp. 237–243. [DOI] [PubMed] [Google Scholar]
- Abramson N, Melton B. Leukocytosis: basics of clinical assessment. Am Fam Physician. 2000;62(9):2053–2060. [PubMed] [Google Scholar]
- Van Zeben D, Hazes J M, Zwinderman A H, Cats A, Van Der Voort E A, Breedveld F C. Annals of the Rheumatic Diseases. 9. Vol. 51. BMJ; 1992. Clinical significance of rheumatoid factors in early rheumatoid arthritis: results of a follow up study. pp. 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani F, Bianchi F B, Lenzi M, Volta U, Pisi E. Journal of Clinical Pathology. 7. Vol. 38. BMJ; 1985. Immunomorphological characterisation of antinuclear antibodies in chronic liver disease. pp. 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin G G, Cardiel M H, Cornejo H, Viveros M E. Prevalence of antinuclear antibodies in 3 groups of healthy individuals: blood donors, hospital personnel, and relatives of patients with autoimmune diseases. J Clin Rheumatol. 2009;15(7):325–329. doi: 10.1097/RHU.0b013e3181bb971b. [DOI] [PubMed] [Google Scholar]
- Coenen Dries, Verschueren Patrick, Westhovens René, Bossuyt Xavier. Clinical Chemistry. 3. Vol. 53. Oxford University Press (OUP); 2007. Technical and Diagnostic Performance of 6 Assays for the Measurement of Citrullinated Protein/Peptide Antibodies in the Diagnosis of Rheumatoid Arthritis; pp. 498–504. [DOI] [PubMed] [Google Scholar]
- Hansell David M, Bankier Alexander A, Macmahon Heber, Mcloud Theresa C, Müller Nestor L, Remy Jacques. Radiology. 3. Vol. 246. Radiological Society of North America (RSNA); 2008. Fleischner Society: Glossary of Terms for Thoracic Imaging; pp. 697–722. [DOI] [PubMed] [Google Scholar]
- Miller Martin R, Hankinson JATS, Brusasco Vito, Burgos F, Casaburi R, Coates A, Crapo R, Enright Pvd, Van Der Grinten CPM, Gustafsson P. European respiratory journal. 2. Vol. 26. Eur Respiratory Soc; 2005. Standardisation of spirometry; pp. 319–338. [DOI] [PubMed] [Google Scholar]
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, Van Der Grinten CPM. European respiratory journal. 3. Vol. 26. Eur Respiratory Soc; 2005. Standardisation of the measurement of lung volumes; pp. 511–522. [DOI] [PubMed] [Google Scholar]
- Ketfi A, Gharnaout M, Saad Ben, H. The plethysmographic reference equations established for adult natives of Eastern Algeria are not applicable to natives of Northern Algeria. Rev Mal Respir. 2019;36(7):870–879. doi: 10.1016/j.rmr.2019.05.042. [DOI] [PubMed] [Google Scholar]
- Ketfi Abdelbassat, Gharnaout Merzak, Bougrida Mohamed, Ben Saad Helmi. Respiratory function technologists/scient. Vol. 13. European Respiratory Society; 2018. The multi-ethnic global lung initiative 2012 (GLI-2012) norms reflect contemporary adult's algerian spirometry; pp. 203023–203023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanjer Philip H, Stanojevic Sanja, Cole Tim J, Baur Xaver, Hall Graham L, Culver Bruce H, Enright Paul L, Hankinson John L, Ip Mary S M, Zheng Jinping, Stocks Janet. European Respiratory Journal. 6. Vol. 40. European Respiratory Society (ERS); 2012. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations; pp. 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Graham L, Filipow Nicole, Ruppel Gregg, Okitika Tolu, Thompson Bruce, Kirkby Jane, Steenbruggen Irene, Cooper Brendan G, Stanojevic Sanja. European Respiratory Journal. 3. Vol. 57. European Respiratory Society (ERS); 2021. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry; pp. 2000289–2000289. [DOI] [PubMed] [Google Scholar]
- Ketfi Abdelbassat, Ben Saad Helmi. Libyan Journal of Medicine. 1. Vol. 17. Informa UK Limited; 2022. The global lung function initiative 2021 (GLI-2021) norms provide mixed results for static lung volumes (SLVs) in Algerian adults; pp. 2059893–2059893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Ben, H. Interpretation of respiratory functional explorations of deficiency and incapacity in adult. Tunis Med. 2020;98(11):797–815. [PubMed] [Google Scholar]
- Saad Ben, H. Review of the current use of global lung function initiative norms for spirometry (GLI-2012) and static lung volumes (GLI-2021) in Great Arab Maghreb (GAM) countries and steps required to improve their utilization. Libyan J Med. 2022;17(1):2031596–2031596. doi: 10.1080/19932820.2022.2031596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojevic Sanja, Kaminsky David A, Miller Martin R, Thompson Bruce, Aliverti Andrea, Barjaktarevic Igor, Cooper Brendan G, Culver Bruce, Derom Eric, Hall Graham L. European Respiratory Journal. 1. Vol. 60. Eur Respiratory Soc; 2022. ERS/ATS technical standard on interpretive strategies for routine lung function tests. [DOI] [PubMed] [Google Scholar]
- Kammoun Rim, Ghannouchi Ines, Rouatbi Sonia, Ben Saad Helmi. Libyan Journal of Medicine. 1. Vol. 13. Informa UK Limited; 2018. Defining and grading an obstructive ventilatory defect (OVD): ‘FEV<sub>1</sub>/FVC lower limit of normal (LLN) vs. <i>Z</i>-score’ and ‘FEV<sub>1</sub> percentage predicted (%pred) vs. <i>Z</i>-score’; pp. 1487751–1487751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhier Z, Bendahhou K, Abdelaziz Ben, Bennani A, M O. Methodological sheet n degrees 1: How to calculate the size of a sample for an observational study. Tunis Med. 2020;98(1):1–7. [PubMed] [Google Scholar]
- Olejnik Stephen, Algina James. Contemporary Educational Psychology. 3. Vol. 25. Elsevier BV; 2000. Measures of Effect Size for Comparative Studies: Applications, Interpretations, and Limitations; pp. 241–286. [DOI] [PubMed] [Google Scholar]
- Reynisdottir Gudrun, Karimi Reza, Joshua Vijay, Olsen Helga V, Hensvold Aase Haj, Harju Anders H, Engström Marianne, Grunewald Johan, Nyren Sven, Eklund Anders, Klareskog Lars, Sköld Carl Magnus, Irinel Catrina Anca. Arthritis & Rheumatology. 1. Vol. 66. Wiley; 2014. Structural Changes and Antibody Enrichment in the Lungs Are Early Features of Anti-Citrullinated Protein Antibody-Positive Rheumatoid Arthritis; pp. 31–39. [DOI] [PubMed] [Google Scholar]
- Ruiz-Esquide Virginia, Gómara María José, Peinado Víctor I, Gómez Puerta José Alfredo, Barberá Joan Albert, Cañete Juan De Dios, Haro Isabel, Sanmartí Raimon. Clinical Rheumatology. 7. Vol. 31. Springer Science and Business Media LLC; 2012. Anti-citrullinated peptide antibodies in the serum of heavy smokers without rheumatoid arthritis. A differential effect of chronic obstructive pulmonary disease? pp. 1047–1050. [DOI] [PubMed] [Google Scholar]
- Perry E, Kelly C, De-Soyza A, Moullaali T J, Eggleton P, Hutchinson D G. Natural history, disease characteristics and autoantibody positivity in patients with bronchiectasis and RA: is the lung an initiating site of autoimmunity in rheumatoid arthritis? Rheumatology. 2013;52:76–83. [Google Scholar]
- Kochbati Samir, Boussema Fatma, Miled Ben, Shili Sarra, Chérif Mohamed, Jemni C, Mzabi H, Chérif O, Daghfous MH, Rokbani L. Bronchiectasis in rheumatoid arthritis. High resolution computed pulmonary tomography. La Tunisie medicale. 2003;81(10):768–773. [PubMed] [Google Scholar]
- Bilgici Ayhan, Ulusoy H, Kuru O, Çelenk Ç, Ünsal M, Danacı M. Rheumatology International. 6. Vol. 25. Springer Science and Business Media LLC; 2005. Pulmonary involvement in rheumatoid arthritis; pp. 429–435. [DOI] [PubMed] [Google Scholar]
- Van De Sande M G H, De Hair M J H, Van Der Leij C, Klarenbeek P L, Bos W H, Smith M D, Maas M, De Vries N, Van Schaardenburg D, Dijkmans B A C, Gerlag D M, Tak P P. Annals of the Rheumatic Diseases. 5. Vol. 70. BMJ; 2011. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase; pp. 772–777. [DOI] [PubMed] [Google Scholar]
- Huang Sicong, He Xintong, Doyle Tracy J, Zaccardelli Alessandra, Marshall Allison A, Friedlander H Maura, Blaustein Rachel B, Smith Elisabeth A, Cui Jing, Iannaccone Christine K, Mahmoud Taysir G, Weinblatt Michael E, Dellaripa Paul F, Shadick Nancy A, Sparks Jeffrey A. Clinical Rheumatology. 12. Vol. 38. Springer Science and Business Media LLC; 2019. Association of rheumatoid arthritis-related autoantibodies with pulmonary function test abnormalities in a rheumatoid arthritis registry; pp. 3401–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer Aryeh, Solomon Joshua J, Du Bois Roland M, Deane Kevin D, Olson Amy L, Fernandez-Perez Evans R, Huie Tristan J, Stevens Allen D, Gill Mary B, Rabinovitch Avi M, Lynch David A, Burns David A, Pineiro Isabel S, Groshong Steve D, Duarte Achcar Rosane D, Brown Kevin K, Martin Richard J, Swigris Jeffrey J. Respiratory Medicine. 7. Vol. 106. Elsevier BV; 2012. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease; pp. 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui Naoki, Enomoto Noriyuki, Suda Takafumi, Kageyama Yasunori, Watanabe Hiroshi, Chida Kingo. Clinical Biochemistry. 13. Vol. 41. Elsevier BV; 2008. Anti-cyclic citrullinated peptide antibodies in lung diseases associated with rheumatoid arthritis; pp. 1074–1077. [DOI] [PubMed] [Google Scholar]
- Kelly C, Chan C, Ahmad Y. RA-related interstitial lung disease: which factors predict its development? Rheumatology. 2013;52:75–75. [Google Scholar]
- Mori Shunsuke, Koga Yukinori, Sugimoto Mineharu. Respiratory Medicine. 11. Vol. 106. Elsevier BV; 2012. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis; pp. 1591–1599. [DOI] [PubMed] [Google Scholar]
- Wilsher Margaret, Voight Louisa, Milne David, Teh Mark, Good Nicola, Kolbe John, Williams Megan, Pui Karen, Merriman Tony, Sidhu Karishma, Dalbeth Nicola. Respiratory Medicine. 10. Vol. 106. Elsevier BV; 2012. Prevalence of airway and parenchymal abnormalities in newly diagnosed rheumatoid arthritis; pp. 1441–1446. [DOI] [PubMed] [Google Scholar]
- Saad Ben, H, Amor Ben, L, Mdalla Ben, Ghannouchi S, I, Essghair Ben, Sfaxi M, R. The importance of lung volumes in the investigation of heavy smokers. Rev Mal Respir. 2014;31(1):29–40. doi: 10.1016/j.rmr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Perez T, Guenard H. Evaluation and follow up of hyperinflation in COPD. Rev Mal Respir. 2009;26(4):482–482. doi: 10.1016/s0761-8425(09)74043-4. [DOI] [PubMed] [Google Scholar]
- O'donnell D. Dynamic lung hyperinflation and its clinical implication in COPD. Rev Mal Respir. 2008;25(10):1305–1318. doi: 10.1016/s0761-8425(08)75094-0. [DOI] [PubMed] [Google Scholar]
- Hogg James C, Chu Fanny, Utokaparch Soraya, Woods Ryan, Elliott W Mark, Buzatu Liliana, Cherniack Ruben M, Rogers Robert M, Sciurba Frank C, Coxson Harvey O, Paré Peter D. New England Journal of Medicine. 26. Vol. 350. Massachusetts Medical Society; 2004. The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease; pp. 2645–2653. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, Crapo R O, Burgos F, Casaburi R. European Respiratory Journal. 5. Vol. 26. European Respiratory Society (ERS); 2005. Interpretative strategies for lung function tests; pp. 948–968. [DOI] [PubMed] [Google Scholar]
- Laveneziana Pierantonio, Albuquerque Andre, Aliverti Andrea, Babb Tony, Barreiro Esther, Dres Martin, Dubé Bruno-Pierre, Fauroux Brigitte, Gea Joaquim, Guenette Jordan A, Hudson Anna L, Kabitz Hans-Joachim, Laghi Franco, Langer Daniel, Luo Yuan-Ming, Neder J Alberto, O'donnell Denis, Polkey Michael I, Rabinovich Roberto A, Rossi Andrea, Series Frédéric, Similowski Thomas, Spengler Christina, Vogiatzis Ioannis, Verges Samuel. European Respiratory Journal. 6. Vol. 53. European Respiratory Society (ERS); 2019. ERS statement on respiratory muscle testing at rest and during exercise; pp. 1801214–1801214. [DOI] [PubMed] [Google Scholar]
- Lee Gina, Walser Tonya C, Dubinett Steven M. Current Opinion in Pulmonary Medicine. 4. Vol. 15. Ovid Technologies (Wolters Kluwer Health); 2009. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer; pp. 303–307. [DOI] [PubMed] [Google Scholar]
- Newkirk Marianna M, Mitchell Simeon, Procino Michael, Li Zhenhong, Cosio Manuel, Mazur Witold, Kinnula Vuokko L, Hudson Marie, Baron Murray, Fritzler Marvin J, El-Gabalawy Hani S. European Journal of Immunology. 4. Vol. 42. Wiley; 2012. Chronic smoke exposure induces rheumatoid factor and anti-heat shock protein 70 autoantibodies in susceptible mice and humans with lung disease; pp. 1051–1061. [DOI] [PubMed] [Google Scholar]
- Yang Deng-Ho H, Tu Chuan-Chou C, Wang Shou-Cheng C, Wei Cheng-Chung C, Cheng Ya-Wen W. Rheumatology International. 7. Vol. 34. Springer Science and Business Media LLC; 2014. Circulating anti-cyclic citrullinated peptide antibody in patients with rheumatoid arthritis and chronic obstructive pulmonary disease; pp. 971–977. [DOI] [PubMed] [Google Scholar]
- Zaccardelli Alessandra, Liu Xinyi, Ford Julia A, Cui Jing, Lu Bing, Chu Su H, Schur Peter H, Speyer Cameron B, Costenbader Karen H, Robinson William H, Sokolove Jeremy, Karlson Elizabeth W, Camargo Carlos A, Sparks Jeffrey A. Arthritis Care & Research. 4. Vol. 73. Wiley; 2021. Elevated Anti–Citrullinated Protein Antibodies Prior to Rheumatoid Arthritis Diagnosis and Risks for Chronic Obstructive Pulmonary Disease or Asthma; pp. 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Won Hong, Kim Song Soo, Shim Seung Cheol, Song Seung Taek, Jung Sung Soo, Kim Jin Hwan, Kim Namkug S, Seo Joon Beom. Lung. 1. Vol. 194. Springer Science and Business Media LLC; 2016. Visual Assessment of Chest Computed Tomography Findings in Anti-cyclic Citrullinated Peptide Antibody Positive Rheumatoid Arthritis: Is it Associated with Airway Abnormalities? pp. 97–105. [DOI] [PubMed] [Google Scholar]
- Grönwall C, Liljefors L, Bang H, Hensvold A H, Hansson M, Mathsson-Alm L. A comprehensive evaluation of the relationship between different IgG and IgA anti- modified protein autoantibodies in rheumatoid arthritis. Front Immunol. 2021;12:627986–627986. doi: 10.3389/fimmu.2021.627986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive John T, Hyatt Robert E. American Review of Respiratory Disease. 3. Vol. 106. American Thoracic Society; 1972. Maximal Expiratory Flow and Total Respiratory Resistance During Induced Bronchoconstriction in Asthmatic Subjects<sup>1–</sup><sup>3</sup>; pp. 366–376. [DOI] [PubMed] [Google Scholar]
- Iyer V N, Parker K O, Schroeder D R, Hyatt R E, Scanlon P D. D53. LUNG AND CHEST WALL MECHANICS. Vol. 139. American Thoracic Society; 2009. The 'Non-Specific' Pulmonary Function Test: Longitudinal Follow up and Outcomes on 1284 Patients. pp. 878–886. [DOI] [PubMed] [Google Scholar]
- Wan Emily S, Fortis Spyridon, Regan Elizabeth A, Hokanson John, Han Meilan K, Casaburi Richard, Make Barry J, Crapo James D, Demeo Dawn L, Silverman Edwin K, Crapo James D, Silverman Edwin K, Make Barry J, Regan Elizabeth A, Beaty Terri, Begum Ferdouse, Castaldi Peter J, Cho Michael, Demeo Dawn L, Boueiz Adel R, Foreman Marilyn G, Halper-Stromberg Eitan, Hayden Lystra P, Hersh Craig P, Hetmanski Jacqueline, Hobbs Brian D, Hokanson John E, Laird Nan, Lange Christoph, Lutz Sharon M, Mcdonald Merry-Lynn, Parker Margaret M, Qiao Dandi, Regan Elizabeth A, Silverman Edwin K, Wan Emily S, Won Sungho, Sakornsakolpat Phuwanat, Prokopenko Dmitry, Al Qaisi Mustafa, Coxson Harvey O, Gray Teresa, Han Meilan K, Hoffman Eric A, Humphries Stephen, Jacobson Francine L, Judy Philip F, Kazerooni Ella A, Kluiber Alex, Lynch David A, Newell John D, Regan Elizabeth A, Ross James C, San Jose Estepar Raul, Schroeder Joyce, Sieren Jered, Stinson Douglas, Stoel Berend C, Tschirren Juerg, Van Beek Edwin, Van Ginneken Bram, Van Rikxoort Eva, Washko George, Wilson Carla G, Jensen Robert, Everett Douglas, Crooks Jim, Moore Camille, Strand Matt, Wilson Carla G, Hokanson John E, Hughes John, Kinney Gregory, Lutz Sharon M, Pratte Katherine, Young Kendra A, Bhatt Surya, Bon Jessica, Han Meilan K, Make Barry, Martinez Carlos, Murray Susan, Regan Elizabeth A, Soler Xavier, Wilson Carla G, Bowler Russell P, Kechris Katerina, Banaei-Kashani Farnoush, Curtis Jeffrey L, Martinez Carlos H, Pernicano Perry G, Hanania Nicola, Alapat Philip, Atik Mustafa, Bandi Venkata, Boriek Aladin, Guntupalli Kalpatha, Guy Elizabeth, Nachiappan Arun, Parulekar Amit, Demeo Dawn L, Hersh Craig, Jacobson Francine L, Washko George, Barr R Graham, Austin John, D’souza Belinda, Pearson Gregory D N, Rozenshtein Anna, Thomashow Byron, Macintyre Neil, Mcadams H Page, Washington Lacey, Mcevoy Charlene, Tashjian Joseph, Wise Robert, Brown Robert, Hansel Nadia N, Horton Karen, Lambert Allison, Putcha Nirupama, Casaburi Richard, Adami Alessandra, Budoff Matthew, Fischer Hans, Porszasz Janos, Rossiter Harry, Stringer William, Sharafkhaneh Amir, Lan Charlie, Wendt Christine, Bell Brian, Foreman Marilyn G, Berkowitz Eugene, Westney Gloria, Bowler Russell, Lynch David A, Rosiello Richard, Pace David, Criner Gerard, Ciccolella David, Cordova Francis, Dass Chandra, D’alonzo Gilbert, Desai Parag, Jacobs Michael, Kelsen Steven, Kim Victor, Mamary A James, Marchetti Nathaniel, Satti Aditi, Shenoy Kartik, Steiner Robert M, Swift Alex, Swift Irene, Vega-Sanchez Maria Elena, Dransfield Mark, Bailey William, Bhatt Surya, Iyer Anand, Nath Hrudaya, Wells J Michael, Ramsdell Joe, Friedman Paul, Soler Xavier, Yen Andrew, Comellas Alejandro P, Hoth Karin F, Newell John, Thompson Brad, Han Meilan K, Kazerooni Ella, Martinez Carlos H, Billings Joanne, Begnaud Abbie, Allen Tadashi, Sciurba Frank, Bon Jessica, Chandra Divay, Fuhrman Carl, Weissfeld Joel, Anzueto Antonio, Adams Sandra, Maselli-Caceres Diego, Ruiz Mario E. American Journal of Respiratory and Critical Care Medicine. 11. Vol. 198. American Thoracic Society; 2018. Longitudinal Phenotypes and Mortality in Preserved Ratio Impaired Spirometry in the COPDGene Study; pp. 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortis Spyridon, Comellas Alejandro, Kim Victor, Casaburi Richard, Hokanson John E, Crapo James D, Silverman Edwin K, Wan Emily S. Scientific Reports. 1. Vol. 10. Springer Science and Business Media LLC; 2020. Low FVC/TLC in Preserved Ratio Impaired Spirometry (PRISm) is associated with features of and progression to obstructive lung disease; pp. 5169–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marott Jacob Louis, Ingebrigtsen Truls Sylvan, Çolak Yunus, Vestbo Jørgen, Lange Peter. American Journal of Respiratory and Critical Care Medicine. 8. Vol. 204. American Thoracic Society; 2021. Trajectory of Preserved Ratio Impaired Spirometry: Natural History and Long-Term Prognosis; pp. 910–920. [DOI] [PubMed] [Google Scholar]
- Aaron Shawn D, Dales Robert E, Cardinal Pierre. Chest. 3. Vol. 115. Elsevier BV; 1999. How Accurate Is Spirometry at Predicting Restrictive Pulmonary Impairment? pp. 869–873. [DOI] [PubMed] [Google Scholar]
- Hyatt Robert E, Cowl Clayton T, Bjoraker Julie A, Scanlon Paul D. Chest. 2. Vol. 135. Elsevier BV; 2009. Conditions Associated With an Abnormal Nonspecific Pattern of Pulmonary Function Tests; pp. 419–424. [DOI] [PubMed] [Google Scholar]
- Mascha E J, Vetter T R. Significance, errors, power, and sample size: The blocking and tackling of statistics. Anesth Analg. 2018;126(2):691–698. doi: 10.1213/ANE.0000000000002741. [DOI] [PubMed] [Google Scholar]
- Kammoun R, Saad Ben, H. From deficiency to handicap in the respiratory field: lung function tests (LFT) norms and quality of life (QOL) questionnaires validated for the Tunisian population. Tunis Med. 2020;98(5):378–395. [PubMed] [Google Scholar]
- Knaz Hend, Anane Ichraf, Guezguez Fatma, Ben Saad Helmi. Physiotherapists. Vol. 37. European Respiratory Society; 2019. Applicability of the Arabic version of the quality of life (QOL) questionnaire (VQ11) in Tunisian patients with COPD; pp. 699–709. [DOI] [PubMed] [Google Scholar]


