Background and epidemiology: Varicella zoster virus (VZV) is a DNA virus belonging to the herpesvirus family. It causes a primary illness commonly known as chickenpox and can also lie latent in the sensory nerve ganglia until it reactivates later in life and causes herpes zoster (shingles). The lifetime risk of VZV infection is about 95%,1 with about 350 000 cases reported annually in Canada. Disease tracking is currently based on passive reporting from 6 provinces and 2 territories, and it likely underestimates the true incidence of infection by a factor of 5 or more.2
Most (90%) reported cases of VZV infection involve uncomplicated cases of chickenpox in children.2 The uncomplicated disease, despite its benign course, carries an economic burden of about $109.2 million annually, with direct medical costs accounting for only about 10% of this; the largest cost driver is lost productivity caused by caregivers' lost work days.3
Severe complications such as superimposed skin infections (group A β-hemolytic streptococci), encephalitis and pneumonia develop in a small proportion of patients. The risk of severe complications from primary VZV infection is much higher in adults than in children. Adults account for only 5% of all annual cases, yet between 1987 and 1996 they represented about 70% of reported VZV-related deaths in Canada.4 Maternal infection during the first 28 weeks of gestation can transmit VZV to the fetus and lead to congenital varicella syndrome.
VZV infection, which is highly contagious, is spread by direct contact with skin lesions or oral secretions; airborne spread can also occur. Those infected are contagious from 1 to 2 days before the onset of rash up until the last lesion has crusted. The incubation period ranges from 10 to 21 days, and there may be prodromal symptoms such as fever, malaise and upper respiratory tract infection. The characteristic lesions appear in successive crops over the first 3 to 4 days and progress from macules to vesicles to pustules to crusted lesions.4 The rash is generally centrally distributed, with lesions concentrated on the trunk, scalp and face.
Diagnosis can be made clinically by the characteristic rash and epidemiologic links such as known exposure to another patient.1 The virus can be isolated from scrapings of the vesicle base during the first 3 to 4 days after the eruption as well as from a variety of serologic antibody tests. A significant increase in serum varicella IgG antibody can retrospectively confirm a diagnosis in immunocompetent people.5
Clinical management: The care given to otherwise healthy individuals who have an uncomplicated course of VZV infection is primarily supportive. Intravenous or oral therapy with acyclovir, valacyclovir, famciclovir or foscarnet can be given. The decision to use antiviral therapy, and the duration and route, will depend on a variety of specific host factors and the extent of infection.5
Prevention and control: Varicella is a notifiable disease. When treating a patient, physicians should follow the recommended precautions for airborne diseases in the infection control guidelines for the physician's office.6 This includes quickly triaging such patients out of the common waiting area and, if possible, seeing them at the end of the day. Health care workers who are not immune to the disease should not enter the room, and routine housekeeping measures as outlined in section 4 of the guidelines should be followed.6 Susceptible health care workers may also consider vaccination — the National Committee on Immunization4 and the Canadian Task Force on Preventive Health Care7 both recommend primary vaccination of healthy people over 12 months of age who are susceptible to the disease. People older than 13 years should receive 2 full doses at least 28 days apart.1
A live attenuated vaccine called the Oka strain was developed in Japan, in the early 1970s. Until June 2000 the only varicella vaccine licensed for use in Canada was highly heat sensitive, but it has been replaced with a vaccine that offers better stability at fridge temperature.
Despite the advent of a more stable vaccine, health care professionals do not universally accept routine administration of the varicella vaccine because it is unclear whether lifelong immunity will develop in children who are vaccinated. It is currently estimated that the vaccine offers about 70%–90% protection against VZV of any severity for at least 7 to 10 years, which is the observation period reported from recent studies.1,8,9,10,11 If the protective effect wanes, a program of universal vaccination could cause a shift in the epidemiology of varicella to the adult population, with a resulting increase in morbidity.3,12 This would put susceptible adults at increased risk of the disease at an age when significant complications are common.12
Tamara Wallington Community Medicine McMaster University
Erica Weir CMAJ
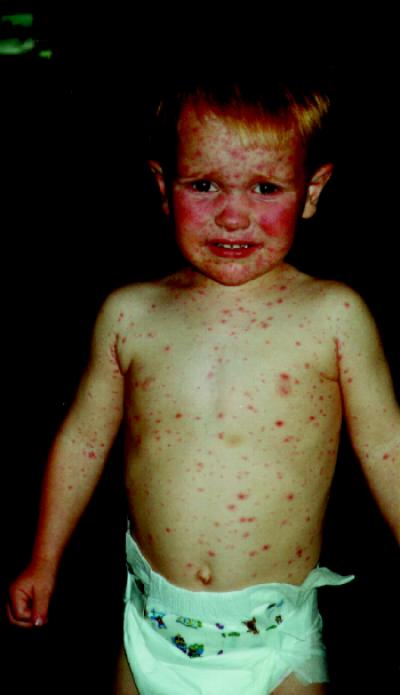
Figure. Varicella, an uncomfortable reality for most children, occasionally leads to more serious complications.
References
- 1.Varicella-zoster virus disease and epidemiology: seeking better control strategies — Part 1. Can Commun Dis Rep 1998;24(24). Available: www.hc-sc.gc.ca/hpb/lcdc/publicat/ccdr/98vol24/dr2424ea.html (accessed 2002 Jan 31). [PubMed]
- 2.Varicella: highlights on current issues and challenges. Paediatr Child Health 1999;4(Suppl C).
- 3.Law B, Fitzsimon C, Ford-Jones L, MacDonald N, Dery P, Vaudry W, et al. Cost of chickenpox in Canada: part 1. Cost of uncomplicated cases. Pediatrics 1999;104:1-6. [DOI] [PubMed]
- 4.National Committee on Immunization. Statement on recommended use of varicella virus vaccine. Can Commun Dis Rep 1999;25(ACS-1). Available: www .hc-sc.gc.ca/hpb/lcdc /publicat /ccdr /99vol25 /25 sup /acs1.html (accessed 2002 Jan 31).
- 5.Report of the Committee on Infectious Diseases. Red book. 25th ed. Elk Grove Village (IL): American Academy of Pediatrics; 2000.
- 6.Infection control in the physician's office. Toronto: College of Physicians and Surgeons of Ontario; 1999.
- 7.Varicella vaccination: recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ 2001;164(13):1888-9. Available: www.cma.ca/cmaj/vol-164/issue-13/1888.asp [PMC free article] [PubMed]
- 8.Weibel RE, Neff BJ, Kuter BJ, Guess HA, Rothenberger CA, Fitzgerald AJ, et al. Live attenuated varicella virus vaccine. Efficacy trial in healthy children. N Engl J Med 1984;310:1409-15. [DOI] [PubMed]
- 9.Asano Y, Nagai T, Miyata T, Yazaki T, Ito S, Yamanishi K, et al. Long-term protective immunity of recipients of the OKA strain of live varicella vaccine. Pediatrics 1985:75:667-71. [PubMed]
- 10.Kuter BJ, Weibel RE, Guess HA, Matthews H, Morton DH, Neff BJ, et al. Oka/Merck varicella vaccine in healthy children: final report of a 2-year efficacy study and 7-year follow-up studies. Vaccine 1991;9:643-7. [DOI] [PubMed]
- 11.Arbeter AM, Starr SE, Plotkin SA. Varicella vaccine studies in healthy children and adults. Pediatrics 1986;78(Suppl):748-56. [PubMed]
- 12.Taylor J. Herd Immunity and the varicella vaccine. Is it a good thing? Arch Pediatr Adolesc Med 2001; 155(4):440-1. [DOI] [PubMed]


