Abstract
Tissue-engineered products (TEPs) consist of engineered cells or tissues produced to regenerate, repair, or replace a dysfunctional, diseased, or absent human tissue. TEPs make up <5% of all advanced therapeutic medicinal products (ATMPs) in clinical trials and received 5.1% of ATMP-designated funding in trials in the European Union (EU) in 2019, highlighting the relatively low proportion of TEPs being developed. The realization of TEPs being marketed has yet to be fulfilled, with few products being approved. Since 2009, 90 TEP-based clinical trials have been undertaken in the EU. Of these 90, 25 were Phase I/II trials, 35 were Phase II, 28 were Phase III, and two were Phase IV trials. This review provides an overview of TEPs in development, identifying musculoskeletal, cardiovascular, and skin/connective tissue disorders as the main therapeutic areas of interest. Commercial sponsors have funded most trials, and a significantly higher proportion of late-phase trials. Furthermore, this study has identified a shift toward the use of allogeneic cells in TEPs and increased activity in the proportion of early phase trials listed. This indicates a renewed interest in TEP development as sponsors adapt to the new regulation, with prospects of more TEP market authorization applications in the future.
Impact Statement
Tissue-engineered products (TEPs) consist of engineered cells or tissues produced to regenerate, repair, or replace a dysfunctional, diseased, or absent human tissue. This article evaluates the regulatory landscape of TEPs and identifies the trends in clinical trial activity in the European Union (EU) since the introduction of Regulation (EC) No 1394/2007. This article identifies trends in TEP development, highlighting the most active member states, commercial involvement, a shift toward the use of allogeneic cells and a renewed interest in TEP development in recent years.
Keywords: tissue-engineered product, advanced therapeutic medicinal products, clinical trial, European Union
Introduction
In Europe, advanced therapeutic medicinal products (ATMPs) include gene therapy, somatic cell therapy, and tissue-engineered products (TEPs). These advanced “biologic” therapies are regulated under Regulation (EC) No 1394/20071 since December 30, 2008. ATMPs must follow this regulation and other relevant legislation and guidelines on medicines to demonstrate quality, safety, and efficacy and good practice requirements, like good manufacturing practice (GMP for ATMPs) and good clinical practice. This legislation ensures that ATMPs are authorized through the centralized procedure in the EU resulting in a single marketing authorization for the whole of the EU. It has been over 10 years since the introduction of the ATMP Regulation and further ATMP-specific legislation and guidance. Its effect on product development and successful use in clinical practice is evident.
The regulatory framework and market authorization overview
TEPs are defined in Chapter 1—Article 2 (EC/1394/2007) as “a product that contains or consists of engineered cells or tissues and that can be used to regenerate, repair, or replace a human tissue.”1 TEPs may contain human/animal cells or tissues, which can be viable or not, and other components might be added (e.g., cellular products, biomolecules, biomaterials, matrices).1 However, products constituted exclusively by nonviable human/animal cells or tissues that do not act principally by pharmacological, metabolic, or immunological action are exempt from this definition and not governed by this regulation.1 If a product that meets the definition of a TEP incorporates as an integral part of the product, a medical device, and if its cells or tissues are viable, then it would be considered a combined ATMP.1 (Article 2 [1 day]).
The European Medicines Agency (EMA) has granted a total of 21 ATMP market authorizations (MA), with five having been withdrawn from the market (two TEPs, two somatic cell therapy medicinal products, and one gene therapy medicinal product [GTMP]).2–7 Of the 19 ATMPS, four TEPs have been granted MA, with two currently remaining on the market. ChondroCelect® produced by TiGenix® (Belgium), a cartilage cell-based product, was the first ATMP to be approved in 2009 (submitted in 2007) as an autologous chondrocyte implantation (ACI) product for the treatment of focal chondral defects.5
MACI® (Vericel), a matrix-induced ACI, is a third-generation ACI product indicated for the repair of focal chondral defects of the knee.4 These two have been withdrawn. Spherox®, a TEP consisting of spheroids of human autologous matrix-associated chondrocytes, was authorized in 2017.8 In 2015, Holoclar® by Chiesi® (Italy) became the first stem cell therapy to receive conditional MA by EMA under Article 2 of EC/507/2006 for limbal stem cell deficiency (LSCD),8 an orphan indication.
Thus far, TEPs make up <5% of all ATMPs in clinical trials and received 5.1% of total financing for ATMPs in trials in Europe in 2019.9 Since trends in ATMP approval have favored mainly gene therapy products, the realization of tissue engineering has yet to be fulfilled, with a smaller proportion of TEPs being approved.9 This review article presents an analysis of TEPs in clinical trials in the EU to explore trends in activities and strategies to adapt to the requirements outlined in EC/1394/2007.
Methods
Search strategy
A search was performed using the public EU Clinical Trials Register, in which the full trial details for all 33,876 trials from January 1, 2008, to December 31, 2021 were searched for TEP inclusion using MS Power Automate software® (Microsoft®). Study details were downloaded in .txt format, processed in Microsoft Excel,® and filtered using MS Power Automate to capture all studies with “Tissue Engineered Product: Yes.”
Where studies were undertaken in multiple member states, geographical data include all clinical sites, while summary data only include the country of sponsor registration. For this article, we have included clinical trials undertaken in United Kingdom (UK) to accurately represent clinical trial activity in the EU to date. The format of data entry into the EudraCT database is highly variable. Thus, standardization of data has been performed for parameters such as funding source, cell type, pharmaceutical form, route of administration, therapeutic area, and active substance.
Data extraction, synthesis, and analysis
One author (K.J.) performed data extraction of the EU Clinical Trials Register and identification of TEP containing trials. Two reviewers (G.R. and Z.B.) independently screened the results and validated the presented data applying the same standards while discrepancies were resolved through discussion until consensus was reached. The following information was extracted: EudraCT number, national competent authority, entry date, title, sponsor, sponsor country, sponsor status, source of funding, country of funding source, orphan drug status, pharmaceutical form, route of administration, medical condition indicated, therapeutic area, rare disease status, study design, active substance, type of ATMP (only TEPs included), and Committee for Advanced Therapies (CAT) classification. All data were processed using Microsoft excel and graphs were generated using GraphPad Prism, Datawrapper© (Germany)10 and RAWGraphs 2.0© (DestinyDesign, Italy).11 Chi-square and Fisher's exact tests (FET) were used where appropriate to assess the relationship between the above variables.
Results and Discussion
TEPs in clinical trials in the European Economic Area
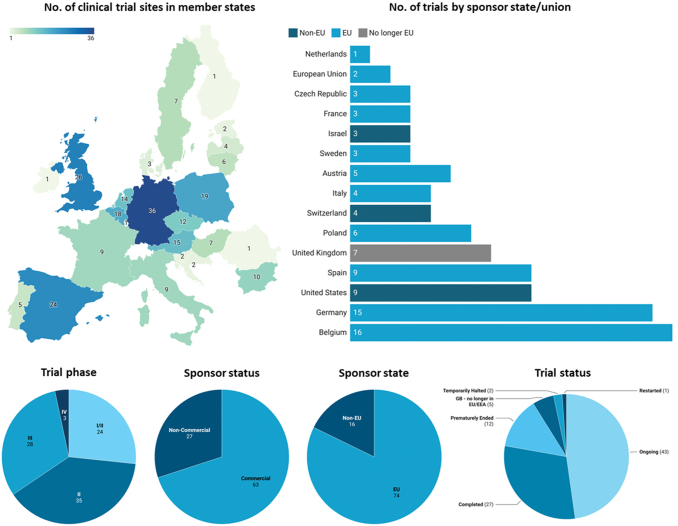
The search strategy yielded 98 clinical trials involving TEPs that had been undertaken in the EU across 243 listed sites. After individual validation of all entries, eight studies were identified as not investigating TEPs and were omitted. Of the remaining 90 trials, 25 were Phase I/II trials, 35 were Phase II, 28 were Phase III, and two were Phase IV trials. At the time of writing, 27 trials are completed, 43 are ongoing, one has been restarted, two have been halted, 12 ended prematurely, and five are taking place in UK for which no follow-up data are recorded by the EU (Fig. 1). One trial included both Phase II and Phase III studies (EudraCT no.: 2011-000595-33), which has been recorded as a Phase III trial for inclusion in statistical analysis.
FIG. 1.
Geographical overview of clinical trial activity investigating TEPs in each Member State according to the EU Clinical Trials register, analyzed on January 22, 2022. The geographical map represents all trial sites across EU states, where some trials contain multiple sites across states. “No. of trials by sponsor state/union” represents the number of individual trials funded by a sponsor in each named jurisdiction. Sponsor state, trial phase, sponsor status, and trial status reflect individual trials with unique EudraCT numbers. Funding sources for TEP clinical trials are divided into sponsors located in EU and non-EU states. Two trials sponsored by the EU through the European Commission and Horizon 2020 listed the EU as the sponsor and thus no state is given. However, while UK is no longer in the EU, it has been included in this graph to accurately represent clinical activity over the last 12 years. Trial-phase status is based on the most recent updated list in the EU Clinical Trials Register. Phase I trials are not publicly available in the EudraCT database, except those including pediatric populations. “Other” in trial status includes two halted trials and one restarted trial. EU, European Union; TEP, tissue-engineered product; UK, United Kingdom. Color images are available online.
There appear to be certain EU member states where clinical development of TEPs is concentrated, such as Germany, Spain, Belgium, Poland, and UK, with more than 18 trials with sites in each state. This may be due to a previous tradition of using TEPs even before the ATMP Regulation came into place (especially in the case of ACI).12 Furthermore, Germany, Spain, Belgium, and UK have a wide landscape of biotech companies developing ATMPs, including TEPs.13
Funding for TEP clinical trials supported by “commercial” sponsors accounted for 70% (63/90) of trials, while the remaining sponsors reported as “noncommercial.” Monetary or material support from biomedical companies accounted for 69% (62/90), while national agencies funded 18% (16/90) and the EU (Horizon 2020, a European Commission initiative) funded four trials (4.4%). Academic institutions, hospital groups, and charity organizations funded the remaining 7.7% (7/90) trials.
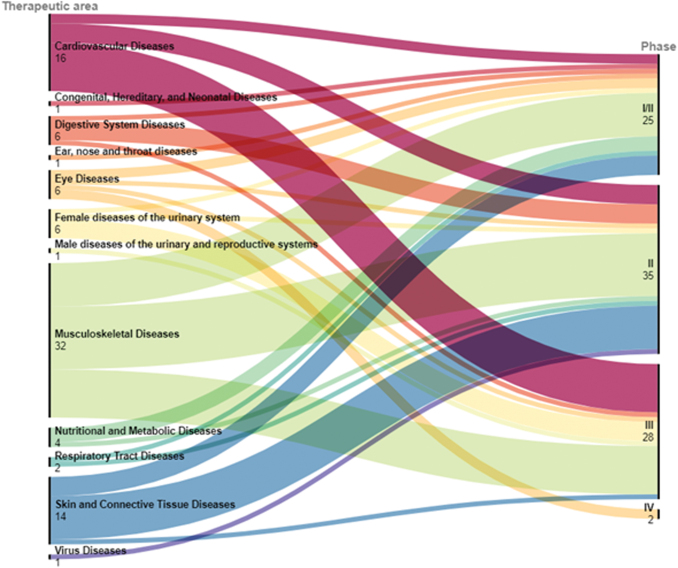
The most common therapeutic areas targeted by TEPs include musculoskeletal disorders, cardiovascular diseases, urinary diseases (incontinence), ocular diseases, skin and connective tissue disorders. This is reflected by the observation that most trials with TEPs are investigating the efficacy in musculoskeletal diseases (32), cardiovascular diseases (16), and skin/connective tissue diseases (14). Of the 14 trials underway in skin/connective tissue disorders, 11 are Phase I/II or II trials that have been started after 2016, while studies in musculoskeletal and cardiovascular diseases have been steadily added since 2009. This indicates an emerging interest in this therapeutic area. Multiple trials have also taken place in eye diseases (6), digestive system (6), and female urinary system indications (6) (Fig. 2). Rare disease indications made up 17% (15/90) of all trials. However, it should be noted that these consist of only 10 different products, 5 of which have a EU orphan drug designation (ODD).
FIG. 2.
Medical conditions under investigation summarized by therapeutic area and trial phase. The number of trials in various therapeutic areas (left) is matched to the trial phase (right), providing an overview of the proportion of therapeutic areas in early- and late-phase trials. The EMA provides updates and opinions on therapeutic areas for approved medicines.42 Many entries have been revised and corrected due to sponsor errors and inaccuracies. Errors include the sponsors listing therapeutic area as “Analytical, Diagnostic and Therapeutic Techniques and Equipment—Surgical Procedures, Operative,” “Body processes—Cell Physiological Phenomena,” and “Not possible to specify” for TEPs that repair focal cartilage defects, which should be listed as “Musculoskeletal Diseases.” EMA, European Medicines Agency. Color images are available online.
Trends in clinical trial characteristics
Cell source and commercial preference
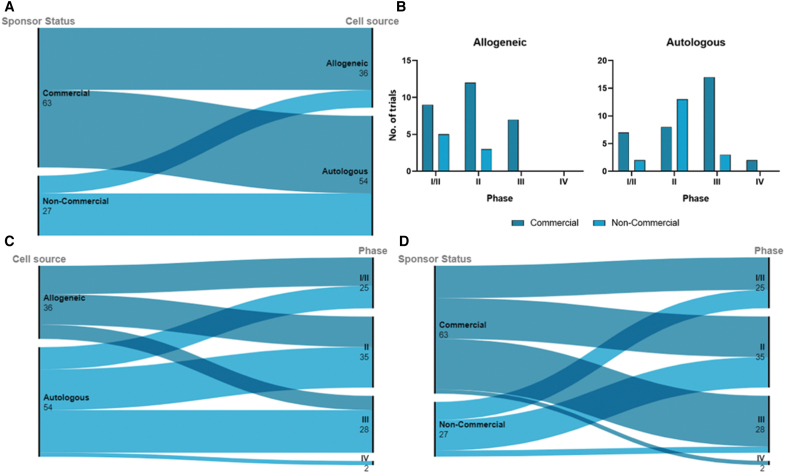
All TEPs in clinical trials in the EU contain either autologous or allogeneic human cells. Sixty percent (54/90) of studies used autologous cells, while 40% were prepared with expanded allogeneic cells (Fig. 3). There are numerous advantages and disadvantages in the regulation and manufacture of autologous over allogeneic cells. Autologous cells incur no immunogenicity on the recipient, who is also the donor. On the other hand, this requires single-patient batches, with lower production capacity and high variability in the final product. The production process is dependent on cell availability from the donor and donor site morbidity.14
FIG. 3.
Distribution of autologous and allogeneic cell use across sponsor types and trial phase. Commercial status is self-reported by sponsor input. (A) Commercial sponsors fund a great proportion of trials using allogeneic cells versus autologous cells. In contrast, noncommercial sponsors funded a greater proportion of trials using autologous cells. (B) Overview of the number of trials in each phase using autologous and allogeneic cells, further divided by sponsor status. (C) Distribution of cell origin in the trial phase. Autologous cells have been used in a greater proportion of late-phase trials. (D) Commercial sponsors have funded a greater proportion of late-phase trials over noncommercial sponsors. Statistical analysis of these data is presented in Table 1. Color images are available online.
Allogeneic cells retain use in a significant proportion of TEP clinical trials, due to their many advantages. Variability can be controlled, making larger batch sizes possible, depending on the possibility of master cell banking.15 Lower production costs and the possibility for off-the-shelf products are of high value in emergency and time-dependent indications,15 increasing the attraction of industry. Recent advances in allogeneic cell therapies have addressed alloreactivity that results in graft versus host disease, for example, T cell receptor deletion in CAR-T cells (GTMP).16 Mechanisms of tolerance induction against immunogenicity have been described in artificial skin substitutes, including chemical reagents and photodynamic therapy and gene transfection approaches to suppress dendritic cell activity.17
Lentiviral vectors have also been used to silence human leukocyte antigen (HLA) class expression to produce low immunogenic endothelial cells, while co-transplantation of mesenchymal stromal cells (MSCs) reduced immune rejection in allogeneic cardiomyocyte transplantation.18,19 Still, the immunogenicity of allogeneic cells is the main challenge for further development. In this analysis, there is a significant association between cell origin and trial phase, where a greater proportion of autologous cell products are in later phases of development than allogeneic cell products (FET, p = 0.0248) (Table 1). This may be due to the historical preference for autologous cells, which are more likely to be at later trial stages than recently developed products using allogeneic cell sources.
Table 1.
Statistical Analysis of Categorical Data of All Tissue-Engineered Product Clinical Trials from 2009 to December 31, 2021
| Commercial status vs. early/late phase | |||
|---|---|---|---|
| Commercial | Noncommercial | Total | |
| Phase I/II and/or II | 36 | 24 | 60 |
| Phase III and or IV | 27 | 3 | 30 |
| Total | 63 | 27 | 90 |
| Fisher's exact test | 0.0034 | ||
| Commercial status vs. cell type | |||
| Commercial | Noncommercial | Total | |
| Allogeneic | 28 | 8 | 36 |
| Autologous | 35 | 19 | 54 |
| Total | 63 | 27 | 90 |
| Fisher's exact test | 0.2427 | ||
| Cell type vs. early/late phase | |||
| Allogeneic | Autologous | Total | |
| Phase I/II and/or II | 29 | 31 | 60 |
| Phase III and/or IV | 7 | 23 | 30 |
| Total | 36 | 54 | 90 |
| Fisher's exact test | 0.0248 | ||
Fisher's exact test was used to determine a significant association between commercial status, cell type, and trial phase (categorized as early or late). There was a statistically significant association between commercial status and trial phase (p = 0.0034) and cell type and trial phase (p = 0.0248), while there was no significant association between commercial status and cell type (p = 0.2427).
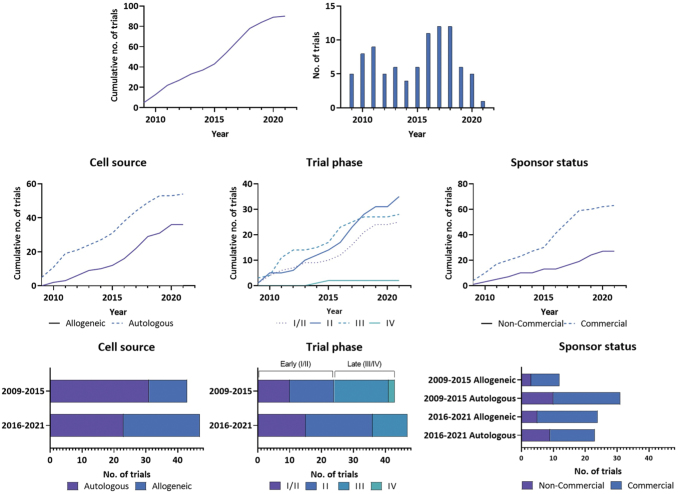
In the past 2 years, the trend shows more trials in allogeneic than autologous products compared to the first 5 years (Fig. 5). Both commercial and noncommercial sponsors fund a similar proportion of allogeneic TEPs, likely due to the ease of processing and manufacture to scale up production; however, this option will depend on the target tissue, how immunogenic this might be, or if the product can be made less immunogenic, such as through HLA typing.20
FIG. 5.
Trends in trial characteristics from 2009 to 2021, inclusive. Characteristics from the first 6 years since regulation introduction (2009–2015) (n = 43) were compared to the last 5 years of trial entries to date (2016–2021) (n = 47). Two different time frames were used to capture a similar number of studies. A significant relationship was identified between time period and cell source (X2 = 5.02, p = 0.0251) and trial phase (X2 = 4.36, p = 0.037). No relationship was observed for the time period and commercial status (p = 0.9288). Color images are available online.
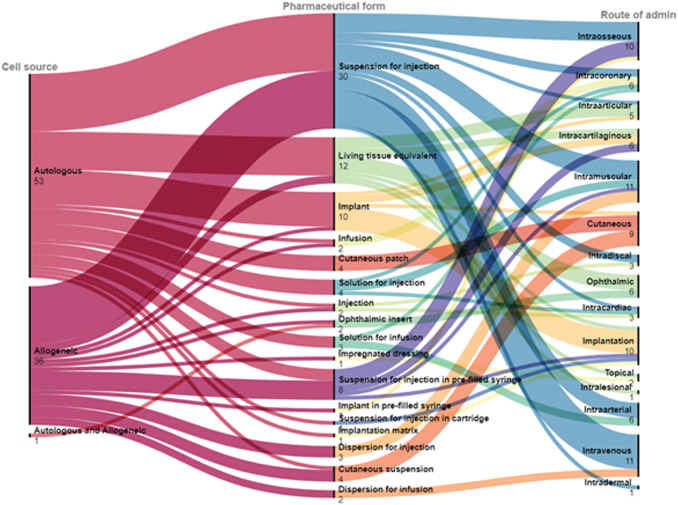
Pharmaceutical form and storage
The European Directorate for the Quality of Medicines (EDQM) & HealthCare database has defined standard terms to cover pharmaceutical dose forms in response to a request from the European Commission.21 Unfortunately, there are still many discrepancies in the data entered by sponsors, varying across studies for the same product. Ambiguity remains with forms such as “implant” encompassing the term “living tissue equivalent,” while the reason to use forms such as “dispersion,” “suspension,” and “solution” with injection and infusion is not transparent and seems to be used interchangeably by sponsors.
Living tissue equivalents are defined as “Cultured, living tissue used for the reconstruction of parts of the body. The tissue may consist of ex vivo expanded cells with an extracellular matrix.”22 Where appropriate, the tissue of origin, such as epidermis, dermis, cartilage, or muscle, must be stated elsewhere in the product information.22 Allogeneic and autologous cells were equally distributed among pharmaceutical forms, delivered through various routes of administration (Fig. 4). This reflects that the cells always need to be in a suspension/solution to retain adequate oxygenation/nutrition across small diffusion gradients.
FIG. 4.
Details of the IMPs used in TEP trials. Autologous and allogeneic cells were used in all trials. The “Standard Terms Database” generated by the EDQM is used to describe the pharmaceutical form and route of administration of IMPs. EDQM, European Directorate for the Quality of Medicines; IMPs, investigational medicinal products. Color images are available online.
Another important aspect is that it appears TEPs usually need to be provided fresh rather than frozen. Therefore, the shelf life (time for long-term storage) mostly including the transport time is usually short (12–72 h). For authorized TEPs, the shelf-life is highly variable, but there is still a maximum limit at about 4 days when cells start to deteriorate and lose function. According to the product EPAR, Spherox may be stored for 72 h between 1°C and 10°C, while ChondroCelect and MACI had proposed shelf lives of 48 h and 6 days (exceptionally long), respectively. Holoclar is recommended to be stored between 15°C and 25°C for a maximum of 36 h.
While freezing requires a chain of custody in a robust cold-chain delivery system, which may carry significant cost implications and increase transport complexity, it is advantageous for increasing shelf life and providing time for full release testing of the product.23 Inability to freeze TEP products, for example, requires adapted sterility testing regimes and negative to date testing with the risk that the product a patient receives might contain microbial contamination at the time of release.
Therefore, the development and application of rapid sterility methods are further encouraged.24 So, where products still have to be provided as fresh/unfrozen, other solutions are developed such as cell banking or a rapidly accessible donor database for a cell source and starting materials or intermediates that can be frozen (e.g., peripheral blood mononuclear cells or MSCs) for on-demand manufacturing to create fresh products. These options can better be realized with allogeneic TEPs, which adds to the advantages to develop allogeneic products rather than autologous.
Orphan drug designation
The EMA facilitates the development and authorization of medicines for rare diseases through ODD, which provides protocol assistance, access to the centralized authorization procedure, and market exclusivity for 10 years upon successful MA with additional incentives for small- and medium-sized enterprises.25 Companies pursuing ODD also benefit from fee reductions for regulatory activities.25 In addition, companies benefit from policies within member states, such as ease of reimbursement versus non-ODD products. France, Germany, and The Netherlands reimburse nearly all ODD products with few exceptions, while less than half of ODD products are reimbursed in UK.26
Despite the regional incentives and market access, the risk in pursuing ODD product development and authorization is high, as similar products may be faster in achieving successful MA. Very few ODDs are found among the TEP clinical trials (only one Orphan TEP is authorized: Holoclar). Nine trials (10%, 9/90) have investigated TEPs with six unique ODDs, while two designations were subsequently withdrawn by the sponsor. These trials include one Phase I/II trial, four Phase II trials, and two Phase III trials. The two Phase IV (postmarketing) trials found in the database are concerning the already marketed product Holoclar, reflecting to fulfill the postapproval obligations to collect additional efficacy and/or safety data to support the conditional MA.
Four active ODDs are in place in therapeutic areas, including eye diseases (LSCD), skin and connective tissue disorders (full-thickness burns and skin defects), female reproductive health (Asherman's syndrome), and acute liver failure. PREOB®, human autologous bone-forming cells, by Bone Therapeutics® SA (Belgium) was granted ODD to treat osteonecrosis of the femoral head, but ODD was withdrawn by the sponsor in 2019.27 An additional product produced by Shire Medical® (Ireland) consisting of human dermal fibroblasts cultured on a bioresorbable polyglactin mesh for the treatment of epidermolysis bullosa (Dermagraft®) had received ODD; however, this has since been withdrawn by request of the sponsor.28 It can be summarized that ODD is not used frequently in TEP development and that usually the therapeutic areas and more specifically the indications are applicable to a broader population.
Sponsor commercial status
Preliminary analysis demonstrates that trials in Phase III/IV are far more likely to have commercial sponsorship than earlier phases, likely due to the financial investment necessary to run these trials and the higher likelihood to be successful in this later stage. There was a significant association between sponsor status and trial phase as commercial sponsors funded a greater proportion of Phase III/IV trials (FET, p = 0.0034) (Table 1).
Classically, phase III trials are so-called “confirmatory” trials, providing the decisive (pivotal) data for a Marketing Authorisation Application (MAA). However, it must be acknowledged that classical trial progression from Phase I to Phase III is rarely followed by ATMPs and Phase II or II/III trials can also provide pivotal data to ATMP MAAs. This is especially the case for Orphan medicinal products, where companies often strive for a conditional MA and where conducting large controlled Phase III trials is unfeasible. For example, Holoclar was authorized based on two retrospective nonrandomized, uncontrolled multicenter observational studies based on an efficacy population of 133 patients.29 Postmarketing data are currently collected in Phase IV trials.
Commercial sponsors have funded 69% of all trials, including 89% (25/28) of Phase III trials and 100% (2/2) of Phase IV trials (one prospective uncontrolled trial and one long-term follow-up trial investigating Holoclar). Of the 25 commercially funded trials, 36% (9/25) were from non-EU sponsors. Noncommercial sponsors of Phase III trials include The Goethe University Frankfurt, Germany, which has sponsored a Phase III trial to investigate the intracoronary application of autologous bone marrow-derived mononuclear cells on mortality in patients with chronic postinfarction heart failure.
This trial was funded by LOEWE, a research promotion program in Hessen, Germany.30 Another Phase III trial funded by the European Commission FP7 program to the value of 5.9 million euro, investigated the use of bone marrow-derived mononuclear cells on mortality in acute myocardial infarction.31 Finally, Horizon 2020 program funded the ORTHOUNION project, which had been developed from an FP-7 project (REBORNE). This included an investment of 5.9 million euros to investigate the use of expanded bone marrow-derived MSCs in nonunion fractures of long bones.32
Critical analysis of clinical trial activity
There are many limitations to the interpretation of this overview of data from clinical trials presented above. This review only captures trials listed on the EU Clinical Trials Register (Phase I/II–IV). Information on Phase I clinical trials is entered in the EU Clinical Trials Register. but not accessible to the public (except Phase I studies. including pediatric populations).33 Therefore, the number of Phase I trials is unknown. It can only be assumed that more trials have been/are performed with ATMP, including TEPs than provided in this study. In addition, this analysis only reflects the state of the database at the time of data extraction (January 22, 2022) and does not account for data cleaning activities by agencies at various intervals.
Several studies investigating TEPs have inaccurate and conflicting information entered into the EU Clinical Register. Throughout the trial data review, information on product classification, study status, and results posting were inconsistent. For example, using terms like (tissue) engineered cells and cell therapy is not always consistent, and might not always fulfill the ATMP regulation's definition of engineered cells requiring “substantial manipulation” or where the intended use of cells or tissues “is not for the same essential function in the recipient as in the donor.” Considering these limitations, the number of studies with TEPs is lower compared with somatic cell therapies and considerably lower than gene therapies (∼159 in 2020).34,35 Despite this, more TEPs than somatic cell therapies have undergone a successful marketing authorization procedure.
In this review, it was observed that there has been a shift in cell sources in recent years as between 2016 and 2021, 51% (24/47) of studies used allogeneic sources, while between 2009 and 2015, only 28% (12/43) of studies used allogeneic cells. There was a significant association between these time periods and the cell source used in clinical trials (X2 = 5.02, p = 0.0251). Commercial sponsors alone have shifted toward allogeneic sources, from 30% (9/30) of commercial studies between 2009 and 2015 to 61% (19/31) of commercial studies using allogeneic cells between 2016 and 2021 (X2 = 6.01, p = 0.014) (Fig. 5).
A significant association between early versus recent time period and trial phase was identified (X2 = 4.36, p = 0.037). A higher proportion of Phase I/II and II clinical trials have been registered in recent years, potentially indicating a renewed interest in the development of TEPs. Trial entries were exceptionally high from 2016 to 2018, increasing the proportion of allogeneic cell sources, the proportion of Phase II trials, and the proportion of trials run by commercial sponsors. It has to be acknowledged that due to the COVID19 pandemic, most probably, a lower amount of trials has been initiated than it might have been expected,36,37 so it is unknown if that trajectory may have continued without the pandemic.
Of the four TEPs that have gained MA under EC/1394/2007, three have the same indication of focal chondral defect repair in the knee. Of these, two have been withdrawn citing poor commercial performance.38,39 Spherox from co.don® AG appears to be gaining market share, having commenced building a second production site in 2017 to meet demand following MA, receiving its manufacturing licence in 2020.40 No information is currently available on the profitability of Spherox; however, over 16,000 patients have already been treated with the product.41 Holoclar remains approved, although this is for a rare disease indication and is unlikely to see widespread adoption due to disease rarity.
Of the four approved products, three had already been on the market in an earlier less defined product version before the introduction of EC/1394/2007, the experience in production and clinical administration of the products providing some advantage to plan their confirmatory trial(s). On the other hand, clinical data that are not systematically collected cannot support an MAA and correctly designed, controlled trials have also been necessary in these cases.
Conclusions
This study has identified trends in clinical trial progression for TEPs, identifying the role of funding source, commercial status, cell origin, updated regulations, and national and international policies on product development and trial progression. Key findings suggest that the following:
Musculoskeletal and cardiovascular remain the therapeutic areas of focus for TEP trial activity, while products for skin and connective tissue disorders are being increasingly investigated in early trials in recent years.
Germany, UK and Spain have hosted the highest number of clinical trial sites.
Commercial sponsors fund most trials, including non-EU sponsors, representing a significant proportion of trial activity.
Commercial sponsors fund far more late-phase trials than noncommercial sponsors. This is no surprise as late-phase trials are much costlier than early-phase trials involving more patients and centers. Also, in this phase, the commercial sponsors usually get into contact with the EMA and national agencies to discuss and streamline the clinical trial design for the purpose of a later MA.
Commercial sponsors also fund about one-third more Phase I/II and II trials than noncommercial sponsors. It can be assumed that some of these commercial sponsors in early phases are small Biotech Companies, including spin-offs from academia.
The rate of new trials being listed each year has not increased significantly since the introduction of EC/1394/2007; however, there appears to be a biphasic trend in the number of trials being listed with a spike in 2010–2011 and again in the 2016–2018 period. The regulation's transition phase for TEPs marketed before 2009 ended in December 2012. This was connected to an incentive omitting payment of the EMA submission fee. It is possible that the first spike is at least partly due to the end of the transition phase with resurgence of activity in new TEP developments seen in 2016, but this interpretation cannot be confirmed. There is another decline in trial entries in 2020–2021, which is likely at least partly due to the COVID-19 pandemic.
There has been a shift toward the use of allogeneic cells in TEPs in recent years.
Increased proportion of the early-phase trial phase has been noted, indicating a renewed interest in the development of TEPs.
As the landscape for TEP development and market access has evolved since the introduction of EC/1394/2007, clinical trial activity has adapted in response to regulatory and economic pressures to maximize the potential for product translation. While trial activity continues to be driven by commercial sponsors, with most activity in a few member states, there is a clear shift toward using allogeneic cells and an increased proportion of early-phase trials by noncommercial sponsors. This trend would be expected to continue as more early trials are listed in the coming years in parallel to existing trials progressing to later phases. Upcoming trials will continue to streamline navigation of regulatory processes to evolve for the benefit of all stakeholders, to realize the translation of TEPs, with prospects of more TEP MA applications in future.
Acknowledgments
The authors would like to acknowledge Dr. Christian K. Schneider (Pharmalex) for reviewing and Dr. Raghvendra Bohara for proofreading this article.
Authors' Contributions
K.J.: conceptualization, methodology, software, and formal analysis. Z.B.: conceptualization, methodology, and validation. G.R.: validation. M.K.B.: validation, review. A.P.: conceptualization, review, and funding acquisition.
Disclosure Statement
Z.B., G.R., and M.K.B. are employed by PharmaLex GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Funding Information
This publication has emanated from research conducted with the financial support of the College of Medicine, Nursing and Health Sciences, NUI Galway, Science Foundation Ireland (SFI), and the European Regional Development Fund (grant no. 13/RC/2073_P2) and the European Commission's Horizon 2020 funding program for the iPSpine project (grant no. 825925).
References
- 1. The European Parliament and the Council of the European Union. Regulation (EC) no 1394/2007 of the European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) no 726/2004. Off J Eur Union 2007;L324/121:1–17. [Google Scholar]
- 2. Warreth S, Harris E. The regulatory landscape for ATMPs in the EU and US: A comparison. Lev 3 2020;15(2):5; doi: 10.21427/pk3v-g445 [DOI] [Google Scholar]
- 3. CAT Monthly Report of Application Procedure, Guidelines and Related Documents and Advance Therapies. May 2021 Meeting; 2021. Available from: www.ema.europa.eu/contact [Last accessed: July 30, 2021].
- 4. Committee for Medicinal Products for Human Use. European Public Assessment Report for MACI. Procedure No. EMEA/H/C/002522/0000. London; 2013. Available from: https://www.ema.europa.eu/en/documents/assessment-report/maci-epar-public-assessment-report_en.pdf [Last accessed: June 1, 2021].
- 5. Committee for Medicinal Products for Human Use. European Public Assessment Report for Chondrocelect. Procedure No. EMEA/H/C/000878. London; 2009. Available from: https://www.ema.europa.eu/en/documents/assessment-report/chondrocelect-epar-public-assessment-report_en.pdf [Last accessed: June 1, 2021].
- 6. Committee for Medicinal Products for Human Use (CHMP). European Public Assessment Report for Zalmoxis. Procedure No. EMEA/H/C/002801/0000; 2016. Available from: www.ema.europa.eu/contact [Last accessed: February 10, 2022].
- 7. Committee for Medicinal Products for Human Use (CHMP). European Public Assessment Report for Provenge. Procedure No. EMEA/H/C/002513/0000; 2013. Available from: www.ema.europa.eu [Last accessed: February 10, 2022].
- 8. First Stem-Cell Therapy Recommended for Approval in EU; 2014. Available from: https://www.ema.europa.eu/en/news/first-stem-cell-therapy-recommended-approval-eu [Last accessed: May 24, 2021].
- 9. Hubert A. ATMPs in Europe—State of Play. Berlin; 2020. Available from: https://alliancerm.org/wp-content/uploads/2020/02/CBX-Meeting-7-Feb-2020-FINAL.pdf [Last accessed: June 1, 2021].
- 10. Lorenz M, Aisch G, Kokkelink D. Datawrapper: Create Charts and Maps [Software]; 2012. Available from: https://www.datawrapper.de/[Last accessed: July 2, 2021].
- 11. Mauri M, Elli T, Caviglia G, et al. RAWGraphs: A visualisation platform to create open outputs. In: ACM International Conference Proceeding Series Association for Computing Machinery; 2017; doi: 10.1145/3125571.3125585 [DOI] [Google Scholar]
- 12. Lindahl A. From gristle to chondrocyte transplantation: Treatment of cartilage injuries. Philos Trans R Soc B Biol Sci 2015;370(1680):20140369; doi: 10.1098/RSTB.2014.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boráň T, Menezes-Ferreira M, Reischl I, et al. Clinical development and commercialization of advanced therapy medicinal products in the European Union: How are the product pipeline and regulatory framework evolving? Hum Gene Ther Clin Dev 2017;28(3):126–135; doi: 10.1089/humc.2016.193 [DOI] [PubMed] [Google Scholar]
- 14. Verboket R, Leiblein M, Seebach C, et al. Autologous cell-based therapy for treatment of large bone defects: From bench to bedside. Eur J Trauma Emerg Surg 2018;44(5):649–665; doi: 10.1007/S00068-018-0906-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbasalizadeh S, Pakzad M, Cabral JMS, et al. Allogeneic cell therapy manufacturing: Process development technologies and facility design options. Expert Opin Biol Ther 2017;17(10):1201–1219; doi: 10.1080/14712598.2017.1354982 [DOI] [PubMed] [Google Scholar]
- 16. Depil S, Duchateau P, Grupp SA, et al. ‘Off-the-shelf’ allogeneic CAR T cells: Development and challenges. Nat Rev Drug Discov 2020;19(3):185–199; doi: 10.1038/s41573-019-0051-2 [DOI] [PubMed] [Google Scholar]
- 17. Dixit S, Baganizi DR, Sahu R, et al. Immunological challenges associated with artificial skin grafts: Available solutions and stem cells in future design of synthetic skin. J Biol Eng 2017;11(1):1–23; doi: 10.1186/S13036-017-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Figueiredo C, Eicke D, Yuzefovych Y, et al. Low immunogenic endothelial cells endothelialize the left ventricular assist device. Sci Rep 2019;9(1):11319; doi: 10.1038/s41598-019-47780-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshida S, Miyagawa S, Toyofuku T, et al. Syngeneic mesenchymal stem cells reduce immune rejection after induced pluripotent stem cell-derived allogeneic cardiomyocyte transplantation. Sci Rep 2020;10(1):4593; doi: 10.1038/s41598-020-58126-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahdi BM. A glow of HLA typing in organ transplantation. Clin Transl Med 2013;2(6):6; doi: 10.1186/2001-1326-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Standard Terms Database | EDQM—European Directorate for the Quality of Medicines. n.d. Available from: https://www.edqm.eu/en/standard-terms-database [Last accessed: February 11, 2022].
- 22. European Dictorate for the Quality of Medicines and Healthcare. Living Tissue Equivalent, 2015. Available from: https://standardterms.edqm.eu/reports/default_pdf/296/full [Last accessed: February 11, 2022].
- 23. Overcoming the Challenges and Complexities of the Personalized Medicine Supply Chain. n.d. Available from: https://logistics.quick.aero/newsroom/overcoming-the-challenges-and-complexities/ [Last accessed: August 13, 2021].
- 24. Guidelines on good manufacturing practice specific to advanced therapy medicinal products document history. EudraLex Rules Gov Med Prod Eur Union 2017;4(1):47–52. [Google Scholar]
- 25. Orphan Incentives. n.d. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/orphan-designation/orphan-incentives [Last accessed: July 24, 2021].
- 26. Czech M, Baran-Kooiker A, Atikeler K, et al. A review of rare disease policies and orphan drug reimbursement systems in 12 Eurasian countries. Front Public Heal 2019;7(1):416; doi: 10.3389/FPUBH.2019.00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. EU/3/07/490; 2019. Available from: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu307490 [Last accessed: July 30, 2021].
- 28. European Medicines Agency. Public Summary of Opinion on Orphan Designation Human Dermal Fibroblasts Cultured on a Bioresorbable Polyglactin Mesh for the Treatment of Epidermolysis Bullosa; 2014. Available from: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/11/873-public-summary-opinion-orphan-designation-human-dermal-fibroblasts-cultured-bioresorbable_en.pdf [Last accessed: September 7, 2021].
- 29. Committee for Medicinal Products for Human Use. European Public Assessment Report for Holoclar. Procedure No. EMEA/H/C/002450/0000; 2014. Available from: https://www.ema.europa.eu/en/documents/assessment-report/holoclar-epar-public-assessment-report_en.pdf [Last accessed: May 31, 2021].
- 30. Assmus B, Alakmeh S, De Rosa S, et al. Improved outcome with repeated intracoronary injection of bone marrow-derived cells within a registry: Rationale for the randomized outcome trial REPEAT. Eur Hear J 2016;37(21):1659–1666; doi: 10.1093/eurheartj/ehv596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Queen Mary University of London. The Effect of Intracoronary Reinfusion of Bone Marrow-Derived Mononuclear Cells (BM-MNC) on All-Cause Mortality in Acute Myocardial Infarction; 2011. Available from: https://cordis.europa.eu/project/id/278967/reporting [Last accessed: July 24, 2021].
- 32. Universidad Autonoma de Madrid. ORTHOpedic Randomized Clinical Trial with Expanded Bone Marrow MSC and Bioceramics versus Autograft in Long Bone NonUNIONs; 2017. Available from: https://cordis.europa.eu/project/id/733288 [Last accessed: July 24, 2021].
- 33. EudraCT & EU-CTR Question and Answer Table; 2017. Available from: https://eudract.ema.europa.eu/help/content/resources/documents/EudraCT FAQ_for publication.pdf [Last accessed: August 13, 2021].
- 34. Ilieva K, Borissov B, Toumi M. Gene therapy randomised clinical trials in Europe—A review paper of methodology and design. J Mark Access Heal Policy 2020;8(1):1847808; doi: 10.1080/20016689.2020.1847808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clinical Trials in Europe: Recent Trends in ATMP Development; 2019. Available from: https://alliancerm.org/wp-content/uploads/2019/10/Trends-in-Clinical-Trials-2019-Final_Digital.pdf [Last accessed: July 30, 2021].
- 36. Hawila N, Berg A. Assessing the impact of COVID-19 on registered interventional clinical trials. Clin Transl Sci 2021;14(3):1147–1154; doi: 10.1111/CTS.13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nishiwaki S, Ando Y. COVID-19 pandemic and trends in clinical trials: A multi-region and global perspective. Front Med 2021;8(1):2800; doi: 10.3389/FMED.2021.812370/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Committee for Medicinal Products for Human Use. Closure of EU Manufacturing Site for MACI. London; 2014. Available from: https://www.ema.europa.eu/en/documents/referral/maci-article-20-procedure-closure-eu-manufacturing-site-maci_en.pdf [Last accessed: June 1, 2021].
- 39. Ronco V, Dilecce M, Lanati E, et al. Price and reimbursement of advanced therapeutic medicinal products in Europe: Are assessment and appraisal diverging from expert recommendations? J Pharm Policy Pract 2021;14(1):1–11; doi: 10.1186/s40545-021-00311-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hildreth C. The New Co.Don Plant for the EU-Approved Advanced Therapy Cartilage Regeneration Treatment Sets a Milestone in Cell Therapy for Commercial Manufacturing; 2021. Available from: https://bioinformant.com/co-don-ag-human-cell-experts/[Last accessed: June 1, 2021].
- 41. Meißner M. Co.Don AG: Technology Transfer Process Gets Green Light from EMA; 2021. Available from: https://www.codon.de/en/unternehmen/news/codon-ag-technologietransfer-ema-seitig-abgeschlossen [Last accessed: June 10, 2021].
- 42. Therapeutic Areas: Latest Updates; 2021. Available from: https://www.ema.europa.eu/en/news-events/therapeutic-areas-latest-updates [Last accessed: August 4, 2021].