Abstract
Background:
The current social and legal landscape is likely to foster the medicinal and recreational use of cannabis. Synthetic cannabinoid use is associated with acute kidney injury (AKI) in case reports; however, the association between natural cannabis use and AKI risk in patients with advanced chronic kidney disease (CKD) is unknown.
Materials and Methods:
From a nationally representative cohort of 102,477 U.S. veterans transitioning to dialysis between 2007 and 2015, we identified 2215 patients with advanced CKD who had undergone urine toxicology (UTOX) tests within a year before dialysis initiation and had inpatient serial serum creatinine levels measured within 7 days after their UTOX test. The exposure of interest was cannabis use compared with no use as ascertained by the UTOX test. We examined the association of this exposure with AKI using logistic regression and inverse probability of treatment weighting with extensive adjustment for potential confounders.
Results:
The mean age of the overall cohort was 61 years; 97% were males, 51% were African Americans, 97% had hypertension, 76% had hyperlipidemia, and 75% were diabetic. AKI occurred in 56% of the cohort, and in multivariable-adjusted analysis, cannabis use (when compared with no substance use) was not associated with significantly higher odds of AKI (odds ratio 0.85, 95% confidence interval 0.38–1.87; p=0.7). These results were robust to various sensitivity analyses.
Conclusions:
In this observational study examining patients with advanced CKD, cannabis use was not associated with AKI risk. Additional studies are needed to characterize the impact of cannabis use on risk of kidney disease and injury.
Keywords: chronic kidney disease, acute kidney injury, end-stage kidney disease, cannabinoids, urine toxicology, multinomial propensity score weights
Introduction
Chronic kidney disease (CKD) is a global public health problem.1–3 Acute kidney injury (AKI) is a risk factor for development of CKD, and experiencing AKI may worsen CKD progression and hasten the development of end-stage kidney disease.4–7 The rise in cannabis use is also a rapidly evolving public health concern that may impact various health problems, including CKD.8 The increasing number of people using cannabis over the past two decades is most likely related to a persistent trend toward legalization of cannabis (both medicinal and recreational) in the United States and worldwide.8,9
In light of the current legal trends, it is expected that cannabis use will continue to increase in the coming years. This is especially the case in older adults with multiple comorbidities10,11 given the growing medicinal applications of this substance and its related components. This patient population will include greater numbers of individuals with chronic diseases.11 In this regard, patients with advanced CKD are often prescribed opioids and non-steroidal anti-inflammatory drugs (NSAIDs) for pain management.12 However, these medications can induce many adverse effects including opioid dependence which may lead to addiction and potential nephrotoxic properties which limits their safe use in CKD.13 Given the potentially less severe adverse effect profile associated with cannabis when compared with the traditional pain control medications, patients with advanced CKD may opt for cannabis-based regimens for their symptoms.8,14 The increased likelihood for exposure of patients with CKD to cannabis highlights the need for a deeper understanding of the real-world effects of cannabis use on kidney outcomes.
The main psychotropic active ingredient in cannabis (tetrahydrocannabinol [THC]) exerts its effects by acting on cannabinoid receptors type 1 (CB1) and type 2 (CB2), which are expressed in multiple organs throughout the body, including the kidneys.11,15–17 These receptors can also be activated by other compounds commonly found in cannabis collectively referred to as cannabinoids (e.g., cannabinol [CBN]),11,17 and endogenous ligands that are commonly referred to as endocannabinoids (ECs). The cannabinoid receptors together with the endogenous ligands that act on these receptors and the machinery involved in their synthesis and breakdown comprise the EC system.11,17,18 There is accumulating evidence that the EC system plays a significant role in maintaining normal homeostasis, and alterations of this system can lead to various pathological conditions including CKD and AKI.11,17,18 Previous research using in vitro and in vivo preclinical models of kidney disease has found that alterations of CB1 and CB2 receptors (e.g., in the localization or expression of CB receptors or downstream signaling subunits of CB receptors) can play a role in the pathogenesis of various renal conditions including AKI. Also, previous preclinical research has shown that inhibition of CB1 receptor and/or activation of CB2 receptor have shown to be renoprotective.11,19–22 Despite the abundance of preclinical data, clinical studies evaluating the impact of cannabis and cannabinoids on kidney disease and injury are limited. More recently, there have been series of case reports linking exposure to synthetic cannabinoids (SCBs) to increased risk of AKI and the need for renal replacement therapy.11,23–26 These reports have raised concern over the impact of cannabis use on kidney function and on the pathogenesis of kidney disease including AKI, especially in patients with pre-existing kidney disease.
There is a paucity of epidemiological studies exploring the association between natural/non-SCBs use and AKI. The aim of this study was to examine the association between cannabis use and the incidence of AKI in a large cohort of patients with advanced CKD. We hypothesized that cannabis use would be associated with higher incidence of AKI.
Materials and Methods
Study population
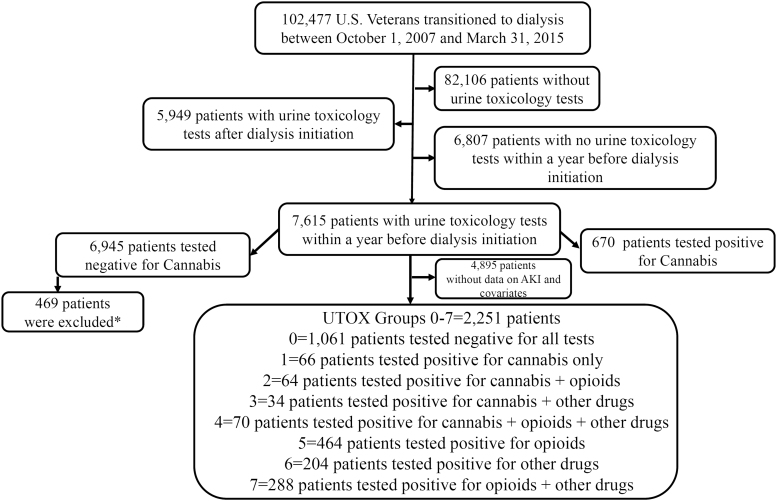
We examined a nationally representative cohort of U.S. veterans with incident end stage renal disease (ESRD) who transitioned to renal replacement therapy from October 1, 2007, through March 31, 2015 (Transition of Care in Chronic Kidney Disease [TC-CKD]).27,28 The TC-CKD cohort consisted of 102,477 U.S. veterans identified from the United States Renal Data System (USRDS).29 Applying various inclusion and exclusion criteria (Supplementary Methods) resulted in a study population of 2251 patients. Figure 1 describes the sample selection criteria.
FIG. 1.
Study population for AKI outcome. *Four hundred sixty-nine patients tested positive for cannabis any time before 365 days of dialysis initiation or after dialysis initiation. AKI, acute kidney injury.
Exposure
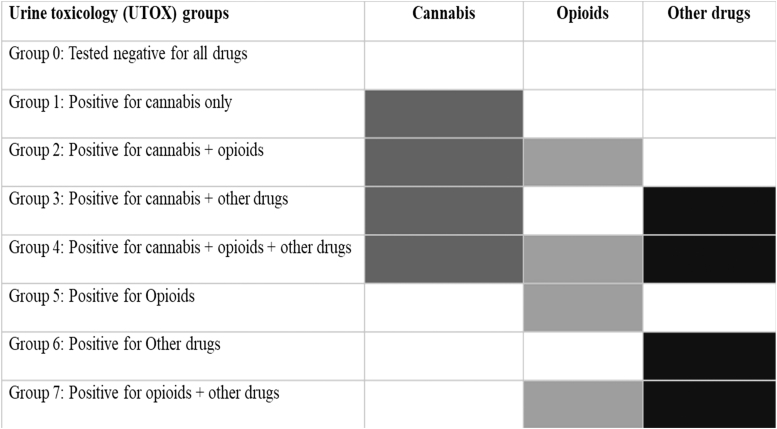
Our primary analysis compared patients with a cannabis-only positive toxicology screen with patients whose toxicology screens were negative for all tested substances. In patients who had undergone a urine toxicology (UTOX) test in the 1-year prelude, cannabis status (positive vs. negative, based on 50 ng/mL cutoff for cannabinoids, CBN, cannabis, and THC) was ascertained by the validated algorithm by Morasco et al.30 Of the 2251 patients, 1061 patients had negative test results for all the toxicology tests and 234 patients tested positive for cannabis. Of the 234 cannabis-positive patients, 66 patients tested positive for cannabis use only. Previous research has shown that cannabis users are more prone to polysubstance use.31 Hence, we further classified the rest of 168 cannabis users as combined users of opioids and/or other drugs. The remaining 956 patients were opioids/other polysubstance users without cannabis exposure and served as positive controls in our analyses (Fig. 2 and Supplementary Methods).
FIG. 2.
Color matrix for the UTOX groups. Description of UTOX groups 0–7. UTOX, urine toxicology.
Covariates
Multivariable models were adjusted for a priori specified variables including sociodemographics, comorbidities, medications, and vital signs, as listed below.8,24,32–35 Data from the USRDS Patient and Medical Evidence file were used to determine patients' baseline information on age, sex, and race at a year before dialysis initiation (1-year prelude). Pre-existing comorbidities (Supplementary Methods) were identified from the VA Inpatient and Outpatient Medical SAS Datasets, and the VA/CMS databases, using International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic and Current Procedural Terminology codes. The Charlson comorbidity index scores were estimated using the Deyo modification for administrative data sets without including kidney disease.36 Smoking information was extracted from VA health factors data.37,38 Vascular access data were obtained from the USRDS Patient and Medical Evidence Form 2728.39 Information about potentially nephrotoxic medication40,41 use (at least one prescription, Supplementary Methods) during the year before dialysis initiation was collected from both VA pharmacy dispensation records and CMS Medicare Part D files.40,42–44 The VA Vital Status file was used to obtain data on systolic blood pressure, diastolic blood pressure, body mass index, and pain score. We used the mean value of all measurements performed within a year before dialysis initiation for each of these variables. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), using outpatient serum creatinine values.45 Baseline eGFR was defined as the intercept estimated from a mixed-effects model of all outpatient eGFR values measured during the last prelude year.
Outcome
The outcome of interest was incidence of AKI within 7 days of the UTOX test. Natural cannabinoids can be detected in the urine as long as 3 days after a single use, 7 days after multiple uses, 14 days in frequent users, and 30 days in heavy daily users.46,47 A 7-day window was used as the estimated time window in which cannabis could be expected to remain in the body following a positive urine test and hence exert biological effects on kidney function. As a sensitivity analysis, we also repeated analyses using a 3-day window for the detection of AKI. AKI was defined per the Kidney Disease Improving Global Outcomes (KDIGO) guidelines.4,48 In the current study, due to the low number of patients in various exposure groups, we only used the binary definition (presence or absence) of AKI as an outcome. For descriptive purposes, we also staged AKI events according to the KDIGO guidelines.
Statistical analyses
Baseline data are presented for the entire cohort and by UTOX group as a number (percent) for categorical variables and mean (standard deviation [SD]) or median (Q1–Q3), as appropriate. Inverse probability of treatment weighting (IPTW) was used to adjust for differences between baseline covariates (listed above). We used generalized boosted modeling, a nonparametric method to calculate the weights with more than two treatment groups (using the “twang” package in R), which is referred to as multinomial propensity score weights.49,50 To correctly interpret IPTW weights as probability weights, we used survey packages/methods.50 We assessed the odds ratios of AKI associated with UTOX groups using logistic regression models (crude and adjusted). Specifically, we used the survey logistic procedure in the SAS software with UTOX group 0 (negative for all tested substances) as reference. We performed various levels of adjustments categorized as main and sensitivity analyses to examine the association between cannabis use and AKI. The main analysis examined the association of cannabis exposure (alone and in combination with opioids or other illicit substances) versus no exposure to any illicit substances with AKI in unadjusted and IPTW weights-adjusted analyses. Details of the various sensitivity analyses are provided in the Supplementary Methods. p-Values of <0.05 were used as a threshold of statistical significance for most statistical analyses. We also performed additional analyses to control for multiple comparisons between the eight UTOX groups (described in detail in the Supplementary Methods). All analyses were conducted in SAS Enterprise Guide v7.1 (SAS Institute, Cary, NC, USA), STATA/MP Version 15 (STATA Corporation, College Station, TX, USA), and R-Studio 1.0.153. The study was approved by the institutional review boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
Results
Baseline characteristics
The mean (SD) age of the overall cohort was 61 years (9); 97% were males, 51% were African Americans, 97% had hypertension, 76% had hyperlipidemia, 75% were diabetic, 54% were current smokers, 92% were analgesics users (68% aspirin users; 46% acetaminophen users; 67% opioid users; no NSAID users), 90% used diuretics, and 56% were prescribed psychiatric medications. Cannabis-only positive patients (UTOX group 1) were more likely to be younger, less likely to be White, and more likely to be smokers (Table 1). The baseline characteristics of the remaining UTOX groups (polysubstance use including/excluding cannabis use) are provided in Supplementary Table S1.
Table 1.
Cohort Baseline Characteristics
| Characteristic | All (N=2251) | Tested negative for all tests (ref) (N=1061) | Tested positive for cannabis alone (N=66) |
|---|---|---|---|
| Demographics | |||
| Mean age (SD), years | 61 (9) | 63 (10) | 57 (7) |
| Males, n (%) | 2184 (97) | 1034 (98) | 63 (96) |
| Race, n (%) | |||
| Whites | 1045 (46) | 575 (54) | 33 (50) |
| African Americans | 1143 (51) | 441 (42) | 32 (49) |
| Other | 63 (3) | 45 (4) | 1 (2) |
| Comorbidities, n (%) | |||
| Chronic pulmonary disease | 1206 (54) | 540 (51) | 25 (38) |
| Liver disease | 634 (28) | 219 (21) | 24 (36) |
| Diabetes | 1686 (75) | 820 (77) | 39 (59) |
| Hyperlipidemia | 1700 (76) | 850 (80) | 39 (59) |
| Hypertension | 2186 (97) | 1030 (97) | 62 (94) |
| PTSD | 467 (21) | 160 (15) | 15 (23) |
| CCI score, median (25th–75th percentile) | 5 (3–7) | 5 (3–7) | 4 (2–5) |
| Access type, n (%) | |||
| AVF | 304 (14) | 153 (14) | 10 (15) |
| AVG | 51 (2) | 17 (2) | 4 (6) |
| Catheter | 1741 (77) | 821 (77) | 47 (71) |
| Other | 14 (1) | 9 (1) | 0 (0) |
| Missing | 141 (6) | 61 (6) | 5 (8) |
| Baseline eGFR mL/min/1.73 m2, mean (SD) | 29 (21) | 28 (21) | 30 (23) |
| Smoking, n (%) | |||
| Never | 570 (25) | 342 (32) | 6 (9) |
| Current | 1212 (54) | 432 (41) | 50 (76) |
| Past | 462 (21) | 282 (27) | 9 (14) |
| Missing | 7 (0) | 5 (0) | 1 (2) |
| Medication use, n (%) | |||
| Analgesics | 2079 (92) | 958 (90) | 57 (86) |
| Psychiatric medications | 1255 (56) | 499 (47) | 36 (55) |
| Antimicrobials | 885 (39) | 407 (38) | 18 (27) |
| Antiretrovirals | 5 (0) | 1 (0) | 1 (2) |
| Cardiovascular medications | 1934 (86) | 934 (88) | 50 (76) |
| Chemotherapeutics | 16 (1) | 6 (1) | 1 (2) |
| Diuretics | 2029 (90) | 949 (89) | 60 (91) |
| Proton pump inhibitors | 1566 (70) | 692 (65) | 48 (73) |
| H2 receptor blockers | 560 (25) | 263 (25) | 12 (18) |
| Warfarin | 240 (11) | 122 (12) | 5 (8) |
| Anticoagulants | 1793 (80) | 838 (79) | 46 (70) |
| Antihistamines | 543 (24) | 222 (21) | 15 (23) |
| Benzodiazepines | 580 (26) | 236 (22) | 15 (23) |
| Vital signs, mean (SD) | |||
| SBP, mmHg | 146 (17) | 146 (17) | 150 (19) |
| DBP, mmHg | 79 (11) | 77 (11) | 83 (11) |
| BMI, kg/m2 | 28 (7) | 29 (7) | 26 (5) |
| Pain score | 2 (2) | 1 (1) | 2 (2) |
AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; CCI, Charlson comorbidity index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; H2 receptors, histamine 2 receptors; IQR, interquartile range; PTSD, post-traumatic stress disorder; SBP, systolic blood pressure; SD, standard deviation.
Association of cannabis use with AKI
Inpatient AKI (definition: 7-day window) occurred in 56% of the overall cohort (N=1270). AKI occurred in 50% of cannabis-alone users (N=33) and in 54% of no substance users (N=569). When applying a 3-day evaluation window to define AKI, the outcome was detected in 1230 patients (55%) of the overall cohort (N=33 [50%] of cannabis-alone users and N=547 [52%] of no substance users). Of 56% (overall cohort) who had AKI, 88% (N=1115) had stage 1, 8% (N=107) had stage 2, and 4% (N=48) had stage 3 AKI.
Table 2 shows the association between cannabis use and AKI risk. In the unadjusted model, cannabis-alone use (vs. no substance use) was not associated with higher odds of AKI (odds ratio [OR] 0.87, 95% confidence interval [CI] 0.53–1.42; p=0.6). In the IPTW weights-adjusted analysis, a similar statistically nonsignificant association was observed (OR 0.85, 95% CI 0.38–1.87; p=0.7). Similar trends of associations were observed in all the sensitivity analyses (incrementally adjusted multivariable analysis, adjusted for winsorized IPTW weights, and doubly robust model).
Table 2.
Association of Cannabis Use with Acute Kidney Injury
| Cannabis use versus negative for all substance use | Unadjusted/Model 1, OR (95% CI) | p | Adjusted (IPTW weights), OR (95% CI) | p |
|---|---|---|---|---|
| Main analysis | 0.87 (0.53–1.42) | 0.6 | 0.85 (0.38–1.87) | 0.7 |
| Sensitivity analyses | ||||
| Double robust estimation | 0.79 (0.34–1.83) | 0.6 | ||
| Adjusted (winsorized IPTW weights) | 0.81 (0.46–1.43) | 0.5 | ||
| DRE (winsorized IPTW weights) | 0.79 (0.34–1.83) | 0.6 | ||
| Incremental analysis (weights not included) | ||||
| Model 2 | 0.84 (0.51–1.39) | 0.5 | ||
| Model 3 | 0.91 (0.55–1.51) | 0.7 | ||
| Model 4 | 0.93 (0.55–1.57) | 0.8 | ||
| Model 5 | 0.83 (0.48–1.41) | 0.5 | ||
IPTW weights are calculated from the variables shown in Table 1. Doubly robust estimation: “doubly robust” means the model includes all the variables used to calculate IPTW weights and weights too. Winsorized weights: Weights adjusted at 90 percentiles, respectively, for each group. Model 1: Unadjusted analysis. Models 2–5 presented here are incrementally adjusted, as follows: Model 2: Model 1+demographics (sex, race, and age). Model 3: Model 2+comorbidities (chronic pulmonary disease, liver disease, diabetes, hyperlipidemia, hypertension, and post-traumatic stress disorder)+access type+smoking. Model 4: Model 3+use of medications (analgesics, psychiatric drugs, antimicrobials, antiretrovirals, cardiovascular medications, chemotherapeutic inhibitors, diuretics, proton pump inhibitors, H2 receptor blockers, warfarin, anticoagulants, and benzodiazepines). Model 5: Model 4+vital signs (mean systolic blood pressure, mean DBP, mean BMI, and mean pain score)+baseline eGFR (eGFR intercept). DRE, doubly robust estimation; IPTW, inverse probability of treatment weighting.
Cannabis use combined with opioids (Supplementary Table S2) or with other drugs (Supplementary Table S3) or with both opioids and other drugs (Supplementary Table S4) showed no significant association with the odds of AKI in both the main and sensitivity analyses.
Opioid use excluding cannabis use (Supplementary Table S5) showed no significant association with AKI. However, polysubstance use excluding cannabis use (Supplementary Tables S6 and S7) was associated with higher odds of AKI. However, none of the associations between AKI and pairwise comparisons of UTOX groups were statistically significant in Tukey's adjustment for pairwise comparisons (Supplementary Fig. S1). Similar trends of association were observed between cannabis/combined cannabis use and AKI when AKI was determined using a 3-day window (Supplementary Tables S8–S14 and Supplementary Fig. S2).
Discussion
In this nationally representative cohort of U.S. veterans with advanced CKD who transitioned to dialysis, we did not observe a statistically significant association between cannabis use and AKI risk, whether modeling cannabis exposure alone or in combination with other drugs. Similar nonsignificant associations were observed for exposure to other types of illicit drugs besides cannabis.
Based on prior studies, the likely targets for the ingredients found in cannabis would be their cognate receptors, CB1 and CB2, which are known to be differentially expressed throughout the tubular epithelial cells of the nephron, interstitial cells, and vasculature in the kidney.11 Similar binding patterns to CB1 and CB2 can be observed with the EC system.11,26 Functionally, increased expression of CB1 or CB2 receptors and their associated activity were detected in various forms of kidney disease and injury.26,51–53 At this time, there is increasing evidence that the activation of CB1 receptors plays a causal role in both acute51 kidney disease54–56 so blockade of the receptor has been shown to ameliorate disease progression. Conversely, CB2 receptor activation has an opposing function to CB1 and elicits protective properties in animal models of AKI, including cisplatin-induced nephrotoxicity57,58 and renal ischemia–reperfusion injury.59–61
Based on the findings of these above-mentioned preclinical studies, we postulated that the exposure to cannabis and its active ingredients would be associated with an increased risk of kidney injury and progression of CKD. However, our findings did not support this hypothesis. The reasons for a lack of association with AKI may be attributed to the distribution pattern and complex activation states of both the CB1 and CB2 in the kidney, where many of the major chemical constituents of cannabis have the capability to activate either of the receptors to varying degrees. Moreover, the effect of cannabis and its active ingredients may have been complicated by the presence of other drugs and underlying disease pathologies. Therefore, relying on the activity pattern or lack thereof for each receptor to predict the overall renal impact can be misleading,62 especially since we could not determine the expression profile of these receptors in the patients being studied. With that said, there is a strong possibility that both CB1 and CB2 are involved in the pathogenic effects seen with SCBs in several published case reports.11,23–26 First, SCBs are known to have much higher potency than naturally occurring THC in cannabis and therefore elicit a much more intense CB activity resulting in renal toxicity. Second, binding of SCBs to CB receptors may lead to a differential intracellular signaling event compared with THC and other ingredients that would normally be found in different amounts. Finally, SCBs are unregulated drugs that may contain other chemical diluents and excipients that may mediate the toxic renal effects leading to AKI. These observations highlight the critical need for clinical and translational studies, which can bridge the gap between preclinical results and relevant patient findings.
A novel aspect of our study is the examination of the association between the combined use of cannabis with opioids or with other illicit drugs, which was not previously studied, although a higher risk of AKI associated with both opioid overdose63 and with exposure to other illicit drugs64 has been reported. The combined use of cannabis with opioids leads to potential synergistic interactions, and studies have shown that while administering lower doses of THC or morphine alone may not be effective in treating pain,8 when the same small doses of both THC and morphine were administered together, they produced a significant reduction in pain.65,66 In our study, we observed a nonsignificant association between the risk of AKI with combined cannabis use and either opioids or other illicit drugs. Possible explanations for the lack of significant associations include differences in the cohorts studied. Also, the combined use of cannabis with opioids/other drugs may result in the consumption of lower doses of each substance compared with individual use of each substance, thus mitigating the adverse effects of opioids and/or other illicit substances.
There are several unique features in the current study that contributed to the strength of this investigation. First, we used UTOX tests to ascertain the use of cannabis, opioids, other/illicit drugs, and the combination of these, using a validated algorithm. Prior literature on the effects of cannabis is primarily based on the self-reported exposure by the participants,24,32–35 which is known to be an inaccurate method from research using patients with chronic pain.67 Besides self-reporting, drug use can be ascertained through biological specimens, including urine, blood, breath, oral fluid, nail, and hair, with the most commonly used drug-testing specimen being urine. Advantages of urine specimens include the noninvasive nature of specimen collection, a higher concentration of the parent drug and its metabolites, and longer drug detection times.46,47 The second strength of our study is the definition of AKI using serial serum creatinine measurements during an inpatient hospitalization following the UTOX screen. To the best of our knowledge, this is the first study to ascertain cannabis/combined cannabis use with opioid/other drugs via UTOX tests and to examine the association between various combinations of exposures and the risk of AKI using IPTW. Furthermore, the current study is also the first study to explore these associations in patients with advanced CKD.
Even with these strengths, we recognize that our study has several limitations. First, even though UTOX tests are more sensitive than self-reported use, there is still a possibility of misclassification due to false positive or negative test results. Second, the low number of AKI events also resulted in limited statistical power, and we were not able to assess the risk of AKI stages. Third, we had no information about frequency of cannabis use, and thus, the degree of exposure to cannabis or dose/level of cannabis cannot be ascertained for the full evaluation period for AKI (7 days). To mitigate this limitation, we repeated analyses after defining AKI using a 3-day post-toxicology test period, which showed similar results. Fourth, as the study cohort was restricted to predominantly male U.S. veterans and all of our patients transitioned to dialysis, our study findings may have limited generalizability. Fifth, as we used observational data for this study, we cannot infer causality. Finally, while we used extensive adjustment for confounders, there remains a possibility of residual confounding by unmeasured covariates.
In summary, this study shows that cannabis use alone or combined with opioids/other drugs was not significantly associated with a risk of AKI. These findings remained robust after extensive adjustment for covariates. Additional clinical studies are needed to better characterize the association of cannabis and cannabinoids with kidney injury and markers of chronic disease in different patient cohorts.
Supplementary Material
Abbreviations Used
- AKI
acute kidney injury
- AVF
arteriovenous fistula
- BMI
body mass index
- CB1
cannabinoid receptors type 1
- CB2
cannabinoid receptors type 2
- CBN
cannabinol
- CKD
chronic kidney disease
- CI
confidence interval
- DBP
diastolic blood pressure
- EC
endocannabinoid
- eGFR
estimated glomerular filtration rate
- H2 receptors
histamine 2 receptors
- IPTW
inverse probability of treatment weighting
- IQR
interquartile range
- NSAID
non-steroidal anti-inflammatory drug
- OR
odds ratio
- SBP
systolic blood pressure
- SCB
synthetic cannabinoid
- SD
standard deviation
- TC-CKD
Transition of Care in Chronic Kidney Disease
- THC
tetrahydrocannabinol
- USRDS
United States Renal Data System
- UTOX
urine toxicology
Authors' Contributions
Research idea and study design: P.K.P., C.P.K., H.M., F.P., C.K., and F.T. Data acquisition: P.K.P., E.S., K.K.-Z., and C.P.K. Data analysis/interpretation: P.K.P., C.P.K., H.M., F.P., C.K., F.T., and K.S. Statistical analysis: P.K.P. Supervision or mentorship: K.K.-Z. and C.P.K. Each author contributed important intellectual content during article drafting or revision, accepts personal accountability for the author's own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors reviewed and approved the final version of this article.
Disclaimer
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs (VA) or the U.S. government. The results of this article have not been published previously in whole or part.
Author Disclosure Statement
C.P.K., H.M., K.K.-Z., and E.S. are employees of the U.S. Department of Veterans Affairs. C.P.K. received honoraria from Abbott, Akebia, Astra Zeneca, Bayer, Cara Therapeutics, Rockwell, and Vifor. K.K.-Z. has received honoraria and/or support from Abbott, Abbvie, ACI Clinical (Cara Therapeutics), Akebia, Alexion, Amgen, ASN (American Society of Nephrology), Astra-Zeneca, Aveo, BBraun, Chugai, Cytokinetics, Daiichi, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, IFKF (International Federation of Kidney Foundations), ISH (International Society of Hemodialysis), International Society of Renal Nutrition & Metabolism (ISRNM), JSDT (Japanese Society of Dialysis Therapy), Hospira, Kabi, Keryx, Kissei, Novartis, OPKO, NIH (National Institutes of Health), NKF (National Kidney Foundations), Pfizer, Regulus, Relypsa, Resverlogix, Dr Schaer, Sandoz, Sanofi, Shire, VA (Veterans' Affairs), Vifor, UpToDate, and ZS-Pharma. J.D.G. reports research funding unrelated to this work provided by Merck & Co., AstraZeneca, and GlaxoSmithKline. The remaining authors declare that they have no relevant financial interests.
Funding Information
This study was supported by grant 5U01DK102163 from the National Institutes of Health (NIH) to K.K.-Z. and C.P.K. and by resources from the U.S. Department of Veterans Affairs. The data reported here have been supplied in part by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Nos. SDR 02-237 and 98-004).
Supplementary Material
Cite this article as: Potukuchi PK, Moradi H, Park F, Kaplan C, Thomas F, Dashputre AA, Sumida K, Molnar MZ, Gaipov A, Gatwood JD, Rhee C, Streja E, Kalantar-Zadeh K, Kovesdy CP (2023) Cannabis use and risk of acute kidney injury in patients with advanced chronic kidney disease transitioning to dialysis, Cannabis and Cannabinoid Research 8:1, 138–147, DOI: 10.1089/can.2021.0044.
References
- 1. Coresh J, Turin TC, Matsushita K, et al. . Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coyne DW, Kovesdy CP. Changing the paradigms for the treatment of chronic kidney disease. Kidney Int Suppl. 2017;7:155–156. [Google Scholar]
- 3. Levey AS, Atkins R, Coresh J, et al. . Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. [DOI] [PubMed] [Google Scholar]
- 4. Grams ME, Sang Y, Coresh J, et al. . Candidate surrogate end points for ESRD after AKI. J Am Soc Nephrol. 2016;27:2851–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishani A, Xue JL, Himmelfarb J, et al. . Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kovesdy CP, Naseer A, Sumida K, et al. . Abrupt decline in kidney function precipitating initiation of chronic renal replacement therapy. Kidney Int Rep 2018;3:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strausser SA, Nakano D, Souma T. Acute kidney injury to chronic kidney disease transition: insufficient cellular stress response. Curr Opin Nephrol Hypertens. 2018;27:314–322. [DOI] [PubMed] [Google Scholar]
- 8. National Academies of Sciences Engineering and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. The National Academies Press: Washington, DC, 2017. [PubMed] [Google Scholar]
- 9. Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T. 2017;42:180–188. [PMC free article] [PubMed] [Google Scholar]
- 10. Han BH, Palamar JJ. Trends in cannabis use among older adults in the United States, 2015-2018. JAMA Intern Med. 2020;180:609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park F, Potukuchi PK, Moradi H, et al. . Cannabinoids and the kidney: effects in health and disease. Am J Physiol Renal Physiol. 2017;313:F1124–F1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu J, Ginsberg JS, Zhan M, et al. . Chronic pain and analgesic use in CKD: implications for patient safety. Clin J Am Soc Nephrol. 2015;10:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coluzzi F. Assessing and treating chronic pain in patients with end-stage renal disease. Drugs. 2018;78:1459–1479. [DOI] [PubMed] [Google Scholar]
- 14. Davison SN, Davison JS. Is there a legitimate role for the therapeutic use of cannabinoids for symptom management in chronic kidney disease? J Pain Symptom Manage. 2011;41:768–778. [DOI] [PubMed] [Google Scholar]
- 15. Borgelt LM, Franson KL, Nussbaum AM, et al. . The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33:195–209. [DOI] [PubMed] [Google Scholar]
- 16. Greydanus DE, Kaplan G, Baxter LE, Sr, et al. Cannabis: the never-ending, nefarious nepenthe of the 21st century: what should the clinician know? Dis Mon. 2015;61:118–175. [DOI] [PubMed] [Google Scholar]
- 17. Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17: 475–490. [DOI] [PubMed] [Google Scholar]
- 19. Janiak P, Poirier B, Bidouard JP, et al. . Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007;72:1345–1357. [DOI] [PubMed] [Google Scholar]
- 20. Jenkin KA, O'Keefe L, Simcocks AC, et al. . Chronic administration of AM251 improves albuminuria and renal tubular structure in obese rats. J Endocrinol. 2015;225:113–124. [DOI] [PubMed] [Google Scholar]
- 21. Jourdan T, Szanda G, Rosenberg AZ, et al. . Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc Natl Acad Sci U S A. 2014;111:E5420–E5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nam DH, Lee MH, Kim JE, et al. . Blockade of cannabinoid receptor 1 improves insulin resistance, lipid metabolism, and diabetic nephropathy in db/db mice. Endocrinology. 2012;153:1387–1396. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease, C. & Prevention. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93–98. [PMC free article] [PubMed] [Google Scholar]
- 24. Ishida JH, Auer R, Vittinghoff E, et al. . Marijuana Use and Estimated Glomerular Filtration Rate in Young Adults. Clin J Am Soc Nephrol. 2017;12:1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barutta F, Bruno G, Mastrocola R, et al. . The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney Int. 2018;94:252–258. [DOI] [PubMed] [Google Scholar]
- 26. Chua JT, Argueta DA, DiPatrizio NV, et al. . Endocannabinoid System and the Kidneys: From Renal Physiology to Injury and Disease. Cannabis Cannabinoid Res. 2019;4:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaipov A, Molnar MZ, Potukuchi PK, et al. . Acute kidney injury following coronary revascularization procedures in patients with advanced CKD. Nephrol Dial Transplant. 2018;34:1894–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sumida K, Dashputre AA, Potukuchi PK, et al. Laxative use in patients with advanced chronic kidney disease transitioning to dialysis. Nephrol Dial Transplant. (2020). [DOI] [PMC free article] [PubMed]
- 29. Saran R, Robinson B, Abbott KC, et al. . US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73:A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morasco BJ, Shull SE, Adams MH, et al. . Development of an Algorithm to Identify Cannabis Urine Drug Test Results within a Multi-Site Electronic Health Record System. J Med Syst. 2018;42:163. [DOI] [PubMed] [Google Scholar]
- 31. Connor JP, Gullo MJ, Chan G, et al. . Polysubstance use in cannabis users referred for treatment: drug use profiles, psychiatric comorbidity and cannabis-related beliefs. Front Psychiatry. 2013;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bundy JD, Bazzano LA, Xie D, et al. . Self-Reported Tobacco, Alcohol, and Illicit Drug Use and Progression of Chronic Kidney Disease. Clin J Am Soc Nephrol. 2018;13:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grubbs V, Vittighoff E, Grimes B, et al. . Mortality and illicit drug dependence among hemodialysis patients in the United States: a retrospective cohort analysis. BMC Nephrol. 2016;17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu C, Papatheodorou SI, Danziger J, et al. . Marijuana Use and Renal Function Among US Adults. Am J Med. 2018;131:408–414. [DOI] [PubMed] [Google Scholar]
- 35. Vupputuri S, Batuman V, Muntner P, et al. . The risk for mild kidney function decline associated with illicit drug use among hypertensive men. Am J Kidney Dis. 2004;43:629–635. [DOI] [PubMed] [Google Scholar]
- 36. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 37. McGinnis KA, Brandt CA, Skanderson M, et al. . Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soohoo M, Moradi H, Obi Y, et al. . Statin Therapy Before Transition to End-Stage Renal Disease With Posttransition Outcomes. J Am Heart Assoc. 2019;8:e011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saleh T, Sumida K, Molnar MZ, et al. . Effect of Age on the Association of Vascular Access Type with Mortality in a Cohort of Incident End-Stage Renal Disease Patients. Nephron. 2017;137:57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perazella MA. Pharmacology behind Common Drug Nephrotoxicities. Clin J Am Soc Nephrol. 2018;13:1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saker BM. Everyday drug therapies affecting the kidneys. Australian Prescriber: Canberra, Australia, Vol. 2019. (2000). [Google Scholar]
- 42. Alsherbiny MA, Li CG. Medicinal Cannabis-Potential Drug Interactions. Medicines (Basel) 6(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lindsey WT, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38:334–343. [DOI] [PubMed] [Google Scholar]
- 44. Overholser BR, Foster DR. Opioid pharmacokinetic drug-drug interactions. Am J Manag Care. 2011;17 Suppl 11:S276–S287. [PubMed] [Google Scholar]
- 45. Levey AS, Stevens LA, Schmid CH, et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moeller KE, Kissack JC, Atayee RS, et al. . Clinical Interpretation of Urine Drug Tests: What Clinicians Need to Know About Urine Drug Screens. Mayo Clin Proc. 2017;92:774–796. [DOI] [PubMed] [Google Scholar]
- 47. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83:66–76. [DOI] [PubMed] [Google Scholar]
- 48. Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Griffin BA, McCaffrey D, Almirall D, et al. . Chasing balance and other recommendations for improving nonparametric propensity score models. J Causal Inference. 2017;5:20150026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McCaffrey DF, Griffin BA, Almirall D, et al. . A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mukhopadhyay P, Pan H, Rajesh M, et al. . CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol. 2010;160:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mukhopadhyay P, Rajesh M, Batkai S, et al. . CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85:773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rajesh M, Batkai S, Kechrid M, et al. . Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barutta F, Corbelli A, Mastrocola R, et al. . Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes. 2010;59:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Udi S, Hinden L, Ahmad M, et al. . Dual inhibition of cannabinoid CB1 receptor and inducible NOS attenuates obesity-induced chronic kidney disease. Br J Pharmacol. 2020;177:110–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Udi S, Hinden L, Earley B, et al. . Proximal Tubular Cannabinoid-1 Receptor Regulates Obesity-Induced CKD. J Am Soc Nephrol. 2017;28:3518–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mukhopadhyay P, Baggelaar M, Erdelyi K, et al. . The novel, orally available and peripherally restricted selective cannabinoid CB2 receptor agonist LEI-101 prevents cisplatin-induced nephrotoxicity. Br J Pharmacol. 2016;173:446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mukhopadhyay P, Rajesh M, Pan H, et al. . Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Rad Biol Med. 2010;48:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pressly JD, Mustafa SM, Adibi AH, et al. . Selective Cannabinoid 2 Receptor Stimulation Reduces Tubular Epithelial Cell Damage after Renal Ischemia-Reperfusion Injury. J Pharmacol Exp Ther. 2018;364:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pressly JD, Soni H, Jiang S, et al. . Activation of the cannabinoid receptor 2 increases renal perfusion. Phys Gen. 2019;51:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nettekoven M, Adam JM, Bendels S, et al. . Novel Triazolopyrimidine-Derived Cannabinoid Receptor 2 Agonists as Potential Treatment for Inflammatory Kidney Diseases. Chem Med Chem. 2016;11:179–189. [DOI] [PubMed] [Google Scholar]
- 62. Soethoudt M, Grether U, Fingerle J, et al. . Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun. 2017;8:13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mallappallil M, Sabu J, Friedman EA, et al. . What do we know about opioids and the kidney? Int J Mol Sci. 2017;18:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lau Hing Yim C, Wong EWW, Jellie LJ, et al. . Illicit drug use and acute kidney injury in patients admitted to hospital with rhabdomyolysis. Intern Med J. 2019;49:1285–1292. [DOI] [PubMed] [Google Scholar]
- 65. Abrams DI, Couey P, Shade SB, et al. . Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844–851. [DOI] [PubMed] [Google Scholar]
- 66. Wiese B. Wilson-Poe AR. Emerging evidence for cannabis' role in opioid use disorder. Cannabis Cannabinoid Res. 2018;3:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reisfield GM, Salazar E, Bertholf RL. Rational use and interpretation of urine drug testing in chronic opioid therapy. Ann Clin Lab Sci. 2007;37:301–314. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.