Abstract
Background:
The majority of radiopharmaceuticals for use in disease detection and targeted treatment undergo a single radioactive transition (decay) to reach a stable ground state. Complex emitters, which produce a series of daughter radionuclides, are emerging as novel radiopharmaceuticals. The need for validation of chemical and radiopurity with such agents using common quality control instrumentation is an area of active investigation. Here, we demonstrate novel methods to characterize 227Th and 223Ra.
Materials and Methods:
A radio-TLC scanner and a γ-counter, two common and widely accessible technologies, as well as a solid-state α-particle spectral imaging camera were evaluated for their ability to characterize and distinguish 227Th and 223Ra. We verified these results through purity evaluation of a novel 227Th-labeled protein construct.
Results:
The γ-counter and α-camera distinguished 227Th from 223Ra, enabling rapid and quantitative determination of radionuclidic purity. The radio-TLC showed limited ability to describe purity, although use under α-particle-specific settings enhanced resolution. All three methods were able to distinguish a pure from impure 227Th-labeled protein.
Conclusions:
The presented quality control evaluation for 227Th and 223Ra on three different instruments can be applied to both research and clinical settings as new alpha particle therapies are developed.
Keywords: α particle therapy, quality control, radio-thin layer chromatography, γ counter
Introduction
Radionuclides have long been used as tools to image and treat cancer. When systemically administered, specific radiopharmaceuticals may localize to sites of disease for diagnostic or therapeutic purposes. Presently, there is a growing interest in α particle emitting radiotherapy for cancer treatment. 223RaCl2 (tradename Xofigo) is the first α particle therapy approved by the U.S. Food and Drug Administration for the treatment of bone metastatic castrate-resistant prostate cancer in men.1,2
Radium-223 (223Ra) is a bone seeker, and it accumulates in sites of active bone turnover, as existing at metastatic bone tumors.3,4 The α particles emitted by 223Ra and its daughters are characterized by a very short track range, irradiating bone metastatic prostate cancer cells and the surrounding tumor microenvironment, while largely sparing healthy tissue from fatal irreversible DNA damage.5
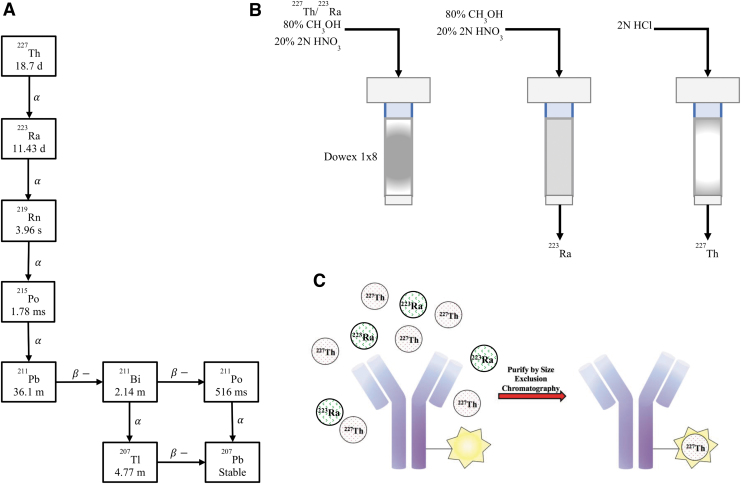
Thorium-227 (227Th) is also currently under investigation in clinical trials (NCT03507452, NCT03724747) for its potential in targeted α therapy. This radionuclide is the parent ion of 223Ra and can be readily chelated and conjugated to various targeting vectors (Fig. 1).6,7 This enables specific targeting of 227Th to sites of soft tissue disease with molecular precision. In addition, its long half-life allows advantages for preparation, transportation, and administration.8
FIG. 1.
(A) Decay chain of 227Ac, which decays into 227Th and 223Ra. (B) Depiction of the anion exchange column used for the purification of 223Ra and 227Th. (C) Antibody radiolabeling with 227Th. 227Th is chelated to an antibody and purified using size exclusion chromatography.
223Ra and 227Th are produced from an 227Ac (21.7 years half-life) source and purified using an ion-exchange resin. Radionuclidic purity may be evaluated by γ-spectroscopy for subsequent labeling and radiopharmaceutical formulation.9 However, the assortment of daughters emitted through concatenated decay of these radionuclides leads to complicated chelation status analyses and characterization of the products, which are essential for reliable quality control of these radiopharmaceutical products. In addition, the complex (γ, β-, and α-particle) decay profiles of 227Th and 223Ra have overlapping emission spectra, further complicating individual radionuclide distinction.10
Although measurement by high purity Germanium detector (HPGe) is a simple method for quality control, and 227Th and 223Ra are easily distinguished, many community care centers and hospitals do not possess such equipment. At present, manufacturer determination of purity, specific activity, and activity is performed at a central manufacturing or compounding site and subsequently transported to medical centers for use.
A growing consideration for the field is how to properly validate novel radiopharmaceuticals for clinical application, specifically radionuclides that produce multiple daughters and long half-lives.11,12 In this study, a series of characterizations on technologies typically accessible for radiopharmaceutical identification and determination of purity was undertaken, including measures from radio-thin layer chromatography (TLC), γ counting, and a solid-state α-particle detector (Table 1).
Table 1.
Most Significant α Particle Energies (Intensity >3%) of the Radioisotopes in the Decay Chain of Thorium-227 and Their Corresponding Intensities
|
227Th |
223Ra |
219Rn |
211Bi |
215Po |
211Po |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy (keV) | Intensity (%) | Energy (keV) | Intensity (%) | Energy (keV) | Intensity (%) | Energy (keV) | Intensity (%) | Energy (keV) | Intensity (%) | Energy (keV) | Intensity (%) |
| 5700.8 | 3.6 | 5433.6 | 1.8 | 6425.0 | 7.5 | 6278.2 | 16.2 | 7386.1 | 100 | 7450.3 | 98.9 |
| 5708.8 | 8.3 | 5539.8 | 8.9 | 6552.6 | 12.9 | 6622.9 | 83.5 | ||||
| 5713.2 | 4.9 | 5606.7 | 25.0 | 6819.1 | 79.4 | ||||||
| 5756.9 | 20.4 | 5716.2 | 51.2 | ||||||||
| 5866.6 | 2.42 | 5747.0 | 8.9 | ||||||||
| 5959.7 | 3.0 | ||||||||||
| 5977.7 | 23.5 | ||||||||||
| 6008.8 | 2.9 | ||||||||||
| 6038.0 | 24.2 | ||||||||||
All energies are recorded in keV.
227Th and 223Ra identification and quantification were demonstrated utilizing γ spectrometry and γ-counting and also an α-particle imaging spectrometer was evaluated. Novel use of a standard radio-TLC scanner for α-particle specific imaging was also evaluated. Through these methods, a quality control protocol for a 227Th-radiolabeled protein construct was developed.
Materials and Methods
All reagents and solvents were purchased from Sigma-Aldrich, unless otherwise noted. 227Th was supplied by the department of energy (Oak Ridge). All buffered solutions were prepared from metal-free water purified using Chelex 100 resin (BioRad). The handling of radioactivity requires specific caution and protection, and certification and protocol approval were provided by the Environmental Health and Safety Department at Washington University in St. Louis (Radioactive Materials Authorization 1169-01). Additional details for methods are found in the Supplementary Data, where indicated.
Characterization of 223Ra and 227Th using radio-TLC
Radium-223 and Thorium-227 were produced from a microgenerator, as previously described.13 Additional details are found in the Supplementary Data. The Bioscan AR-2000 Imaging Scanner (Eckert and Ziegler Radiopharma, Inc.) was used to measure the decay of 223Ra, 227Th, and a mixture of both isotopes. Purified 223Ra (0.025–0.1 μCi, 925–3700 Bq, 2–5 μL) was spotted in triplicate on 2.5 × 2.5 cm squares of Whatman paper and was not migrated (3 mm, Schleicher and Schuell).
A similar process was conducted for pure 227Th and a cospotted mixture of 223Ra and 227Th. Each paper strip was covered in cellophane wrap and read on the radio-TLC for 1 min at a high voltage setting (1500 V) and for 5 min at a low voltage setting (1000 V). Placing each square at a similar position on the scanner, these measurements were completed daily covering an entire half-life of 223Ra (11.4 days) or 227Th (18.7 days), with the initial reading occurring immediately after purification.
The counts per minute (CPM) data were exported from the Bioscan software (version 3.13), and the total area under the curve was calculated using Riemann sums for each time point. The average sum was plotted and fit to a one-phase decay curve using Graphpad Prism.
Quantitative readings of 223Ra and 227Th using γ counting
The Wizard2 Gamma Counter (PerkinElmer) was used to measure the decay of 223Ra and 227Th over an entire half-life. In brief, purified 223Ra, 227Th, and a cospotted mixture (0.03–0.1 μCi, 1110–3700 Bq, 1–5 μL) were pipetted on 1 × 1 cm squares of 3 mm Whatman paper in triplicate. Each paper was then inserted into a 12 × 75 mm disposable culture tube and read on the γ counter for 60 s daily. The samples were counted over the full energy window from 100 to 2000 keV.
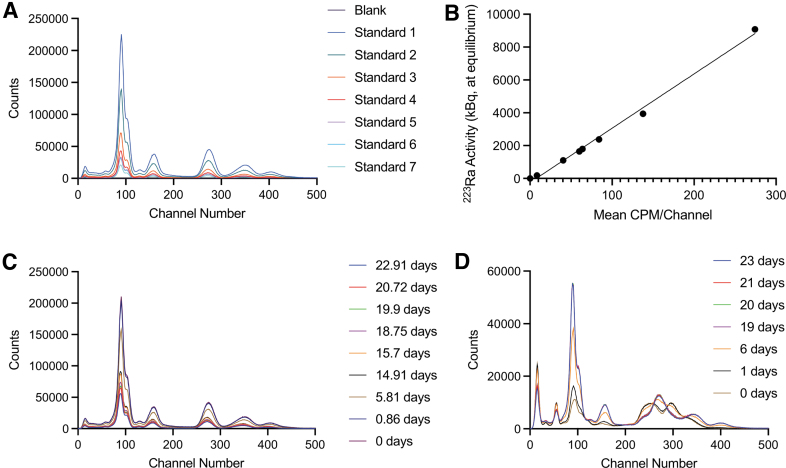
For quantification of activity amounts, the γ-well counter and HPGe system were calibrated using a National Institute of Standards and Technology (NIST) traceable standard of 223Ra. Defined volumes of the standard were aliquoted to form a dilution series, and each volume was weighed using a precision microbalance for accuracy (XP205; Mettler Toledo). The standards ranged from 0 to 9 kBq, and the full spectrum of γ-emissions was captured (2048 channels). CPM were plotted over activity, and linearity at each measured time point was r2 = 0.992. The same undertaking was achieved using quantitative HPGe measurements using the 154 keV spectral line for 223Ra, producing a linear response of r2 = 0.997.
A method was developed to separate 223Ra and 227Th activity amounts using the automated sample-changing γ-counter (Fig. 2). Spectral measurements of 223Ra (at secular equilibrium with its daughters) and freshly purified 227Th (<10 min) were acquired daily for 25 days, providing time for ingrowth of daughter and attendant emissions. Quantitation of the 227Th portion to absolute (Bq) activity amounts was made using the absolute NIST quantification of the 223Ra component and the known relative amounts of each radioisotope in the mixed spectra, calculated using the Bateman equations.14
FIG. 2.
(A) National Institute of Standards and Technology calibrated dilution series for 223Ra standards. (B) Quantitation of activity from traceable 223Ra source summed across channels. (C) Measurements of a 223Ra source at equilibrium over time, days indicated. (D) Measurements of 227Th over time. Ingrowth of 223Ra and daughters is noted. CPM, counts per minute.
Taken together, these measurements allowed two basis functions (for 227Th and 223Ra with daughters) to be defined, each in units of counts per spectral bin per Bq. Additional details are found in the Supplementary Data.
223Ra and 227Th readings using Minipix α spectral detection
The Minipix spectral imaging camera (300 μm silicon; ADVACAM) was used to characterize the α decay of 223Ra and 227Th. Approximately 0.025 μCi (925 Bq) of purified 223Ra or 227Th was spotted on 3 × 3 cm squares of 3 mm Whatman paper. In addition, ∼0.025 μCi (925 Bq) of purified 223Ra or 227Th was spotted on a 3 × 3 cm square of a solid aluminum support and allowed to air dry. The clustering tool on the PIXET PRO software (ADVACAM, version 1.4.10.704) was used to collect the global cluster volume of each detection event. For the measurement of 223Ra and 227Th α decay, samples were placed on top of the α particle imaging spectrometer, ∼0.7 cm from the detector, and measured daily over the period of one half-life for ∼15 min. The data were exported from PIXET PRO and analyzed as counts per energy bin (keV) using Graphpad Prism.
Radionuclide separation using N,N,N′,N′-tetra-n-octyldiglycolamide-coated chromatographic paper
227Th (0.012 μCi, 444 Bq) and 223Ra (0.007 μCi, 259 Bq) sources were cospotted on a 100 × 10 mm strip of chromatography paper impregnated with N,N,N′,N′-tetra-n-octyldiglycolamide (DGA; TrisKem International) and migrated in a mobile phase of 1 M HNO3. The migrated strip was wrapped in cellophane and read using the radio-TLC under two high-voltage settings (1500 and 1000 V). Strips were measured at 5 min, 1 h, and 3 h postmigration, with an acquisition time of 5 min at 1500 V and 10 min at 1000 V. A duplicate DGA migration was separated into three equal sections and measured on the HPGe detector immediately after separation for radioisotopic identification.
227Th-radiolabeled protein characterization
A protein conjugated to a 227Th chelator of the hydroxypyridinone ligand (HOPO) class was purified by size exclusion spin centrifugation (Zeba column; ThermoFisher Scientific). Chelation status was analyzed by TLC before and after purification. Both purified and unpurified samples of the 227Th-labeled protein were spotted in triplicate on silica chromatographic paper and migrated in a mobile phase of 50 mM diethylenetriamine pentaacetate and assessed by TLC scanner at high-voltage settings of 1000 and 1500 V. Following this reading, the strips were cut in half, and both sets were measured using the α particle imaging spectrometer. Each half was then measured on the γ counter for 60 s.
Results
The overall goal was to develop quality control methods for characterization of targeted α-particle therapy constructs. For each source purified by the microgenerator column, radioisotopic purity was evaluated using the HPGe (Supplementary Fig. S1).13 Measurement by HPGe is a straightforward method for distinguishing 227Th from 223Ra, but many secondary and tertiary clinics, including academic medical centers, do not routinely use such equipment. The authors, therefore, tested the capacity of more readily available technologies to measure and report purity of an antibody-conjugated radiotherapeutic.
Radio-TLC scanner
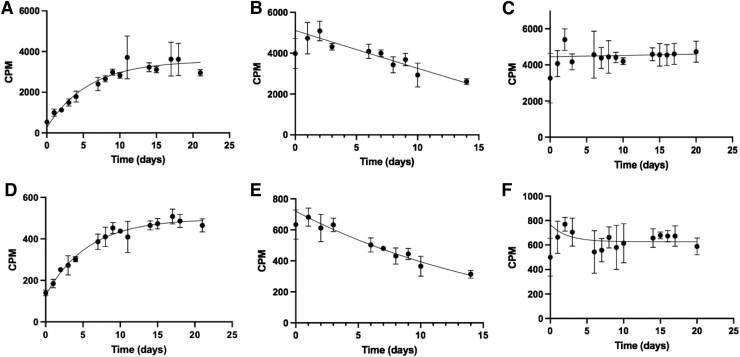
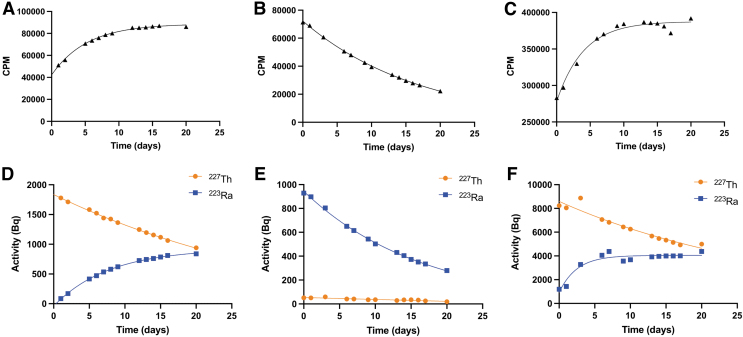
The authors first evaluated a TLC scanner, the most widely deployed tool for radiolabeled agents, to measure the decay of 223Ra and 227Th using a novel α-particle specific setting. The differences in CPM acquisition for 227Th and 223Ra between the default high-voltage setting (1500 V) and a secondary setting (1000 V) were examined to report mixed ionizing radiation or α-particle specific emissions, respectively. A 227Th source measured at 1500 V showed an increase in CPM over time, gradually stagnating in slope near its transient equilibrium (Fig. 3A). This increase in CPM over time can be attributed to the ingrowth of its daughter isotopes, specifically 223Ra.
FIG. 3.
Readings at 1500 V (A–C) and at 1000 V (D–F) of each radioisotope and a mixed source spotted on cellulose support, measured over time. (A, D) 227Th CPM were plotted and fit according to a one-phase exponential decay. (B, E) 223Ra CPM were plotted and fit to a one-phase exponential decay. (C, F) Mixed source of 223Ra and 227Th. For all curves, the initial point is excluded from the exponential fitting to allow the system to reach equilibrium. Error bars indicate standard deviation between triplicates. CPM, counts per minute.
Measured under similar parameters, 223Ra showed a profile moderately fitted over a standard one-phase decay (r2 = 0.5810; Fig. 3B). This poor fit is most likely due to the ingrowth of 223Ra daughters. The cospotted mixture of 227Th and 223Ra produced a fitted curve with minimal changes in CPM over the half-life of 227Th (Fig. 3C), again demonstrating the limitations of the system in measuring an isotopic mixture.
At lower bias voltage, β-particles will not produce detectable ionization events, whereas higher energy α-particles will produce electrons suitable for signal amplification, allowing for α-particle specific readings at 1000 V (Fig. 3D–F). The observed curve profiles were similar at 1000 and 1500 V for the mixture of 227Th/223Ra and for 227Th alone. However, the count plot for 223Ra matched a more accurate one-phase decay despite the presence of daughters (r2 = 0.8611).
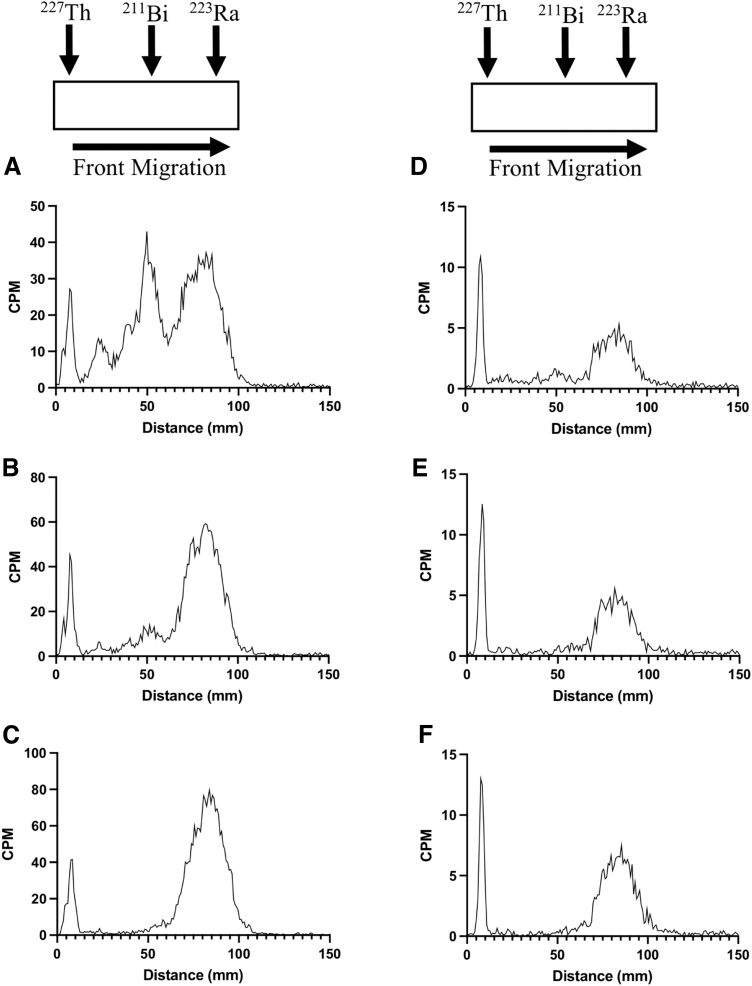
The authors were interested to see how these results would compare with measurements of a migrated TLC strip separating 223Ra from 227Th using the default and α particle specific settings. DGA-impregnated TLC paper was used to separate 227Th from 223Ra by ionic discrimination. Immediately after migration, three distinct peaks were recorded at the 1500 V setting (Fig. 4A), whereas two peaks were present when measured at 1000 V (Fig. 4D). Approximately 1 h later, the middle peak had decreased significantly (Fig. 4B), and by 3 h postmigration, this peak disappeared (Fig. 4C). The peaks at the front and base of the strip were not observed to decrease over this timeframe.
FIG. 4.
A strip of DGA-coated chromatographic iTLC paper was spotted with a mixture of 223Ra/227Th at 10 mm and migrated with a mobile phase of 1 M HNO3 up to 100 mm. TLC readings over high voltage 1500 V at 20 min (A), 1 h (B), and 3 h postmigration (C). TLC readings of the same mixture at 1000 V at 5 min (D), 1 h (E), and 3 h postmigration (F). The middle peak seen at both high-voltage settings immediately after purification gradually decreases until it completely disappears 3 h postmigration. CPM, counts per minute; DGA, N,N,N′,N′-tetra-n-octyldiglycolamide; iTLC, instant thin layer chromatography; TLC, thin layer chromatography.
To verify the radioisotopic identity of each peak, a second strip was migrated, and each section was measured using the HPGe detector (Supplementary Fig. S1D–F). These measurements suggested that [227Th]Th4+ remained at the bottom of the strip, [223Ra]Ra2+ migrated to the top, and 211Bi (3+ charge) (half-life = 2.14 min) was present in the middle. Based on these results, the radio-TLC has a limited ability to quantitatively describe 227Th and 223Ra, although the 1000 V setting enhances resolution and increases the accuracy of measurements.
Sodium iodide γ counting
Another widely available instrument for radiopharmaceutical quality control is the γ-counter. Although highly sensitive to γ emissions, distinction of 227Th and 223Ra is challenging due to overlapping emissions (Supplementary Fig. S2A). The CPM acquired over a full window of energy spectra (100–2000 keV) for 227Th and 223Ra across all channels (open window) presents similar measurement profiles to those acquired under the radio-TLC scanner (Fig. 5A, B). The mixture of the two radioisotopes on the γ-counter shows a trend that is somewhat similar to that of 227Th (Fig. 5C). This is because a higher activity of 227Th than 223Ra was initially spotted on the paper.
FIG. 5.
Total CPM acquired in an open window channel measured for 227Th (A), 223Ra (B), and a mixture (C); Deconvoluted activity (Bq) calculated for a pure 227Th sample (D) showing 227Th decay fitting a single-phase decay (R2 = 0.9980), as well as 223Ra ingrowth. (E) Activity (Bq) for 223Ra fit with a single-phase decay (R2 = 0.9986). (F) 227Th and 223Ra mixed sample activity (Bq) plot. CPM, counts per minute.
To use this ubiquitous tool for radiopharmaceutical evaluation of 227Th and 223Ra, a method to deconvolute each energy spectrum was developed and the counts to report activities for each radioisotope were calibrated, enabling absolute quantification (Supplementary Fig. S2B). The method was tested by measuring an initially pure 227Th sample. In this study, a highly accurate one-phase decay curve (r2 > 0.99) was observed while separately recording the ingrowth of 223Ra (Fig. 5D).
As expected, pure 223Ra reveals the predicted one-phase decay (r2 > 0.99; Fig. 5E). The profiles of 227Th and 223Ra in the cospotted mixture showed the expected exponential decay for 227Th while recording 223Ra ingrowth (227Th: r2 = 0.9294; Fig. 5F). Together, this evaluation demonstrates the utility of this method to reliably separate the 223Ra and 227Th γ spectra to quantitate activities accurately.
α Particle spectral detection
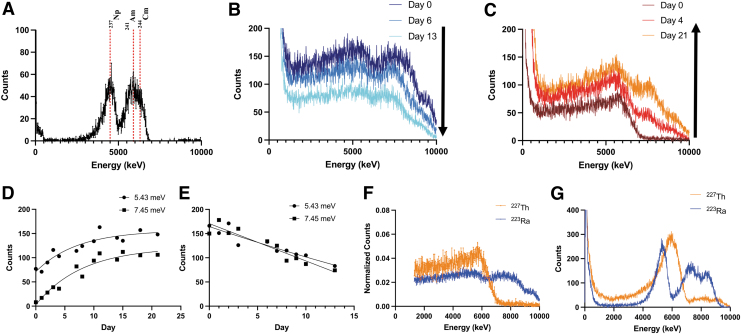
227Th and 223Ra emit α particles at characteristic energies, and isotope identification based upon these high-energy particles can be used for radiopharmaceutical evaluation. The authors were interested to use the Minipix spectral imaging camera for this purpose, a robust and mobile system for localized α-particle detection (Table 2). The α spectra of pure 227Th and 223Ra were evaluated and it was found that they were significantly different, allowing for differentiation between the two (Fig. 6F).
Table 2.
Specifications for Instrumentation Examined in These Methods
| Bioscan—1500 V | Bioscan—1000 V | γ Counter | Minipix | |
|---|---|---|---|---|
| Detected radiation | All ionizing radiation | α particles | γ | All ionizing radiation |
| Detection mechanism | P-10 ionizing gas | P-10 ionizing gas | Well-type NaI detector | Si solid-state semiconductor (256 × 256 pixels) |
| Detection timea | 1 min | 5 min | 1 min | 15 min |
| Detection resolution | 3 mmb | 3 mmb | <30%b | Spatial: 9 lp/mm Energy: 0.8 keV (THL) and 2 keV (ToT) |
| Software | WinScan | WinScan | Wizard2 data analyzer | PixetPRO |
| Portable | No | No | No | Yes |
For samples >0.025 μCi.
For most γ emitters.
THL, threshold level; ToT, time-over-threshold.
FIG. 6.
(A) A 241Am/237Np/244Cm standard source read using Minipix spectral imaging camera. From left to right, the three peaks correspond to the α-particles emitted by 237Np, 241Am, and 244Cm. (B, C) 223Ra and 227Th imaging spectra over one complete half-life. (D, E) Plotted counts acquired at 5.43 and 7.45 MeV for pure 227Th (D) and 223Ra (E). 5.43 MeV depicts the most significant α particle contribution emitted by 223Ra, and 7.45 MeV corresponds to the α particle released by 211Po and relates to the second peak seen in the graphs. Both increase over time for 227Th and decrease for 223Ra. (F) For the mixed spectra, the day 0 measurements of the radionuclides from (B) and (C) were normalized and plotted together. (G) The α spectra of 227Th and 223Ra plus daughters when spotted on an aluminum support.
Qualitatively, 223Ra shows two very spread peaks at ∼5500 and 7500 keV (Fig. 6B). The peak seen at 5500 keV corresponds to the α particles that are released by 223Ra (Table 2). The higher energy α-particles are most likely contributed by the daughters of 223Ra, specifically 215Po (7386.1 keV) and 210Po (7450.3 keV) and energies are collated in Table 2. Over 13 days, total counts in the 223Ra sample peaks decreased by approximately one half, corresponding to its half-life (Fig. 6E).
Unlike 223Ra, pure 227Th showed a single distinct peak at ∼5500 keV (Fig. 6C). Over time, the counts of this peak increase (Fig. 6D), due to 223Ra ingrowth, which emits α particles close in energy (Table 2). The emergence of a second peak at 7500 keV again corresponds to the high energy α particles emitted by the daughters of 223Ra.
The activity spotted on Whatman paper demonstrated low-energy resolution of individual α-particle spectra. This may be due to attenuation in air, by the required chromatography support and angular dispersion. Acquisitions were not collimated, as it produced no significant reduction in the energy distribution of individual peaks (not shown). When a pure 223Ra or 227Th source was spotted on a solid aluminum support, however, the energy resolution increased significantly (Fig. 6G). Here, comparative assessment of α-spectral detection permitted the differentiation of 227Th and 223Ra.
227Th-radiolabeled protein quality control
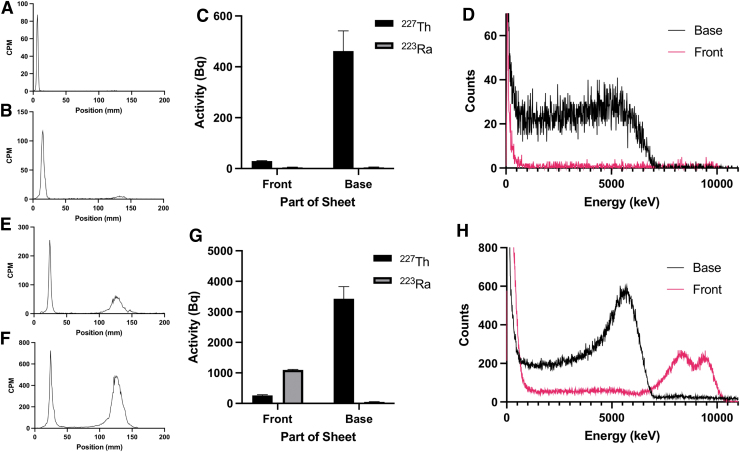
The previously presented analytical methods were applied to characterize a purified and nonpurified 227Th-radiolabeled protein. After migration on silica-coated chromatography paper, samples were first measured by radio-TLC. For the purified sample, only a single peak at the base of the strip was read, while the unpurified sample presented two peaks, suggesting the presence of free radioisotopes (Fig. 7A, B, E, F). These results demonstrate the value of the radio-TLC to evaluate the purity of a sample after it is migrated by TLC, but also its inability to identify specific radionuclides.
FIG. 7.
227Th-labeled antibody migrated on silica-coated chromatographic iTLC with DTPA (10 mM). Purified (A–D) and nonpurified (E–H) material were compared using radio-TLC scanner (A, B, E, F), γ counting (C, G), and α spectral imaging (D, H). The TLC scanner of purified and nonpurified 227Th-labeled protein was acquired immediately after migration at a high-voltage setting of 1000 V (A, E) and 1500 V (B, F), showing a main peak at the spotted area characteristic of 227Th-labeled material and migration of unchelated 223Ra at the top of the strip. (C, G) Purified (C) and unpurified (G) 227Th-labeled protein measured using γ counting. 227Th was found primarily nonmigrated at the bottom of the strip, confirming protein labeling. 223Ra was detected at the top of the strip for the unpurified material (G); (D, H) α spectral imaging of the purified (D) and nonpurified (H) 227Th-labeled antibody showed 227Th α emission at the bottom of the strip for both materials and 223Ra contribution at the top of the strip for the nonpurified material. CPM, counts per minute; DTPA, diethylenetriamine pentaacetate; iTLC, instant thin layer chromatography; TLC, thin layer chromatography.
The authors next measured the 227Th-radiolabeled protein using the γ counting protocol on a bisected sample. The purified protein showed only 227Th, which was present primarily at the bottom of the TLC strip; this contrasts to the unpurified material for which the majority of activity at the front was identified as 223Ra (Fig. 7C, G). The radiolabeling efficiency (92%) and radiochemical purity (>98%) for the purified compound were significant. The samples were also read on the Minipix spectral imaging camera. The purified sample depicts 227Th at the origin with nothing detected at the solvent front. Conversely, the unpurified sample reveals 223Ra at the front of the strip (Fig. 7D, H).
Discussion
The ability to accurately analyze a radiolabeled pharmaceutical is critical in both development and translation of new radiopharmaceuticals. With increasing interest in the field of radiopharmaceuticals for therapeutic use, the classical instrumentation utilized for radiotracer evaluation may be insufficient to assess the purity of agents that involve concatenated decay products with complex emission profiles.3,13 The evaluation of several technologies was conducted to determine the capacity of widely available or affordable systems for these purposes. These results demonstrate the ability to quantitatively discriminate between 227Th and 223Ra utilizing a γ-counter, as well as the practical applications of these methods for the quality assessment of radiolabeled antibodies.
One common quality control approach for radiopharmaceutical evaluation is TLC migration read using a P-10 gas imaging scanner. The system is, by manufacturer default, operated at a setting of 1500 V. These results show that at 1500 V, the imaging scanner presents low sensitivity for measuring the decay of complex emitters. The measured decay of 223Ra presented an r2 of only 0.5810 to an exponential fit. This is likely explained by the presence of its daughters, which emit X-rays, γ-rays, electrons, and α-particles for which the system has variable sensitivities.
The same observation is noted for pure 227Th, which did not follow a one-phase decay. This increase in signal before equilibrium can be explained by the ingrowth of 223Ra, which presents a comparable half-life (11.4 days) and strong emitted γ rays (324 keV, 3.64%; 269 keV, 13.30%; 154 keV, 6.02%; 144 keV, 3.47%) and has increased detection through ionization of the P-10 gas source. When lowering the high-voltage setting to 1000 V, the authors found that the accuracy of measurement increased substantially for 223Ra (r2 = 0.8611).
A different migration profile was recorded for a mixture of 223Ra and 227Th when the scanner was used at a setting of 1500 V as compared to at 1000 V. At the lower voltage setting, the spatial resolution of peaks was enhanced, at the cost of longer acquisition duration. As both time and resolution are important considerations, the application of the radio-TLC will determine which high-voltage setting is appropriate. These observations suggest the limitations of the imaging scanner to quantitatively describe complex emitting isotopes, but the utility is apparent in qualitatively determining impurities in a migrated sample.
The total counts obtained from γ-counting readings under an open energy window setting (100–2000 keV) showed similar results to those of the radio-TLC. However, 223Ra values demonstrated an accurate one-phase decay (r2 = 0.9993). This increase in accuracy when read on the γ-counter is due to the selective measurement of γ-rays. The γ-energy spectra of 227Th and 223Ra share significant regions of overlap and are, therefore, challenging to distinguish. Measurements of each pure radioisotope presented classical one-phase decay with time, with an r2 > 0.99 for both 227Th and 223Ra sources over the course of 20 days. Both the mixture and pure 227Th capture the ingrowth of 223Ra.
The absolute activity values first begin to plateau ∼15 days postpurification, consistent with the transient equilibrium (19 days). The deconvolution method employed here enhances the quantification capabilities of the γ-counter, enabling the distinction of 227Th from 223Ra and can be applied in research or clinical settings for purity assessment as well as an exact activity amount of each isotope in a mixture. This method may be useful for clinical quality control applications, which currently employ high-resolution γ-spectroscopy.15
As another analytical method for measuring the emitted α-particles from 227Th and 223Ra, detection using the Minipix solid-state semiconductor was investigated. The results show that this method was also able to differentiate between 227Th and 223Ra. Pure 227Th can be isolated by a single peak close to 6000 keV, which is consistent with the reported α particles (Table 2). Over time, the main peak observed shifts slightly downward in energy and increases in counts as 223Ra grows into the mixture, and a second peak closer to 8000 keV appears.
This second high-energy peak becomes apparent in as little as a day postpurification, demonstrating the utility of this method to differentiate between pure and impure 227Th. Pure 223Ra measured over time shows two peaks, with the lower peak corresponding to 223Ra and the upper peak to its higher energy α-emitting daughters. Both peaks decrease in overall counts over the course of one half-life to approximately half that of the day 0 measurement, consistent with the natural decay of the radioisotope. As demonstrated here, it is possible to qualitatively measure and distinguish 227Th and 223Ra by α-spectrometry.
The authors did observe significant spreading of individual peaks when measuring both 227Th and 223Ra. The high number of emitted α-particles (Table 2) and the distance that the α-particles must travel through the air before reaching the detector (0.7 cm) contribute to this observation. Using an aluminum support in place of the filter paper improved the energy resolution of 223Ra and 227Th samples. This implies the dispersion of α-particle energies was due to absorption within the paper. From these results, the authors note that a solid support may provide improved α-particle spectrometry-based assessment of radiopharmaceuticals.
Finally, the practicality of these methods using a 227Th-labeled protein drug was shown, and the important clinical applications of this study for other radiopharmaceutical products incorporating complex emitters were demonstrated, such as 223Ra and 225Ac, among others. The authors showed the ability to distinguish between the purified and nonpurified construct utilizing all three previously described methods. The radio-TLC at both high-voltage settings clearly demonstrates the presence of two chemically different species in the unpurified sample, but the authors were unable to identify individual radioisotopes by TLC evaluation.
To further characterize each sample, the upper and lower sections of the TLC strip were measured using the γ-counter and the counts per channel of energy were processed using the deconvolution method. The activity of 227Th and 223Ra in each sample was quantified, and the authors determined that the majority of the impurity that migrated to the front of the strip in the unpurified sample was free 223Ra. This is a validation of the γ-counting method to quickly and accurately determine the purity of a prepared radiopharmaceutical. The final results were confirmed by α-spectral analysis and HPGe measurement.
A final consideration is the improvements that these methods can make to current quality control practices. Currently, measurement by HPGe is the gold standard for discrimination between 227Th and 223Ra. However, this equipment is expensive and not accessible at all research and care facilities, and these methods propose three instruments that are widely available. With the exception of the γ-counter, the instruments are unable to provide exact quantification of activity amounts but provide a measurement of qualitative purity assessment. The γ-counter can provide quantitative activity amounts for the radioisotope of interest and the impurity, and is also broadly available, leading to the possibility for more accurate and accessible quality control of complex-emitting radiopharmaceuticals.
Conclusion
The development and clinical evaluation of 227Th and 223Ra therapies place new demands on radiochemists and pharmacists to ensure safe and effective use. As the field evolves to regulate and handle these potent agents, the authors have investigated widely deployed, robust, and affordable methods for their quality control.16 The data demonstrate an accessible method for assessing 227Th and 223Ra radiopharmaceutical purity using a γ-counter after discrimination of each isotope.
Radio-TLC and α-particle spectrometer imaging may prove useful for evaluating constituents and purity of a sample, but they may not be ideally suited for evaluation of complex mixtures of isotopes. Overall, this evaluation of two emerging radionuclides for α-therapy treatment underlines technological developments toward improved quality assurance for radiopharmaceuticals with concatenated decays and complex emission profiles.
Supplementary Material
Authors' Contributions
A.H. contributed to writing (lead), conceptualization (lead), formal analysis (lead), methodology development (equal), review, and editing (equal). W.J. was involved in writing (support), formal analysis (support), methodology development (support), review, and editing (equal). N.B. and L.S. carried out methodology development (support). P.L. carried out methodology development (equal). M.S.L. was involved in conceptualization (support), methodology development (equal), review, and editing (equal). B.J.B. carried out methodology development (equal), review, and editing (equal). H.Z. was in charge of conceptualization (support) and methodology development (support). R.L.W. carried out conceptualization (support), review, and editing (support). D.S.A. took care of writing (equal), conceptualization (lead), formal analysis (equal), methodology development (equal), review, and editing (equal). D.L.J.T. was in charge of writing (equal), conceptualization (equal), formal analysis (equal), methodology development (equal), review, and editing (equal).
Disclosure Statement
There are no existing financial conflicts.
Funding Information
The study herein was funded in part by the National Cancer Institute at the National United States Institutes of Health R01CA229893 (D.L.J.T.), R01CA201035 (D.L.J.T.) and R01CA240711 (D.L.J.T.). Isotopes used in this research were supplied in part by the United States Department of Energy Office of Science, Isotope Program, Office of Nuclear Physics.
Supplementary Material
References
- 1. Den RB, George D, Pieczonka C, et al. Ra-223 treatment for bone metastases in castrate-resistant prostate cancer: Practical management issues for patient selection. Am J Clin Oncol 2019;42(4):399–406; doi: 10.1097/COC.0000000000000528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poeppel TD, Handkiewicz-Junak D, Andreeff M, et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2018;45(5):824–845; doi: 10.1007/s00259-017-3900-4 [DOI] [PubMed] [Google Scholar]
- 3. Abou DS, Ulmert D, Doucet M, et al. Whole-body and microenvironmental localization of radium-223 in naïve and mouse models of prostate cancer metastasis. J Natl Cancer Inst 2016;108(5):djv380; doi: 10.1093/jnci/djv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parker C, Heinrich D, Helle SI, et al. Alpha emitter Radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213–223; doi: 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 5. Jiang W, Ulmert D, Simons BW, et al. The impact of age on radium-223 distribution and an evaluation of molecular imaging surrogates. Nucl Med Biol 2018;62–63:1–8; doi: 10.1016/j.nucmedbio.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramdahl T, Bonge-Hansen HT, Ryan OB, et al. An efficient chelator for complexation of thorium-227. Bioorg Med Chem Lett 2016;26(17):4318–4321; doi: 10.1016/j.bmcl.2016.07.034 [DOI] [PubMed] [Google Scholar]
- 7. Larsen RH, Borrebaek J, Dahle J, et al. Preparation of 227Th labeled radioimmunoconjugates, assessment of serum stability and antigen binding ability. Cancer Biother Radiopharm 2007;22(3):431–437; doi: 10.1089/cbr.2006.321 [DOI] [PubMed] [Google Scholar]
- 8. Liberal FDC, O'Sullivan JM, McMahon SJ, et al. Targeted alpha therapy: Current clinical applications. Cancer Biother Radiopharm 2020;35(6):404–417; doi: 10.1089/cbr.2020.3576 [DOI] [PubMed] [Google Scholar]
- 9. Bruland O, Nilsson S, Fisher DR, et al. High-linear energy transfer irradiation targeted to skeletal metastases by the α-Emitter 223Ra: Adjuvant or alternative to conventional modalities? Clin Cancer Res 2006;12(20):6250s–6257s; doi: 10.1158/1078-0432.CCR-06-0841 [DOI] [PubMed] [Google Scholar]
- 10. Smith T, Kearfott KJ. Practical considerations for gamma ray spectroscopy with NaI(Tl). Health Phys 2018;114(1):94–106; doi: 10.1097/HP.0000000000000804 [DOI] [PubMed] [Google Scholar]
- 11. Marcus CS. How should the FDA review diagnostic radiopharmaceuticals? J Nuclear Med 2018;59(6):868–870; doi: 10.2967/jnumed.117.200337 [DOI] [PubMed] [Google Scholar]
- 12. Schwarz SW, Clarke B.. Perspective on how the FDA should review diagnostic pharmaceuticals. J Nuclear Med 2018;59(6):865–867; doi: 10.2967/jnumed.117.204446 [DOI] [PubMed] [Google Scholar]
- 13. Abou DS, Pickett J, Mattson JE, et al. A Radium-223 microgenerator from cyclotron-produced trace Actinium-227. Appl Radiat Isot 2017;119:36–42; doi: 10.1016/j.apradiso.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bateman H. The solution of a system of differential equations occurring in the theory of radioactive transformations. Proc Cambridge Phil Soc 1910;15(V):423–427. [Google Scholar]
- 15. Assessment Report—Xofigo®. European Medicines Agency. 2013. Available from: https://www.ema.europa.eu/en/documents/assessment-report/xofigo-epar-public-assessment-report_en.pdf [Last accessed October 2021].
- 16. Hagemann UB, Wickstroem K, Hammer S, et al. Advances in precision oncology: Targeted Thorium-227 conjugates as a new modality in targeted alpha therapy. Cancer Biother Radiopharm 2020;35(7):497–510; doi: 10.1089/cbr.2020.3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.