Abstract
Background:
Δ9-Tetrahydrocannabinol (THC), the main intoxicating component of cannabis, can cause cognitive and psychomotor impairment. Whether this impairment is still present many hours or even days after THC use requires clarification. Possible “next day” effects are of major significance in safety-sensitive workplaces. We therefore conducted a systematic review of studies investigating the “next day” effects of THC.
Methods:
Studies that measured performance on safety-sensitive tasks (e.g., driving, flying) and/or neuropsychological tests >8 h after THC (or cannabis) use using interventional designs were identified by searching two online databases from inception until March 28, 2022. Risk of bias (RoB) was evaluated using the relevant Cochrane tools. Results were described in terms of whether THC had a significant effect on performance relative to the primary comparator (i.e., placebo or baseline, as appropriate).
Results:
Twenty studies (n=458) involving 345 performance tests were reviewed. Most studies administered a single dose of THC (median [interquartile range]: 16 [11–26] mg) and assessed performance between >12 and 24 h post-treatment. N=209/345 tests conducted across 16 published studies showed no “next day” effects of THC. Nine of these 16 studies used randomized, double-blind, placebo-controlled designs. Half (N=8) had “some” RoB, and half (N=8) had a “high” RoB. Notably, N=88 of these 209 tests failed to demonstrate “acute” (i.e., <8 h post-treatment) THC-induced impairment. N=12/345 tests conducted across five published studies indicated negative (i.e., impairing) “next day” effects of THC. None of these five studies used randomized, double-blind, placebo-controlled designs and all were published >18 years ago (four, >30 years ago). Three had “some” RoB, and two had a “high” RoB. A further N=121/345 tests indicated “unclear” “next day” effects of THC with insufficient information provided to assess outcomes. The remaining N=3/345 tests indicated positive (i.e., enhancing) “next day” effects of THC.
Conclusions:
Some lower quality studies have reported “next day” effects of THC on cognitive function and safety-sensitive tasks. However, most studies, including some of higher quality, have found no such effect. Overall, it appears that there is limited scientific evidence to support the assertion that cannabis use impairs “next day” performance. Further studies involving improved methodologies are required to better address this issue.
Keywords: cannabis, THC, cannabinoids, impairment, cognitive function, driving
Introduction
Two hundred million people use cannabis each year.1 This includes those using cannabis for its euphorigenic effects (i.e., so-called “recreational” users) and, increasingly, those using it to treat medical conditions such as chronic pain, insomnia, and anxiety.2
The potential harms associated with cannabis use have been debated over many decades. One ongoing concern is that the major cannabis constituent, Δ9- tetrahydrocannabinol (THC), can induce intoxication and impair cognitive and psychomotor performance (e.g., reaction time, working memory, divided attention),3 increasing the risk of error, accident, and injury when operating a motor vehicle or engaging in other safety-sensitive tasks.4–6 Indeed, epidemiological studies suggest that “THC-positive” drivers are between ∼1.1 and 1.4 times more likely to become crash-involved than other drivers.7
The duration of THC-induced impairment, or length of time an individual should wait following cannabis use before performing safety-sensitive tasks, is a critical issue. A recent meta-regression analysis3 concluded that there was a “window of impairment” extending from ∼3 to 10 h after THC use, with the exact duration dependent on the following: (1) dose: higher THC doses produced longer lasting impairment; (2) route of administration: oral THC produced longer lasting impairment than inhaled THC (e.g., smoked, vaporized), owing to the fact that gastrointestinal absorption is slower than pulmonary absorption8,9; and (3) regularity of cannabis use: occasional cannabis users became more impaired than regular cannabis users (who appear to be more tolerant to the impairing effects of THC10). This review did not, however, include performance tests conducted >12 h after THC use.
Some government agencies and experts in occupational safety caution that THC-induced impairment may persist for >24 h and recommend that individuals avoid performing safety-sensitive tasks for at least this long after cannabis use.11,12 This can impact upon those who are reliant on driving for their work and/or family life, and upon individuals employed in safety-sensitive positions (e.g., transit and construction workers, defense personnel), who may use cannabis “off-duty” (e.g., in the evening, on the weekend) to treat conditions such as insomnia and chronic pain. However, such advice does not appear to have been informed by a comprehensive review of the scientific evidence.
We therefore conducted a systematic review to better understand the “next day” (i.e., >8 h) effects of THC use on cognitive function and safety-sensitive tasks.
Methods
The methods of this review were developed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Version 6.2, 2021).13
Literature search
Relevant studies were identified by searching the online databases Scopus and Web of Science (Thomas Reuters) from inception until March 28, 2022, using the Boolean expression in Supplementary File S1. Two investigators (D.M. and A.S.) independently screened all titles and abstracts against the following inclusion criteria: (1) English language; (2) full-length article; (3) original research; (4) interventional design; and (5) THC administration. Suitable records were then screened for eligibility by full text (see “Eligibility criteria” section). The final decision to include (or discard) a study was made between these two investigators; discrepancies were resolved in discussion with a third investigator (I.S.M.). One investigator (D.M.) also hand-searched the reference lists of the included publications and two previous reviews3,14 to ensure all relevant articles were captured.
Eligibility criteria
Studies that measured performance on “safety-sensitive” tasks (e.g., simulated or on-road driving performance, simulated aeroplane flying) and/or discrete neuropsychological tests > 8 h post (last)-THC (or cannabis) use using an interventional experimental design (any)15 were eligible for inclusion. The >8-h interval was selected to represent a typical overnight “recovery” period16 and to minimize overlap with a previous review investigating the shorter-term effects of THC (i.e., ≤12 h).3 No “upper limit” was imposed. All participant populations (e.g., clinical, “healthy”) and comparator conditions (e.g., placebo, baseline) were accepted. However, studies were excluded if THC was co-administered with another treatment (excluding placebo treatments, other cannabinoids or cannabis constituents, tobacco, or participants' usual medication) or if results were reported in another included article. Only full-length, English-language, original research articles published in scientific journals were accepted.
Note that if a study contained multiple “intervention arms,” more than one of which was eligible for inclusion, the separate “arms” were treated as discrete “studies,” termed trials, identifiable by the additional letters (e.g., a–d) in the citation.
Performance outcomes
All objective outcomes measured on safety-sensitive tasks and discrete neuropsychological tests >8 h post-THC administration were accepted. Outcomes measured ≤8 h post-THC administration (on eligible performance tests) were also included. Indeed, these data were used to determine whether the performance tests administered >8 h post-treatment were sensitive to the “acute” (i.e., <8 h post-treatment) effects of THC.
Quality assessment
Risk of bias (RoB) in included studies was evaluated by two independent assessors (D.M. and A.S.) using (1) the Revised Cochrane Risk of Bias tool (RoB 2.0)17 and (2) the RoB 2.0 for crossover trials,18 as appropriate. Both tools examine five potential sources of bias, that is, bias arising from (1) the randomization process; (2) deviations from the intended intervention; (3) missing outcome data; (4) measurement of the outcome; and (5) selective outcome reporting. The latter also examines bias arising from period or carryover effects. Both tools generate an overall “risk rating” (i.e., “low risk,” “some concerns,” “high risk”).
Data extraction
The extracted data included the following: (1) study design; (2) participant characteristics (e.g., age, sex, body weight, health status, cannabis use behavior); (3) treatment characteristics (e.g., type, composition, route of administration, THC dose); (4) task characteristics (e.g., test, outcomes, number of assessments, length of time between THC administration and the performance test[s]); and (5) standardization procedures employed, that is, the methods used to control participants' pre-trial and “within-trial” (i.e., up until the >8 h post-treatment assessment) sleep behavior and cannabis, alcohol, caffeine, and other psychoactive drug use. The latter were considered important as they have been shown to influence cognitive and psychomotor performance.3,19–21
Data synthesis
The results of the included studies were synthesized qualitatively, that is, described in terms of whether THC was found to have a statistically significant effect (i.e., p<0.05) on each performance test (i.e., any one of its outcome measures) relative to the primary comparator, taken as placebo in placebo-controlled trials and baseline (i.e., pre-treatment) elsewhere. If an outcome was analyzed within a complex model (e.g., including three or more treatments and[or] other factors, e.g., time) and no main effect of treatment or relevant interaction(s) was observed, the effect was assumed to be nonsignificant. If a main effect of treatment or relevant interaction was observed, statistical significance was ascertained on the basis of post-hoc comparisons.
The results of post-hoc comparisons on main effects of treatment that included a time parameter were generalized across all included time points unless the individual time points were compared by treatment or the comparison incorporated baseline (i.e., pre-treatment) data (in the latter case, the comparison was considered ambiguous). If post-hoc comparisons were not performed, or there was any ambiguity in the reported result, the statistical significance of the effect was not presented in this review. Meta-analysis was not performed as studies often failed to report (or graph) the information required to calculate an effect estimate (most studies [80%] were also published >10 years ago [65%, >20 years ago], making it difficult to retrieve the missing data).
Each neuropsychological test was reviewed and categorized into one of the following cognitive domains as previously demonstrated by McCartney et al3 and shown in Supplementary Table S1: (1) divided attention; (2) executive function; (3) information processing; (4) tracking performance; (5) reaction time; (6) motor function; (7) sustained attention; (8) working memory; (9) perception; (10) learning and(or) memory; and (11) spatial reasoning.
The terms used to describe participants' cannabis use behavior (e.g., daily, weekly–daily, monthly, etc.) are also as per McCartney et al3 and defined in Supplementary Table S2. These categories were further collapsed into two main groupings: regular cannabis users (which included populations of daily users, weekly users, weekly–daily users) and other cannabis users (all other populations) to aid in synthesizing the available literature.
Note that the length of time between THC administration and the beginning of the performance test was calculated from: (1) the last THC exposure if more than one dose was administered before the performance test; and (2) the beginning of the “battery” if multiple tests were administered in succession and their individual start times were not reported.
Results
Overview of included studies
Twenty studies (n=458 participants; 79% male, excluding studies that did not report the sex of their participants) were included in this systematic review. These studies administered a total of 345 performance tests (i.e., across all trials and time points >8 h post-treatment). The study selection process is detailed in Supplementary File S1.
The characteristics of the included studies are summarized in Table 1. Briefly, most studies used randomized (N=11) or “nonrandomized” (i.e., randomization was not reported; N=5) double-blind, placebo-controlled designs; however, three were single blind and one used a “pre-/post-treatment” design. All included “healthy” participants, only (i.e., no studies of clinical populations were eligible for inclusion). Other (i.e., mostly occasional) cannabis users and populations with an average age ≤30 years were studied more often than regular (i.e., weekly, or more often) cannabis users and those with an average age >30 years, respectively (Table 1). Most studies administered THC by smoking (N=13); the remainder did so through oral ingestion (N=7) (all, but three22–24 gave a single dose of THC).
Table 1.
Characteristics of Included Studies
| Studies (N) or participants (n) | Citations | |
|---|---|---|
| Study design | ||
| Randomized, DB, PC | N=11 | 23,25,26,28,29,31,34–37,39 |
| Nonrandomized,a DB, PC | N=5 | 22,27,30,32,38 |
| Nonrandomized, SB,b PC | N=3 | 24,33,40 |
| Pre-/post-trial | N=1 | 41 |
| Participant characteristics | ||
| Male | n=297 | — |
| Female | n=79 | — |
| Sex not specified | n=82 (N=4) | 31,32,40,41 |
| Average age ≤30 years | N=15 | 22–24,26,28–31,34–39,41 |
| Average age >30 years | N=4 | 25,31,32,40 |
| Average age not specified | N=2 | 27,33 |
| “Regular” cannabis usersc | N=4 | 22,28,29,37 |
| “Other” cannabis usersc | N=16 | 23–27,30–36,38–41 |
| Healthy population | N=20 | 22–41 |
| Treatment characteristics | ||
| Smoked cannabis or THC | N=13 | 22–24,28–31,33,35,37,38,40,41 |
| Ingested cannabis or THC | N=7 | 25–27,32,34,36,39 |
| THC dose unknown | N=5 | 22–24,30,35 |
| THC dose (mg) (median [IQR]) | 16 [11–26]d | — |
| Type of performance teste | ||
| Divided attention | N=6 | 22,25–27,30,33 |
| Executive function | N=4 | 23,30,34,35 |
| Information processing | N=11 | 22–28,30,33,34,36 |
| Tracking performance | N=1 | 33 |
| Reaction time | N=5 | 22,23,27,34,35 |
| Motor function | N=3 | 28,30,35 |
| Sustained attention | N=4 | 27,28,34,37 |
| Working memory | N=6 | 22,23,30,34–36 |
| Perception | N=3 | 22,24,30 |
| Learning and(or) memory | N=9 | 22–25,27,28,30,34,35 |
| Spatial reasoning | N=1 | 35 |
| Driving performance | N=4 | 29,37–39 |
| Flying performance | N=3 | 31,40,41 |
| Unknown | N=2 | 32,36 |
| Time of performance test | ||
| >8 to 12 h Post-treatment | N=7 | 22,24–27,33,37 |
| >12 to 24 h Post-treatment | N=16 | 23,25,28–41 |
| >24 to 48 h Post-treatment | N=8 | 23,26,28,29,31,34,35,40 |
| ≤8 h Post-treatment | N=18 | 23–26,28–41 |
| “Recovery” conditions | ||
| Supervised | N=8 | 22–24,27,30,35,36,39 |
| Unsupervised | N=10 | 26,28,29,31–34,38,40,41 |
| Unclear or not specified | N=2 | 25,37 |
Includes studies that did not indicate whether randomization was performed.
Includes studies that did not indicate whether researchers were blinded.
As defined in “Data synthesis” section.
Across all trials where the THC dose is known.
Includes those administered >8 h post-treatment, only.
DB, double blind; IQR, interquartile range; PC, placebo controlled; SB, single blind; THC, Δ9-tetrahydrocannabinol.
The median (interquartile range [IQR]) (last) THC dose was 16 [11–26] mg (where reported; N=15). Two types of “safety-sensitive task” (simulated driving and flying) and a wide range of neuropsychological tests were administered. The number of tests conducted between >8–12, >12–24, and >24–48 h post-treatment was 98, 158, and 89, respectively. Eight studies supervised their participants throughout the >8 h “recovery” period; the remainder (N=12) allowed them to leave the laboratory between assessments. All appeared to assess performance the day following THC administration (i.e., the “next day” or longer). (Note that only the 12-, 10-, and 10-h assessments conducted in Schoedel et al,25 Ménétrey et al,26 and Nicholson et al,27 respectively, are presented in both the current and former3 review).
Risk of bias
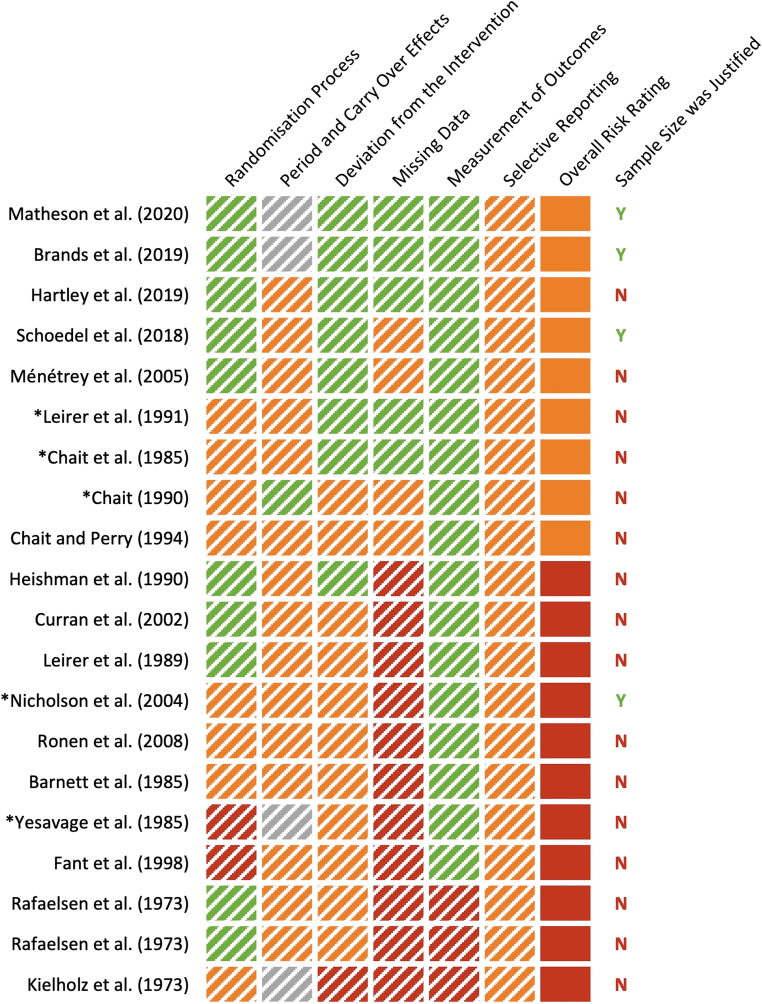
The results of the RoB assessment are detailed in Supplementary File S2 and summarized in Figure 1. None of the included studies demonstrated an overall “low risk” of bias, although two, Matheson et al28 and Brands et al,29 received “low risk” ratings on four out of the five RoB domains assessed. Nine studies were found to have “some concerns,” and 11 had a “high risk” of bias. The most common problems were RoB arising from (1) missing outcome data; (2) selective outcome reporting; and (3) carryover effects—with studies often failing to indicate whether any participant discontinued in the trial, analyze their data in accordance with a pre-specified plan, and report the number of participants assigned to each treatment order. Only four studies justified their chosen sample size.
FIG. 1.
Risk of bias as assessed using the Revised Cochrane Risk of Bias tool (RoB 2.0)17 and the RoB 2.0 for crossover trials18 (as appropriate). Green: low risk of bias; orange: some concerns; red: high risk of bias; gray: not applicable (not a crossover trial); N: No; Y: Yes. *Studies that detected significant detrimental effects of THC on “next day” performance (see Table 2). See Supplementary File S2 for full assessment.
Standardization procedures
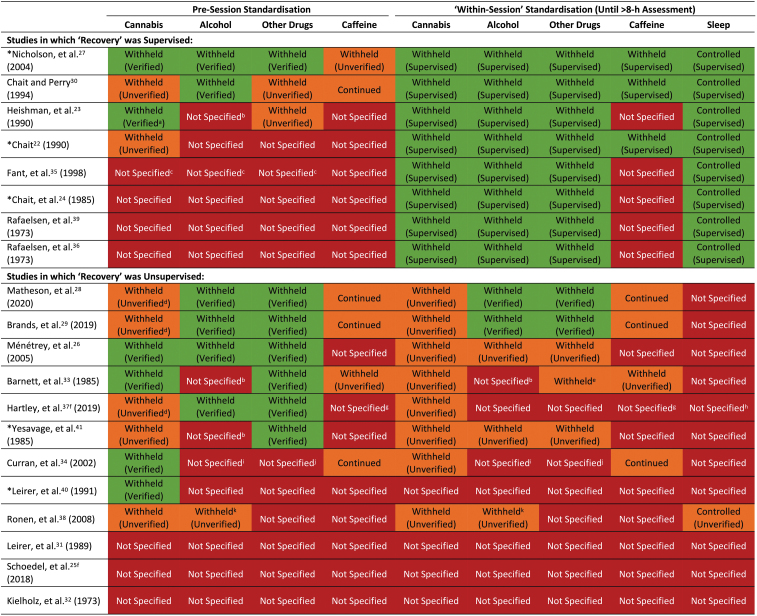
The “standardization procedures” employed, that is, methods used to control participants' pre-trial and “within-trial” (i.e., up until the >8 h post-treatment assessment) sleep behavior and cannabis, alcohol, caffeine, and other drug use, are summarized in Fig. 2. Studies that supervised their participants throughout the >8-h recovery period (N=8) achieved better within-trial standardization than those that did not (N=12). However, the latter tended to achieve better pre-trial standardization with most (N=9) controlling at least one pre-trial condition. Nicholson et al27 and Chait and Perry30 implemented the most robust standardization procedures; followed by Matheson et al28 and Brands et al.29 Three studies failed to report implementing any standardization procedure.25,31,32
FIG. 2.
Standardization procedures employed in included studies. A substance was considered “withheld” if participants were instructed to avoid using it for ≥24 h (12 h for caffeine) or if abstinence was “verified.” Abstinence was considered “verified” if participants returned a negative “drug test”; that is, a breath test (alcohol), urine screen (other drugs), or blood test (caffeine). Pre-session cannabis abstinence was only “verified” if participants returned a negative blood or urine screen; furthermore, within-session cannabis abstinence was only “verified” if participants were supervised until the >8 h post-treatment assessment. Within-session alcohol and other drug use were also assumed to be “verified” if participants were supervised until this assessment (but could otherwise be demonstrated through a drug test). A substance was considered “continued” if participants were instructed to continue using it as usual. Sleep was considered “controlled” if participants were supervised until the >8 h post-treatment assessment or instructed to obtain sufficient sleep. Adherence to the latter was considered “verified” if an objective measure of sleep quality or duration was obtained. aUrine 11-COOH-THC concentrations were <20 ng∙mL−1. bParticipants were instructed to avoid using “drugs”; however, it is unclear whether this included alcohol. cParticipants were retained in the laboratory for 2 weeks; however, the pre-session one standardization procedures were not specified. dShort-term cannabis abstinence cannot be verified in a population of regular users. eIt is unclear if this was verified. f“Recovery” was assumed to be unsupervised. gIndividuals with high habitual caffeine intakes were excluded. hIndividuals with sleep disorders were excluded. iIndividuals with high habitual alcohol intakes were excluded. jIndividuals who used psychedelic drugs were excluded. kAlcohol intake was restricted to one glass. *Studies that observed significant negative effects of THC on “next day” performance (see Table 2).
“Next Day” effects of THC
The results of the included studies are described below and detailed in Table 2. Note that the studies that administered multiple performance tests can appear in multiple “subsections” (e.g., if they observed negative “next day” effects on some tests, but not others).
Table 2.
Characteristics and Results of Included Trials (>8-h Treatment, only)
| Citation | Study design | Participants | Usual cannabis use behavior | Treatment | THC dose (mg) | Performance test | Outcomes | Time since last THC use | Effect of THC (compared to placebo unless otherwise stated) |
|---|---|---|---|---|---|---|---|---|---|
| Matheson et al 28 _a (2020) | Randomized; DB; PC (BSD) |
C: 30 (21 M); 22±2 years I: 31 (18 M); 22±2 years |
Weekly–daily | Smoked cannabis cigarettes (562±170 mg; 12.5% THC) (<0.5% CBD) |
70.3±21.3 a | Grooved pegboard task | Time to complete (DH) Time to complete (non-DH) |
24 & 48 h | No significant effect |
| DSST | Number of completed trials Number of correct trials Reaction time |
24 & 48 h | THC ↑ number of correct trials at 48 h | ||||||
| CPT | Percent omission errors Percent commission errors Hit rate Hit rate variability Detectability |
24 & 48 h | No significant effect | ||||||
| HVLT-R | Immediate recall Total recall Learning score Delayed recall Percent retained True positives False positive Discrimination index |
24 & 48 h | No significant effect | ||||||
| Matheson et al 28 _b (2020) | Randomized; DB; PC (BSD) |
C: 30 (21 M); 22±2 years I: 30 (26 M); 22±2 years |
Weekly–daily | Smoked cannabis cigarettes (752±131 mg; 12.5% THC) (<0.5% CBD) |
94.0±16.4 a | Grooved pegboard task | Time to complete (DH) Time to complete (non-DH) |
24 & 48 h | No significant effect |
| DSST | Number of completed trials Number of correct trials Reaction time |
24 & 48 h | THC ↑ number of correct trials at 48 h. | ||||||
| CPT | Percent omission errors Percent commission errors Hit rate Hit rate variability Detectability |
24 & 48 h | No significant effect | ||||||
| HVLT-R | Immediate recall Total recall Learning score Delayed recall Percent retained True positives False positive Discrimination index |
24 & 48 h | No significant effect | ||||||
| Brands et al 29 _a (2019) | Randomized; DB; PC (BSD) |
C: 30 (21 M); 22±2 years I: 31 (18 M); 22±2 years |
Weekly–daily | Smoked cannabis cigarettes (562±170 mg; 12.5% THC) (<0.5% CBD) |
70.3±21.3 a | Simulated driving | SDLP Speed |
24 & 48 h | No significant effect |
| Simulated driving (dual task) | SDLP Speed |
24 & 48 h | No significant effect | ||||||
| Brands et al 29 _b (2019) | Randomized; DB; PC (BSD) |
C: 30 (21 M); 22±2 years I: 30 (26 M); 22±2 years |
Weekly–daily | Smoked cannabis cigarettes (752±131 mg; 12.5% THC) (<0.5% CBD) |
94.0±16.4 a | Simulated driving | SDLP Speed |
24 & 48 h | THC ↓ SDLP at 48 h |
| Simulated driving (dual task) | SDLP Speed |
24 & 48 h | No significant effect | ||||||
| Hartley et al 37 _a (2019) | Randomized; DB; PC (WSD) | 15 M; 22±3 years | Weekly | Smoked cannabis cigarettes (9.8% THC; 1 g tobacco) (<0.1% CBD and CBN) |
10 | Simulated driving | SDLP | 12 & 24 h | No effect b |
| PVT | Reciprocal reaction time | 12 & 24 h | No effect b | ||||||
| Hartley et al 37 _b (2019) | Randomized; DB; PC (WSD) | 15 M; 22±3 years | Weekly | Smoked cannabis cigarettes (9.8% THC; 1 g tobacco) (<0.1% CBD and CBN) |
30 | Simulated driving | SDLP | 12 & 24 h | No effect b |
| PVT | Reciprocal reaction time | 12 & 24 h | No effect b | ||||||
| Hartley et al 37 _c (2019) | Randomized; DB; PC (WSD) | 15 M; 22±3 years | Daily | Smoked cannabis cigarettes (9.8% THC; 1 g tobacco) (<0.1% CBD and CBN) |
10 | Simulated driving | SDLP | 12 & 24 h | No effect b |
| PVT | Reciprocal reaction time | 12 & 24 h | No effect b | ||||||
| Hartley et al 37 _d (2019) | Randomized; DB; PC (WSD) | 15 M; 22±3 years | Daily | Smoked cannabis cigarettes (9.8% THC; 1 g tobacco) (<0.1% CBD and CBN) |
30 | Simulated driving | SDLP | 12 & 24 h | No effect b |
| PVT | Reciprocal reaction time | 12 & 24 h | No effect b | ||||||
| Schoedel et al 25 _a (2018) | Randomized; DB; PC (WSD) c | 43 (31 M) d ; 38±9 years | Infrequent–daily | THC capsules | 10 | Divided attention task | Tracking accuracy | 12 & 24 h | No significant effect |
| HVLT-R | Delayed recall Percent retained |
12 & 24 h | No relevant analysis e | ||||||
| DSST | Number of completed trials Number of incorrect trials |
12 & 24 h | No relevant analysis e | ||||||
| Schoedel et al 25 _b (2018) | Randomized; DB; PC (WSD) c | 43 (31 M) d ; 38±9 years | Infrequent–daily | THC capsules | 30 | Divided attention task | Tracking accuracy | 12 & 24 h | No significant effect |
| HVLT-R | Delayed recall Percent retained |
12 & 24 h | No relevant analysis e | ||||||
| DSST | Number of completed trials Number of incorrect trials |
12 & 24 h | No relevant analysis e | ||||||
| Ronen et al 38 (2008) | DB; PC (WSD) | 14 (10 M); 22±2 years | Monthly–weekly | Smoked THC cigarettes | 17 | Simulated driving | RMS lane position RMS speed Speed RMS steering deviations Reaction time (dual task) |
24 h | No significant effect f |
| Ménétrey et al 26 _a (2005) | Randomized; DB; PC (WSD) | 8 M g ; range: 22–30 years | Unclear | Hemp milk decoction | 16.5 | Road sign test | Time to complete | 10 & 25 h | Ambiguous h |
| Divided attention task | Tracking accuracy Number of errors Reaction time |
10 & 25 h | Ambiguous h | ||||||
| Ménétrey et al 26 _b (2005) | Randomized; DB; PC (WSD) | 8 M g ; range: 22–30 years | Unclear | Hemp milk decoction | 45.7 | Road sign test | Time to complete | 10 & 25 h | Ambiguous h |
| Divided attention task | Tracking accuracy Number of errors Reaction time |
10 & 25 h | Ambiguous h | ||||||
| Ménétrey et al 26 _c (2005) | Randomized; DB; PC (WSD) | 8 M g ; range: 22–30 years | Unclear | THC capsules | 20 | Road sign test | Time to complete | 10 & 25 h | Ambiguous h |
| Divided attention task | Tracking accuracy Number of errors Reaction time |
10 & 25 h | Ambiguous h | ||||||
| Nicholson et al 27 _a (2004) | DB; PC (WSD) | 8 (4 M); range 21–34 years | Current nonusers | Oromucosal spray | 15 | Word memory recall | Immediate recall Delayed recall |
10 h | THC ↓ immediate and delayed recall at 10 h |
| Digit memory recall | Reaction time Number of errors |
10 h | No significant effect | ||||||
| 6-Letter memory recall | Reaction time Number of errors |
10 h | No significant effect | ||||||
| DSST | Number of completed trials | 10 h | No significant effect | ||||||
| Multi-attribute task | System monitoring RT System monitoring RA Communications RT Communications RA Resource management RT Resource management RA Tracking accuracy |
10 h | No significant effect | ||||||
| Choice reaction time task | Reciprocal reaction time Number of errors |
10 h | No significant effect | ||||||
| Sustained attention task | Reaction time Number of errors |
10 h | No significant effect | ||||||
| Nicholson et al 27 _b (2004) | DB; PC (WSD) | 8 (4 M); range 21–34 years | Current nonusers | Oromucosal spray (5 mg CBD) |
5 | Word memory recall | Immediate recall Delayed recall |
10 h | No significant effect |
| Digit memory recall | Reaction time Number of errors |
10 h | THC ↑ reaction time at 10 h | ||||||
| 6-Letter memory recall | Reaction time Number of errors |
10 h | No significant effect | ||||||
| DSST | Number of completed trials | 10 h | No significant effect | ||||||
| Multi-attribute task | System Monitoring RT System Monitoring RA Communications RT Communications RA Resource management RT Resource management RA Tracking accuracy |
10 h | No significant effect | ||||||
| Choice reaction time task | Reciprocal reaction time Number of errors |
10 h | No significant effect | ||||||
| Sustained attention task | Reaction time Number of errors |
10 h | No significant effect | ||||||
| Nicholson et al 27 _c (2004) | DB; PC (WSD) | 8 (4 M); range 21–34 years | Current nonusers | Oromucosal spray (15 mg CBD) |
15 | Word memory recall | Immediate recall Delayed recall |
10 h | No significant effects |
| Digit memory recall | Reaction time Number of errors |
10 h | No significant effects | ||||||
| 6-Letter memory recall | Reaction time Number of errors |
10 h | No significant effect | ||||||
| DSST | Number of completed trials | 10 h | No significant effect | ||||||
| Multi-attribute task | System monitoring RT System monitoring RA Communications RT Communications RA Resource management RT Resource management RA Tracking accuracy |
10 h | No significant effect | ||||||
| Choice reaction time task | Reciprocal reaction time Number of errors |
10 h | No significant effect | ||||||
| Sustained attention task | Reaction time Number of errors |
10 h | No significant effect | ||||||
| Curran et al 34 _a (2002) | Randomized; DB; PC (WSD) | 15 M; 24±2 years | Unclear | THC capsules | 7.5 | Buschkel selective reminding task | Immediate recall Delayed recall |
24 & 48 h | Ambiguous |
| RVIPT | Proportion of hits Reaction time |
24 & 48 h | No significant effect | ||||||
| Baddeley reasoning task | Reaction time Number of errors |
24 & 48 h | No significant effect | ||||||
| Subtract serial sevens task | Reaction time Number of errors |
24 & 48 h | No significant effect | ||||||
| Choice reaction time task | Reaction time Number of errors |
24 & 48 h | No significant effect | ||||||
| Digit cancellation task | Time to complete (ST) Number of errors (ST) Time to complete (DT) Number of errors (DT) |
24 & 48 h | No significant effects | ||||||
| Simple reaction time task | Reaction time | 24 & 48 h | No significant effect | ||||||
| Curran et al 34 _b (2002) | Randomized; DB; PC (WSD) | 15 M; 24±2 years | Unclear | THC capsules | 15 | Buschkel selective reminding task | Immediate recall Delayed recall |
24 & 48 h | Ambiguous |
| RVIPT | Proportion of hits Reaction time |
24 & 48 h | No significant effect | ||||||
| Baddeley reasoning task | Reaction time Number of errors |
24 & 48 h | No significant effect | ||||||
| Subtract serial sevens task | Reaction time Number of errors |
24 & 48 h | No significant effect | ||||||
| Choice reaction time task | Reaction time Number of errors |
24 & 48 h | No significant effect | ||||||
| Digit cancellation task | Time to complete (ST) Number of errors (ST) Time to complete (DT) Number of errors (DT) |
24 & 48 h | No significant effect | ||||||
| Simple reaction time task | Reaction time | 24 & 48 h | No significant effect | ||||||
| Fant et al 35 _a (1998) | Randomized; DB; PC (WSD) | 10 M; 27 years, range: 24–31 years |
Monthly–weekly | Smoked cannabis cigarettes (1.8% THC) |
“Eight Puffs” (dose unknown) |
Smooth-pursuit eye movements | Central speed (fixed) Central speed (varied) Peripheral speed (fixed) Peripheral speed (varied) |
23, 24 & 25 h | Ambiguous i |
| Circular lights task | Number of correct responses | 23, 24 & 25 h | No significant effect | ||||||
| Serial addition and subtraction task | Number of correct responses Percent correct responses Reaction time |
23, 24 & 25 h | No significant effect | ||||||
| Digit recall task | Number of correct responses Percent correct responses Reaction time |
23, 24 & 25 h | No significant effect | ||||||
| Logical reasoning task | Number of correct responses Percent correct responses Reaction time |
23, 24 & 25 h | No significant effect | ||||||
| Mannequin task | Number of correct responses Percent correct responses Reaction time |
23, 24 & 25 h | No significant effect | ||||||
| Fant et al 35 _b (1998) | Randomized; DB; PC (WSD) | 10 M; 27 years, range: 24–31 years |
Monthly–weekly | Smoked cannabis cigarettes (3.6% THC) |
“Eight Puffs” (dose unknown) |
Smooth-pursuit eye movements | Central speed (fixed) Central speed (varied) Peripheral speed (fixed) Peripheral speed (varied) |
23, 24 & 25 h | Ambiguous i |
| Circular lights task | Number of correct responses | 23, 24 & 25 h | No significant effect | ||||||
| Serial addition and subtraction task | Number of correct responses Percent correct responses Reaction time |
23, 24 & 25 h | No significant effect | ||||||
| Digit recall task | Number of correct responses Percent correct responses Reaction time |
23, 24 & 25 h | No significant effect | ||||||
| Logical reasoning task | Number of correct responses Percent correct responses Reaction time |
23, 24 & 25 h | No significant effect | ||||||
| Mannequin task | Number of correct responses Percent correct responses Reaction time |
23, 24 & 25 h | No significant effect | ||||||
| Chait and Perry 30 (1994) | DB; PC (WSD) | 14 (10 M); 25 years, range: 21–34 years |
Monthly–daily | Smoked cannabis cigarettes (3.6% THC) |
“Eight Puffs” (dose unknown) |
Time production task | Time interval (30 sec) Time interval (60 sec) Time interval (120 sec) |
11 & 18 h | No significant effect |
| Standing steadiness task | Standing time | 11 & 18 h | No significant effect | ||||||
| DSST | Number of trials attempted Number of correct trials Percent correct |
11 & 18 h | No significant effect | ||||||
| Backward digit span task | Digit span | 11 & 18 h | No significant effect | ||||||
| Logical reasoning task | NS | 11 & 18 h | No significant effect | ||||||
| Visual divided attention task | Hit rate Reaction time Secondary target errors Number of false alarms |
11 & 18 h | No significant effect | ||||||
| Free recall task | Immediate recall | 11 & 18 h | No significant effect | ||||||
| Leirer et al 40 (1991) | “Blinded” j ; PC (WSD) | 9 (Sex NS); 31 years, range: 24–40 years | Unclear | Smoked cannabis cigarettes | 20 | Simulated flying | Performance score | 24 & 48 h | THC ↓ performance at 24 h |
| Chait 22 (1990) | DB; PC (WSD) | 12 (9 M); 21 years, range: 18–26 years | Weekly–daily | Smoked cannabis cigarettes (800–900 mg; 2.1% THC) |
“Eight Puffs”
k
(dose unknown) |
Time production task | Time interval | 12, 12 & 12 h k | THC ↓ time interval (all days) l,m |
| Simple reaction time task | NS | 12, 12 & 12 h k | No significant effect | ||||||
| Forward digit span task | Digit span | 12, 12 & 12 h k | No significant effect | ||||||
| Visual divided attention task | Reaction time Number of misses Number of false alarms Reaction time variability |
12, 12 & 12 h k | THC ↑ reaction time (all days) l | ||||||
| Choice reaction time task | NS | 12, 12 & 12 h k | No significant effect | ||||||
| Backward digit span task | Digit span | 12, 12 & 12 h k | THC ↓ digit span on day 1 | ||||||
| DSST | NS | 12, 12 & 12 h k | No significant effect | ||||||
| Buschkel selective Reminding task | NS | 12, 12 & 12 h k | No significant effect | ||||||
| Heishman et al 23 _a (1990) | Randomized; DB; PC (WSD) | 3 M; range 27–29 years | Unclear | Smoked cannabis cigarettes (2.57% THC) |
“1×Cigarette” (dose unknown) | Two letter search task | Number of trials attempted Number of correct trials Percent correct |
23, 25, 27, 29 & 31 h | Results not adequately reported |
| Logical reasoning task | Number of trials attempted Number of correct trials Percent correct |
23, 25, 27, 29 & 31 h | Results not adequately reported | ||||||
| Digit recall task | Number of trials attempted Number of correct trials Percent correct |
23, 25, 27, 29 & 31 h | Results not adequately reported | ||||||
| Serial addition and subtraction task | Number of trials attempted Number of correct trials Percent correct |
23, 25, 27, 29 & 31 h | Results not adequately reported | ||||||
| Circular lights task | Number of correct responses | 23, 25, 27, 29 & 31 h | Results not adequately reported | ||||||
| Heishman et al 23 _b (1990) | Randomized; DB; PC (WSD) | 3 M; range 27–29 years | Unclear | Smoked cannabis cigarettes (2.57% THC) |
“2×Cigarette” (dose unknown) n | Two letter search task | Number of trials attempted Number of correct trials Percent correct |
19, 21, 23, 25 & 27 h | Results not adequately reported |
| Logical reasoning task | Number of trials attempted Number of correct trials Percent correct |
19, 21, 23, 25 & 27 h | Results not adequately reported | ||||||
| Digit recall task | Number of trials attempted Number of correct trials Percent correct |
19, 21, 23, 25 & 27 h | Results not adequately reported | ||||||
| Serial addition and subtraction task | Number of trials attempted Number of correct trials Percent correct |
19, 21, 23, 25 & 27 h | Results not adequately reported | ||||||
| Circular lights task | Number of correct responses | 19, 21, 23, 25 & 27 h | Results not adequately reported | ||||||
| Heishman et al 23 _c (1990) | Randomized; DB; PC (WSD) | 2 M; range 27–29 years | Unclear | Smoked cannabis cigarettes (2.57% THC) |
“4×Cigarette” (dose unknown) o | Two letter search task | Number of trials attempted Number of correct trials Percent correct |
19, 21, 23, 25 & 27 h | Results not adequately reported |
| Logical reasoning task | Number of trials attempted Number of correct trials Percent correct |
19, 21, 23, 25 & 27 h | Results not adequately reported | ||||||
| Digit recall task | Number of trials attempted Number of correct trials Percent correct |
19, 21, 23, 25 & 27 h | Results not adequately reported | ||||||
| Serial addition and subtraction task | Number of trials attempted Number of correct trials Percent correct |
19, 21, 23, 25 & 27 h | Results not adequately reported | ||||||
| Circular lights task | Number of correct responses | 19, 21, 23, 25 & 27 h | Results not adequately reported. | ||||||
| Leirer et al 31 _a (1989) | Randomized; DB; PC (WSD) | 9 (Sex NS); 26 years, range: 18–29 years | Unclear | Smoked cannabis cigarettes | 10 | Simulated flying | Performance score (calm) Performance score (turbulent) |
24 & 48 h | No significant effect |
| Leirer et al 31 _b (1989) | Randomized; DB; PC (WSD) | 9 (Sex NS); 26 years, range: 18–29 years | Unclear | Smoked cannabis cigarettes | 20 | Simulated flying | Performance score (calm) Performance score (turbulent) |
24 & 48 h | No significant effect |
| Leirer et al 31 _c (1989) | Randomized; DB; PC (WSD) | 9 (Sex NS); 38 years, range: 30–48 years | Unclear | Smoked cannabis cigarettes | 10 | Simulated flying | Performance score (calm) Performance score (turbulent) |
24 & 48 h | No significant effect |
| Leirer et al 31 _d (1989) | Randomized; DB; PC (WSD) | 9 (Sex NS); 38 years, range: 30–48 years | Unclear | Smoked cannabis cigarettes | 20 | Simulated flying | Performance score (calm) Performance Score (turbulent) |
24 & 48 h | No significant effect |
| Barnett et al 33 _a (1985) | “Blinded” j ; PC (WSD) | 8 M; range: 22–33 years | Unclear | Smoked cannabis cigarettes (700 mg; 1% THC) |
100 μg·kg−1
(6.8–7.3 mg) |
Visual search task | Reaction time | 10, 12 & 23 h | No effect b |
| Divided attention task | Reaction time Tracking accuracy |
10, 12 & 23 h | No effect b | ||||||
| Critical tracking task | Tracking accuracy | 10, 12 & 23 h | No effect b | ||||||
| Barnett et al 33 _b (1985) | “Blinded” j ; PC (WSD) | 8 M; range: 22–33 years | Unclear | Smoked cannabis cigarettes (700 mg; 1% THC) |
200 μg·kg−1
(14–15 mg) |
Visual search task | Reaction time | 10, 12 & 23 h | No effect b |
| Divided attention task | Reaction time Tracking accuracy |
10, 12 & 23 h | No effect b | ||||||
| Critical tracking task | Tracking accuracy | 10, 12 & 23 h | No effect b | ||||||
| Barnett et al 33 _c (1985) | “Blinded” j ; PC (WSD) | 8 M; range: 22–33 years | Unclear | Smoked cannabis cigarettes (700 mg; 1% THC) |
250 μg·kg−1
(17–18 mg) |
Visual search task | Reaction Time | 10, 12 & 23 h | No effect b |
| Divided attention task | Reaction time Tracking accuracy |
10, 12 & 23 h | No effect b | ||||||
| Critical tracking task | Tracking accuracy | 10, 12 & 23 h | No effect b | ||||||
| Chait et al 24 _a (1985) | “Blinded” j ; PC (WSD) | 13 M; 25 years, range: 21–35 years |
Infrequent–daily | Smoked cannabis cigarettes (1 g; 2.9% THC) |
“Ten Puffs” (dose unknown) |
Card sorting task | Time to complete (simple) Time to complete (suit) |
9.5 h | No significant effect |
| Free recall task | Immediate recall | 9.5 h | No significant effect | ||||||
| DSST | Number of correct trials | 9.5 h | No significant effect | ||||||
| Time production task | Time interval (10 sec) Time interval (30 sec) |
9.5 h | THC ↑ time interval (10 & 30 sec) at 9.5 h compared to target | ||||||
| Chait et al 24 _b (1985) | “Blinded” j ; PC (WSD) | 6 M; age NS | Unclear | Smoked cannabis cigarettes (1 g; 2.9% THC) |
“Five Puffs” (dose unknown) |
Card sorting task | Time to complete (simple) Time to complete (suit) |
9.5 h | Results not reported |
| Free recall task | Immediate recall | 9.5 h | Results not reported | ||||||
| DSST | Number of correct trials | 9.5 h | Results not reported | ||||||
| Time production task | Time interval (10 sec) Time interval (30 sec) |
9.5 h | Results not reported | ||||||
| Yesavage et al 41 (1985) | Pre-/post-trial | 10 (Sex NS); 29 years | Unclear | Smoked cannabis cigarettes | 19 | Simulated flying | Distance off-center on landing Lateral deviation Vertical deviation Aileron (number of changes) Aileron (mean size) Elevations (number of changes) Elevations (mean size) Number of throttle changes |
24 h | THC ↑ distance off-center on landing, lateral deviation, aileron (number of changes), aileron (mean size) and elevations (mean size) at 24 h compared to baseline |
| Rafaelsen et al 39 _a (1973) | Randomized; DB; PC (WSD) | 8 M; range: 21–29 years | Unclear | Oral cannabis (baked into cake) |
8 | Simulated driving | Brake time Start time Number of gear changes Mean speed |
∼15 h | No significant effect p |
| Rafaelsen et al 39 _b (1973) | Randomized; DB; PC (WSD) | 8 M; range: 21–29 years | Unclear | Oral cannabis (baked into cake) |
12 | Simulated driving | Brake time Start time Number of gear changes Mean speed |
∼15 h | No significant effect p |
| Rafaelsen et al 39 _c (1973) | Randomized; DB; PC (WSD) | 8 M; range: 21–29 years | Unclear | Oral cannabis (baked into cake) |
12 | Simulated driving | Brake time Start time Number of gear changes Mean speed |
∼15 h | No significant effect p |
| Rafaelsen et al 39 _d (1973) | Randomized; DB; PC (WSD) | 8 M; range: 21–29 years | Unclear | Oral cannabis (baked into cake) |
16 | Simulated driving | Brake time Start time Number of gear changes Mean speed |
∼15 h | No significant effect p |
| Rafaelsen et al 36 _a (1973) | Randomized; DB; PC (WSD) | 8 M; range: 21–29 years | Unclear | Oral cannabis (baked into cake) |
8 | Digit span task (direction NS) | Digit span | ∼15 h | No significant effect p |
| Addition test | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Subtract serial sevens task | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Finger labyrinths task | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Bourdon's cancellation test | Number of letters scanned Number of errors |
∼15 h | No significant effect p | ||||||
| Rafaelsen et al 36 _b (1973) | Randomized; DB; PC (WSD) | 8 M; range: 21–29 years | Unclear | Oral cannabis (baked into cake) |
12 | Digit span task (direction NS) | Digit span | ∼15 h | No significant effect p |
| Addition test | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Subtract serial sevens task | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Finger labyrinths task | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Bourdon's cancellation test | Number of letters scanned Number of errors |
∼15 h | No significant effect p | ||||||
| Rafaelsen et al 36 _c (1973) | Randomized; DB; PC (WSD) | 8 M; range: 21–29 years | Unclear | Oral cannabis (baked into cake) |
12 | Digit span task (direction NS) | Digit span | ∼15 h | No significant effect p |
| Addition test | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Subtract serial sevens task | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Finger labyrinths task | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Bourdon's cancellation test | Number of letters scanned Number of errors |
∼15 h | No significant effect p | ||||||
| Rafaelsen et al 36 _d (1973) | Randomized; DB; PC (WSD) | 8 M; range: 21–29 years | Unclear | Oral cannabis (baked into cake) |
16 | Digit span task (direction NS) | Digit span | ∼15 h | No significant effect p |
| Addition test | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Subtract serial sevens task | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Finger labyrinths task | Time to complete Number of errors |
∼15 h | No significant effect p | ||||||
| Bourdon's cancellation test | Number of letters scanned Number of errors |
∼15 h | No significant effect p | ||||||
| Kielholz et al 32 _a (1973) | DB; PC (BSD) | 54 q (Sex NS); 34 years | Unclear | THC capsules | 350 μg·kg−1
(∼24.5 mg) r |
Tapping task | Taps (comfortable) (right) Taps (comfortable) (left) Taps (fast) (right) Taps (fast) (left) |
17.5 h | Results not adequately reported |
| Spiral rotor task | NS | 17.5 h | Results not adequately reported | ||||||
| The compensation apparatus | NS | 17.5 h | Results not adequately reported | ||||||
| The tracking apparatus | Reaction time Frequency of pedal pressure |
17.5 h | Results not adequately reported | ||||||
| Kielholz et al 32 _b (1973) | DB; PC (BSD) | 54 q (Sex NS); 34 years | Unclear | THC capsules | 400 μg·kg−1
(∼28 mg) r |
Tapping task | Taps (comfortable) (R) Taps (comfortable) (L) Taps (fast) (R) Taps (fast) (L) |
17.5 h | Results not adequately reported |
| Spiral rotor task | NS | 17.5 h | Results not adequately reported | ||||||
| The compensation apparatus | NS | 17.5 h | Results not adequately reported | ||||||
| The tracking apparatus | Reaction time Frequency of pedal pressure |
17.5 h | Results not adequately reported | ||||||
| Kielholz et al 32 _c (1973) | DB; PC (BSD) | 54 q (Sex NS); 34 years | Unclear | THC capsules | 450 μg·kg−1
(∼31.5 mg) r |
Tapping task | Taps (comfortable) (right) Taps (comfortable) (left) Taps (fast) (right) Taps (fast) (left) |
17.5 h | Results not adequately reported |
| Spiral rotor task | NS | 17.5 h | Results not adequately reported | ||||||
| The compensation apparatus | NS | 17.5 h | Results not adequately reported | ||||||
| The tracking apparatus | Reaction time Frequency of pedal pressure |
17.5 h | Results not adequately reported |
All “Effects of THC” are in comparison to placebo unless otherwise stated; comparisons to baseline are only reported when those to placebo were not conducted or not reported. Significant effects are in bold text.
Cigarettes were smoked ad libitum.
The authors modeled the “behavioral pharmacokinetics” of THC rather than investigating its effect at specific times post-treatment; however, their modeling still suggests impairment resolves within 8 h.
Although “double blinded,” participants had to demonstrate a capacity to distinguish between THC and placebo (in a “Quantification Phase”) to be eligible for inclusion.
Only 35 of these participants were included in the analyses investigating THC's effects on cognitive function.
Only the “minimum” and “maximum” performance scores were presented and subjected to statistical analysis.
Compared to “20 minutes post-placebo” (as performance was not assessed 24 h post-placebo).
It is unclear whether six or eight participants completed the cognitive function tests.
It is unclear how the time parameter was handled in these statistical analyses (see also ‘ “Next Day” effects of THC’ section).
The authors indicate that THC decreased pursuit speeds at 1.75 h, but do not clearly describe its effects at the other time points.
The authors do not state whether a single- or double-blind design was used.
Participants completed a total of five smoking periods involving “eight puffs” each: (1) 9 PM Friday; (2) 3 PM Saturday; (3) 9 PM Saturday; (4) 3 PM Sunday; and (5) 9 PM Sunday; cognitive function was assessed 12 h after each evening (9 PM) smoking period.
Main effect of treatment across all 3 days.
This effect is described as “negative” in this article (since any change in time production could indicate “impairment”); however, it is worth noting that participants were closer to the target time on THC than placebo.
The first cigarette was administered 4 h before the second.
The first two cigarettes were administered 4 h before the second two.
We presume these comparisons are against placebo.
Total number across all four treatment groups.
Value estimated at a body weight of 70 kg.
BSD, between-subject design; C, control group; CBD, cannabidiol; CBN, cannabinol; CPT, continuous performance test; DB, double blind; DH, dominant hand; DSST, digit symbol substitution test; DT, double target; HVLT-R, Hopkins Verbal Learning Test Revised; I, intervention group; L, left; M, male participants; NS, not specified; PC, placebo controlled; PVT, psychomotor vigilance task; R, right; R, correlation coefficient; RA, response accuracy; RT, reaction time; RVIPT, rapid visual information processing task; SB, single blind; SDLP, standard deviation of lane position; ST, single target; WSD, within subject design.
No “Next Day” effects
A total of 180 neuropsychological tests and 29 safety-sensitive tasks showed no “next day” effect of THC (N=18 divided attention25,27,30,33; N=12 executive function30,34,35; N=32 information processing22,24,27,28,30,33,34,36; N=6 tracking performance33; N=23 reaction time22,27,34,35; N=6 motor function28,30; N=19 sustained attention27,28,34,37; N=22 working memory22,30,34–36; N=2 perception30; N=26 learning and(or) memory22,24,27,28,30,35; N=6 spatial reasoning35; N=8 unknown36; N=20 simulated driving29,37–39; and N=9 simulated flying31,40). Seventy, 82, and 28 of these 180 neuropsychological tests and 4, 17, and 8 of these 29 safety-sensitive tasks were conducted between >8–12, >12–24, and >24–48 h post-treatment, respectively.
No “next day” effect was observed across a total of 16 published studies.22,24,25,27–31,33–40 Most of these 16 studies (N=9) used randomized double-blind, placebo-controlled designs25,28,29,31,34–37,39 (N=4 nonrandomized double blind22,27,30,38 and N=3 nonrandomized single blind24,33,40), involved other cannabis users (N=12),24,25,27,30,31,33–36,38–40 and administered THC by smoking (N=11).22,24,28–31,33,35,37,38,40 The median [IQR] THC dose was 15 [10–20] mg (where reported; N=1225,27–29,31,33,34,36–40).
With respect to RoB, half of these 16 studies were rated as having “some concerns” (N=8),22,24,25,28–30,37,40 and the other half had a “high risk” of bias (N=8).27,31,33–36,38,39 Of those with “some concerns,” two received “low risk” ratings on four of the five RoB domains assessed28,29 and three employed “robust” standardization procedures.28–30
Negative “Next Day” effects
A total of 10 neuropsychological tests conducted between >8 and 12 h post-treatment and two safety-sensitive tasks conducted 24 h post-treatment indicated negative (i.e., impairing) “next day” effects of THC (N=2 learning and[or] memory27; N=4 perception22,24; N=1 working memory22; N=3 divided attention22; and N=2 simulated flying40,41).
These negative “next day” effects were observed across a total of five published studies.22,24,27,40,41 None of these studies used randomized double-blind, placebo-controlled designs (N=2 nonrandomized double-blind22,27; N=2 nonrandomized single-blind24,40; and N=1 pre-/post-treatment design41). Most involved other cannabis users (N=4)24,27,40,41 and administered THC by smoking (N=4).22,24,40,41 THC doses were 5, 15, 19, and 20 mg (where reported; N=327,40,41).
With respect to RoB, three of these five studies were rated as having “some concerns,”22,24,40 and two had a “high risk” of bias.27,41 Of those with “some concerns,” none employed “robust” standardization procedures.
Positive “Next Day” effects
Two neuropsychological tests and one safety-sensitive task, all administered 48 h post-treatment, indicated positive (i.e., enhancing) “next day” effects of THC (N=2 information processing28 and N=1 simulated driving29).
These positive “next day” effects were observed across two published studies28,29 conducted in the same investigation: a randomized double-blind, placebo-controlled trial. Participants were regular cannabis users and smoked either 70.3±21.3 or 94.0±16.4 mg THC ad libitum.
With respect to RoB, both studies were rated as having “some concerns”—but received “low risk” ratings on four of the five domains assessed.28,29 Both also employed “robust” standardization procedures.
Unclear “Next Day” effects
A total of 121 performance tests indicated “unclear” or ambiguous “next day” effects of THC (i.e., insufficient information was provided to accurately determine the result) (Table 2). These unclear “next day” effects were observed across a total of seven published studies,23–26,32,34,35 three of which reported all of their relevant results (N=99 performance tests) in a manner that was of limited use to the current review. First, Ménétrey et al26 reported using a Kruskal-Wallis test to compare cognitive function data across four different treatments. This is problematic as these data were collected at seven different time points (plus baseline) and the authors do not explain how the time parameter was handled in their analyses.
Second, Heishman et al23 were unable to perform statistical analyses as only three participants completed their trial (and only two completed treatment arm “c”). Third, the results of Kielholz et al32 were poorly described and could not be reliably interpreted. These studies and tests were retained for completeness, but will not be discussed further.
“Acute Effects” of THC
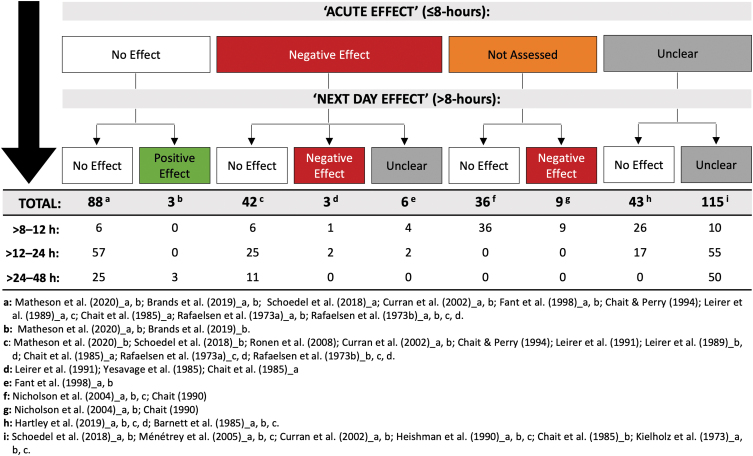
It is important to consider whether the 345 performance tests administered >8 h post-treatment also demonstrated “acute” (i.e., <8 h post-treatment) effects of THC. Indeed, a lack of impairment at, say, 24 h is a more definitive illustration of no “next day” effects on a performance test if impairment had been evident on that same test at shorter durations following THC (i.e., <8 h post-treatment). The relevant results are detailed in Supplementary File S1 and summarized in Figure 3. We note the following: only 20% (N=42) of the tests that showed no “next day” effects of THC also demonstrated “acute” effects (i.e., initial impairment). Most did not (42%; N=88). The remainder either did not assess (17%; N=36) or adequately describe (21%; N=43) the acute effects of THC.
FIG. 3.
A total of 345 performance tests were administered >8 h post-treatment. This figure shows the number demonstrating “no effect,” a “positive effect,” a “negative effect,” and an “unclear effect” of THC (i.e., on any one of its outcome measures) >8–12, >12–24, and >24–48 h post-treatment. Counts are further subcategorized based on whether the performance test also demonstrated “no effect,” a “negative effect,” or an “unclear effect” “acutely” (i.e., anytime ≤8 h post-treatment) (or if acute effects were not assessed). Unused subgroupings were omitted from this figure. Note: reductions in driving speed ≤8 h post-treatment were not considered either “positive” or “negative” and were therefore omitted from this analysis. See Table 2 and Supplementary Table S3 for full results. THC, Δ9-tetrahydrocannabinol.
Discussion
This systematic review found little by way of high-quality scientific evidence to support the assertion that cannabis use impairs “next day” performance. Indeed, of the 345 performance tests reviewed, only 12 indicated negative (i.e., impairing) “next day” effects of THC. Notably, the five studies that observed these effects were all published >18 years ago (four, >30 years ago) and found to have significant methodological limitations.
Only two investigations: the flight simulator studies of Leirer et al40 and Yesavage et al41 provided any evidence of THC-induced impairment persisting beyond 12 h. Both studies administered ∼20 mg THC to a poorly characterized participant population (i.e., their cannabis use behavior and sex were not reported) by smoking (cannabis) and reported impairment 24 h post-treatment. However, they also employed suboptimal designs (i.e., “pre-/post-treatment” and nonrandomized, single blind, placebo controlled) and inadequate standardization procedures (Fig. 2), one indicating a “high risk” of bias (due to missing outcome data and the randomization process employed).41 It can further be assumed that flight simulator technology was very rudimentary at this stage in history (i.e., ∼1990) and noted that these “next day” effects were not replicated in a third flight simulator study (employing a superior randomized, double-blind, placebo-controlled design) conducted by the same group of authors.31
Three additional investigations Nicholson et al,27 Chait et al,24 and Chait22 reported impaired cognitive performance between >8 and 12 h after THC use. Again, however, each of these studies employed suboptimal designs (Table 2) and had either a “high risk” of bias (due to missing outcome data)27 or inadequate standardization procedures22,24 (Fig. 2); two also involved an unknown dose of THC.22,24 Of further note is the fact that many of the effects observed across these three studies (N=4 out of 10)—and the only effect observed in Chait et al24—were on “time production” tests (i.e., during which participants estimate when a given amount of time has elapsed, e.g., 120 sec). These tests may be of limited relevance to driving and workplace safety. In addition, time estimations were often closer to the target on THC than placebo (i.e., arguably enhanced).22
The remaining “negative” effects could be due, in part, to certain methodological factors. For example, the oromucosal THC (5 and 15 mg) preparation used in Nicholson et al27 would be expected to elicit longer lasting impairment than inhaled THC.3,42 Chait22 also utilized an unusually demanding treatment protocol in which participants completed five separate “smoking sessions” over a 48-h period. Overall, however, these “next day” effects did not appear to be associated with a specific methodological factor (e.g., dose, route of administration or whether regular or occasional users were assessed) and should be interpreted with caution.
The “next day” effects of alcohol use have also received some scientific attention. Indeed, a recent meta-analysis showed that “alcohol hangover” had a small to moderate detrimental effect on cognitive performance (e.g., sustained attention, psychomotor speed, short-/long-term memory).43 The “next day” effects of THC use could not be quantified in this review as studies often failed to report the information required to calculate an effect estimate. However, the small number of significant effects observed would suggest that a THC “hangover” is unlikely to be more impairing than an alcohol hangover, which is generally tolerated among drivers and individuals employed in safety-sensitive positions.
A total of 209 performance tests conducted across 16 published studies showed no “next day” effects of THC.22,24,25,27–31,33–40 Most of these 16 studies used randomized double-blind, placebo-controlled designs (N=9),25,28,29,31,34–37,39 but still had methodological limitations. Indeed, half had a “high risk” of bias (often due to missing outcome data)27,31,33–36,38,39 and most used inadequate standardization procedures22,24,25,27,31,33–40 (Fig. 2). In addition, only three justified their chosen sample sizes (Fig. 1) (and none used noninferiority analysis to test the specific hypothesis that THC does not impair “next day” performance44).
One additional concern is that 42% of the tests showing no “next day” effects of THC also failed to demonstrate “acute” (i.e., <8 h post-treatment) THC-induced impairment (Fig. 3). This is important as “next day” effects seem unlikely to occur in the absence of initial impairment, which could reflect the use of lower THC doses and/or tests or cognitive domains that are relatively insensitive to the effects of THC. The collective results of these 16 studies should therefore be interpreted with some degree of caution.
Nevertheless, two recent studies, both finding no “next day” effects of THC, were identified as having employed good-quality research methods: Matheson et al28 and Brands et al.29 These studies were conducted within the same investigation: a randomized double-blind, placebo-controlled trial in which participants (weekly–daily cannabis users) smoked either 70.3±21.3 or 94.0±16.4 mg THC (cannabis) ad libitum. Both studies had “some” RoB—but received “low risk” ratings on four of the five domains assessed (Fig. 1).
They also justified their chosen sample size (n=91) (Fig. 1) and employed relatively robust standardization procedures (Fig. 2). Motor function, learning and(or) memory, information processing, sustained attention, and simulated driving performance were not impaired 24 or 48 h post-treatment in these investigations. Some positive (i.e., enhancing) effects were unexpectedly observed 48 h post-treatment. In addition, only learning and(or) memory demonstrated “acute” (i.e., <8 h post-treatment) impairment. However, these findings provide some confirmation that high doses of inhaled THC are unlikely to impair “next day” performance in regular cannabis users.
Further high-quality studies investigating the “next day” effects of THC in both occasional and medicinal cannabis uses are, of course, required, as are studies involving the administration of oral THC. Until the results of such studies become available, there remains some justification for a cautious regulatory approach. However, policy makers should bear in mind that the implementation of very conservative workplace regulations can have serious consequences (e.g., termination of employment with a positive drug test) and impact the quality of life of individuals who are required to abstain from medicinal cannabis use to treat conditions such as insomnia or chronic pain for fear of a positive workplace or roadside drug test.
The following factors might also be considered in future studies of this nature. First, while most of the studies conducted to date have administered a single dose of THC, many individuals (in particular, regular cannabis users) do not consume THC in this manner under real-world conditions. High-quality studies involving daily users of medical and nonmedical cannabis would therefore be valuable. Second, performance on safety-sensitive tasks (e.g., driving, flying) and neuropsychological tests may be susceptible to “practice” (learning) and “fatigue” (loss of motivation) effects over time, and these might be better controlled in future studies. Indeed, in addition to masking “acute” effects of THC, practice effects might be attenuated under the influence of THC such that “next day” effects appear to be present.
Conclusion
A small number of lower-quality studies have observed negative (i.e., impairing) ‘next day’ effects of THC on cognitive function and safety-sensitive tasks. However, higher-quality studies, and a large majority of performance tests, have not. Overall, it appears that there is limited scientific evidence to support the assertion that cannabis use impairs ‘next day’ performance. However, further research, in particular, studies involving both occasional and medicinal cannabis users and oral THC administration, is strongly recommended.
Supplementary Material
Abbreviations Used
- IQR
interquartile range
- RoB
risk of bias
- THC
Δ9-tetrahydrocannabinol
Authors' Contributions
All authors (D.M., A.S., and I.S.M.) contributed to the conception and design of the research project; D.M. and A.S. completed data acquisition; and all authors contributed to the interpretation of the research data, were involved in drafting and critically revising the article, and approved the final submitted version.
Author Disclosure Statement
ISM is a consultant to Kinoxis Therapeutics and Psylo Ltd and has received a speakers honorarium from Janssen and consultancy fees from the Medical Cannabis Industry Association. He holds a number of patents for cannabinoid and non-cannabinoid therapeutics. ISM also acts as an expert witness in legal cases where issues of cannabis-induced impairment may be relevant. AS has received consulting fees from the Medical Cannabis Industry Association and GlaxoSmithKline Consumer Healthcare Australia Pty Ltd trading as Haleon. DM has received consulting fees from the Medical Cannabis Industry Association.
Funding Information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. D.M., A.S.S., and I.S.M. receive salary support from the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded center for medicinal cannabis research at the University of Sydney.
Supplementary Material
Cite this article as: McCartney D, Suraev A, McGregor IS (2023) The “Next Day” effects of cannabis use: a systematic review, Cannabis and Cannabinoid Research 8:1, 92–114, DOI: 10.1089/can.2022.0185.
References
- 1. United Nations: Office on Drugs and Crime. World Drug Report 2021. Vienna, Austria, 2021. [Google Scholar]
- 2. Lintzeris N, Mills L, Suraev A, et al. Medical cannabis use in the Australian community following introduction of legal access: The 2018–2019 Online Cross-Sectional Cannabis as Medicine Survey (CAMS-18). Harm Reduct J 2020;17(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCartney D, Arkell T, Irwin C, et al. Determining the magnitude and duration of acute Δ9-tetrahydrocannabinol (Δ9-THC)-induced driving and cognitive impairment: A systematic and meta-analytic review. Neurosci Biobehav Rev 2021;126:175–193. [DOI] [PubMed] [Google Scholar]
- 4. Rogeberg O, Elvik R. Response to Li, et al. (2017): Cannabis use and crash risk in drivers. Addiction 2017;112(7):1316. [DOI] [PubMed] [Google Scholar]
- 5. Rogeberg O. A meta-analysis of the crash risk of cannabis-positive drivers in culpability studies—avoiding interpretational bias. Accid Anal Prev 2019;123:69–78. [DOI] [PubMed] [Google Scholar]
- 6. Rogeberg O, Elvik R. The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction 2016;111(8):1348–1359. [DOI] [PubMed] [Google Scholar]
- 7. Arkell T, McCartney D, McGregor I. Medical cannabis and driving. Aust J Gen Pract 2021;50(6):357–362. [DOI] [PubMed] [Google Scholar]
- 8. Spindle T, Cone E, Schlienz N, et al. Acute pharmacokinetic profile of smoked and vaporized cannabis in human blood and oral fluid. J Anal Toxicol 2019;43(4):233–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vandrey R, Herrmann E, Mitchell J, et al. Pharmacokinetic profile of oral cannabis in humans: Blood and oral fluid disposition and relation to pharmacodynamic outcomes. J Anal Toxicol 2017;41(2):83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colizzi M, Bhattacharyya S. Cannabis use and the development of tolerance: A systematic review of human evidence. Neurosci Biobehav Rev 2018;93:1–25. [DOI] [PubMed] [Google Scholar]
- 11. Occupational and Environmental Medical Association of Canada. Position Statement on the Implications of Cannabis Use for Safety-Sensitive Work. 2018. Available from: https://oemac.org/wp-content/uploads/2018/09/Position-Statement-on-the-Implications-of-cannabis-use.pdf [last accessed: November 14, 2022].
- 12. Beckson M, Hagtvedt R, Els C. Cannabis use before safety-sensitive work: What delay is prudent? Neurosci Biobehav Rev 2022;133:104488. [DOI] [PubMed] [Google Scholar]
- 13. Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochran; 2021. [Google Scholar]
- 14. Pope Jr H, Gruber A, Yurgelun-Todd D. The residual neuropsychological effects of cannabis: The current status of research. Drug Alcohol Depend 1995;38(1):25–34. [DOI] [PubMed] [Google Scholar]
- 15. Aggarwal R, Ranganathan P. Study designs: Part 4. Interventional studies. Perspect Clin Res 2019;10(3):137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kronholm E, Härmä M, Hublin C, et al. Self-reported sleep duration in Finnish general population. J Sleep Res 2006;15(3):276–290. [DOI] [PubMed] [Google Scholar]
- 17. Higgins J, Savović J, Page M, et al. Chapter 8: Assessing Risk of Bias in a Randomized Trial. In: Cochrane Handbook for Systematic Reviews of Interventions Version 62 (updated February 2021). (Higgins J, Thomas J, Chandler J, et al. eds.) Cochran; 2021. [Google Scholar]
- 18. Higgins J, Eldridge S, Li T. Chapter 23: Including Variants on Randomized Trials. In: Cochrane Handbook for Systematic Reviews of Interventions Version 62 (updated February 2021). (Higgins J, Thomas J, Chandler J, et al. eds.) Cochran; 2021. [Google Scholar]
- 19. Goel N, Rao H, Durmer J, et al. Neurocognitive consequences of sleep deprivation. Semin Neurol 2009;29(4):320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moskowitz H, Florentino D. A Review of the Literature on the Effects of Low Doses of Alcohol on Driving-Related Skills 2000 (DOT-HS-809-028; NTIS-PB2000105778). Department of Transportation: Washington, DC, USA; 2000. [Google Scholar]
- 21. Irwin C, Khalesi S, Desbrow B, et al. Effects of acute caffeine consumption following sleep loss on cognitive, physical, occupational and driving performance: A systematic review and meta-analysis. Neurosci Biobehav Rev 2020;108:877–888. [DOI] [PubMed] [Google Scholar]
- 22. Chait LD. Subjective and behavioral effects of marijuana the morning after smoking. Psychopharmacology 1990;100(3):328–333. [DOI] [PubMed] [Google Scholar]
- 23. Heishman S, Huestis M, Henningfield J, et al. Acute and residual effects of marijuana: Profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav 1990;37(3):561–565. [DOI] [PubMed] [Google Scholar]
- 24. Chait L, Fischman M, Schuster C. “Hangover” effects the morning after marijuana smoking. Drug Alcohol Depend 1985;15(3):229–238. [DOI] [PubMed] [Google Scholar]
- 25. Schoedel K, Szeto I, Setnik B, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: A randomized, double-blind, controlled trial. Epilepsy Behav 2018;88:162–171. [DOI] [PubMed] [Google Scholar]
- 26. Ménétrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoids levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol 2005;29(5):327–338. [DOI] [PubMed] [Google Scholar]
- 27. Nicholson A, Turner C, Stone B, et al. Effect of Δ-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol 2004;24(3):305–313. [DOI] [PubMed] [Google Scholar]
- 28. Matheson J, Mann R, Sproule B, et al. Acute and residual mood and cognitive performance of young adults following smoked cannabis. Pharmacol Biochem Behav 2020;194:172937. [DOI] [PubMed] [Google Scholar]
- 29. Brands B, Mann R, Wickens C, et al. Acute and residual effects of smoked cannabis: Impact on driving speed and lateral control, heart rate, and self-reported drug effects. Drug Alcohol Depend 2019;205:107641. [DOI] [PubMed] [Google Scholar]
- 30. Chait L, Perry J. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology 1994;115(3):340–349. [DOI] [PubMed] [Google Scholar]
- 31. Leirer VO, Yesavage J, Morrow D. Marijuana, aging, and task difficulty effects on pilot performance. Aviat Space Environ Med 1989;60(12):1145–1152. [PubMed] [Google Scholar]
- 32. Kielholz P, Hobi V, Ladewig D, et al. An experimental investigation about the effect of cannabis on car driving behaviour. Pharmacopsychiatry 1973;6(01):91–103. [DOI] [PubMed] [Google Scholar]
- 33. Barnett G, Licko V, Thompson T. Behavioral pharmacokinetics of marijuana. Psychopharmacology 1985;85(1):51–56. [DOI] [PubMed] [Google Scholar]
- 34. Curran V, Brignell C, Fletcher S, et al. Cognitive and subjective dose-response effects of acute oral Δ 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 2002;164(1):61–70. [DOI] [PubMed] [Google Scholar]
- 35. Fant R, Heishman S, Bunker E, et al. Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav 1998;60(4):777–784. [DOI] [PubMed] [Google Scholar]
- 36. Rafaelsen L, Christrup H, Bech P, et al. Effects of cannabis and alcohol on psychological tests. Nature 1973;242(5393):117–118. [DOI] [PubMed] [Google Scholar]
- 37. Hartley S, Simon N, Larabi A, et al. Effect of smoked cannabis on vigilance and accident risk using simulated driving in occasional and chronic users and the pharmacokinetic-pharmacodynamic relationship. Clin Chem 2019;65(5):684–693. [DOI] [PubMed] [Google Scholar]
- 38. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev 2008;40(3):926–934. [DOI] [PubMed] [Google Scholar]
- 39. Rafaelsen O, Bech P, Christiansen J, et al. Cannabis and alcohol: Effects on simulated car driving. Science 1973;179(4076):920–923. [DOI] [PubMed] [Google Scholar]
- 40. Leirer VO, Yesavage J, Morrow D. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med 1991;62(3):221–227. [PubMed] [Google Scholar]
- 41. Yesavage J, Leirer O, Denari M, et al. Carry-over effects of marijuana intoxication on aircraft pilot performance: A preliminary report. Am J Psychiatry 1985;142(11):1325–1329. [DOI] [PubMed] [Google Scholar]
- 42. Karschner EL, Darwin WD, Goodwin RS, et al. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 2011;57(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gunn C, Mackus M, Griffin C, et al. A systematic review of the next-day effects of heavy alcohol consumption on cognitive performance. Addiction 2018;113(12):2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCartney D, Suraev A, Doohan P, et al. Effects of cannabidiol on simulated driving and cognitive performance: A dose-ranging randomised controlled trial. J Psychopharmacol [Epub ahead of print]; DOI: 10.1177/02698811221095356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.