Abstract
Objective:
We examined growth trajectories of hippocampal volume (HV) in early childhood in a longitudinal cohort of male and female participants with autism spectrum disorder (ASD) and typically developing (TD) individuals, and investigated HV in those with large brains. Relations between factors potentially associated with hippocampal size and growth were investigated.
Method:
Participants received 1 to 3 structural magnetic resonance imaging scans between ages 25 and 80 months (unique participants: ASD, n =200; TD, n =110; total longitudinal scans, n = 593). HV growth during this period was examined using mixed-effects linear models. Associations between early HV and growth rates, and IQ and adaptive functioning, were evaluated.
Results:
After accounting for cerebral hemisphere volume, male participants exhibited larger left and right HV than female participants. Hippocampal growth rates did not differ by sex. In children with larger hemisphere volumes, male and female participants with ASD had relatively larger HV than TD participants of similar hemisphere volume. This effect was present in a broader group than only those with disproportionate megalencephaly (male participants with large cerebral volumes relative to body size). Right hippocampi were larger than left hippocampi in both groups and sexes. Right versus left volume differences were greater for ASD. After adjusting for hemisphere volume, male participants with ASD showed a significant positive association between right hippocampal growth and adaptive behavior.
Conclusion:
HV was relatively greater in ASD in analyses adjusting for hemisphere volume, whereas only subtle differences were observed in HV and growth between participants with ASD and TD participants in unadjusted analyses, suggesting that ASD involves atypical coupling between HV and brain size.
Keywords: hippocampus, longitudinal, IQ, neurogenesis, adaptive functioning
The hippocampus, or, more properly, the hippocampal formation, is one part of a medial temporal lobe memory system that includes a number of cytoarchitectonically distinct regions including the dentate gyrus, hippocampus proper, subiculum, and entorhinal cortex.1 As the magnetic resonance imaging (MRI) data that we report in this article cannot differentiate these regions and, in fact, includes at least the first 3 of these, we will use the more widely used term hippocampus as an umbrella for all of these regions. The hippocampus has been proposed to play roles in a number of cognitive processes including memory, spatial navigation, and the emotional experience of stress.2 In particular, it appears to be critically involved in binding constituent elements of experiences into meaningful contextually laden episodic memories.3 Furthermore, the hippocampus and its associated cortices have been implicated in inferential reasoning, future thinking, decision making, problem solving, emotion processing, creative thinking, and choice-guided behavior, all of which enables us “to act optimally in and on the world,” and to “navigate life in all its beautiful complexity.”4
Environmental enrichment, learning challenging new tasks, nurturing caregivers, and exercise have been associated with increased neuronal density and volume of the hippocampus in animal models.5 A similar phenomenon has been observed in humans, including London taxi drivers, who, after learning the highly complex city street map, demonstrate enlarged posterior hippocampal growth relative to controls.6 Increased hippocampal growth is observed in adults who exercise,7 and children receiving higher levels of early maternal support, which is then predictive of larger hippocampal volume (HV) later in development.8 Associations between hippocampal or hippocampal subregion volumes and cognitive skills also have been observed in children, in whom higher volumes have been associated with more developed overall cognitive abilities, expressive language, and memory skills.9–11
Recent research has begun to highlight factors affecting HV during typical development. For example, the right hippocampus is consistently reported to have a larger volume than the left hippocampus.12–14 Several longitudinal and large shared dataset-based studies also have suggested that the hippocampus grows rapidly in childhood and during puberty, after which the growth rate levels off into middle age, and then eventually decreases in older age.11
Furthermore, studies have shown that typically developing (TD) female participants have smaller overall hippocampal volumes than male participants, although these differences typically disappear after accounting for smaller brain volumes in female participants.9,12,15,16 However, several cross-sectional studies suggest sex differences in the developmental trajectories of the human hippocampus, including faster rates of hippocampal growth16 and earlier estimated peak volumes for female participants.
We know less about the development of the hippocampus in individuals with autism spectrum disorder (ASD). Several influential, albeit small, cross-sectional studies of children,10,17 and adolescents18,19 have found generalized enlargement in ASD relative to TD individuals. However, studies focusing on older and broader age ranges have not always found this to be the case.20–23
The goal of the current study was to examine hippocampal growth across early childhood (aged 25–80 months) in male and female participants with ASD and TD individuals in a large recent longitudinal cohort. Our primary analyses investigated HV size and growth in both groups with and without adjusting for brain hemisphere volume. We hypothesized that, even after adjusting for cerebral hemisphere volume, the hippocampus would be larger in both girls and boys with ASD. Given suggestions that many boys with ASD have large bodies and brains,24 as a secondary analysis we also examined HV in a previously defined phenotype of male participants with large brains relative to their heights at 2 to 3 years of age, which has been called disproportionate megalencephaly (DM).25,26This group has shown unique patterns of neural alterations from children with ASD without DM and TD controls,25–27 as well as clinically meaningful patterns of IQ development over the course of early childhood suggesting a poorer prognosis.28,29 Finally, given previous findings about the association between hippocampal size and growth and cognitive abilities and other skills in TD individuals,9–11 we predicted that IQ/DQ and adaptive functioning would be positively associated with HV in all groups.
METHOD
Participants
Participants included in the current study were children from the Autism Phenome Project (APP) and from the Girls with Autism–Imaging Neurodevelopment (GAIN) study cohorts with 1 to 3 structural MRI scans between 25 and 80 months of age. The APP is a longitudinal study of children with ASD that aims to identify meaningful phenotypic subgroups based on biological and behavioral features. The GAIN study has an identical study design to APP and enriches the predominantly male APP cohort with additional female participants. Participants enrolled in APP/GAIN when they were 2 to 3.5 years of age (time 1 [T1]; meanage = 38.2 months, SD = 6.5) and completed a comprehensive battery of behavioral assessments and an MRI scan. Approximately 1 year later, participants were invited to return for an MRI scan (T2: meanage = 51.2 months, SD = 6.6), and again at approximately 5 years of age for an MRI scan and a behavioral assessment (T3: meanage = 64.0, SD = 6.1). Participants with ASD were recruited from Northern California Regional Centers, local clinics, and community events, whereas TD controls were recruited through community events and other participants. All participants endorsed English as the primary language spoken at home, and did not have primary sensory impairments (eg, visual impairments, hearing difficulties), known neurological conditions, or any contraindications for MRI. TD participants were born at full term, and had no reported developmental concerns or first-degree family members with ASD. Three included girls were taking selective serotonin reuptake inhibitors (SSRIs) (2 at T1 and 1 at T3). Two boys with nonidiopathic ASD (1 with a CHD8 mutation and 1 with Fragile X syndrome) were excluded.
An ASD diagnosis was confirmed at study entry by trained clinical psychologists using the Autism Diagnostic Observation Scale-G (ADOS-G)30 or ADOS-2,31 and the Autism Diagnostic Interview-Revised32 and DSM-IV-TR criteria.33 All ASD participants met criteria outlined by the Collaborative Program of Excellence in Autism (CPEA).34 TD participants were screened for ASD using the Social Communication Questionnaire (SCQ),35 and participants with elevated scores (SCQ ≥11) were further evaluated using the ADOS-G or ADOS-2 to rule out ASD. All participants within the current sample met TD and ASD inclusion criteria at both T1 and T3. Boys with ASD were further characterized by the presence BEGIN (ASD-DM) or absence (ASD-N) of DM at T1, which is defined as a ratio of total cerebral volume to height that is at least 1.5 SD above the mean of TD boys.25–27 Study procedures were approved by the University of California Davis institutional review board, and informed consent was obtained from parents at each visit.
Additional behavioral assessment included direct observation and parent report measures of cognitive ability (T1: Mullen Scales of Early Learning [MSEL])36 T3: Differential Ability Scales-II (DAS-II).37 At T1, cognitive skills were estimated using the MSEL. Ratio developmental quotient was calculated by averaging age equivalents from the Fine Motor, Visual Reception, Expressive Language, and Receptive Language subscales, dividing by the child’s chronological age and multiplying by 100. At T3, cognitive ability was estimated using the General Conceptual Ability composite from the Early Years Form of the DAS-II. Children who were unable to achieve a basal score on the DAS-II completed the MSEL instead, and ratio DQ was calculated using the procedure outlined at T1. At T1 and T3, parents completed the Vineland Adaptive Behavior Scales-II Parent/Caregiver Rating form38 to measure adaptive functioning.
Participant behavioral characteristics at T1 and T3 are summarized in Table 1. Additional demographic information, including family income, parental educational attainment, race, and ethnicity is presented in Supplement 1 and Table S1, available online. More detailed information about these measures, which are commonly used in the assessment of children with ASD and in TD individuals, is reported in Supplement 1, available online.
TABLE 1.
Participant Behavioral Descriptive Information by Diagnosis and Sex
| TD-M (n = 60) |
ASD-M (n = 137) |
TD-F (n = 50) |
ASD-F (n = 63) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| T1 | ||||||||||||
| VDQ | 59 | 105.4 | 12.1 | 137 | 57.3 | 27.0 | 50 | 109.7 | 13.5 | 62 | 59.2 | 29.2 |
| NVDQ | 59 | 102.6 | 14.4 | 137 | 72.9 | 19.3 | 50 | 109.6 | 13.3 | 62 | 72.1 | 19.9 |
| VABS-II ABC | 54 | 110.4 | 13.3 | 126 | 77.3 | 11.2 | 43 | 111.2 | 12.1 | 57 | 70.8 | 10.2 |
| ADOS CSS | 135 | 7.5 | 1.6 | 63 | 7.5 | 1.8 | ||||||
| ADI | ||||||||||||
| Communication total | 137 | 9.6 | 2.7 | 63 | 10.3 | 2.8 | ||||||
| Social total | 136 | 16.8 | 4.1 | 63 | 18.1 | 4.0 | ||||||
| Behavior total | 136 | 5.5 | 2.0 | 63 | 5.4 | 1.9 | ||||||
| T3 | ||||||||||||
| VIQ | 23 | 110.1 | 9.9 | 43 | 77.6 | 35.2 | 17 | 113.6 | 9.9 | 13 | 78.9 | 34.4 |
| NVIQ | 23 | 110.3 | 10.9 | 43 | 87.5 | 29.5 | 17 | 111.1 | 12.3 | 13 | 83.5 | 36.7 |
| VABS ABC | 23 | 111.6 | 10.8 | 39 | 77.9 | 17.1 | 16 | 115.7 | 12.4 | 12 | 70.3 | 10.8 |
| ADOS CSS | 44 | 7.0 | 2.3 | 13 | 6.7 | 2.2 | ||||||
Note: ADI = Autism Diagnostic Interview–Revised; ADOS CSS = Autism Diagnostic Observation Schedule, Calibrated Severity Score; ASD = autism spectrum disorder; ASD-F = ASD female participants; ASD-M = ASD male participants; NVIQ = nonverbal IQ; NVDQ = nonverbal developmental quotient; T1 = time 1; T3 = time 3; TD = typically developing; TD-F = TD female participants; TD-M = TD male participants; VIQ = verbal IQ; VDQ = verbal developmental quotient; VABS-II ABC = Vineland Adaptive Behavior Scales–II Adaptive Behavior Composite.
Neuroimaging
MRI was carried out at the University of California Davis Imaging Research Center using a 3T Siemens TIM Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany) with an 8-channel head coil. MRI scans were conducted during natural nocturnal sleep. Our scanning success rates were high, at 88.1%, 90.6%, and 89.6% at T1, T2, and T3 respectively. T1-weighted 3-dimensional sagittal MPRAGE scans (TR = 2170 milliseconds, TE = 4.86 milliseconds, matrix size = 256 × 256, slice thickness = 1.0 mm, isotropic voxel size = 1 mm) were acquired for each child. T2-weighted images were also collected and reviewed by a pediatric neuroradiologist to rule out clinically significant findings.
A calibration phantom (ADNI MAGPHAN, Phantom Laboratory, Inc, Salem, NY) was scanned at the end of each MRI session using an MPRAGE pulse sequence matched to the study sequence and using the same landmark and shim as the participant to ensure accurate measurement of spatial characteristics of the MRI volume. A 3-dimensional image distortion map was derived and used to correct for hardware-induced geometric distortion (Image Owl, Inc, Salem, NY, as previously described in Nordahl et al.29).
Image Processing
Participants’ distortion corrected structural MRI images were visually inspected slicewise for motion, grainy images, ringing, or Gibbs artifact that blurred the boundaries (contrast) between gray matter and white matter tissues. If any of these artifacts was present on ≥1 2-dimensional image slice, the entire 3-dimensional structural MRI scan did not pass inspection. Eight scans were excluded due to motion artifacts (1 ASD at T2, 3 TD and 5 ASD at T3). Approved quality-checked MRIs were deidentified by removing any identifying participant information from the image header and “defacing” the image by stripping the face (without removing any brain tissue).
The distortion corrected, anonymized, and defaced MRIs were then uploaded to MRICloud (https://mricloud.org).39 The images were segmented using the fully automated MRICloud T1-Segmentation pipeline, which is based on the Diffeomorphic Multi-Atlas Likelihood Fusion (DMALF) algorithm and uses Large Deformation Diffeomorphic Metric Mapping (LDDMM) for image registration.40 For each participant, the multi-atlas was selected based on the participant’s age in years at the time of the scan. For 2- and 3-year-olds, the multi-atlases were optimized using data from our site at each age group and 13 pediatric atlases. These 2 multi-atlas sets developed for our cohort (UC Davis 2 Years and UC Davis 3 Years) are available on MRICloud. For children ≥4 years of age, the Pediatric 4–8 Years multi-atlas (based on 10 pediatric atlases) was selected. The whole-brain segmentation output for each participant was downloaded and visually inspected for segmentation quality. Four scans were excluded for poor whole-brain segmentation (criteria for poor fit include the following: exclusion of any brain tissue from the segmentation, or inclusion of skull or cerebrospinal fluid within a cortical brain region). In addition, visual inspection of hippocampal volumes was conducted, and 10 hippocampal volumes were removed from the dataset for overinclusion of surrounding tissue and/or cerebrospinal fluid or for exclusion of hippocampal tissue. Participants with positive seizure history were excluded from the current analyses (T1, n = 6; T2, n = 4; T3, n = 9) as well 2 male ASD participants with benign incidental MRI findings (temporal lobe cyst, posterior fossa cyst). For each child, bilateral hippocampus volumes and cerebral hemisphere volumes were extracted and used for statistical analyses. To enhance the ability to compare current results with those in the literature, we report all findings before and after including hemisphere volumes in the models to adjust for overall brain size. The final sample consisted of 593 scans from 310 unique children (ASD, n = 200; TD, n = 110).
Analytic Approach
Our analytic framework was based on mixed effects linear models.41 This highly flexible approach takes into account the correlated structure of the data due to repeated assessments of an individual over time or conditions (eg, left and right hippocampal and hemisphere volumes); it can accommodate missing and unequally spaced observations, and can account for the effects of covariates such as sex, side, or hemisphere volume. It produces valid inferences under the assumption that data are missing at random. We first examined growth models of HV in male and female participants with ASD and TD individuals. Left and right HV growth was modeled separately because asymmetry in hippocampal volumes is well documented, as described above. Each model included fixed effects for sex, diagnosis, linear and quadratic effect of age (in months, centered at 38 months), the interaction between sex and age, the interaction between diagnosis and age, and random effects for child and time. The random effect for child addressed the problem of participants having different baseline HV; the second random effect allowed for the estimation of variation in the speed of HV growth. To facilitate interpretation, age was centered at 38 months, the average age at T1. Thus, intercept values represent estimated hippocampus volumes at 38 months of age for male TD participants (reference group).
A second set of models was fit to examine trajectories of HV after adjustment for hemisphere volumes, by adding to the models described above terms for hemisphere volume and the interaction between hemisphere volume and diagnosis. Children-specific predicted HV at 38 months (intercepts) and HV linear growth rates (slopes) were estimated from the mixed effects models that included cerebral hemisphere volume. To adjusted and unadjusted models, we added terms for DM status and the interactions of DM status and age and hemisphere, to investigate whether boys with the ASD-DM neurophenotype exhibited different patterns of hippocampal growth from ASD boys without DM neurophenotype.
Next, we examined differences in hippocampal asymmetry across early childhood in ASD relative to same-sex controls. For this analysis, we used data from both left and right hippocampi and fitted a mixed -effects linear model with fixed effects for side, sex, diagnosis, linear and quadratic effect of age, hemisphere (left/right), and the interactions of age with sex and diagnosis, and of diagnosis with hemisphere.
Finally, Pearson correlations were conducted to examine associations between individual participants’ predicted HV at 38 months (intercept), HV linear growth rates (slope) from adjusted models, and T1 developmental and adaptive functioning for each sex and diagnostic group (ASD male, TD male, ASD female, TD female). For this family of 32 tests, the Benjamini–Hochberg procedure with a false discovery rate of 0.05 was applied using SAS Proc Multtest to control for multiple testing. All analyses were conducted in SAS (Version 9.4; SAS Institute, Cary, NC). All tests were 2-sided, and p-values <.05 were considered statistically significant.
RESULTS
Mean hippocampal and cerebral hemisphere volumes for female and male ASD and TD children across measurement times are presented in Table 2. A comparison of the results of the current study with raw hippocampal means from 22 other studies of male participants with ASD, TD individuals, and individuals with other neurodevelopmental disorders from birth to 18 years of age suggests that our findings are consistent with the extant literature (see Supplement 1 and Figure S1, available online).
TABLE 2.
Summary Statistics for Age, Hippocampal, and Hemisphere Volumes at T1, T2, and T3 (mm3) by Diagnosis and Sex
| TD-M (n = 60) |
ASD-M (n = 137) |
TD-F (n = 50) |
ASD-F (n = 63) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| T1 | ||||||||||||
| Age (mo) | 60 | 37.0 | 6.4 | 137 | 37.7 | 6.2 | 50 | 38.8 | 6.8 | 63 | 39.6 | 6.5 |
| L HV | 58 | 3,359.6 | 289.9 | 135 | 3,435.4 | 329.0 | 47 | 3,202.7 | 275.8 | 63 | 3221.8 | 351.2 |
| R HV | 58 | 3,541.1 | 286.6 | 135 | 3,653.4 | 352.8 | 47 | 3,390.3 | 288.5 | 63 | 3,438.1 | 348.2 |
| L hemisphere | 56 | 509,233.4 | 42,155.2 | 137 | 530,477.1 | 42,132.0 | 50 | 480,205.7 | 34,980.2 | 63 | 489,476.8 | 46,033.6 |
| R hemisphere | 56 | 516,058.6 | 42,158.3 | 137 | 538,973.9 | 41,830.3 | 50 | 483,933.1 | 35,870.5 | 63 | 493,670.1 | 45,429.9 |
| T2 | ||||||||||||
| Age (mo) | 43 | 50.4 | 6.5 | 81 | 50.1 | 6.3 | 28 | 51.8 | 6.1 | 28 | 54.7 | 7.3 |
| L HV | 43 | 3,584.8 | 289.6 | 81 | 3,647.6 | 366.0 | 28 | 3,387.9 | 278.0 | 28 | 3,466.8 | 329.4 |
| R HV | 43 | 3,773.1 | 300.8 | 81 | 3,863.2 | 364.7 | 28 | 3,576.9 | 268.0 | 28 | 3,663.8 | 318.7 |
| L hemisphere | 43 | 544,535.1 | 45,730.4 | 81 | 564,425.2 | 45,588.4 | 28 | 511,082.6 | 40,316.9 | 28 | 516,097.9 | 42,636.9 |
| R hemisphere | 43 | 550,376.6 | 45,373.3 | 81 | 570,366.1 | 45,577.5 | 28 | 514,474.0 | 41,922.1 | 28 | 520,072.2 | 40,647.1 |
| T3 | ||||||||||||
| Age (mo) | 29 | 61.6 | 5.1 | 50 | 63.8 | 5.8 | 20 | 65.5 | 6.8 | 13 | 67.7 | 6.3 |
| L HV | 29 | 3,786.0 | 272.8 | 50 | 3,849.3 | 361.8 | 20 | 3,593.5 | 344.4 | 13 | 3,605.4 | 397.5 |
| R HV | 29 | 3,905.7 | 243.7 | 50 | 4,046.6 | 405.4 | 20 | 3,725.9 | 303.8 | 13 | 3,758.0 | 433.3 |
| L hemisphere | 29 | 563,830.4 | 40,644.3 | 50 | 593,898.4 | 43,707.8 | 20 | 532,488.3 | 35,573.8 | 13 | 533,039.8 | 45,662.0 |
| R hemisphere | 29 | 566,987.2 | 40,125.9 | 50 | 597,425.9 | 43,129.3 | 20 | 535,659.9 | 36,030.4 | 13 | 535,762.8 | 44,505.9 |
Note: Hippocampus and hemisphere volumes are expressed in cubic millimeters (mm3). ASD = autism spectrum disorder; ASD-F = ASD female participants; ASD-M = ASD male participants; HV = hippocampal volume; L = left; R = right; T1 = time 1; T2 = time 2; T3 = time 3; TD = typically developing; TD-F = TD female participants; TD-M = TD male participants.
Hippocampal Growth Trajectories
Effect of Sex and Diagnosis.
Hippocampal growth trajectories for the study participants with 95% CIs from the unadjusted models are illustrated in Figure 1.
FIGURE 1.
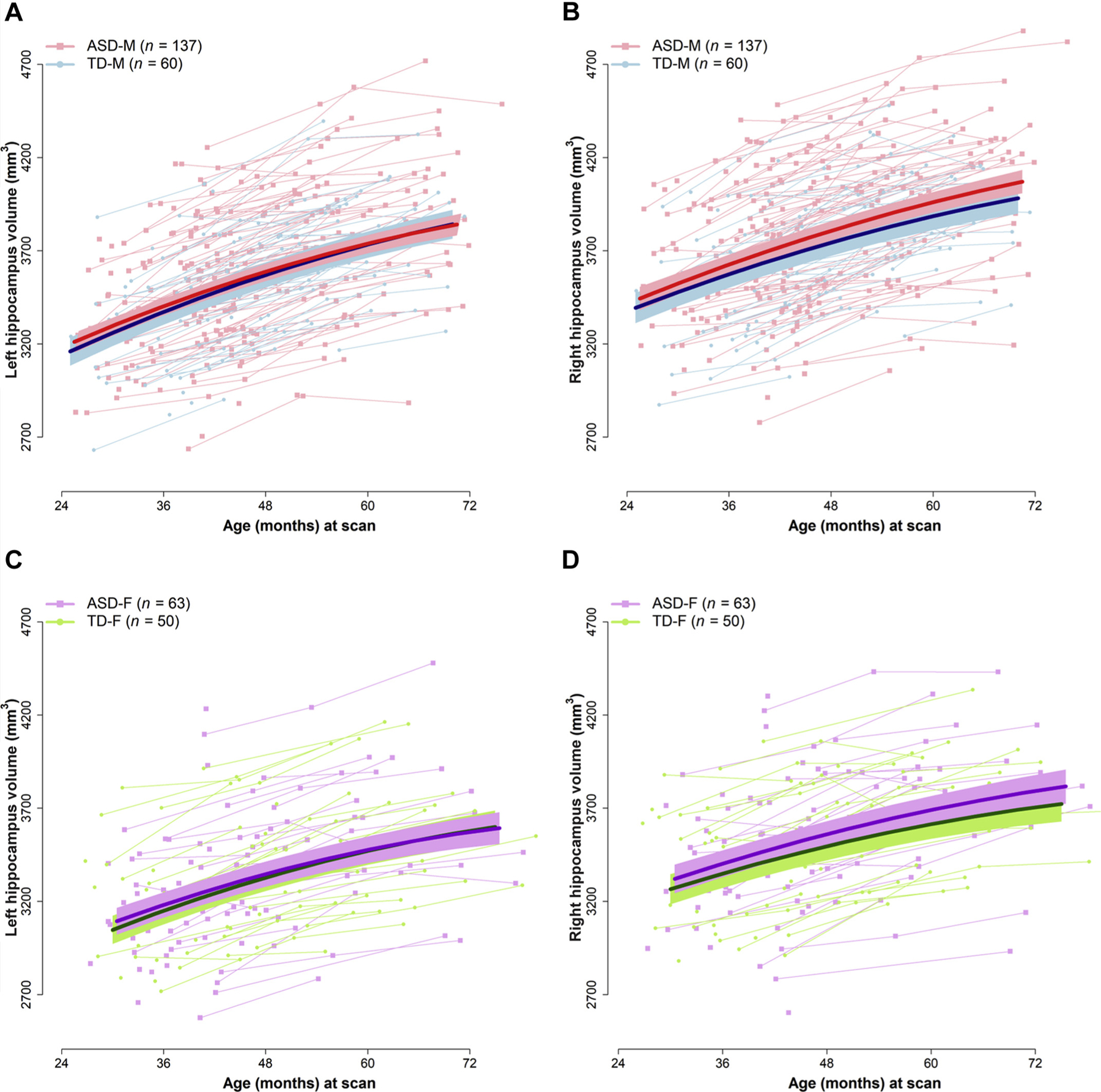
Left and Right Hippocampal Volume Trajectories in Boys (A, B) and Girls (C, D) With Autism Spectrum Disorder (ASD) and Those Typically Developing (TD)
Note: Straight lines represent estimated volumes from the unadjusted models; highlighted areas along each line represent 95% CIs from the unadjusted models. TD-F = typically developing female. Please note color figures are available online.)
Male participants exhibited larger left 38-month HV than female participants (estimate = 226.0 mm3, p < 0.001) and faster linear HV growth (estimate = 1.7 mm3 per month, p = 0.055) (see Supplement 1 and Table S2, available online). After adjusting for left hemisphere volume within the growth model (Table 3), the effect of sex on intercept attenuated but remained significant (estimate = 67.2 mm3, p = .04), indicating that boys had larger left hippocampal volumes even after accounting for cerebral hemisphere volumes (as a proxy for overall brain size). However, the effect of sex on growth attenuated after including hemisphere within the model, indicating similar rates of left HV linear growth within male and female children over the time course of MRI scans acquired in this study.
TABLE 3.
Summary of the Mixed-Effects Models Examining the Effect of Diagnostic Group, Sex, and Corresponding Hemisphere Volume on Left and Right Hippocampal Volume (mm3)
| Left |
Right |
|||||
|---|---|---|---|---|---|---|
| Effect | Estimate | SE | p | Estimate | SE | p |
| Intercept | 3,336.26 | 28.33 | <.001 | 3,512.23 | 31.05 | <.001 |
| Sex (female) | −67.24 | 32.46 | .04 | −57.79 | 35.55 | .11 |
| Age (months) | 8.65 | 1.37 | <.001 | 7.37 | 1.51 | <.001 |
| ASD diagnosis | −24.06 | 30.90 | .44 | −−15.16 | 33.90 | .66 |
| Hemisphere volume (cm3) | 3.06 | 0.46 | <.001 | 2.73 | 0.51 | <.001 |
| Age × sex (female) | −0.75 | 0.82 | .36 | −1.16 | 0.96 | .23 |
| Age × ASD diagnosis | −3.33 | 1.35 | .01 | −1.93 | 1.46 | .19 |
| Age × age | −0.03 | 0.03 | .19 | −0.02 | 0.03 | .61 |
| Hemisphere × ASD diagnosis | 1.19 | 0.54 | .03 | 1.62 | 0.59 | .007 |
Note: Age was centered at 38 months and hemisphere at 498.91 cm3. Thus, the intercept represents predicted volume for 38-month-old typically developing male participants with 498.91 cm3 hemisphere volume. ASD = autism spectrum disorder; SE = standard error.
Similar to the pattern observed in left volumes, higher right HV at 38 months (estimate = 228.9 mm3, p < .001) and faster linear growth rates of right HV were noted in male participants (estimate = 1.9 mm3 per month, p = .053). Including hemisphere volume in the model decreased the effects of sex on intercept and growth.
In unadjusted analyses, diagnostic group did not account for significant variance in left or right HV, intercept, or growth, indicating that participants with ASD had baseline volumes and rates of growth similar to those of same-sex TD participants (see Supplement 1 and Table S2, available online). In the analyses accounting for cerebral hemisphere volumes, a significant interaction between diagnosis and hemisphere volume was detected in both left and right models (estimate = 1.2 cm3 in left, 1.6 in right, p = .03 and .007, respectively) (Table 3). This indicated that in individuals with larger hemisphere volumes, male and female children with ASD had relatively larger HV than same-age and same-sex TD children of similar hemisphere volume, whereas in individuals with smaller hemisphere volumes, male and female children with ASD had relatively smaller HV than same-age and same-sex TD children of similar hemisphere volume. This is illustrated in Supplement 1, Figure S2 and Table S3, available online.
Effect of Diagnosis and DM Status on HV Among Male Participants.
Tables S4 and S5, available online, present descriptive statistics for behavioral and brain measures, including those for male participants with ASD-DM. To examine hippocampal development in the ASD-DM neurophenotype, additional mixed-effects linear models examined the effect of diagnosis and ASD-DM status by adding terms for DM status and its interaction with age and hemisphere to the models examined previously. In these models, the ASD-DM effect can be interpreted as the estimated difference in volume between ASD-DM boys and same-age ASD boys without DM. The interactions of ASD-DM with age and hemisphere can be interpreted as the difference in linear rate of growth and hemisphere effect, respectively, between ASD-DM boys and same-age ASD boys without DM. In unadjusted analyses, the boys with ASD-DM had significantly larger left (estimate = 320.3 mm3, p < .001) and right HV (estimate = 368.2 mm3, p < .001) and faster left HV growth (estimate = 4.3 mm3 per month, p = .006) than ASD boys without DM. However (see Supplement 1 and Table S6, available online), including cerebral hemisphere volumes in the growth models significantly attenuated these effects (p = .78, .80, and .38, respectively). The interactions between hemisphere volumes and DM status were also not statistically significant (p = .92 and .80, respectively).
Right and Left HV Asymmetry.
The results of the asymmetry analyses are detailed in Supplement 1 and Table S7, available online. On average, right HV was larger than left HV, irrespective of diagnostic status and sex. Analyses indicated an overall significant effect of sex, such that male demonstrated larger right and left HV relative to female participants (estimate = 71 mm3, p = .03). A significant side-by-diagnosis interaction was observed (p = .03), with larger differences between right and left HV observed in individuals with ASD (estimate = 186 mm3, p < .001), relative to TD individuals (estimate = 160 mm3, p < .001).
Correlations With Behavior
We combined each child’s random effects with the average predicted slope and intercept to produce child-specific intercept (estimated HV at 38 months), and linear slope (HV growth) estimates. We then examined associations between behavioral data at T1 and these child-specific measures. Behavioral measures from T1 were used, given the larger sample size available at this time point. For these analyses, we used the person-specific intercepts and linear slopes for the participants with at least 2 MRI scans (N = 184). Given the small size (n = 17) of the ASD-DM group, we did not examine it separately. Contrary to our hypothesis, higher T1 DQ was not associated with larger right or left HV in TD-M. In male participants with ASD, DQ was positively associated with faster left and right hippocampal growth (r = 0.21, p = .057 and r = 0.25, p = .02, respectively). However, these associations did not remain significant following correction for multiple comparisons (Table 4). We next investigated associations between adaptive functioning and HV and growth in early childhood. We found a positive association between right HV growth in ASD-M and the Vineland Adaptive Behavior Composite at T1 (r = 0.39, p = .02 after multiple comparisons adjustment) (see Figure S3, available online). For ASD female participants, this association was negative and did not survive correction for multiple comparisons (r = −0.40, p = .04). Significant associations with DQ and adaptive behavior were not observed for the other groups.
TABLE 4.
Pearson Correlations Between Developmental Functioning, Adaptive Behavior, and Child-Specific Hippocampal Intercepts and Linear Slopes Across Groups
| T1 | Vineland | ||
|---|---|---|---|
| Group | DQ | ABC | |
| TD-male (n = 43) | Left intercept | 0.28 | −0.11 |
| Left linear slope | 0.21 | 0.10 | |
| Right intercept | 0.24 | −0.14 | |
| Right linear slope | 0.22 | −0.13 | |
| ASD-male (n = 84) | Left intercept | 0.03 | −0.01 |
| Left linear slope | 0.21 | 0.15 | |
| Right intercept | 0.14 | 0.10 | |
| Right linear slope | 0.25a | 0.39b,c | |
| TD-female (n = 28) | Left intercept | 0.11 | 0.11 |
| Left linear slope | 0.13 | 0.14 | |
| Right intercept | 0.10 | 0.001 | |
| Right linear slope | 0.20 | 0.28 | |
| ASD-male (n = 84) | Left intercept | −0.11 | −0.20 |
| Left linear slope | −0.03 | −0.18 | |
| Right intercept | −0.21 | −0.36 | |
| Right linear slope | −0.06 | −0.40a | |
Note: Child-specific hippocampal intercepts and linear slopes were derived from the models that adjusted for cerebral hemisphere volume presented in Table 3. Participants with 2+ scans were included. ABC = Adaptive Behavior Scales–II Adaptive Behavior Composite; ASD = autism spectrum disorder; ASD-F = ASD female participants; ASD-M = ASD male participants; DQ = developmental quotient; T1 = time 1; TD = typically developing; TD-F = TD female participants; TD-M = TD male participants.
Uncorrected p values <.05.
Uncorrected p values <.01.
Significant following correction for conducting 32 tests using Benjamini-Hochberg procedure with a false discovery rate of 0.05.
DISCUSSION
We examined hippocampal volume and growth trajectories in early childhood (ages 25–80 months) in a large, recently assessed longitudinal cohort of male and female children with ASD and TD children, with an emphasis on understanding HV in those with megalencephaly. When examining findings for the whole ASD group, male and female participants with ASD and TD participants had similar HV and rates of growth. However, supportive of the notion that normative coupling between hippocampal size and brain size is disrupted in ASD, in our main model that considered the influence of the interaction between hemisphere volume and diagnosis on HV, we found that HV in children with ASD was relatively larger in those with large brains and relatively smaller in those with small brains, as compared to same-age and same-sex TD children. A secondary analysis of male ASD-DM participants compared to those with ASD-N showed similar but nonsignificant effects, suggesting that the pattern of findings was present not only in this small group of male participants but more generally in the ASD group. Male participants, both ASD and TD, exhibited larger early hippocampal volumes than female participants and faster HV growth. After adjusting HV to account for cerebral hemisphere volume, male participants still exhibited significantly larger left HV than female participants, although hippocampal growth rates did not differ by sex. Right hippocampi were larger than left hippocampi in all groups examined. ASD participants evidenced more pronounced hippocampal asymmetry, such that the right versus left difference in hippocampal volumes was greater for the ASD group. Finally, male participants with ASD showed a significant positive association between right hippocampal growth rates and adaptive behavior.
There is a consensus in the autism field that ASD has multiple etiologies and subtypes. This has hampered efforts to understand the basic biology of ASD, and to engage in efforts to design targeted treatments. Here we illustrate how a subset of children with ASD, namely, those with large cerebral hemisphere volumes and proportionately larger HV, may have obscured the characterization of early hippocampal size and development in ASD. Contrary to the popular view that individuals with ASD have generally enlarged hippocampi, this is true only for a subset of these individuals.
Somewhat surprisingly, we found that the asymmetry in volume between right and left hippocampi, albeit in the normative direction, was more pronounced in the ASD versus the TD group. This is in contrast to literature that has documented atypical lateralization in samples of children with ASD.42 It bears mentioning that clinical groups do not always exhibit the normative pattern of asymmetry. There may be a reduction in the normative right>left asymmetry over the course of Alzheimer’s disease.43 In addition, a recent study in adults with type 2 diabetes showed that the left hippocampus was larger than the right, and that the right hippocampus was more susceptible to atrophy, with its volume associated with cognitive impairment.44 Although hippocampal asymmetry is not yet well understood, it is known that in nonhuman primates, ventral hippocampal commissural connections are extremely sparse, suggesting a lack of direct communication between the left and right hippocampi of higher mammals.1 It has been proposed that the functions of the right and left hippocampus are consistent with those of the hemispheres, such that there is left hemispheric dominance for language encoding and right-sided involvement in visuospatial processing.45 Indeed, a recent functional MRI study of pattern separation showed greater recruitment of the left hippocampus when semantic information was more relevant, and right hippocampus when visuospatial information was more important.46 An intriguing possibility is that accentuated right-left hippocampal asymmetry in ASD may be associated with the distinct memory profile found in persons with the disorder, which includes a relative weakness in episodic versus semantic memory and better visuospatial versus verbal skills.47
We expected to find faster rates of growth in female participants within this sample, in line with previous studies of TD children and theory that posits faster growth rates in girls due to the presence of estrogen receptors in the hippocampus and increased estrogen in female individuals with the onset of puberty. Within our sample of children aged 25 to 80 months, boys demonstrated faster growth. It is possible that this finding reflects the young age (pre-pubertal status) of our sample. A recent large, cross-sectional study with an age range similar to ours (4–8 years) found that male participants had larger hippocampi than female participants, but there were no age-by-sex interactions over the period for subfields of the hippocampus including the head and body.48 More longitudinal studies of individuals of age ranges comparable to those in the Riggins et al. study and ours may be required to resolve discrepancies in the field.
The current study had several limitations. First, although ours is a relatively large longitudinal sample, as was evidenced by the magnitude of associations between IQ/DQ and HV and growth that did not survive correction for multiple comparisons, we lacked sufficient statistical power to test the association of early HV and growth rate with a broader range of factors that may be associated with higher hippocampal volumes and growth rates (eg, amount of early intervention, antidepressant medication use, parental education, and family income). Similarly, it would be instructive to examine the effect of stress on neuroplasticity in the hippocampus, as the hippocampus is arguably the most stress sensitive region of the brain,49 and as stress can suppress neurogenesis, alter dendritic architecture, and lead to atrophy of the hippocampus, which could have contributed to our findings of relatively smaller hippocampi in individuals with ASD and smaller hemisphere volumes. A second limitation of the current study was that it focused on volumes and growth rates of the hippocampus proper, and did not investigate the roles of related cortices or neural networks, as has been suggested as a more comprehensive approach.2 Similarly, there has been increased interest in examining the volumes and development of hippocampal subregions (ie, head, body, tail) and subfields (CA 1–3, dentate gyrus, subiculum) in children and adolescents and their association with cognitive functioning.48,50 It was not possible to address these factors in the current study.
In conclusion, this large longitudinal study of hippocampal growth in individuals with ASD from 25 to 80 months of age showed that there are many similarities and some differences in the growth of the hippocampus in girls and boys with ASD and TD children. Larger HV after normalization by hemisphere volume relative to same-sex controls was observed male and female participants with ASD and large hemisphere volumes. Early adaptive functioning in male participants with ASD was associated with faster hippocampal growth, highlighting a possible transactional relation between children’s early functioning and later neurodevelopmental outcomes. Furthermore, intriguing findings of enhanced right-to-left hippocampal asymmetry, and the relatively larger than typical hippocampi in participants with larger hemisphere volumes and relatively smaller-than-typical hippocampi in participants with smaller hemisphere volumes, which both are indicative of disrupted normative coupling between hippocampal size and brain size, merit further investigation, given the unique memory profile found in ASD.
Supplementary Material
Acknowledgments
During the conduct of this work, Dr. Reinhardt was supported by the Autism Research Training Program (T32 MH073124); Dr. Solomon was supported by R01MH106518 and R01MH103284; Dr. Amaral was supported by R01MH103371, T32 MH073124, and the Simons Foundation; Dr. Nordahl was supported by R01MH104438; Dr. Rogers was supported by T32 MH073124, HRSA 1T73MC30113–01-02, NIH 1 R01 MH100030–05, and IES R324A150211; Dr. Ghetti was supported by R21HD088928; Dr. Iosif was supported by the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125); and Dr. Libero was supported by the UC President’s Postdoctoral Fellowship.
This work was presented at the International Society for Autism Research Annual Meeting; May 9L12, 2018; Rotterdam, Netherlands.
Dr. Reinhardt wrote the first draft of the manuscript. Drs. Solomon, Ghetti, and Amaral designed the study. Drs. Solomon and Amaral oversaw all aspects of the data collection, analysis, and writing of the manuscript. Drs. Nordahl and Ghetti contributed to data analysis and interpretation. All authors read and commented on all manuscript drafts and approved the final version. All authors agree to be responsible for the integrity of the findings.
Dr. Iosif provided statistical support as part of the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125). Drs. Iosif and Ferrer served as the statistical experts for this research.
The authors would like to acknowledge Susumu Mori, PhD, of Johns Hopkins University and Zhipeng Hou, BS, of Northwestern University, for their assistance in developing the pediatric atlases for this study. The authors would also like to thank Cory Coleman, BS, of the University of California, Davis, for his assistance with data processing and Alexa Hetchman, BS, and Caroline Gohring, BA, of the University of California, Davis, who helped with MRI data acquisition and processing. The authors would also like to thank the families that participated in the Autism Phenome Project and GAIN study.
Footnotes
Disclosure: Dr. Amaral has served as a consultant for Stemina Biomarkers Discovery and Axial Therapeutics. Drs. Reinhardt, Iosif, Libero, Heath, Rogers, Ferrer, Nordahl, Ghetti, and Solomon have reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Vanessa P. Reinhardt, University of California, Davis.
Ana-Maria Iosif, University of California, Davis.
Lauren Libero, University of California, Davis.
Brianna Heath, University of California, Davis.
Sally J. Rogers, University of California, Davis.
Emilio Ferrer, University of California, Davis.
Christine Nordahl, University of California, Davis, MIND Institute, Davis, California.
Simona Ghetti, University of California, Davis.
David Amaral, University of California, Davis, MIND Institute, Davis, California.
Marjorie Solomon, University of California, Davis, MIND Institute, Davis, California, UC Davis Imaging Research Center, Davis, California.
REFERENCES
- 1.Amaral DG, Lavanex P. Hippocampal neuroanatomy. In: Andersen P, Morris R, Amaral DG, O’Keefe J, Bliss T, eds. The Hippocampus Book. New York, NY: Oxford; 2007. [Google Scholar]
- 2.Eichenbaum H, Amaral DG, Buffalo EA, et al. Hippocampus at 25. Hippocampus 2016; 26:1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yonelinas AP. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res 2013; 254:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen NJ. Navigating life. Hippocampus 2015; 25:704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahati K, Bhagya V, Christofer T, Sneha A, Shankaranarayana Rao BS. Enriched environment ameliorates depression-induced cognitive deficits and restores abnormal hippocampal synaptic plasticity. Neurobiol Learn Mem 2016; 134:379–391. [DOI] [PubMed] [Google Scholar]
- 6.Woollett K, Maguire EA. Acquiring “the Knowledge” of London’s layout drives structural brain changes. Curr Biol 2011; 21:2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, Ward PB. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage 2018; 166:230–238. [DOI] [PubMed] [Google Scholar]
- 8.Luby JL, Belden A, Harms MP, Tillman R, Barch DM. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc Natl Acad Sci U S A 2016; 113:5742–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JK, Nordahl CW, Amaral DG, Lee A, Solomon M, Ghetti S. Assessing hippocampal development and language in early childhood: evidence from a new application of the Automatic Segmentation Adapter Tool. Hum Brain Mapp 2015; 36:4483–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci 2004; 24:6392–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamnes CK, Bos MGN, van de Kamp FC, Peters S, Crone EA. Longitudinal development of hippocampal subregions from childhood to adulthood. Dev Cogn Neurosci 2018; 30:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uematsu A, Matsui M, Tanaka C, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One 2012; 7: e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu S, Pruessner JC, Coupe P, Collins DL. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. Neuroimage 2013; 74: 276–287. [DOI] [PubMed] [Google Scholar]
- 14.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol 1996; 366:223–230. [DOI] [PubMed] [Google Scholar]
- 15.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21: 1185–1201. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore JH, Shi F, Woolson SL, et al. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex 2012; 22:2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002; 59:184–192. [DOI] [PubMed] [Google Scholar]
- 18.Groen W, Teluij M, Buitelaar J, Tendolkar I. Amygdala and hippocampus enlargement during adolescence in autism. J Am Acad Child Adolesc Psychiatry 2010; 49:552–560. [DOI] [PubMed] [Google Scholar]
- 19.Hasan KM, Walimuni IS, Frye RE. Global cerebral and regional multimodal neuroimaging markers of the neurobiology of autism: development and cognition. J Child Neurol 2013; 28:874–885. [DOI] [PubMed] [Google Scholar]
- 20.Haar S, Berman S, Behrmann M, Dinstein I. Anatomical abnormalities in autism? Cereb Cortex 2016; 26:1440–1452. [DOI] [PubMed] [Google Scholar]
- 21.Murphy CM, Deeley Q, Daly EM, et al. Anatomy and aging of the amygdala and hippocampus in autism spectrum disorder: an in vivo magnetic resonance imaging study of Asperger syndrome. Autism Res 2012; 5:3–12. [DOI] [PubMed] [Google Scholar]
- 22.Hazlett HC, Poe MD, Lightbody AA, et al. Trajectories of early brain volume development in fragile X syndrome and autism. J Am Acad Child Adolesc Psychiatry 2012; 51:921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shou XJ, Xu XJ, Zeng XZ, et al. A volumetric and functional connectivity MRI study of brain arginine-vasopressin pathways in autistic children. Neurosci Bull 2017; 33: 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chawarska K, Campbell D, Chen L, Shic F, Klin A, Chang J. Early generalized overgrowth in boys with autism. Arch Gen Psychiatry 2011; 68:1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libero LE, Schaer M, Li DD, Amaral DG, Nordahl CW. A longitudinal study of local gyrification index in young boys with autism spectrum Disorder. Cereb Cortex 2019; 29: 2575–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaral DG, Li D, Libero L, et al. In pursuit of neurophenotypes: the consequences of having autism and a big brain. Autism Res 2017; 10:711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libero LE, Nordahl CW, Li DD, Ferrer E, Rogers SJ, Amaral DG. Persistence of megalencephaly in a subgroup of young boys with autism spectrum disorder. Autism Res 2016; 9:1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordahl CW, Braunschweig D, Iosif AM, et al. Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain Behav Immun 2013; 30:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nordahl CW, Lange N, Li DD, et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A 2011; 108: 20195–20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30:205–223. [PubMed] [Google Scholar]
- 31.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule: ADOS-2 Los Angeles: Western Psychological Services; 2012. [Google Scholar]
- 32.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24: 659–685. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. Text Revised. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 34.Lainhart JE, Bigler ED, Bocian M, et al. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am J Med Genet A 2006; 140:2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 36.Mullen EM. Mullen Scales of Early Learning Circle Pines, MN: American Guidance Service, Inc.; 1995. [Google Scholar]
- 37.Elliott CD. Differential Ability Scales (2nd ed). San Antonio, TX: Pearson; 2007. [Google Scholar]
- 38.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Beahavior Scales, Second Edition San Antonio, TX: Pearson; 2005. [Google Scholar]
- 39.Mori S, Wu D, Ceritoglu C, Li Y, et al. MRICloud: delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Comput Sci Eng 2016; 18:21–35. [Google Scholar]
- 40.Tang X, Crocetti D, Kutten K, et al. Segmentation of brain magnetic resonance images based on multi-atlas likelihood fusion: testing using data with a broad range of anatomical and photometric profiles. Frontiers in neuroscience 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982; 38: 963–974. [PubMed] [Google Scholar]
- 42.Lindell AK, Hudry K. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychol Rev 2013; 23: 257–270. [DOI] [PubMed] [Google Scholar]
- 43.Barnes J, Scahill RI, Frost C, Schott JM, Rossor MN, Fox NC. Increased hippocampal atrophy rates in AD over 6 months using serial MR imaging. Neurobiol Aging 2008; 29: 1199–1203. [DOI] [PubMed] [Google Scholar]
- 44.Milne NT, Bucks RS, Davis WA, et al. Hippocampal atrophy, asymmetry, and cognition in type 2 diabetes mellitus. Brain Behav 2018; 8:e00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy J The mammalian brain and the adaptive advantage of cerebral asymmetry. Ann N Y Acad Sci 1977; 299:264–272. [DOI] [PubMed] [Google Scholar]
- 46.Motley SE, Kirwan CB. A parametric investigation of pattern separation processes in the medial temporal lobe. J Neurosci 2012; 32:13076–13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon M, McCauley JB, Iosif AM, Carter CS, Ragland JD. Cognitive control and episodic memory in adolescents with autism spectrum disorders. Neuropsychologia 2016; 89:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riggins T, Geng F, Botdorf M, Canada K, Cox L, Hancock GR. Protracted hippocampal development is associated with age-related improvements in memory during early childhood. Neuroimage 2018; 174:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci 1985; 5:1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lavenex P, Banta Lavenex P. Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav Brain Res 2013; 254:8–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


