Objectives
The aim of this study was to examine the effect and safety of biological agents for lupus nephritis (LN).
Methods
PubMed, EMBASE, and the Cochrane Library databases were searched from their inception up to November 2021. The outcomes were overall response, complete remission, proteinuria, renal activity index, and adverse events (AEs). Only randomized controlled trials (RCTs) were included.
Results
Nine RCTs (1645 patients) were included. The RCTs evaluated abatacept (n = 2), belimumab (n = 1), obinutuzumab (n = 1), atacicept (n = 1), IL-2 (n = 1), ocrelizumab (n = 1), and rituximab (n = 2). The use of biological agents was associated with higher likelihoods of achieving an overall response (relative risk [RR], 1.26; 95% confidence interval [CI], 1.15–1.39; p < 0.001; I2 = 14.3%; pQ = 0.301) and a complete response (RR, 1.33; 95% CI, 1.16–1.54; p < 0.001; I2 = 41.8%; pQ = 0.056). The use of biological agents was not associated with improvements in the urinary protein-to-creatinine ratio (weighted mean difference, 3.83; 95% CI, −3.71 to 11.38; p = 0.319; I2 = 99.4%; pQ < 0.001). The use of biological agents in patients with LN was also not associated with an increased risk of any AEs (RR, 1.01; 95% CI, 0.98–1.04; p = 0.519; I2 = 0.0%; pQ = 0.533), serious AEs (RR, 0.95; 95% CI, 0.82–1.09; p = 0.457; I2 = 0.0%; pQ = 0.667), grade >3 AEs (RR, 0.91; 95% CI, 0.67–1.22; p = 0.522; I2 = 0.0%; pQ = 0.977), infections (RR, 1.09; 95% CI, 0.99–1.20; p = 0.084; I2 = 0.0%; pQ = 0.430), and deaths (RR, 0.67; 95% CI, 0.36–1.24; p = 0.200; I2 = 0.0%; pQ = 0.439). The meta-regression analysis showed that follow-up duration and the sample size did not influence the complete response rate, whereas publications in 2012 to 2014 influence the rate compared with 2015 to 2020.
Conclusions
Biological agents seem to be effective and safe for managing patients with LN.
Key Words: biologic agents, lupus nephritis, meta-analysis
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disorder of connective tissue characterized by autoantibodies that target nuclear antigens, remissions, and flares, and a highly variable clinical presentation, disease course, and prognosis.1–5 Therefore, SLE is characterized by an unpredictable disease course and periods of remission and flare, leading to organ damage and mortality.1–5
The treatment goals of SLE include minimizing organ damage, preventing flares during periods of stability, and optimizing health-related quality of life.1–5 The standard management of SLE is based on hydroxychloroquine, glucocorticoids, methotrexate, azathioprine, mycophenolate mofetil, and cyclophosphamide.1–7 In addition, novel biological agents are being developed (targeting the lymphocytes, accessory molecules, and cytokines) to enhance the therapeutic efficacy when combined with standard therapies.8 With those novel treatments, the 5- and 10-year survival rates now reach 95% and 91%, respectively.4,5
Lupus nephritis (LN) occurs in approximately 40% of SLE patients, mostly within 5 years from the SLE diagnosis, and presents a rate of progression to end-stage renal disease of 4.3% to 10.1%.9 Patients with LN have a poorer prognosis than patients with SLE without kidney involvement, reported at 5% to 25% at 5 years.10,11 Still, there is uncertainty regarding the effect and safety of biological agents for LN. A randomized controlled trial (RCT) confirmed that, although rituximab therapy led to more responders and greater reductions in anti-dsDNA and C3/C4 levels, it did not improve clinical outcomes after 1 year of treatment.12 The combination of rituximab with mycophenolate mofetil and corticosteroids did not result in any new or unexpected safety signals.12 Another controlled trial confirmed that, in patients with active LN, more patients who received belimumab plus standard therapy had a primary efficacy renal response than those who received standard therapy alone.13
Therefore, given the conflicting results, a higher level of evidence was required. Therefore, this meta-analysis aimed to examine the effect and safety of biological agents for LN. The results could help guide treatments and future guidelines for the management of LN.
MATERIALS AND METHODS
Literature Search
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines.14,15 The search parameters were designed based on the PICOS principle.16 PubMed, EMBASE, and the Cochrane Library databases were searched from their inception up to November 2021 for potentially eligible studies, using the MeSH terms of “lupus nephritis” and “biological factors,” as well as relevant key words (Supplementary Table S1, http://links.lww.com/RHU/A480). The literature search was conducted independently by 2 investigators (L.W. and S.C.). Their results were compared, and the differences were solved by discussion until a consensus was reached. The reference lists of the included articles were searched for additional potentially eligible studies.
Eligibility Criteria
The inclusion criteria were (1) patients with a diagnosis of LN, (2) the intervention included biological agents, (3) the outcomes were overall response, complete remission, proteinuria, renal activity index, and adverse events (AEs), and (4) RCT. The exclusion criteria were (1) conference abstract, case report, meta-analysis, review, animal study, or protocol; (2) language not in English; (3) the full text could not be obtained; or (4) no data could be used from the report.
Data Extraction and Quality Assessment
The data were extracted by 2 independent investigators (P.C. and Y.Z.). Disagreements were resolved by a third investigator (S.C.). The retrieved data included the names of the authors, year, time, country, patient characteristics (sample size, age, and male proportion), intervention, control, dosage, follow-up, and outcomes. The RCTs were evaluated using the Cochrane risk bias tool.17 The quality assessment was performed independently by 2 investigators independently (P.C. and F.H.). Differences were solved by discussion.
Statistical Analysis
All analyses were performed using STATA SE 14.0 (StataCorp, College Station, TX). Relative risks (RRs), weighted mean differences, and the corresponding 95% confidence intervals (CIs) were used to compare the outcomes. Statistical heterogeneity among the included studies was calculated using Cochran Q test and the I2 index. An I2 > 50% and pQ < 0.10 indicated high heterogeneity. The random-effects model was used when high heterogeneity was observed among studies. Otherwise, the fixed-effects model was applied. The p values <0.05 were considered statistically significant.17 The potential publication bias was assessed using Begg and Egger tests. Sensitivity analyses were performed to identify individual study effects on pooled results and test the reliability of the results. Furthermore, we performed meta-regression to assess the influence of different study factors on the complete response rates of LN to biological agents. Subgroup analyses were conducted to explore the source of heterogeneity among subgroups of publication time.
RESULTS
Identification of Eligible Studies
Figure 1 presents the study selection process. The initial search yielded 310 records, and 212 were excluded before the screening. Then, 98 records were screened, and 79 were excluded. Nineteen reports were sought for retrieval, and 10 could not be retrieved. Nine reports were assessed for eligibility and were included in this meta-analysis.
FIGURE 1.
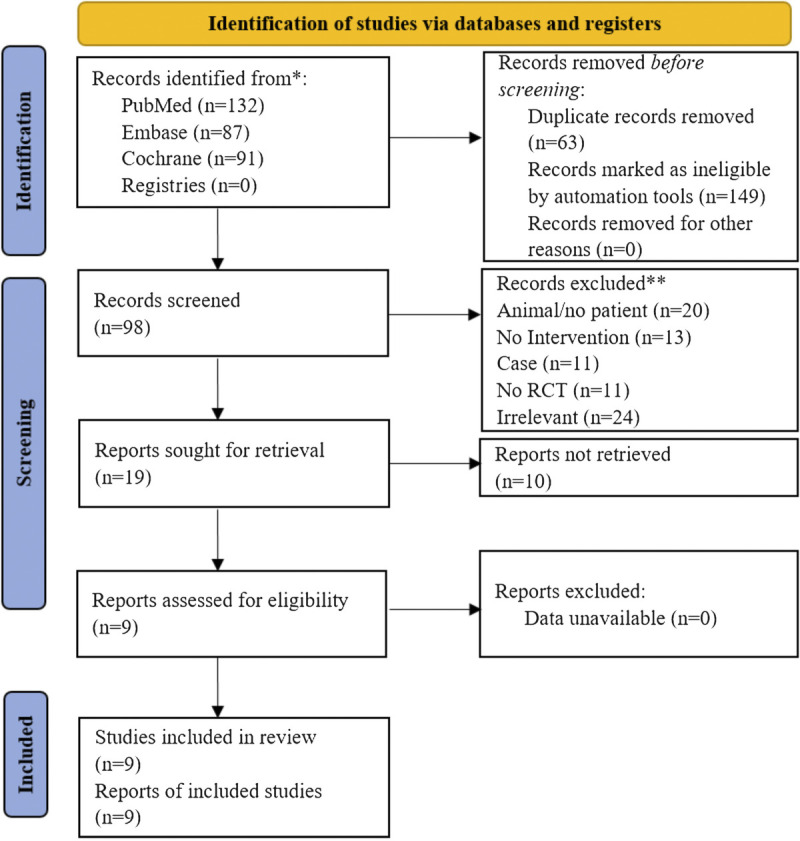
Study selection process.
Study Characteristics and Quality Assessment
Supplementary Table S2 (http://links.lww.com/RHU/A480) presents the characteristics of the included studies. The 9 RCTs12,13,18–24 included 1645 patients. The RCTs evaluated abatacept,18,19 belimumab,13 obinutuzumab,20 atacicept,21 IL-2,22 ocrelizumab,23 and rituximab.12,24 The controls groups included placebo,12,13,18–22 standard of care,23 and cyclophosphamide.24
Supplementary Table S3 (http://links.lww.com/RHU/A480) presents the quality evaluation using the Cochrane tool for RCTs. Seven RCTs scored 6 points,12,18,19,21–24 and 2 RCTs scored 7 points.13,20
Overall Response
Eight RCTs presented overall response data.12,13,18–20,22–24 The use of biological agents was associated with a higher likelihood of achieving an overall response (RR, 1.26; 95% CI, 1.15–1.39; p < 0.001; I2 = 14.3%; pQ = 0.301) (Fig. 2).
FIGURE 2.
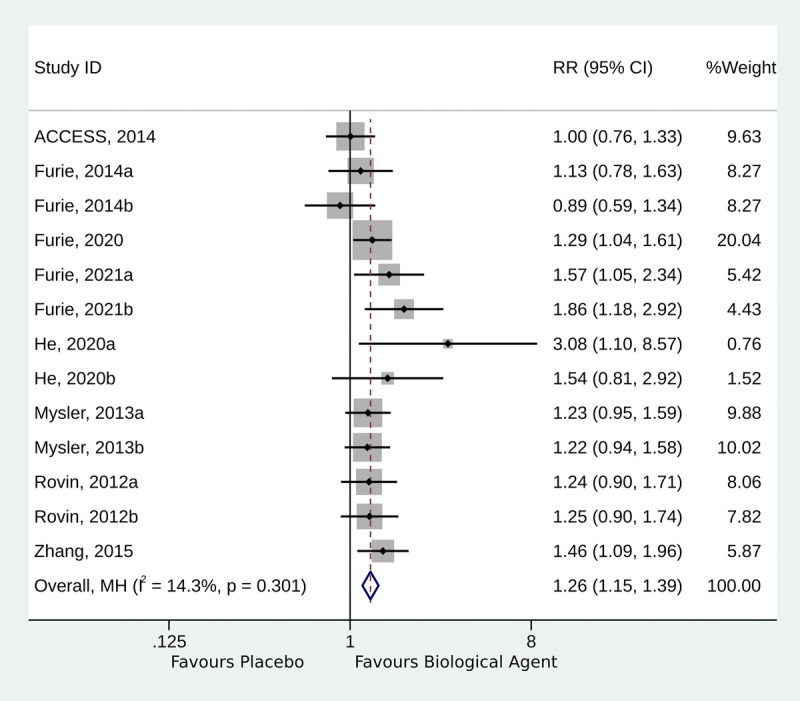
Forest plot of the overall response.
Complete Response
Eight RCTs presented overall response data.12,13,18–20,22–24 The use of biological agents was associated with a higher likelihood of achieving a complete response compared with the control intervention (RR, 1.33; 95% CI, 1.16–1.54; p < 0.001; I2 = 41.8%; pQ = 0.056) (Fig. 3). The studies were subgrouped according to those published in 2012 to 2014,12,18,19,23 2015 to 2020,13,22,24 and 2021.20 The studies published in 2012 to 2014 did not show a higher likelihood of complete response (RR, 1.06; 95% CI, 0.89–1.27; p = 0.518; I2 = 0.0%; pQ = 0.936), whereas significant complete responses were observed for those in 2015 to 2020 (RR, 1.91; 95% CI, 1.45–2.53; p < 0.001; I2 = 49.2%; pQ = 0.116) and 2021 (RR, 1.69; 95% CI, 1.14–2.50; p < 0.001; I2 = 0.0%; pQ = 0.679) (Supplementary Fig. S1, http://links.lww.com/RHU/A480).
FIGURE 3.
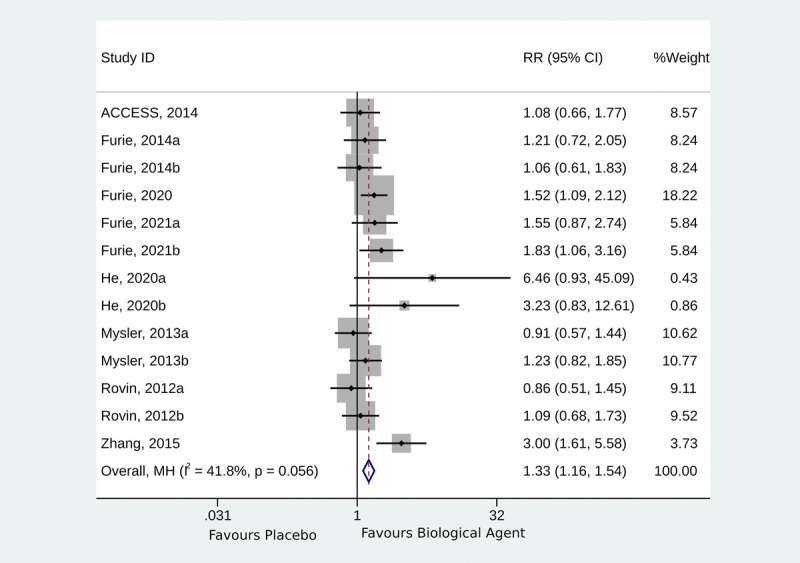
Forest plot of complete response.
Urinary Protein-to-Creatinine Ratio
Two studies reported the urinary protein-to-creatinine ratio.18,21 The use of biological agents was not associated with improvements in the urinary protein-to-creatinine ratio (weighted mean difference, 3.83; 95% CI, −3.71 to 11.38; p = 0.319; I2 = 99.4%; pQ < 0.001) (Fig. 4).
FIGURE 4.
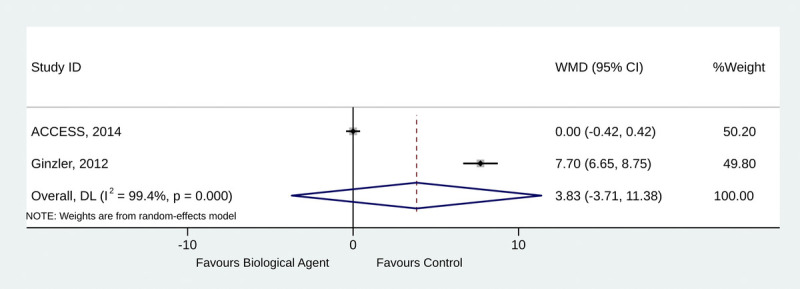
Forest plot of urinary protein-to-creatinine ratio.
Safety
In the analysis, the AEs showed that the use of biological agents in patients with LN was not associated with an increased risk of any AEs (RR, 1.01; 95% CI, 0.98–1.04; p = 0.519; I2 = 0.0%; pQ = 0.533) (Fig. 5A), serious AEs (RR, 0.95; 95% CI, 0.82–1.09; p = 0.457; I2 = 0.0%; pQ = 0.667) (Fig. 5B), grade >3 AEs (RR, 0.91; 95% CI, 0.67–1.22; p = 0.522; I2 = 0.0%; pQ = 0.977) (Fig. 5C), infections (RR, 1.09; 95% CI, 0.99–1.20; p = 0.084; I2 = 0.0%; pQ = 0.430) (Fig. 5D), and deaths (RR, 0.67; 95% CI, 0.36–1.24; p = 0.200; I2 = 0.0%; pQ = 0.439) (Fig. 5E).
FIGURE 5.
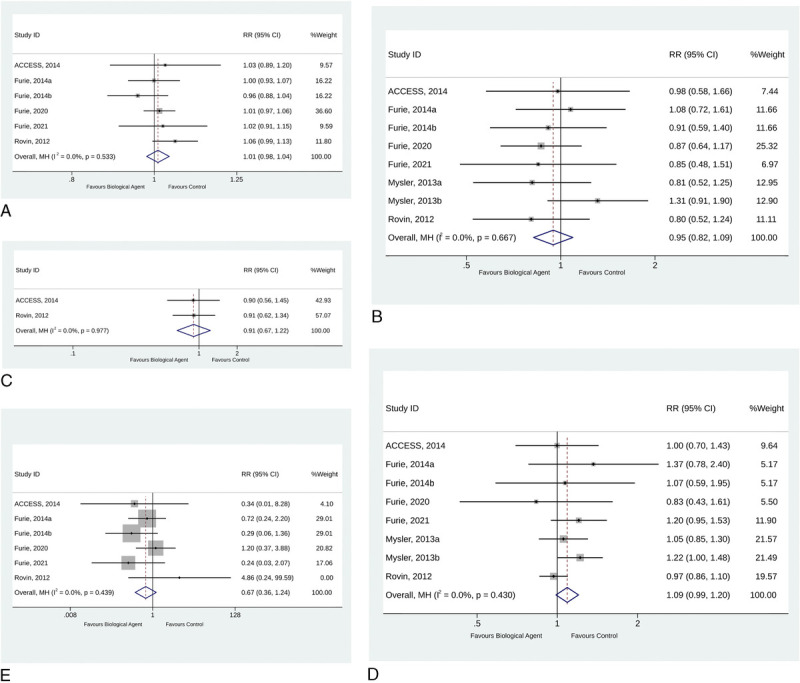
Forest plot of Adverse Events (AEs). A, Any Adverse Events (AEs). B, Serious Adverse Events (AEs). C, Grade 3 or higher Adverse Events (AEs). D, Infection. E, Deaths.
Publication Bias
The publication bias analyses showed no significant publication bias when considering the overall response (Begg test, p = 0.200; Egger test, p = 0.121). The publication bias analyses showed publication bias when considering the complete response (Begg test, p = 0.017; Egger test; p = 0.117), but no publication bias was observed according to publication years (Supplementary Fig. S2, http://links.lww.com/RHU/A480).
Sensitivity Analysis
The sensitivity analyses based on the overall response (Supplementary Fig. S3, http://links.lww.com/RHU/A480), and the complete response (Supplementary Fig. S4, http://links.lww.com/RHU/A480) showed that the results were robust.
Meta-Regression of Complete Response
Supplementary Figure S5 (http://links.lww.com/RHU/A480) presents the meta-regression analysis of the factors that could affect the complete response to biological agents in patients with LN. Follow-up duration and the sample size did not influence the complete response rate, whereas publication in 2012 to 2014 influenced the rate compared with 2015 to 2020.
DISCUSSION
Biological agents are recommended to manage SLE,4,5,8 but their use in patients with LN yielded conflicting results.12,13,18–24 Therefore, this meta-analysis aimed to examine the effect and safety of biological agents for LN. The results suggest that biological agents seem to be effective and safe for managing patients with LN. However, the use of biological agents was not associated with improvements in the urinary protein-to-creatinine ratio, contrary to previous studies.25 Still, this failure to lower the protein-to-creatinine ratio is consistent with Rovin et al.12 Still, because the 2 included studies that examined that point displayed high heterogeneity, no conclusion can be drawn regarding the protein-to-creatinine ratio.
Kidney lesions in SLE progress from mild to end-stage renal disease and are a major cause of morbidity and mortality in patients with SLE.1,2,7 For now, the guidelines and expert consensuses for managing LN recommend immunosuppression (cyclophosphamide, mycophenolate mofetil, calcineurin inhibitors, leflunomide, and mTOR inhibitors), anti-inflammatory treatments, and therapies targeting autoimmunity.26–28 Biological agents such as belimumab, rituximab, obinutuzumab, and abatacept can be used for induction therapy in selected patients with LN.26–28 Still, more recent biological agents are not included in the guidelines. The present meta-analysis suggested that the more recent RCTs (≥2015) that tested more recent biological agents had better efficacy than the older agents (2012–2014). Such results could both be due to better agents and to more effectively selected patients. This could suggest improvements in patient selection with time, as well as improvements in concomitant standard therapy. Still, a previous meta-analysis of biological agents in SLE, including 5 RCTs in LN, showed no significant effect of the included agents, except for belimumab; on the other hand, all agents had significant steroid-sparing effects, suggesting that this outcome could be included in future trials.29 Steroids can have significant AEs and affect the patients' quality of life.30
This meta-analysis showed that the use of biological agents was safe, without differences in AEs compared with the control groups. It was previously observed with rituximab in LN.25
This study has limitations. First, a meta-analysis is an imperfect analysis because it inherits the limitations and biases of all included studies. Still, a meta-analysis provides a general trend in larger sample sizes and across multiple study populations. Second, unpublished articles could not be retrieved. Third, the definition of complete and partial response used in each RCT was not the same, will cause data heterogeneity. Finally, high heterogeneity was observed in several analyses because of the different agents, doses, and follow-up times used in the different studies.
CONCLUSIONS
This meta-analysis of 9 RCTs suggests that biological agents seem to be effective and safe for managing patients with LN. These results could help guide the treatment of patients with LN, design future clinical trials, and delineate future guidelines for the management of LN.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions: P.C. and Y.Z. carried out the studies, participated in collecting data, and drafted the manuscript. L.W. and S.C. performed the statistical analysis and participated in its design. P.C. and F.H. participated in acquisition, analysis, or interpretation of data and drafted the manuscript. All authors read and approved the final manuscript.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.jclinrheum.com).
Contributor Information
Pang Chen, Email: chenpang_2007@126.com.
Yadong Zhou, Email: 280230485@qq.com.
Lianghua Wu, Email: 123340857@qq.com.
Shihan Chen, Email: 853286544@qq.com.
REFERENCES
- 1.Fanouriakis A Kostopoulou M Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–745. [DOI] [PubMed] [Google Scholar]
- 2.Mosca M Tani C Aringer M, et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis. 2010;69:1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun. 2019;96:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oku K, Atsumi T. Systemic lupus erythematosus: nothing stale her infinite variety. Mod Rheumatol. 2018;28:758–765. [DOI] [PubMed] [Google Scholar]
- 5.Gergianaki I, Bortoluzzi A, Bertsias G. Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2018;32:188–205. [DOI] [PubMed] [Google Scholar]
- 6.van Vollenhoven RF Mosca M Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. 2014;73:958–967. [DOI] [PubMed] [Google Scholar]
- 7.Hahn BH McMahon MA Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012;64:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mok CC. Biological and targeted therapies of systemic lupus erythematosus: evidence and the state of the art. Expert Rev Clin Immunol. 2017;13:677–692. [DOI] [PubMed] [Google Scholar]
- 9.Gasparotto M Gatto M Binda V, et al. Lupus nephritis: clinical presentations and outcomes in the 21st century. Rheumatology (Oxford). 2020;59:v39–v51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh SV Almaani S Brodsky S, et al. Update on lupus nephritis: core curriculum 2020. Am J Kidney Dis. 2020;76:265–281. [DOI] [PubMed] [Google Scholar]
- 11.Moroni G Vercelloni PG Quaglini S, et al. Changing patterns in clinical-histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann Rheum Dis. 2018;77:1318–1325. [DOI] [PubMed] [Google Scholar]
- 12.Rovin BH Furie R Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. 2012;64:1215–1226. [DOI] [PubMed] [Google Scholar]
- 13.Furie R Rovin BH Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383:1117–1128. [DOI] [PubMed] [Google Scholar]
- 14.PRISMA 2020. J Clin Epidemiol. 2021;134:A5–A6. [DOI] [PubMed] [Google Scholar]
- 15.Swartz MK. PRISMA 2020: an update. J Pediatr Health Care. 2021;35:351. [DOI] [PubMed] [Google Scholar]
- 16.Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 2010;31:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT Thomas J Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. London, United Kingdom: Cochrane Collaboration; 2020. [Google Scholar]
- 18.Access Trial Group . Treatment of lupus nephritis with abatacept: the abatacept and cyclophosphamide combination efficacy and safety study. Arthritis Rheumatol. 2014;66:3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furie R Nicholls K Cheng TT, et al. Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol. 2014;66:379–389. [DOI] [PubMed] [Google Scholar]
- 20.Furie RA Aroca G Cascino MD, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022;81:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginzler EM Wax S Rajeswaran A, et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. 2012;14:R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J Zhang R Shao M, et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2020;79:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mysler EF Spindler AJ Guzman R, et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum. 2013;65:2368–2379. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Zhao Z, Hu X. Effect of rituximab on serum levels of anti-C1q and antineutrophil cytoplasmic autoantibodies in refractory severe lupus nephritis. Cell Biochem Biophys. 2015;72:197–201. [DOI] [PubMed] [Google Scholar]
- 25.Zhong Z Li H Zhong H, et al. Clinical efficacy and safety of rituximab in lupus nephritis. Drug Des Devel Ther. 2019;13:845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO . Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;2021:S1–S276. [DOI] [PubMed] [Google Scholar]
- 27.Fanouriakis A Kostopoulou M Cheema K, et al. 2019 update of the joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79:713–723. [DOI] [PubMed] [Google Scholar]
- 28.Parikh SV, Rovin BH. Current and emerging therapies for lupus nephritis. J Am Soc Nephrol. 2016;27:2929–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oon S Huq M Godfrey T, et al. Systematic review, and meta-analysis of steroid-sparing effect, of biologic agents in randomized, placebo-controlled phase 3 trials for systemic lupus erythematosus. Semin Arthritis Rheum. 2018;48:221–239. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Irastorza G Danza A Perales I, et al. Prednisone in lupus nephritis: how much is enough? Autoimmun Rev. 2014;13:206–214. [DOI] [PubMed] [Google Scholar]


