Abstract
Purpose:
The aim of this study was to use acoustic and kinematic speech measures to characterize type of motor speech impairment—apraxia of speech (AOS) versus dysarthria—in individuals with four-repeat tauopathy (4RT)–associated syndromes, including nonfluent variant primary progressive aphasia (nfvPPA), primary progressive AOS (PPAOS), corticobasal syndrome (CBS), and progressive supranuclear palsy syndrome (PSPs).
Method:
Twenty patient participants were recruited and stratified into two groups: (a) a motor-speech–impaired group of individuals with nfvPPA, PPAOS, CBS, or PSPs and suspected 4RT pathology (“MSI+”) and (b) a non–motor-speech-impaired group of individuals with logopenic variant primary progressive aphasia (“MSI−”). Ten healthy, age-matched controls also participated in the study. Participants completed a battery of speech tasks, and 15 acoustic and kinematic speech measures were derived. Quantitative speech measures were grouped into feature categories (“AOS features,” “dysarthria features,” “shared features”). In addition to quantitative speech measures, two certified speech-language pathologists made independent, blinded auditory-perceptual ratings of motor speech impairment. A principal component analysis (PCA) was conducted to investigate the relative contributions of quantitative features.
Results:
Quantitative speech measures were generally concordant with independent clinician ratings of motor speech impairment severity. Hypothesis-driven groupings of quantitative measures differentiated predominantly apraxic from predominantly dysarthric presentations within the MSI+ group. PCA results provided additional evidence for differential profiles of motor speech impairment in the MSI+ group; heterogeneity across individuals is explained in large part by varying levels of overall severity—captured by the shared feature variable group—and degree of apraxia severity, as measured by the AOS feature variable group.
Conclusions:
Quantitative features reveal heterogeneity of MSI in the 4RT group in terms of both overall severity and subtype of MSI. Results suggest the potential for acoustic and kinematic speech assessment methods to inform characterization of motor speech impairment in 4RT-associated syndromes.
Supplemental Material:
Four-repeat tauopathies (4RT) are a subclass of frontotemporal lobar degeneration tauopathies characterized by neuronal and glial cytoplasmic inclusions of a type of hyperphosphorylated tau protein with four spliced copies of the microtubule-binding repeat domain (Dickson et al., 2011). The clinical syndromes that are associated with the 4RT pathologies are complex and characterized by a wide range of cognitive, linguistic, and motor symptoms (Josephs et al., 2011; Kouri et al., 2011; VandeVrede et al., 2020). Corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) are the most common of the 4RT pathologies (Josephs et al., 2011). CBD and PSP pathology can manifest as a variety of specific clinical syndromes including corticobasal syndrome (CBS), PSP syndrome (PSPs), nonfluent variant primary progressive aphasia (nfvPPA), or primary progressive apraxia of speech (PPAOS; Armstrong et al., 2013; Höglinger et al., 2017; Josephs et al., 2006; Olney et al., 2017). Because of these many-to-one relationships between clinical syndrome and underlying pathology, associative links between pathology and syndrome have been difficult to establish (Grossman, 2012; Rohrer et al., 2010). Therefore, for all tauopathy-associated syndromes, it is valuable to identify specific clinical signs that may relate more reliably to one underlying pathology or the other. Behavioral phenotyping based on clinical symptoms and signs as well as neuroimaging remain the primary means of premortem diagnosis of 4RT-associated syndromes since pathological diagnoses can only be confirmed at autopsy. This diagnostic challenge makes research into reliable, potentially diagnostic clinical signs critical in this population, especially as targeted, protein-specific clinical trials emerge.
Motor speech impairment is one clinical sign that has been cited in previous literature to be an early indicator of tau-positive pathology and of 4RT specifically (Josephs, 2008; Montembeault et al., 2018; Ogar et al., 2007; Santos-Santos et al., 2016). In clinical settings, motor speech impairments are often dichotomously classified as an apraxia of speech (AOS) or a dysarthria, with the former characterized by speech symptoms consistent with problems with planning or programming of speech movements and the latter by speech symptoms consistent with weakness, dyscoordination, or paresis of the speech musculature (Duffy, 2013). Prior research suggests that the type of motor speech impairment may vary depending on the presenting tau-associated syndrome. There is, for example, overwhelming evidence for the salience of AOS in nfvPPA—indeed, it is one of two diagnostic inclusion criteria (Gorno-Tempini et al., 2011)—as well as mounting evidence of dysarthria and comorbid AOS/dysarthria presentations in the nfvPPA population (Caso et al., 2014; Duffy et al., 2014; Poole et al., 2017). More recently, consensus has been building in support of a related but distinct diagnostic category—PPAOS—that is diagnosed when AOS is the initial and only presenting symptom (i.e., no concomitant language impairment, thus precluding a progressive aphasia designation) of a neurodegenerative process (Josephs et al., 2012). A robust body of research has characterized AOS in this population (Duffy et al., 2014, 2015; Poole et al., 2017; Utianski et al., 2018); dysarthria too has been noted to co-occur, particularly in later stages of disease progression (Duffy et al., 2015; Josephs et al., 2014). In contrast to nfvPPA and PPAOS, relatively less is known about motor speech impairments in CBS or PSPs. Extant literature suggests AOS and orobuccal apraxia to be more common in CBS compared with PSPs, whereas dysarthria is very common in PSPs (Duffy et al., 2014). With regard to PSPs, Clark and colleagues recently reported that, across a large cohort including six variants of PSPs, fully 88% had motor speech impairment, with a majority exhibiting dysarthric speech symptoms and a minority exhibiting symptoms of AOS (Clark et al., 2021).
Emerging research suggests that differentiating between the different motor speech subtypes will be informative for improving predictions of underlying pathology. A study looking at a group of nfvPPA patients with a postmortem pathological diagnosis of PSP (nfv-PSP) or CBD (nfv-CBD) found early dysarthric features to be one factor useful for identifying nfv-PSP as compared with nfv-CBD (Santos-Santos et al., 2016). This finding extends prior work associating motor speech impairment with tauopathies generally (Deramecourt et al., 2010; Duffy et al., 2014; Josephs et al., 2006) and suggests that specific motor speech impairments may be associated with different underlying pathologies within the 4RT family. Recent studies have indicated, for example, that separating out prosodic versus phonetic speech features may be important both because of the existence of naturally occurring prosodic and phonetic subtypes within AOS (Mailend & Maas, 2021; Takakura et al., 2019; Utianski et al., 2018) and also because these motor speech subgroups seem to have important implications for predicting clinical progression (Duffy et al., 2021; Whitwell et al., 2017) as well as divergent underlying pathologic correlates (García et al., 2022; Josephs et al., 2021).
Despite the emerging evidence demonstrating the importance of more granular classification of motor speech impairments in 4RT-associated syndromes, differentiating AOS from dysarthria has been a long-standing scientific and clinical challenge (Weismer & Green, 2015). Perhaps the most significant barrier to differential diagnosis is the degree of overlap of diagnostic features. Although AOS and dysarthria appear to be associated with lesions or atrophy at different cortical and subcortical locations (Weismer & Green, 2015), surface speech features often cannot be attributed uniquely to a motor planning/programming versus motor execution deficit (Maassen et al., 2007). For instance, reduced rate of speech and sound distortions are the two most commonly cited diagnostic inclusion features for AOS, as determined in a recent review of AOS-related research studies since 2007 (Allison et al., 2020). These same features, however, are also widely cited in the dysarthria literature as being common characteristics of most types of dysarthria (Clark et al., 2014; Darley et al., 1969; Duffy, 2013; Mefferd et al., 2014; Rong et al., 2015). There is, therefore, a critical need to identify speech features that can be more reliably mapped to either AOS or dysarthria.
Another major barrier to differential diagnosis of motor speech impairment subtypes is the reliance on perceptual judgment of speech features. Diagnosis of motor speech impairment remains dependent mainly on clinician judgment, which can be time-consuming, requires extensive rater training, and, most importantly, is not always reliable (Kent, 1996). Prior research has demonstrated added value for quantitative speech measures for the identification of motor speech impairment (Allison et al., 2017; Cordella et al., 2017; Green et al., 2018; Rowe et al., 2021). Quantitative measures have included speech and articulation rate (Ash et al., 2013; Sajjadi et al., 2012; Wilson et al., 2009), pairwise variability for vowel duration (Ballard et al., 2014; Basilakos et al., 2017; Vergis et al., 2014), vowel space metrics (Turner et al., 1995; Whitfield & Goberman, 2014), and kinematic measures of articulator movement (Green, Yunusova, et al., 2013; Rong et al., 2012; Yunusova et al., 2010). However, most prior work has focused on identifying motor speech impairment (cf. phonological or other higher-level language impairment) and not on distinguishing between motor speech subtypes. It remains unclear the extent to which quantitative features could be useful for characterizing AOS-specific versus dysarthria-specific impairments. It is also crucial to investigate whether diagnostic models based on hypothesis-driven groupings of quantitative speech measures (e.g., a priori multivariate models) may more accurately distinguish apraxia from dysarthria than do models based on a single quantitative speech measure.
In this study, we focus on characterizing the type(s) of motor speech impairment as it occurs in 4RT-associated syndromes. We aim to (a) identify acoustic and kinematic markers of motor speech impairment in individuals with nfvPPA, PPAOS, CBS, and PSPs and compare these with clinician-rated auditory-perceptual measures for the same individuals and (b) use these acoustic/kinematic measures to better characterize the type of motor speech impairment (i.e., AOS, dysarthria), specifically by deriving individual profiles of motor speech impairment. We predict that the results will demonstrate heterogenous profiles of motor speech impairment within the 4RT syndrome group, with both apraxic and dysarthric features present and characterizable using a combination of acoustic and kinematic measures.
Method
Participants
Patient Participants
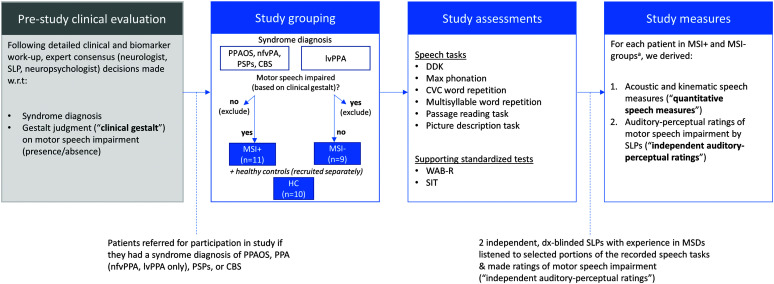
Twenty patient participants were recruited from the Massachusetts General Hospital Frontotemporal Disorders (MGH FTD) Unit. The process we followed to identify appropriate patients, assign study groupings, and assess participants is shown in Figure 1. Briefly, the process began with a prestudy clinical evaluation, during which a multidisciplinary team (including a highly trained and specialized neurologist, a speech-language pathologist [SLP], and a neuropsychologist) assessed the patient using a previously described clinical battery (Sapolsky et al., 2010, 2014) and rendered a diagnosis according to syndrome-specific published diagnostic criteria. Patients were referred for participation in the current study if they received a diagnosis of one of four syndromes: PPAOS (Josephs et al., 2012), primary progressive aphasia (PPA; Gorno-Tempini et al., 2011), CBS (Armstrong et al., 2013), or PSP (Höglinger et al., 2017). Before being referred to the current study, patients diagnosed with PPA were subgrouped into nonfluent (nfvPPA) or logopenic (lvPPA) variants, according to current consensus criteria; patients with semantic variant PPA were excluded from the current study. The lvPPA group served as a disease control group, wherein only patients who demonstrated language, but not speech, impairment were included in the study; additionally, the most common underlying pathology for this group of patients is Alzheimer's disease (AD) neuropathologic changes, the hallmarks of which are amyloid-beta plaques and mixed three-repeat/four-repeat tau neurofibrillary tangles (all cases in this report had positron emission tomography imaging and/or cerebrospinal fluid biomarkers supportive of this diagnosis). In accordance with lvPPA diagnostic criteria (Gorno-Tempini et al., 2011), all participants demonstrated impaired repetition and lexical retrieval (primary inclusion criteria), as well as phonological impairment evidenced by spontaneous speech errors (one of four secondary inclusion criteria). Also as part of the prestudy clinical evaluation, judgments on the presence/absence of motor speech impairment were made by evaluating SLPs in the MGH FTD Unit, each of whom had extensive expertise in the assessment and diagnosis of these syndromes. This initial clinical judgment (henceforth referred to as the “clinical gestalt”) on the presence/absence of motor speech impairment was used in the current study to inform patient groupings, as described below.
Figure 1.
Flowchart detailing the process by which study participants were recruited, grouped, and assessed in the current study, as well as study-specific measures that form the basis of study results. CBS = corticobasal syndrome; CVC = consonant–vowel–consonant; DDK = diadochokinesis; HC = healthy controls; lvPPA = logopenic variant primary progressive aphasia; nfvPPA = nonfluent variant primary progressive aphasia; PPA = primary progressive aphasia; PPAOS = primary progressive apraxia of speech; PSPs = progressive supranuclear palsy syndrome; SIT = Speech Intelligibility Test; SLP = speech-language pathologist; WAB-R = Western Aphasia Battery–Revised. aInitial syndrome diagnoses (e.g., PPAOS, nfvPPA) and subgroupings (MSI+, MSI−) are treated as the diagnostic ground truth in the current study and are maintained even in cases where subsequent study-specific analyses—either quantitative speech measures and/or blinded auditory-perceptual ratings—did not agree with these initial designations.
Patient Grouping
In accordance with study aims and hypotheses, patients diagnosed with PPAOS, nfvPPA, CBS, or PSPs were grouped together to form an umbrella 4RT group. Patients in the 4RT group additionally had to show evidence of at least a mild motor speech impairment of any type, as determined by initial clinical gestalt judgment. Thus, following patient stratification based on syndrome diagnosis and clinically determined presence/absence of motor speech impairment, we had two resultant subgroups: (a) a motor-speech–impaired group of individuals with nfvPPA, PPAOS, CBS, or PSP and suspected 4RT pathology (hereafter “MSI+”) and (b) a non–motor-speech-impaired group of individuals with lvPPA and suspected AD pathology (hereafter “MSI−”). Individuals not meeting both of these respective inclusion criteria (i.e., individuals with nfvPPA, CBS, or PSP without motor speech impairment; individuals with lvPPA with motor speech impairment) were not included in the current study (see Figure 1, second panel). Initial syndrome diagnoses (e.g., PPAOS, nfvPPA) and subgroupings (MSI+, MSI−) were treated as the diagnostic “ground truth” in the current study and were maintained even in cases where subsequent study-specific analyses—such as quantitative speech analysis and/or independent auditory-perceptual ratings (both described below)—did not agree with these initial designations. Syndrome diagnoses and gestalt clinical judgments were made by consensus of several domain experts following a detailed in-person evaluation, who had available not only behavioral data but also extensive standardized testing, imaging, and biomarker information that was not part of the current study.
Healthy Control Participants
Ten neurologically healthy, age-matched control participants were recruited through the MGH Institute of Health Professions Speech and Feeding Disorders Laboratory. All control participants spoke American English as their primary language; passed a pure-tone hearing screen in at least one ear at 35 dB HL at 1, 2, and 4 kHz; and reported no history of speech, language, hearing, or neurological disorder.
Ethics approval and patient consent. This study was approved by the Partners Human Research Committee, the institutional review board of Partners HealthCare (IRB Protocol #2016P001594). All participants provided written informed consent before participating in the study.
Assessment Procedure
Speech Tasks and Elicitation Procedure
All study participants participated in a standardized data collection protocol that included (a) a diadochokinesis (DDK) task in which participants were asked to produce maximum alternating motion rates (AMRs; e.g., /bʌbʌbʌ/) and sequential motion rates (SMRs; e.g., /bʌdʌgʌ/); (b) a maximum phonation task in which participants were asked to produce the sustained vowel /ah/ for as long as possible on a single breath; (c) a consonant–vowel–consonant (CVC) word repetition task in which participants were asked to produce three repetitions each of the tokens /bit/, /bæt/, and /but/; (d) a multisyllabic word repetition task—taken from the Sydney Language Battery (Savage et al., 2013)—in which participants were asked to produce two repetitions each of a set of five words with a weak–strong stress pattern (i.e., <banana>, <computer>, <potato>, <pagoda>, <thermometer>) and five words with strong–weak stress pattern (i.e., <stethoscope>, <butterfly>, <bicycle>, <dinosaur>, <caterpillar>); (e) a passage reading task in which participants read aloud the Bamboo Passage; and (f) a picture description task in which participants were asked to describe the Western Aphasia Battery (WAB) picnic scene.
For all tasks, participants interacted with a computerized platform (E-Prime; Psychology Software Tools) that visually presented each token (English orthography) and cued repetition of that token with a “go” light. For real-word tokens (e.g., CVC task), an accompanying picture was presented to facilitate repetition. For all tokens, prerecorded audio from a male speaker of American English was also played upon stimulus presentation, before initiation of the “go” signal. Token order was randomized per trial block for tasks with multiple blocks (i.e., CVC, multisyllable word repetition). Participants' responses were recorded using a professional-quality head-mounted microphone (Countryman B3P4FF05B) positioned approximately 5 cm from the mouth.
In addition to audio recording, speech biomechanic data were continuously recorded for all tasks using a three-dimensional (3D) electromagnetic articulography device (Wave, Northern Digital, Inc.). A sampling rate of 100 Hz was used. Sensors were placed on the center of the forehead, middle of the jaw, midline on the upper lip, and midline on the lower lip using medical tape. Two additional sensors were placed on the tongue (midline): one on the tongue blade (1 cm posterior from the tongue tip) and one on the tongue body (4 cm posterior from the tongue tip). Tongue sensors were adhered using periodontal glue (PeriAcryl High Viscosity). The head sensor was a 6–degree-of-freedom (DOF) sensor, whereas all remaining sensors were 5-DOF. To remove movement of the head, the articulatory positional data were expressed relative to the 6-DOF head sensor.
Supporting Standardized Testing
To enable a more complete characterization of patients' speech, language, and cognitive profiles, all patient participants completed a short battery of standardized assessments. These assessments included the Montreal Cognitive Assessment (Nasreddine et al., 2005), the Western Aphasia Battery–Revised (WAB-R; Kertesz, 2007), and a reading screen (including the WAB Word–Picture Choice Matching and Boston Diagnostic Aphasia Examination Basic Oral Word Reading) to ensure patients' ability to comply with the experimental protocol. Demographic information and summary clinical characteristics are given for all patient participants in Table 1. Lastly, all participants, including healthy controls, also completed the Speech Intelligibility Test (SIT; Yorkston et al., 2007), which is a gold-standard measure of intelligibility that consists of 11 randomly selected sentences ranging from five to 15 words per sentence.
Table 1.
Summary of demographic and clinical characteristics.
| Group | Case | Clinical phenotype | Age (years) | Age of onset (years) | Gender | Education (years) | CDR | MoCA | WAB-R AQ |
|---|---|---|---|---|---|---|---|---|---|
| MSI− | 1 | lvPPA | 71 | 65–69 | F | 14 | 0.5 | 21 | 86.2 |
| 2 | lvPPA | 68 | 65–69 | F | 12 | 0 | 23 | 83 | |
| 3 | lvPPA | 73 | 60–64 | M | 16 | 0.5 | 15 | 81.1 | |
| 4 | lvPPA | 71 | 65–69 | M | 16 | 0.5 | 19 | 81.4 | |
| 5 | lvPPA | 69 | 60–64 | M | 16 | 0.5 | 13 | 75.2 | |
| 6 | lvPPA | 72 | 65–69 | M | 12 | — | 12 | 78.1 | |
| 7 | lvPPA | 56 | 50–54 | F | 16 | 1 | 14 | 69.8 | |
| 8 | lvPPA | 71 | 60–64 | F | 16 | 0.5 | 20 | 75 | |
| 9 | lvPPA | 77 | 70–74 | M | 12 | 1 | — | 61.2 | |
| MSI+ | 10 | PPAOS | 70 | 65–69 | M | 19 | 0.5 | 26 | 96.6 |
| 11 | nfvPPA | 70 | 65–69 | F | 14 | 0.5 | 22 | 81.2 | |
| 12 | CBS | 51 | 45–49 | F | 14 | 0.5 | 25 | 86.4 | |
| 13 | PSP | 61 | 55–59 | F | 14 | 0.5 | 22 | 93.8 | |
| 14 | nfvPPA | 76 | 70–74 | F | 16 | 0 | 25 | 89.1 | |
| 15 | PSP | 70 | 65–69 | M | 16 | 1 | 16 | 86.4 | |
| 16 | PPAOS | 74 | 70–74 | M | 20 | 0 | 28 | 96.6 | |
| 17 | PPAOS | 73 | 70–74 | F | 16 | 0 | 28 | 94.5 | |
| 18 | PPAOS | 65 | 60–64 | F | 15 | 0.5 | 27 | 96.4 | |
| 19 | CBS | 76 | 65–69 | F | 14 | 0.5 | 27 | 81.8 | |
| 20 | nfvPPA | 72 | 65–69 | F | 18 | 0.5 | 6 | 54.4 |
Note. Em dashes indicate data not available. CDR is scored on a common interval scale: 0 = no impairment, 0.5 = very mild impairment, 1 = mild impairment, 2 = moderate impairment, and 3 = severe impairment. CDR = (global) clinical dementia rating; MoCA = Montreal Cognitive Assessment (score range: 0 [worst] to 30 [best]); WAB-R AQ = Western Aphasia Battery–Revised Aphasia Quotient (a weighted summary score indicating overall aphasia severity, score range: 0 [severe aphasia] to 100 [no aphasia]); MSI− = non–motor-speech-impaired group of individuals with logopenic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia; MSI+ = motor-speech–impaired group of individuals with nfvPPA, PPAOS, CBS, or PSP and suspected four-repeat tauopathy pathology; F = female; M = male; PPAOS = primary progressive apraxia of speech; nfvPPA = nonfluent variant primary progressive aphasia; CBS = corticobasal syndrome; PSP = progressive supranuclear palsy.
Quantitative Speech Measures
Acoustic Measures
Acoustic analyses were conducted using Praat software (Boersma & Weenink, 2018). Six acoustically derived measures were extracted from participants' speech samples, including (a) duration of sustained vowel, measured in milliseconds from the maximum phonation task; (b) smoothed cepstral peak prominence (CPPS), measured using a Praat script (Maryn & Weenink, 2015) run on audio from both the maximum phonation (middle 3 s) and passage reading tasks (first two sentences); (c) formant centralization ratio, measured using the formula given by Sapir et al. (2010), with formant measurements extracted using a supervised semi-automatic script (McCloy, 2012/2018); (d) pairwise variability index for vowel duration for weak–strong words, calculated by comparing the first versus second vowel durations of each weak–strong word token (e.g., banana, computer) from the multisyllabic word repetition task and then averaged across the word set; (e) pairwise variability index for vowel duration for strong–weak words, calculated by comparing the first versus second vowel durations of each strong–weak word token (e.g., bicycle, stethoscope) and then averaged across the word set; and (f) articulation rate, calculated as the number of syllables over the duration of speaking time in the picture description task (Cordella et al., 2017, 2019; Wilson et al., 2010). Detailed derivations—including relevant formulae—for each measure are listed in Table 2.
Table 2.
Speech tasks and resultant quantitative speech measures.
| Task | Tokens | Measure | Abbreviation | Acoustic vs. kinematic | Derivation |
|---|---|---|---|---|---|
| DDK | AMR: /bʌbʌbʌ/ SMR: /bʌdʌgʌ/ |
Duration, AMR | dur_AMR | Kinematic | Auto-extract all variables (Rong et al., 2018) |
| Duration, SMR | dur_SMR | ||||
| # cycles, AMR | ncyc_AMR | Kinematic | |||
| # cycles, SMR | ncyc_SMR | ||||
| Maximum velocity, AMR | vel_AMR | Kinematic | |||
| Spatiotemporal index, SMR | sti_SMR | Kinematic | |||
| Rate, AMR | rate_AMR | Kinematic | Formula: |
||
| Rate, SMR | rate_SMR | ||||
| Rate, AMR vs. SMR | rate_AMR.SMR | Formula: |
|||
| Maximum phonation | /ah/ | Duration | dur_ah | Acoustic | Auto-extract duration following manual TextGrid parsing (Podgornik, 2011) |
| Cepstral peak prominence | cpp | Acoustic | Auto-extract cpps (Maryn & Weenink, 2015) | ||
| CVC repetition | /bit/, /bæt/, /but/ (×3, randomized) |
Formant centralization ratio | fcr | Acoustic | Supervised extraction of F1, F2 following manual TextGrid parsing (McCloy, 2012/2018) Formula: |
| Multisyllabic word repetition | Weak–strong: <banana>, <computer>, <potato>, <pagoda>, <thermometer> (×2, randomized) |
Pairwise variability index, weak–strong | pvi_ws | Acoustic | Auto-extract duration following manual TextGrid parsing of the first (V1) and second vowels (V2; (Podgornik, 2011) Formula: 100 |
| Strong–weak: <stethoscope>, <butterfly>, <bicycle>, <dinosaur>, <caterpillar> (×2, randomized) |
Pairwise variability index, strong–weak | pvi_sw | |||
| Picture description | WAB picnic scene picture | Articulation rate | articrate | Acoustic | Auto-extract total speech duration; manual syllable count (Green et al., 2004) Formula: |
Note. DDK = diadochokinesis; AMR = alternating motion rate; SMR = sequential motion rate; CVC = consonant–vowel–consonant; WAB = Western Aphasia Battery.
Kinematic Measures
Articulatory kinematic data were preprocessed using a custom MATLAB-based program, Speech Movement Analysis for Speech and Hearing research (Green, Wang, & Wilson, 2013). Files were trimmed to exclude all extraneous movements of articulators before and after task performance. All kinematic analyses were focused exclusively on lip movement, as measured by the 3D Euclidean distance between the upper lip and the lower lip. Five kinematic measures were derived, including (a) total duration, measured for both the AMR and SMR DDK tasks; (b) number of cycles, measured for both AMR and SMR DDK tasks; (c) rate, calculated as number of cycles per duration, for AMR and SMR tasks; (d) spatiotemporal index (STI; i.e., variability in movement trajectory, as measured by the cumulative sum of standard deviations across individual cycles; Smith et al., 1995), measured for the SMR task only; and (e) maximum velocity, measured for the AMR task only (see Table 2). STI was measured for SMR because the increased sequencing demands of this task (cf. AMR) are more likely to elicit atypical spatiotemporal coupling patterns characteristic of AOS. By contrast, maximum velocity was measured for AMR so as not to confound this intended measure of articulator speed with sequencing task demands. All kinematic measures were extracted using a MATLAB-based algorithmic approach developed by Rong et al. (2018). The algorithm first uses automatic peak detection to identify individual cycles and then automatically extracts 21 lip movement features, including the five selected for analysis in this study, which were hypothesized to be sensitive to the presence of dysarthria and/or AOS.
Reliability of Quantitative Speech Measures
Acoustic and kinematic variables were remeasured for 20% of participants (two participants each in the healthy control, MSI+, and MSI− groups) by an independent second rater in order to calculate interrater reliability. The second independent rater was trained in acoustic and kinematic analysis and blinded to group membership. Reliability for each measure was assessed using interclass correlation coefficients (ICCs) to index agreement between the first and second raters. Following established guidelines for ICC model selection (Koo & Li, 2016; McGraw & Wong, 1996), we calculated ICC(C,1). This ICC type is a two-way random effects model measuring the degree of consistency between raters. This ICC type assumes a single rater, as that is the measurement protocol in actual application (i.e., in practice, acoustic and kinematic measurements would be calculated by a single rater and not an average of multiple raters). ICCs were in the good-to-excellent range for all 15 acoustic and kinematic variables, ranging from .79 (formant centralization ratio) to 1.0 (duration of sustained vowel) for variables that required at least partial manual parsing or decision making (e.g., demarcation of start/end boundaries, supervision of a semi-automated Praat script). CPPS was calculated via fully automated, unsupervised script, and thus, interrater agreement for this variable was exact (ICC = 1.0). ICCs and associated 95% confidence intervals are shown for all variables in Supplemental Material S1.
Quantitative Speech Measure Grouping
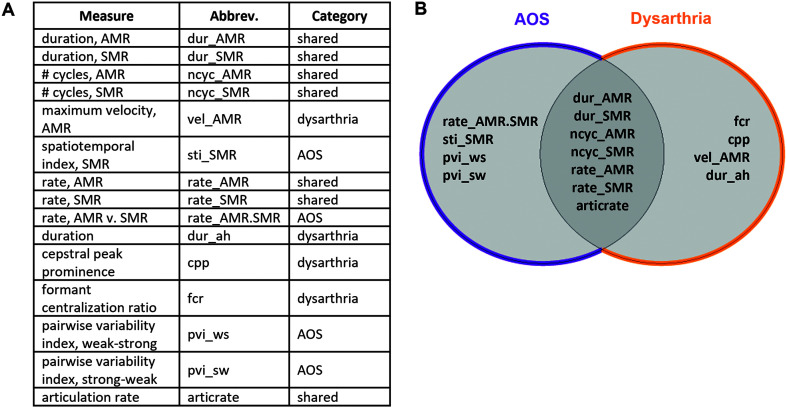
We first grouped the 15 quantitative variables according to whether they were hypothesized to capture speech deficits specific to AOS (“AOS features”) or dysarthria (“dysarthria features”), or could plausibly reflect either (“shared features”). We based these feature groupings on findings from prior research associating specific features with AOS and/or dysarthria. AOS features included those related to equal/excess stress patterns (e.g., pairwise variability index), movement variability (e.g., STI), and difficulty with sound sequencing (e.g., relative rate of AMR vs. SMR tasks; Allison et al., 2020; Ballard et al., 2014; Basilakos et al., 2017; Duffy, 2013; Haley et al., 2012, 2021; Mauszycki et al., 2005; Vergis et al., 2014). Dysarthria features included those related to vowel space reduction (e.g., formant centralization ratio), abnormal vocal quality (e.g., CPPS), reduced articulator speed (e.g., maximum velocity of articulators), and respiratory insufficiency (e.g., sustained vowel duration; Allison et al., 2017; Fletcher et al., 2017; Kim et al., 2011; Lee et al., 2014; Šimek & Rusz, 2021). Shared features included more general indicators of motor speech impairment (e.g., slowed rate, reduced speech amount) not uniquely attributable to either AOS or dysarthria. Figure 2 illustrates the grouping schema for all quantitative variables. Importantly, assignment of speech features to AOS and/or dysarthria groupings was based primarily on the available literature demonstrating the utility of a given feature to identify AOS or dysarthria, typically compared with a healthy control population, and is limited by a lack of available literature that reports both sensitivity and specificity metrics with regard to differentiating AOS and dysarthria (Allison et al., 2020). For this reason, we also used a data-driven approach (see Principal Component Analysis section) wherein individual quantitative features were entered into an analysis without any grouping assumptions.
Figure 2.
Classification and grouping schema for quantitative speech measures. (A) Listing of all quantitative speech measures, including abbreviation and category assignment. (B) Graphical representation of feature category assignment, indicating “AOS features” (left), “shared features” (overlap), and “dysarthria features” (right). AMR = alternating motion rate; AOS = apraxia of speech; SMR = sequential motion rate.
Quantitative Speech Measure z-Score Derivation
All patients' individual scores for each of the 15 variables were converted to z scores with reference to the mean and standard deviation (SD) of the healthy controls. For variables known to vary significantly by gender, including formant and voice-related measures, the z-score reference was the gender-matched healthy control mean/standard deviation. All z scores were expressed as an absolute value in order to equate deviations from normal in either direction. For each individual, a |z| mean was calculated per category (i.e., AOS, dysarthria, shared) by averaging |z| scores of the component variables in that category. A mean |z| score ≥ 2—reflecting extreme ends (±2.5%) of the normal distribution—in any feature category was the cutoff score used to determine the presence/absence of overall motor speech impairment for each patient based on quantitative speech features. The mean |z| across all features was used as a quantitative proxy for overall motor speech impairment when (a) comparing group means between the MSI+ and MSI− groups and (b) correlating with standardized measures of intelligibility from the SIT.
Independent Auditory-Perceptual Ratings
SLP Raters
Two certified, licensed SLPs made ratings of motor speech impairment for each study participant. Both SLP raters had extensive clinical and/or research training in assessing motor speech disorders in adults. For one rater, this training included over a decade of clinical experience in acute, hospital-based settings, including assessment and treatment of motor speech disorders across a wide variety of disease presentations (e.g., poststroke aphasia, Parkinson's disease, amyotrophic lateral sclerosis). For the other rater, training included 8 years of intensive clinical and research training working primarily with individuals with a variety of neurological conditions resulting in motor speech disorders (i.e., amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, stroke). SLP raters made judgments independent of each other and blinded to the diagnosis (e.g., nfvPPA, PPAOS) and subgrouping (MSI+, MSI−) of individuals. Importantly, these SLPs were not the same as those administering the in-clinic speech (i.e., clinical gestalt) assessment that was used to evaluate study inclusion/exclusion criteria and subgroup patients into MSI+ and MSI− study groups.
Rating Procedure
Ratings of motor speech impairment were made using an online survey created in REDCap (Harris et al., 2009). Supplemental Material S2 shows the REDCap survey as it appeared to raters. As part of the online survey, raters completed a brief training module in which they listened to researcher-selected speech samples chosen to exemplify (a) AOS, (b) dysarthria, and (c) comorbid AOS and dysarthria. Two exemplars were chosen per category, one representing a mild severity presentation and the second representing a moderate/severe presentation. Definitions of AOS and dysarthria (Duffy, 2013) were also provided as part of the training.
After completing the training, the first part of the rating procedure instructed raters to listen to blinded speech samples for each participant, including the picture description and DDK (AMR + SMR) tasks. Raters were asked to rate overall speech impairment on a 0–3 scale (0 = no impairment, 0.5 = questionable/very mild, 1 = mild, 2 = moderate, 3 = severe), with operational definitions provided per severity category (see Supplemental Material S2). This rating rubric was modeled after the Progressive Aphasia Severity Scale, a similar clinician rating scale used to rate symptom severity in progressive aphasia (Sapolsky et al., 2014). For any participants rated ≥ 0.5 (i.e., indicating presence of speech impairment), SLPs were asked to provide follow-up ratings indicating the type of motor speech impairment (AOS, dysarthria, comorbid, other) and severity (questionable/very mild, mild, moderate, severe). This embedded branching logic is displayed in Supplemental Material S3. SLPs also estimated intelligibility (0–100 using a visual analog scale) and indicated which speech features—selected from a researcher-provided list of common apraxia and dysarthria features—were most salient for a given participant.
In the second part of the rating procedure, which was conducted on patient participants only, raters were provided with more detailed samples of recorded audio/video on which to judge more fine-grained aspects of motor speech impairment. The results of this extended rating procedure are beyond the scope of the current study, but importantly, the full procedure for all study participants took each SLP rater approximately 8–10 total hours. To address potential confounds of rater fatigue and order effects, participant order was randomized per rater. Although raters had the option to complete all ratings in one sitting, both opted to distribute the ratings across two to three separate sittings (no more than 1 day apart). Raters were instructed to fully complete ratings for a given participant before concluding a rating session. The training module and exemplar samples were available during each rating session.
Reliability of Auditory-Perceptual Ratings
Interrater and intrarater reliability were measured using weighted Cohen's kappa statistics. To derive intrarater reliability, clinicians rerated four out of 30 total participants (including both patients and healthy controls randomly selected from the pool of speakers). Interrater agreement was calculated across all participants since both raters independently rated each participant. Interrater agreement between the two raters was good for overall motor speech severity (weighted Cohen's κ = 0.82) and fair for AOS severity (κ = 0.58) and dysarthria severity (κ = 0.54). Intrarater agreement was excellent overall (Rater 1: κ = 0.89, 0.86, and 1.00; Rater 2: κ = 1.00, 0.86, and 1.00—for overall motor speech, AOS, and dysarthria severity, respectively).
Intelligibility Assessment
In addition to auditory-perceptual ratings of motor speech impairment, a standardized assessment of speech intelligibility (i.e., the SIT) was also administered to all study participants. A research assistant listened to the recorded audio of the SIT and transcribed what she heard using English orthography. The research assistant was unfamiliar with the participants and was blinded to diagnosis and subgrouping. She had modest prior experience listening to disordered speech for laboratory projects focused on individuals with amyotrophic lateral sclerosis. Scores on the SIT were derived, expressed as a percentage of words correctly transcribed. Although transcriptions for the SIT can be variable across raters, several prior studies have reported strong intrarater and interrater reliability for orthographic transcription of the SIT (Stipancic et al., 2018, 2021). The SIT is used in this study as a secondary measure against which quantitative and auditory-perceptual results are compared. Specifically, the mean |z| score across all quantitative features per individual was correlated (Spearman's rho) with individual SIT intelligibility scores to assess the validity of the quantitative measures to capture the overall severity of motor speech impairment. Auditory-perceptual ratings of overall motor speech impairment were also correlated with SIT intelligibility scores for comparison.
Principal Component Analysis
In addition to deriving individual profiles of motor speech impairment, we used a data-driven principal component analysis (PCA) to investigate the relative contributions of each quantitative feature toward explaining variance of speech performance across all study participants. Individual raw scores per quantitative speech variable were entered into a PCA, implemented in R (Version 4.0.2; R Core Team, 2021). Individual scores were standardized and centered, and individual orthogonal components were extracted. Factors with eigenvalues < 1 were excluded from results. Sample size adequacy was assessed using both Bartlett's test of sphericity and the Kaiser–Meyer–Olkin (KMO) measure.
Summary of Study Analyses
Because we hypothesized heterogenous profiles of motor speech impairment in the MSI+ group, our analyses focused on characterizing individual patients with quantitative speech measures, independent auditory-perceptual ratings, and the congruence of these two approaches for identifying motor-speech–impaired individuals and their predominant impairment (i.e., AOS vs. dysarthria). Secondarily, we also evaluated the validity of both quantitative and auditory-perceptual measures to identify overall motor speech impairment severity compared with a gold-standard measure of intelligibility (i.e., transcription of the SIT). Finally, we employed a data-driven PCA using all quantitative speech measures as input, free of assumptions about individual diagnoses or subgroup assignments.
Results
Quantitative Speech Results
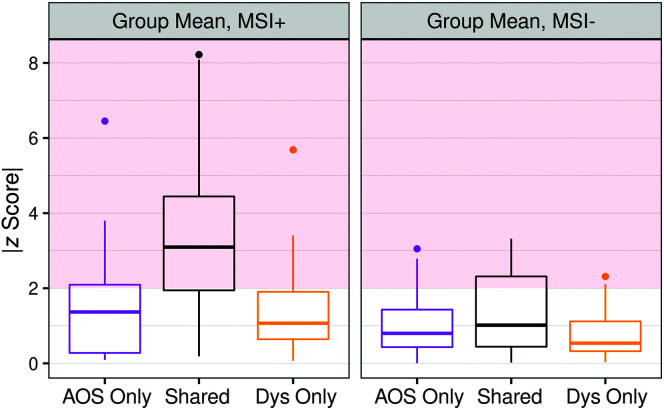
Analysis of the z-score transformed quantitative speech measures (which were grouped into “shared,” “AOS,” and “dysarthria” features, as described in the Method section) suggests significantly greater levels of overall motor speech impairment—as measured by the mean |z| of all features—in the MSI+ group (M |z| = 2.64, SD = 1.85) than in the MSI− group (M |z| = 1.15, SD = 0.93), t(428) = 10.19, p < .001. Ten out of 11 patients in the MSI+ group were identified as being motor speech impaired, defined as having |z| mean > 2 in the any feature category. Within the MSI− group, only two patients were identified as being motor speech impaired (i.e., |z| mean > 2 in the any feature category). t Tests comparing the MSI+ versus MSI− groups revealed that the MSI+ group had significantly greater quantitative AOS severity, t(211) = 7.07, p < .001, as well as dysarthria severity, t(215) = 7.33, p < .001, compared with the MSI− group. For these analyses, AOS severity was calculated as the mean |z| of AOS + shared features, whereas dysarthria severity was calculated as the mean |z| of dysarthria + shared features. Figure 3 shows the mean |z| scores for each of the three feature categories, in the MSI+ as compared with MSI− group. Supplemental Materials S5 and S6 show the mean |z| scores per individual, in the MSI+ and MSI− groups, respectively.
Figure 3.
Quantitative profiles of motor speech impairment across each of the three feature categories for the MSI+ group as compared with the MSI− group. Red shaded area at |z| ≥ 2 indicates cutoff for atypical (relative to healthy control mean) values. AOS = apraxia of speech; Dys = dysarthria.
Within the MSI+ group, quantitative speech features showed that AOS was the predominant impairment (i.e., mean |z| AOS > mean |z| dysarthria) for seven of these patients, with syndromic diagnoses of PPAOS (three patients), nfvPPA (two patients), and CBS (two patients). Dysarthria was the predominant impairment for the remaining four patients, who had syndromic diagnoses of PSP (two patients), nfvPPA (one patient), and PPAOS (one patient). Table 3 summarizes the quantitative speech results per individual and by group.
Table 3.
Summary of quantitative acoustic and kinematic speech measures.
| Group | Case | Syndrome dx | Mean |z|, all features | Mean |z|, shared features | Mean |z|, AOS features | Mean |z|, dysarthria features | Quantitative MSI predominance (AOS + , Dys + ) a |
|---|---|---|---|---|---|---|---|
| MSI− | 1 | lvPPA | 0.64 | 0.56 | 0.68 | 0.87 | — |
| 2 | lvPPA | 0.70 | 0.55 | 1.05 | 0.72 | — | |
| 3 | lvPPA | 1.24 | 1.94 | 0.78 | 0.34 | — | |
| 4 | lvPPA | 1.75 | 2.51 | 1.47 | 0.43 | — | |
| 5 | lvPPA | 0.91 | 0.87 | 1.04 | 0.89 | — | |
| 6 | lvPPA | 0.84 | 1.13 | 0.46 | 0.48 | — | |
| 7 | lvPPA | 1.59 | 2.15 | 1.24 | 0.98 | — | |
| 8 | lvPPA | 1.20 | 1.18 | 1.59 | 0.87 | — | |
| 9 | lvPPA | 1.05 | 0.89 | 1.39 | 1.00 | — | |
| Group M (SD), MSI− | 1.15 (0.93) | 1.31 (1.00) | 1.01 (0.8) | 0.74 (0.57) | — | ||
| MSI+ | 10 | PPAOS | 1.64 | 2.64 | 0.16 | 1.08 | Dys+ |
| 11 | nfvPPA | 2.51 | 3.34 | 2.67 | 2.23 | AOS+ | |
| 12 | CBS | 1.48 | 1.31 | 3.43 | 1.98 | AOS+ | |
| 13 | PSP | 0.95 | 1.26 | 0.32 | 1.20 | Dys+ | |
| 14 | nfvPPA | 1.85 | 2.76 | 1.40 | 1.18 | AOS+ | |
| 15 | PSP | 2.46 | 3.63 | 0.68 | 1.43 | Dys+ | |
| 16 | PPAOS | 2.61 | 4.46 | 1.30 | 0.34 | AOS+ | |
| 17 | PPAOS | 2.16 | 3.60 | 1.01 | 0.77 | AOS+ | |
| 18 | PPAOS | 2.48 | 3.77 | 1.41 | 1.04 | AOS+ | |
| 19 | CBS | 3.76 | 4.74 | 3.52 | 2.21 | AOS+ | |
| 20 | nfvPPA | 3.94 | 4.98 | 3.18 | 8.38 | Dys+ | |
| Group M (SD), MSI+ | 2.64 (1.85) | 3.30 (1.81) | 1.46 (1.29) | 1.38 (1.13) | — | ||
Note. Em dashes indicate data not reported. dx = diagnosis; AOS = apraxia of speech; Dys = dysarthria; lvPPA = logopenic variant primary progressive aphasia; MSI+ = motor-speech–impaired group of individuals with nfvPPA, PPAOS, CBS, or PSP and suspected four-repeat tauopathy pathology; PPAOS = primary progressive apraxia of speech; MSI− = non–motor-speech-impaired group of individuals with logopenic variant primary progressive aphasia; nfvPPA = nonfluent variant primary progressive aphasia; CBS = corticobasal syndrome; PSP = progressive supranuclear palsy.
Predominant impairment based on quantitative speech measures, rated for the MSI+ group only. Predominance determined from a ratio of mean |z|, AOS features to mean |z|, dysarthria features. AOS + (|z|AOS > |z|dys) indicates predominant apraxic impairment; Dys + (|z|dys > |z|AOS) indicates predominant dysarthric impairment.
Independent Auditory-Perceptual Rating Results
All 11 patients in the MSI+ group were rated as having some degree of overall motor speech impairment based on the averaged scores of the two SLP raters (mean severity = 1.61, SD = 1.01, range: 0.25–3). Ten out of 11 (91%) patients were designated as having some degree of AOS (mean severity = 1.07, SD = 0.81, range: 0–2.5); 10 out of 11 (91%) were also identified as having some degree of dysarthria (mean severity = 1.18, SD = 1.19, range: 0–3). Of the 11 MSI+ patients, six were designated as having primary AOS (i.e., mean rated AOS severity > mean rated dysarthria severity), four were designated as having primary dysarthria (i.e., mean rated dysarthria severity > mean rated AOS severity), and one was designated as having no predominance for either AOS or dysarthria (i.e., mean rated dysarthria severity = mean rated AOS severity). In the primary AOS subgroup, the motor speech features that were noted as present for a majority of patients included slowed rate (6/6 patients), inconsistent sound distortions (4/6 of patients), speech initiation difficulty (4/6 patients), and syllable segregation (4/6 patients). In the primary dysarthria group, features noted as present for a majority of patients included slowed rate (4/4 patients) and consistent sound distortions (3/4 patients).
All nine patients in the MSI− group were also rated by clinicians as having some degree of overall motor speech impairment (mean severity = 0.69, SD = 0.49, range: 0.25–1.5), despite being designated by traditional clinical evaluation (i.e., initial gestalt clinical judgment used for subgrouping into the MSI− group) as having absent motor speech impairment. All patients in this group were designated as apraxic (mean severity = 0.86, SD = 0.64, range: 0.25–1.5). Additionally, four individuals in the MSI− group were rated as questionably dysarthric, wherein one clinician rater endorsed the presence of a mild dysarthria; none of these patients were unanimously rated as dysarthric by both clinicians. A summary of independent auditory-perceptual results—as well as SIT scores—are summarized per individual and by group in Table 4. Additional details on these rating results are given in Supplemental Material S4.
Table 4.
Summary of auditory-perceptual ratings of motor speech impairment.
| Group | Case | Syndrome dx | SIT score (%) | Overall MSI severity | AOS severity | Dysarthria severity | Auditory-perceptual MSI predominance (AOS + , Dys + ) a |
|---|---|---|---|---|---|---|---|
| MSI− | 1 | lvPPA | 100 | 0.75 | 1 | 0 | — |
| 2 | lvPPA | 99 | 0.25 | 0.25 | 0 | — | |
| 3 | lvPPA | 98 | 0.25 | 0.25 | 0 | — | |
| 4 | lvPPA | 95 | 1 | 1 | 0.5 | — | |
| 5 | lvPPA | 98 | 1 | 1.5 | 0.5 | — | |
| 6 | lvPPA | 96 | 0.25 | 0.25 | 0 | — | |
| 7 | lvPPA | 92 | 0.5 | 0.75 | 0 | — | |
| 8 | lvPPA | 99 | 0.75 | 1.25 | 0.5 | — | |
| 9 | lvPPA | 95 | 1.5 | 1.5 | 0.5 | — | |
| Group M (SD), MSI− | 97.1 (2.6) | 0.69 (0.49) | 0.86 (0.64) | 0.22 (0.43) | — | ||
| MSI+ | 10 | PPAOS | 91 | 0.75 | 0.5 | 0.75 | Dys+ |
| 11 | nfvPPA | 94 | 1.5 | 1.5 | 0.5 | AOS+ | |
| 12 | CBS | 71 | 2 | 2 | 0.25 | AOS+ | |
| 13 | PSP | 97 | 0.25 | 0.25 | 0 | AOS+ | |
| 14 | nfvPPA | 94 | 0.75 | 0.75 | 0.25 | AOS+ | |
| 15 | PSP | 5 | 3 | 0 | 3 | Dys+ | |
| 16 | PPAOS | 100 | 1.5 | 1 | 0.75 | AOS+ | |
| 17 | PPAOS | 98 | 1 | 1 | 0.5 | AOS+ | |
| 18 | PPAOS | 96 | 1 | 0.75 | 0.75 | = | |
| 19 | CBS | 71 | 3 | 2.5 | 3 | Dys+ | |
| 20 | nfvPPA | 2 | 3 | 1.5 | 3 | Dys+ | |
| Group M (SD), MSI+ | 74.3 (36.6) | 1.61 (1.01) | 1.07 (0.81) | 1.18 (1.19) | — | ||
| Healthy controls (HC) | HC1 | — | 100 | 0 | 0 | 0 | — |
| HC2 | — | 100 | 0 | 0 | 0 | — | |
| HC3 | — | 95 | 0 | 0 | 0 | — | |
| HC4 | — | 100 | 0 | 0 | 0 | — | |
| HC5 | — | 100 | 0 | 0 | 0 | — | |
| HC6 | — | 98 | 0 | 0 | 0 | — | |
| HC7 | — | 97 | 0 | 0 | 0 | — | |
| HC8 | — | 97 | 0.5 | 0 | 0.25 | — | |
| HC9 | — | 99 | 0.25 | 0.25 | 0 | — | |
| HC10 | — | 99 | 0 | 0 | 0 | — | |
| Group M (SD), HC | 98.6 (1.6) | 0.08 (0.18) | 0.03 (0.11) | 0.03 (0.11) | — | ||
Note. Em dashes indicate data not reported. dx = diagnosis; SIT = Speech Intelligibility Test; AOS = apraxia of speech; Dys = dysarthria; MSI− = non -motor-speech-impaired group of individuals with logopenic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia; MSI+ = motor-speech–impaired group of individuals with nfvPPA, PPAOS, CBS, or PSP and suspected four-repeat tauopathy pathology; PPAOS = primary progressive apraxia of speech; nfvPPA = nonfluent variant primary progressive aphasia; CBS = corticobasal syndrome; PSP = progressive supranuclear palsy.
Predominant impairment based on independent auditory-perceptual ratings, rated for the MSI+ group only. Predominance determined from a ratio of mean (across two speech-language pathologist raters) scores for AOS to dysarthria severity. “AOS+” (i.e., mean rated severity AOS > Dys) indicates predominant apraxic impairment; “Dys+” (i.e., mean rated severity Dys > AOS) indicates predominant dysarthric impairment; “=” (i.e., mean rated severity AOS = Dys) indicates no predominance of either apraxia or dysarthria.
Comparing Independent Auditory-Perceptual Ratings and Quantitative Results
There was moderately strong agreement in predominant impairment as determined separately by independent auditory-perceptual rating and quantitative feature z scores: eight out of 11 patients in the MSI+ group were classified into the same predominant impairment category (e.g., AOS+, Dys+) using these two different and independent approaches (see Table 5). Two patients were categorized in opposite categories, although notably these cases of disagreement occurred in the context of very mild or questionable impairment on the one hand (Case 13) or very severe comorbid impairment on the other (Case 19). One patient was not assigned a clinician-based predominance category because severity scores were exactly equal for AOS and dysarthria; therefore, the agreement between clinician ratings and quantitative z scores could not be assessed in this case.
Table 5.
Comparison of study-determined MSI predominance: quantitative speech measures versus auditory-perceptual ratings (MSI+ group only).
| Group | Case | Syndrome dx | Quantitative MSI predominance (AOS + , Dys + ) a | Auditory-perceptual MSI predominance (AOS + , Dys + ) b |
|---|---|---|---|---|
| MSI+ | 10 | PPAOS | Dys + | Dys + |
| 11 | nfvPPA | AOS + | AOS + | |
| 12 | CBS | AOS + | AOS + | |
| 13 | PSP | Dys+ | AOS+ | |
| 14 | nfvPPA | AOS + | AOS + | |
| 15 | PSP | Dys + | Dys + | |
| 16 | PPAOS | AOS + | AOS + | |
| 17 | PPAOS | AOS + | AOS + | |
| 18 | PPAOS | AOS + | = | |
| 19 | CBS | AOS+ | Dys+ | |
| 20 | nfvPPA | Dys + | Dys + |
Note. Bold text indicates agreement (bold = agree; nonbold = disagree) between predominance rating given by quantitative features as compared with auditory-perceptual rating. Italicized text (Case 18) indicates that agreement could not be assessed because auditory-perceptual ratings did not establish a predominance of either AOS or dysarthria. dx = diagnosis; AOS = apraxia of speech; Dys = dysarthria; MSI+ = motor-speech–impaired group of individuals with nfvPPA, PPAOS, CBS, or PSP and suspected four-repeat tauopathy pathology; PPAOS = primary progressive apraxia of speech; nfvPPA = nonfluent variant primary progressive aphasia; CBS = corticobasal syndrome; PSP = progressive supranuclear palsy.
Predominant impairment based on quantitative speech features, rated for the MSI+ group only. Predominance determined from a ratio of mean |z|, AOS features to mean |z|, dysarthria features. AOS + (|z|AOS > |z|dys) indicates predominant apraxic impairment; Dys + (|z|dys > |z|AOS) indicates predominant dysarthric impairment.
Predominant impairment based on independent auditory-perceptual ratings, rated for the MSI+ group only. Predominance determined from a ratio of mean (across two speech-language pathologist raters) scores for AOS to dysarthria severity. “AOS+” (i.e., mean rated severity AOS > Dys) indicates predominant apraxic impairment; “Dys+” (i.e., mean rated severity Dys > AOS) indicates predominant dysarthric impairment; “=” (i.e., mean rated severity AOS = Dys) indicates no predominance of either apraxia or dysarthria.
Overall, the quantitative feature scores were more specific for identifying motor speech impairment than were the independent auditory-perceptual ratings. Within the MSI− group, only two patients were identified as being motor speech impaired (i.e., mean z score ≥ 2 in any feature category) using the quantitative feature approach, compared with all nine patients identified by clinicians as having motor speech impairment.
When clinician ratings and quantitative feature z scores (specifically mean |z| for all features) were compared individually with standardized intelligibility scores from the SIT, results revealed a moderately strong inverse relation to intelligibility for quantitative measures (r = −.60, p = .005) and a strong inverse relation to intelligibility for clinician ratings (r = −.80, p < .001). This finding suggests that intelligibility may account for much of the variance in clinician severity ratings but that quantitative measures may be somewhat less dependent on overall speech intelligibility as rated by clinicians.
PCA Results
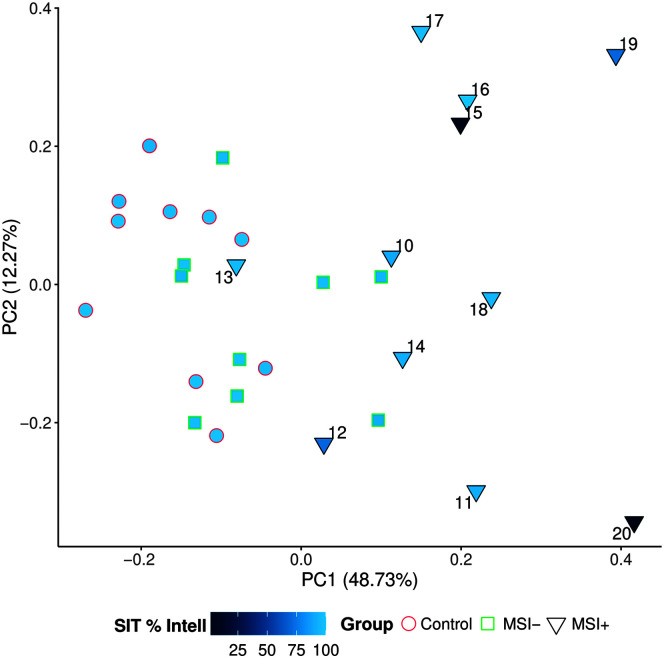
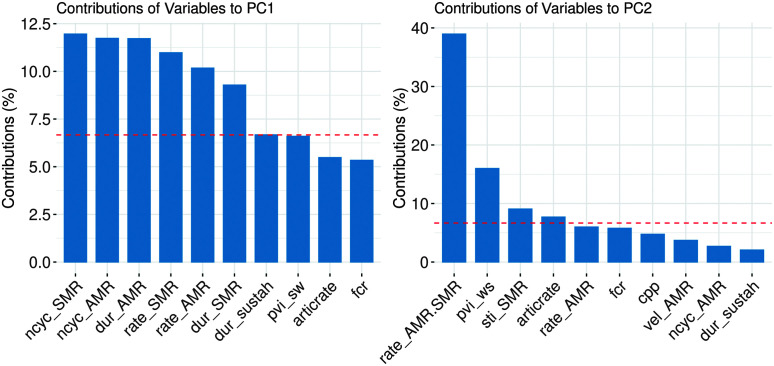
Statistical analysis of sample adequacy for the PCA revealed correlations between individual measures to be sufficient (Bartlett's test = 441.8, p < .001) and the overall sample size to be adequate for the PCA (KMO = 0.65). Nevertheless, due to the relatively small sample size in the current analysis, we consider the following results exploratory. Following the exclusion of PCA component factors with eigenvalues < 1, four individual component factors resulted from the PCA, which together accounted for 77% of the total variance (Component 1 [C1]: 48.73%, Component 2 [C2]: 12.27%, Component 3: 8.78%, Component 4: 6.85%). We focused our analysis on the first two component factors (explaining 61% of the variance), as shown in Figure 4, with group and SIT intelligibility scores superimposed. For patients in the MSI+ group, case number is also displayed. We further investigated the loading onto each of these two component factors as a way of interpreting which of the quantitative measures are driving dispersion in the data. Figure 5 shows the top 10 measures that load onto C1 and C2, respectively. For C1, measures that load heavily include DDK-based amount and rate variables (i.e., duration, number of cycles, and rate for the AMR and SMR tasks) that are all features in the “shared” category and would be expected to be affected in both AOS and dysarthria. We thus interpret C1 to capture “Overall Severity.” For C2, the heaviest loading measures include relative rate of AMR:SMR, pairwise variability index (for weak–strong words), STI, and articulation rate. Because at least three of four (i.e., all except articulation rate) of these measures are considered primary AOS features, we consider C2 to capture “AOS Severity.”
Figure 4.
Principal component analysis results show stratification of MSI+ patients and reflect within-group heterogeneity in severity and speech features. Gray arrows indicate individual loadings for each quantitative variable. Group membership was connoted by shape and outline color, fill shaded based on Sentence Intelligibility Test (SIT) scores (%). For the MSI+ group only, individual patients are indicated with case numbers for cross-referencing purposes. PC1 = Component 1 (% variance explained); PC2 = Component 2 (% variance explained).
Figure 5.
Individual speech measures load differentially on Components 1 and 2, reflecting “Overall Severity” (C1) and “AOS Severity” (C2). Reference dashed red line indicates expected value (%) if all variable contributions were uniform. PC1 = Component 1; PC2 = Component 2; AMR = alternating motion rate; SMR = sequential motion rate.
Discussion
In this exploratory study, we profiled quantitative and clinician-rated auditory-perceptual features of motor speech impairment in a group of patients with suspected 4RT neuropathology. Quantitative speech measures were generally in concordance with independent auditory-perceptual ratings of motor speech impairment severity, and higher quantitative speech z scores (i.e., more disordered) were associated with reduced intelligibility. Moreover, results indicate that hypothesis-driven groupings of quantitative measures suggest differentiation between predominantly apraxic versus predominantly dysarthric presentations within the MSI+ group. Results from an exploratory PCA provide additional evidence for differential profiles of motor speech impairment in the MSI+ group; heterogeneity across individuals is explained in large part by varying levels of overall severity—captured by the shared feature variable group—and degree of apraxia severity, as measured by the AOS feature variable group.
Quantitative Speech Measures Differentiate Speech From Language Impairment
Differentiating speech impairment from language impairment is a long-standing clinical challenge in populations with comorbid impairments, including many 4RT-associated syndromes (Josephs et al., 2012). The challenge of comorbid speech and language presentations in PPA is well known, but recent literature has also documented significant language impairments in PSPs and CBS as well (Catricalà et al., 2019; Peterson et al., 2021). In the current study, WAB-R Aphasia Quotient (AQ) scores (see Table 1) showed that all nine patients within the MSI− group had aphasia (i.e., AQ < 93.8), as did six of 11 patients in the MSI+ group, including individuals with diagnoses of nfvPPA, PSPs, and CBS. Of particular importance is the nature of aphasic deficit the MSI− group: Consistent with their syndromic diagnosis of lvPPA, all nine patients in this group had a phonological impairment. Prior work in the PPA literature specifically has identified the challenge in differentiating phonological impairment common in the lvPPA subgroup from AOS common in the nfvPPA subgroup because these two impairment types share similar surface speech features (e.g., inconsistent sound distortions, difficulty in sequencing sounds; Ballard et al., 2014; Cordella et al., 2017; Haley et al., 2021).
The independent auditory-perceptual rating findings further illustrate this challenge, as all nine patients in the MSI− group were rated as being mildly motor speech impaired and further subclassified as mildly apraxic by two expert SLP raters. By contrast, using a standard z-score cut point (z ≥ 2) for the quantitative speech measures, only two of the nine patients in the MSI− group were identified as motor speech impaired. Importantly, in order to be assigned to the MSI− group at study outset, patients were evaluated in a clinical setting by a multidisciplinary team that included at least one SLP with expertise in neurodegenerative syndromes and were determined by consensus to have absent motor speech impairment. Therefore, current findings make clear a point of disagreement between the initial clinical classifications—which we treated as ground truth for reasons detailed in the Method section—and the blinded auditory-perceptual ratings made by independent SLP raters in our study. We interpret this apparent inconsistency as highlighting the limitations of subjective ratings, which have been shown in prior literature to be prone to high false-positive rates of nearly 40% for identifying motor speech impairment among even neurotypical older control participants (Allison et al., 2017). False-positive identification of motor speech impairment is likely to occur at even higher rates in the presence of phonological impairment, as is the case in lvPPA. Indeed, in the current study, false-positive identification of motor speech impairment was markedly more common for the MSI− group (9/9 identified as motor speech impaired, corresponding to a 100% false-positive rate) compared with the healthy control group (2/10 identified as motor speech impaired, corresponding to a 20% false-positive rate).
Both independent auditory-perceptual ratings and quantitative speech measures appeared to effectively identify motor speech impairment in the MSI+ group. The presence of motor speech impairment was questionable for one of the 11 MSI+ patients (Case 13) based on both the quantitative and independent auditory-perceptual measures. For this patient, quantitative scores did not pass the threshold to constitute motor speech impairment. In addition, one of the SLPs assigned a normal speech rating. Overall, results suggest that both independent auditory-perceptual speech ratings made by clinicians and quantitative speech measures have the potential for high sensitivity in identifying motor speech impairment but that quantitative measures appear more promising in terms of specificity (cf. phonological impairment). We believe the low specificity of the blinded auditory-perceptual ratings for identifying motor speech impairment—and specifically the tendency for even these highly trained raters to endorse motor speech impairment among individuals with phonological impairment—reflects the essential difficulty of differentiating motor speech (especially apraxic) features from phonologic errors in connected speech.
Acoustic and Kinematic Speech Measures Capture Motor Speech Impairment Severity
Besides identifying the presence of motor speech impairment, it is important for candidate measures to reliably capture the severity of that impairment for accurate clinical staging and/or monitoring of disease progression. Results of the current study show that quantitative speech measures are related to intelligibility scores (used here as a proxy for motor speech impairment severity) such that more extreme quantitative scores are associated with reduced intelligibility. Although not unexpected, this result provides confirmatory evidence that quantitative measures of motor speech impairment are meaningfully and reliably related to a well-established perceptual construct of severity, namely, intelligibility.
It is also interesting to consider, however, the MSI+ individuals whose quantitative speech scores did not correlate well with intelligibility (i.e., outliers to the general trend). These cases appeared to follow two patterns where either (a) quantitative features did not capture the patients' specific and unique motor speech deficits or (b) the patient was perceptibly motor speech impaired but highly intelligible. Case 12 is a good example of the first pattern, where quantitative measures revealed only a mild impairment based on the mean |z| of all features despite markedly reduced intelligibility. This finding suggests that although the quantitative features used in this study reflect impairments across a broad range of motor speech impairments including AOS and many dysarthria subtypes, they are not universal and may not account fully for atypical motor speech presentations. The clinician ratings shed light on Case 12: At least one rater indicated the possibility of a fluency disorder appearing alongside a more traditional AOS presentation, which has been previously documented in individuals with CBS (Silbergleit et al., 2009). Cases 11 and 17 are good examples of the second pattern wherein motor-speech–impaired individuals have maintained intelligibility. In both cases, clinician ratings agreed with quantitative results that motor speech impairment was present, although quantitative features identified a greater severity of motor speech impairment compared with clinician ratings. This finding supports the efficacy of quantitative speech measures for detecting speech changes prior to declines in intelligibility. It also suggests that the relationship between motor speech severity and intelligibility is likely not linear and that small changes in severity may have little impact on intelligibility in mild stages but large effects in moderate–severe stages (Rong et al., 2015; Stipancic et al., 2021). Likewise, impairment subtype may matter: Cases 11 and 17 both carry a diagnosis of PPAOS, and independent auditory-perceptual ratings from the current study (see Supplemental Material S4) highlight several prosodic features as most salient to their speech presentations. It could be the case that individuals with prosodic-predominant (cf. phonetic-predominant) AOS maintain intelligibility to a greater degree, as articulatory precision may be preserved longer.
Quantitative Feature Groupings Dissociate to Reveal Heterogeneity in Motor Speech Subtypes Within the 4RT Group
A major aim of the current study was to profile the range of motor speech impairments in 4RT-associated syndromes to identify and distinguish between AOS and dysarthria. Results suggest that, within our small sample of patients, quantitative feature groupings (i.e., “shared,” “AOS,” “dysarthria”) did reveal differential profiles of motor speech impairment and, therefore, can give an indication of whether a particular patient is primarily apraxic versus dysarthric. For the majority of patients in the MSI+ group, the designation of predominant impairment agreed with the designation derived from independent auditory-perceptual ratings made by trained SLPs. For the two patients for whom there was disagreement regarding predominance, the disagreement was likely because the motor speech impairment was either very mild or very severe and therefore difficult to subtype. Case 13 is an example of a patient with a questionable or very mild motor speech impairment for whom quantitative speech measures did not meet the |z| score ≥ 2 criterion and for whom at least one SLP rater assigned as having no motor speech impairment. Thus, the disagreement regarding motor speech predominance likely reflects a paucity of observable and/or measurable speech features on which to judge AOS versus dysarthria. Case 19 is an example of a patient with severe overall motor speech impairment and severe, co-occurring AOS and dysarthria. Disagreement over predominance, in this case, may be attributable to the preponderance of salient features of both AOS and dysarthria. Further work will be required to determine whether there is a quantitative difference threshold that would help with the classification of predominant AOS versus dysarthria. Future work may also consider whether the difficulty of diagnostic distinction between predominant AOS versus dysarthria is conditioned by the subtype of either AOS or dysarthria, as the degree of featural overlap may range from modest (e.g., prosodic AOS and hypokinetic dysarthria) to substantial (e.g., prosodic AOS and spastic dysarthria).
Results from the exploratory PCA suggest that a data-driven approach—based on quantitative speech features and free of assumptions about patient group membership (i.e., MSI−, MSI+) or quantitative feature category (i.e., “AOS,” “dysarthria,” “shared”)—can capture heterogeneity of motor speech impairment in the MSI+ group. Results also shed light on which specific features are accounting for such heterogeneity. We identified that DDK-based amount and rate variables (i.e., duration, number of cycles, and rate for the AMR and SMR tasks) appeared to be a reliable proxy of overall severity, which explains much of the variability in our small sample. This is consistent with the original, hypothesis-driven assignment of these measures to the shared feature grouping based on the expectation of reduction in these measures in more severe cases of motor speech impairment regardless of impairment subtypes (i.e., AOS vs. dysarthria). Among MSI+ individuals, features such as relative rate of AMR:SMR, pairwise variability index (for weak–strong words), STI, and articulation rate show promise in separating out individuals with more and less prominent apraxia features (e.g., Cases 15, 16, and 17 as compared with Cases 11, 12, and 20); those with less prominent apraxic features tend to be those with prominent dysarthria. This data-driven grouping is thus also consistent with the initial assignment of these measures as AOS measures. Importantly though, AOS severity does not entirely explain the variance along the second PCA dimension, as illustrated by Case 19. Interestingly, this is also one of the cases in which there was disagreement between quantitative features' scores and clinician ratings over the predominance of AOS versus dysarthria. Cases such as these also point to the possibility of AOS subtypes (e.g., phonetic vs. prosodic; Utianski et al., 2018), which would not necessarily be well captured in the current study since our apraxia features were overwhelmingly prosodic.
Unlike apraxia features, dysarthria-specific features—including measures of formant centralization, cepstral peak prominence, maximum articulatory velocity, and maximum phonation duration—do not dissociate as robustly and specifically in the current study, which may indicate overlap between the dysarthria and shared feature groupings. This likely reflects the fact that dysarthria subtypes are more varied, and it is thus more difficult to identify a limited number of individual quantitative measures that capture the full range of potential dysarthric presentations. Additional work is required to elucidate the presence and characteristics of dysarthria in 4RT.
At this time, the literature on motor speech impairment in 4RT-associated syndromes is primarily focused on PPAOS and nfvPPA, with an emerging attention to PSPs and CBS. Taken together, this literature increasingly suggests that (a) speech/language features are important for diagnostic subgrouping across the spectrum of 4RT-associated syndromes and (b) motor speech features can be quite heterogeneous and often include both apraxic and dysarthric elements and even varied subtypes with AOS and dysarthria (Clark et al., 2021; Duffy et al., 2021; García et al., 2022; Josephs et al., 2021; Santos-Santos et al., 2016; Utianski et al., 2018). Preliminary results in the current study suggest the potential for quantitative speech measures to identify and characterize these heterogenous profiles of motor speech impairment among individuals diagnosed with 4RT-associated syndromes. Results are also informative in terms of evaluating the limits of predefined diagnostic categories, a point best illustrated by Case 10. Case 10 is an individual diagnosed with PPAOS on the account of an isolated motor-speech–predominant presentation in the absence of aphasia (WAB-R AQ = 96.6), cognitive, or gross motor symptoms. Notably, this same presentation does not preclude an nfvPPA diagnosis since current consensus criteria require either only a motor speech impairment or agrammatism for inclusion in this diagnostic category (Gorno-Tempini et al., 2011). However, a look at Case 10's motor speech profile, which is in agreement with the independent auditory-perceptual ratings from the SLPs, shows that they have dysarthria and no evidence of apraxia. An open question remains then of how best to account for progressive motor speech prominent presentations that are characterized by primary dysarthria rather than AOS. In any case, fine-grained characterization of motor speech impairment is likely to be of considerable value toward the goal of more reliable motor speech phenotyping (e.g., Rowe et al., 2021).
In the larger scope of the literature on 4RT-associated syndromes, fine-grained quantitative speech analysis also stands to inform ongoing efforts by several groups to more reliably link surface behaviors such as speech to underlying pathology (e.g., 4RT) and, perhaps even more importantly, to specific loci and patterns of spread of neurodegeneration. These efforts will increasingly involve not only highly sophisticated approaches to characterizing the biological disease profile but also the addition of detailed acoustic and/or kinematic speech analysis, particularly among speech-involved clinical phenotypes (see, e.g., García et al., 2022; Josephs et al., 2021; Utianski et al., 2021).
Limitations
This exploratory study was intended to elucidate the diversity of motor speech impairment subtypes across 4RT-associated syndromes. Our inclusion of individuals with varying syndrome diagnoses (i.e., PPAOS, nfvPPA, CBS, PSPs), while allowing us the opportunity to explore heterogeneity in tau-related motor speech impairment, prevents us from making more definitive conclusions about which subtypes of motor speech impairment are more likely to occur in each of these syndromes. Specifically, we acknowledge that, within the MSI+ group, the intragroup subclassifications based on syndrome diagnoses are quite small (i.e., four PPAOS, three nfvPPA, two PSPs, two CBS). For this reason, we have focused our analyses on both the individual patient level and broad between-groups comparisons between the MSI− and MSI+ groups. We do not attempt to draw any conclusions about syndrome-specific patterns of motor speech impairment but rather focus interpretation on the utility of quantitative features to detect different types of motor speech impairment among individuals with 4RT-associated syndromes. Future work is needed to evaluate the utility of these measures to characterize syndrome-specific patterns of impairment beyond the individual level that is the focus of the current study.
The data-driven analysis provided evidence for the utility of quantitative measures for differentiating among individuals with different types of motor speech impairment. Additional research is required to determine the generalizability of these findings to other samples. Data-driven approaches are a promising way to evaluate the diagnostic potential of many individual candidate quantitative measures, without relying on predefined feature categories (“AOS,” “dysarthria,” “shared”) into which individual features may not fit neatly. We acknowledge, for instance, that certain measures may be sensitive, but not necessarily specific, to a given impairment type (e.g., pairwise variability index being reduced in dysarthria and AOS; Liss et al., 2009). Another improvement on the current data-driven analysis would be to include more candidate quantitative features for analysis to ensure that analyses capture even more fine-grained patterns of impairment, such as the differentiation of prosodic versus phonetic AOS. In the current study, our AOS-specific quantitative features were overwhelmingly prosodic in nature and thus may have biased results toward the identification of prosodic-predominant (cf. phonetic-predominant) AOS presentations.
Lastly, in the current study, we compared quantitative speech measures with both blinded auditory-perceptual ratings of motor speech impairment and a gold-standard measure of speech intelligibility in order to provide information on how well quantitative measures align with these more commonly used metrics for identifying motor speech impairment. These metrics are a useful starting point of comparison that highlight potential advantages for quantitative features. Future work should improve on the rating procedures used in this pilot study (i.e., only two SLPs for auditory-perceptual ratings, one transcriber for SIT), which may have been influenced by variability across raters and transcribers. Although interrater reliability for auditory-perceptual ratings was fair to good in this study, a more methodologically robust rating and transcription procedure would nonetheless be needed in order to draw more definitive conclusions about the relative advantages and disadvantages of quantitative assessment versus subjective clinician ratings of motor speech impairment.
Author Contributions
Claire Cordella: Conceptualization (Equal), Data curation (Lead), Formal analysis (Lead), Funding acquisition (Equal), Validation (Equal), Writing – original draft (Lead), Writing – review & editing (Lead). Sarah E. Gutz: Formal analysis (Supporting), Validation (Equal), Writing – review & editing (Supporting). Marziye Eshghi: Conceptualization (Supporting), Formal analysis (Supporting), Methodology (Supporting), Writing – review & editing (Supporting). Kaila L. Stipancic: Formal analysis (Supporting), Methodology (Supporting), Writing – review & editing (Supporting). Megan Schliep: Formal analysis (Supporting), Methodology (Supporting), Writing – review & editing (Supporting). Bradford C. Dickerson: Conceptualization (Supporting), Funding acquisition (Equal), Resources (Supporting), Writing – review & editing (Supporting). Jordan R. Green: Conceptualization (Equal), Data curation (Supporting), Formal analysis (Supporting), Funding acquisition (Equal), Resources (Lead), Software (Lead), Supervision (Lead), Writing – review & editing (Supporting).
Data Availability Statement
De-identified data used for the current analysis will be made available upon reasonable request by any qualified investigator.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health (Grants R01DC013547and K24DC016312 to J. R. G., Grant R01DC014296 to B. C. D., and Grant F31DC015703 to C. C.). In addition to funding sponsors, the authors wish to thank patients and family members for their participation, as well as clinical staff at the Massachusetts General Hospital Frontotemporal Disorders Unit. They are also grateful for specific contributions to data collection and analysis by Brian Richburg, Rajeshwari Jakkam, and Katelynn Getchell.
Funding Statement
This work was funded by the National Institutes of Health (Grants R01DC013547and K24DC016312 to J. R. G., Grant R01DC014296 to B. C. D., and Grant F31DC015703 to C. C.).
References
- Allison, K. M. , Cordella, C. , Iuzzini-Seigel, J. , & Green, J. R. (2020). Differential diagnosis of apraxia of speech in children and adults: A scoping review. Journal of Speech, Language, and Hearing Research, 63(9), 2952–2994. https://doi.org/10.1044/2020_JSLHR-20-00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison, K. M. , Yunusova, Y. , Campbell, T. F. , Wang, J. , Berry, J. D. , & Green, J. R. (2017). The diagnostic utility of patient-report and speech-language pathologists' ratings for detecting the early onset of bulbar symptoms due to ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 18(5–6), 358–366. https://doi.org/10.1080/21678421.2017.1303515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, M. J. , Litvan, I. , Lang, A. E. , Bak, T. H. , Bhatia, K. P. , Borroni, B. , Boxer, A. L. , Dickson, D. W. , Grossman, M. , Hallett, M. , Josephs, K. A. , Kertesz, A. , Lee, S. E. , Miller, B. L. , Reich, S. G. , Riley, D. E. , Tolosa, E. , Tröster, A. I. , Vidailhet, M. , & Weiner, W. J. (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology, 80(5), 496–503. https://doi.org/10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash, S. , Evans, E. , O'Shea, J. , Powers, J. , Boller, A. , Weinberg, D. , Haley, J. , McMillan, C. , Irwin, D. J. , & Rascovsky, K. (2013). Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology, 81(4), 329–336. https://doi.org/10.1212/WNL.0b013e31829c5d0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard, K. J. , Savage, S. , Leyton, C. E. , Vogel, A. P. , Hornberger, M. , & Hodges, J. R. (2014). Logopenic and nonfluent variants of primary progressive aphasia are differentiated by acoustic measures of speech production. PLOS ONE, 9(2), e89864. https://doi.org/10.1371/journal.pone.0089864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilakos, A. , Grigori, Y. , den Ouden, D. B., Daniel, F. , Chris, R. , Lynda, F. , & Julius, F. (2017). A multivariate analytic approach to the differential diagnosis of apraxia of speech. Journal of Speech, Language, and Hearing Research, 60(12), 3378–3392. https://doi.org/10.1044/2017_JSLHR-S-16-0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma, P. , & Weenink, D (2018). Praat: Doing phonetics by computer (Version 6.0.44). http://www.praat.org/
- Caso, F. , Mandelli, M. L. , Henry, M. , Gesierich, B. , Bettcher, B. M. , Ogar, J. , Filippi, M. , Comi, G. , Magnani, G. , Sidhu, M. , Trojanowski, J. Q. , Huang, E. J. , Grinberg, L. T. , Miller, B. L. , Dronkers, N. , Seeley, W. W. , & Gorno-Tempini, M. L. (2014). In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology, 82(3), 239–247. https://doi.org/10.1212/WNL.0000000000000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catricalà, E. , Boschi, V. , Cuoco, S. , Galiano, F. , Picillo, M. , Gobbi, E. , Miozzo, A. , Chesi, C. , Esposito, V. , Santangelo, G. , Pellecchia, M. T. , Borsa, V. M. , Barone, P. , Garrard, P. , Iannaccone, S. , & Cappa, S. F. (2019). The language profile of progressive supranuclear palsy. Cortex, 115, 294–308. https://doi.org/10.1016/j.cortex.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Clark, H. M. , Duffy, J. R. , Whitwell, J. L. , Ahlskog, J. E. , Sorenson, E. J. , & Josephs, K. A. (2014). Clinical and imaging characterization of progressive spastic dysarthria. European Journal of Neurology, 21(3), 368–376. https://doi.org/10.1111/ene.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, H. M. , Utianski, R. L. , Ali, F. , Botha, H. , Whitwell, J. L. , & Josephs, K. A. (2021). Motor speech disorders and communication limitations in progressive supranuclear palsy. American Journal of Speech-Language Pathology, 30(3S), 1361–1372. https://doi.org/10.1044/2020_AJSLP-20-00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordella, C. , Dickerson, B. C. , Quimby, M. , Yunusova, Y. , & Green, J. R. (2017). Slowed articulation rate is a sensitive diagnostic marker for identifying non-fluent primary progressive aphasia. Aphasiology, 31(2), 241–260. https://doi.org/10.1080/02687038.2016.1191054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordella, C. , Quimby, M. , Touroutoglou, A. , Brickhouse, M. , Dickerson, B. C. , & Green, J. R. (2019). Quantification of motor speech impairment and its anatomic basis in primary progressive aphasia. Neurology, 92(17), e1992–e2004. https://doi.org/10.1212/WNL.0000000000007367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley, F. L. , Aronson, A. E. , & Brown, J. R. (1969). Differential diagnostic patterns of dysarthria. Journal of Speech and Hearing Research, 12(2), 246–269. https://doi.org/10.1044/jshr.1202.246 [DOI] [PubMed] [Google Scholar]
- Deramecourt, V. , Lebert, F. , Debachy, B. , Mackowiak-Cordoliani, M. A. , Bombois, S. , Kerdraon, O. , Buée, L. , Maurage, C.-A. , & Pasquier, F. (2010). Prediction of pathology in primary progressive language and speech disorders. Neurology, 74(1), 42–49. https://doi.org/10.1212/WNL.0b013e3181c7198e [DOI] [PubMed] [Google Scholar]
- Dickson, D. W. , Kouri, N. , Murray, M. E. , & Josephs, K. A. (2011). Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). Journal of Molecular Neuroscience, 45(3), 384–389. https://doi.org/10.1007/s12031-011-9589-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, J. R. (2013). Motor speech disorders: Substrates, differential diagnosis, and management. Elsevier Health Sciences. [Google Scholar]
- Duffy, J. R. , Strand, E. A. , Clark, H. , Machulda, M. , Whitwell, J. L. , & Josephs, K. A. (2015). Primary progressive apraxia of speech: Clinical features and acoustic and neurologic correlates. American Journal of Speech-Language Pathology, 24(2), 88–100. https://doi.org/10.1044/2015_AJSLP-14-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, J. R. , Strand, E. A. , & Josephs, K. A. (2014). Motor speech disorders associated with primary progressive aphasia. Aphasiology, 28(8–9), 1004–1017. https://doi.org/10.1080/02687038.2013.869307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, J. R. , Utianski, R. L. , & Josephs, K. A. (2021). Primary progressive apraxia of speech: From recognition to diagnosis and care. Aphasiology, 35(4), 560–591. https://doi.org/10.1080/02687038.2020.1787732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, A. R. , McAuliffe, M. J. , Lansford, K. L. , & Liss, J. M. (2017). Assessing vowel centralization in dysarthria: A comparison of methods. Journal of Speech, Language, and Hearing Research, 60(2), 341–354. https://doi.org/10.1044/2016_JSLHR-S-15-0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García, A. M. , Welch, A. E. , Mandelli, M. L. , Henry, M. L. , Lukic, S. , Prioris, M. J. T. , Deleon, J. , Ratnasiri, B. M. , Puls, D. L. L. , Miller, B. L. , Seeley, W. , Vogel, A. P. , & Gorno-Tempini, M. L. (2022). Automated detection of speech timing alterations in autopsy-confirmed non-fluent/agrammatic variant primary progressive aphasia. Neurology, 99(5), e500–e511. https://doi.org/10.1212/WNL.0000000000200750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini, M. L. , Hillis, A. E. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. , Ogar, J. M. , Rohrer, J. D. , Black, S. , Boeve, B. F. , Manes, F. , Dronkers, N. F. , Vandenberghe, R. , Rascovsky, K. , Patterson, K. , Miller, B. L. , Knopman, D. S. , Hodges, J. R. , Mesulam, M. M. , & Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, J. R. , Allison, K. M. , Cordella, C. , Richburg, B. D. , Pattee, G. L. , Berry, J. D. , Macklin, E. A. , Pioro, E. P. , & Smith, R. (2018). Additional evidence for a therapeutic effect of dextromethorphan/quinidine on bulbar motor function in persons with ALS: A quantitative speech analysis. British Journal of Clinical Pharmacology, 84(12), 2849–2856. https://doi.org/10.1111/bcp.13745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, J. R. , Beukelman, D. R. , & Ball, L. J. (2004). Algorithmic estimation of pauses in extended speech samples of dysarthric and typical speech. Journal of Medical Speech-Language Pathology, 12(4), 149–154. [PMC free article] [PubMed] [Google Scholar]
- Green, J. R. , Wang, J. , & Wilson, D. L. (2013). SMASH: A tool for articulatory data processing and analysis. Interspeech, 1331–1335. https://doi.org/10.21437/Interspeech.2013-353 [Google Scholar]
- Green, J. R. , Yunusova, Y. , Kuruvilla, M. S. , Wang, J. , Pattee, G. L. , Synhorst, L. , Zinman, L. , & Berry, J. D. (2013). Bulbar and speech motor assessment in ALS: Challenges and future directions. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 14(7–8), 494–500. https://doi.org/10.3109/21678421.2013.817585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman, M. (2012). The non-fluent/agrammatic variant of primary progressive aphasia. The Lancet Neurology, 11(6), 545–555. https://doi.org/10.1016/S1474-4422(12)70099-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley, K. L. , Jacks, A. , de Riesthal, M. , Abou-Khalil, R. , & Roth, H. L. (2012). Toward a quantitative basis for assessment and diagnosis of apraxia of speech. Journal of Speech, Language, and Hearing Research, 55(5), S1502–S1502. https://doi.org/10.1044/1092-4388(2012/11-0318) [DOI] [PubMed] [Google Scholar]
- Haley, K. L. , Jacks, A. , Jarrett, J. , Ray, T. , Cunningham, K. T. , & Gorno-Tempini, M. L., & Henry, M. L. (2021). Speech metrics and samples that differentiate between nonfluent/agrammatic and logopenic variants of primary progressive aphasia. Journal of Speech, Language, and Hearing Research, 64(3), 754–775. https://doi.org/10.1044/2020_JSLHR-20-00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, P. A. , Taylor, R. , Thielke, R. , Payne, J. , Gonzalez, N. , & Conde, J. G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. https://doi.org/10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger, G. U. , Respondek, G. , Stamelou, M. , Kurz, C. , Josephs, K. A. , Lang, A. E. , Mollenhauer, B. , Müller, U. , Nilsson, C. , Whitwell, J. L. , Arzberger, T. , Englund, E. , Gelpi, E. , Giese, A. , Irwin, D. J. , Meissner, W. G. , Pantelyat, A. , Rajput, A. , van Swieten, J. C. , …. Movement Disorder Society–Endorsed PSP Study Group. (2017). Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Movement Disorders, 32(6), 853–864. https://doi.org/10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. (2008). Frontotemporal dementia and related disorders: Deciphering the enigma. Annals of Neurology, 64(1), 4–14. https://doi.org/10.1002/ana.21426 [DOI] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Clark, H. M. , Utianski, R. L. , Strand, E. A. , Machulda, M. M. , Botha, H. , Martin, P. R. , Pham, N. T. T. , Stierwalt, J. , Ali, F. , Buciuc, M. , Baker, M. , Fernandez De Castro, C. H. , Spychalla, A. J. , Schwarz, C. G. , Reid, R. I. , Senjem, M. L. , Jack, C. R. , … Whitwell, J. L. (2021). A molecular pathology, neurobiology, biochemical, genetic and neuroimaging study of progressive apraxia of speech. Nature Communications, 12(1), 3452. https://doi.org/10.1038/s41467-021-23687-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Gunter, J. L. , Schwarz, C. G. , Reid, R. I. , Spychalla, A. J. , Lowe, V. J. , Jack, C. R. , & Whitwell, J. L. (2014). The evolution of primary progressive apraxia of speech. Brain, 137(10), 2783–2795. https://doi.org/10.1093/brain/awu223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Master, A. V. , Lowe, V. J. , Jack, C. R. , & Whitwell, J. L. (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135(5), 1522–1536. https://doi.org/10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Hodges, J. R. , Snowden, J. S. , Mackenzie, I. R. , Neumann, M. , Mann, D. M. , & Dickson, D. W. (2011). Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathologica, 122(2), 137–153. https://doi.org/10.1007/s00401-011-0839-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Petersen, R. C. , Knopman, D. S. , Boeve, B. F. , Whitwell, J. L. , Duffy, J. R. , Parisi, J. E. , & Dickson, D. W. (2006). Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology, 66(1), 41–48. https://doi.org/10.1212/01.wnl.0000191307.69661.c3 [DOI] [PubMed] [Google Scholar]
- Kent, R. D. (1996). Hearing and believing. American Journal of Speech-Language Pathology, 5(3), 7–23. https://doi.org/10.1044/1058-0360.0503.07 [Google Scholar]
- Kertesz, A. (2007). Western Aphasia Battery–Revised. PsychCorp. [Google Scholar]
- Kim, Y. , Kent, R. D. , & Weismer, G. (2011). An acoustic study of the relationships among neurologic disease, dysarthria type, and severity of dysarthria. Journal of Speech, Language, and Hearing Research, 54(2), 417–429. https://doi.org/10.1044/1092-4388(2010/10-0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo, T. K. , & Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. https://doi.org/10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri, N. , Whitwell, J. L. , Josephs, K. A. , Rademakers, R. , & Dickson, D. W. (2011). Corticobasal degeneration: A pathologically distinct 4R tauopathy. Nature Reviews Neurology, 7(5), 263–272. https://doi.org/10.1038/nrneurol.2011.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Hustad, K. C. , & Weismer, G. (2014). Predicting speech intelligibility with a multiple speech subsystems approach in children with cerebral palsy. Journal of Speech, Language, and Hearing Research, 57(5), 1666–1678. https://doi.org/10.1044/2014_JSLHR-S-13-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss, J. M. , White, L. , Mattys, S. L. , Lansford, K. , Lotto, A. J. , Spitzer, S. M. , & Caviness, J. N. (2009). Quantifying speech rhythm abnormalities in the dysarthrias. Journal of Speech, Language, and Hearing Research, 52(5), 1334–1352. https://doi.org/10.1044/1092-4388(2009/08-0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen, B. , Kent, R. , Peters, H. , & van Lieshout, P . (2007). Speech motor control: In normal and disordered speech. Oxford University Press. [Google Scholar]
- Mailend, M.-L. , & Maas, E. (2021). To lump or to split? Possible subtypes of apraxia of speech. Aphasiology, 35(4), 592–613. https://doi.org/10.1080/02687038.2020.1836319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryn, Y. , & Weenink, D. (2015). Objective dysphonia measures in the program Praat: Smoothed cepstral peak prominence and acoustic voice quality index. Journal of Voice, 29(1), 35–43. https://doi.org/10.1016/j.jvoice.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Mauszycki, S. C. , Dromey, C. , & Wambaugh, J. L. (2005, May 31–June 4). Variability in apraxia of speech: A perceptual, acoustic, and kinematic analysis of stop consonants [Paper presentation]. Clinical Aphasia Conference, Sanibel Island, FL, United States. http://aphasiology.pitt.edu/1572/ [Google Scholar]
- McCloy, D . (2018). Praat scripts for streamlining manual measurements in acoustic analysis: Drammock/praat-semiauto. https://github.com/drammock/praat-semiauto (Original work published 2012)
- McGraw, K. O. , & Wong, S. P. (1996). Forming inferences about some intraclass correlation coefficients. Psychological Methods, 1(1), 30–46. https://doi.org/10.1037/1082-989X.1.1.30 [Google Scholar]
- Mefferd, A. S. , Pattee, G. L. , & Green, J. R. (2014). Speaking rate effects on articulatory pattern consistency in talkers with mild ALS. Clinical Linguistics & Phonetics, 28(11), 799–811. https://doi.org/10.3109/02699206.2014.908239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montembeault, M. , Brambati, S. M. , Gorno-Tempini, M. L. , & Migliaccio, R. (2018). Clinical, anatomical, and pathological features in the three variants of primary progressive aphasia: A review. Frontiers in Neurology, 9. https://doi.org/10.3389/fneur.2018.00692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Bédirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , Cummings, J. L. , & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Ogar, J. M. , Dronkers, N. F. , Brambati, S. M. , Miller, B. L. , & Gorno-Tempini, M. L. (2007). Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Disease & Associated Disorders, 21(4), S23–S30. https://doi.org/10.1097/WAD.0b013e31815d19fe [DOI] [PubMed] [Google Scholar]
- Olney, N. T. , Spina, S. , & Miller, B. L. (2017). Frontotemporal dementia. Neurologic Clinics, 35(2), 339–374. https://doi.org/10.1016/j.ncl.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, K. A. , Patterson, K. , & Rowe, J. B. (2021). Language impairment in progressive supranuclear palsy and corticobasal syndrome. Journal of Neurology, 268(3), 796–809. https://doi.org/10.1007/s00415-019-09463-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgornik, S . (2011). Get duration, pitch, formants. https://depts.washington.edu/phonlab/resources/get-pitch-formants.praat
- Poole, M. L. , Brodtmann, A. , Darby, D. , & Vogel, A. P. (2017). Motor speech phenotypes of frontotemporal dementia, primary progressive aphasia, and progressive apraxia of speech. Journal of Speech, Language, and Hearing Research, 60(4), 897–911. https://doi.org/10.1044/2016_JSLHR-S-16-0140 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Rohrer, J. D. , Rossor, M. N. , & Warren, J. D. (2010). Apraxia in progressive nonfluent aphasia. Journal of Neurology, 257(4), 569–574. https://doi.org/10.1007/s00415-009-5371-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, P. , Loucks, T. , Kim, H. , & Hasegawa-Johnson, M. (2012). Relationship between kinematics, F2 slope and speech intelligibility in dysarthria due to cerebral palsy. Clinical Linguistics & Phonetics, 26(9), 806–822. https://doi.org/10.3109/02699206.2012.706686 [DOI] [PubMed] [Google Scholar]
- Rong, P. , Yunusova, Y. , Richburg, B. , & Green, J. R. (2018). Automatic extraction of abnormal lip movement features from the alternating motion rate task in amyotrophic lateral sclerosis. International Journal of Speech-Language Pathology, 20(6), 610–623. https://doi.org/10.1080/17549507.2018.1485739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, P. , Yunusova, Y. , Wang, J. , & Green, J. R. (2015). Predicting early bulbar decline in amyotrophic lateral sclerosis: A speech subsystem approach. Behavioural Neurology, 2015, 183027. https://doi.org/10.1155/2015/183027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, H. P. , Stipancic, K. L. , Lammert, A. C. , & Green, J. R. (2021). Validation of an acoustic-based framework of speech motor control: Assessing criterion and construct validity using kinematic and perceptual measures. Journal of Speech, Language, and Hearing Research, 64(12), 4736–4753. https://doi.org/10.1044/2021_JSLHR-21-00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi, S. A. , Patterson, K. , Tomek, M. , & Nestor, P. J. (2012). Abnormalities of connected speech in the non-semantic variants of primary progressive aphasia. Aphasiology, 26(10), 1219–1237. https://doi.org/10.1080/02687038.2012.710318 [Google Scholar]
- Santos-Santos, M. A. , Mandelli, M. L. , Binney, R. J. , Ogar, J. , Wilson, S. M. , Henry, M. L. , Hubbard, H. I. , Meese, M. , Attygalle, S. , Rosenberg, L. , Pakvasa, M. , Trojanowski, J. Q. , Grinberg, L. T. , Rosen, H. , Boxer, A. L. , Miller, B. L. , Seeley, W. W. , & Gorno-Tempini, M. L. (2016). Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurology, 73(6), 733–742. https://doi.org/10.1001/jamaneurol.2016.0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir, S. , Ramig, L. O. , Spielman, J. L. , & Fox, C. (2010). Formant centralization ratio (FCR): A proposal for a new acoustic measure of dysarthric speech. Journal of Speech, Language, and Hearing Research, 53(1), 114–125. https://doi.org/10.1044/1092-4388(2009/08-0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky, D. , Bakkour, A. , Negreira, A. , Nalipinski, P. , Weintraub, S. , Mesulam, M.-M. , Caplan, D. , & Dickerson, B. C. (2010). Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology, 75(4), 358–366. https://doi.org/10.1212/WNL.0b013e3181ea15e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky, D. , Domoto-Reilly, K. , & Dickerson, B. C. (2014). Use of the Progressive Aphasia Severity Scale (PASS) in monitoring speech and language status in PPA. Aphasiology, 28(8–9), 993–1003. https://doi.org/10.1080/02687038.2014.931563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage, S. , Hsieh, S. , Leslie, F. , Foxe, D. , Piguet, O. , & Hodges, J. R. (2013). Distinguishing subtypes in primary progressive aphasia: Application of the Sydney language battery. Dementia and Geriatric Cognitive Disorders, 35(3–4), 208–218. https://doi.org/10.1159/000346389 [DOI] [PubMed] [Google Scholar]
- Silbergleit, A. K. , Feit, H. , & Silbergleit, R. (2009). Neurogenic stuttering in corticobasal ganglionic degeneration: A case report. Journal of Neurolinguistics, 22(1), 83–90. https://doi.org/10.1016/j.jneuroling.2008.06.003 [Google Scholar]
- Šimek, M. , & Rusz, J. (2021). Validation of cepstral peak prominence in assessing early voice changes of Parkinson's disease: Effect of speaking task and ambient noise. The Journal of the Acoustical Society of America, 150(6), 4522–4533. https://doi.org/10.1121/10.0009063 [DOI] [PubMed] [Google Scholar]
- Smith, A. , Goffman, L. , Zelaznik, H. N. , Ying, G. , & McGillem, C. (1995). Spatiotemporal stability and patterning of speech movement sequences. Experimental Brain Research, 104(3), 493–501. https://doi.org/10.1007/BF00231983 [DOI] [PubMed] [Google Scholar]
- Stipancic, K. L. , Palmer, K. M. , Rowe, H. P. , Yunusova, Y. , Berry, J. D. , & Green, J. R. (2021). “You say severe, I say mild”: Toward an empirical classification of dysarthria severity. Journal of Speech, Language, and Hearing Research, 64(12), 4718–4735. https://doi.org/10.1044/2021_JSLHR-21-00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipancic, K. L. , Yunusova, Y. , Berry, J. D. , & Green, J. R. (2018). Minimally detectable change and minimal clinically important difference of a decline in sentence intelligibility and speaking rate for individuals with amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 61(11), 2757–2771. https://doi.org/10.1044/2018_JSLHR-S-17-0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura, Y. , Otsuki, M. , Sakai, S. , Tajima, Y. , Mito, Y. , Ogata, A. , Koshimizu, S. , Yoshino, M. , Uemori, G. , Takakura, S. , & Nakagawa, Y. (2019). Sub-classification of apraxia of speech in patients with cerebrovascular and neurodegenerative diseases. Brain and Cognition, 130, 1–10. https://doi.org/10.1016/j.bandc.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Turner, G. S. , Tjaden, K. , & Weismer, G. (1995). The influence of speaking rate on vowel space and speech intelligibility for individuals with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 38(5), 1001–1013. https://doi.org/10.1044/jshr.3805.1001 [DOI] [PubMed] [Google Scholar]
- Utianski, R. L. , Duffy, J. R. , Clark, H. M. , Strand, E. A. , Botha, H. , Schwarz, C. G. , Machulda, M. M. , Senjem, M. L. , Spychalla, A. J. , Jack, C. R. , Petersen, R. C. , Lowe, V. J. , Whitwell, J. L. , & Josephs, K. A. (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and Language, 184, 54–65. https://doi.org/10.1016/j.bandl.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski, R. L. , Martin, P. R. , Hanley, H. , Duffy, J. R. , Botha, H. , Clark, H. M. , Whitwell, J. L. , & Josephs, K. A. (2021). A longitudinal evaluation of speech rate in primary progressive apraxia of speech. Journal of Speech, Language, and Hearing Research, 64(2), 392–404. https://doi.org/10.1044/2020_JSLHR-20-00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeVrede, L. , Ljubenkov, P. A. , Rojas, J. C. , Welch, A. E. , & Boxer, A. L. (2020). Four-repeat tauopathies: Current management and future treatments. Neurotherapeutics, 17(4), 1563–1581. https://doi.org/10.1007/s13311-020-00888-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergis, M. K. , Ballard, K. J. , Duffy, J. R. , McNeil, M. R. , Scholl, D. , & Layfield, C. (2014). An acoustic measure of lexical stress differentiates aphasia and aphasia plus apraxia of speech after stroke. Aphasiology, 28(5), 554–575. https://doi.org/10.1080/02687038.2014.889275 [Google Scholar]
- Weismer, G. , & Green, J. (2015). Speech production in motor speech disorders: Lesions, models, and a research agenda. In Redford M. (Ed.), The handbook of speech production. Wiley Blackwell. https://doi.org/10.1002/9781118584156.ch14 [Google Scholar]
- Whitfield, J. A. , & Goberman, A. M. (2014). Articulatory–acoustic vowel space: Application to clear speech in individuals with Parkinson's disease. Journal of Communication Disorders, 51, 19–28. https://doi.org/10.1016/j.jcomdis.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Whitwell, J. L. , Weigand, D. , Duffy, R. , Clark, M. , Strand, A. , Machulda, M. , Spychalla, J. , Senjem, L. , Jack, R. , & Josephs, K. A. (2017). Predicting clinical decline in progressive agrammatic aphasia and apraxia of speech. Neurology, 89(22), 2271–2279. https://doi.org/10.1212/WNL.0000000000004685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. M. , Henry, M. L. , Besbris, M. , Ogar, J. M. , Dronkers, N. F. , Jarrold, W. , Miller, B. L. , & Gorno-Tempini, M. L. (2010). Connected speech production in three variants of primary progressive aphasia. Brain, 133(7), 2069–2088. https://doi.org/10.1093/brain/awq129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. M. , Ogar, J. M. , Laluz, V. , Growdon, M. , Jang, J. , Glenn, S. , Miller, B. L. , Weiner, M. W. , & Gorno-Tempini, M. L. (2009). Automated MRI-based classification of primary progressive aphasia variants. NeuroImage, 47(4), 1558–1567. https://doi.org/10.1016/j.neuroimage.2009.05.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston, K. M. , Beukelman, D. R. , Hakel, M. , & Dorsey, M. (2007). Speech Intelligibility Test [Computer software] . Institute for Rehabilitation Science and Engineering at Madonna Rehabilitation Hospital. [Google Scholar]
- Yunusova, Y. , Green, J. , Lindstrom, M. , Ball, L. , Pattee, G. , & Zinman, L. (2010). Kinematics of disease progression in bulbar ALS. Journal of Communication Disorders, 43(1), 6–20. https://doi.org/10.1016/j.jcomdis.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data used for the current analysis will be made available upon reasonable request by any qualified investigator.