Abstract
Background:
Donation after cardiac death(DCD) has been proposed as an avenue to expand the liver donor pool.
Methods:
We examined factors associated with nonrecovery of DCD livers using UNOS data from 2015 to 2019.
Results:
There 265 non-recovered potential(NRP) DCD livers. Blood type AB (7.8% vs. 1.1%) and B (16.9% vs. 9.8%) were more frequent in the NRP versus actual donors (p < 0.001). The median driving time between donor hospital and transplant center was similar for NRP and actual donors (30.1 min vs. 30.0 min; p = 0.689), as was the percentage located within a transplant hospital (20.8% vs. 20.9%; p = 0.984).The donation service area(DSA) of a donor hospital explained 27.9% (p = 0.001) of the variability in whether a DCD liver was recovered.
Conclusion:
A number of potentially high quality DCD donor livers go unrecovered each year, which may be partially explained by donor blood type and variation in regional and DSA level practice patterns.
Keywords: Liver transplant, DCD, UNOS, Geography, Organ donation
1. Introduction
As of May 2020, there are 12,739 patients awaiting liver transplantation in the United States, with more than 12,000 candidates added to the waiting list every year since 2016.1 Although deceased donor liver transplants (LT) have increased every year from 6,010 in 2012 to 8,372 in 2019,1 this still represents a significant shortage of available livers for transplant. The consequences of this shortage are deadly, with individual centers commonly reporting waitlist mortality rates of 20% or more.2,3 This has led to recognition that reliance solely upon organ donors following brain death determination (DBD) is insufficient to meet the growing need for transplantation.4
Increased utilization of organs from donation after cardiac death (DCD) donors has been suggested as one potential means to make-up for the shortfall in available livers.3,5 Several centers experienced in DCD liver transplantation have demonstrated good outcomes,3,6–10 and accepting a DCD liver has been shown to provide a survival advantage over awaiting a DBD liver.11 Recognition of the potential for DCD donors to partially ameliorate the organ shortage has resulted in an overall increase in DCD donors in the United States, with the total number of DCD donors increasing from 642 (8% of all donors) in 2006 to 1684 (17% of all donors) in 2017.8 Unfortunately, the increase in DCD donation overall has not translated into widespread adoption of DCD liver transplantation. Between 65% and 75% of DCD livers recovered for transplant have been ultimately discarded every year from 2006 through 2018.12
A recent survey of United States liver transplant centers found that 23% of responding centers categorically do not use DCD livers. Of the remaining centers that continue to perform DCD liver transplantation, 53% stated they would only accept locally allocated DCD livers, and 31% responded that they would not accept DCD livers that required a flight. Travel cost and perceived likelihood of arrest within acceptable limits of warm ischemia time (WIT) were also cited as considerations in whether to pursue a given DCD liver donor offer.13 Utilization of DCD livers is also highly concentrated to a few high-volume centers. Although the majority of US centers performed at least 1 DCD liver transplant from 2013 through 2017, 17.5% of all DCD liver transplants during this period were performed by just 3 centers, while 46% were performed by 11 centers.14 Given the apparent continued lack of enthusiasm for DCD liver transplantation despite an overall increase in DCD donation, we hypothesized that a significant untapped pool of potential DCD livers exists. We further hypothesized that distance from centers performing DCD LT would be a significant factor in whether the liver from a DCD donor was pursued. Herein, we describe a retrospective analysis of United Network for Organ Sharing (UNOS) data to test these hypotheses.
2. Methods
2.1. Study population and data sources
Adult DCD organ donors from 2015 onward contained in the March 2019 UNOS Standard Transplant Analysis and Research (STAR) file were analyzed. Donors from whom a liver was not recovered were identified by liver disposition code 3, indicating that the liver was not recovered and remained with the donor. Amongst this cohort, suitable liver donors were identified by the following criteria: age under 60, body mass index (BMI) ≤ 35, AST and ALT ≤2 times the upper limit of normal, total bilirubin within normal limits, hepatitis B virus (HBV) core antibody and nucleic acid testing (NAT) negative, hepatitis C virus (HCV) antibody and NAT negative, no recent (e.g., past 6 months) history of heavy alcohol use, and interval from withdrawal of life support to crossclamp ≤30 min. Further exclusions were made if the reason given for the liver not being recovered indicated that the liver was evaluated for transplant but found to be unsuitable, time constraints precluded allocation of the liver, or medical examiner restrictions precluded liver recovery. Donors fulfilling the above criteria were defined as nonrecovered potential (NRP) DCD liver donors. A second cohort of nonrecovered potential liver donors was subsequently identified using a more liberal set of criteria. The liberal criteria for NRP liver donors mirrored the more conservative criteria outlined above, but without the restriction on history of alcohol use or HCV antibody or NAT positivity. Comparison was made to DCD donors resulting in a liver which was transplanted, identified by liver disposition code 6 (transplanted) in the STAR file and identification of a recipient record corresponding to the donor.
2.2. Analytical methods
NRP DCD liver donors were compared to actual DCD liver donors on baseline characteristics using the Wilcoxon-Mann-Whitney test for continuous variables and chi-squared analysis for categorical variables. In order to estimate recipient outcomes should the livers from NRP donors be transplanted, a subset of actual liver donors was selected by matching to the NRP donors using propensity scores. Propensity scores were calculated using multivariable logistic regression with the following covariates: age, race, gender, blood type, history of diabetes, history of IV drug use, cause of death, HBV core antibody, HBV surface antigen, HBV NAT, HCV antibody, HCV NAT, history of recent heavy alcohol use, HIV NAT, body mass index, height, weight, AST, ALT, total bilirubin, and the interval from withdrawal of life support to crossclamp.
After calculation of propensity scores, matching was carried out in a 1:1 nearest neighbor fashion based on the logit of the propensity score. The matching algorithm was “greedy” in that, once a match was made, it was not broken. In order to prevent poor matches from being made, a caliper width equal to 0.2 times the pooled standard deviation of the logit propensity score for the entire cohort was imposed. Matched pairs with a difference in logit propensity score greater than the caliper were discarded. Residual differences in covariates between groups after propensity matching were assessed using the formulas for standardized differences as proposed by Austin.15 Standardized differences <0.1 in absolute value are generally considered to be insignificant in terms of introducing residual confounding.16 The reason for using standardized differences in this setting was to minimize the effect of the smaller size of the propensity matched cohort compared to the overall cohort, which would reduce the power of traditional significance tests and potentially mask important covariate imbalances. Recipient survival following transplant from the matched liver donor cohort was calculated according to the Kaplan-Meier method and compared to recipient survival from the remaining unmatched cohort of DCD livers in the study period using the log-rank test. Multivariable Cox proportional hazards regression was then performed to compare survival adjusted for recipient factors that were different for the matched and unmatched donor cohorts at the p < 0.1 level on univariable analysis. Donor factors were not included in the multivariable model as the goal was to see if there was a survival difference in recipients of grafts from the two different donor populations; thus, adjusting for donor factors would not have been appropriate. The proportional hazards assumption was verified by visual inspection of survival curves. The above analyses were performed using both the original and more liberal definitions of NRP liver donors defined in the study population section.
The influence of UNOS region and donation service area (DSA) in which the donor hospital was located, as well as the travel time from the donor hospital to the nearest transplant center, on whether livers were recovered for transplant was subsequently investigated using the more strict definition of NRP liver donors (e.g. cohort that excluded donors with history of alcohol use or HCV antibody or NAT positivity). The percentage of missed opportunities for DCD liver donation in each UNOS region and donation service area (DSA) was calculated as:
The addresses for donor hospitals and transplant centers were obtained from the Centers for Medicare Services hospital general information file17 and manual web search. The driving time and distance between each donor hospital and the nearest transplant center was calculated using ArcGIS (Esri, Redlands CA). Only transplant centers performing at least one DCD liver transplant during the study period were considered. The locations of the donor and transplant hospitals, as well as driving routes, were mapped using ArcGIS. The requirement to fly for donor procurement was determined by a driving time of greater than 2 h from the donor hospital to the nearest transplant center. This time was chosen empirically based on practice patterns at the authors’ centers.
Multivariable, multilevel logistic regression was performed with missed opportunity for liver donation using the stricter definition of NRP liver donors (ie. whether the donor was a NRP vs. actual liver donor) as the dependent variable and driving time between the donor hospital and the nearest transplant center as the main effect. The models were additionally adjusted for donor age, blood type, diabetes, and cause of death as fixed effects based on results of the univariable analysis. Separate models were fitted with either DSA or UNOS region as random intercepts. The percent of variability in whether a liver was recovered explained by variance in either DSAs or UNOS regions was determined by the calculation of the intraclass correlation coefficient (ICC) using the following equation: , where is the variance of the random intercept.18,19 Donors in Hawaii and Alaska were not included in this analysis due to the impossibility or extreme infeasibility of driving to a transplant center performing DCD liver transplant from these areas. There were no DCD donors in Puerto Rico during the study period. Association between the percentage of missed opportunities in a DSA and the percent of DCD livers exported from the DSA was assessed using linear regression. Continuous variables were summarized by median and interquartile range (IQR) while categorical variables were summarized as counts and percentage.
There was less than 5% missing data for all variables, indicating minimal risk of bias due to missing data.20 As such, complete case analysis was performed with the following exception: a category was created for both HIV and HCV NAT results to indicate missing results due to donors occurring prior to routine inclusion of NAT testing for these diseases in UNOS Data (3/31/15 for HCV and 4/20/2016 for HIV). It should also be noted that 4 recipients in the survival comparison did not have survival times recorded in the data, presumably due to proximity of their transplant to the end of recorded follow-up. These patients were excluded from survival analysis. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
There were 3087 DCD donors from whom the liver was not recovered during the study period. Of these, 314 met the initial screening criteria for NRP liver donors. After exclusions made based on provided reasons for not recovering the liver, there were 154 nonrecovered potential liver donors remaining. In comparison, there were 1,890 DCD donors resulting in a liver recovered and transplanted during the study period. In 87% (n = 134) of the NRP donors, at least one non-hepatic organ was recovered and transplanted. Specifically, 74.7% (n = 115) NRP donors had both kidneys transplanted while an additional 117% (n = 18%) had one kidney transplanted. Additionally, 2 (1.3%) donated 1 lung which was transplanted and 4 (2.6%) donated 2 lungs which were transplanted. No hearts, intestines, or pancreata were transplanted from NRP donors. The median age of the nonrecovered potential donors was higher than the actual donors (48 vs. 34; p < 0.001), though transaminases and bilirubin were significantly lower in the NRP donors (Table 1). Notably, there was an increased frequency of blood type AB (7.8% vs. 1.1%; p <0.001) and B (16.9% vs. 9.8%; p <0.001) in the NRP donor cohort compared to actual donors. Significantly more NRP donors underwent withdrawal of life support at night or on weekends compared to actual donors (Table 1). Only 2 (1.3%) of NRP donors had liver biopsies compared to 400 (21.2%) of actual donors. This imbalance likely reflects our cohort selection criteria, as donors ruled out for biopsy findings were not included in the NRP cohort. The remaining comparison between the NRP and actual donor cohort is summarized in Table 1.
Table 1.
Characteristics of non-recovered potential versus actual donation after cardiac death liver donors.
| Non-Recovered Potential DCDs (n = 154) | Actual DCDs (n = 1,890) | P-value | |
|---|---|---|---|
|
| |||
| Age | 48.0 (33.0–55.0) | 34.0 (24.0–47.0) | <0.001 |
| Male Gender | 102 (66.2%) | 1295 (68.5%) | |
| Height (cm) | 174.5 (165.1–180.3) | 173.0 (165.1–180.0) | 0.779 |
| Weight (kg) | 81.1 (69.6–95.5) | 79.5 (68.0–92.1) | 0.322 |
| BMI (mg/m2) | 27.5 (23.8–31.5) | 26.4 (23.0–30.6) | 0.138 |
| AST | 38.5 (25–58) | 54.0 (34.0–87.0) | <0.001 |
| ALT | 33 (20–48) | 43.0 (25.0–78.0) | <0.001 |
| Total Bilirubin (mg/dl) | 0.5 (0.3–0.7) | 0.6 (0.4–0.9) | <0.001 |
| Diabetes | 19 (12.3%) | 144 (7.6)% | 0.104 |
| Ethnicity | |||
| Caucasian | 127 (82.5%) | 1442 (76.3%) | 0.539 |
| African American | 11 (7.1%) | 182 (9.6%) | |
| Hispanic | 13 (8.4%) | 209 (11.1%) | |
| Asian | 2 (1.3%) | 35 (1.9%) | |
| Native American/Other | 1 (0.7%) | 22 (1.2%) | |
| ABO | |||
| A | 54 (35.1%) | 771 (40.8%) | <0.001 |
| AB | 12 (7.8%) | 20 (1.1%) | |
| B | 26 (16.9%) | 185 (9.8%) | |
| O | 62 (40.3%) | 914 (48.4%) | |
| Cause of Death | |||
| Anoxia | 65 (44.2%) | 994 (52.6%) | 0.002 |
| CVA | 32 (20.8%) | 279 (14.8%) | |
| Trauma | 43 (27.9%) | 541 (28.6%) | |
| Other | 14 (9.1%) | 76 (4.0%) | |
| IV Drug Use | 16 (10.4%) | 255 (13.5%) | 0.466 |
| Withdrawal to Crossclamp Interval (min) | 24.0 (20.0–27.0) | 23.0 (19.0–26.0) | 0.065 |
| Weekend Recovery | 51 (33.1%) | 485 (25.7%) | 0.043 |
| Night (1700–0600) Recovery | 99 (64.3%) | 974 (51.5%) | 0.002 |
Propensity score matching yielded a cohort of 144 donors each drawn from the NRP and actual donor pools. The median age of the matched actual donors was 46, with normal transaminases and bilirubin levels. Summary of the balance of characteristics between the actual and NRP liver donors after matching is presented in Table 2. AB blood type, anoxia, and trauma as causes of death remained slightly out of balance after matching. Recipient survival at 6 months, 1 year, and 2 years from transplant using livers from the matched actual donor cohort was 94.5%, 92.4%, and 90.5% compared to 94.0%, 91.0%, and 86.6% for the unmatched actual donor cohort (p = 0.553; Fig. 1). Graft survival at 6 months, 1 year, and 2 years from transplant using livers from the matched actual donor cohort was 89.0%, 86.0%, and 84.2% compared to 91.1%, 87.0%, and 81.7% for the unmatched actual cohort (p = 0.998). Recipient characteristics for the matched and unmatched donor cohorts are presented in Supplemental Table 1. After adjusting for relevant recipient factors, recipient survival (HR 0.825; 95% CI 0.457–1.487; p = 0.513) and graft survival (HR 0.955; 95% CI 0.602–1.515; p = 0.846) remained similar for the matched versus unmatched donor cohorts.
Table 2.
Comparison of balance between a propensity score matched cohort of nonrecovered potential and actual donation after cardiac death liver donors. Standardized differences greater than 0.1 in absolute value are considered significant and highlighted in bold type.
| Non-Recovered Potential DCDs (n = 144) | Actual DCDs (n = 144) | Standardized Difference | |
|---|---|---|---|
|
| |||
| Age | 48.0 (34.0–55.0) | 46.0 (35.5–54.5) | −0.01 |
| Male Gender | 95 (66.0%) | 97 (67.4%) | −0.03 |
| Height | 174.5 (165.1–180.0) | 175.0 (167.8–180.2) | −0.06 |
| Weight | 80.9 (70.0–95.4) | 83.1 (67.7–97.9) | −0.06 |
| BMI | 27.5 (23.7–31.6) | 27.3 (23.5–31.5) | −0.05 |
| AST | 38.5 (25.0–58.5) | 36.0 (23.0–48.0) | 0.07 |
| ALT | 33.0 (20.0–48.5) | 28.0 (16.0–41.0) | 0.08 |
| Total Bilirubin | 0.5 (0.4–0.7) | 0.5 (0.3–0.7) | −0.01 |
| Diabetes | 18 (12.5%) | 17 (11.9%) | 0.02 |
| Ethinicity | |||
| Caucasian | 118 (81.9%) | 120 (83.3%) | −0.04 |
| African American | 11 (7.6%) | 8 (5.6%) | 0.08 |
| Hispanic | 12 (8.3%) | 13 (9.0%) | −0.02 |
| Asian | 2 (1.4%) | 2 (1.4%) | 0.00 |
| Native American/Other | 1 (0.7%) | 1 (0.7%) | 0.00 |
| ABO | |||
| A | 52 (36.1%) | 54 (37.5%) | −0.03 |
| AB | 10 (6.9%) | 5 (3.5%) | 0.16 |
| B | 24 (16.7%) | 21 (14.6%) | 0.06 |
| O | 58 (40.3%) | 64 (44.4%) | −0.08 |
| Cause of Death | |||
| Anoxia | 64 (44.4%) | 54 (37.5%) | 0.14 |
| CVA | 31 (21.5%) | 35 (24.3%) | −0.07 |
| Trauma | 37 (25.7%) | 44 (30.6%) | 0.11 |
| Other | 12 (8.3%) | 11 (7.6%) | 0.03 |
| IV Drug Use | 16 (11.1%) | 16 (11.1%) | 0.00 |
| Withdrawal to Crossclamp Interval (minutes) | 24.0 (20.0–27.0) | 22.0 (19.0–22.5) | 0.06 |
| HIV NAT | |||
| Negative | 139 (96.5%) | 136 (94.4%) | 0.09 |
| Not Done | 2 (1.4%) | 3 (2.1%) | −0.05 |
| Prior to Routine Collection | 3 (2.1%) | 5 (3.5%) | −0.08 |
| Hepatitis C NAT | |||
| Negative | 141 (97.9%) | 139 (96.5%) | 0.08 |
| Prior to Routine Collection | 3 (2.1%) | 5 (3.5%) | −0.08 |
| Heavy Alcohol Use | |||
| No | 140 (97.2%) | 138 (95.8%) | 0.07 |
| Unknown | 4 (2.8%) | 6 (4.2%) | −0.08 |
Fig. 1.
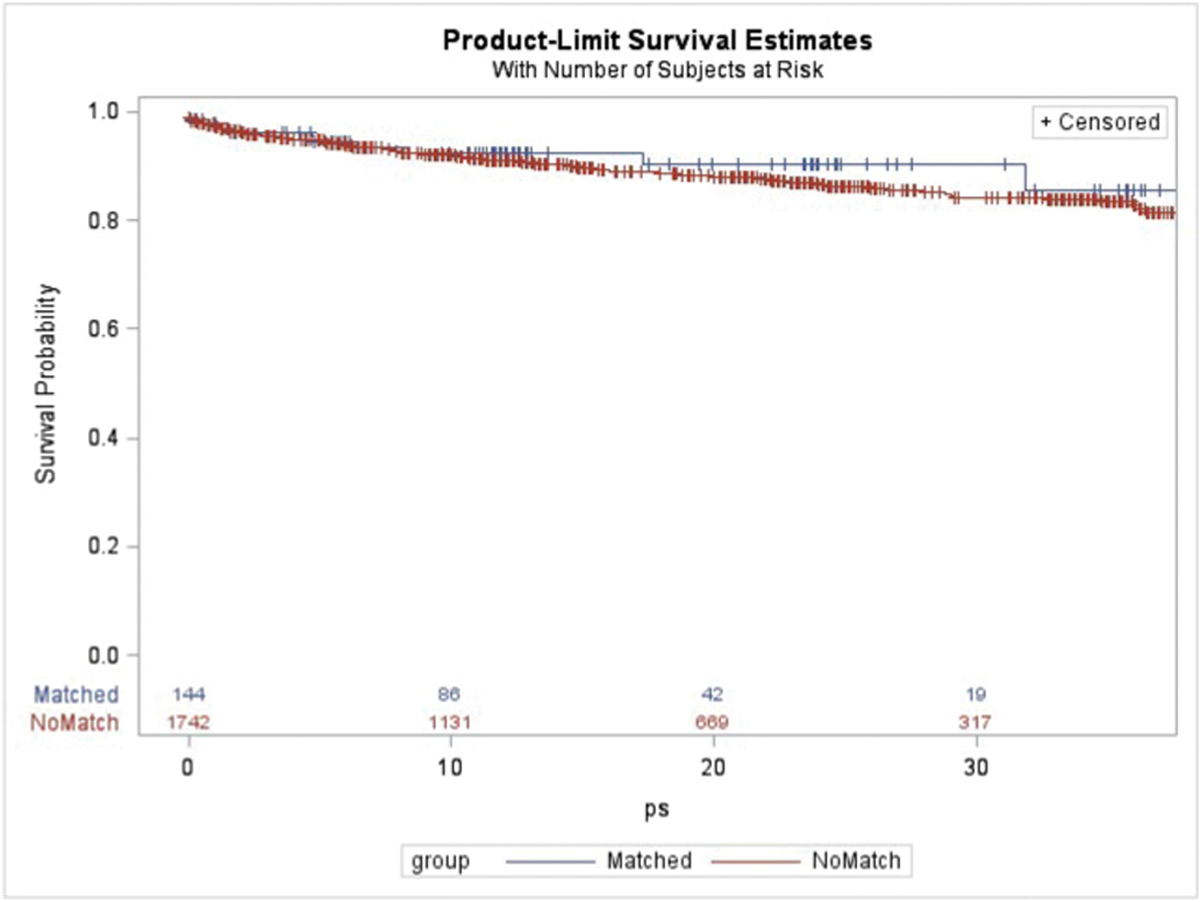
Kaplan-Meier recipient survival using donors matched to the nonrecovered potential donor cohort versus the unmatched remaining donation after cardiac death donors.
Using the more liberal definition for NRP liver donors, there were 493 donors who met the inclusion criteria. After making exclusions based on reasons given for why the liver was not recovered, there were 265 NRP liver donors in the study period. Comparison between these 265 NRP donors and the 1,890 actual liver donors is summarized in Table 3. Propensity score matching yielded a cohort of 245 donors each drawn from the NRP and actual donor pools. Balance between covariates after matching is summarized in Table 4. Recipient survival at 6 months, 1 year, and 2 years from transplant using livers from the matched actual donor cohort was 93.6%, 91.7%, 88.9% vs. 94.1%, 91.0%, and 86.6% for the unmatched actual donor cohort (p = 0.968; Fig. 2). Graft survival at 6 months, 1 year, and 2 years from transplant using livers from the matched actual donor cohort was 91.0%, 85.9%, and 80.4% vs. 91.0%, 87.1%, and 82.0% for the unmatched actual donor cohort (p = 0.536). Recipient characteristics for the matched and unmatched donor cohorts are presented in Supplemental Table 2. After adjusting for relevant recipient factors, recipient survival (HR 1.018; 95% CI 0.665–1.558; p = 0.934) and graft survival (HR 1.130; 95% CI 0.801–1.593; p = 0.486) again remained similar for the matched versus unmatched donor cohorts.
Table 3.
Characteristics of non-recovered potential versus actual donation after cardiac death liver donors using more liberal inclusion and exclusion criteria.
| Non-Recovered Potential DCDs (n = 265) | Actual DCDs (n = 1,890) | P-value | |
|---|---|---|---|
|
| |||
| Age | 48.0 (34.0–55.0) | 34.0 (24.0–47.0) | <0.001 |
| Male Gender | 192 (72.5%) | 1295 (68.5%) | 0.195 |
| Height (cm) | 175.3 (168.0–182.0) | 173.0 (165.1–180.0) | 0.007 |
| Weight (kg) | 81.6 (70.8–95.2) | 79.5 (68.0–92.1) | 0.104 |
| BMI (mg/m2) | 26.9 (23.6–31.0) | 26.4 (23.0–30.6) | 0.439 |
| AST | 42.0 (27.0–58.0) | 54.0 (34.0–87.0) | <0.001 |
| ALT | 33.0 (20.0–47.0) | 43.0 (25.0–78.0) | <0.001 |
| Total Bilirubin (mg/dl) | 0.5 (0.3–0.7) | 0.6 (0.4–0.9) | <0.001 |
| Diabetes | 26 (9.8%) | 144 (7.6)% | 0.426 |
| Ethinicity | 0.069 | ||
| Caucasian | 220 (83.0%) | 1442 (76.3%) | |
| African American | 17 (6.4%) | 182 (9.6%) | |
| Hispanic | 21 (7.9%) | 209 (11.1%) | |
| Asian | 2 (0.8%) | 35 (1.9%) | |
| Native American/Other | 5 (1.9%) | 22 (1.2%) | |
| ABO | <0.001 | ||
| A | 97 (36.6%) | 771 (40.8%) | |
| AB | 20 (7.6%) | 20 (1.1%) | |
| B | 38 (14.3%) | 185 (9.8%) | |
| O | 110 (41.5%) | 914 (48.4%) | |
| Cause of Death | 0.003 | ||
| Anoxia | 121 (45.7%) | 994 (52.6%) | |
| CVA | 54 (20.4%) | 279 (14.8%) | |
| Trauma | 70 (26.4%) | 541 (28.6%) | |
| Other | 20 (7.6%) | 76 (4.0%) | |
| IV Drug Use | 47 (17.7%) | 255 (13.5%) | 0.159 |
| Withdrawal to Crossclamp Interval (min) | 24.0 (20.0–27.0) | 23.0 (19.0–26.0) | 0.003 |
| Weekend Recovery | 77 (29.1%) | 485 (25.7%) | 0.238 |
| Night Recovery | 172 (64.9%) | 974 (51.5%) | <0.001 |
Table 4.
Comparison of balance between a propensity score matched cohort of nonrecovered potential and actual donation after cardiac death liver donors using more liberal criteria to define non-recovered potential donors. Standardized differences greater than 0.1 in absolute value are considered significant and highlighted in bold.
| Non-Recovered Potential DCDs (n = 245) | Actual DCDs (n = 245) | Standardized Difference | |
|---|---|---|---|
|
| |||
| Age | 48.0 (34.0–55.0) | 46.0 (37.0–54.0) | −0.02 |
| Male Gender | 179 (73.1%) | 181 (73.9%) | −0.02 |
| Height | 175.3 (168.0–182.0) | 175.0 (168.0–182.9) | −0.05 |
| Weight | 81.2 (71.1–95.0) | 80.7 (70.7–92.3) | −0.05 |
| BMI | 26.9 (23.6–31.0) | 26.3 (23.5–30.7) | −0.05 |
| AST | 43.0 (28.0–58.0) | 36.0 (25.0–51.0) | 0.07 |
| ALT | 33.0 (20.0–46.0) | 28.0 (18.0–40.0) | 0.09 |
| Total Bilirubin | 0.5 (0.3–0.7) | 0.5 (0.3–0.7) | 0.03 |
| Diabetes | 26 (10.6%) | 27 (11.0%) | −0.01 |
| Ethinicity | |||
| Caucasian | 203 (82.9%) | 197 (80.4%) | 0.06 |
| African American | 16 (6.5%) | 17 (6.9%) | −0.02 |
| Hispanic | 20 (8.2%) | 22 (9.0%) | −0.03 |
| Asian | 2 (0.8%) | 2 (0.8%) | 0.00 |
| Native American/Other | 4 (1.6%) | 7 (2.9%) | −0.08 |
| ABO | |||
| A | 92 (37.6%) | 98 (40.0%) | −0.05 |
| AB | 10 (4.1%) | 8 (3.3%) | 0.04 |
| B | 37 (15.1%) | 36 (14.7%) | 0.01 |
| O | 106 (43.3%) | 103 (42.0%) | 0.02 |
| Cause of Death | |||
| Anoxia | 115 (46.9%) | 116 (47.4%) | −0.01 |
| CVA | 51 (20.8%) | 55 (22.5%) | −0.04 |
| Trauma | 63 (25.7%) | 56 (22.9%) | 0.07 |
| Other | 16 (6.5%) | 18 (7.4%) | −0.03 |
| IV Drug Use | 45 (18.4%) | 41 (16.7%) | 0.04 |
| Withdrawal to Crossclamp Interval (minutes) | 24.0 (20.0–27.0) | 23.0 (19.0–27.0) | −0.03 |
| HIV NAT | |||
| Negative | 236 (96.3%) | 234 (95.5%) | 0.04 |
| Not Done | 2 (0.8%) | 1 (0.4%) | 0.05 |
| Prior to Routine Collection | 7 (2.9%) | 10 (4.1%) | −0.07 |
| Hepatitis C NAT | |||
| Positive | 21 (8.6%) | 17 (6.9%) | 0.06 |
| Negative | 217 (88.6%) | 218 (89.0%) | −0.01 |
| Prior to Routine Collection | 7 (2.9%) | 10 (4.1%) | −0.07 |
| Hepatitis C Serology Positive | 32 (13.1%) | 24 (9.8%) | 0.10 |
| Heavy Alcohol Use | |||
| Yes | 76 (31.0%) | 73 (29.8%) | 0.03 |
| No | 163 (66.5%) | 165 (67.4%) | −0.02 |
| Unknown | 6 (2.5%) | 7 (2.9%) | −0.03 |
Fig. 2.
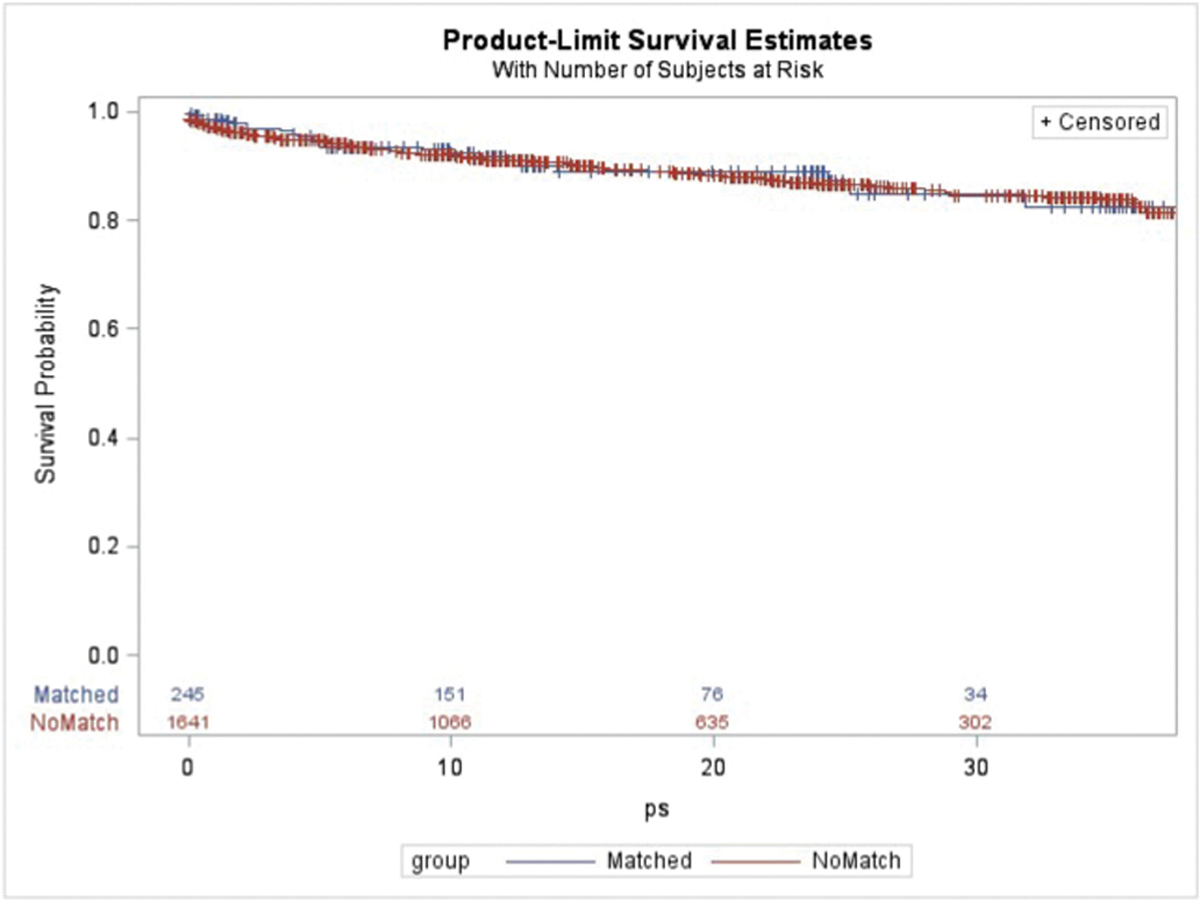
Kaplan-Meier recipient survival using donors matched to the potential donor cohort constructed using more liberal criteria versus the unmatched remaining donation after cardiac death donors.
Geographic analysis demonstrated that the percentage of missed opportunities by UNOS region ranged from 2.0% to 24.5% (Fig. 3). At the DSA level, the percentage of missed opportunities ranged from 0% to 31.8% in the continental US (Fig. 4), and this percentage did not correlate with the percentage of actual DCD livers which were exported versus transplanted locally (R2 = 0.031, p = 0.188; Fig. 5). The median driving time was not different for NRP (30.1 min, IQR 2.9–76.3) and actual liver donors (30.0 min, IQR 3.6–90.2 min; p = 0.618). The percentage of donors outside driving distance was also similar for NRP (15.7%) and actual (16.9%; p = 0.689) donors. The locations of donor hospitals and transplant centers in the study are presented in an interactive online map at https://arcg.is/nHKe4.
Fig. 3.
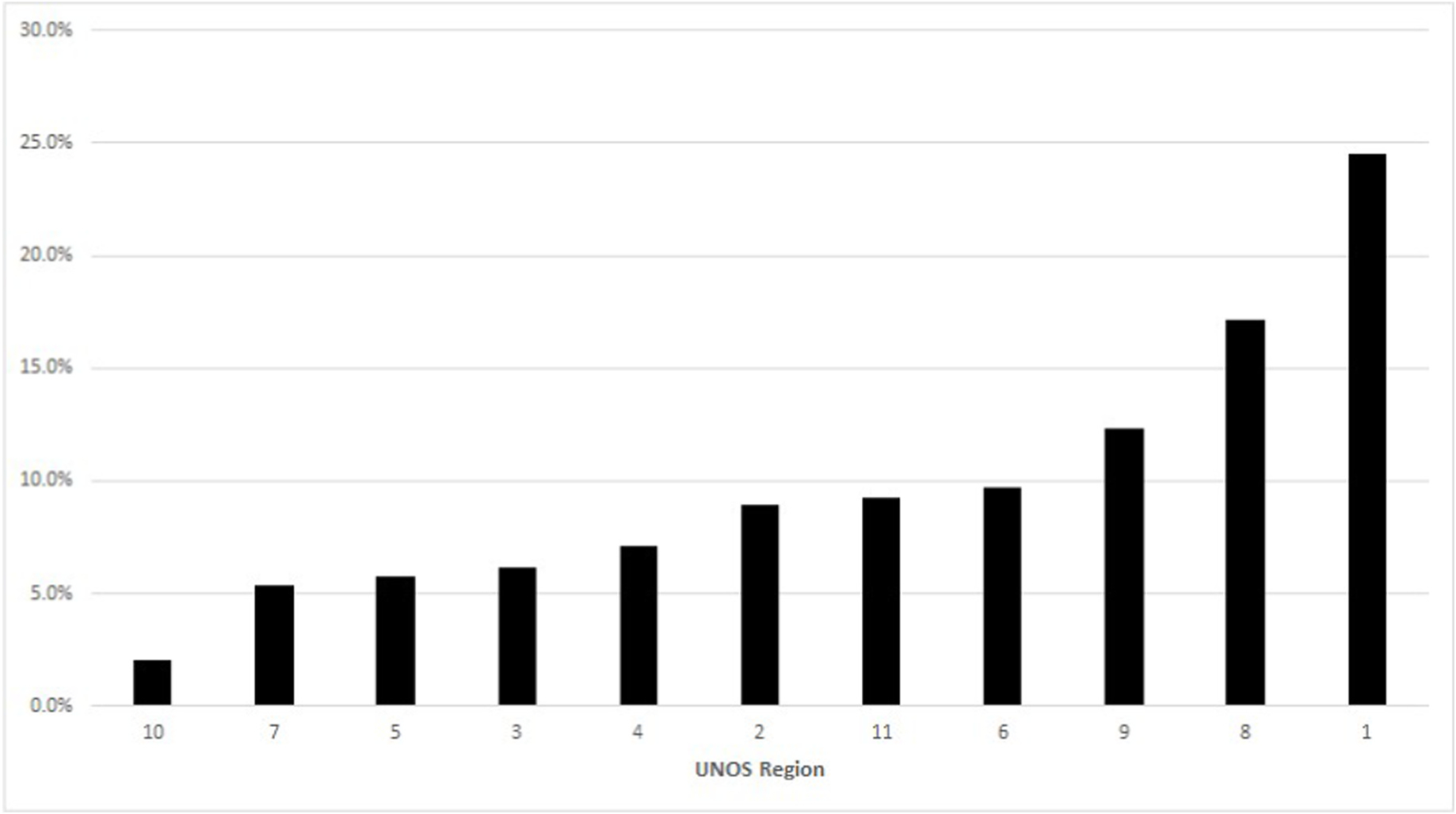
Percentage of missed opportunities for donation after cardiac death liver recovery by UNOS region.
Fig. 4.
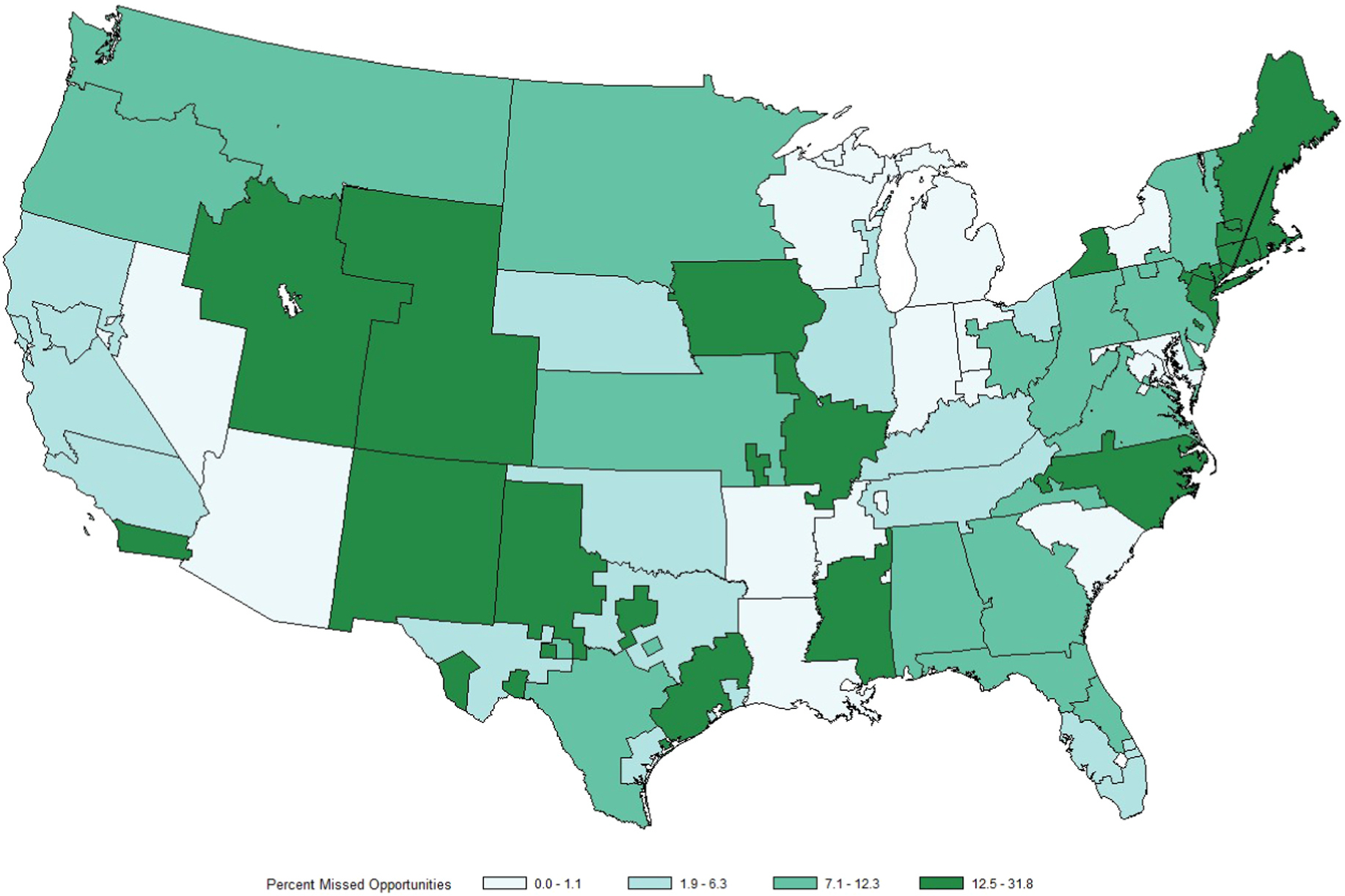
Map of the 56 continental DSAs shaded by percentage of missed opportunities for donation after cardiac death liver recovery.
Fig. 5.
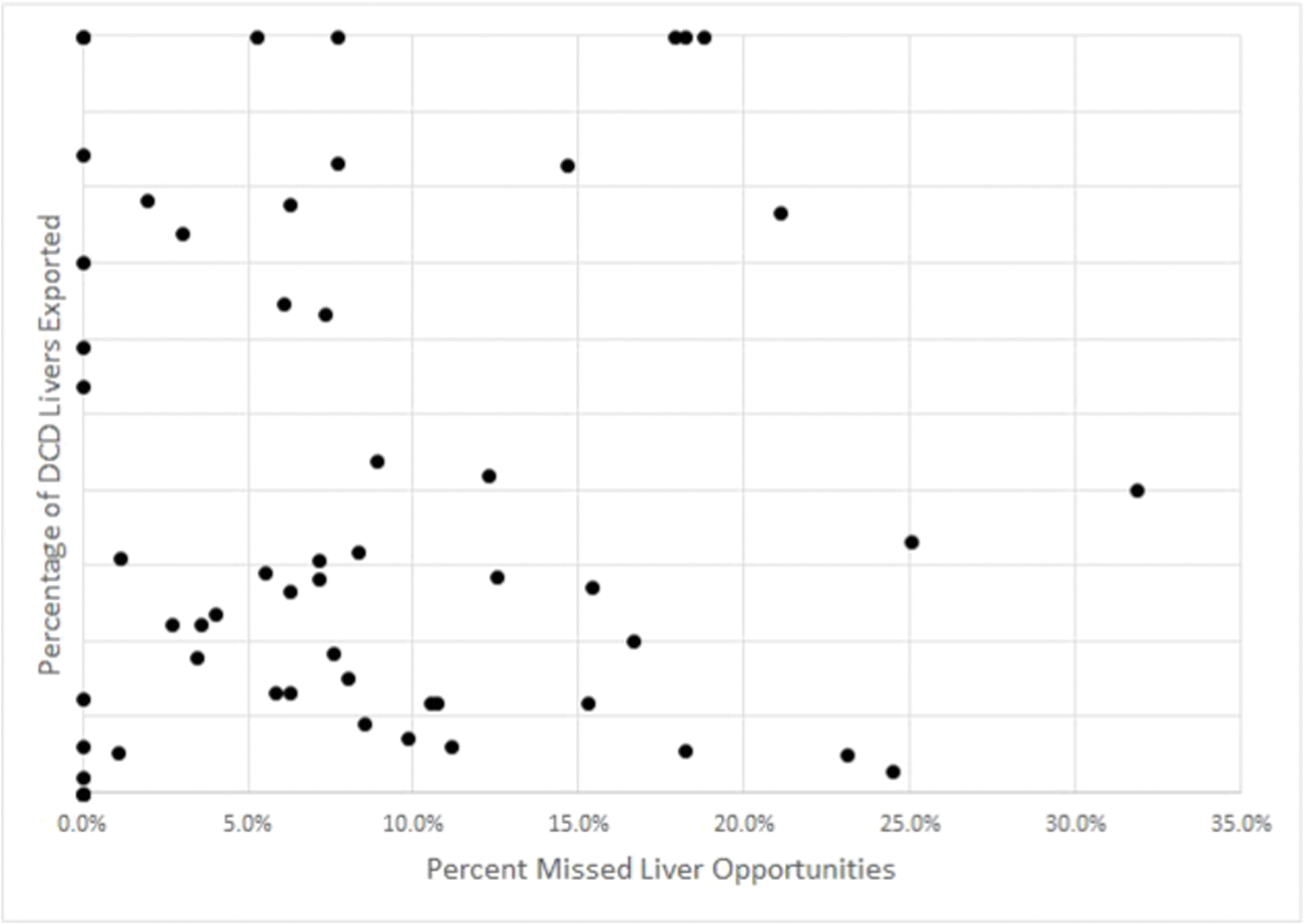
Plot of percentage of missed opportunities after cardiac death liver recovery (x-axis) by DSA versus percentage of donation after cardiac death livers exported by DSA (y-axis)
Online Map: https://arcg.is/nHKe4. Interactive map depicting the locations of hospitals performing DCD liver transplants during the study period (red dots), hospitals with recovered DCD livers (blue dots), and hospitals with non-recovered potential DCD livers (green dots). Click the icon to select layers representing each of the three hospital types above.
Notably, 20.8% of NRP donors and 20.9% (p = 0.984) of actual donors were located in transplant centers. Of the NRP donors located in transplant centers, 21.9% had blood type AB or A compared to 25.4% located in hospitals without a transplant center (p = 0.680).
Multivariable analysis with random intercept for UNOS region indicated that donor age, blood types AB and B, and “other” as a cause of death were significantly associated with being a NRP rather than actual donor (Table 5). The ICC associated with UNOS region on the multilevel logistic model was 0.154 (variance 0.599; p = 0.026), indicating that 15.4% of variation in whether a donor was a NRP vs. actual donor is explained by regional level variation. The model with random intercepts for DSA demonstrated the same significant predictors as the regional random intercept model (Table 6). The ICC for DSA was 0.279 (variance 1.274; p = 0.001), indicating that 27.9% of variation was explained by DSA level variation. Driving time was not a significant predictor in either model.
Table 5.
Multivariable logistic regression model for non-recovered potential versus actual donation after cardiac death liver donors with random intercepts for UNOS region. The probability modeled is that the liver is not recovered. The intraclass correlation coefficient for UNOS region was 0.154. CI: Confidence interval. P values significant at p < 0.05 are in bold.
| Odds Ratio | Lower 95% CI | Upper 95% CI | P-value | |
|---|---|---|---|---|
|
| ||||
| Age (per year) | 1.067 | 1.050 | 1.084 | <0.001 |
| Driving Time (per hour) | 1.009 | 0.867 | 1.174 | 0.907 |
| ABO type (versus tye O) | ||||
| A | 1.000 | 0.672 | 1.489 | 0.998 |
| AB | 15.520 | 6.577 | 36.622 | <0.001 |
| B | 2.052 | 1.211 | 3.477 | 0.008 |
| Donor diabetes | 1.428 | 0.817 | 2.497 | 0.211 |
| Donor Cause of Death (versus anoxia) | ||||
| Cerebrovascular accident | 1.219 | 0.752 | 1.976 | 0.421 |
| Head Trauma | 1.426 | 0.925 | 2.197 | 0.108 |
| Other | 2.759 | 1.358 | 5.604 | 0.005 |
Table 6.
Multivariable logistic regression model for non-recovered potential versus actual donation after cardiac death liver donors with random intercepts for donation service area. The probability modeled is that the liver is not recovered. The intraclass correlation coefficient for donation service area was 0.279. CI: Confidence interval. P values significant at p < 0.05 are in bold.
| Odds Ratio | Lower 95% CI | Upper 95% CI | P-value | |
|---|---|---|---|---|
|
| ||||
| Age (per year) | 1.071 | 1.054 | 1.089 | <0.001 |
| Driving Time (per hour) | 0.996 | 0.842 | 1.179 | 0.967 |
| ABO type (versus tye O) | ||||
| A | 1.021 | 0.677 | 1.540 | 0.920 |
| AB | 20.601 | 8.115 | 52.297 | <0.001 |
| B | 2.178 | 1.256 | 3.775 | 0.006 |
| Donor diabetes | 1.484 | 0.827 | 2.662 | 0.185 |
| Donor Cause of Death (versus anoxia) | ||||
| Cerebrovascular accident | 1.232 | 0.742 | 2.045 | 0.419 |
| Head Trauma | 1.400 | 0.894 | 2.193 | 0.142 |
| Other | 4.086 | 1.906 | 8.760 | <0.001 |
4. Discussion
In this analyses we found 154 donor livers that were discarded despite evidence in the data to suggest that they could have been transplanted with excellent results. Using more lenient criteria, this number increases to 265 non-recovered potential donor livers. Each of these cases represents a potentially missed opportunity to save a life and alleviate suffering. Because these estimates are based on analysis of donors who were actually taken to the OR for organ recovery, we believe that the estimates presented above are actually quite conservative. For organ procurement organizations in areas where transplant programs do not routinely utilize DCD livers, there is little incentive to pursue potential DCD liver donors unless there is potential for donation of other organs. Potential donors that aren’t pursued never make it into national level data on organ donation, and aren’t included in the widely used eligible death metric upon which organ procurement organizations are evaluated. In some donation service areas, the true number of potential donors is double the number of eligible deaths.21 When considering these facts, the number of non-recovered potential liver donors is likely much higher than we have estimated in this study.
Reasons for reluctance to pursue DCD liver donors can be divided into three broad categories: concern over clinical outcomes, workload, and financial considerations. Perhaps one of the more feared complications specific to DCD liver transplantation is development of ischemic cholangiopathy (IC).22–26 Absence of an expedient rescue pathway for retransplantation in patients developing IC was quoted as a significant barrier to greater use of DCD livers by 74% of responding centers in a recent survey.13 In addition to patient suffering induced by IC, there is also a significant financial cost incurred in the management of biliary strictures, which have been estimated to add an incremental cost of over $80,000 per patient.27,28 It is important to note that rates of IC reported vary widely in the literature, ranging from 7.0%29,30 up to 44%,23 with lower rates typically found in more recent studies. Since specific complication rates were not reported in UNOS data, the majority of the literature on IC rates comes from single center studies. The first report from the multicenter Improving DCDD Outcomes in Liver Transplantation (IDOL) consortium published in 2017 demonstrated an 11.8% rate of IC,31 further confirming improved outcomes with DCD transplantation in the current era.
We intentionally chose conservative criteria to define the cohort of NRP donors in order to minimize the likelihood that unfavorable donor characteristics backed by evidence would be a reason for failure to recover the liver. We recognize that the upper limit of age we set (59 years) in defining a high quality donor is above thresholds set by earlier single center studies as a risk factor for worse outcomes23,24; however, we believe that current literature supports this choice. In the IDOL consortium report, 21.3% of the donors were in the 50–59 year old strata, and age was not found to be significantly associated with IC.31 In a study by the three Mayo Clinic programs specifically examining DCD donors age 50 or older, there was no difference in graft survival or ischemic cholangiopathy between the 50 and over and under 50 year old donor cohorts.7 The UK DCD risk score, which was validated using UNOS data, includes donors up to age 59 in the low risk category.32 Finally, the analysis of outcomes in recipients of liver transplants from DCD donors matched to the NRP donor cohort in the present study further confirms our choice of selection criteria regarding quality of the NRP donors.
Cold ischemia time is another oft-cited risk factor for inferior outcomes in DCD liver transplantation,7,32 with 6–8 h being commonly cited thresholds for optimal outcomes. If concern for prolonged cold ischemia time was a major factor into the decision not to pursue liver donation from the potential donor cohort, then we would expect to see a greater proportion of donor hospitals located far from a transplant center in the group of donors where the liver was not recovered. Instead, we found that the median driving time from the donor hospital to the nearest transplant center for the NRP donors was only 30 min, and 75% of NRP donors were within a 76-min drive of the nearest transplant center performing DCD liver transplantation. These distances were statistically (and numerically, for that matter) strikingly similar to those seen in the actual cohort. As such, we have to conclude that potential for prolonged cold ischemia time was likely not a major factor for failure to recover livers from the NRP donor cohort. A donor’s clinical history may lead to suspicion of a diseased liver that is not necessarily reflected in laboratory parameters. An obese donor with a history of diabetes, for example, would be at increased risk for hepatic steatosis, and the presence of these additional risk factors may tip the balance away from pursuing a DCD liver. Notably, our study did not find any significant difference in diabetes or BMI between the NRP and actual donor cohorts.
Having determined that unfavorable donor characteristics and cold ischemia times were unlikely to be the reason for discard of these seemingly high quality donor livers, we must turn to workload and financial considerations. Warm ischemia times of greater than 30 min are widely considered to convey unacceptable risk for DCD liver transplantation.13,32 In a recent single center study, Montgomery and colleagues found that only 47.9% of accepted DCD liver donors ultimately resulted in liver transplantation. The high number of “dry runs” in their study translated into 218 additional miles that were traveled per successful DCD liver in comparison to travel per successful donation after brain death liver.33 Although reimbursement arrangements vary widely, it is common for centers and recovery surgeons to be paid significantly less when a liver is not recovered from a donor. We are even aware of some organ procurement organizations (OPOs) that do not reimburse recovery surgeons at all for dry runs, which serves to further hamper enthusiasm for pursuing a donor not expected to expire in time for donation.
When considering the time and resources that must be invested to pursue a potential donor, it is understandable that programs in some cases may not wish to pursue a potential donor liver if they believe the donor is unlikely to expire within an acceptable timeframe. The fact that the NRP liver donors in this study all donated other organs somewhat mitigates this explanation as, by definition, a recovery team was already being dispatched to the donor. Other time and resource expenditures exist outside of those directly involved in the recovery operation, however, including admitting a recipient to the hospital, mobilizing an OR team, and potentially arranging for separate air transportation for the liver in the case of a remote donor hospital. The logistical challenges are potentially greater when donor recovery takes place during a night or weekend, when less personnel are available to assist with these frequently complex cases. These difficulties may explain our finding that the percentage of NRP donors is greater for donors undergoing withdrawal of life support at night or on a weekend. Alternatively, given the decreased number of organs procured from the NRP donors, centers may not have given these donors priority for a daytime recovery to minimize disruption to normal OR operations.
Our multilevel model revealed that 27.6% of the variation in whether a donor liver was pursued could be explained by the donation service area (DSA) of the donor hospital. The fact that non-hepatic organs were recovered from these donors means that the OPOs involved were willing to pursue the donor, so the decision to not pursue the livers from these donors likely lies with the local and regional transplant centers. In fact, some of the DSAs with the highest rate of missed opportunities for liver donation identified in this study are the territory of OPOs with high overall percentages of DCD donation. Wide center level variation in DCD utilization has been previously described,14 lending further credence to the idea that transplant center practice patterns play a large role in the variation seen in the current study. Multicenter collaborations such as the Improving DCDD Outcomes in Liver Transplantation (IDOL) consortium31 may help in disseminating best practices and improving DCD utilization by centers that have traditionally utilized DCD organs infrequently or not at all. Visual inspection of Fig. 4 demonstrates that some areas with higher percentages of missed DCD opportunities correspond to regions with higher allocation MELD scores. This finding is in keeping with Hobeika and colleagues’ observation that high allocation MELD scores at transplant do not necessarily translate into more aggressive DCD utilization.14
There are a number of limitations to this study. Without having the actual match run data for each donor, we can’t definitively say that there were suitable recipients available for all NRP donors identified in the study, nor do we know how far down the match run the host OPO went in attempting to allocate the liver. As Croome and colleagues have previously noted, “when local centers do not routinely pursue these organs, OPOs may falsely assume that other regional and national transplant programs also would not be interested”.5 Without match run level data, we don’t know how aggressively host OPOs pursued allocation beyond the initial local centers. We also don’t have data that would inform estimation of the probability that the donors would expire such as reflexes, respiratory rate, and sedation. Such information would have provided valuable insight into the possible decision-making processes leading to declining to pursue the liver. Predicting which donors will expire has long been a challenge, with a number of scoring systems devised that all are relatively limited in utility.34 A marginal donor that may be pursued if an expeditious arrest is expected may wind up being declined if a more drawn out agonal period is anticipated.
With these limitations in mind, we have found in this study a relatively small, but significant, pool of seemingly high quality DCD livers which were buried with their donors due to lack of interest in pursuing them. Some reasons for the lack of enthusiasm regarding these livers can be gleaned from the data, most notably the donor blood type. The potential donors in this study were significantly enriched with ABO type B and AB compared to the cohort of donors where the liver was procured. Given decreased competition on the waitlist for these blood types, it indeed may not be in the best interest of some blood type B and AB candidates to accept a DCD liver when their likelihood of getting a DBD liver is higher than if they had a more common blood type. This is particularly the case if their MELD scores are low. With the current shortage of pilots and high cost of charter aircraft, it is also reasonable that centers may not wish to pursue donors requiring a fly-out if the likelihood of expiration in an acceptable timeframe is thought to be low. More concerning is the 1 in 5 potential donors located in hospitals with a transplant center. It is one thing to be unwilling or unable to pay for an airplane and potentially risk the safety of a team to pursue a donor thought to have low potential to yield a transplantable organ, it is quite another to be unwilling to walk down the hallway. We recognize that the pool of organs represented in this study represents only a fraction of the shortfall between the number of patients needing a transplant and the number of available organs. Nonetheless, these organs could have saved the lives of a significant number of patients across the country. At a time when we are overhauling our allocation system to fly more livers over longer distances than at any time in the past in order to get organs to those in need, it remains incumbent upon all of us to be as aggressive as possible in pursuing the organs in our own back yards.
Supplementary Material
Acknowledgement
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Funding sources
This work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K08DK125769 (PI: Cannon). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- LT
Liver Transplant
- DCD
Donation after cardiac death
- WIT
Warm ischemia time
- UNOS
United Network for Organ Sharing
- STAR
Standard Transplant Analysis and Research
- BMI
Body mass index
- HBV
Hepatitis B virus
- NAT
Nucleic acid testing HCV Hepatitis C virus
- NRP
Nonrecovered potential
- DSA
Donation service area
- ICC
Intraclass correlation coefficient
- IQR
Interquartile range
- IDOL
Improving DCDD outcomes in liver transplantation
- OPO
Organ procurement organization Acknowledgement
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to declare relative to the contents of this manuscript.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjsurg.2022.05.001.
References
- 1.OPTN. Organ Procurement and transplantation network national data. Accessed 05/04/2020, https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/.
- 2.Northup PG, Intagliata NM, Shah NL, Pelletier SJ, Berg CL, Argo CK. Excess mortality on the liver transplant waiting list: unintended policy consequences and Model for End-Stage Liver Disease (MELD) inflation. Hepatology. 2015;61(1):285–291. 10.1002/hep.27283. Jan. [DOI] [PubMed] [Google Scholar]
- 3.Kollmann D, Sapisochin G, Goldaracena N, et al. Expanding the donor pool: donation after circulatory death and living liver donation do not compromise the results of liver transplantation. Liver Transplant. 2018;24(6):779–789. 10.1002/lt.25068. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manyalich M, Nelson H, Delmonico FL. The need and opportunity for donation after circulatory death worldwide. Curr Opin Organ Transplant. 2018;23(1):136–141. 10.1097/MOT.0000000000000486. Feb. [DOI] [PubMed] [Google Scholar]
- 5.Croome KP, Lee DD, Keaveny AP, Taner CB. Noneligible donors as a strategy to decrease the organ shortage. Am J Transplant. 2017;17(6):1649–1655. 10.1111/ajt.14163. Jun. [DOI] [PubMed] [Google Scholar]
- 6.Scalea JR, Redfield RR, Foley DP. Liver transplant outcomes using ideal donation after circulatory death livers are superior to using older donation after brain death donor livers. Liver Transplant. 2016;22(9):1197–1204. 10.1002/lt.24494. Sep. [DOI] [PubMed] [Google Scholar]
- 7.Croome KP, Mathur AK, Lee DD, et al. Outcomes of donation after circulatory death liver grafts from donors 50 Years or older: a multicenter analysis. Transplantation. 2018;102(7):1108–1114. 10.1097/TP.0000000000002120. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihaylov P, Mangus R, Ekser B, et al. Expanding the donor pool with the use of extended criteria donation after circulatory death livers. Liver Transplant. 2019;25(8):1198–1208. 10.1002/lt.25462. Aug. [DOI] [PubMed] [Google Scholar]
- 9.Grewal HP, Willingham DL, Nguyen J, et al. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver Transplant. 2009;15(9):1028–1035. 10.1002/lt.21811. Sep. [DOI] [PubMed] [Google Scholar]
- 10.Laing RW, Scalera I, Isaac J, et al. Liver transplantation using grafts from donors after circulatory death: a propensity score-matched study from a single center. Am J Transplant. 2016;16(6):1795–1804. 10.1111/ajt.13699. Jun. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R, Allen E, Richards JA, et al. Survival advantage for patients accepting the offer of a circulatory death liver transplant. J Hepatol. 2019;70(5):855–865. 10.1016/j.jhep.2018.12.033. May. [DOI] [PubMed] [Google Scholar]
- 12.Kwong A, Kim WR, Lake JR, et al. OPTN/SRTR 2018 annual data report: liver. Jan Am J Transplant. 2020;20(suppl s1). 10.1111/ajt.15674, 193–299. [DOI] [PubMed] [Google Scholar]
- 13.Sher L, Quintini C, Fayek SA, et al. Attitudes and barriers to the use of donation after cardiac death livers: comparison of a United States transplant center survey to the united network for organ sharing data. Liver Transplant. 2017;23(11):1372–1383. 10.1002/lt.24855. Nov. [DOI] [PubMed] [Google Scholar]
- 14.Hobeika MJ, Menser T, Nguyen DT, Beal LL, Zajac S, Graviss EA. United States donation after circulatory death liver transplantation is driven by a few high-utilization transplant centers. Am J Transplant. 2020;20(1):320–321. 10.1111/ajt.15629. Jan. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. 10.1080/00273171.2011.568786. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. Apr. [DOI] [PubMed] [Google Scholar]
- 17.CMS. Hospital General information. Accessed 02/2020, https://data.medicare.gov/Hospital-Compare/Hospital-General-Information/xubh-q36u.
- 18.Bittermann T, Hubbard RA, Lewis JD, Goldberg DS. The use of induction therapy in liver transplantation is highly variable and is associated with posttransplant outcomes. Am J Transplant. 2019;19(12):3319–3327. 10.1111/ajt.15513. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med. 2017;36(20):3257–3277. 10.1002/sim.7336. Sep. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. 10.1177/096228029900800102. Mar. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg D, Kallan MJ, Fu L, et al. Changing metrics of organ procurement organization performance in order to increase organ donation rates in the United States. Am J Transplant. 2017;17(12):3183–3192. 10.1111/ajt.14391. Dec. [DOI] [PubMed] [Google Scholar]
- 22.Tang JX, Na N, Li JJ, Fan L, Weng RH, Jiang N. Outcomes of controlled donation after cardiac death compared with donation after brain death in liver transplantation: a systematic review and meta-analysis. Transplant Proc. 2018;50(1):33–41. 10.1016/j.transproceed.2017.11.034. Jan - Feb. [DOI] [PubMed] [Google Scholar]
- 23.Jay CL, Lyuksemburg V, Kang R, et al. The increased costs of donation after cardiac death liver transplantation: caveat emptor. Ann Surg. 2010;251(4):743–748. 10.1097/SLA.0b013e3181d3d3da. Apr. [DOI] [PubMed] [Google Scholar]
- 24.Foley DP, Fernandez LA, Leverson G, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253(4):817–825. 10.1097/SLA.0b013e3182104784. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meurisse N, Vanden Bussche S, Jochmans I, et al. Outcomes of liver transplantations using donations after circulatory death: a single-center experience. Transplant Proc. 2012;44(9):2868–2873. 10.1016/j.transproceed.2012.09.077. Nov. [DOI] [PubMed] [Google Scholar]
- 26.Skaro AI, Jay CL, Baker TB, et al. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Oct Surgery. 2009;146(4):543–552. 10.1016/j.surg.2009.06.052.; discussion 552–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhutiani N, Jones JM, Wei D, et al. A cost analysis of early biliary strictures following orthotopic liver transplantation in the United States. Clin Transplant. 2018;32(10), e13396. 10.1111/ctr.13396. Oct. [DOI] [PubMed] [Google Scholar]
- 28.Jones JM, Bhutiani N, Wei D, Goldstein L, Jones CM, Cannon RM. A literature-based cost analysis of tissue plasminogen activator for prevention of biliary stricture in donation after circulatory death liver transplantation. Am J Surg. 2018;216(5):959–962. 10.1016/j.amjsurg.2018.04.004. Nov. [DOI] [PubMed] [Google Scholar]
- 29.Vanatta JM, Dean AG, Hathaway DK, et al. Liver transplant using donors after cardiac death: a single-center approach providing outcomes comparable to donation after brain death. Exp Clin Transplant. 2013;11(2):154–163. 10.6002/ect.2012.0173. Apr. [DOI] [PubMed] [Google Scholar]
- 30.Firl DJ, Hashimoto K, O’Rourke C, et al. Impact of donor age in liver transplantation from donation after circulatory death donors: a decade of experience at Cleveland Clinic. Liver Transplant. 2015;21(12):1494–1503. 10.1002/lt.24316. Dec. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg DS, Karp SJ, McCauley ME, et al. Interpreting outcomes in DCDD liver transplantation: first report of the multicenter IDOL consortium. Transplantation. 2017;101(5):1067–1073. 10.1097/TP.0000000000001656. May. [DOI] [PubMed] [Google Scholar]
- 32.Schlegel A, Kalisvaart M, Scalera I, et al. The UK DCD Risk Score: a new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol. 2018;68(3):456–464. 10.1016/j.jhep.2017.10.034. Mar. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery JR, Highet A, Hobeika MJ, Englesbe MJ, McElroy LM. Going the distance for procurement of donation after circulatory death livers for transplantation-Does reimbursement reflect reality? Clin Transplant. 2020;34(2), e13780. 10.1111/ctr.13780. Feb. [DOI] [PubMed] [Google Scholar]
- 34.Cannon RM. Prediction of organ donation after circulatory death: in search of a better crystal ball. Transplantation. 2021;105(6):1165–1166. 10.1097/TP.0000000000003431. Jun 1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


