Abstract
Gastric adenocarcinomas are lesions that raise important issues in clinical practice, due to their incidence and biological behavior. Over time, various systems have been used for classifying and grading of gastric adenocarcinomas, in the hope of increasing the diagnostic accuracy. In this study we statistically analyzed 112 cases of gastric adenocarcinomas in relation to different classification and grading systems, in order to identify their efficacy and concordance in the histopathological diagnosis. The results indicated a significant association of the Lauren and World Health Organization 2019 classifications and also between these and the three-tier and two-tier grading systems, which supports their practical utility in establishing the diagnosis and assessment of the tumor aggressiveness, for the differentiated therapy.
Keywords: Gastric adenocarcinoma , histopathological type , degree of differentiation , Lauren classification , WHO classification
Introduction
Gastric adenocarcinomas represent the most frequent gastric malignant tumors, which are generally characterized by an aggressive biological behaviour [1].
Although the incidence of these tumors has decreased in recent years due to the screening programs and eradication of Helicobacter pylori infection, they still hold the fifth place in the world when within human malignant neoplasms [2].
Moreover, the mortality rate is relatively high, ranking third as a cause of death from cancer [1].
Over time, there have been various systems for classifying and grading of the gastric adenocarcinomas.
The most used classification was the one proposed by Lauren P. in 1965, who divided the gastric malignant epithelial tumors into five types, respectively intestinal, diffuse, mixed, indeterminate and not defined [3].
The latest classification was elaborated by the WHO (World Health Organization) working group for gastric tumors in 2019, which introduced new histopathological types of gastric carcinomas, upgrading the existing ones in terms of correspondence [4].
Previous published research indicated the practical superiority of the Lauren classification, but currently, the WHO 2019 classification is being adopted because it provides more detailed information regarding the histopathological features of epithelial malignancies [5, 6].
Another important parameter for assessing the aggressiveness of gastric tumors is represented by the degree of differentiation.
Also, for this parameter, several grading systems were used, formed by two or three-tiers of assessment.
Most of the studies performed up until 2019 were conducted by utilizing the three-tier system, respectively poorly, moderate and well differentiated.
Subsequently, the WHO working group for gastric tumors indicated the use of the two-tier grading system, respectively low and high-grade, which revealed a superior inter-and intraobservatory concordance of the diagnosis, as well as a higher correspondence in relation with tumor aggressiveness 4, 6, 7].
However, the behavior of gastric adenocarcinomas remains unpredictable, an aspect which requires a permanent improvement of the histopathological assessment criteria.
In this study we analyzed the gastric adenocarcinomas by comparative reporting to the classical grading and classification systems Lauren and WHO in order to establish their concordance.
Materials and Methods
The study included 112 cases of gastric adenocarcinomas diagnosed in the Pathology Department of the Clinical Emergency County Hospital Craiova during 4 years (2017-2020).
The biological material was represented by surgical total gastrectomy specimens which were fixed in 10% formalin, processed by the classic paraffin embedding technique and stained with hematoxylin-eosin.
The classification of lesions was performed separately according to the Lauren classification, the latest WHO classification, the three-tier grading system (well differentiated-G1, moderate differentiated-G2, poorly differentiated-G3) and the two-tier grading system (low, high) [3, 4, 8].
The evaluation for the two classification and grading systems was performed separately by two experienced pathologists who participated in the study.
The obtained data was collected, stored and processed using Microsoft Office Excel 2010 function and the SPSS 12 (Statistical Package for the Social Sciences) software.
The statistical analysis followed the concordance of the used classification and grading systems by highlighting the differences in distribution within the tumor groups.
Within our scientific research, the ethical aspects were respected based on the patients informed consent, the study being endorsed by the Local Ethics Committee (no.151/24.09.2021).
Results
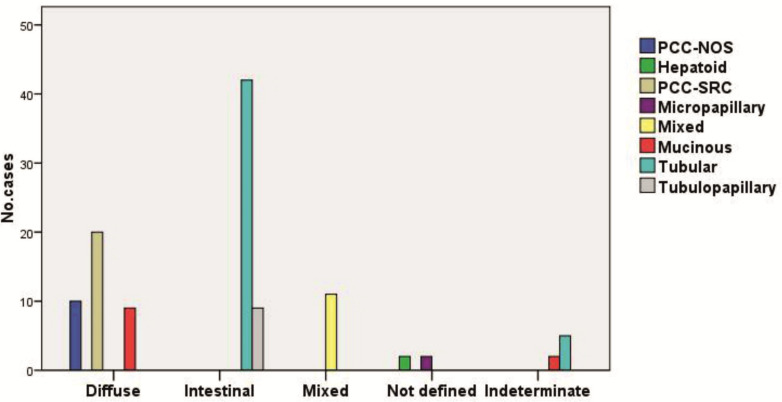
The study included 112 gastric adenocarcinomas, and following the histopathological type analysis in relation to the Lauren classification system, it was observed that the intestinal type was the most common, being identified in 51 cases (45.5%).
Compared to the WHO system, these tumors corresponded in 42 cases to the tubular type and 9 cases to the tubulopapillary type (Table 1).
Table 1.
Distribution of cases according to the histopathological type.
|
Lauren classification (no. cases) |
WHO 2019 classification (no. cases) |
|
Intestinal (51) |
Tubular (42) |
|
Tubulopapillary (9) | |
|
Diffuse (39) |
PCC-SRC (20) |
|
PCC-NOS (10) | |
|
Mucinous (9) | |
|
Mixed (11) |
Mixed (11) |
|
Not defined (4) |
Hepatoid (2) |
|
Micropapillary (2) | |
|
Indeterminate (7) |
Mucinous (2) |
|
Tubular (5) |
Regarding the frequency in Lauren classification, on the second place were identified the diffuse gastric adenocarcinomas in 39 cases (34.8%), tumors which in relation to the WHO classification were represented by poorly cohesive carcinomas with signet-ring cell type (PCC-SRC) in 20 cases, poorly cohesive non-signet-ring cell type carcinomas (PCC-NOS) in 10 cases and mucinous carcinomas in 9 cases (Table 1).
In both histopathological classification systems, the same 11 cases (9.8%) of mixed gastric adenocarcinomas were identified (Table 1).
In Lauren classification, there were 4 cases (3.6%) of not defined gastric adenocarcinoma type.
Compared to the WHO system, these tumors were represented by two cases of hepatoid type and 2 cases of micropapillary type of gastric adenocarcinoma (Table 1).
The indeterminate type was identified in 7 cases (6.3%), of which 2 cases corresponded to the mucinous type and 5 cases to the tubular type within the WHO classification (Table 1).
In this study, the statistical analysis of the classification systems indicated a significant association, expressed by a high concordance of the encountered histopathological types (p<0.001, χ2 test) (Figure 1).
Figure 1.
Distribution of cases in relation to the Lauren and WHO 2019 classification systems
In this study, each histopathological type presented a few particular aspects.
Thus, tubular gastric adenocarcinomas were identified in 42% of cases, most of them being low grade, according to the latest histopathological criteria.
These cases presented tubular/glandular architecture with branched, tortuous and anastomosed structures (Figure 2A).
Figure 2.
Gastric adenocarcinoma, HE staining, x200. A. Tubular adenocarcinoma. B. PCC-SRC.
C. PCC-NOS. D. Mucinous adenocarcinoma. E. Mixed adenocarcinoma. F. Hepatoid adenocarcinoma.
The cases of tubulopapillary gastric adenocarcinoma were present in 8% of cases, most of them being of low grade and were characterized by a mixed tubular and papillary architecture with obvious fibrovascular connective cores.
PCC-SRC cases were present in 17.9% of cases, most of them being of high grade and characterized by the exclusively or primarily presence of cells filled with cytoplasmic mucin droplets and peripheral placed nucleus, with discohesive architecture or glandular lace-like or microtrabecular patterns (Figure 2B).
PCC-NOS was identified in 8.9% of cases and all lesions were of high grade.
The tumor cells composing this type of carcinoma were cells with intensely eosinophilic cytoplasm, rare signet-ring cells and cells resembling lymphocytes and histiocytes (Figure 2C).
The mucinous type was identified in 9.8% of cases, of which most were high grade tumors.
In this type it was observed that the presence of extracellular mucin pools was more than 50% of the tumor area.
Moreover, there were present glandular, papillary structures, isolated or small nests of signet-ring cells or other cell type surrounded by mucin (Figure 2D).
The mixed type of gastric adenocarcinoma was identified in 9.8% of cases, of which most were high grade tumors and characterized by the presence of at least two histopathological components, more frequently with tubular and discohesive aspects (Figure 2E).
Micropapillary and hepatoid gastric adenocarcinomas were high grade tumors, each being present in 1.8% of cases.
The micropapillary type was characterized by the presence of small papillary structures without fibrovascular cores, while in the hepatoid type there were observed large polygonal tumor cells with eosinophilic cytoplasm similar to hepatocytes, with marked nuclear atypia, mostly arranged in cords (Figure 2F).
According to the three-tier grading system, most tumors classified within the Lauren classification were poorly differentiated (G3), respectively in 60 cases (53.6%).
Of these, 33 cases were of diffuse type, 11 cases of intestinal type, 7 cases of mixed type, 4 cases of not defined type and 5 cases of indeterminate type.
In comparison, moderately differentiated (G2) carcinomas were present in 44 cases (39.3%) and well-differentiated (G1) in 8 cases (7.1%) (Table 2).
Table 2.
Distribution of cases according to histopathological type and degree of differentiation within Lauren system
|
Lauren classification |
Three-tier grading system |
||
|
G1 |
G2 |
G3 |
|
|
Intestinal |
7 |
33 |
11 |
|
Diffuse |
1 |
5 |
33 |
|
Mixed |
0 |
4 |
7 |
|
Not defined |
0 |
0 |
4 |
|
Indeterminate |
0 |
2 |
5 |
|
Total |
8 |
44 |
60 |
Compared to the two-tier grading system, G3 tumors were high grade lesions and G1/G2 tumors were low grade lesions.
On the other hand, conforming to the three-tier grading system, most cases in the WHO classification were poorly differentiated (G3), observed in 60 cases (53.6%).
In this group, there were identified 17 cases of PCC-SRC, 13 cases of tubular type, 10 cases of PCC-NOS type, 8 cases of mucinous type, 7 of mixed type, 2 cases of micropapillary type, 2 cases of hepatoid type and a single tubulopapillary case.
By comparison, moderately differentiated carcinomas (G2) were present in 44 cases (39.3%), most being tubular or tubulopapillary, while well differentiated carcinomas (G1) were found in 8 cases (7.1%), most being of tubular type (Table 3).
Table 3.
Distribution of cases according to histopathological type and degree of differentiation within WHO system.
|
WHO classification |
Three-tier grading system |
||
|
G1 |
G2 |
G3 |
|
|
Tubular |
6 |
28 |
13 |
|
Tubulopapillary |
1 |
7 |
1 |
|
PCC-SRC |
0 |
3 |
17 |
|
PCC-NOS |
0 |
0 |
10 |
|
Micropapillary |
0 |
0 |
2 |
|
Mixed |
0 |
4 |
7 |
|
Mucinous |
1 |
2 |
8 |
|
Hepatoid |
0 |
0 |
2 |
|
Total |
8 |
44 |
60 |
The relation between the WHO classification and the two-tier grading system was the same as in the case of Lauren classification, respectively G3 tumors were high grade lesions and G1/G2 were low grade lesions.
The analysis of the case distribution in relation with the analyzed classification and grading systems indicated a significant association of both classifications with the three-tier grading system (p<0.001, χ2 test), and also with the two-tier system (p<0.001, χ2 test), which supports the effectiveness in establishing the diagnosis and prognosis of the lesions (Figure 3 A-D).
Figure 3.
Distribution of cases according to the Lauren classification (A,B) and WHO 2019 (C,D)
and the three-tier (A,C) and two-tier (B,D) grading systems; G1 (well differentiated);
G2 (moderate differentiated); G3 (poorly differentiated); LG (low grade); HG (high grade).
Discussions
Gastric adenocarcinomas represent important oncological lesions due to their incidence, mortality and morbidity, which have generated interest in developing a well-established system for diagnosis, classification and therapeutic management [5].
Over time, various histopathological classification systems were developed for gastric adenocarcinomas.
The most known and used classification systems are the ones proposed by Lauren P. in 1965 and the most recent one developed by the WHO working group on gastrointestinal malignancies and published in 2019 [3, 4, 5].
The classification developed by Lauren P. introduced two main types of gastric adenocarcinomas, respectively intestinal and diffuse type, as well as other types that have lower incidence [3].
On the other hand, the WHO 2019 system has developed a more elaborate classification based on the histopathological and molecular characteristics of gastric adenocarcinomas, including the rare types that are identified in Lauren's classification as indeterminate or not defined gastric adenocarcinomas [3, 4].
There were also proposed other classification systems for the diagnosis of gastric adenocarcinomas, such as the system introduced by Goseki N in 1992, which classified the lesions into four categories using the degree of differentiation of glandular structures and the amount of mucin present in tumor cells [9].
Moreover, Ming SC proposed in 1977 a classification that relates to the growth pattern of the tumor and the degree of invasion, whereas Nakamura K elaborated in 1968 a classification that divided gastric adenocarcinomas into differentiated, undifferentiated and unclassified types [4,10, 11].
However, many studies indicated the practical superiority and prognostic concordance of the classifications proposed by Lauren P. and WHO [5, 12].
Data from the literature report that the most common histopathological type of gastric adenocarcinoma is the intestinal type [12, 13].
Also, similar data was obtained by Sarrugarte LA et al., indicating that in the majority of cases, the intestinal gastric adenocarcinomas were moderately differentiated [12].
On the other hand, Chen YC et al. reported that the most intestinal cases were of low grade [14].
Similarly, these results were also obtained in the study conducted by Tang D. et al. in 2021 [15].
The present study identified most cases of gastric adenocarcinomas as intestinal type (45.5%), most of them being moderately differentiated in the three-tire grading system, and low grade in the two-tier grading system.
The tubular and tubulopapillary types are considered to correspond to the intestinal type in the Lauren classification.
Tubular adedenocarcinomas were identified in more cases then tubulopapillary lesions (47% vs. 8%), most of both types having low grade differentiation.
Reports in the literature support the predominance of tubular type and high grade of differentiation [16].
In 2021, Tang D et al. reported that the diffuse type of gastric adenocarcinomas were in the second place as frequency, and most cases were of high grade, data which is also supported by Ning FL et al. research report [15, 17].
Similar aspects were identified in our study regarding both the frequency of cases of diffuse gastric adenocarcinoma (34.8%) and the degree of differentiation.
In the WHO classification the PCC-SRC and PCC-NOS types correspond to the diffuse type [4].
Multiple studies reported a predominance of the PCC-NOS cases over PCC-SRC, in both types most of them being poorly differentiated [12, 18].
On the other hand, in our study we identified a higher percentage of PCC-SRC cases (17.9%) compared with PCC-NOS (8.9%).
The degree of differentiation of both types of gastric adenocarcinoma was similar to the data in literature.
In the present study, we identified 11 cases (9.8%) of mucinous type, most of them being of high grade and classified in the Lauren classification system as diffuse or indeterminate type.
The mucinous gastric adenocarcinoma is characterized by the presence of mucin pools and an aggressive biological behavior in early stages, but with a more favorable prognosis compared to PCC-SRC [18].
Tseng CH et al. reported an incidence of 3.5% for mucinous gastric adenocarcinomas, whereas Lim SW et al. observed the lesions in 6.5% of cases, the majority being of high grade [20, 21].
In both classification systems, the mixed type gastric adenocarcinomas are defined by the association of at least two histopathological types, which may have varying degrees of differentiation [22, 23].
In our study, we observed 11 cases (9.8%) of mixed type, the majority being of high grade.
In the present study, we identified two cases (1.8%) of high grade hepatoid gastric adenocarcinoma.
Similar data from literature reported an incidence below 1% of cases, most of them presenting a low grade of differentiation [24, 25, 26].
The micropapillary type is a newly studied histopathological type, with low incidence and poor prognosis due to the high rate of lymphatic dissemination [13].
Guzinska-Ustymowicz K et al. reported that most cases of micropapillary lesions are moderately differentiated [27].
In the present study, the micropapillary type was identified in two cases (1.8%), both of them being of high grade.
The studies conducted until our days have indicated a correspondence between various types of gastric adenocarcinomas and the degree of tumor differentiation.
Thus, while tubular or tubulopapillary carcinomas are mostly of low grade, PCC-SRC, PCC-NOS, micropapillary and hepatoid are predominantly of high grade, and for the mixed ones, the aspect of differentiation is variable in relation to the existing tumor components [16, 18, 21, 28].
The assessment of the differentiation degree for gastric adenocarcinomas is an important step in implementing a suitable therapeutic protocol.
Multiple studies have shown a possible association between the differentiation degree of gastric adenocarcinomas and the depth of invasion, the presence of metastases and cancer stage [28, 29].
Consequently, the tumor differentiation degree is an important criterion in the assessment of patient’s prognosis [28, 30].
The aspects were confirmed in our study, in which we found statistically significant associations of the two classification systems with each of the grading systems used, which suggests the usefulness of these systems in assessing the tumor aggressiveness.
Conclusions
The study indicated a high concordance of the classification and grading systems in gastric adenocarcinomas, which supports their effectiveness in the elaboration of an accurate diagnosis for lesions in past and present.
At the same time, both grading systems prove useful for assessing tumor aggression and implicitly the prognosis of patients, in the context of the need for a differentiated oncological treatment.
Conflict of interests
None to declare
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Cavatorta O, Scida S, Miraglia C, Barchi A, Nouvenne A, Leandro G, Meschi T, De' Angelis, Di Mario. Epidemiology of gastric cancer and risk factors. Acta Biomed. 2018;89(8-S):82–87. doi: 10.23750/abm.v89i8-S.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 4.WHO Classification of Tumours: Digestive System Tumours. Vol. 5 International Agency for Research on Cancer, World Health Organization, International Academy of Pathology, Elder DE; New York : 2019. [Google Scholar]
- 5.Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol. 2014;20(19):5679–5684. doi: 10.3748/wjg.v20.i19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez RS, Raza A, Propst R, Adeyi O, Bateman J, Sopha SC, Shaw J, Auerbach A. Recent Advances in Digestive Tract Tumors: Updates From the 5th Edition of the World Health Organization "Blue Book". Arch Pathol Lab Med. 2021;145(5):607–626. doi: 10.5858/arpa.2020-0047-RA. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, Cho MY, Sohn JH, Kang DY, Park CK, Kim WH, Jin SY, Kim KM, Chang HK, Yu E, Jung ES, Chang MS, Joo JE, Joo M, Kim YW, Park DY, Kang YK, Park SH, Han HS, Kim YB, Park HS, Chae YS, Kwon KW, Chang HJ; Diagnosis of gastric epithelial neoplasia: Dilemma for Korean pathologists. World J Gastroenterol. 2011;17(21):2602–2610. doi: 10.3748/wjg.v17.i21.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. IARC Press ; Lyon : 2010. [Google Scholar]
- 9.Goseki N, Takizawa T, Koike M. Differences in the mode of the extension of gastric cancer classified by histological type: new histological classification of gastric carcinoma. Gut. 1992;33(5):606–612. doi: 10.1136/gut.33.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ming SC. Gastric carcinoma. A pathobiological classification. Cancer. 1977;39(6):2475–2485. doi: 10.1002/1097-0142(197706)39:6<2475::aid-cncr2820390626>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968;59(3):251–258. [PubMed] [Google Scholar]
- 12.Sarriugarte LA, García AE, Martínez IL, Gutiérrez GO, Álvarez AI, Guerra LM, Calle BM, Colina AA. From Lauren's diffuse gastric cancer to WHO's poorly cohesive carcinoma. Clinicopathological and prognostic characteristics. Rev Esp Enferm Dig. 2021;113(5):324–331. doi: 10.17235/reed.2020.7184/2020. [DOI] [PubMed] [Google Scholar]
- 13.Hu B, El Hajj, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, Wu CW, Li AF, Shyr YM, Huang KH. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol Oncol Res. 2016;22(1):197–202. doi: 10.1007/s12253-015-9996-6. [DOI] [PubMed] [Google Scholar]
- 15.Tang D, Ni M, Zhu H, Cao J, Zhou L, Shen S, Peng C, Lv Y, Xu G, Wang L, Zou X. Differential prognostic implications of gastric adenocarcinoma based on Lauren's classification: a Surveillance, Epidemiology, and End Results (SEER)-based cohort study. Ann Transl Med. 2021;9(8):646–646. doi: 10.21037/atm-20-7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H, Fang C, Chen L, Shi J, Fan X, Zou X, Huang Q. Worse Prognosis in Papillary, Compared to Tubular, Early Gastric Carcinoma. J Cancer. 2017;8(1):117–123. doi: 10.7150/jca.17326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ning FL, Zhang NN, Wang J, Jin YF, Quan HG, Pei JP, Zhao Y, Zeng XT, Abe M, Zhang CD. Prognostic value of modified Lauren classification in gastric cancer. World J Gastrointest Oncol. 2021;13(9):1184–1195. doi: 10.4251/wjgo.v13.i9.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roviello F, Marano L, Ambrosio MR, Resca L, D'Ignazio A, Petrelli F, Petrioli R, Costantini M, Polom K, Macchiarelli R, Biviano I, Marrelli D. Signet ring cell percentage in poorly cohesive gastric cancer patients: A potential novel predictor of survival. Eur J Surg Oncol. 2022;48(3):561–569. doi: 10.1016/j.ejso.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Tang CT, Chen Y, Zeng C. Prognostic analysis of gastric signet ring cell carcinoma and mucinous carcinoma: a propensity score-matched study and competing risk analysis. Aging (Albany NY) 2020;12(21):22059–22077. doi: 10.18632/aging.104048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng CH, Fang WL, Huang KH, Chen MH, Chao Y, Lo SS, Li AF, Wu CW, Shyr YM. The clinicopathological characteristics and genetic alterations of mucinous carcinoma of the stomach. J Chin Med Assoc. 2020;83(2):141–147. doi: 10.1097/JCMA.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 21.Lim SW, Kim DY, Kim YJ, Kim SK. Clinicopathologic features of mucinous gastric carcinoma. Dig Surg. 2002;19(4):286–290. doi: 10.1159/000064583. [DOI] [PubMed] [Google Scholar]
- 22.Yang S, Gu X, Tao R, Huo J, Hu Z, Sun F, Ni J, Wang X. Relationship between histological mixed-type early gastric cancer and lymph node metastasis: A systematic review and meta-analysis. PLoS One. 2022;17(4):e0266952–e0266952. doi: 10.1371/journal.pone.0266952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JN, Wang QW, Zhang QW, Tang ZR, Li XB. Poorly differentiated is more significant than signet ring cell component for lymph node metastasis in mixed-type early gastric cancer: a retrospective study from a large-volume hospital. Surg Endosc. 2021;35(4):1558–1565. doi: 10.1007/s00464-020-07532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Wang R, Zhang W, Zhuang W, Wang M, Tang C. Clinicopathological and prognostic characteristics of hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract. 2014;2014:140587–140587. doi: 10.1155/2014/140587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baek SK, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic characteristics and treatment outcomes of hepatoid adenocarcinoma of the stomach, a rare but unique subtype of gastric cancer. BMC Gastroenterol. 2011;11:56–56. doi: 10.1186/1471-230X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CY, Yeh HC, Hsu CM, Lin WR, Chiu CT. Clinicopathologial features of gastric hepatoid adenocarcinoma. Biomed J. 2015;38(1):65–69. doi: 10.4103/2319-4170.126860. [DOI] [PubMed] [Google Scholar]
- 27.Guzinska-Ustymowicz K, Niewiarowska K, Pryczynicz A. Invasive micropapillary carcinoma: a distinct type of adenocarcinomas in the gastrointestinal tract. World J Gastroenterol. 2014;20(16):4597–4606. doi: 10.3748/wjg.v20.i16.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Chen J, Liu S, Shi H, Guan W, Ji C, Guo T, Zheng H, Guan Y, Ge Y, He J, Zhou Z, Yang X, Liu T. Assessment of histological differentiation in gastric cancers using whole-volume histogram analysis of apparent diffusion coefficient maps. J Magn Reson Imaging. 2017;45(2):440–449. doi: 10.1002/jmri.25360. [DOI] [PubMed] [Google Scholar]
- 29.Cambruzzi E, Azeredo AM, Kronhart A, Foltz KM, Zettler CG, Pêgas KL. The presence of metastases in regional lymph nodes is associated with tumor size and depth of invasion in sporadic gastric adenocarcinoma. Arq Bras Cir Dig. 2014;27(1):18–21. doi: 10.1590/S0102-67202014000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adachi Y, Yasuda K, Inomata M, Sato K, Shiraishi N, Kitano S. Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer. 2000;89(7):1418–1424. [PubMed] [Google Scholar]