PURPOSE
We sought to investigate whether enzalutamide (ENZA), without concurrent androgen deprivation therapy, increases freedom from prostate-specific antigen (PSA) progression (FFPP) when combined with salvage radiation therapy (SRT) in men with recurrent prostate cancer after radical prostatectomy (RP).
PATIENTS AND METHODS
Men with biochemically recurrent prostate cancer after RP were enrolled into a randomized, double‐blind, phase II, placebo-controlled, multicenter study of SRT plus ENZA or placebo (ClinicalTrials.gov identifier: NCT02203695). Random assignment (1:1) was stratified by center, surgical margin status (R0 v R1), PSA before salvage treatment (PSA ≥ 0.5 v < 0.5 ng/mL), and pathologic Gleason sum (7 v 8‐10). Patients were assigned to receive either ENZA 160 mg once daily or matching placebo for 6 months. After 2 months of study drug therapy, external-beam radiation (66.6‐70.2 Gy) was administered to the prostate bed (no pelvic nodes). The primary end point was FFPP in the intention-to-treat population. Secondary end points were time to local recurrence within the radiation field, metastasis‐free survival, and safety as determined by frequency and severity of adverse events.
RESULTS
Eighty-six (86) patients were randomly assigned, with a median follow-up of 34 (range, 0-52) months. Trial arms were well balanced. The median pre-SRT PSA was 0.3 (range, 0.06-4.6) ng/mL, 56 of 86 patients (65%) had extraprostatic disease (pT3), 39 of 86 (45%) had a Gleason sum of 8-10, and 43 of 86 (50%) had positive surgical margins (R1). FFPP was significantly improved with ENZA versus placebo (hazard ratio [HR], 0.42; 95% CI, 0.19 to 0.92; P = .031), and 2-year FFPP was 84% versus 66%, respectively. Subgroup analyses demonstrated differential benefit of ENZA in men with pT3 (HR, 0.22; 95% CI, 0.07 to 0.69) versus pT2 disease (HR, 1.54; 95% CI, 0.43 to 5.47; Pinteraction = .019) and R1 (HR, 0.14; 95% CI, 0.03 to 0.64) versus R0 disease (HR, 1.00; 95% CI, 0.36 to 2.76; Pinteraction = .023). There were insufficient secondary end point events for analysis. The most common adverse events were grade 1-2 fatigue (65% ENZA v 53% placebo) and urinary frequency (40% ENZA v 49% placebo).
CONCLUSION
SRT plus ENZA monotherapy for 6 months in men with PSA-recurrent high-risk prostate cancer after RP is safe and delays PSA progression relative to SRT alone. The impact of ENZA on distant metastasis or survival is unknown at this time.
INTRODUCTION
Prostate cancer is the second leading cause of cancer deaths in men in the United States. According to American Cancer Society estimates, > 268,000 men will be diagnosed with prostate cancer in the United States, and nearly 35,000 will die of the disease in 2022.1 Most men who ultimately die of prostate cancer die from metastatic castrate-resistant disease,2 but interestingly, of the approximately 35,000 men who die of metastatic castrate-resistant disease per year, the majority of these men originally present with localized prostate cancer and experience subsequent recurrence.1 Failure of definitive local therapy typically results in a rising level of prostate-specific antigen (PSA) with no detectable metastases, also known as biochemical recurrence (BCR).3-5
CONTEXT
Key Objective
Does next-generation androgen receptor blockade with enzalutamide (ENZA) enhance postoperative salvage radiotherapy (SRT) biochemical outcomes (freedom from prostate-specific antigen progression [FFPP])?
Knowledge Generated
Treatment with ENZA was demonstrated to improve FFPP over placebo when combined with SRT and have a favorable toxicity profile in patients treated in the setting of biochemically recurrent prostate cancer. ENZA demonstrated an 18% absolute improvement in 2-year FFPP and, to our knowledge, is the first randomized trial to examine next-generation androgen receptor blockade with SRT.
Relevance
Early androgen blockade is safe and delays biochemical progression. This intervention should be discussed with patients requiring SRT.
Importantly, men who experience BCR after prostatectomy still represent potentially curable patients with salvage radiation therapy (SRT).3-5 A wealth of retrospective and prospective data suggest that SRT can improve biochemical control rates and in select studies even shows prevention of future metastatic recurrence and death from prostate cancer.6-10 Biochemical control is best achieved when SRT is given at lower PSA levels (PSA < 0.5 ng/mL), so-called early SRT, which has become the modern-day standard.9,10
Androgen receptor (AR) signaling promotes dysregulated growth in the majority of untreated prostate cancers.11 Androgen deprivation therapy (ADT), a form of hormonal therapy, via medical or surgical castration4,5 can induce apoptosis or senescence in prostate cancer cells,11 radiosensitizes prostate cancer cells by downregulation of DNA repair pathways,12,13 and prevents compensatory radiation-induced AR signaling.14 Low historical control rates with SRT alone prompted the conduct of the prospective studies, NRG/RTOG 96-0115 and GETUG-016,16 that ultimately showed benefits to the addition of hormonal therapy to SRT. These studies help to form the basis for treatment guideline recommendations to consider hormonal therapy with SRT.3-5 However, ADT is associated with numerous adverse effects resulting in metabolic sequelae (adverse changes in fat body mass and lipid and glycemic profiles), decreases in bone mineral density, erectile dysfunction, and decreases in patient-reported quality of life.17
A potential hormonal alternative to ADT is direct AR blockade. First-generation AR inhibition with high-dose bicalutamide has been used in combination with SRT in the NRG/RTOG 96-01 trial.15 However, NRG/RTOG 96-01 showed an increase in grade 3-5 cardiovascular and neurologic events, and in a subsequent analysis, patients who had low PSA before SRT trended toward worse survival.18 These findings suggest that in this subset of patients, the oncologic benefits observed in the overall study population are eclipsed by toxicities derived from the combinatorial approach. Enzalutamide is a second-generation oral AR blocker that unlike previous agents does not display any agonist properties, inhibits translocation of the ligand-receptor complex into the nucleus, and appears to radiosensitize better than ADT.19 Enzalutamide significantly prolongs survival in prostate cancer patients with metastatic castration-sensitive prostate cancer20,21 and CRPC.22-24 Finally, provocative phase II data in ADT-naive prostate cancer patients with enzalutamide alone achieved high PSA response rates with efficacy similar to ADT, but in contrast to ADT, bone mineral density remained stable, and metabolic variables and global health status were not substantially affected.25 These factors make enzalutamide potentially an ideal candidate for combination with SRT in men with BCR after surgery. To evaluate this concept, we conducted a multi-institutional, double-blind, placebo-controlled, randomized, phase II trial, termed the SALV-ENZA trial, testing the efficacy and safety of enzalutamide monotherapy with SRT in men with high-risk prostate cancer and BCR after prostatectomy.
PATIENTS AND METHODS
Study Design and Participants
This multicenter, randomized, phase II trial was approved by the Johns Hopkins University institutional review board (JHU IRB; ClinicalTrials.gov identifier: NCT02203695). Patients were recruited from seven institutions across the United States. Patients had to be at least age 18 years and have provided informed consent. Eligibility criteria included histologically confirmed adenocarcinoma of the prostate, primary treatment with radical prostatectomy, and freedom from prior malignancies for at least 3 years (with the exception of nonmelanoma skin cancers and superficial urothelial cancers). Pathological Gleason sum had to be 8-10 or Gleason 7 with either pT3 (extracapsular extension or seminal vesicle invasion) or R1 disease (positive margins); and there had to be node-negative disease (pN0) at the time of surgery and lack radiographic or clinical evidence of local/regional tumor recurrence. Patients had to have nonmetastatic disease (M0) on a CT of the abdomen and pelvis and whole-body radionuclide 99Tc bone scan (or sodium fluoride PET scan) within 3 months of study entry. Patients could not have received prior hormonal therapy (luteinizing hormone-releasing hormone agonist, antiandrogen, or both), and serum testosterone was > 150 ng/dL.
Evidence of biochemical (PSA) relapse after prostatectomy was required and defined as one rise in PSA above a baseline detectable value (≥ 0.05 ng/mL) using measurements taken at least 4 weeks apart and all within 12 months of study entry. Laboratory requirements for study entry included absolute PSA level > 0.05 and < 0.7 ng/mL and noncastrate levels of serum testosterone (≥ 150 ng/dL). Other eligibility factors included an Eastern Cooperative Oncology Group (ECOG) status of 0-1, life expectancy ≥ 3 years, ability to swallow the study drug whole as a tablet/capsule, and agreement to using two forms of birth control during the study period and for 3 months after the last administration of the study drug. Participants were also counseled about the positive survival results of the NRG/RTOG 96-01 clinical trial and elected to forgo treatment with high-dose bicalutimide.15
Exclusion criteria included active second malignancies; primary treatment with radiation therapy; concurrent use of other antiandrogens, estrogen-like agents, 5α-reductase inhibitors, other anticancer agents, or treatments; and the use of systemic corticosteroids equivalent to prednisone 10 mg/day or higher. Patients were also excluded if they had a history of seizure or any condition that might predispose them to seizures or a history of loss of consciousness or transient ischemic attack within 12 months. Patients taking medications which lower seizure threshold or which may have adverse interactions with enzalutamide were also excluded. Other disqualifying conditions included serious concurrent illness, active major infections, and certain clinically significant cardiovascular diseases. Patients with radiographic or clinical evidence of regional tumor nodal recurrence or radiographic evidence of distant metastasis were also ineligible.
Random Assignment
Patients were randomly assigned to receive either enzalutamide or placebo in a 1:1 ratio. Random assignment was stratified by center, surgical margin status (R0 v R1), PSA before salvage treatment (PSA ≥ 0.5 v < 0.5 ng/mL), and pathologic Gleason sum (7 v 8-10). A minimization approach was used to balance assignment between treatment arms. Treatment assignments were blinded to the patient and all investigators and members of the study team, except the coordinating site staff responsible for enrollment/random assignment and pharmacy staff responsible for drug dispensing at each site.
Procedures
After random assignment, patients began a 6-month treatment period. This consisted of 180 days of either placebo or enzalutamide 160 mg by mouth once daily, with compliance monitored via pill count/diary. SRT was started on day 61 and ended on day 120, given as 66.6-70.2 Gy at 1.8 Gy fractions Monday through Friday for 37-39 fractions. Patients had visits every 30 days during the treatment period and underwent the following: review of concurrent medications, physical examination, ECOG performance status, adverse events (AEs) evaluation, laboratory tests (CBC with differential, PSA, testosterone, comprehensive chemistry panel), quality-of-life questionnaires, and a review of the pill diary. After 6 months of treatment, patients entered a follow-up period where they were seen every 3 months lasting up to 42 months after end of treatment or until evidence of treatment failure. For the first 24 months, follow-up consisted of review of concurrent medications, physical examination, ECOG performance status, AEs evaluation, laboratory tests (CBC, PSA, testosterone, and comprehensive chemistry panel), and quality-of-life questionnaires. The remaining follow-up visits consisted only of PSA checks.
Exploratory analysis of tissue specimens (derived from prostatectomy, when available) were subjected to transcriptome profiling using the Decipher assay. Formalin-fixed paraffin-embedded tissue samples were from freshly cut tissue slides or punch biopsies from submitted radical prostatectomy blocks or were from archived unstained tissue slides. The highest-grade tumor focus was identified, and tumor RNA was extracted after macrodissection guided by a histologic review of a matched hematoxylin and eosin slide. Specimen selection, RNA extraction, and microarray hybridization were performed in a Clinical Laboratory Improvement Amendments–certified laboratory (Veracyte, San Diego, CA). Quality control was performed using Affymetrix Power Tools, and normalization was performed using the Single Channel Array Normalization (SCAN) algorithm. Each sample was required to meet prespecified criteria from tumor sampling, RNA extraction, cDNA amplification, and a series of microarray quality control metrics as described previously.26
Outcomes
The primary outcome measure was freedom from PSA progression (FFPP), defined as the time from random assignment to the date of PSA progression or if there was no progression at the time of analysis, the last PSA measurement. In patients who achieved an undetectable PSA value (defined as ≤ 0.1 ng/mL), PSA progression was defined as a detectable PSA value (≥ 0.2 ng/mL) that was confirmed by a second consecutive PSA value obtained ≥ 8 weeks later which was higher (and ≥ 0.4 ng/mL). In patients who did not achieve an undetectable PSA level, PSA progression was defined as a 0.2 ng/mL increase from nadir that was confirmed by a second consecutive PSA value obtained ≥ 8 weeks later which was higher. The date of PSA progression was the first date of PSA rise from nadir or baseline. Secondary outcomes included local recurrence within the radiation field (confirmed via pathology), metastasis-free survival rates, and safety, feasibility, and tolerability as assessed by NCI CTCAE v4, EPIC survey, and accrual. For AE analysis when multiple of the same event occurred, we only considered one occurrence at the maximum reported grade to include in the analysis. We only considered AEs that were possibly, probably, or definitely attributable to the study drug and analyzed those together.
Statistical Analyses
The study used a randomized phase II design to detect an improvement in FFPP with treatment with enzalutamide and salvage radiation versus placebo and salvage radiation. The primary analysis was performed on an intention-to-treat basis. Kaplan-Meier curves for FFPP per arm were constructed. Cox regression was used to estimate the hazard ratio (HR) stratified according to surgical margin (R0 v R1), Gleason sum (7 v 8-10), and baseline PSA level (PSA ≥ 0.5 v < 0.5 ng/mL). Subgroup analyses were conducted for pathological T stage, surgical margin, Gleason score, baseline PSA, age, and race. On the basis of the largest multi-institutional SRT series published at the time in which patients received SRT followed by observation until the PSA reached ≥ 0.2 ng/mL above the post-SRT nadir, the 2-year FFPP was approximately 60% with SRT.27 We expected an absolute 20% improvement with SRT plus enzalutamide (2-year FFPP 80%) over SRT alone (2-year FFPP 60%), corresponding to a HR of 0.44. We assumed an accrual time of 60 months, with 21 months of additional follow-up time with a hard stop to trial accrual in February 2020 and analysis in December 2021 (as mandated by the commercial sponsor). It required 39 total PSA-progression events to ensure 90% power to detect a HR of 0.44, using a one-sided log-rank test at a 0.1 significance level. Accounting for 15% nonevaluable or dropout patients before PSA progression events, the trial planned to randomly assign 96 patients (48 patients in each arm). We were unable to meet 96 accruals and did not reach 39 events by our predetermined hard stop deadline of December 2021 for analysis. The trial was terminated, and the data cutoff for this analysis was December 31, 2021. Although not meeting the target event number reduced the power at the planned effect size, it did not inflate the type I error. Thus, the P value threshold to claim efficacy remained at one-sided 0.1. The reported P values are two-sided, and the statistical analysis was performed using R (version 4.1.0). Investigation of biomarker-by-treatment interaction effect on FFPP between treatment arms was conducted in an exploratory fashion using the Genomics Resource Information Database (GRID), a library of locked gene expression signatures and classifiers as described previously.28
RESULTS
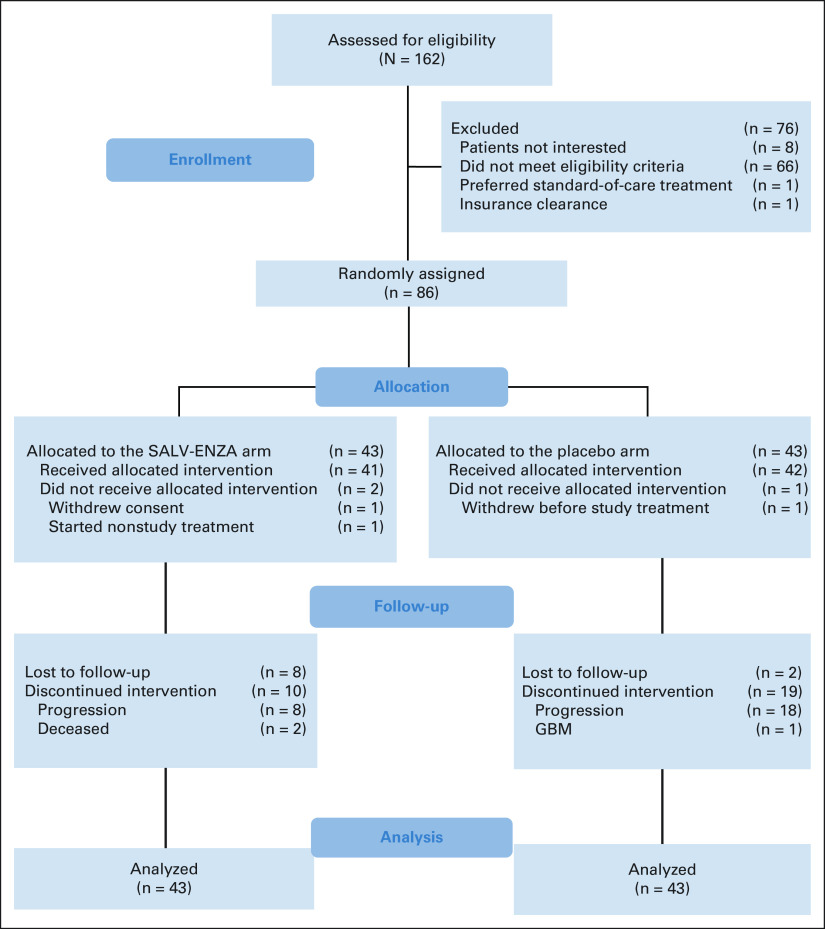
Between April 16, 2015, and February 25, 2020, 86 patients were randomly assigned to receive either salvage radiation plus enzalutamide or placebo (Fig 1). The majority were White (90%) while 9% were African American. One patient from the placebo arm withdrew consent before starting treatment, and two withdrew from the enzalutamide arm (one withdrew consent and one started a nonstudy treatment); these patients were censored for analysis at the time of random assignment. All remaining patients received their study-allocated treatments. Patient characteristics are summarized in Table 1. The median baseline pre-SRT PSA level was 0.3 (range, 0.06-4.6) ng/mL, 56 of 86 patients (65%) had extraprostatic disease (pT3), 39 of 86 (45%) had Gleason sum 8-10, and 43 of 86 (50%) had positive surgical margins (R1). Trial arms were well-balanced across stratification factors.
FIG 1.
CONSORT diagram of the SALV-ENZA trial. GBM, glioblastoma multiforme.
TABLE 1.
Baseline Characteristics for the SALV-ENZA Trial

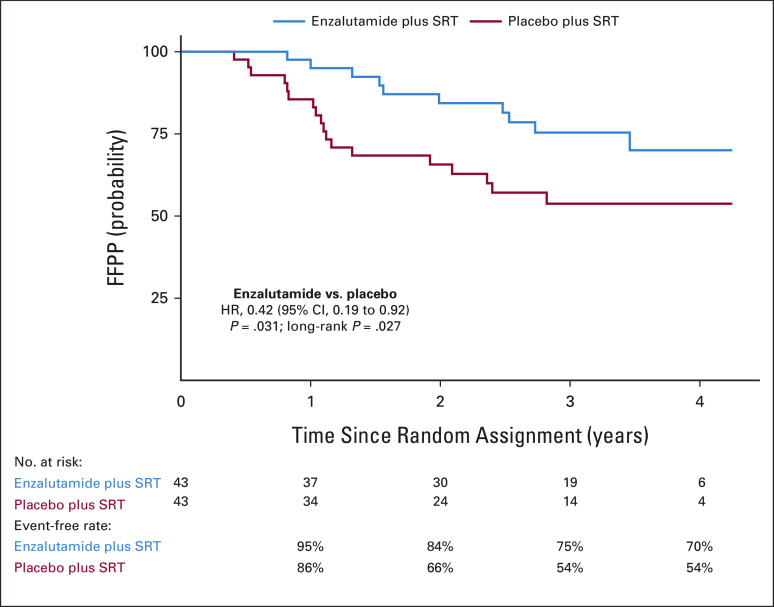
The median follow-up time for the entire cohort was 34 months (range, 0-52 months). At the time of study closure, 10 patients on the enzalutamide arm experienced PSA progression at 9-39 months after random assignment. On the placebo arm, 18 patients experienced PSA progression, from 4-33 months after random assignment. This translated into a 58% relative improvement in FFPP with the addition of enzalutamide to SRT (HR, 0.42; 95% CI, 0.19 to 0.92; P = .031), on the basis of the analysis stratified by surgical margin status, Gleason sum, and baseline PSA (Fig 2). The difference in 2-year FFPP was 84% versus 66%, respectively (log-rank, P = .027). Figure 3 depicts the subgroup analyses which suggest differential benefit of enzalutamide in men with pT3 (HR, 0.22; 95% CI, 0.07 to 0.69) versus pT2 disease (HR, 1.54; 95% CI, 0.43 to 5.47; Pinteraction = .019) and R1 (HR, 0.14; 95% CI, 0.03 to 0.64) versus R0 disease (HR, 1.00; 95% CI, 0.36 to 2.76; Pinteraction = .023). There were no documented local recurrences and only one metastasis event at the time of analysis and thus did not represent enough events for reporting. In the enzalutamide arm, one patient died from developing pancreatic cancer and one additional patient died of unknown causes. In the placebo arm, two patients developed other cancers (one glioblastoma multiforme and one squamous cell carcinoma of the tonsil), but no deaths were reported.
FIG 2.
Kaplan-Meier curves of PSA progression-free survival (FFPP) by enzalutamide versus placebo arms. FFPP, freedom from prostate-specific antigen progression; PSA, prostate-specific antigen.
FIG 3.
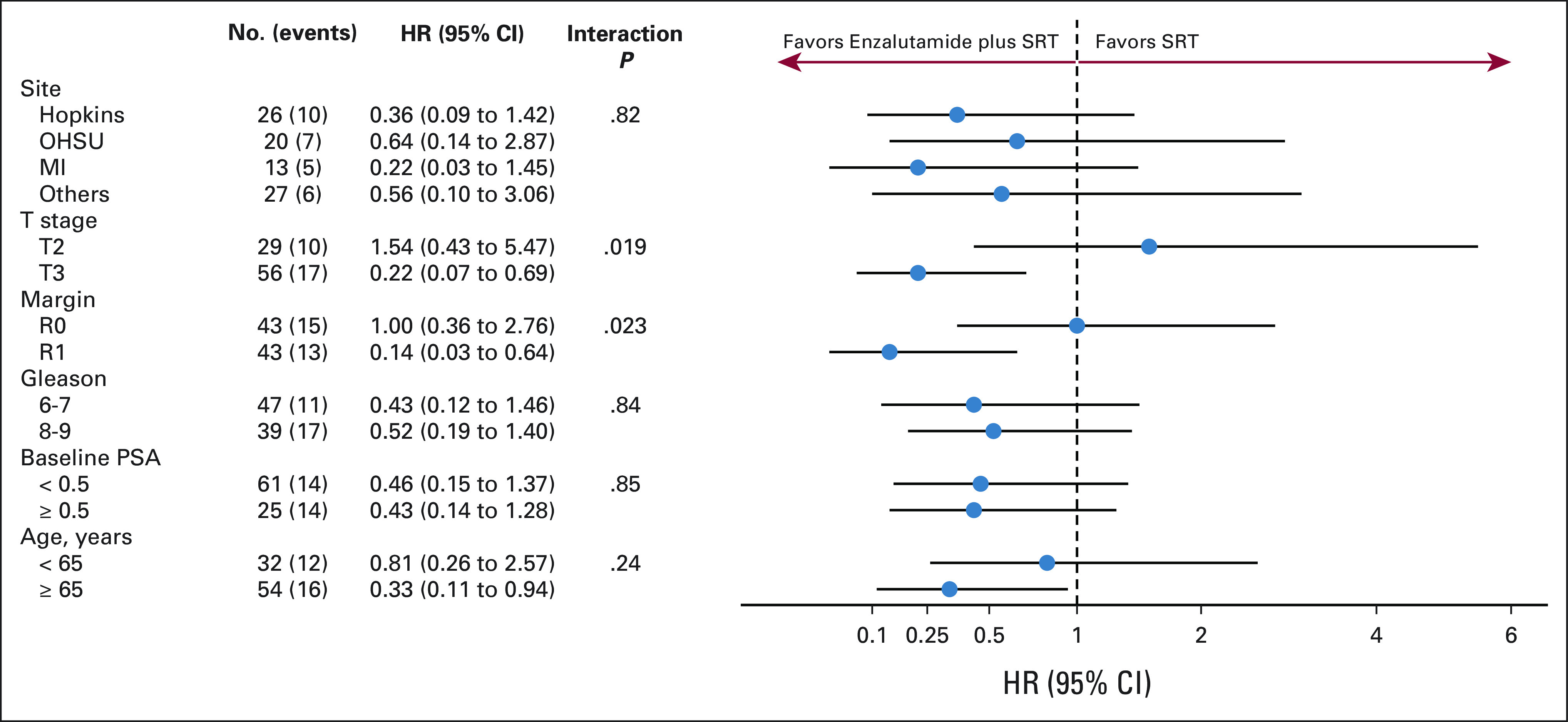
Forest plots of subgroup analysis for FFPP of enzalutamide versus placebo arms. Pathologic T-stage (pT3 v pT2) and surgical margin status (R1 v R0) displayed differential benefit from the addition of enzalutamide to salvage radiation. FFPP, freedom from prostate-specific antigen progression; HR, hazard ratio; MI, University of Michigan; OHSU, Oregon Health & Science University; PSA, prostate-specific antigen; SRT, salvage radiotherapy.
Table 2 shows the most common AEs observed in the study. There were three grade 3 AEs in the enzalutamide arm (nocturia, lymphopenia, and back pain). No AEs higher than grade 3 were reported in the enzalutamide arm. In the placebo arm, seven patients reported grade 3 AEs: erectile dysfunction, fall, humerus fracture, hypertension (two patients), nocturia, and back pain. Grade 1-2 fatigue (28 [65%] on enzalutamide v 23 [53%] on placebo) and urinary frequency/urgency (17 [40%] on enzalutamide v 21 [49%] on placebo) were the most common. Other common grade 1-2 AEs in the enzalutamide arm were diarrhea (13 patients), nocturia (13 patients), breast/nipple pain (11 patients), and sexuality alteration (12 patients). In the placebo arm, common grade 1-2 AEs were diarrhea (11 patients), erectile dysfunction (nine patients), nocturia (13 patients), sexuality alteration (nine patients), and urinary incontinence (10 patients). Grade 1-2 breast/nipple pain and nausea were more common in the enzalutamide versus placebo arm (P < .01). We also observed testosterone elevations for men taking enzalutamide that returned to baseline levels after enzalutamide cessation, as anticipated (Data Supplement, online only).
TABLE 2.
Common Adverse Events by Grade and Treatment Arm
Forty-four (44) radical prostatectomy samples were evaluable for transcriptomic profiling analysis. Baseline characteristics of this genomic-evaluable cohort remained well-balanced with the overall trial cohort (Data Supplement). Although molecular analyses were underpowered, several hypothesis-generating trends were observed (Fig 4). For instance, a greater relative benefit of enzalutamide (ENZA) over placebo was seen in patients with high (v intermediate/low) Decipher genomic classifier risk group, ERG-positive (v ERG-negative) status, luminal (v basal) subtype, activated (v low) CD8 T-cell signature, and intact TP53 (v TP53-altered) status (Data Supplement).
FIG 4.
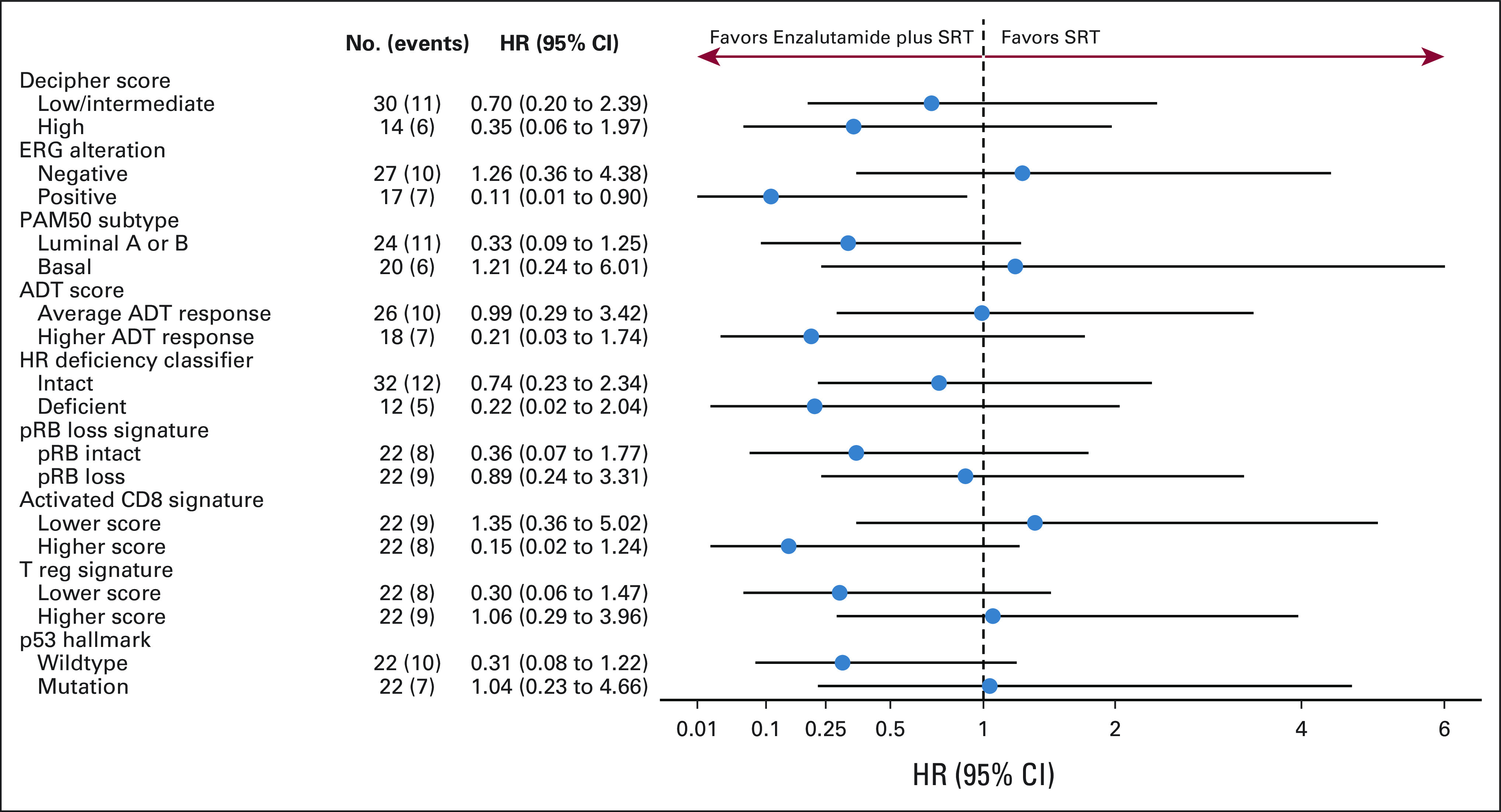
Forest plots of gene expression signature subgroup analysis for FFPP of enzalutamide versus placebo arms. Selected signatures included Decipher risk group,26 ERG alteration,29 PAM50 subtype,30 ADT score,31 homologous recombination deficiency classifier,32 RB1 loss signature,33 Activated CD8 and T reg signatures,34 and p53 Hallmarks of Cancer signature.35 For RB1 loss signature (loss v intact), activated CD8 (higher v lower) T reg (higher v lower) signatures, and p53 hallmarks of cancer (higher v lower) signature values were split into ≤ median and > median for categorical survival analysis. Higher RB1 loss signatures scores were shown to predict DNA-level biallelic loss of RB. Higher activated CD8 activity and T reg signature scores characterize the intratumoral immune levels for activated CD8 and regulatory T cells, respectively. Higher p53 hallmark signature scores predict p53 tumor suppressor pathway activity. ADT, androgen deprivation therapy; FFPP, freedom from prostate-specific antigen progression; HR, hazard ratio; SRT, salvage radiotherapy.
DISCUSSION
To our knowledge, this is the first randomized trial testing the efficacy of next-generation AR blockade with SRT in men with BCR prostate cancer after prostatectomy. We demonstrate an 18% absolute benefit in 2-year FFPP and 58% relative improvement in FFPP with 6 months of enzalutamide combined with SRT in the absence of ADT. In addition, we observed a favorable side effect profile for enzalutamide that was very similar to placebo. Our planned subgroup analyses suggested a differential benefit of enzalutamide in patients with more aggressive pathologic features, specifically extraprostatic (pT3) or margin-positive (R1) disease.
The only other published randomized trials of hormonal therapy in the SRT setting are NRG/RTOG 96-01,15 GETUG-16,16 and NRG/RTOG 05-34.36 With long-term follow-up, the two former trials demonstrated the positive effect of hormonal therapy for preventing metastatic disease and, in the case of NRG/RTOG 96-01, in delaying death. The SALV-ENZA trial reported here differs in several ways from these other published randomized trials. First, relative to RTOG 96-01, more contemporary patients with lower pre-SRT PSA levels were studied. Second, modern radiotherapy dose and techniques for SRT were employed. Third, only 6 months of enzalutamide was used, whereas in RTOG 96-01, two years of high-dose bicalutamide was examined. Fourth, SALV-ENZA included only men with pN0 and only treated the prostate bed in comparison with NRG/RTOG 05-34 which directly tested the question of elective nodal irradiation in this disease space. These other trials also did not show a differential benefit in pT3 or R1 disease with the addition of hormonal therapy to SRT. The pT3 and R1 factors denote the likelihood of local disease, and the added benefit of enzalutamide may thus point toward local radiosensitization. Most importantly, we tested the second-generation AR blocker, enzalutamide (in the absence of ADT), in combination with SRT as opposed to GETUG-16 and NRG/RTOG 05-34 which used ADT. Finally, the single-arm STREAM trial treated 38 biochemically recurrent patients with SRT plus ADT and enzalutamide and demonstrated a good safety profile and a 2-year progression-free survival rate of 65%, suggesting promising efficacy, but this is limited by the single-arm nature of the study and is not readily comparable with SALV-ENZA given the inclusion of pathologically node-positive patients.37
There are a number of ongoing randomized trials testing next-generation AR inhibitors which broadly have similar enrollment criteria to SALV-ENZA. Most similar to our study is the NRG GU006 (BALANCE; ClinicalTrials.gov identifier: NCT03371719) phase II study, randomly assigning men with BCR to SRT plus placebo versus SRT plus apalutamide, stratified by the mRNA-based PAM50 genomic classifier. Additional ongoing randomized studies include the RTOG 3506 (STEEL; ClinicalTrials.gov identifier: NCT03809000: SRT plus ADT v SRT plus ADT and enzalutamide) and FORMULA-509 (ClinicalTrials.gov identifier: NCT03141671: SRT plus ADT v SRT plus ADT and abiraterone/apalutamide). When reported, these studies should provide additional clarity to the benefit of hormonal therapy with SRT and will provide further context for the potential advantages we observed with AR blockade alone with SRT and an impression of the incremental benefit of ADT. Furthermore, transcriptome analyses of these trials will be useful to validate our exploratory analysis that found differential response to AR inhibition on the basis of the biological characteristics of the tumor.
Limitations of our trial include the small sample size susceptible to imbalances such as patients lost to follow-up, the slower-than-expected accrual, inability to accrue to our predetermined goal, and time-limited study design (because of sponsor restrictions). In addition, FFPP is not a surrogate for overall survival in this patient population,38 and our relatively short follow-up time to date made it impossible to assess many of our secondary end points such as metastasis-free survival which may be a better surrogate end point for overall survival.39,40 Strengths of the SALV-ENZA study include the multi-institutional, double-blind, placebo-controlled randomized design; enrollment of patients that reflect current practice patterns; and use of relevant SRT doses and modern techniques. Therefore, despite the limitations, we did demonstrate in a rigorous fashion the safety of enzalutamide with SRT and efficacy in the form of FFPP when combining this potent AR blocker with SRT.
In conclusion, we demonstrate that SRT plus enzalutamide monotherapy for men with PSA-recurrent high-risk prostate cancer after prostatectomy was safe and delayed PSA progression relative to SRT alone. However, these data are insufficient to change clinical practice at this time. The impact of enzalutamide on distant metastasis or survival is unknown and should be explored further in a larger trial ideally also addressing the incremental benefit of the addition of ADT.
ACKNOWLEDGMENT
We would like to thank the following team members for their contribution to the success of this on-going trial: Helen Kim, Terry Caldwell, Loretta Hollifield, Ella-Mae Shupe, Riley McIntyre, Natasha Raman, Megan Kummerlowe, Iyah Chen, Matt Gaver, James Huang, and Dana Kaplin. The funding bodies had no role in the design or execution of the study; data collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript. PTT was funded by an anonymous foundation, Movember Foundation-Distinguished Gentlemen's Ride-Prostate Cancer Foundation, and the NIH/NCI (U01CA212007, U01CA231776, and U54CA273956) and DoD (W81XWH-21-1-0296).
Sponsor: Academic sponsor: Sidney Kimmel Comprehensive Cancer Center; Investigator Initiated Johns Hopkins University IND Exempt trial IND #: 121891. Commercial sponsor: Astellas Pharma Global Development, Inc and Pfizer, Inc, who both provided funding, enzalutamide, and matching placebo.
Phuoc T. Tran
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Reflexion Medical
Consulting or Advisory Role: Astellas Pharma, Regeneron, GenomeDx, Reflexion Medical, Dendreon, Noxopharm, Janssen, Myovant Sciences, AstraZeneca, Bayer Health
Research Funding: Astellas Pharma (Inst), Reflexion Medical (Inst), Bayer Health (Inst)
Patents, Royalties, Other Intellectual Property: Compounds and Methods of Use in Ablative Radiotherapy. Patent filed 3/9/2012. PCT/US2012/028475. PCT/WO/2012/122471
Travel, Accommodations, Expenses: Reflexion Medical
Daniel Y. Song
Consulting or Advisory Role: Isoray, BioProtect
Research Funding: Candel Therapeutics, BioProtect
Arthur Y. Hung
Research Funding: Janssen Oncology (Inst)
Jason W.D. Hearn
Consulting or Advisory Role: The Dedham Group
James A. Proudfoot
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Ryan Phillips
Uncompensated Relationships: Veracyte
Tamara Lotan
Consulting or Advisory Role: Janssen Oncology
Research Funding: Ventana Medical Systems, DeepBio, AIRA Matrix (Inst), Exact Sciences
Channing J. Paller
Consulting or Advisory Role: Dendreon, Omnitura, Exelixis
Research Funding: Lilly (Inst)
Catherine H. Marshall
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: McGraw-Hill Education, Dendreon, Bayer, Obseva
Patents, Royalties, Other Intellectual Property: McGraw Hill—textbook royalties
Travel, Accommodations, Expenses: Bayer
Mark Markowski
Honoraria: Clovis Oncology, Exelixis
Samuel Denmeade
Research Funding: Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Patent licensed to Pfizer receive patent maintenance fees
Michael Carducci
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Pfizer, Exelixis, Acrivon, AstraZeneca, Sanofi
Research Funding: Pfizer (Inst), Arcus Biosciences (Inst), Merck (Inst), Celgene/Bristol Myers Squibb (Inst)
Mario Eisenberger
Leadership: Veru
Stock and Other Ownership Interests: Veru
Honoraria: Merck Sharp & Dohme, Bristol Myers Squibb, Seattle Genetics, Bayer
Travel, Accommodations, Expenses: Veru
Theodore L. DeWeese
Stock and Other Ownership Interests: Digital Harmonic
Patents, Royalties, Other Intellectual Property: patent pending on computer algorithm in radiation therapy planning
Curtiland Deville
Consulting or Advisory Role: Blue Earth Diagnostics, AstraZeneca
Elai Davicioni
Employment: GenomeDx, Decipher Biosciences, Veracyte
Leadership: GenomeDx, Decipher Biosciences, Veracyte
Stock and Other Ownership Interests: GenomeDx, Decipher Biosciences, Veracyte
Patents, Royalties, Other Intellectual Property: Cancer Diagnostics Using Biomarkers 20140066323
Travel, Accommodations, Expenses: GenomeDx, Veracyte
Elisabeth I. Heath
Honoraria: Bayer, Seattle Genetics, Sanofi, AstraZeneca, ECOR1 Capital, Genzyme, Janssen, MJH Healthcare Holdings, LLC, Team 9, Vereo Communications, HSC Acquisition, Astellas Pharma, Caris Life Sciences, Johnson & Johnson/Janssen
Consulting or Advisory Role: Bayer, Sanofi, AstraZeneca, Astellas Pharma, Bristol Myers Squibb, Janssen, Seattle Genetics
Speakers' Bureau: Sanofi
Research Funding: Tokai Pharmaceuticals (Inst), Seattle Genetics (Inst), Agensys (Inst), Dendreon (Inst), Genentech/Roche (Inst), Millennium (Inst), Celldex (Inst), Inovio Pharmaceuticals (Inst), Celgene (Inst), Merck (Inst), AstraZeneca (Inst), Esanik (Inst), Zenith Epigenetics (Inst), Oncolys BioPharma (Inst), Curemeta (Inst), Bristol Myers Squibb (Inst), eFFECTOR Therapeutics (Inst), Fortis (Inst), Astellas Pharma (Inst), Medivation (Inst), Ignyta (Inst), Synta (Inst), Caris Life Sciences (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Merck Sharp & Dohme (Inst), Plexxikon (Inst), Corcept Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Bayer (Inst), Modra Pharmaceuticals (Inst), Pellficure (Inst), Champions Oncology (Inst), AIQ Solutions (Inst), Novartis (Inst), Janssen Research & Development (Inst), Mirati Therapeutics (Inst), Peloton Therapeutics (Inst), Daiichi Sankyo Inc (Inst), Calibr (Inst), Eisai (Inst), Pharmacyclics (Inst), Five Prime Therapeutics (Inst), Arvinas (Inst), BioXCel therapeutics (Inst), Calithera Biosciences (Inst), Corvus Pharmaceuticals (Inst), Exelixis (Inst), Gilead Sciences (Inst), Harpoon therapeutics (Inst), Roche (Inst), ITeos Therapeutics (Inst), Pfizer (Inst), POINT Biopharma (Inst)
Travel, Accommodations, Expenses: Caris Life Sciences
Other Relationship: Caris Centers of Excellence
Neil B. Desai
Consulting or Advisory Role: Boston Scientific
Speakers' Bureau: Ultimate Medical Academy
Research Funding: Boston Scientific
Travel, Accommodations, Expenses: Boston Scientific
Uncompensated Relationships: Bayer, Janssen Oncology
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1620445
Daniel E. Spratt
Honoraria: Varian Medical Systems
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Boston Scientific, Bayer, Blue Earth Diagnostics, Varian Medical Systems
Research Funding: Janssen (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/869226
Felix Feng
Stock and Other Ownership Interests: Artera
Consulting or Advisory Role: Janssen Biotech, Astellas Pharma, SerImmune, Foundation Medicine, Exact Sciences, Bristol Myers Squibb, Varian Medical Systems, Novartis, Roivant, Bayer, BlueStar Genomics, Myovant Sciences, Tempus, Artera
Research Funding: Zenith Epigenetics
Tomasz M. Beer
Employment: Exact Sciences
Stock and Other Ownership Interests: Arvinas, Salarius Pharmaceuticals, Exact Sciences
Consulting or Advisory Role: Arvinas, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Constellation Pharmaceuticals, GRAIL, Janssen, Pfizer, Sanofi, AbbVie, Sapience Therapeutics, Amgen, Dantari Pharmaceuticals, GlaxoSmithKline
Research Funding: Alliance Foundation Trials (Inst), Astellas Pharma (Inst), Boehringer Ingelheim (Inst), Corcept Therapeutics (Inst), Endocyte (Inst), Freenome (Inst), GRAIL (Inst), Harpoon Therapeutics (Inst), Janssen Research & Development (Inst), Medivation (Inst), Sotio (Inst), Theraclone Sciences (Inst), Zenith Epigenetics (Inst), Bayer (Inst)
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology, Amgen, Bayer, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Celgene, Constellation Pharmaceuticals, Curium Pharma, Lilly, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Tempus, Orion, AIkido Pharma
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer, Amgen, Astellas Pharma, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Constellation Pharmaceuticals, Curium Pharma, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Medivation, Tempus, Orion, AIkido Pharma
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst), Celgene, Clovis Oncology
Patents, Royalties, Other Intellectual Property: Co-inventor of a biomarker technology that has been licensed to Qiagen
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
No other potential conflicts of interest were reported.
CLINICAL TRIAL INFORMATION
P.T.T. and K.L. contributed equally to this work. P.T.T. and E.S.A. are cosenior authors.
DATA SHARING STATEMENT
Individual deidentified participant data that underlie the results reported in this article will be shared as will the individual study protocol. The data will become available beginning 1 year following and for 3 years following publication to researchers with a methodologically sound proposal to achieve aims in previously said sound proposal. Proposals should be directed toward the corresponding authors. Data will be available in our University's data warehouse but without researcher support other than deposited metadata.
AUTHOR CONTRIBUTIONS
Conception and design: Phuoc T. Tran, Arthur Y. Hung, Matthew P. Deek, Mark Markowski, Michael Carducci, Mario Eisenberger Theodore L. DeWeese, Hao Wang, Emmanuel S. Antonarakis
Financial support: Phuoc T. Tran
Administrative support: Phuoc T. Tran, Stephen Greco
Provision of study materials or patients: Phuoc T. Tran, Daniel Y. Song, Arthur Y. Hung, Jason W.D. Hearn, Tamara Lotan, Shirl Dipasquale, Samuel Denmeade, Michael Carducci, Elai Davicioni, Stanley L. Liauw, Elisabeth I. Heath, Daniel E. Spratt, Tomasz M. Beer, Emmanuel S. Antonarakis
Collection and assembly of data: Phuoc T. Tran, Kathryn Lowe, Daniel Y. Song, Arthur Y. Hung, Jason W.D. Hearn, Steven Miller, Tamara Lotan, Catherine H. Marshall, Shirl Dipasquale, Samuel Denmeade, Michael Carducci, Matthew Orton, Curtiland Deville, Elai Davicioni, Stanley L. Liauw, Stephen Greco, Neil B. Desai, Daniel E. Spratt, Felix Feng, Tomasz M. Beer, Emmanuel S. Antonarakis
Data analysis and interpretation: Phuoc T. Tran, Hua-Ling Tsai, Daniel Y. Song, Arthur Y. Hung, Jason W.D. Hearn, James A. Proudfoot, Matthew P. Deek, Ryan Phillips, Channing J. Paller, Catherine H. Marshall, Mark Markowski, Michael Carducci, Mario Eisenberger Theodore L. DeWeese, Curtiland Deville, Elai Davicioni, Elisabeth I. Heath, Felix Feng, Hao Wang, Tomasz M. Beer, Emmanuel S. Antonarakis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Randomized Study of Salvage Radiation Therapy Plus Enzalutamide or Placebo for High-Risk Prostate-Specific Antigen Recurrent Prostate Cancer After Radical Prostatectomy: The SALV-ENZA Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Phuoc T. Tran
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Reflexion Medical
Consulting or Advisory Role: Astellas Pharma, Regeneron, GenomeDx, Reflexion Medical, Dendreon, Noxopharm, Janssen, Myovant Sciences, AstraZeneca, Bayer Health
Research Funding: Astellas Pharma (Inst), Reflexion Medical (Inst), Bayer Health (Inst)
Patents, Royalties, Other Intellectual Property: Compounds and Methods of Use in Ablative Radiotherapy. Patent filed 3/9/2012. PCT/US2012/028475. PCT/WO/2012/122471
Travel, Accommodations, Expenses: Reflexion Medical
Daniel Y. Song
Consulting or Advisory Role: Isoray, BioProtect
Research Funding: Candel Therapeutics, BioProtect
Arthur Y. Hung
Research Funding: Janssen Oncology (Inst)
Jason W.D. Hearn
Consulting or Advisory Role: The Dedham Group
James A. Proudfoot
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Ryan Phillips
Uncompensated Relationships: Veracyte
Tamara Lotan
Consulting or Advisory Role: Janssen Oncology
Research Funding: Ventana Medical Systems, DeepBio, AIRA Matrix (Inst), Exact Sciences
Channing J. Paller
Consulting or Advisory Role: Dendreon, Omnitura, Exelixis
Research Funding: Lilly (Inst)
Catherine H. Marshall
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: McGraw-Hill Education, Dendreon, Bayer, Obseva
Patents, Royalties, Other Intellectual Property: McGraw Hill—textbook royalties
Travel, Accommodations, Expenses: Bayer
Mark Markowski
Honoraria: Clovis Oncology, Exelixis
Samuel Denmeade
Research Funding: Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Patent licensed to Pfizer receive patent maintenance fees
Michael Carducci
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Pfizer, Exelixis, Acrivon, AstraZeneca, Sanofi
Research Funding: Pfizer (Inst), Arcus Biosciences (Inst), Merck (Inst), Celgene/Bristol Myers Squibb (Inst)
Mario Eisenberger
Leadership: Veru
Stock and Other Ownership Interests: Veru
Honoraria: Merck Sharp & Dohme, Bristol Myers Squibb, Seattle Genetics, Bayer
Travel, Accommodations, Expenses: Veru
Theodore L. DeWeese
Stock and Other Ownership Interests: Digital Harmonic
Patents, Royalties, Other Intellectual Property: patent pending on computer algorithm in radiation therapy planning
Curtiland Deville
Consulting or Advisory Role: Blue Earth Diagnostics, AstraZeneca
Elai Davicioni
Employment: GenomeDx, Decipher Biosciences, Veracyte
Leadership: GenomeDx, Decipher Biosciences, Veracyte
Stock and Other Ownership Interests: GenomeDx, Decipher Biosciences, Veracyte
Patents, Royalties, Other Intellectual Property: Cancer Diagnostics Using Biomarkers 20140066323
Travel, Accommodations, Expenses: GenomeDx, Veracyte
Elisabeth I. Heath
Honoraria: Bayer, Seattle Genetics, Sanofi, AstraZeneca, ECOR1 Capital, Genzyme, Janssen, MJH Healthcare Holdings, LLC, Team 9, Vereo Communications, HSC Acquisition, Astellas Pharma, Caris Life Sciences, Johnson & Johnson/Janssen
Consulting or Advisory Role: Bayer, Sanofi, AstraZeneca, Astellas Pharma, Bristol Myers Squibb, Janssen, Seattle Genetics
Speakers' Bureau: Sanofi
Research Funding: Tokai Pharmaceuticals (Inst), Seattle Genetics (Inst), Agensys (Inst), Dendreon (Inst), Genentech/Roche (Inst), Millennium (Inst), Celldex (Inst), Inovio Pharmaceuticals (Inst), Celgene (Inst), Merck (Inst), AstraZeneca (Inst), Esanik (Inst), Zenith Epigenetics (Inst), Oncolys BioPharma (Inst), Curemeta (Inst), Bristol Myers Squibb (Inst), eFFECTOR Therapeutics (Inst), Fortis (Inst), Astellas Pharma (Inst), Medivation (Inst), Ignyta (Inst), Synta (Inst), Caris Life Sciences (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Merck Sharp & Dohme (Inst), Plexxikon (Inst), Corcept Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Bayer (Inst), Modra Pharmaceuticals (Inst), Pellficure (Inst), Champions Oncology (Inst), AIQ Solutions (Inst), Novartis (Inst), Janssen Research & Development (Inst), Mirati Therapeutics (Inst), Peloton Therapeutics (Inst), Daiichi Sankyo Inc (Inst), Calibr (Inst), Eisai (Inst), Pharmacyclics (Inst), Five Prime Therapeutics (Inst), Arvinas (Inst), BioXCel therapeutics (Inst), Calithera Biosciences (Inst), Corvus Pharmaceuticals (Inst), Exelixis (Inst), Gilead Sciences (Inst), Harpoon therapeutics (Inst), Roche (Inst), ITeos Therapeutics (Inst), Pfizer (Inst), POINT Biopharma (Inst)
Travel, Accommodations, Expenses: Caris Life Sciences
Other Relationship: Caris Centers of Excellence
Neil B. Desai
Consulting or Advisory Role: Boston Scientific
Speakers' Bureau: Ultimate Medical Academy
Research Funding: Boston Scientific
Travel, Accommodations, Expenses: Boston Scientific
Uncompensated Relationships: Bayer, Janssen Oncology
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1620445
Daniel E. Spratt
Honoraria: Varian Medical Systems
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Boston Scientific, Bayer, Blue Earth Diagnostics, Varian Medical Systems
Research Funding: Janssen (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/869226
Felix Feng
Stock and Other Ownership Interests: Artera
Consulting or Advisory Role: Janssen Biotech, Astellas Pharma, SerImmune, Foundation Medicine, Exact Sciences, Bristol Myers Squibb, Varian Medical Systems, Novartis, Roivant, Bayer, BlueStar Genomics, Myovant Sciences, Tempus, Artera
Research Funding: Zenith Epigenetics
Tomasz M. Beer
Employment: Exact Sciences
Stock and Other Ownership Interests: Arvinas, Salarius Pharmaceuticals, Exact Sciences
Consulting or Advisory Role: Arvinas, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Constellation Pharmaceuticals, GRAIL, Janssen, Pfizer, Sanofi, AbbVie, Sapience Therapeutics, Amgen, Dantari Pharmaceuticals, GlaxoSmithKline
Research Funding: Alliance Foundation Trials (Inst), Astellas Pharma (Inst), Boehringer Ingelheim (Inst), Corcept Therapeutics (Inst), Endocyte (Inst), Freenome (Inst), GRAIL (Inst), Harpoon Therapeutics (Inst), Janssen Research & Development (Inst), Medivation (Inst), Sotio (Inst), Theraclone Sciences (Inst), Zenith Epigenetics (Inst), Bayer (Inst)
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology, Amgen, Bayer, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Celgene, Constellation Pharmaceuticals, Curium Pharma, Lilly, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Tempus, Orion, AIkido Pharma
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer, Amgen, Astellas Pharma, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Constellation Pharmaceuticals, Curium Pharma, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Medivation, Tempus, Orion, AIkido Pharma
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst), Celgene, Clovis Oncology
Patents, Royalties, Other Intellectual Property: Co-inventor of a biomarker technology that has been licensed to Qiagen
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A: Cancer statistics, 2022. CA Cancer J Clin 72:7-33, 2022 [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Morris MJ, Stadler WM, et al. : Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 34:1402-1418, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spratt DE, Dess RT, Zumsteg ZS, et al. : A systematic review and framework for the use of hormone therapy with salvage radiation therapy for recurrent prostate cancer. Eur Urol 73:156-165, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Cornford P, van den Bergh RCN, Briers E, et al. : EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: Treatment of relapsing and metastatic prostate cancer. Eur Urol 79:263-282, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Schaeffer E, Srinivas S, Antonarakis ES, et al. : NCCN guidelines insights: Prostate cancer, version 1.2021. J Natl Compr Canc Netw 19:134-143, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Trock BJ, Han M, Freedland SJ, et al. : Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 299:2760-2769, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stish BJ, Pisansky TM, Harmsen WS, et al. : Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol 34:3864-3871, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Hwang WL, Tendulkar RD, Niemierko A, et al. : Comparison between adjuvant and early-salvage postprostatectomy radiotherapy for prostate cancer with adverse pathological features. JAMA Oncol 4:e175230, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vale CL, Fisher D, Kneebone A, et al. : Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 396:1422-1431, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tendulkar RD, Agrawal S, Gao T, et al. : Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol 34:3648-3654, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Coulter JB, Song DY, DeWeese TL, Yegnasubramanian S: Mechanisms, challenges, and opportunities in combined radiation and hormonal therapies. Semin Radiat Oncol 32:76-81, 2022 [DOI] [PubMed] [Google Scholar]
- 12.Polkinghorn WR, Parker JS, Lee MX, et al. : Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 3:1245-1253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin JF, Schiewer MJ, Dean JL, et al. : A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov 3:1254-1271, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spratt DE, Evans MJ, Davis BJ, et al. : Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res 75:4688-4696, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shipley WU, Seiferheld W, Lukka HR, et al. : Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 376:417-428, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrie C, Magne N, Burban-Provost P, et al. : Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): A 112-month follow-up of a phase 3, randomised trial. Lancet Oncol 20:1740-1749, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Narayan V, Ross AE, Parikh RB, et al. : How to treat prostate cancer with androgen deprivation and minimize cardiovascular risk: A therapeutic tightrope. JACC CardioOncol 3:737-741, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dess RT, Sun Y, Jackson WC, et al. : Association of presalvage radiotherapy PSA levels after prostatectomy with outcomes of long-term antiandrogen therapy in men with prostate cancer. JAMA Oncol 6:735-743, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghashghaei M, Niazi TM, Heravi M, et al. : Enhanced radiosensitization of enzalutamide via schedule dependent administration to androgen-sensitive prostate cancer cells. Prostate 78:64-75, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Davis ID, Martin AJ, Stockler MR, et al. : Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 381:121-131, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. : ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 37:2974-2986, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scher HI, Fizazi K, Saad F, et al. : Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187-1197, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Beer TM, Armstrong AJ, Rathkopf DE, et al. : Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424-433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain M, Fizazi K, Saad F, et al. : Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med 378:2465-2474, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tombal B, Borre M, Rathenborg P, et al. : Long-term antitumor activity and safety of enzalutamide monotherapy in hormone naive prostate cancer: 3-Year open label followup results. J Urol 199:459-464, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Dal Pra A, Ghadjar P, Hayoz S, et al. : Validation of the Decipher genomic classifier in patients receiving salvage radiotherapy without hormone therapy after radical prostatectomy—An ancillary study of the SAKK 09/10 randomized clinical trial. Ann Oncol 33:950-958, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Stephenson AJ, Scardino PT, Eastham JA, et al. : Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol 23:7005-7012, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spratt DE, Alshalalfa M, Fishbane N, et al. : Transcriptomic heterogeneity of androgen receptor activity defines a de novo low AR-active subclass in treatment naive primary prostate cancer. Clin Cancer Res 25:6721-6730, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomlins SA, Alshalalfa M, Davicioni E, et al. : Characterization of 1577 primary prostate cancers reveals novel biological and clinicopathologic insights into molecular subtypes. Eur Urol 68:555-567, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao SG, Chang SL, Erho N, et al. : Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol 3:1663-1672, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karnes RJ, Sharma V, Choeurng V, et al. : Development and validation of a prostate cancer genomic signature that predicts early ADT treatment response following radical prostatectomy. Clin Cancer Res 24:3908-3916, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiner AB, Liu Y, McFarlane M, et al. : A transcriptomic model for homologous recombination deficiency in prostate cancer. Prostate Cancer Prostatic Dis 25:659-665, 2022 [DOI] [PubMed] [Google Scholar]
- 33.Chen WS, Alshalalfa M, Zhao SG, et al. : Novel RB1-loss transcriptomic signature is associated with poor clinical outcomes across cancer types. Clin Cancer Res 25:4290-4299, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charoentong P, Finotello F, Angelova M, et al. : Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep 18:248-262, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Liberzon A, Birger C, Thorvaldsdottir H, et al. : The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1:417-425, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollack A, Karrison TG, Balogh AG, et al. : The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): An international, multicentre, randomised phase 3 trial. Lancet 399:1886-1901, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bitting RL, Healy P, George DJ, et al. : Phase II trial of enzalutamide and androgen deprivation therapy with salvage radiation in men with high-risk prostate-specific antigen recurrent prostate cancer: The STREAM trial. Eur Urol Oncol 4:948-954, 2021 [DOI] [PubMed] [Google Scholar]
- 38.Jackson WC, Tang M, Schipper MJ, et al. : Biochemical failure is not a surrogate end point for overall survival in recurrent prostate cancer: Analysis of NRG Oncology/RTOG 9601. J Clin Oncol 40:3172-3179, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gharzai LA, Jiang R, Wallington D, et al. : Intermediate clinical endpoints for surrogacy in localised prostate cancer: An aggregate meta-analysis. Lancet Oncol 22:402-410, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie W, Regan MM, Buyse M, et al. : Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol 35:3097-3104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual deidentified participant data that underlie the results reported in this article will be shared as will the individual study protocol. The data will become available beginning 1 year following and for 3 years following publication to researchers with a methodologically sound proposal to achieve aims in previously said sound proposal. Proposals should be directed toward the corresponding authors. Data will be available in our University's data warehouse but without researcher support other than deposited metadata.