PURPOSE
To assess whether reirradiation (re-RT) and concurrent bevacizumab (BEV) improve overall survival (OS) and/or progression-free survival (PFS), compared with BEV alone in recurrent glioblastoma (GBM). The primary objective was OS, and secondary objectives included PFS, response rate, and treatment adverse events (AEs) including delayed CNS toxicities.
METHODS
NRG Oncology/RTOG1205 is a prospective, phase II, randomized trial of re-RT and BEV versus BEV alone. Stratification factors included age, resection, and Karnofsky performance status (KPS). Patients with recurrent GBM with imaging evidence of tumor progression ≥ 6 months from completion of prior chemo-RT were eligible. Patients were randomly assigned 1:1 to re-RT, 35 Gy in 10 fractions, with concurrent BEV IV 10 mg/kg once in every 2 weeks or BEV alone until progression.
RESULTS
From December 2012 to April 2016, 182 patients were randomly assigned, of whom 170 were eligible. Patient characteristics were well balanced between arms. The median follow-up for censored patients was 12.8 months. There was no improvement in OS for BEV + RT, hazard ratio, 0.98; 80% CI, 0.79 to 1.23; P = .46; the median survival time was 10.1 versus 9.7 months for BEV + RT versus BEV alone. The median PFS for BEV + RT was 7.1 versus 3.8 months for BEV, hazard ratio, 0.73; 95% CI, 0.53 to 1.0; P = .05. The 6-month PFS rate improved from 29.1% (95% CI, 19.1 to 39.1) for BEV to 54.3% (95% CI, 43.5 to 65.1) for BEV + RT, P = .001. Treatment was well tolerated. There were a 5% rate of acute grade 3+ treatment-related AEs and no delayed high-grade AEs. Most patients died of recurrent GBM.
CONCLUSION
To our knowledge, NRG Oncology/RTOG1205 is the first prospective, randomized multi-institutional study to evaluate the safety and efficacy of re-RT in recurrent GBM using modern RT techniques. Overall, re-RT was shown to be safe and well tolerated. BEV + RT demonstrated a clinically meaningful improvement in PFS, specifically the 6-month PFS rate but no difference in OS.
INTRODUCTION
Glioblastoma (GBM) is the most common primary brain tumor in adults.1 Despite optimal treatment with surgery, chemotherapy, and radiation (RT), the median survival remains approximately 12-16 months. Nearly all patients relapse at a median of 8 months with frequently devastating neurologic consequences.1 Management of recurrent gliomas is particularly challenging given the paucity of effective treatment options. The absence of clear evidence of survival benefit has led to diverse treatment strategies.
CONTEXT
Key Objective
What is the role of reirradiation in recurrent glioblastoma (GBM)?
Knowledge Generated
To our knowledge, NRG Oncology/RTOG1205 is the first prospective, randomized multi-institutional study to evaluate the safety and efficacy of reirradiation added to the standard of care bevacizumab (BEV) in recurrent GBM. The addition of RT to BEV did not demonstrate a benefit in overall survival compared with the control arm but did show statistically significant improvement in the predefined secondary end point of 6-month progression-free survival.
Relevance (I.K. Mellinghoff)
Radiation plays an important role in the initial treatment of brain tumors. The current study shows that a second course of radiation with concurrent BEV is well tolerated and prolongs progression-free survival in patients with recurrent GBM. Although this treatment did not prolong overall survival, local disease control remains an important goal in this disease with limited treatment options.*
*Relevance section written by JCO Associate Editor Ingo K. Mellinghoff, MD.
Radiation significantly improves overall survival (OS) in newly diagnosed GBM.2 With limited treatment options, reirradiation (re-RT) is increasingly offered to select patients with localized recurrence. Retrospective data suggest that re-RT is safe and well-tolerated and provides improved disease control. re-RT may delay disease progression, thereby reducing chronic steroid use and potentially decreasing neurologic symptoms. Several retrospective studies have also shown safety and putatively improved outcomes in select patients.3-6 Despite careful patient selection, the benefit of re-RT remains unclear. Given the heterogeneous nature of recurrent GBM, the impact of meta-analyses on establishing benefit is limited. Therefore, a prospective, randomized multi-institutional study was conducted to address this important clinical question.
Vascular proliferation is a notable feature of GBM, and several trials targeting the vascular endothelial growth factor (VEGF) have been conducted.7 Anti-VEGF therapy has been shown to inhibit new vessel growth causing vascular regression and normalization.8 Antiangiogenic therapies rapidly normalize leaky abnormal tumor vessels, decreasing vasogenic edema. Reduced dependence on corticosteroids may significantly improve a patient's quality of life (QOL).9 In 2009, bevacizumab (BEV), a humanized monoclonal antibody that targets VEGF, gained US Food and Drug Administration approval, on the basis of progression-free survival (PFS) improvement, without categorical improvement in OS.10 Several trials have evaluated the safety and efficacy of BEV alone or in combination with chemotherapy. A single-institution phase II trial demonstrated increased response and prolonged 6-month PFS.11,12 The median OS of patients with GBM was 9.2 months. Phase II trials have evaluated BEV in combination with chemotherapy agents, including temozolomide, irinotecan, and nitrosoureas without improved efficacy.9-18
The clinical rationale for combining BEV with re-RT is several-fold. Preclinical studies have demonstrated that antiangiogenic agents may target radioresistant and highly tumorigenic cancer stem cells by disrupting vascular niches harboring cancer stem cells.19,20 Vascular normalization can decrease tumor hypoxia, which is one possible mechanism for radioresistance. Addition of BEV may reduce the toxicity associated with re-RT by reducing the risk of radiation necrosis.21 A single prospective trial suggested increased efficacy with BEV and re-RT with durable disease control and improved OS.22 Several retrospective series have reported on the safety of this approach.23,24 Thus, the combination of BEV and RT in recurrent GBM may increase the therapeutic ratio through enhancement of the RT response, increase the anti-VEGF effect of BEV using RT to target resistant glioma stem-cell–like cells, and lower the incidence of radiation necrosis and CNS toxicity.
To our knowledge, NRG Oncology/RTOG1205 was designed as the first prospective, multi-institutional phase II randomized study to evaluate the role of re-RT in recurrent GBM using modern RT techniques. The primary objective of NRG Oncology/RTOG1205 was to determine if re-RT in combination with BEV improved OS compared with BEV alone. Secondary end points included PFS, 6-month PFS, objective response, and acute and late treatment-related toxicity rates (Common Terminology Criteria for Adverse Events version 4 [CTCAE v4]).
METHODS
Study Schema
NRG Oncology/RTOG1205 was a randomized phase II trial as proposed by Rubinstein et al25 to determine whether BEV + re-RT (experimental arm) would improve OS compared with BEV alone (control arm). Patients were randomly assigned 1:1 using permuted block, after stratification by age (< 50 years v ≥ 50 years), Karnofsky performance status (KPS; 60 v 70-80 v 90-100), and recent re-resection (yes v no/biopsy only).26
Eligibility Criteria
Eligibility criteria included recurrent GBM with histopathologically confirmed or unequivocal imaging evidence of tumor progression within 21 days of study registration. Prior cranial RT was completed at least 6 months before study entry unless one or more of the following criteria was met: (1) histologic evidence of tumor progression; (2) new areas of recurrent tumor outside the original RT fields; or (3) advanced imaging including positron emission tomography, magnetic resonance (MR) spectroscopy, or MR perfusion imaging consistent with true progression, obtained ≥ 90 days from RT completion and ≤ 28 days of study entry.
Inclusion criteria were modified after slower-than-anticipated patient accrual to allow for patient enrollment with up to three relapses, a KPS of ≥ 60, and recurrent tumors ≤ 6 cm. Multifocal recurrence was no longer excluded, provided that the composite tumor volume was ≤ 6 cm. All patients provided written informed consent and received protocol-specified care and follow-up at a member site (see the Protocol [online only] for additional details).
Treatment
Radiation.
re-RT dose was 35 Gy in 10 fractions, using 3D conformal RT, intensity-modulated RT, or protons. Gross target volume (GTV) was defined as enhancing tumor using computed tomography and/or magnetic resonance imaging images or postoperative resection cavity if no residual enhancing tumor was noted. A planning target volume (PTV) expansion of at least 3 mm was used. PTV margins of ≤ 5 mm required the treating institution to obtain prior image-guided RT credentialing and daily image-guided RT. Details regarding specific dose limits are described.
BEV.
BEV was administered at a dose of 10 mg/kg once in every 2 weeks until disease progression. The initial cycle of BEV was initiated within 14 days of registration. Patients randomly assigned to the BEV and re-RT arm received an initial induction BEV dose (day 1) followed by concurrent BEV and re-RT at the next dose (day 14), and then once every 14 days until disease progression.
Outcomes
The primary end point was OS, defined as the interval from random assignment to death because of any cause. Secondary end points were objective response, PFS, defined as the interval from random assignment to progression or death, whichever occurred first, 6-month PFS, treatment-related adverse events (AEs), and evidence of grade 3 or greater acute or delayed CNS toxicity.
Statistical Methods
The primary objective was to determine whether BEV + re-RT improves OS compared with BEV alone. The null hypothesis is that the OS for both arms is 9 months, on the basis of NRG/RTOG 0625.11,27
The alternative hypothesis is that the BEV + re-RT arm will have an improvement in OS of 13 months.22 With 160 eligible participants, there is an 80% power to detect a 31% reduction in the hazard ratio (HR) to 0.69 at the significance level of 0.10 (one-sided), as this was a randomized phase II signal-seeking trial. Analysis was to be performed when 135 events (deaths) had been reported. Guarding against an ineligibility rate of ≤ 10%, the target accrual was 178 participants. The study included an interim toxicity and interim futility analysis.
OS and PFS rates were estimated using the Kaplan-Meier method, with patients censored at their last known follow-up time, and differences between treatment arms were tested using the log-rank test.28,29 Progression was ascertained by the local investigator on the basis of the MacDonald criteria. Multivariable analyses using stepwise selection were performed using the Cox proportional hazard model with the stratification variables as covariates to assess the adjusted treatment effect on OS and PFS.30 Interactions between the treatment arm and stratification factors were run, and if significant (P < .20), subgroup analyses were conducted. CIs presented are 80% to match the type I error in the study design. Acute (occurring within 90 days from the end of treatment) and delayed (occurring after 90 days from the end of treatment) CNS toxicities, graded with CTCAE v4, were assessed. Differences in observed severities of toxicities and objective responses between treatment arms were tested using chi-square tests. For secondary end points, two-sided tests with a significance level of 0.05 were used.
RESULTS
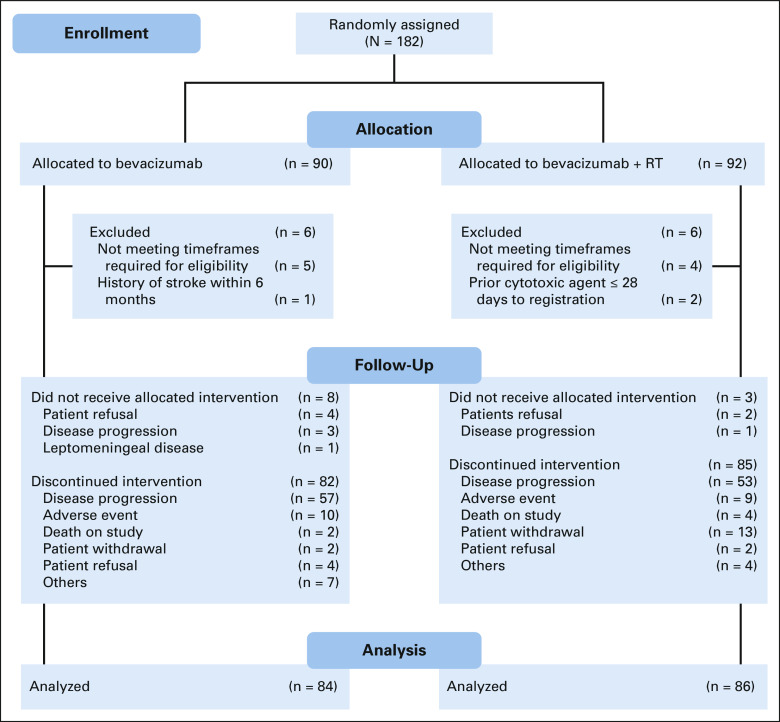
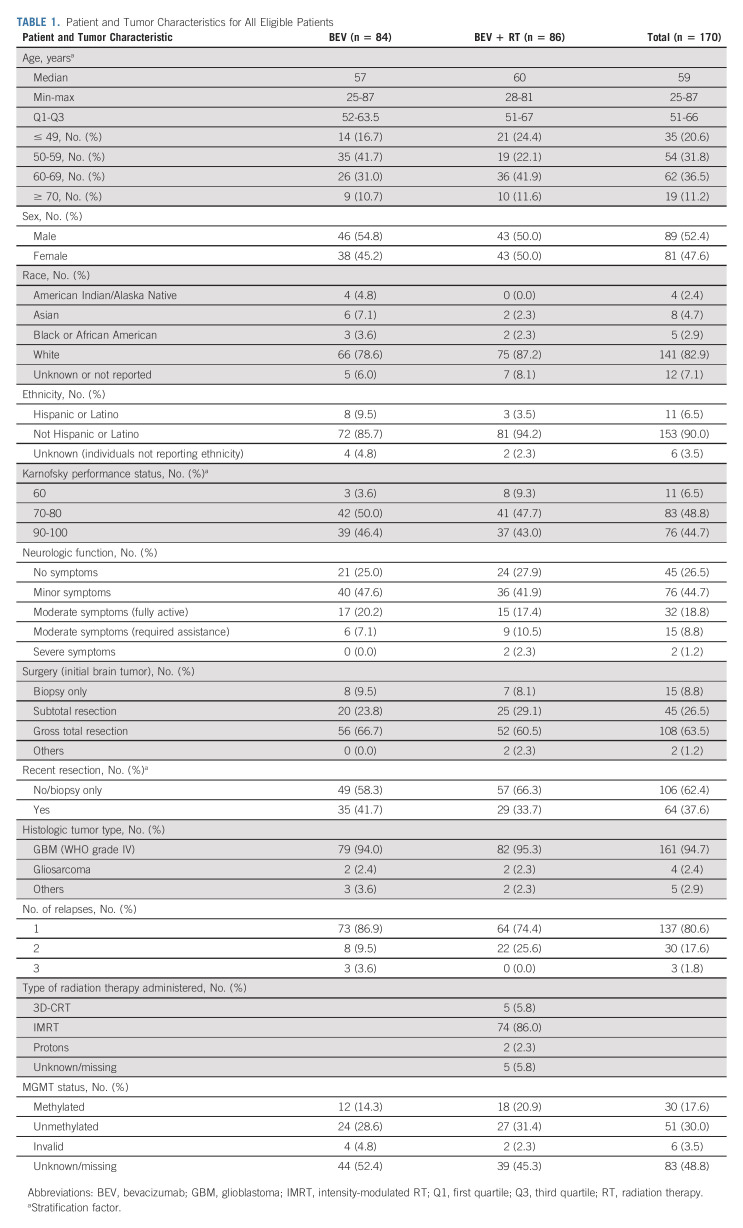
Between December 20, 2012, and April 28, 2016, 182 patients from 90 institutions were randomly assigned. Twelve patients were subsequently found to be ineligible (Fig 1). Of the remaining 170 patients, 84 were randomly assigned to the control arm and 86 to the experimental arm. Eleven patients (6.5%) received no protocol treatment (eight on the control arm and three on the experimental arm) mainly because of disease progression or patient refusal (Fig 1). Pretreatment characteristics including KPS 60 (eight v three patients), moderate/severe neurologic symptoms (11 v 6 patients), and treatment at second/third relapse (22 v 11 patients) were higher in the BEV + RT arm (Table 1). MGMT methylation status from the primary tumor was available in 81 patients, with methylation rates of 30% in the BEV arm and 38% in the BEV + RT arm.
FIG 1.
CONSORT diagram. RT, radiation therapy.
TABLE 1.
Patient and Tumor Characteristics for All Eligible Patients
Primary Outcome
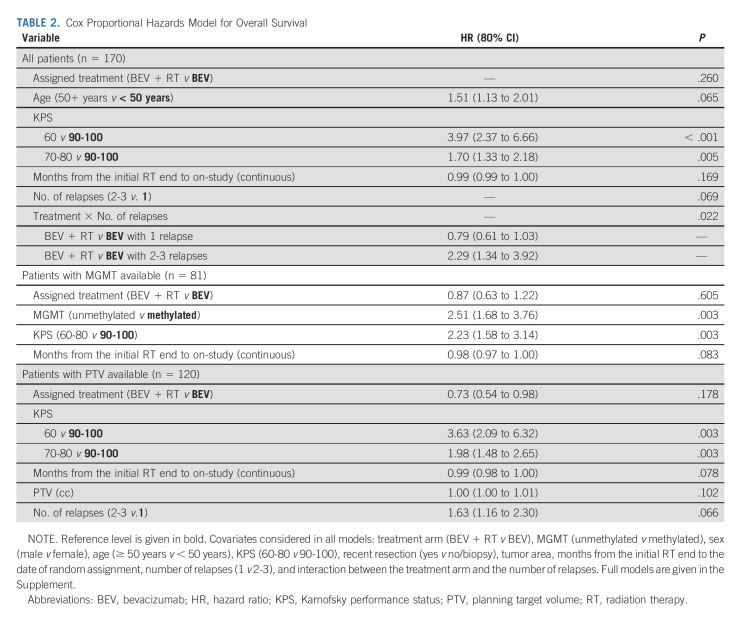
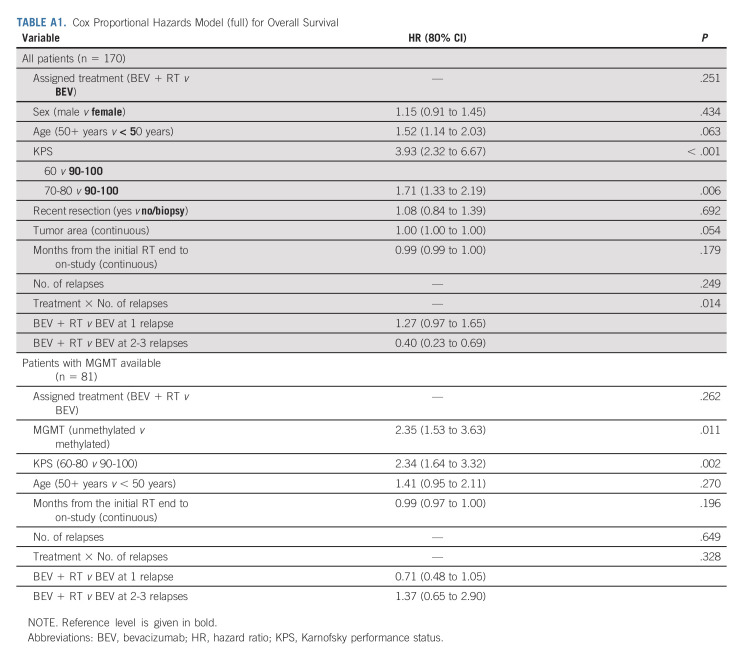
The median OS for the control arm was 9.7 months (80% CI, 9.0 to 11.2) and 10.1 months (95% CI, 9.5 to 11.3) for the experimental arm (HR, 0.98; 80% CI, 0.79 to 1.23, one-sided P value = .46), Figure 2. Thirty-five (20.6%) eligible and randomly assigned patients were censored at the time of this analysis. The median follow-up is 12.8 (min-max: 0.03-52.8) months. Recurrent GBM was the cause of death (approximately 85%). Twelve patients on the BEV arm received re-RT as salvage therapy. Multivariable Cox models revealed that older age (HR, 1.51; 80% CI, 1.13 to 2.01; P = .065) and lower KPS (60 v 90-100: HR, 3.97; 80% CI, 2.37 to 6.66; P < .001 and 70-80 v 90-100: HR, 1.70; 80% CI, 1.33 to 2.18; P = .005) were associated with worse OS (Table 2A and Appendix Table A1A, online only).
FIG 2.
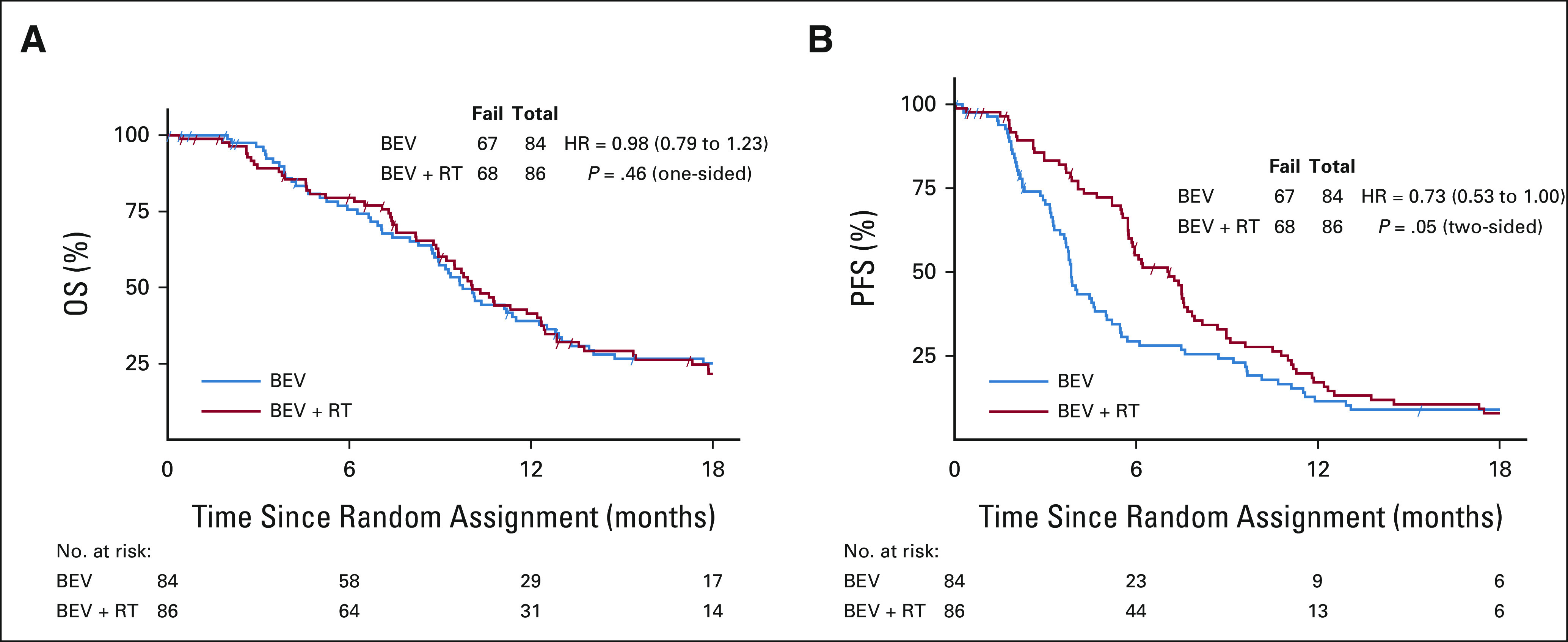
(A) OS and (B) PFS by treatment arm. CIs for OS are 80% and 95% for PFS. BEV, bevacizumab; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; RT, radiation therapy.
TABLE 2.
Cox Proportional Hazards Model for Overall Survival
Significant tests for interaction were noted for KPS and surgery. The only significant difference between arms was noted for the KPS 90-100 subgroup, in which the BEV + RT arm showed improved survival (HR = 0.67; 95% CI, 0.40 to 1.13; P = .13). Multivariable models were performed for patients with available primary tumor MGMT promoter methylation status. Prognostic factors associated with worse OS included unmethylated promoter methylation status (HR, 2.51; 80% CI, 1.68 to 3.76; P = .003) and lower KPS (60-80 v 90-100: HR, 2.23; 80% CI, 1.58 to 3.14; P = .003; Table 2B and Appendix Tables A1B and A2C, online only).
Secondary End Points
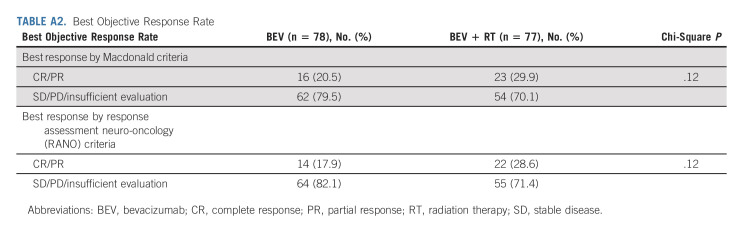
Best objective responses by the treatment arm were based on both Macdonald31 and Response Assessment Neuro-Oncology (RANO)32 criteria (Appendix Table A2). Radiographic response relied on investigator assessment. Using the MacDonald criteria, the median PFS for the control versus experimental arms was 3.8 versus 7.1 months, respectively (HR, 0.73; 95% CI, 0.53 to 1.00; P = .05). The 6-month PFS was 29.1% (95% CI, 19.1 to 39.1) versus 54.3% (95% CI, 43.5 to 65.1; P = .001) in favor of BEV + RT. Analysis using RANO criteria revealed similar results.
Treatment AEs
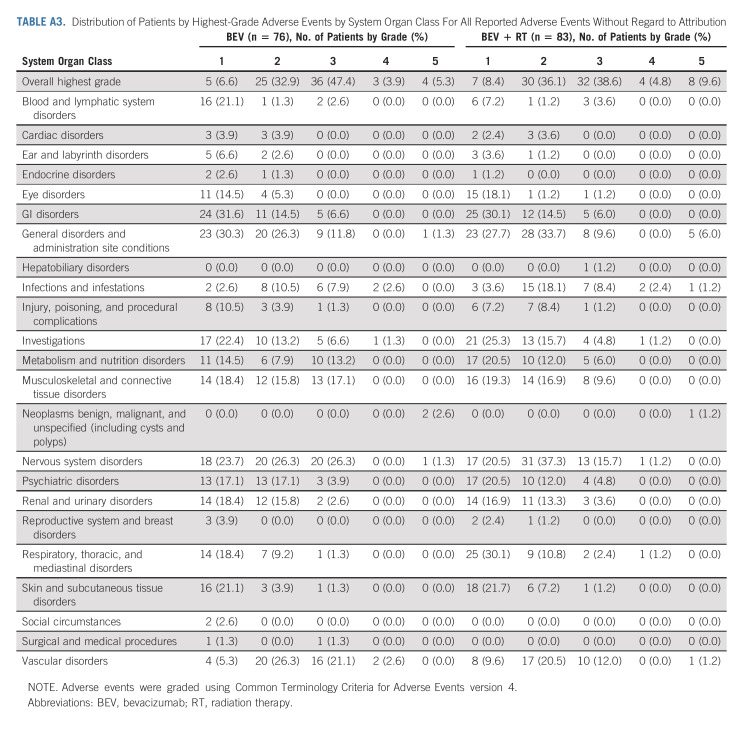
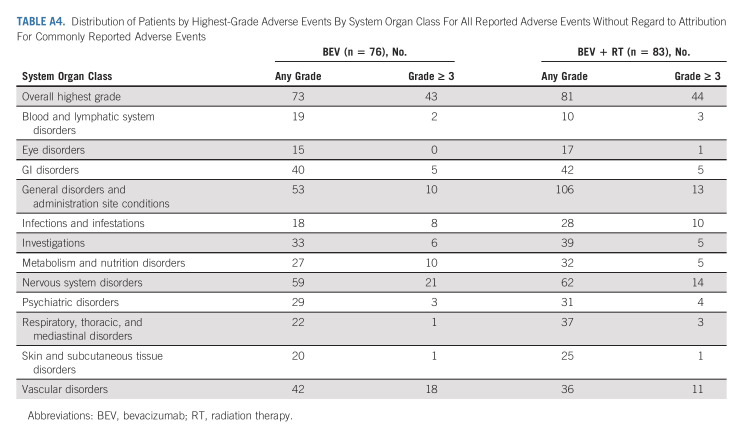
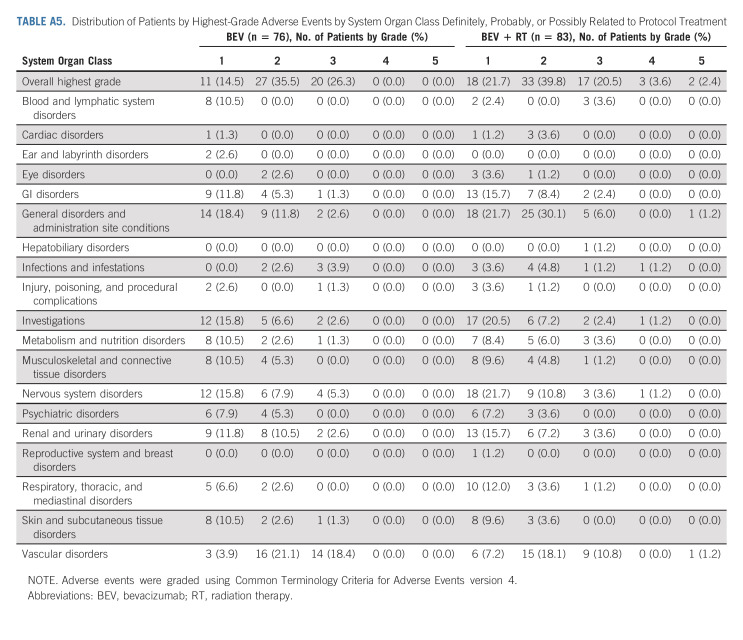
AEs were scored using CTCAE v4. In eligible patients who received protocol treatment there were four patients (5.3%) in the control arm and eight (9.6%) in the experimental arm with reported grade 5 AEs all but two patients were deemed to be either unrelated or unlikely related to protocol treatment (Appendix Tables A3‐A5, online only). One death was related to intratumoral hemorrhage in the BEV + RT arm reported as possibly related to protocol treatment, and the other was death not otherwise specified reported as probably related to protocol treatment. All four patients (4.8%) in the experimental arm reported acute grade 3+ treatment-related CNS AEs, whereas no patient had reported delayed grade 3+ treatment-related CNS AEs.
Centralized Protocol Review
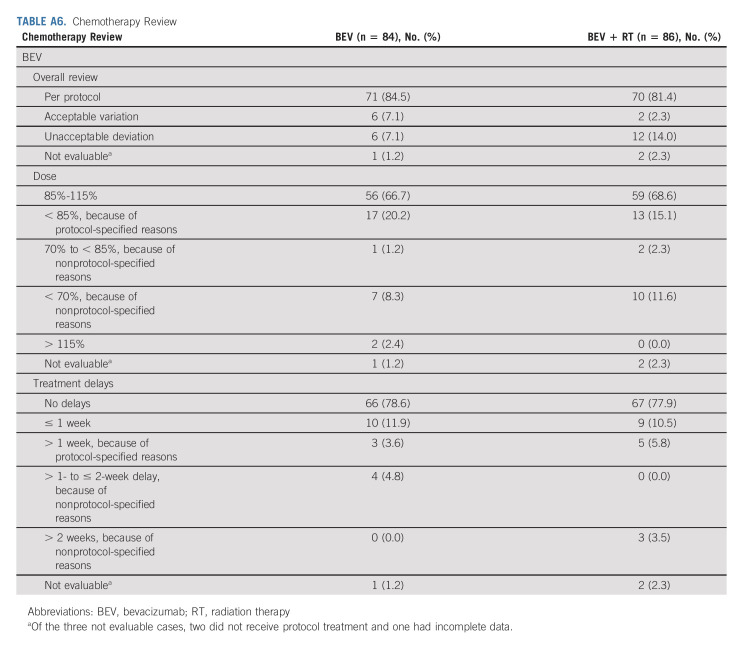
Centralized review was undertaken to ascertain protocol compliance (Appendix Table A6, online only). The median number of BEV cycles completed in the experimental arm was three (range, 1-16) and two (range, 1-17) in the control arm. Approximately 10% of the patients discontinued BEV because of an AE, side effects, or complications.
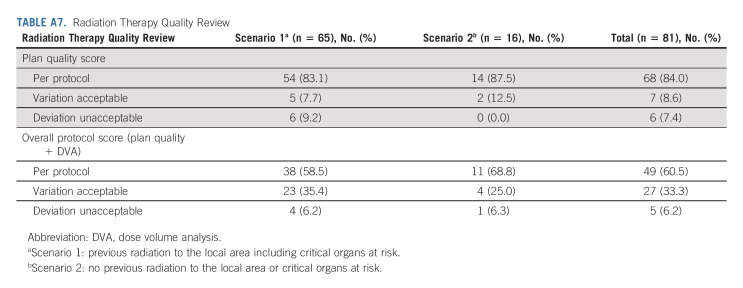
Detailed information on RT planning reviews revealed that 76 of 81 (93.8%) met the minimum quality standard, 60.5% followed the, and 33.3% had an acceptable deviation (Appendix Table A7, online only). The median gross target volume for the re-RT arm was 18 cc (min-max: 0.5-208 cc). The median PTV was 54 cc (min-max: 4-412 cc). Protocol shortcomings included the following: tumor size > 6 cm, evidence of multifocal disease, leptomeningeal or subependymal tumor spread, and RT plans with considerable underdosage of the tumor because of its location near critical structures. Of note, treatment planning MR imaging revealed recurrent, enhancing tumor in nine (36%) cases, despite reported GTR.
DISCUSSION
Few effective salvage treatment options for recurrent glioma exist, and no well-defined standard of care is universally accepted. BEV represents one relatively recently approved approach, and although it yields meaningful PFS improvement, OS improvement remains elusive.33
re-RT has long been proposed as a safe and effective therapy.3,34-37 Advances in RT techniques including fractionated stereotactic radiotherapy, heavy particles, and intensity-modulated RT have enabled increasingly conformal treatment, reducing the likelihood of acute and late CNS toxicity.4-6,38-40 A recent meta-analysis of re-RT revealed a 12-month OS rate of 36% (95% CI, 32 to 40) and a 6-month PFS rate of 43% (95% CI, 35 to 50).41 Previous studies have reported improved 6-month functional status and reduction or discontinuation of corticosteroid usage.3,35,42-46 Consensus around treatment guidelines for recurrent glioma has proved to be elusive in part because of a lack of randomized, prospective trials and retrospective studies published without an established control group.46,47
NRG Oncology/RTOG1205 was a prospective, multi-institutional randomized trial undertaken to confirm the safety of concurrent BEV and re-RT 35 Gy in 10 fractions. The primary objective was to evaluate OS with BEV alone versus concomitant BEV and re-RT in BEV-naïve recurrent GBM. To this end, the study failed to establish a significant survival benefit. Results demonstrated a median OS of 10.1 months for the experimental arm and 9.7 months for the control arm. Multivariable analysis revealed that younger age, improved KPS, and methylated MGMT promoter status were associated with better OS.
NRG Oncology/RTOG1205 confirmed improvement in PFS and 6-month PFS rates for the concomitant BEV + re-RT arm compared with BEV alone. The median PFS was 7.1 and 3.8 months, respectively. The 6-month PFS rate remains an important end point for recurrent GBM, whereas no prior therapeutic trials have demonstrated an OS benefit. Disease progression remains a key event in driving QOL deterioration. Preserving neurologic function and QOL without the need for chronic steroid use remains highly relevant. The historic assumption from multiple recurrent GBM trials is that the 6-month PFS is consistently around 15%. Many trials are powered to detect an approximately 13%-15% improvement on this baseline (28%-30%).48,49 In 2017, BEV was granted regular approval, following results of EORTC trial 26101, a randomized study of 432 patients with recurrent GBM comparing lomustine versus lomustine plus BEV.33 No difference in OS was noted between BEV + lomustine versus lomustine-alone arms. Patient characteristics were similar with a median age of 57 years (59 years in NRG Oncology/RTOG1205), 44% had a WHO performance status of 0 (KPS, 90-100 in 45% of NRG Oncology/RTOG1205), and 56% had a largest tumor diameter of ≤ 40 mm. Median PFS was improved in the BEV + lomustine arm (4.2 v 1.5 months; HR, 0.52; 95% CI, 0.41 to 0.64). Again, the median PFS in NRG Oncology/RTOG1205 was 3.8 months on the BEV arm and 7.1 months for BEV + re-RT.
Secondary analyses of salvage therapies for recurrent GBM have revealed trends toward better survival in those receiving salvage therapy compared with those who did not.50 re-RT combined with systemic therapy was reported in 10% of patients (64 patients). The median OS was 9.7 months (range, 6.5-14.6). In multivariable analysis, KPS ≥ 70% (P < .01), re-RT for first recurrence (P = .02), longer time interval to re-RT (P < .01), and smaller PTVs (P < .05) were significant prognostic factors. Retrospective analyses provide similar confirmation that re-RT remains a safe, reasonable, and effective treatment option for patients with recurrent GBM with excellent KPS and limited volume recurrence.3,4,34,36,37,41,46,47
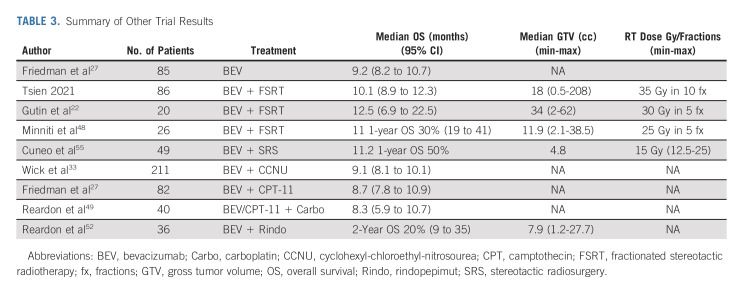
Strategies for salvage treatment of recurrent glioma have evolved largely without consensus. Poor outcomes, significant patient heterogeneity including genetically diverse subclones within the tumor, and a complex glioma tumor microenvironment capable of sustaining stem cell-like tumor cells that are frequently RT-resistant may provide some explanation for this pattern.51 The blood brain barrier remains an ever-present obstacle to the delivery of potentially effective drug therapy. Despite randomized chemotherapy studies including novel targeted agents, no systemic therapy has shown significant improvement in OS for recurrent GBM (Table 3). Novel therapies such as a targeted immunotherapeutic approach with rindopepimut, an antiepidermal growth factor (EGFRvIII) cancer vaccine, in combination with BEV in recurrent GBM demonstrated a disappointing 6-month PFS of 28%.52 Several studies evaluating BEV and re-RT including fractionated stereotactic radiotherapy and stereotactic radiosurgery (SRS) studies suggested promising efficacy (Table 3).22,53-55
TABLE 3.
Summary of Other Trial Results
re-RT using modern, SRS techniques delivers higher doses of RT to a considerably more precise target volume, in one to five fractions, and with a steep dose gradient beyond the tumor. A retrospective study of 49 patients with recurrent GBM receiving SRS and concurrent BEV reported a median OS of 10 months. Smaller treatment volume correlated with improved outcomes.55 Future studies will determine the role of re-RT using SRS techniques in combination with novel agents including checkpoint inhibitors to prevent recurrence by eliciting a more durable immune response.56
Rethinking re-RT treatment target volumes combined with new approaches to RT delivery is another potential avenue. Fractionated SRT schedules (using ≤ 3.5 Gy/fraction) allow treatment of larger tumor volumes particularly near critical eloquent structures. Prospective studies evaluating targeting FLAIR should be considered.57 re-RT strategies that target only enhancing tumor regions need to better account for the diffuse, infiltrative, and often nonenhancing pattern of recurrent GBM growth.58
NRG Oncology/RTOG1205 revealed key challenges in demonstrating an OS benefit in a prospective, randomized multi-institutional trial in the salvage setting. Selection of appropriate patients for re-RT remains crucial.5,6,58-60 In response to low accrual, NRG Oncology/RTOG1205 was amended to broaden eligibility, resulting in the inclusion of a significant number of patients less likely to benefit from focal, re-RT because of extensive disease burden. With limited third-line and fourth-line salvage options available, several patients on the control arm ultimately received salvage re-RT. Additional molecular markers including MGMT methylation and IDH mutation status should be considered as potential stratification factors.
In conclusion, optimal treatment for patients with recurrent GBM remains controversial. Although the combination of re-RT and BEV did not significantly improve OS for patients with BEV-naive recurrent GBM, NRG Oncology/RTOG1205 confirmed meaningful improvement in PFS, including the 6-month PFS rate, with concurrent re-RT and BEV compared with BEV alone, which most patients consider clinically beneficial. This is especially true when considering that treatment was safe and well-tolerated with no delayed CNS treatment–related toxicities. re-RT remains a reasonable option for patients with small volume of recurrence, methylated MGMT promoter status, and good KPS. re-RT should not be withheld on the basis of age as treatment remains safe and results in comparable outcomes. Future cooperative group studies should consider prospectively evaluating the neurocognitive, symptom burden and QOL benefit of salvage interventions in this patient population.
APPENDIX
TABLE A1.
Cox Proportional Hazards Model (full) for Overall Survival
TABLE A2.
Best Objective Response Rate
TABLE A3.
Distribution of Patients by Highest-Grade Adverse Events by System Organ Class For All Reported Adverse Events Without Regard to Attribution
TABLE A4.
Distribution of Patients by Highest-Grade Adverse Events By System Organ Class For All Reported Adverse Events Without Regard to Attribution For Commonly Reported Adverse Events
TABLE A5.
Distribution of Patients by Highest-Grade Adverse Events by System Organ Class Definitely, Probably, or Possibly Related to Protocol Treatment
TABLE A6.
Chemotherapy Review
TABLE A7.
Radiation Therapy Quality Review
Christina I. Tsien
Speakers' Bureau: Varian Medical Systems
Travel, Accommodations, Expenses: Zeiss
Stephanie L. Pugh
Research Funding: Pfizer (Inst), Millennium (Inst)
Adam P. Dicker
Stock and Other Ownership Interests: OncoHost
Consulting or Advisory Role: EMD Serono, Janssen, Roche, Cybrexa Therapeutics, OncoHost, Third Bridge, Accordant, Alcimed, Orano Med, IBA, Genentech, Deallus, CVS, Hengrui Pharmaceutical, Voluntis
Patents, Royalties, Other Intellectual Property: We recently filed a patent "Doped BEO Compounds for Optically Stimulated Luminescence (OSL) and Thermoluminescence (TL) Radiation Dosimetry"
Expert Testimony: Wilson Sonsini
Travel, Accommodations, Expenses: OncoHost
Other Relationship: European Commission
Uncompensated Relationships: Google, Dreamit Ventures
Jeffrey J. Raizer
Employment: Astellas Pharma
Stock and Other Ownership Interests: Agenus, Exicure, Cancer Action Now
Martha M. Matuszak
Consulting or Advisory Role: Varian Medical Systems
Research Funding: Varian Medical Systems
Other Relationship: Blue Cross Blue Shield of Michigan
Enrico C. Lallana
Employment: The Permanente Medical Group
Stock and Other Ownership Interests: The Permanente Medical Group
Open Payments Link: https://openpaymentsdata.cms.gov/physician/483578
Jiayi Huang
Research Funding: Cantex Pharmaceuticals, Pfizer/EMD Serono
Lorraine Portelance
Research Funding: ViewRay (Inst)
Travel, Accommodations, Expenses: RefleXion Medical
John T. Hamm
Consulting or Advisory Role: Meda Pharmaceuticals
Research Funding: AbbVie (Inst), Amgen (Inst), ARMO BioSciences (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Boston Biomedical (Inst), BrightPath Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Cancer Research and Biostatistics (Inst), Celgene (Inst), Clovis Oncology (Inst), Daiichi Sankyo (Inst), Dana-Farber Cancer Institute (Inst), Deciphera (Inst), Lilly (Inst), EMD Serono (Inst), EpicentRx (Inst), Exact Sciences (Inst), Roche (Inst), Five Prime Therapeutics (Inst), G1 Therapeutics (Inst), GBG Forschungs (Inst), Genentech (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst), Gynecologic Oncology Group (Inst), Halozyme (Inst), Immunicum (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Jiangsu Hengrui Medicine (Inst), Johnson & Johnson (Inst), MedImmune (Inst), Merck (Inst), Moffitt (Inst), National Surgical Adjuvant Breast and Bowel Project (Inst), Nektar (Inst), Novartis (Inst), OncoMed (Inst), Pfizer (Inst), PharmaMar (Inst), Plexxikon (Inst), Pronova (Inst), Sarah Cannon Research Institute (Inst), Seattle Genetics (Inst), SWOG (Inst), Spectrum Pharmaceuticals (Inst)
Arif N. Ali
Leadership: Northwest Georgia Radiation Oncology, LLC
Stock and Other Ownership Interests: Northwest Georgia Radiation Oncology
Michelle M. Kim
Consulting or Advisory Role: Blue Earth Diagnostics (Inst)
Research Funding: EpicentRx (Inst), Blue Earth Diagnostics (Inst)
Travel, Accommodations, Expenses: Capital Health
Minesh P. Mehta
Leadership: Oncoceutics
Stock and Other Ownership Interests: Chimerix
Consulting or Advisory Role: Karyopharm Therapeutics, Mevion Medical Systems, ZappRx, Sapience Therapeutics, Xoft
Patents, Royalties, Other Intellectual Property: WARF patent 14/934,27, Topical Vasoconstrictor Preparations and Methods for Protecting Cells During Cancer Chemotherapy and Radiotherapy
Uncompensated Relationships: Xcision Medical Systems, ViewRay
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 1183
PRIOR PRESENTATION
Presented at the 2019 American Society for Radiation Oncology meeting, Chicago, IL, September 17, 2019; and the 2019 Society for Neuro‐Oncology meeting, Phoenix, AZ, November 22, 2019.
SUPPORT
Supported by grants U10CA180868 (NRG Oncology Operations) and U10CA180822 (NRG Oncology SDMC) from the National Cancer Institute (NCI).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Christina I. Tsien, Adam P. Dicker, Jeffrey J. Raizer, Martha M. Matuszak, Minesh P. Mehta
Administrative support: Minesh P. Mehta
Provision of study materials or patients: Adam P. Dicker, Jeffrey J. Raizer, Jiayi Huang, Nimisha Deb, Lorraine Portelance, John L. Villano, John T. Hamm, Arif N. Ali, Michelle M. Kim, Minesh P. Mehta
Collection and assembly of data: Stephanie L. Pugh, Adam P. Dicker, Jeffrey J. Raizer, Martha M. Matuszak, Enrico C. Lallana, Jiayi Huang, Ozer Algan, Nimisha Deb, Lorraine Portelance, John L. Villano, John T. Hamm, Kevin S. Oh, Arif N. Ali, Scott M. Lindhorst
Data analysis and interpretation: Stephanie L. Pugh, Adam P. Dicker, Jeffrey J. Raizer, Martha M. Matuszak, Jiayi Huang, John T. Hamm, Michelle M. Kim, Scott M. Lindhorst, Minesh P. Mehta
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
NRG Oncology/RTOG1205: A Randomized Phase II Trial of Concurrent Bevacizumab and Reirradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Christina I. Tsien
Speakers' Bureau: Varian Medical Systems
Travel, Accommodations, Expenses: Zeiss
Stephanie L. Pugh
Research Funding: Pfizer (Inst), Millennium (Inst)
Adam P. Dicker
Stock and Other Ownership Interests: OncoHost
Consulting or Advisory Role: EMD Serono, Janssen, Roche, Cybrexa Therapeutics, OncoHost, Third Bridge, Accordant, Alcimed, Orano Med, IBA, Genentech, Deallus, CVS, Hengrui Pharmaceutical, Voluntis
Patents, Royalties, Other Intellectual Property: We recently filed a patent "Doped BEO Compounds for Optically Stimulated Luminescence (OSL) and Thermoluminescence (TL) Radiation Dosimetry"
Expert Testimony: Wilson Sonsini
Travel, Accommodations, Expenses: OncoHost
Other Relationship: European Commission
Uncompensated Relationships: Google, Dreamit Ventures
Jeffrey J. Raizer
Employment: Astellas Pharma
Stock and Other Ownership Interests: Agenus, Exicure, Cancer Action Now
Martha M. Matuszak
Consulting or Advisory Role: Varian Medical Systems
Research Funding: Varian Medical Systems
Other Relationship: Blue Cross Blue Shield of Michigan
Enrico C. Lallana
Employment: The Permanente Medical Group
Stock and Other Ownership Interests: The Permanente Medical Group
Open Payments Link: https://openpaymentsdata.cms.gov/physician/483578
Jiayi Huang
Research Funding: Cantex Pharmaceuticals, Pfizer/EMD Serono
Lorraine Portelance
Research Funding: ViewRay (Inst)
Travel, Accommodations, Expenses: RefleXion Medical
John T. Hamm
Consulting or Advisory Role: Meda Pharmaceuticals
Research Funding: AbbVie (Inst), Amgen (Inst), ARMO BioSciences (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Boston Biomedical (Inst), BrightPath Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Cancer Research and Biostatistics (Inst), Celgene (Inst), Clovis Oncology (Inst), Daiichi Sankyo (Inst), Dana-Farber Cancer Institute (Inst), Deciphera (Inst), Lilly (Inst), EMD Serono (Inst), EpicentRx (Inst), Exact Sciences (Inst), Roche (Inst), Five Prime Therapeutics (Inst), G1 Therapeutics (Inst), GBG Forschungs (Inst), Genentech (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst), Gynecologic Oncology Group (Inst), Halozyme (Inst), Immunicum (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Jiangsu Hengrui Medicine (Inst), Johnson & Johnson (Inst), MedImmune (Inst), Merck (Inst), Moffitt (Inst), National Surgical Adjuvant Breast and Bowel Project (Inst), Nektar (Inst), Novartis (Inst), OncoMed (Inst), Pfizer (Inst), PharmaMar (Inst), Plexxikon (Inst), Pronova (Inst), Sarah Cannon Research Institute (Inst), Seattle Genetics (Inst), SWOG (Inst), Spectrum Pharmaceuticals (Inst)
Arif N. Ali
Leadership: Northwest Georgia Radiation Oncology, LLC
Stock and Other Ownership Interests: Northwest Georgia Radiation Oncology
Michelle M. Kim
Consulting or Advisory Role: Blue Earth Diagnostics (Inst)
Research Funding: EpicentRx (Inst), Blue Earth Diagnostics (Inst)
Travel, Accommodations, Expenses: Capital Health
Minesh P. Mehta
Leadership: Oncoceutics
Stock and Other Ownership Interests: Chimerix
Consulting or Advisory Role: Karyopharm Therapeutics, Mevion Medical Systems, ZappRx, Sapience Therapeutics, Xoft
Patents, Royalties, Other Intellectual Property: WARF patent 14/934,27, Topical Vasoconstrictor Preparations and Methods for Protecting Cells During Cancer Chemotherapy and Radiotherapy
Uncompensated Relationships: Xcision Medical Systems, ViewRay
No other potential conflicts of interest were reported.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987-996, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Walker MD, Green SB, Byar DP, et al. : Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 303:1323-1329, 1980 [DOI] [PubMed] [Google Scholar]
- 3.Laing RW, Warrington AP, Graham J, et al. : Efficacy and toxicity of fractionated stereotactic radiotherapy in the treatment of recurrent gliomas (phase I/II study). Radiother Oncol 27:22-29, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Fogh ShannonE, Andrews DavidW, Glass Jon, et al. : Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J Clin Oncol 28:3048-3053, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarria P, Navarria P, Minniti G, et al. : Re-irradiation for recurrent glioma: Outcome evaluation, toxicity and prognostic factors assessment. A multicenter study of the Radiation Oncology Italian Association (AIRO). J Neurooncol 142:59-67, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Combs SE, Niyazi M, Adeberg S, et al. : Re-irradiation of recurrent gliomas: Pooled analysis and validation of an established prognostic score-report of the Radiation Oncology Group (ROG) of the German Cancer Consortium (DKTK). Cancer Med 7:1742-1749, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain RK, Tong RT, Munn LL: Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res 67:2729-2735, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hicklin DJ, Ellis LM: VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer 8:579-591, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Vredenburgh JJ, Cloughesy T, Samant M, et al. : Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist 15:1329-1334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreisl TN, Kim L, Moore K, et al. : Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740-745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vredenburgh JJ, Desjardins A, James E, et al. : Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722-4729, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Vredenburgh JJ, Desjardins A, Herndon JE II, et al. : Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253-1259, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hasselbalch B, Lassen U, Hansen S, et al. : Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: A phase II trial. Neurooncology 12:508-516, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sathornsumetee S, Desjardins A, Vredenburgh JJ, et al. : Phase II trial of bevacizumab plus erlotinib for patients with recurrent malignant gliomas: Final results. J Clin Oncol 28, 2010. (suppl; abstr 2055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reardon DA, Desjardins A, Vredenburgh JJ, et al. : Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: A phase II study. Br J Cancer 101:1986-1994, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhoeff JJC, Lavini C, van Linde ME, et al. : Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol 21:1723-1727, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Taal W, Oosterkamp HM, Walenkamp AM, et al. : Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol 15:943-953, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Field KM, Simes J, Nowak AK, et al. : Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol 17:1504-1513, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovinga KE, Shimizu F, Wang R, et al. : Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells 28:1019-1029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C, Heijn M, di Tomaso E, et al. : Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res 60:5565, 2000 [PubMed] [Google Scholar]
- 21.Levin VA, Bidaut L, Hou P, et al. : Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79:1487-1495, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutin PH, Iwamoto FM, Beal K, et al. : Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 75:156-163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flieger M, Ganswindt U, Schwarz SB, et al. : Re-irradiation and bevacizumab in recurrent high-grade glioma: An effective treatment option. J Neurooncol 117:337-345, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Schnell O, Thorsteinsdottir J, Fleischmann DF, et al. : Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol 130:591-599, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein LV, Korn EL, Freidlin B, et al. : Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol 23:7199-7206, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Zelen M: The randomization and stratification of patients to clinical trials. J Chronic Dis 27:365-375, 1974 [DOI] [PubMed] [Google Scholar]
- 27.Friedman HS, Prados MD, Wen PY, et al. : Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733-4740, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 29.Mantel N: Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163, 1966 [PubMed] [Google Scholar]
- 30.Cox DR: Regression models and life-tables. J R Stat Soc Ser B (Methodol) 34:187-220, 1972 [Google Scholar]
- 31.Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG: Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277-1280, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Wen PY, Macdonald DR, Reardon DA, et al. : Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol 28:1963-1972, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Wick W, Gorlia T, Bendszus M, et al. : Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377:1954-1963, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Hudes RS, Corn BW, Werner-Wasik M, et al. : A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys 43:293-298, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Kim HK, Thornton AF, Greenberg HS, et al. : Results of re-irradiation of primary intracranial neoplasms with three-dimensional conformal therapy. Am J Clin Oncol 20:358-363, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Grosu AL, Weber WA, Franz M, et al. : Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys 63:511-519, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Lederman G, Wronski M, Arbit E, et al. : Treatment of recurrent glioblastoma multiforme using fractionated stereotactic radiosurgery and concurrent paclitaxel. Am J Clin Oncol 23:155-159, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Eberle F, Lautenschläger S, Engenhart-Cabillic R, et al. : Carbon ion beam reirradiation in recurrent high-grade glioma. Cancer Manag Res 12:633-639, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miwa K, Matsuo M, Ogawa S, et al. : Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol 9:181, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Combs SE, Steck I, Schulz-Ertner D, et al. : Long-term outcome of high-precision radiotherapy in patients with brain stem gliomas: Results from a difficult-to-treat patient population using fractionated stereotactic radiotherapy. Radiother Oncol 91:60-66, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Kazmi F, Kazmi F, Soon Y, et al. : Re-irradiation for recurrent glioblastoma (GBM): A systematic review and meta-analysis. J Neurooncol 142:79-90, 2019 [DOI] [PubMed] [Google Scholar]
- 42.Nieder C, Astner ST, Mehta MP, et al. : Improvement, clinical course, and quality of life after palliative radiotherapy for recurrent glioblastoma. Am J Clin Oncol 31:300-305, 2008 [DOI] [PubMed] [Google Scholar]
- 43.VanderSpek L, Fisher B, Bauman G, Macdonald D: 3D conformal radiotherapy and cisplatin for recurrent malignant glioma. Can J Neurol 35:57-64, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Wick W, Krendyukov A, Junge K, et al. : Longitudinal analysis of quality of life following treatment with asunercept plus reirradiation versus reirradiation in progressive glioblastoma patients. J Neurooncol 145:531-540, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coomans M, Dirven L, Aaronson NK, et al. : The added value of health-related quality of life as a prognostic indicator of overall survival and progression-free survival in glioma patients: A meta-analysis based on individual patient data from randomised controlled trials. Eur J Cancer 116:190-198, 2019 [DOI] [PubMed] [Google Scholar]
- 46.Howard SP, Krauze A, Chan MD, et al. : The evolving role for re-irradiation in the management of recurrent grade 4 glioma. J Neurooncol 134:523-530, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Scoccianti S, Francolini G, Carta GA, et al. : Re-irradiation as salvage treatment in recurrent glioblastoma: A comprehensive literature review to provide practical answers to frequently asked questions. Crit Rev Oncol Hematol 126:80-91, 2018 [DOI] [PubMed] [Google Scholar]
- 48.Minniti G, Agolli L, Falco T, et al. : Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. J Neurooncol 122:559-566, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Reardon DA, Desjardins A, Peters KB, et al. : Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol 107:155-164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi W, Scannell Bryan M, Gilbert MR, et al. : Investigating the effect of reirradiation or systemic therapy in patients with glioblastoma after tumor progression: A secondary analysis of NRG oncology/radiation therapy oncology group trial 0525. Int J Radiat Oncol Biol Phys 100:38-44, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bao S, Wu Q, McLendon RE, et al. : Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756-760, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Reardon DA, Desjardins A, Vredenburgh JJ, et al. : Rindopepimut with bevacizumab for patients with relapsed EGFRvIII-expressing glioblastoma (ReACT): Results of a double-blind randomized phase II trial. Clin Cancer Res 26:1586-1594, 2020 [DOI] [PubMed] [Google Scholar]
- 53.Iwamoto FM, Abrey LE, Beal K, et al. : Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 73:1200-1206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabrera AR, Cuneo KC, Vredenburgh JJ, et al. : Stereotactic radiosurgery and bevacizumab for recurrent glioblastoma multiforme. J Natl Compr Canc Netw 10:695-699, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Cuneo KC, Vredenburgh JJ, Sampson JH, et al. : Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 82:2018-2024, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JE, Patel MA, Mangraviti A, et al. : Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res 23:124-136, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bovi JA, Prah MA, Retzlaff AA, et al. : Pulsed reduced dose rate radiotherapy in conjunction with bevacizumab or bevacizumab alone in recurrent high-grade glioma: Survival outcomes. Int J Radiat Oncol Biol Phys 108:979-986, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown TJ, Brennan MC, Li M, et al. : Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol 2:1460-1469, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Post CCB, Kramer MCA, Smid EJ, et al. : Patterns of re-irradiation for recurrent gliomas and validation of a prognostic score. Radiother Oncol 130:156-163, 2019 [DOI] [PubMed] [Google Scholar]
- 60.Nieder C, Molls M: Validation of graded prognostic assessment index for patients with brain metastases: In regard to sperduto et al. (int J radiat oncol biol phys 2008;70:510-514). Int J Radiat Oncol Biol Phys 72:1619, 2008; author reply 1619 [DOI] [PubMed] [Google Scholar]