Abstract
Purpose:
Although stereotactic body radiation therapy (SBRT) is effective in early stage non-small cell lung cancer (NSCLC), typically 10–15% of patients will fail regionally and 20–25% distantly. We evaluate a novel circulating tumor cell (CTC) assay as a prognostic marker for increased risk of recurrence following SBRT.
Experimental Design:
92 subjects (median age 71y) with T1a (64%), T1b (23%), or T2a (13%) stage I NSCLC treated with SBRT were prospectively enrolled. CTCs were enumerated by utilizing a GFP-expressing adenoviral probe that detects the elevated telomerase activity in cancer cells. CTC “positivity” was defined as 1.3 GFP-positive cells/mL of peripheral blood. Samples were obtained before, during, and serially up to 24m after treatment. SBRT was delivered to a median dose of 50Gy (range, 40–60Gy), mostly commonly in 4–5 fractions (92%).
Results:
38 of 92 subjects (41%) had a positive CTC test prior to SBRT. A cutoff of ≥5 CTCs/mL before treatment defined favorable (n=78) and unfavorable (n=14) prognostic groups. Increased risk of nodal (p=0.04) and distant (p=0.03) failure was observed in the unfavorable group. Within 3m following SBRT, CTCs continued to be detected in 10 of 35 (29%) subjects. Persistent detection of CTCs was associated with increased risk of distant failure (p=0.04) and trend toward increased regional (p=0.08) and local failure (p=0.16).
Conclusions:
Higher pre-treatment CTCs and persistence of CTCs post-treatment is significantly associated with increased risk of recurrence outside the targeted treatment site. This suggests that CTC analysis may potentially identify patients at higher risk for regional or distant recurrences and who may benefit from either systemic therapy and/or timely locoregional salvage treatment.
Keywords: biomarkers, stereotactic body radiation therapy, circulating tumor cells, lung cancer, recurrent disease
Translational Relevance
SBRT has been a substantially impactful treatment innovation in recent years, bringing effective and convenient treatment to many patients with early stage lung cancer. Current studies, however, make clear that although many patients with early stage NSCLC are effectively treated by SBRT, regional and distant recurrence rates remain high with long-term follow-up, approaching 40% or more. Recent evidence, too, suggests an unexpectedly low pathologic response rate following SBRT. Detection of persistent disease and local recurrences is made difficult by post-radiation changes around the treated areas, and nodal and distant metastases may not be identified using current radiographic methods for months or years after initial presentation. We have developed a novel telomerase-based CTC assay and demonstrate that patients elevated counts of CTCs prior to the start of SBRT, as well as those patients for whom CTCs remained persistently detectable after SBRT, are associated with increased regional and distant recurrence. Furthermore, increases in CTCs after achieving a post-SBRT nadir provided almost two years of lead time notice of tumor progression or recurrence over conventional imaging. Use of this CTC assay may translate clinically by helping to identify subsets of patients who may maximally benefit from systemic therapy after SBRT for early stage NSCLC, and to help monitor for tumor recurrence or progression.
INTRODUCTION
Approximately 230,000 people in the United States will be diagnosed with lung cancer each year, accounting for nearly 1 in 8 new cancer diagnoses. Seven in eight lung cancer patients are diagnosed with non-small cell lung cancer (NSCLC), of which 16% are diagnosed with early stage disease (Miller, Cancer Statistics 2019).1,2 The proportional incidence of early stage disease is expected to continue to increase with the expansion of low-dose CT screening, now identifying tumors that would have previously gone undetected until a more advanced or metastatic stage.3,4
Those diagnosed with early stage non-small cell lung cancer (ES-NSCLC) are now more likely to receive definitive treatment with the increased utilization of minimally invasive surgeries and hypofractionated radiotherapy, leading to a decrease in the number of untreated patients and an improvement in overall survival.5,6 The increase in definitive therapy and overall survival in recent years has been driven most by the emergence of stereotactic body radiation therapy (SBRT) – also referred to as stereotactic ablative radiotherapy (SABR) – due to its convenience, safety, and excellent local control.7–10 Consistently encouraging outcomes with SBRT have led to several large-scale efforts to compare SBRT with surgery, which at present is considered the standard of care.11 Unfortunately, multiple trials have terminated early and failed to complete accrual due to physician and patient biases. Chang et. al. reported the combined experience in two of these trials in the high-risk population that not only suggested that oncologic outcomes between the SBRT and surgery were similar but also supported SBRT being safer, as the immediate survival was significantly higher with SBRT than surgery that was thought to be related, in large part, to a reduction in morbidity and mortality with SBRT.9 This report was met with significant criticism due to the very limited numbers of enrolled patients, but it has further increased the enthusiasm for SBRT and suggested equipoise of these two approaches.12
However, results from a recent phase II trial with ES-NSCLC undergoing neoadjuvant SBRT followed by a planned surgery revealed a pathologic complete response rate of only 60%, a figure that is substantially below the overall expected >90% local control rate usually reported with SBRT.13 It is possible that the complete pathologic response rate may increase with the passage of additional time, as the replicative ability of live tumor cells may be permanently inhibited by radiation therapy but additional time is required for dying tumor cells to be cleared and to manifest the full antitumor effects of radiation therapy. On the other hand, if the pathologic response is truly lower than historically believed, it may be very difficult to identify progressive or persistent local disease early enough to allow for surgical or other local salvage therapy due to the substantial radiation fibrosis that develops after SBRT. Furthermore, the continued presence of live tumor cells may pose a potential reservoir that may influence local, regional, and distant metastatic rates. Conventional imaging with CT or PET/CT lack sensitivity for detecting live tumor cells and, in the case of PET, can have a substantial false positive rate following SBRT.
We recently reported that a telomerase-based circulating tumor cell (CTC) assay that detects live tumor cells usefully complemented conventional imaging in patients treated for presumed ES-NSCLC lacking pathologic tissue for confirmed diagnosis. In this work, we investigated the application of this CTC assay method in a larger cohort of patients, including those with pathologically-confirmed ES-NSCLC treated with SBRT, hypothesizing that detection and serial monitoring of CTCs may be a useful prognostic marker for detecting an increased risk of recurrence following SBRT.
METHODS AND MATERIALS
Circulating Tumor Cell Assay
The telomerase-based CTC assay employed in this study has been effective for patients with breast cancer, glioma, melanoma, and NSCLC.14–21 The assay consists of an adenoviral-based probe that expresses green fluorescent protein (GFP) driven by a human telomerase reverse transcriptase promoter element in live cells with increased telomerase activity, and which are then detected and enumerated by fluorescence microscopy. Increased telomerase activity is characteristic of most tumor cells and forestalls senescence; conversely, it is not seen in almost all normal cells. Preclinical validation of the assay defined the threshold for CTC “positivity” at 1.3 GFP-expressing cells per mL of processed blood.
Analysis of Patient Samples
Patients with ES-NSCLC (T1–2aN0M0) undergoing SBRT were considered for enrollment on this prospective trial, including both those subjects with biopsy-proven disease and those with a presumed, clinically diagnosed NSCLC (AJCC 7th edition). The clinical diagnosis of NSCLC was determined by multidisciplinary consensus for those subjects who were not eligible for biopsy due to co-morbidities or who had indeterminate pathology results. Factors such as smoking status, lesion size, the radiographic appearance of lesions (i.e. spiculations, lack of calcifications), lesion growth on serial imaging, and PET avidity were taken into consideration to determine clinically diagnosed lesions as ES-NSCLC, in keeping with recent guidelines from our group.22 Patients were ineligible if they had a prior non-lung malignancy within the previous 5 years with the exception of non-melanoma skin cancer. For those subjects with a previous history of NSCLC, cases were review by the principal investigator to include only those cured of their initial disease and currently presenting with a new primary. Following identification, subjects were prospectively approached to participate in an institutional review board–approved biomarker trial (Clinicaltrials.gov number, NCT02135679) and provided written informed consent for participation.
For each patient, a pre-treatment (pre-RT) peripheral blood sample was collected at initial consult or simulation for radiation therapy. In patients with two pre-RT samples, with the second draw commonly occurring just prior to the first SBRT fraction, the greater of the two values were used in pre-RT analysis. On-treatment (on-RT) blood samples were collected between the delivery of the first fraction of radiation therapy and the completion of the full SBRT treatment course. Post-treatment (post-RT) blood samples were obtained at follow-up intervals of 1, 3, 6, 12, 18 and 24 months. All care provider and laboratory personnel were blinded to CTC quantitative and qualitative analysis.
Treatment and Follow-Up
SBRT was delivered to all subjects to a median dose of 50 Gy (range, 50–60 Gy) most commonly in 4 or 5 (92%) fractions (range, 4–20); median biologically effective dose (α/β = 10) was 100 Gy (range, 78–112.5 Gy). Seven patients (8%) received a less aggressive fractionation (>5 fractions) for larger, centrally/ultracentrally located tumors. Following treatment, patients were monitored for disease control via computed tomography (CT) or positron emission tomography (PET)/CT imaging every 3 months in year 1 and every 3 to 6 months thereafter. Local failure was defined as tumor growth following initial shrinkage or progression at the local site on 2 consecutive scans. Nodal failure was defined as recurrence at the hilar, ipsilateral or contralateral mediastinum or supraclavicular lymph nodes. Distant metastasis was defined as tumor recurrence in the contralateral lung or outside the hemithorax. To differentiate metachronous primary tumors from recurrent disease, criteria from Martini and Melamed was applied.23 In all cases, pathologic confirmation of recurrent disease was obtained when possible. Date of death was determined by death certificates or institutional medical records.
Statistical Analysis
Descriptive statistics were used to evaluate demographic characteristics, disease details, and longitudinal CTC trends. Chi-square for categorical variables and student t-tests for continuous variables were performed to investigate differences in clinicopathologic details between populations.
As it has been reported that high levels of CTCs are prognostic of outcome - rather than just the absence or presence of CTCs24 - we sought to test whether there was a threshold for baseline CTC values that was likewise prognostic of disease outcome in this patient population. In order to determine a threshold for baseline CTC values, a series of CTC values between 2 and 7 were tested through analysis of receiver operating characteristic curves.
Overall survival was calculated from the start of SBRT to the date of last follow-up at our institution or death from any cause. Time to recurrence was calculated from the start date of SBRT to the first occurrence of disease recurrence (local, nodal, or distant disease) censored at last follow-up. Kaplan-Meier survival analysis and Cox multivariate regression analysis explored the relative effect of covariates on recurrence and survival outcomes, with the latter controlling for history of previous NSCLC, tumor size, and pre-RT PET scan SUVmax (Supplementary Table S1). Variable inclusion for multivariate regression was established with a p-value cut off <0.20 in univariate comparison of CTC-detectable and undetectable cohorts. Receiver operating characteristic curves were generated for a series of baseline CTC values between 2 and 7 to identify a threshold value prognostic of disease recurrence. Statistical analysis was performed using STATA 14 (StataCorp, College Station, TX). Statistical significance was defined as p<0.05, and all tests were 2-sided.
RESULTS
Subject Demographics and Disease Characteristics
A total of 92 subjects with ES-NSCLC were enrolled between August 2013 and October 2018 and were eligible for analysis. Subjects were predominantly female (58%), Caucasian (80%), and current (20%) or former (75%) smokers with a median 40 pack-year smoking history (range, 0–200 years). Median age was 71 years (range 55–93 years). Subjects had T1a (64%), T1b (23%), or T2a (13%) NSCLC with median tumor size of 1.7 cm (range, 0.5–5 cm). Pathology confirmation was available for the diagnosis of ES-NSCLC in 40% (n=37) of the subjects, with 22 subjects (24%) having adenocarcinoma and 15 (16%) having squamous cell biopsy-proven disease; 60% of the population, in contrast, had clinically diagnosed disease (Table 1).
Table 1.
Patient demographics and clincopathologic details for entire cohort and according to CTC-detectability
| Total (n=92) | CTC-Detectable (n=38) | CTC-Undetectable (n=54) | p-value | ||
|---|---|---|---|---|---|
| Gender | 0.37 | ||||
| Male | 39 (42%) | 14 (37%) | 25 (46%) | ||
| Female | 53 (58%) | 24 (63%) | 29 (54%) | ||
| Age | 0.52 | ||||
| Median (IQR) | 71 (66–78) | 71 (66–80) | 71 (65–78) | ||
| Range | 55–93 | 59–93 | 55–91 | ||
| Race | 0.53 | ||||
| White | 74 (80%) | 33 (87%) | 41 (76%) | ||
| African-American | 14 (15%) | 4 (10%) | 10 (19%) | ||
| Asian | 1 (1%) | 0 (0%) | 1 (2%) | ||
| Other | 3 (3%) | 1 (3%) | 2 (4%) | ||
| Body Mass Index | 0.62 | ||||
| Median (IQR) | 26 (23–31) | 27 (23–30) | 26 (23–31) | ||
| Range | 16–43 | 19–38 | 16–43 | ||
| Smoking Status | 0.85 | ||||
| Former | 69 (75%) | 29 (76%) | 40 (74%) | ||
| Current | 19 (20%) | 7 (18%) | 12 (22%) | ||
| Never | 4 (5%) | 2 (5%) | 2 (4%) | ||
| Pack-years | 0.90 | ||||
| Median (IQR) | 40 (25–68) | 40 (25–69) | 40 (25–60) | ||
| Range | 0–200 | 0–200 | 0–165 | ||
| Tumor Size (cm) | 0.03 | ||||
| Median (IQR) | 1.7 (1.2–2.2) | 1.4 (1.2–2.0) | 1.8 (1.2–2.4) | ||
| Range | 0.5–5 | 0.5–3.9 | 0.9–5.0 | ||
| Tumor SUV | 0.14 | ||||
| Median (IQR) | 4.3 (2.5–8.4) | 3.9 (2.2–7.8) | 4.8 (2.5–9.9) | ||
| Range | 0.8–19.9 | 0.8–13.3 | 0.8–19.9 | ||
| AJCC Stage | 0.69 | ||||
| IA | 81 (88%) | 34 (89%) | 47 (87%) | ||
| IB | 11 (12%) | 4 (11%) | 7 (13%) | ||
| T Stage | 0.11 | ||||
| 1a | 59 (64%) | 29 (76%) | 30 (56%) | ||
| 1b | 21 (23%) | 5 (13%) | 16 (30%) | ||
| 2a | 12 (13%) | 4 (10%) | 8 (15%) | ||
| Histology | 0.22 | ||||
| Adenocarcinoma | 22 (24%) | 7 (18%) | 15 (28%) | ||
| Squamous cell | 15 (16%) | 9 (24%) | 6 (11%) | ||
| Not pathologically confirmed | 55 (60%) | 22 (58%) | 33 (61%) | ||
| Previous NSCLC | <0.01 | ||||
| Yes | 17 (19%) | 12 (32%) | 5 (9%) | ||
| No | 75 (81%) | 26 (68%) | 49 (91%) | ||
Clinicopathologic details and survival outcomes between those subjects with biopsy-proven disease and clinically diagnosed disease were compared. No differences were observed between gender, race, BMI, smoking history, previous history of NSCLC, or baseline CTC detectability (all p>0.05); however, those subjects with a clinical diagnosis of ES-NSCLC were younger (median age, 69 years versus 74 years) and with smaller tumors (median size, 1.4 cm versus 2.0 cm) than those with pathologically-diagnosed disease (Supplementary Table S2). Freedom from local, nodal, and distant recurrence and overall survival between these populations all were equivalent (Supplementary Figure S3A–D). Given the similarities between these populations, we determined it reasonable to combine these groups for the remainder of the analysis, as has been typical for SBRT analyses.25
CTC Detection Prior to Radiation Therapy
Of the total population, 38 of 92 subjects (41%) had a positive CTC test (range, 0 to 209 CTCs/mL) prior to SBRT. We will herein refer to this cohort as those with “CTC-detectable” disease. Correlations between clinicopathologic details and baseline CTC levels were explored (Table 1). Gender, age, race, BMI, smoking history, tumor SUVmax, AJCC stage, T stage, and histology were not associated with CTC-detectable disease.
With a median follow-up of 24 months (range, 2–62 months), the 2-year local, regional and distant control rates for the entire group were 90%, 86%, and 87%, respectively, and the 2-year overall survival was 80% (Supplementary Figure S4A–D).
Thirty-one of 38 (82%) CTC-detectable subjects and 41 of 54 (76%) of CTC-undetectable subjects were alive at the time of analysis. No difference in the incidence of local (p=0.68), nodal (p=0.91), or distant (p=0.73) recurrence between CTC-detectable and undetectable cohorts were observed (Supplementary Figure S5A–C). Additionally, no difference in overall survival was found between cohorts (p=0.37), with estimated 2-year overall survival rates of 86% (95% CI 69%-94%) in the CTC-detectable cohort and 75% (95% CI 59%-86%) in the CTC-undetectable cohort (Supplementary Figure S5D).
The Prognostic Significance of High CTC Numbers Prior to SBRT
A pre-RT cutoff of ≥5 CTCs/mL demonstrated the maximal area under the curve in predicting any event of recurrence (AUC = 0.64, specificity=90%), therein defining favorable (n=78) and unfavorable (n=14) prognostic groups by baseline CTC. Decreased freedom from nodal (HR 3.92, p=0.04) and distant (HR 4.29, p=0.03) failure were observed in the unfavorable cohort, with 2-year actuarial rates in the unfavorable versus favorable cohorts of 84% versus 87% nodal failure-free (Figure 1B) and 76% versus 89% distant failure-free (Figure 1C). In contrast, the unfavorable CTC cohort was not significantly associated with decreased freedom from local failure (HR 2.5, p=0.15) or overall survival (HR 1.0, p=0.95), with 2-year rates in the unfavorable versus favorable cohorts of 89% versus 92% (Figure 1A) and 86% versus 78%, respectively (Figure 1D). These results suggested that CTC counts of 5 or more prior to SBRT was significantly associated with increased nodal and distant failures after the completion of SBRT. Prior history of NSCLC was not associated with recurrence or survival outcomes in our multivariate analysis (Supplementary Table S1).
Figure 1.
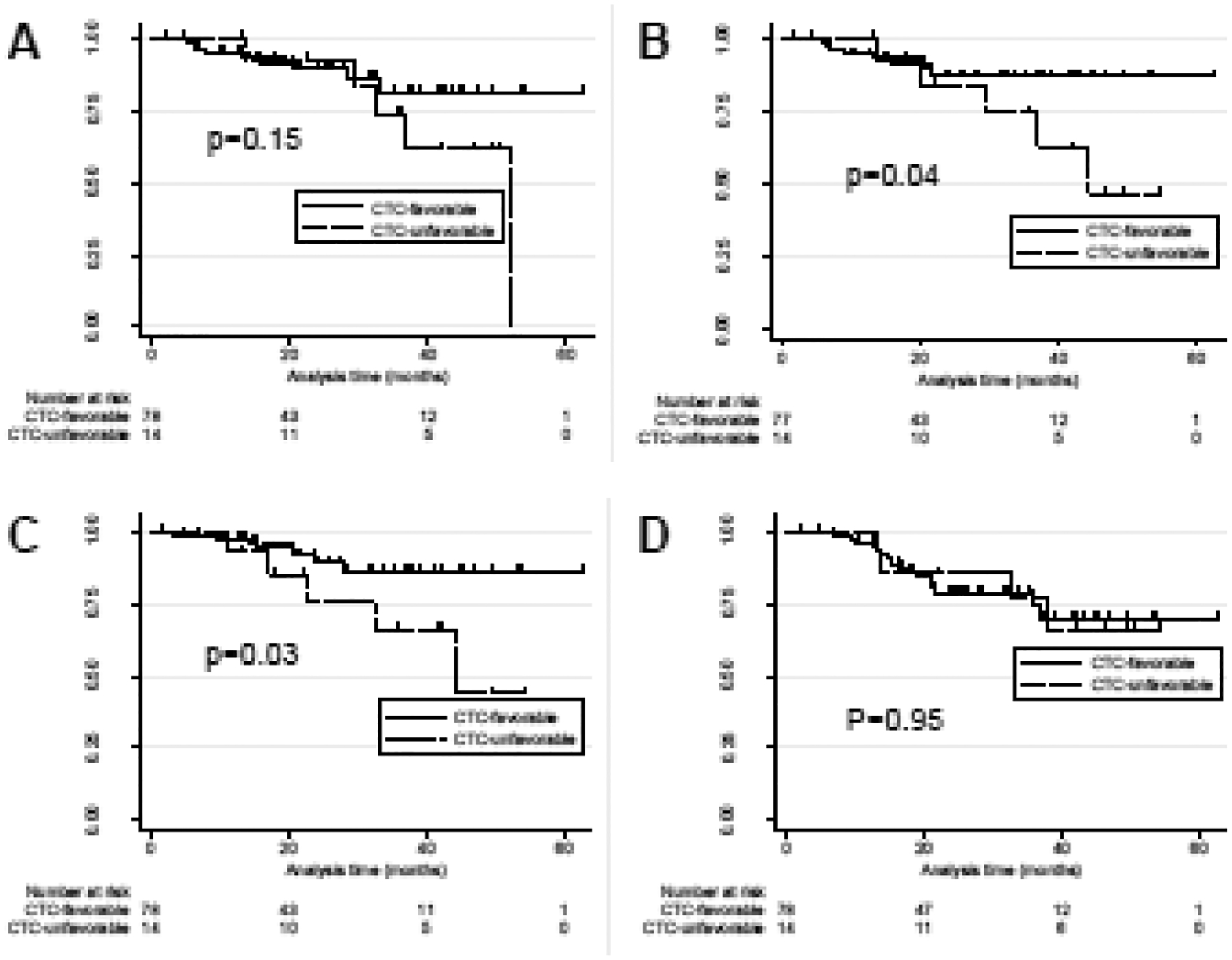
Kaplan Meier incidence of A) freedom from local disease, B) freedom from nodal disease, C) freedom from distant disease, and D) overall survival for subjects with favorable versus unfavorable CTC profiles
The Prognostic Significance of Persistent CTCs Following Completion of SBRT
In most patients, CTCs counts rapidly fell after SBRT, commonly reaching nadir by the 1 months post-SBRT CTC evaluation. Trends in CTC counts of the CTC-detectable population across time are shown in Figure 2. Median pre-RT CTC count (n=38) was 3.9 CTCs per mL (IQR, 2–14.4 CTCs per mL). Median on-RT CTC count was 0.5 CTCs per mL (n=35; IQR 0.1–6.1 CTCs per mL). Median post-RT counts at 1-month (n=25) and 3-months (n=30) were both 0.5 CTCs per mL.
Figure 2.
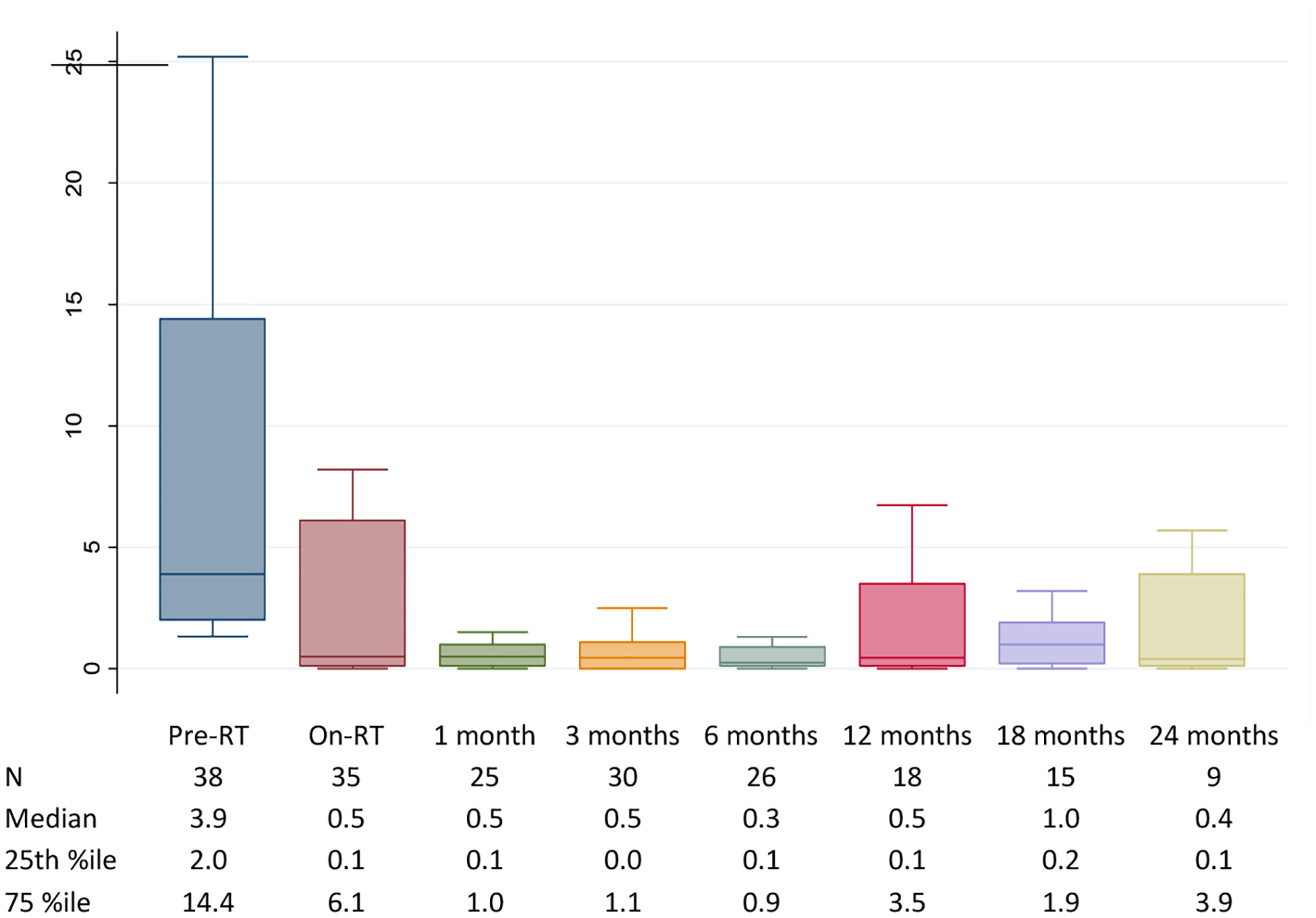
Longitudinal CTC trends
Post-RT CTC trends relating to clinical outcomes were explored. We assessed if there was any association between outcome and continued detection of CTCs in the early post-treatment period, as defined as a blood draw taken within 3 months of completion of treatment and typically corresponding with the first scheduled follow-up imaging (at 3 months post-SBRT). Of the 35 CTC-detectable subjects who had a CTC test drawn in the early post-treatment period, 10 (29%) had at least one positive CTC result. A positive CTC test in the immediate post-treatment time period was associated with an increased risk of distant recurrence (HR 15.1, p=0.04) in the CTC-persistent versus CTC-negative cohorts, with 2-year freedom from distant recurrence of 68% versus 100% (Figure 3C). Trends towards increased risk of local (HR 4.3, p=0.16) and nodal recurrence (HR 11.9, p=0.08) were observed in the CTC-persistent versus CTC-negative cohorts, with 2-year rates of freedom from local and nodal recurrence of 88% versus 100% (Figure 3A) and 77% versus 100% (Figure 3B), respectively. No association in overall survival was observed between CTC-persistent versus CTC-negative cohorts (HR 1.3, p=0.81), with 2-year overall survival rates of 89% versus 92% (Figure 3D).
Figure 3.
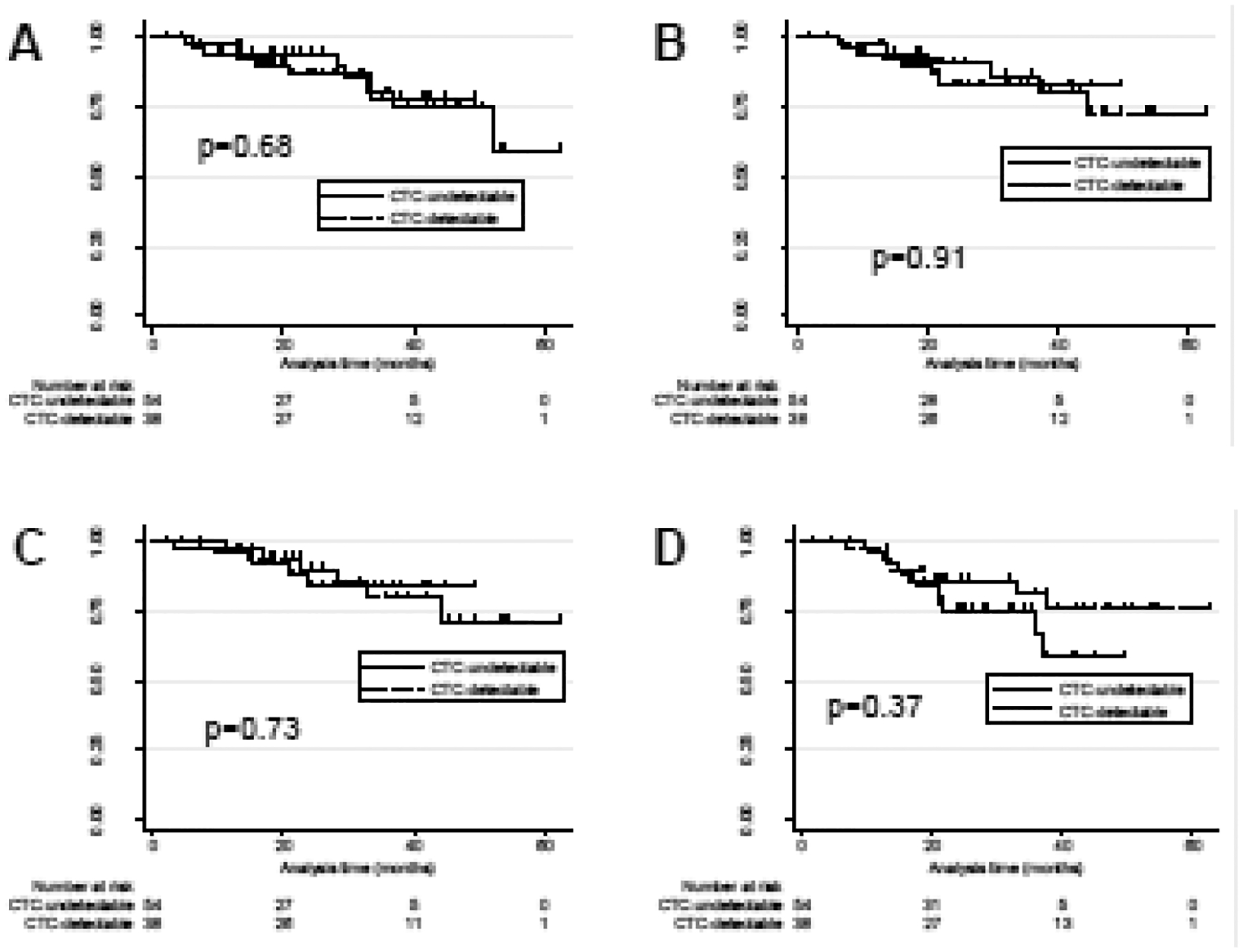
Kaplan Meier incidence of A) freedom from local disease, B) freedom from nodal disease, C), freedom from distant disease, and D) overall survival in subjects with continued detection of CTCs (“CTC-persistent) versus CTC resolution (CTC-negative) in the early post-treatment era (≤3m).
Table 2 provides a summary of clinical details, disease outcomes, and CTC results in subjects who experienced recurrent disease. Of the 10 CTC-detectable subjects who experienced recurrence, 8 (80%) had at least one positive CTC result during follow-up. In all cases, the positive CTC test preceded pathologic diagnosis of recurrence with median lead time of 22 months (range, 1–42 months). In contrast, 17 of 19 (89%) CTC-detectable subjects who had persistently negative CTC tests in follow-up remained free of recurrence in follow-up (p<0.01).
Table 2.
Clinical details, disease outcomes, and CTC results in subjects who experienced recurrent disease
| Subject # | Previous history of NSCLC | Tumor size (cm) | Histology | Pre-RT SUVmax | Post-RT SUVmax | Total dose (Gy) / fractions | Biological Equivalent Dose (Gy) | Local recurrence | Nodal recurrence | Distant recurrence | Vital status | Total follow-up | Pre-RT CTC counta | Max. CTC count within 3 mo. post-RTb | Time of first positive CTC test in follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | No | 1.6 | None | 4.5 | 2.1 | 5000/4 | 112.5 | 28.2 mo. | - | - | Alive | 37.4 mo. | 4.0 | 1.1 | 26.8 mo. |
| 97 | No | 2.1 | None | 2.5 | 5.4 | 5000/4 | 112.5 | 33.3 mo. | - | - | Alive | 33.8 mo. | 0.4 | 2.0 | 2.9 mo. |
| 26 | No | 1.8 | Adenocarcinoma | 3.3 | 3.6 | 5000/5 | 100 | 52.0 mo. | - | - | Alive | 54.4 mo. | 54.2 | 0.5 | 10.5 mo. |
| 136 | Yes | 3.7 | Squamous cell | 9.6 | 10 | 5000/5 | 100 | 6.3 mo. | 6.3 mo. | - | Deceased | 18.8 mo. | 1.5 | - | - |
| 6 | Yes | 1.7 | Adenocarcinoma | 5.1 | 0.8 | 5000/5 | 100 | - | 7.0 mo. | - | Deceased | 12.8 mo. | 0.4 | 2.8 | 1.3 mo. |
| 168 | No | 4.7 | Squamous cell | 19.1 | - | 6000/15 | 84 | 13.2 mo. | 13.2 mo. | - | Deceased | 14.9 mo. | 0.1 | 11.6 | 3 mo. |
| 33 | Yes | 1.2 | None | 0.8 | - | 5000/5 | 100 | 37.0 mo. | 37.0 mo. | - | Alive | 41.9 mo. | 70.1 | 51.6 | 2.9 mo. |
| 143 | No | 3.6 | Squamous cell | 19.1 | 0.8 | 5000/5 | 100 | 5.2 mo. | 8.9 mo. | 3.2 mo. | Alive | 24.3 mo. | 0.2 | 0.1 | - |
| 147 | No | 3 | Adenocarcinoma | 11.5 | 0.8 | 5000/4 | 112.5 | - | 21.7 mo. | 9.4 mo. | Alive | 24.8 mo. | 0 | 14.3 | 3.3 mo. |
| 94 | No | 3 | Adenocarcinoma | 7.8 | 0.8 | 5400/11 | 80.5 | 13.7 mo. | 13.7 mo. | 11.4 mo. | Deceased | 13.8 mo. | 6.8 | 10.8 | 0.6 mo. |
| 142 | No | 3.5 | Adenocarcinoma | 17.2 | - | 6000/15 | 84 | 15.9 mo. | 15.9 mo. | 14.5 mo. | Deceased | 21.5 mo. | 0 | 1.7 | 3.5 mo. |
| 71 | No | 2.3 | Adenocarcinoma | 5.4 | 5.2 | 5000/4 | 112.5 | 6.2 mo. | 6.2 mo. | 15.3 mo. | Deceased | 21.3 mo. | 0 | 0.1 | - |
| 20 | No | 3 | None | 9.6 | 2.8 | 5000/5 | 100 | 29.6 mo. | 29.6 mo. | 17.2 mo. | Deceased | 37.8 mo. | 22.9 | 1.5 | 1 mo. |
| 62 | No | 1.8 | None | 7.2 | 7.4 | 5000/4 | 112.5 | 20.7 mo. | 20.7 mo. | 20.7 mo. | Deceased | 35.9 mo. | 0 | 0.1 | - |
| 84 | No | 1.3 | Adenocarcinoma | 3.9 | 1.4 | 5000/5 | 100 | - | - | 22.6 mo. | Alive | 46.5 mo. | 8.8 | 20.0 | 0.9 mo. |
| 126 | No | 1.6 | None | 3.6 | 1.6 | 5000/4 | 112.5 | 7.8 mo. | - | 24.0 mo. | Alive | 25.1 mo. | 0.5 | 0.1 | - |
| 50 | Yes | 3.2 | None | 3.1 | 5.4 | 5000/4 | 112.5 | - | - | 28.1 mo. | Alive | 43.3 mo. | 2.9 | 0.2 | - |
| 11 | Yes | 2 | None | 12.7 | 5.4 | 5000/5 | 100 | 32.8 mo. | 19.9 mo. | 32.8 mo. | Deceased | 32.8 mo. | 5.2 | 5.9 | 3.1 mo. |
| 25 | No | 1.6 | None | 1.7 | 0.8 | 5000/5 | 100 | - | 44.2 mo. | 44.2 mo. | Alive | 50.3 mo. | 6.0 | 0.1 | 9.9 mo. |
bold, black=CTC unfavorable (CTC>=5)
bold, red=CTC positive (CTC>=1.3)
DISCUSSION
The adoption of SBRT for ES-NSCLC by the thoracic oncology community has improved local control and overall survival compared with conventionally fractionated radiotherapy. Five-year follow-up results from multi-center clinical trials, as well as from our own institution, provide reassurance regarding durable local control and limited rates of long-term complications with SBRT.8,26
There are two observations, however, that currently provoke concern. First, despite overall excellent local control, many patients will eventually show regional and distant progression of disease after SBRT. The substantial regional and distant progression rates contribute to 5-year disease-free and overall survival of only 26% and 40%, respectively, in the long-term update of the NRG Oncology RTOG 0236 trial of SBRT for medically inoperable Stage 1 NSCLC.8 The second concern arises out of surgical pathology examination of SBRT-treated tumors that show persistent tumor cells, as discussed in the Introduction.13 However, hematoxylin and eosin staining cannot distinguish between replicatively-viable versus quiescent cancer cells. It is likely that many of these radiation-treated persistent cells may have lost replicative ability, and that the number of tumor cells would continue to dwindle with additional time as dead and dying tumor cells are cleared. On the other hand, surviving persistent tumor cells theoretically may undergo additional genetic alterations that enable regional or distant metastasis.
These two concerns suggest that a subset of ES-NSCLC patients may benefit from additional treatment. This may include salvage surgery for those few patients with local only recurrences (and who are fit enough for surgery), reirradiation for isolated locally recurrent disease or regionally recurrent disease, or systemic chemotherapy/immunotherapy to forestall regional and distant disease.27,28 However, the same factors that render patients medically inoperable may also render systemic treatment riskier. Many patients treated with SBRT continue to be disease-free and will not needed any subsequent therapy, so uniform consideration for systemic therapy added to SBRT will lead to overtreatment in many. An assay that accurately denotes the subset of SBRT-treated patients who would most benefit from additional treatment would be greatly welcomed and could improve survival. Ideally, the assay could be safely performed in all patients (i.e. noninvasive), involve minimal or no discomfort, and can be performed sequentially in order to accommodate changes in the tumor’s biology or genetic makeup. CTC assays may fulfill such roles. Having such an assay available for stratification of treatment-intensification in the early-stage setting is timely in light of the recently initiated Phase III randomized control trial (PACIFIC-4) that aims to assess the efficacy of durvalumab following definitive SBRT for unresected clinical stage I/II lymph node-negative NSCLC.29 Furthermore, given the difficulty in determining SBRT-induced fibrotic lung changes from local recurrences in many patients, CTCs may also be an innovative strategy for identifying patients with or without local recurrences prior to or in place of biopsy, especially for patients who are at higher risks from biopsy.
The telomerase-based CTC assay has recently been shown to be effective in providing substantial lead-time notice of progression of disease in both early- and locally-advanced NSCLC over conventional radiographic imaging. The advanced lead-time notice averaged about six months for locally advanced disease and almost two years for the pilot group of presumed ES-NSCLC (for whom pathologic tissue was not available).14,30 In this study, the analysis has been extended to a larger group of patients including both presumed and tissue-confirmed ES-NSCLC. It was found that both a high number of CTCs prior to SBRT and the persistence of CTCs after SBRT were significantly associated with increased distant recurrence after the completion of SBRT, and that an increase in CTCs after achieving a post-treatment nadir was associated with a median lead time notice of 22 months over conventional imaging in detecting recurrences.
While we did not observe a difference in overall survival in both the CTC favorable/unfavorable and persistent/negative comparisons, we suspect our subjects have not yet been followed long enough to detect such a difference. Local, nodal, and distant failure are 18, 16, and 19 months, respectively - quite proximal to median follow-up of 24 months in this population. Those patients with recurrence are likely within a window where recurrent disease has not contributed to mortality and/or they are reaping benefit from salvage therapies. Follow-up after this population has matured further will clarify this hypothesis.
This study does have some notable limitations. First, while overall study compliance was quite high, some specimens were not able to be obtained in later follow-up time points. Second, a substantial proportion of patients did not have CTCs detected at baseline despite having a known cancer diagnosis. However, we are especially encouraged that those patients in whom CTCs were detectable prior to SBRT but which were rendered durably undetectable after treatment appeared to do particularly well, with two-year freedom from local and nodal recurrence of 100%. The CTC assay may, therefore, help identify that subset of patients who do well with SBRT alone, and who do not need adjuvant systemic chemo- or immunotherapy.
Furthermore, although this is the largest analysis of CTCs performed in non-metastatic NSCLC to date, addition investigation is indicated, and these provocative results merit confirmation in a larger trial, perhaps that incorporates adjuvant chemotherapy and/or immunotherapy for select patients with unfavorable CTC characteristics. In this study, high pre-SBRT CTC counts and persistence of CTCs were both associated with regional/distant recurrence. Patients with these high or persistent counts may benefit from adjuvant systemic therapy to achieve improved local and regional control. The convenience and short treatment time required for SBRT has the additional advantage of not unduly delaying subsequent chemotherapy or immunotherapy. Finally, while one may speculate that persistence of CTCs after SBRT may be associated with persistence of viable tumor cells within the primary tumor, occult micrometastases may also be sources of CTCs. This hypothesis would most optimally be tested in a trial that incorporates planned lobectomy after SBRT, although such a trial may face difficulty in accrual given the widespread acceptance and increasing availability of SBRT as a standard initial treatment among both medical inoperable and also operable ES-NSCLC patients.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the Department of Radiation Oncology’s Clinical Research Division and Clinical Research Coordinators for excellent assistance with the clinical trial described. The authors acknowledge Yasunari Kashihara and Yasuo Urata of Oncolys Biopharma, Inc for graciously supplying the adenoviral vector used in this research.
Funding:
This work was supported by the following grants from National Institutes of Health: grant R01 CA201071 from the National Cancer Institute and grant K08 NS076548-01 from the National Institute of Neurological Disorders and Stroke (JFD).
Footnotes
Conflict of Interest: University of Pennsylvania has submitted a patent application based on a component of the technology presented in this article. SMH, GDK, and JFD are co-founders of Liquid Biotech, USA. GDK had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Melissa A. Frick, Department of Radiation Oncology, Stanford University School of Medicine; Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA
Steven J. Feigenberg, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA
Samuel Jean-Baptiste, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA.
Louise Aguarin, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA.
Amberly Mendes, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA.
Chimbu Chinniah, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA.
Sam Swisher-McClure, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA.
Abigail T. Berman, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA
William P. Levin, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA
Keith A. Cengel, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA
Stephen M. Hahn, Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, TX
Jay F. Dorsey, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA
Charles B. Simone, II, Department of Radiation Oncology, New York Proton Center, New York, New York.
Gary D. Kao, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 3.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infante M, Lutman FR, Cavuto S, et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer. 2008;59(3):355–363. doi: 10.1016/j.lungcan.2007.08.040 [DOI] [PubMed] [Google Scholar]
- 5.Kapadia NS, Valle LF, George JA, et al. Patterns of Treatment and Outcomes for Definitive Therapy of Early Stage Non-Small Cell Lung Cancer. Ann Thorac Surg. 2017;104(6):1881–1888. doi: 10.1016/j.athoracsur.2017.06.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28(35):5153–5159. doi: 10.1200/JCO.2010.30.0731 [DOI] [PubMed] [Google Scholar]
- 7.Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med. 2015;3(13):172. doi: 10.3978/j.issn.2305-5839.2015.07.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018;4(9):1263–1266. doi: 10.1001/jamaoncol.2018.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. doi: 10.1016/S1470-2045(15)70168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93(5):989–996. doi: 10.1016/j.ijrobp.2015.07.2278 [DOI] [PubMed] [Google Scholar]
- 11.Choi JI, Simone CB 2nd. Stereotactic body radiation therapy versus surgery for early stage non-small cell lung cancer: clearing a path through an evolving treatment landscape. Journal of Thoracic Disease. 2019;1(1):S1360-S1365-S1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veterans Affairs Lung Cancer Surgery Or Stereotactic Radiotherapy (VALOR). NLM identifier: NCT02984761. Available from: https://clinicaltrials.gov/ct2/show/NCT02984761. Accessed June 19, 2019.
- 13.Palma DA, Nguyen TK, Louie AV, et al. Measuring the Integration of Stereotactic Ablative Radiotherapy Plus Surgery for Early-Stage Non-Small Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA Oncol. February 2019. doi: 10.1001/jamaoncol.2018.6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frick MA, Kao GD, Aguarin L, et al. Circulating Tumor Cell Assessment in Presumed Early Stage Non-Small Cell Lung Cancer Patients Treated with Stereotactic Body Radiation Therapy: A Prospective Pilot Study. Int J Radiat Oncol Biol Phys. 2018;102(3):536–542. doi: 10.1016/j.ijrobp.2018.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SJ, Masago A, Tamaki Y, et al. A novel approach using telomerase-specific replication-selective adenovirus for detection of circulating tumor cells in breast cancer patients. Breast Cancer Res Treat. 2011;128(3):765–773. doi: 10.1007/s10549-011-1603-2 [DOI] [PubMed] [Google Scholar]
- 16.Kojima T, Hashimoto Y, Watanabe Y, et al. A simple biological imaging system for detecting viable human circulating tumor cells. J Clin Invest. 2009;119(10):3172–3181. doi: 10.1172/JCI38609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maida Y, Kyo S, Sakaguchi J, et al. Diagnostic potential and limitation of imaging cancer cells in cytological samples using telomerase-specific replicative adenovirus. International Journal of Oncology. 2009;34(6):1549–1556. [DOI] [PubMed] [Google Scholar]
- 18.MacArthur KM, Kao GD, Chandrasekaran S, et al. Detection of Brain Tumor Cells in the Peripheral Blood by a Telomerase Promoter-Based Assay. Cancer Res. 2014;74(8):2152–2159. doi: 10.1158/0008-5472.CAN-13-0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju M, Kao GD, Steinmetz D, et al. Application of a telomerase-based circulating tumor cell (CTC) assay in bladder cancer patients receiving postoperative radiation therapy. Cancer Biology & Therapy. 2014;15(6):683–687. doi: 10.4161/cbt.28412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu MJ, Cooke M, Steinmetz D, et al. A novel approach for the detection and genetic analysis of live melanoma circulating tumor cells. PLoS ONE. 2015;10(3):e0123376. doi: 10.1371/journal.pone.0123376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorsey JF, Kao GD, MacArthur KM, et al. Tracking Viable Circulating Tumor Cells (CTCs) in the Peripheral Blood of Non-Small Cell Lung Cancer Patients Undergoing Definitive Radiation Therapy: Pilot Study Results. Cancer. 2015;121(1):139–149. doi: 10.1002/cncr.28975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman AT, Jabbour SK, Vachani A, et al. Empiric Radiotherapy for Lung Cancer Collaborative Group multiinstitutional evidence-based guidelines for the use of empiric stereotactic body radiation therapy for non-small cell lung cancer without pathologic confirmation. Translational Lung Cancer Research. 2019;8(1):5-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70(4):606–612. [PubMed] [Google Scholar]
- 24.Krebs MG, Sloane R, Priest L, et al. Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients With Non–Small-Cell Lung Cancer. JCO. 2011;29(12):1556–1563. doi: 10.1200/JCO.2010.28.7045 [DOI] [PubMed] [Google Scholar]
- 25.Verstegen NE, Lagerwaard FJ, Hashemi SMS, Dahele M, Slotman BJ, Senan S. Patterns of Disease Recurrence after SABR for Early Stage Non–Small-Cell Lung Cancer: Optimizing Follow-Up Schedules for Salvage Therapy. Journal of Thoracic Oncology. 2015;10(8):1195–1200. doi: 10.1097/JTO.0000000000000576 [DOI] [PubMed] [Google Scholar]
- 26.Schonewolf CA, Heskel M, Doucette A, et al. Five-year Long-term Outcomes of Stereotactic Body Radiation Therapy for Operable Versus Medically Inoperable Stage I Non-small-cell Lung Cancer: Analysis by Operability, Fractionation Regimen, Tumor Size, and Tumor Location. Clin Lung Cancer. 2019;20(1):e63–e71. doi: 10.1016/j.cllc.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 27.Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7(5):295–301. doi: 10.1016/j.prro.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 28.Chao HH, Berman AT, Simone CB 2nd, et al. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12(2):281–292. doi: 10.1016/j.jtho.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 29.AstraZeneca. Durvalumab vs Placebo Following Stereotactic Body Radiation Therapy in Early Stage Non-small Cell Lung Cancer Patients (PACIFIC-4). Available from: https://clinicaltrials.gov/ct2/show/NCT03833154. Accessed October 17, 2019.
- 30.Chinniah C, Aguarin L, Cheng P, et al. Early Detection of Recurrence in Patients with Locally Advanced Non-small Cell Lung Cancer via Circulating Tumor Cell Analysis. Clinical Lung Cancer. 2019;0(0). doi: 10.1016/j.cllc.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


