Abstract
New work on DNA polymerase λ highlights its remarkable flexibility. This fits with the generally adaptable nature of the DNA-repair process in which this enzyme is involved — nonhomologous end-joining — which allows this mechanism to handle diverse types of broken DNA ends in order to restore the duplex structure, albeit with a loss of information at the join.
The major pathway for joining broken DNA ends in nondividing eukaryotic cells is called nonhomologous end-joining, and abbreviated NHEJ1. In dividing cells, the other major repair pathway is homology-directed repair (HDR), which requires long stretches of usually perfect homology between a template DNA strand and the broken strand (longer than 25 base pairs for single-strand annealing, or more than 100 bp for homologous recombination) (Fig. 1). When the term ‘NHEJ’ was originally coined by James Haber2, it was meant to distinguish such long stretches of homology from the more insignificant few base pairs of random homology that may be encountered between any two broken DNA ends, which was later called terminal-end microhomology. In NHEJ, the extent of microhomology between two random DNA ends is usually 0 to 4 bp — simply because increasingly longer lengths of homology are increasingly less probable. However, double-strand breaks (DSBs) in repetitive genomic sequences can be longer and still joined by NHEJ, as in immunoglobulin class switch recombination3. Moreover, NHEJ can occur in experimental systems in which the broken DNA ends have longer microhomology, and even occurs in handicapped experimental situations in which some NHEJ components must substitute for others4–6.
Fig. 1 |. Choice of pathway for double-strand-break repair.

Double-strand breaks (DSBs) in DNA (top) can be repaired by nonhomologous end-joining (NHEJ), alternative end-joining (aEJ), single-strand annealing (SSA) or homologous recombination (HR) (bottom). The name NHEJ was adopted by the DNA-repair field to distinguish this pathway from repair that requires extensive DNA homology (HR and SSA). In NHEJ, lengths of terminal microhomology of between 1 and 4 bp are very common. The NHEJ pathway does not require extensive end resection (although the Artemis–DNA-PKcs complex can carry out some nucleolytic resection of typically less than 20 nucleotides), and the ends are protected from deeper resection by binding of the Ku heterodimer (Ku70–Ku80) ends and of other proteins, including histones. By contrast, in the other DSB-repair pathways, extensive 5’-to-3’ resection of regions of the duplex is carried out by carboxy-terminal binding protein interacting protein (CtIP) and the MRN (MRE11–RAD50–NBS1) complex, generating stretches of single-stranded DNA at DNA ends for aEJ, SSA and HR. (SSA typically requires more than 50 bp of microhomology, whereas the requirement for aEJ is generally less than 20 bp.) Poly(ADP-ribose) polymerase 1 (PARP1) and pol θ are important for most aEJ events (as mentioned by Chandramouly et al.8). For SSA and HR, Bloom syndrome RecQ-like helicase (BLM) and exonuclease 1 (EXO1) account for additional resection, and replication protein A (RPA) binds to single-stranded DNA to promote the SSA and HR pathways. In SSA, RAD52-mediated annealing of homologous sequences is key, and XPF/ERCC1 cuts the remaining 3’-nonhomologus single-stranded DNA prior to ligation by DNA ligase 1. In HR, by contrast, RAD51-mediated strand exchange through an association with BRCA1, BRCA2 and RAD54 is essential.
NHEJ is the major pathway for DSB repair because it can handle the joining of DNA ends that are quite incompatible in their sequences or in their overhang configurations (Fig. 2). For example, one end with a 3’-overhang and another with a 5’-overhang can be processed by the NHEJ nuclease (a complex comprising the Artemis and DNA-PKcs proteins), or possibly by other nucleases, to render them more compatible. The same is true of two DNA ends that lack any terminal microhomology. But in random DNA sequences, almost all ends have some degree of possible hydrogen bonding, even if it is recessed one or two nucleotides from the end. Any such hydrogen bonding between broken DNA ends may permit some stabilization and therefore sufficient transient alignment. Some of this hydrogen bonding may even be noncanonical, as it is merely subserving transient end-to-end approximation 7. Moreover, DNA polymerases can fill in at 5’-overhangs or add bases randomly at 3’-overhangs (in a terminal transferase manner) to generate new microhomology1.
Fig. 2 |. General features of NHEJ.
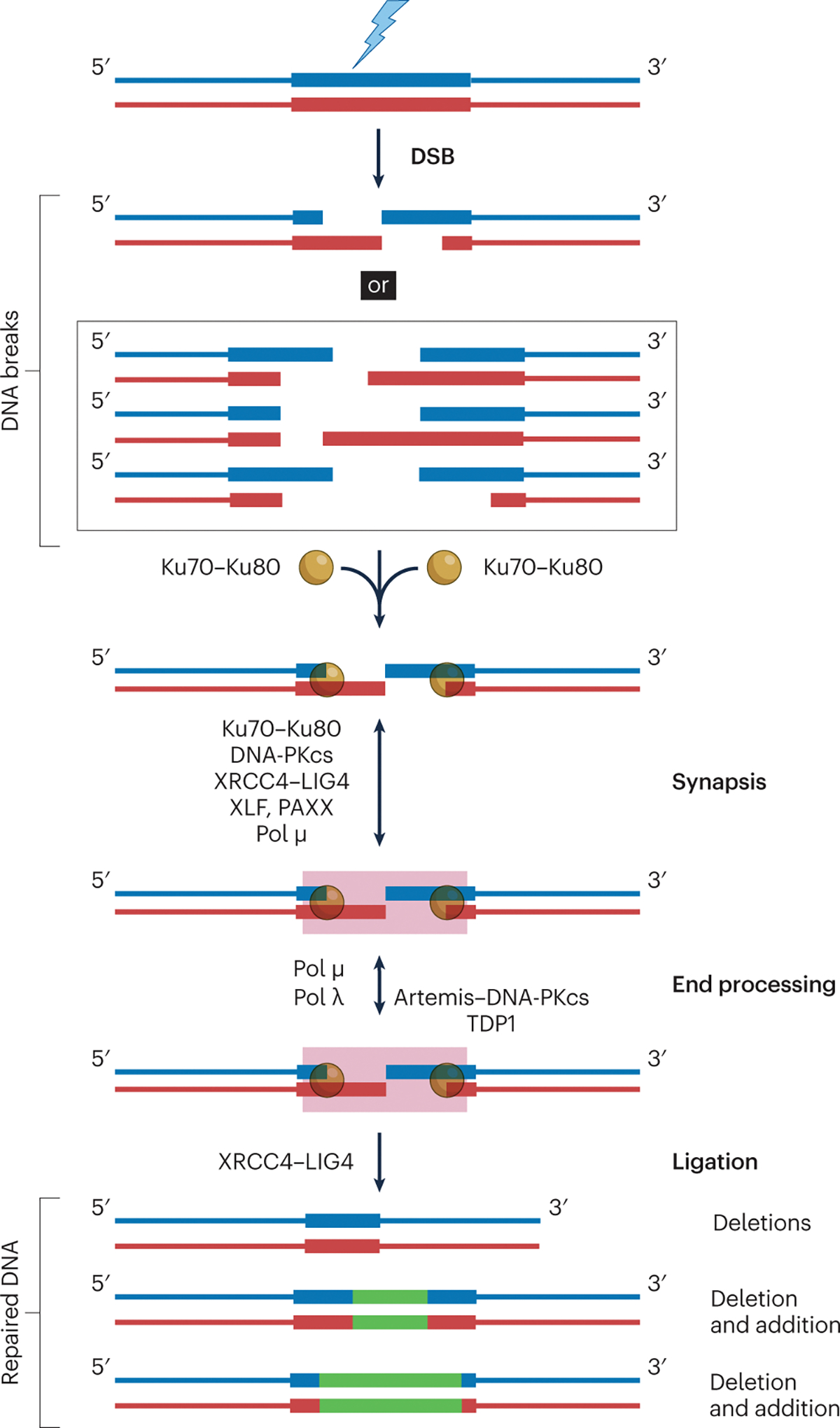
If the choice of repair pathway leads to NHEJ, then Ku70–Ku80 binds to the broken ends, followed by recruitment of other NHEJ proteins. The biochemical steps include synapsis (which ensures that the DNA ends remain in proximity), end processing and final ligation. Two mechanisms exist for NHEJ synapsis. One depends on Ku70–Ku80, XRCC4–LIG4, XLF and/or PAXX; DNA-PKcs is dispensable in this case. The other depends on pol μ (ref. 7) or pol λ (ref. 9), and requires at least one base pair of microhomology. Next, the DNA ends that are incompatible for direct ligation by DNA ligase IV (LIG4) are processed by either the Artemis nuclease or the polymerases (pol μ, λ). Here, tyrosyl DNA phosphodiesterase 1 (TDP1) and Artemis can remove 3’phosphoglycolates (which block ligation and can be generated at DSBs caused by ionizing radiation). Details of the subsequent NHEJ end additions by pol X polymerases (pink box) are in Fig. 3 (resection during NHEJ is not shown, but occurs at most DNA ends and is predominantly due to Artemis–DNA-PKcs). The initial causes of natural DSBs (such as ionizing radiation) almost always result in alterations of junctional information, even before end modification by NHEJ polymerases and nucleases. This usually leads to diverse repair junctions. NHEJ evolved to restore structural integrity, not to restore the original information content by copying from another duplex (as in HR). Precise joining products are sometimes observed, but less often, especially when the ends are compatible for direct ligation 201, 202. Green lines within repaired DNA represent newly added nucleotides. The box on the right shows that NHEJ is flexible and iterative. The flexibility means that the two DNA ends can be covalently ligated via several NHEJ pathways. XRCC4–LIG4 can ligate each strand regardless of the other (reviewed in ref. 13). Artemis–DNA-PKcs can trim the overhangs to expose complementary regions. Pol μ and pol λ can add nucleotides either to create microhomology or to fill in the gap to facilitate strand ligation. The iterative nature allows multiple rounds of revision.
For nearly all NHEJ joining events, XRCC4–DNA ligase IV (X4L4) carries out the ligation. Exceptions to this are observed in cells lacking XRCC4 or ligase IV and concomitantly when there is time for either of the other two ligases (ligases I and III, L1 andL3) to ligate either the top or the bottom strand of the DNA duplex3. This may occur in repetitive sequences when the top-strand and bottom-strand nicks are far enough apart to permit L1 or L3 to ligate either of the two strands. Once the top or bottom strand is ligated, then ligation of the antiparallel strand can be achieved by any ligase. Within cells, joining by L1 or Ll3 occurs more slowly than the normal repair of such breaks by X4L4 (ref. 3). For some processes — or for slowly or nondividing cells — this may not cause a viability problem.
When a low level of less-efficient joining of DSBs was found to occur in cells lacking X4 or L4, it was called alternative end-joining (aEJ, sometimes also known as alt-EJ). Richard Pomerantz has done pioneering work on DNA polymerase θ, and it became clear that much or most alternative end-joining was due to pol θ extending DNA lengths from one broken end to the other, with subsequent ligation by L1 or L3. Such ligation can occur when the two nicks are not close together, as these nicks are essentially treated as isolated cuts. For most aEJ, longer lengths of microhomology (usually 3 to 8 bp) are used. The term theta-mediated end-joining (TMEJ) is yet another designation for aEJ in cases where pol θ is involved. The term microhomology-mediated end-joining (MMEJ) does not make it clear which pathway is used, as microhomology is involved in both NHEJ and aEJ. Moreover, end stabilization by microhomology may be seen in experimental systems in which enzymes from the two different pathways participate; however, such events are likely to be extremely rare in cells, and require genetic knockouts that are not viable in animals.
Now, in this issue of Nature Structural and Molecular Biology, Pomerantz and colleagues describe previously unknown enzymatic activities of DNA polymerase λ at long 3’-overhangs8. In a biochemical system, the authors (Chandramouly et al.) are able to see alignment of DNA ends by microhomology, followed by joining. Their 5–12-nucleotide 3’-overhangs include ones with substantial microhomology (4–6 bp) that is interspersed within the single-stranded 3’-overhang. The authors show that this process occurs not only in their biochemical system using purified protein, but also in cells that lack ligase IV. Of note, in cells, this occurs with 6–8 bp of microhomology and within long 3’-overhangs.
The paper by Chandramouly et al.8 is important for demonstrating the remarkably flexible nature of pol λ. More broadly, it illustrates that, given sufficient microhomology, several different polymerases may be able to polymerize across a DSB junction. In this particular case, the nicks in the top and bottom strands are far enough apart that any of the three eukaryotic DNA ligases could ligate one or both strands to complete the process4–6.
These findings come at an interesting time (Fig. 3). Kaminski et al.9 have shown recently, using biochemical and NHEJ cellular systems, that pol λ can align and join two DNA ends with only 1 or 2 bp of microhomology within 2–4-nucleotide 3’-overhangs. Moreover, Zhao et al.10 have found using a biochemical system that pol μ (which, together with pols βand λ, is a member of the pol X family) can bring two broken strands into perfect end-to-end alignment suitable for ligation. For the synapsis by pol λ9 and pol μ10, only the polymerase and 1 bp of microhomology are needed; in both cases, X4L4 could ligate the DSB. Therefore, any of a number of flexible polymerases can carry out DNA-end synapsis and microhomology-mediated end-alignment in order to achieve ligation, usually (but not exclusively) by X4L4. This last point was presaged by the earlier findings that, if L4 is missing or catalytically inactive, then L1 (in yeast) or L3 (in mammalian cells) can substitute4–6.
Fig. 3 |. Additions by pol X family polymerases at DNA ends.

The figure shows effects of polymerases on different end configurations. a, Pol μ and TdT (not shown; expressed only in early lymphoid cells) can add nucleotides to a blunt end in a template-independent manner. b, Pol μ and pol λ can fill-in gaps at the 3’-recessed DNA end. c, Pol μ and pol λ can add nucleotides to blunt ends in a template-dependent manner (such that the preferentially added nucleotides are complementary to the terminal bases at the other DNA end). d, Pol μ and pol λ can fill in gaps at junctions. e, Pol μ, TdT and pol λ can carry out templated in trans synthesis for overhangs with short regions of base pairing at the termini; that is, the polymerases can use a second 3’-overhang as a template for nucleotide addition. Pol μ and TdT have greater activity than pol λ here. f, Pol μ and TdT can add nucleotides to the 3’-noncomplementary overhangs in a template-dependent manner. g, The 3’primer end (blue) can slip 5’, allowing pol μ or λ to generate of direct repeats. Pol λ may have greater activity here. h, When pol μ or TdT adds nucleotides in a template-independent manner, this newly generated overhang might fold back and allow continued synthesis by pol μ or λ. This template-independent addition and fold-back synthesis might generate inverted repeats at NHEJ junctions, as illustrated. The events in e, f are most relevant to the new studies8,9.
The work by Chandramouly et al. 8 highlights the significance of flexibility amongst DNA polymerases for the repair of DSBs. I would not designate a new version of aEJ on the basis of these studies. Pol μ and pol λ are important components of the NHEJ pathway, as Kaminski et al.9 point out. However, if one artificially creates an experimental situation in which there is long microhomology on 3’-overhangs in cells lacking X4L4, then one can observe the enzyme flexibility that permits pol λ to cross the junction, followed by L1- or L3-mediated ligation.
More broadly, however, this plasticity amongst Pol X enzymes is in line with the broader theme of the iterative and flexible nature of the NHEJ pathway11. This feature of NHEJ has existed since its earliest form in prokaryotes12. In eukaryotes, the number of enzymes and their flexibility have increased to handle all types of broken-end configurations, including damaged 5’- and 3’-overhangs, blunt ends, and even the hairpin ends that are central during V(D)J recombination in vertebrates. Structural biology is enhancing our understanding of NHEJ, and it is tempting to emphasize the more-rigid, static aspect of NHEJ proteins. But it is important to note that each experimental method has its limitations, and that structural snapshots of a dynamic process may omit some intermediate structures. Moreover, multiple rounds of nuclease, polymerase and ligase action at a single DSB are more difficult to convey in anything other than the complicated DNA sequences seen at the final repaired DNA or chromosome junctions. As in both NHEJ and the much less common aEJ, the DNA duplex and overall chromosome structure are restored, but some sequence information is almost always lost and altered at the junction. I coined the term ‘information scar’ in 2008 for most NHEJ products13. Some authors have abbreviated this as a ‘scar’, which I would disagree with, as it implies that there is an alteration in the duplex structure, which there is not. Only the sequence information is altered.
More than anything, the study by Chandramouly et al.8 illustrates that aEJ is not one pathway. As Pomerantz and others have shown, pol θ and PARP1 are key components of the major form of aEJ (reviewed in ref. 14). But in experimental systems, when L4 and pol θ are missing, other enzymes — often from NHEJ — can be used to repair a DSB. These artificial systems do not reflect common situations in nature, but they do highlight the remarkable flexibility of the enzymes of the NHEJ pathway.
Acknowledgements
The author is supported by the National Interests of Health (NIH), grants GM118009 and CA100504.
Footnotes
Competing interests
The author declares no competing interests.
References
- 1.Zhao B, Rothenberg E, Ramsden DA & Lieber MR Nature Rev. Mol. Cell Biol. 21, 765–781 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore JK & Haber JE Mol. Cell. Biol. 16, 2164–2173 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han L & Yu KJ Exp. Med. 205, 2745–2753 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiruvella KK, Liang Z, Birkeland SR, Basrur V & Wilson TE PLoS Genet. 9, e1003599 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottarel J et al. J. Cell Biol. 200, 173–186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff NJ et al. Nucleic Acids Res. 50, 11058–11071 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao B et al. Nat. Commun. 10, 3588 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandramouly G et al. Nat. Struct. Mol. Biol. 10.1038/s41594-022-00895-4 (2022). [DOI] [Google Scholar]
- 9.Kaminski AM et al. Nat. Commun. 13, 3806 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B, Watanabe G & Lieber MR Nucleic Acids Res. 48, 3605–3618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieber MR, Ma Y, Pannicke U & Schwarz K DNA Repair 3, 817–826 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Gu J & Lieber MR Genes Dev. 22, 411–415 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieber MR J. Biol. Chem. 283, 1–5 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Ramsden DA, Carvajal-Garcia J & Gupta GP Nat. Rev. Mol. Cell Biol. 23, 125–140 (2022). [DOI] [PubMed] [Google Scholar]


