Reason for posting: Health Canada warns that some over-the-counter supplements used to increase energy and promote weight loss and bodybuilding pose a serious risk to health.1 The warning applies to supplements containing the herb ephedra or its alkaloid derivative, ephedrine; in Canada, ephedrine is authorized for use only as a nasal decongestant.
More than 60 adverse events — suicide, psychotic episodes, seizure, stroke and cardiovascular events ranging from hypertension to myocardial infarction — have been reported in Canada.2 Other recognized adverse effects include anxiety, tremors, headaches, insomnia and flushing.3 An independent analysis of 140 adverse events reported to the US Food and Drug Administration (FDA) between June 1997 and March 1999 found that 47% involved the cardiovascular system and 18% the central nervous system (CNS).4 Health Canada has recalled unapproved herbal preparations containing more than the recommended dose of ephedrine.1,2,3,5 In addition, the manufacturers of approved commercial higher-dose preparations are being asked to submit data demonstrating their safety and efficacy.2
The drugs: Ephedra sinica, also known as ma huang, is the source of the alkaloid ephedrine. Other related ephedra alkaloids not encompassed by the current advisory include pseudoephedrine, norpseudoephedrine, methylephedrine and norephedrine (also known as phenylpropanolamine, a nasal decongestant taken off the market in 2001 because of its association with hemorrhagic stroke6). In traditional Asian medicines, ephedra-based products are sometimes used as bronchodilators.
Ephedrine is a sympathomimetic drug prescribed as a nasal decongestant, with properties similar to epinephrine; its effects on both a- and b-adrenergic receptors and its central effects resemble those of amphetamines (see figure).7 The adverse effects of ephedrine are hypothesized to be related to coronary artery constriction, vasospasm, shortening of cardiac refractory periods allowing re-entrant cardiac arrhythmias, hypertension -induced subarachnoid hemorrhage, cerebral artery vasoconstriction and sympathomimetic -induced platelet activation.4
Products containing ephedrine are not to be used by people with heart disease, hypertension, diabetes, thyroid disease, enlarged prostate, anxiety and restlessness or glaucoma, people taking monoamine oxidase inhibitors or women who are pregnant or lactating.
Ephedrine often exists in unapproved herbal preparations that also contain caffeine (sometimes called “herbal ecstasy”) and ASA. These combination products may augment ephedrine's cardiac and CNS effects8 and should not be used.3
Hundreds of unapproved products containing ephedra or ephedrine exist, including ma huang, E. sinica, Sida cordifolia and epitonin.3 Approved preparations, mostly nasal decongestants, range in dose from 0.13 to 25 mg per tablet. Health Canada recommends that the dose be restricted to 8 mg of ephedrine per dose and 32 mg per day, for a period no longer than 7 days.1
What to do: Physicians should ask about the use of over-the-counter herbal and dietary supplements, particularly if patients want to lose weight, improve their exercise tolerance, seek extended periods of wakefulness (such as long-distance truck drivers) or use traditional remedies. Products containing ephedra or ephedrine are currently marketed only as nasal decongestants, and health claims pertaining to appetite suppression and other supposed benefits are not allowed. Enquiries about concurrent sources of caffeine (e.g., green tea, cola nut, yerba mate and yohimbe3) may also be warranted. Patients using products containing ephedra or ephedrine, especially in doses above the recommended limits, should be warned of the potential serious adverse events. Health Canada also recommends that patients use only approved products — those with a drug identification number (DIN).
Eric Wooltorton Editorial Fellow, CMAJ Barbara Sibbald Associate Editor (News), CMAJ
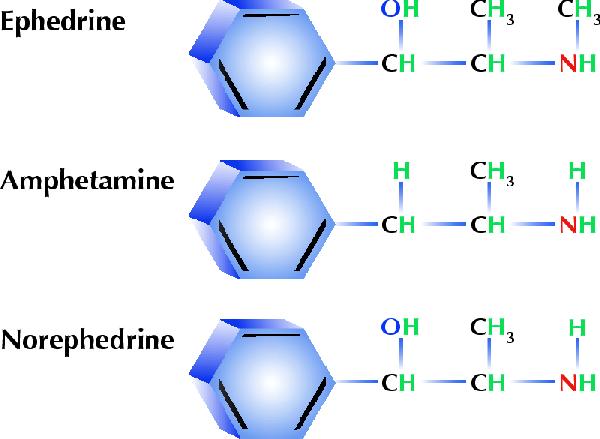
Figure. The molecular structure of ephedrine is similar to that of amphetamine and the related ephedra-alkaloid and metabolite norephedrine (phenylpropanolamine). Photo by: Julie-Line Danis
References
- 1.Health Canada requests recall of certain products containing Ephedra/ephedrine. Ottawa: Health Canada; 2002 Jan 9. Available: www.hc-sc.gc.ca/english/protection/warnings/2002/2002_01e.htm (accessed 2002 Feb 5).
- 2.Ephedra/ephedrine — frequently asked questions. Ottawa: Health Canada; Jan 2002. Available: www .hc-sc.gc.ca/english/protection/warnings /2002 /2002 _01 ebk2.htm (accessed 2002 Feb 5).
- 3.Advisory not to use products containing Ephedra or ephedrine. Ottawa: Health Canada; 2001 Jun 14. Available: www.hc-sc.gc.ca/english/protection/warnings/2001/2001_67e.htm (accessed 2002 Feb 5).
- 4.Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med 2000;343:833-8. [DOI] [PubMed]
- 5.Sibbald B. Voluntary recall of ephedra products not enough, MD says. CMAJ 2002;166(2):225. Available: www.cma.ca/cmaj/vol-166/issue-2/0225.asp [PMC free article] [PubMed]
- 6.Health Canada withdraws drug products containing phenylpropanolamine (PPA) from the market. Ottawa: Health Canada; 2001 May 30. Available: www .hc -sc.gc.ca/english/protection/warnings /2001 /2001 _61 e .htm (accessed 2002 Feb 5).
- 7.Ephedrine HCl. In: Compendium of pharmaceuticals and specialties. 36th ed. Ottawa: Canadian Pharmacists Association, 2001.
- 8.Smith M. Ma huang. In: Herbs: everyday reference for health professionals. Ottawa: Canadian Pharmacists Association and CMA; 2000. p. 153-5.


