Abstract
Background
The COVID-19 pandemic has greatly impacted people's lifestyles and changed the delivery of health interventions, especially interventions for community-dwelling older people with sarcopenia.
Objective
To summarize the components and explore the effectiveness of home-based interventions for improving sarcopenia and other health-related outcomes among community-dwelling older people with sarcopenia.
Design
Systematic review and meta-analysis.
Methods
The Cochrane Library, Scopus, EMBASE, Web of Science, CINAHL, Medline (via PubMed), and PsycINFO were searched for relevant papers published from January 1, 2010 to March 29, 2022. Only papers written in English were included. The modified version of Cochrane's risk-of-bias tool was used to assess the risks of bias in the included studies. The template for intervention description and replication checklist was used to summarize the intervention components. The mean difference (MD) or standard mean difference with a 95 % confidence interval (CI) was used to determine the effect size of studies using the same or different measuring methods. Random-effects models were in meta-analyses to pool the effects of home-based interventions on the included outcomes.
Results
After detailed screening and exclusion, 11 randomized controlled trials including 1136 older people with sarcopenia were included in our analyses. Three categories of home-based interventions were identified: exercise interventions, nutritional interventions, and combined exercise and nutritional interventions. The overall analysis of the outcomes (e.g., appendicular skeletal muscle mass index, lean mass, body fat mass, handgrip strength, and gait speed), showed that the effects of home-based exercise interventions were inconclusive. Compared with passive controls, home-based exercise interventions significantly improved knee extension strength (MD = 0.56 kg, 95 % CI: 0.09, 1.03, p = 0.020) and reduced the time required to complete the Timed Up and Go Test (MD = −1.41 s, 95 % CI: −2.28, −0.54, p = 0.001). Home-based nutritional interventions were effective in improving appendicular skeletal muscle mass (MD = 0.25 kg, 95 % CI: 0.02, 0.49, p = 0.030), gait speed (MD = 0.06 m/s, 95 % CI: 0.03, 0.09, p = 0.0001), and quality of life in terms of both the physical component summary (MD = 13.54, 95 % CI: 0.73, 26.34, p = 0.040) and mental component summary scores (MD = 8.69, 95 % CI: 2.98, 14.41, p = 0.003).
Conclusion
Home-based exercise interventions have the potential to improve muscle strength and physical function, while home-based nutritional interventions are effective in increasing muscle mass, physical function, and quality of life. Both of these can be applied at home during and after the COVID-19 pandemic to alleviate sarcopenia and improve health-related outcomes in community-dwelling older people.
Keywords: Sarcopenia, Home-based, Exercise, Nutrition, COVID-19
1. Introduction
Sarcopenia is a progressive and generalized skeletal muscle disorder characterized by an accelerated decline in muscle mass, muscle strength, and physical function (Cruz-Jentoft and Sayer, 2019). It is estimated that, currently, >120 million older adults are living with sarcopenia globally, and due to rapid aging, this number is expected to double by 2050 (Mayhew et al., 2019; World Health Organization, 2021). The World Health Organization has classified sarcopenia as an independent disease with the code ICD-10-CM (M62.84) (Cao and Morley, 2016). Compared with healthy aging, sarcopenia significantly increases the risks of various health-related adverse events, such as falling by 3.23 times, functional decline by 3.03 times, fracture by 3.75 times (Beaudart et al., 2017), hospitalization by 2.07 times (Yang et al., 2019), and all-cause mortality by 2.20 times (Nakamura et al., 2021). Moreover, a recent study found that it also greatly undermines the psychological health of older people (Chang et al., 2017). Sarcopenia is positively associated with increased healthcare costs and utilization of medical resources (Bruyère et al., 2019), leading to shortages of health professionals and resources, especially during the COVID-19 pandemic.
Currently, there are no approved medications to prevent or treat sarcopenia (Kwak and Kwon, 2019). Lifestyle interventions, particularly exercise and nutritional interventions, have been shown to be effective in alleviating the symptoms of sarcopenia (Cruz-Jentoft and Sayer, 2019; Wu et al., 2021). However, the COVID-19 pandemic has profoundly changed people's lifestyle, causing considerable reductions in physical activity, increases in sedentary behavior (Ammar et al., 2020; Schuch et al., 2022), and adoption of unhealthy eating behaviors (Ammar et al., 2020). These changes greatly accelerate the loss of muscle mass and decline in physical function in older people, which are key characteristics of sarcopenia (Cruz-Jentoft and Sayer, 2019). In addition, the COVID-19 pandemic has disrupted the way research is conducted, given the reduced chance to interact with participants in person and the increased reliance on digital technologies (Hale-Gallardo et al., 2022). This has increased the risk of leaving behind those who lack digital literacy or are too unwell to use such tools (Hale-Gallardo et al., 2022), such as older people with sarcopenia. Moreover, studies have shown that people who have recovered from COVID-19 may have long-term symptoms that cannot be explained, known as “long COVID” (Peter et al., 2022). The two main symptoms of “long COVID”, extreme fatigue and breathlessness (Institute for Health Metrics and Evaluation, 2022), reduce the individual's ability to exercise and increase their time spent being physically inactive, which further greatly accelerate the development of sarcopenia (Peter et al., 2022). Thus, it is imperative to find practical ways to prevent and treat sarcopenia in the real world during and after the COVID-19 pandemic.
Given that they already have accelerated muscle loss, older people with sarcopenia are more vulnerable to unexpected perturbations than healthy individuals and thus have greatly elevated risks of being infected and having further muscle atrophy during the COVID-19 pandemic and other infectious disease pandemics (Kirwan et al., 2020). Pragmatic and acceptable strategies are urgently needed to alleviate the symptoms of sarcopenia and improve the intrinsic ability to remain healthy in this population during and after the COVID-19 pandemic. Studies have suggested that older people with sarcopenia prefer staying at home to going out or participating in center-based activities because of reduced mobility and physical function (Lin et al., 2022). A study also indicated that older people prefer doing exercise in a familiar environment, such as home, rather than in a formal group setting (Dorresteijn et al., 2012). Thus, home-based interventions are preferable to and more practical than center-based interventions for this population, especially during pandemics when in-person interactions with participants are risky and restricted.
However, the effectiveness of current home-based interventions in alleviating sarcopenia is still unclear (Sen et al., 2021). For example, although some studies have found that home-based exercise interventions improve the physical performance of older people with sarcopenia (Sen et al., 2021), this contradicted the findings of Chang and his colleagues (Chang et al., 2021). Furthermore, although many systematic reviews (SRs) of sarcopenia have shown that interventions (i.e., exercise and nutritional supplements) can alleviate the symptoms (i.e., improve physical function and muscle strength) (Bao et al., 2020; Wu et al., 2021; Yoshimura et al., 2017), all of these SRs included a mix of older people with sarcopenia living in various settings (namely nursing homes, hospitals, and communities). Older people living in different settings have considerable differences in terms of health backgrounds, levels of functional ability, and factors influencing the effects of interventions (Essery et al., 2017; Farrance et al., 2016). For example, older people in nursing homes are likely to be malnourished and depressed, while community-dwelling older people, mainly those with frailty and sarcopenia, are likely to be physically inactive (Papadopoulou et al., 2020). Thus, it is necessary to develop an effective intervention to alleviate sarcopenia among community-dwelling older people and facilitate their “aging in place.”
Home-based interventions, defined as structured programs conducted in informal, flexible settings, typically in a participant's home or a nearby place (Ashworth et al., 2005), are more convenient and practical than center-based interventions and are preferred by older people with sarcopenia (Thiebaud et al., 2014). Home-based interventions with no or minimal supervision or equipment are cost-effective and can relieve the shortage of medical resources (Chaabene et al., 2021), reduce the risk of transmission of the virus that causes COVID-19 by reducing the need to go out, and provide health professionals with an opportunity to understand participants' needs specific to their unique home environment context (Kreider et al., 2022). These home-based interventions are applicable not only during the COVID-19 pandemic but also during other infectious disease pandemics or pandemic-free periods.
Despite these advantages of home-based interventions, they have some barriers, and low adherence is a major concern (Thiebaud et al., 2014). An SR demonstrated that the rate of adherence to home-based exercise among older people was 58 % (Mañas et al., 2021). Among the few studies that have specifically focused on home-based interventions for community-dwelling older people with sarcopenia, the interventions can be classified as home-based exercise interventions (Sen et al., 2021; Tsekoura et al., 2018), home-based nutritional interventions (Björkman et al., 2020; Lin et al., 2021), and mind–body physical activity interventions [i.e., yoga (Pandya, 2019) and Tai Chi (Zhu et al., 2019)]. However, due to the different control groups used in these studies (usual care, active care, and center-based interventions) and limited studies on specific types of intervention, the effectiveness and optimal types of home-based interventions to treat sarcopenia are still unclear. Moreover, to our knowledge, no SR and meta-analysis has investigated home-based non-pharmacological interventions in community-dwelling older people with sarcopenia, except one SR on frailty (Clegg et al., 2012) that has several limitations, such as a small number of studies included (only one or two studies on some outcomes), lack of validated criteria to define frailty, and the need for updating (the literature search was up to February 2010).
In addition to physical health, sarcopenia also affects psychological health, which has been greatly undermined by the COVID-19 pandemic (Sepúlveda-Loyola et al., 2020). A review of 15 articles (Chang et al., 2017) demonstrated that sarcopenia was positively associated with depression after adjusting for potential confounders, including age, sex, cognitive performance, and physical activity. It is highly recommended that interventions include components addressing psychological health, especially during and after the COVID-19 pandemic, as mental health issues (e.g., anxiety and depression) are also key symptoms of “long COVID” (Institute for Health Metrics and Evaluation (IHME), 2022). Nevertheless, few home-based intervention studies have evaluated the psychological health of community-dwelling older people with sarcopenia (Pinheiro et al., 2020), and no SR has evaluated the effectiveness of non-pharmacological home-based interventions in improving diverse physical and psychological health indicators among older people with sarcopenia.
Therefore, the aim of this SR and meta-analysis was to evaluate the effects of non-pharmacological home-based interventions on sarcopenia and other health-related indicators (e.g., psychological well-being) to provide practical and acceptable strategies to treat sarcopenia in community-dwelling older people during and after the COVID-19 pandemic. The objectives of this SR and meta-analysis were: 1) to summarize the common components of non-pharmacological home-based interventions targeting community-dwelling older people with sarcopenia; 2) to explore the effectiveness of non-pharmacological home-based interventions on both physical and psychological outcomes in this population; and 3) to evaluate the adherence to non-pharmacological home-based interventions in this population.
2. Methods
An SR and meta-analysis was conducted by following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses, 2009).
2.1. Eligibility criteria
2.1.1. Inclusion criteria
The inclusion criteria were defined according to the population, intervention, comparison, outcome, and study design (PICOS) framework:
2.1.1.1. Population
This review included only studies that included community-dwelling older people aged 60 years or older who were diagnosed with sarcopenia based on established criteria, such as the European Working Group on Sarcopenia in Older People (EWGSOP) criteria (Cruz-Jentoft et al., 2019) and the Asian Working Group for Sarcopenia (AWGS) criteria (Chen et al., 2020).
2.1.1.2. Intervention
Home-based intervention is defined as a structured program conducted in informal, flexible settings, typically a participant's home or place with clear objectives (Anderson et al., 2017; Ashworth et al., 2005). Therefore, only interventions that met all of the following criteria were included in this SR: (1) they were non-pharmacological; (2) they were structured programs with clear objectives for sarcopenia; and (3) the whole process of the intervention was conducted at home or in another informal place (e.g., in the common areas associated with the participants' residential buildings).
2.1.1.3. Comparison
Studies in which the control groups received passive controls (i.e., usual care, waitlist, and placebo) or active controls (i.e., nutritional supplement and group-based exercise) were included.
2.1.1.4. Outcomes
Studies that assessed the symptoms of sarcopenia as the primary outcomes, including muscle mass (e.g., skeletal muscle mass and the skeletal muscle mass index), muscle strength (e.g., handgrip strength and knee flexion strength), and physical function (e.g., gait speed), and other health-related outcomes as secondary outcomes, especially psychological health (i.e., depression and anxiety) and others (such as quality of life [QoL]), were included.
2.1.1.5. Study design
Only relevant randomized controlled trials (RCTs) and quasi-experimental studies (e.g., pre-test, post-test, and non-equivalent comparison group studies) were considered for inclusion in this review. Only papers written in English were included to avoid translation and understanding bias.
2.1.2. Exclusion criteria
Studies were excluded if sarcopenia was not age-related but was caused by cancer, cachexia, surgery, or other pathological conditions, or the participants were hospital patients or older people who resided in nursing homes rather than in communities. Studies of interventions conducted in centers such as gyms or health care facilities (i.e., hospitals, community health care centers, and nursing homes), or those combined with center-based interventions were excluded. Studies published only as abstracts or not published were excluded due to insufficient data.
2.2. Search strategy
The Cochrane Library, Scopus, EMBASE, Web of Science, CINAHL, Medline (via PubMed), and PsycINFO were searched for relevant studies published from January 1, 2010 to March 29, 2022, as the widely accepted definition of sarcopenia was first introduced in 2010 (Cruz-Jentoft et al., 2010). The search terms were (sarcopenia (mesh) OR sarcopen* OR sarcopaen*) AND (home OR communit* OR house OR education OR walking OR diet* OR yoga OR “tai chi” OR qigong OR Wuqinxi). Forward and backward citation searches were manually conducted to find potential studies that were not identified by database searches. Clinical trial registries (https://clinicaltrials.gov/), ProQuest Dissertation & Thesis, and Conference Proceedings Citation Index were searched for gray literature. The draft literature search strategy and results were reviewed by an experienced librarian, which enabled us to revise our search strategy and ensure its quality. The references of included studies were also searched to find relevant papers.
2.3. Study selection and data extraction
All references were imported into the reference management software EndNote20. After removing duplications, two reviewers (ML and PK) independently screened potential papers via two steps: screening of titles and abstracts, and screening of full-text articles according to the eligibility criteria. Any disagreements were resolved by discussion and consultation with a third reviewer (YF) to reach a consensus. The two reviewers extracted and recorded all relevant information according to a standardized data extraction form that had been piloted on two of the included studies. The following data were extracted: first author, title, year, country, demographic data (age, sex, and setting), methodological data (sample size, blinding, group design, intervention duration, assessment tools, and assessment time points), and outcome data (i. components of sarcopenia such as the skeletal muscle mass index, handgrip strength, gait speed, and knee strength; ii. sarcopenia-related outcomes such as QoL, activities of daily living, depression, and anxiety; and iii. drop-out and compliance rates). The details of the interventions were extracted according to the template for intervention description and replication (TIDieR) checklist (Hoffmann et al., 2016).
The mean (M) and standard deviation (SD) values for each outcome before and after the intervention were extracted from each study, and the equations from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2021) were used to derive SD values when these were not reported. When there were multiple papers with the same study protocol or registration number, the data were only extracted and recorded once. The authors of individual studies were contacted to provide additional information or data when necessary. Any disagreements were resolved by discussion or consultation with the third reviewer.
2.4. Risk of bias assessment
Although we intended to include both RCTs and quasi-experimental studies, the studies included in this SR were all RCTs. Therefore, the modified version of Cochrane's risk-of-bias tool (RoB-2) was used to assess the risks of bias (Sterne et al., 2019) across five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain for each study was judged as “a low risk of bias,” “some concerns,” or “a high risk of bias” using the intrinsic algorithms of RoB-2. Thereafter, studies were classified as having a “low risk of bias” if they had a low risk of bias in all domains, “some concerns” if they had some concerns in at least one domain but did not have a high risk of bias in any domain, and “high risk of bias” if they had a high risk of bias in at least one domain or some concerns in multiple domains (Sterne et al., 2019). All assessments were completed by the two reviewers independently, and any disagreements were resolved by discussion with a third reviewer.
2.5. Statistical analysis
To compare the effects of home-based interventions with those of control interventions, meta-analyses of the primary outcomes (e.g., the skeletal muscle mass index [SMI], lean mass, body fat mass, body mass index (BMI), handgrip strength, and gait speed) and secondary outcomes (e.g., QoL) were conducted. Subgroup analyses were performed on the available data (e.g., the SMI, lean mass, body fat mass, BMI, and handgrip strength) according to the type of control group: passive control or active control. For outcomes not included in the meta-analyses (e.g., the score of the Mini Nutritional Assessment), the information was narratively analyzed and synthesized.
The data reported at baseline and post-intervention were analyzed. In the meta-analyses, mean differences (MDs) with 95 % confidence intervals (CIs) were used to determine the effect sizes for studies with the same measurement methods, while standard mean differences (SMDs) with 95 % CIs were calculated for studies with different measurement methods. Random-effects models were used to synthesize the data because of inevitable clinical heterogeneity (Sterne et al., 2019). No funnel plot was generated because of the small number of studies included. The meta-analyses were conducted using Review Manager 5.3.5 (Cochrane Collaboration, 2019).
Heterogeneity was assessed using the chi-square test and I2 statistic. A chi-square test p value < 0.1 was considered to indicate the existence of heterogeneity. I2 values of 0 %–30 %, >30 %–50 %, >50 %–75 %, and >75 %–100 % were considered to represent small, moderate, substantial, and considerable heterogeneity, respectively (Sterne et al., 2019).
3. Results
3.1. Study selection
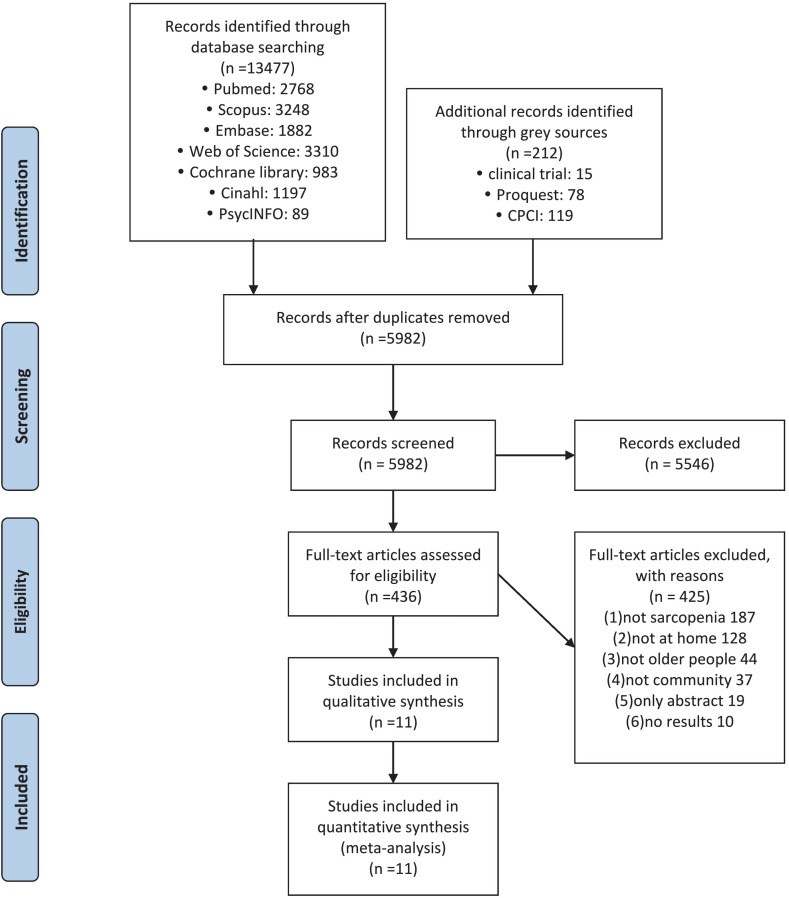
A total of 13,689 studies were identified from our search. Of these, 5982 papers remained after removing duplicates. After title and abstract screening, 436 studies were subjected to full-text screening, including those that mentioned sarcopenia many times in the abstracts, as we wanted to check whether these papers had subgroup analyses of older people with sarcopenia to ensure that we did not miss any relevant papers. Finally, after excluding papers that did not include subgroup analyses of older people with sarcopenia (n = 187), did not focus on home-based interventions (n = 128), did not focus on older people (n = 44), did not focus on community-dwelling older people (n = 37), were only published as abstracts (n = 19), and were only clinical trials with no results (n = 10), 11 studies were included in this SR and meta-analysis for both qualitative and quantitative syntheses. The paper selection process is shown in Fig. 1 .
Fig. 1.
PRISMA Flowchart showing the literature search and study selection.
3.2. Study characteristics
3.2.1. Study design
Although we intended to include both RCTs and quasi-experimental studies, the final 11 included studies were all RCTs (Table 1 ). These RCTs were conducted from 2010 to 2021 in Greece (Tsekoura et al., 2018), Turkey (Sen et al., 2021), Japan (Maruya et al., 2016), mainland China (two RCTs) (Bo et al., 2019; Li et al., 2021), Taiwan (two RCTs) (Chang et al., 2021; Lin et al., 2021), Finland (Björkman et al., 2020), Iran (Nasimi et al., 2021), Mexico (Alemán-Mateo et al., 2012) and eight countries across Europe and North America (Cramer et al., 2016). Eight RCTs had two arms, two RCTs had three arms, and the remaining RCT had four arms.
Table 1.
The characteristics of the included studies.
| Author | Year | Design | Country/region | Population | Intervention duration | Sample size | Intervention design (number, age) | Control design (number, age) | Outcome domain and measurement |
|---|---|---|---|---|---|---|---|---|---|
| Tsekoura (Tsekoura et al., 2018) | 2018 | 3-Arms RCT | Greece | ≥60 years living independently in the community with (pre)sarcopenia | 3 months | 54 |
|
Usual care (N = 18, 72.89±8.31) | BMI, SMI, fat-free mass, calf circumference, handgrip strength, knee extension strength, knee flexion strength, TUGT, 4 m test, 5 times chair stand test, QoL |
| Sen (Sen et al., 2021) | 2021 | 2-Arms RCT | Turkey | Community-dwelling older adult aged 65–80 years and diagnosed with sarcopenia | 3 months | 90 | Home-based exercise (N = 46, 73±4.8) | Usual care (N = 44, 72.7±5) | TUGT, QoL, 6MWT, BBS |
| Maruya (Maruya et al., 2016) | 2016 | 2-Arms RCT | Japan | ≥60 years old people living in the community with (pre)sarcopenia | 6 months | 40 | Home-based exercise (N = 26, 69.2±5.6) | Usual care (N = 14, 68.5±6.2) | BMI, SMI, Body fat, handgrip, duration of single leg standing, gait speed, knee extension strength, GLFS-25, QoL, pre-sarcopenia, sarcopenia |
| Li (Li et al., 2021) | 2021 | 4-Arms RCT | China | ≥60 years old people with sarcopenia and have normal cognition or only mild cognitive disturbance | 12 weeks | 169 |
|
Usual care (N = 33, 72.91±6.29) | ASM, SMI, ASM/weight, ASM/BMI, ASM:fat ratio, fat mass, percentage of fat mass, WHR, VFA, handgrip strength |
| Chang (Chang et al., 2021) | 2021 | 2-Arms RCT | Taiwan | >65 years old people with sarcopenia | 12 weeks | 57 | Home-based exercise (N = 28, 75.7±5.9) | Group-based exercise (N = 29, 74.3±5.8) | Lean mass of upper extremities, lean mass of lower extremities, lean mass of the trunk, total lean mass, total fat content, handgrip strength, gait speed, 30-second chair stand test, 2-minute step test |
| Björkman (Björkman et al., 2020) | 2020 | 3-Arms RCT | Finland | ≥75 years old people with sarcopenia | 12 months | 178 |
|
Usual care (N = 50, 83.7±5.1) | SMI, hand grip strength, SPPB, CSPPS |
| Nasimi (Nasimi et al., 2021) | 2021 | 2-Arms RCT | Iran | ≥65 years old people with sarcopenia | 12 weeks | 64 | Fortified yogurt (N = 33, 71±3.33) | Active control (N = 31, 69±7.03) | BMI, SMI, total lean mass, ALM, fat mass, waist circumference, calf circumference, handgrip strength, gait speed, PCS score, MCS score, MNA score, IPAQ score, vitamin D, IGF-1, hs-CRP, insulin, HOMA-IR |
| Lin (Lin et al., 2021) | 2021 | 2-Arms RCT | Taiwan | ≥65 years old people with sarcopenia | 12 weeks | 56 | Protein supplement (N = 28, 72.5±5.75) | Usual care (N = 28, 73.8±8.11) | Body weight, BMI, fat mass, fat free mass, ASM, SMI, handgrip strength, gait speed, total energy, carbohydrate, fat and protein intake |
| Cramer (Cramer et al., 2016) | 2016 | 2-Arms RCT | 8 countries across Europe and North America | ≥65 years old people with sarcopenia and malnutrition | 24 weeks | 328 | High protein supplement (N = 163, 77±7.40) | Low protein supplement (N = 165, 77±7.40) | BMI, body weight, fat mass, single left leg muscle mass, sum of left and right legs muscle mass |
| Bo (Bo et al., 2019) | 2019 | 2-Arms RCT | China | Aged 60–85 years old people with sarcopenia | 6 months | 60 | Protein supplement (N = 30, 73.23±6.52) | Usual care (N = 30, 74.38±5.94) | Body weight, BMI, fat mass, AMM, RSMI, handgrip strength, gait speed, time to complete 5 stands, time to stand up, SF-36 MCS score, SF-36 PCS score, C-reactive protein, albumin, total protein, LDL-C, HDL-C, total cholesterol, triglyceride, IGF-1, TNF-a, IL-2, IL-6, vitamin D3, vitamin E, energy, protein, fat and carbohydrates intake |
| Alemán-Mateo (Alemán-Mateo et al., 2012) | 2010 | 2-Arms RCT | Mexico | ≥60 years old people with sarcopenia | 3 months | 40 | Ricotta cheese supplement (N = 20, 75.4±5.0) | Usual care (N = 20, 76.7±5.8) | Body weight, ASM, Lean body mass (arms and legs), Body fat, truncal fat, lean body mass, total body mass, handgrip strength, IGF-1, insulin, HOMA-IR, glucose, hemoglobin, total cholesterol, triglycerides, creatinine, uric acid, urea, GFR, microalbumin |
Abbreviations: 6MWT, 6-Minute Walking Test; ASM, appendicular skeletal muscle mass; BBS, Berg Balance Scale; CSPPS, Continuous Summary Physical; GLFS-25, the 25-question Geriatric Locomotive Functional Scale; HDL-C, high density lipoprotein cholesterol; HOMA-IR, insulin resistance; IGF-1, insulin like growth factor-1; IPAQ, International Physical Activity Questionnaire; LDL-C, low density lipoprotein cholesterol; MCS, Mental Component Summery; PCS, Physical Component Summery; MNA, Mini-Nutritional Assessment; QoL, quality of life; SF-36, Short-Form 36-Item health survey; SMI, skeletal muscle mass index; SPPB, Short Physical Performance Battery; TNF-a, tumor necrosis factor-∝; TUGT, Time Up and Go Test; VFA, visceral fat area; WHR, waist-hip ratio.
3.2.2. Participants and diagnosis of sarcopenia
A total of 1136 participants (aged 60–89 years) were included in the 11 RCTs, ranging from 40 to 328 in each individual study. All RCTs included older people of both sexes with sarcopenia, and the age criteria were older than 65 years in four RCTs (Chang et al., 2021; Cramer et al., 2016; Lin et al., 2021; Nasimi et al., 2021), older than 60 years in four RCTs (Alemán-Mateo et al., 2012; Li et al., 2021; Maruya et al., 2016; Tsekoura et al., 2018), older than 75 years in one RCT (Björkman et al., 2020), 65–80 years in one RCT (Sen et al., 2021) and 60–80 years in one RCT (Bo et al., 2019) (Table 1). The diagnostic criteria for sarcopenia varied in each study (Appendix Table 1) and were classified into three types: AWGS 2014 sarcopenia diagnostic criteria (used in five RCTs), EWGSOP 2010 criteria (used in three RCTs), and other criteria (used in three RCTs). Two RCTs also included older people with pre-sarcopenia, which was defined as low muscle mass in both studies (Maruya et al., 2016; Tsekoura et al., 2018).
3.2.3. Description of interventions
Details of the home-based non-pharmacological interventions for sarcopenia are provided in Appendix Table 2 according to the TIDieR checklist (Hoffmann et al., 2016). The interventions were categorized into three types: home-based exercise intervention (Chang et al., 2021; Maruya et al., 2016; Sen et al., 2021; Tsekoura et al., 2018), home-based nutritional intervention (Alemán-Mateo et al., 2012; Björkman et al., 2020; Bo et al., 2019; Cramer et al., 2016; Lin et al., 2021; Nasimi et al., 2021), and home-based combined exercise and nutritional intervention (Li et al., 2021). Although we found some studies of mind-body interventions for sarcopenia in our preliminary search, the limited studies did not meet our inclusion criteria (Pandya, 2019; Zhu et al., 2019).
The home-based exercise interventions included RE, aerobic exercise (walking), balance exercise, and gait training. The duration of the exercise interventions ranged from 12 weeks to 6 months, the duration of each session varied from 30 min to 90 min, and the frequency of sessions ranged from once daily to twice weekly. The intensity of exercise interventions was moderate in two studies (Chang et al., 2021; Tsekoura et al., 2018), light in one study (Sen et al., 2021), and not mentioned in two studies (Li et al., 2021; Maruya et al., 2016). For modes of home-based exercise delivery, one RCT used guidebooks, face-to-face instructions, and phone calls for each participant during a 12-week intervention (Tsekoura et al., 2018); three RCTs only used self-learning material [guidebooks (Maruya et al., 2016), brochures, and CDs (Chang et al., 2021; Li et al., 2021)]; and one RCT did not mention any material or instruction (Sen et al., 2021). The control groups were classified into passive controls, such as usual care in two RCTs (Maruya et al., 2016; Sen et al., 2021), and active controls, such as group-based exercise in one RCT (Chang et al., 2021).
The home-based nutritional interventions included nutritional supplements and dietary food supplements (yogurt and ricotta cheese) available as powders or sachets. In all of these nutritional interventions, participants were asked to consume these supplements with their regular diet (i.e., breakfast, lunch, or dinner). The supplements in five of the seven RCTs involving nutritional interventions contained multiple nutrients, such as protein, vitamin D, leucine, vitamin C, vitamin E, and hydroxy beta-methylbutyrate (Bo et al., 2019; Cramer et al., 2016; Li et al., 2021; Lin et al., 2021; Nasimi et al., 2021). Protein was included in all nutritional interventions (all seven studies), and its amount varied from 12.8 g to 47.1 g per day. The duration of the nutritional interventions ranged from 12 weeks to 12 months, and the frequency of taking the supplements varied from one to three times a day. The control groups were categorized as passive controls, such as usual care or placebo in four RCTs (who received dietary advice or an isocaloric supplement) (Alemán-Mateo et al., 2012; Björkman et al., 2020; Bo et al., 2019; Lin et al., 2021), and active controls, such as reduced supplementation in two RCTs (Cramer et al., 2016; Nasimi et al., 2021).
3.2.4. Outcomes
All of the included outcomes were classified into four types: body composition, muscle strength, physical function, and others (QoL, inflammatory factors, and other biochemical indicators) (Table 1); notably, none of the included RCTs assessed psychological health. Body composition (i.e., the SMI, fat-free mass, and body fat), muscle strength (i.e., handgrip strength and knee strength), and physical function (i.e., gait speed, time to complete the Timed Up and Go Test [TUGT], and time to complete the chair stand test) were evaluated in 10, 9, and 8 RCTs, respectively. QoL was evaluated in five RCTs using various tools (i.e., the European Quality of Life Five Dimension [EQ-5D] instrument, the 12/36-Item Short Form Health Survey [SF-12/36], the Sarcopenia Quality of Life [SarQoL] instrument and a numeric scale). Biomechanical indicators (i.e., insulin-like growth factor 1, high-sensitivity C-reactive protein, and insulin) were measured in only three RCTs (Alemán-Mateo et al., 2012; Bo et al., 2019; Lin et al., 2021).
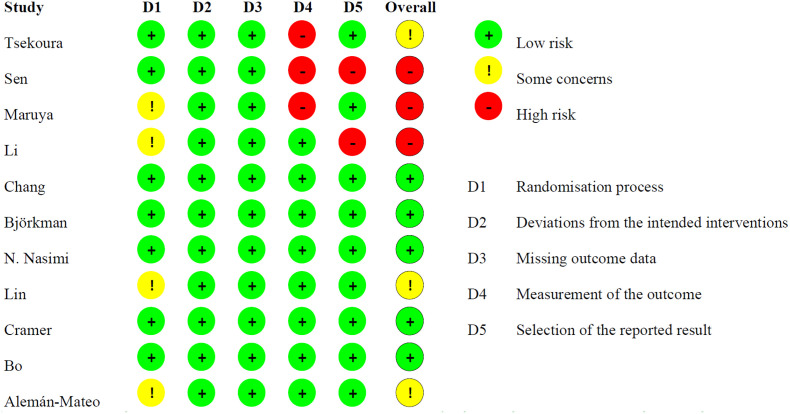
3.3. Risk of bias
The results of risk-of-bias assessments using the Cochrane RoB-2 tool are presented in Fig. 2 . Randomization concealment was not mentioned in four RCTs (Alemán-Mateo et al., 2012; Li et al., 2021; Lin et al., 2021; Maruya et al., 2016). Three RCTs did not describe the blinding of outcome assessors (Maruya et al., 2016; Sen et al., 2021; Tsekoura et al., 2018). Two RCTs had a risk of reporting bias because the researchers only gave the data of some but not all outcomes mentioned in their protocols (Li et al., 2021; Sen et al., 2021).
Fig. 2.
Risk of bias summary of the included studies.
3.4. Overall effects of home-based exercise interventions on primary outcomes
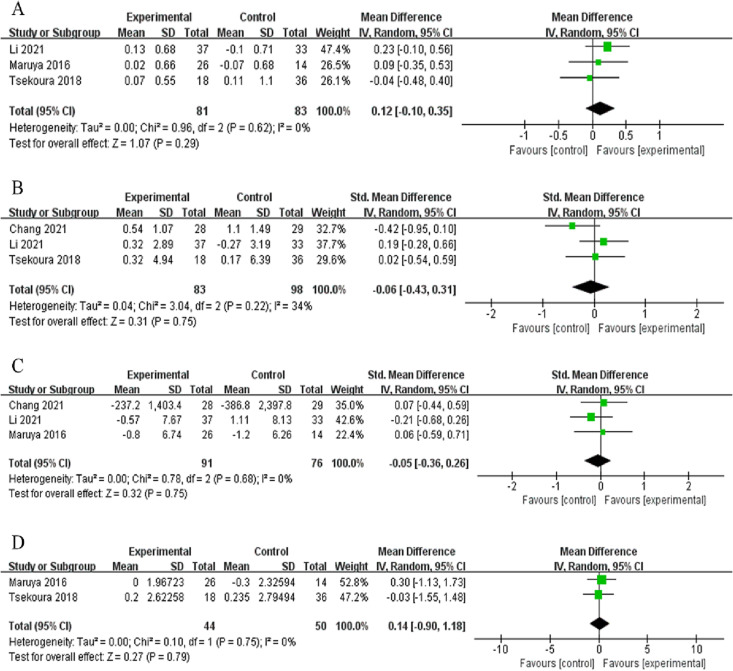
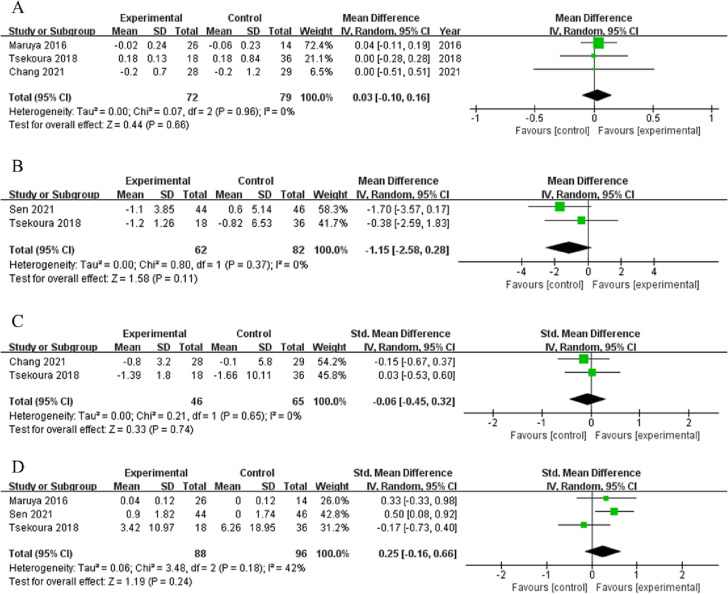
Among studies that evaluated the effects of home-based exercise interventions on body composition outcomes, three studies provided data on the SMI (n = 81), three on lean mass (n = 83), three on body fat (n = 91), and two on the BMI (n = 44). The home-based exercise interventions increased the SMI by 0.12 kg/m2 compared with the controls (95 % CI: −0.10, 0.35), but the difference was not statistically significant (p = 0.29, I2 = 0 %, Fig. 3A). However, home-based exercise significantly increased the SMI compared with usual care in one individual study(p = 0.0018, 95 % CI: 0.043, 0.446) (Li et al., 2021), but had no overall effects on lean mass, body fat mass, and BMI (SMD = −0.06, 95 % CI: −0.43, 0.31, p = 0.75, I2 = 34 %; SMD = −0.05, 95 % CI: −0.36, 0.26, p = 0.75, I2 = 0 %; MD = 0.14 kg/m2, 95 % CI: −0.90, 0.18, p = 0.79, I2 = 0 %, Fig. 3B, C, and D, respectively).
Fig. 3.
Overall effect of home-based exercise on body composition: SMI (A), lean mass (B), body fat mass (C), BMI (D).
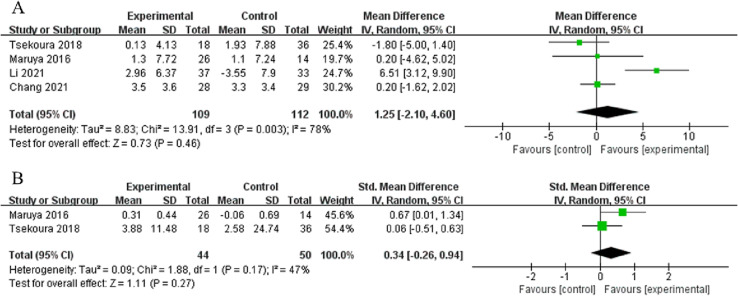
Among studies that evaluated the effects of home-based exercise interventions on muscle strength, four studies provided data on handgrip strength (n = 109) and two on knee extension strength (n = 44). Although these two outcomes showed increasing trends in the intervention groups, the trends were not statistically significant compared with the controls (handgrip strength: MD = 1.25 kg, 95 % CI: −2.10, 4.60, p = 0.46, I2 = 78 %; knee extension strength: MD = 0.34 kg, 95 % CI: −0.26, 0.94, p = 0.27, I2 = 47 %, Fig. 4A and B, respectively). However, handgrip strength increased significantly in the intervention group compared with the control group (p < 0.001, 95 % CI: 2.904, 8.732) in one study (Li et al., 2021) and from pre- to post-intervention within the intervention group in two studies (Chang et al., 2021; Maruya et al., 2016).
Fig. 4.
Overall effect of home-based exercise on muscle strength: handgrip strength (A) and knee extension strength (B).
Among studies that evaluated the effects of home-based exercise interventions on physical function outcomes, three studies provided data on gait speed (n = 72), two on time to complete the TUGT (n = 62), and two on time to complete the chair stand test (n = 65). In the home-based exercise intervention groups, gait speed showed an increasing trend (MD = 0.03 m/s, 95 % CI: −0.10, 0.16, p = 0.66, I2 = 0 %, Fig. 5 ) and times to complete the TUGT and chair stand test showed decreasing trends, but these trends were not statistically significant compared with the controls (MD = −1.15 s, 95 % CI: −2.58, 0.28, p = 0.11, I2 = 0 %; MD = −0.06 s, 95 % CI: −0.45, 0.32, p = 0.74, I2 = 0 %, respectively, Fig. 5B and C, respectively). However, gait speed increased significantly in the intervention groups compared with the control groups in two RCTs (Maruya et al., 2016; Tsekoura et al., 2018) and from pre- to post-intervention in one RCT (Chang et al., 2021).
Fig. 5.
Overall effect of home-based exercise on physical function: gait speed (A), TUGT (B), chair stand (C) and others: quality of life (D).
3.5. Overall effects of home-based exercise interventions on secondary outcomes
Regarding the effects of home-based exercise interventions on secondary outcomes, sufficient data for meta-analysis were available only for QoL (three studies, n = 88). The heterogeneity for this outcome was quite high among the studies (I2 = 42 %, p = 0.18), and the overall intervention effect on this outcome was not significant (SMD = 0.25, 95 % CI = −0.16, 0.66, p = 0.24, Fig. 5D). However, QoL in the intervention groups significantly increased compared with the control groups in two individual RCTs (Sen et al., 2021; Tsekoura et al., 2018).
3.6. Overall effects of home-based nutritional interventions on primary outcomes
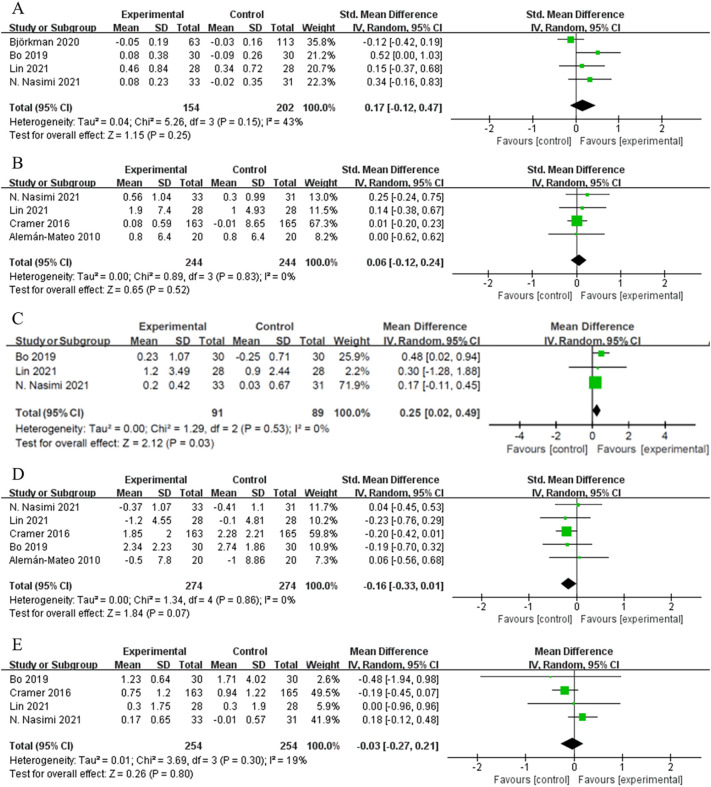
Among studies that evaluated the effects of home-based nutritional interventions on body composition outcomes, four studies provided data on the SMI (n = 154), four on lean mass (n = 244), three on the ALM (n = 91), four on the BMI (n = 254), and five on body fat (n = 274). Home-based nutritional interventions increased the SMI by 0.17 compared with the controls (SMD 95 % CI: −0.12, 0.47, I2 = 43 %), but the increase was not statistically significant (p = 0.250, Fig. 6A). However, in one RCT, the SMI significantly increased after 6 months of home-based protein supplementation (MD = 0.18 kg/m2, 95 % CI: 0.01, 0.35, p = 0.040) compared with placebo (Bo et al., 2019). Two RCTs showed significant increases in the SMI from pre- to post-intervention within the intervention groups, but not in comparison with the control groups (Lin et al., 2021; Nasimi et al., 2021). No change in lean mass was reported after the nutritional interventions (SMD = 0.06, 95 % CI: −0.12, 0.24, I2 = 0 %, p = 0.520, Fig. 6B), but the appendicular skeletal muscle mass (ASM) increased significantly compared with the controls (MD = 0.25 kg, 95 % CI: 0.02, 0.49, I2 = 0 %, p = 0.03, Fig. 6C). Fat mass showed a decreasing trend in the intervention groups compared with the control groups, but the trend was not statistically significant (SMD = −0.16, 95 % CI: −0.33, 0.01, I2 = 0 %, p = 0.070); similar results were found for the BMI (MD = −0.03 kg/m2, 95 % CI: −0.27, 0.21, I2 = 19 %, p = 0.080; Fig. 6C and D, respectively).
Fig. 6.
Overall effect of home-based nutritional interventions on body composition: SMI (A), lean mass (B), ASM (C), body fat mass (D) and BMI (E).
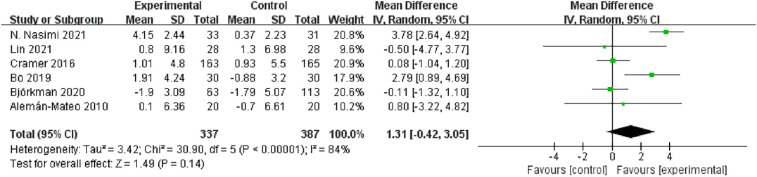
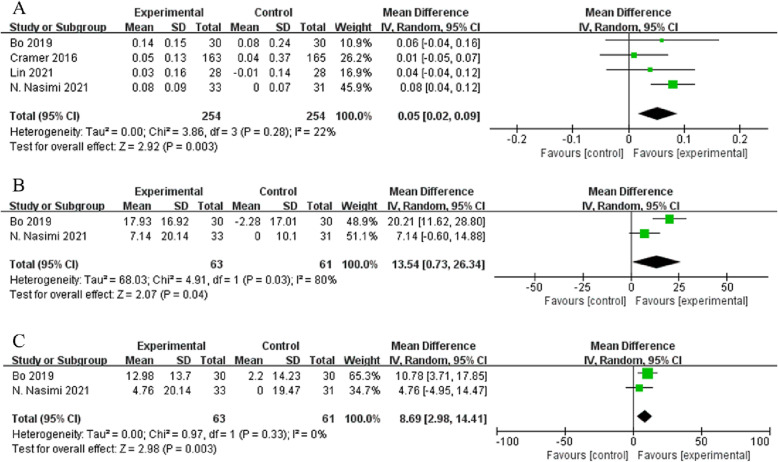
Among muscle strength outcomes, sufficient data for meta-analysis were only available for handgrip strength (six studies, n = 337). Overall, a slight non-significant increase was found in this outcome compared with the controls (MD = 1.31 kg, 95 % CI: −0.42, 3.05, I2 = 84 %, p = 0.140, Fig. 7 ). However, handgrip strength significantly increased in the intervention groups compared with the control groups in two individual RCTs (Bo et al., 2019; Nasimi et al., 2021). Among physical function outcomes, data were only available for gait speed in four studies (n = 254), which showed a significant increase by 0.05 m/s compared with the controls (95 % CI: 0.02, 0.09, p = 0.003, Fig. 8A).
Fig. 7.
Overall effect of home-based nutritional interventions on handgrip strength.
Fig. 8.
Overall effect of home-based nutritional interventions on gait speed (A) and quality of life, PCS (B) and MCS (C).
3.7. Overall effects of home-based nutritional interventions on secondary outcomes
Regarding secondary outcomes, physical component summary (PCS) and mental component summary (MCS) scores were available only in two studies (n = 63). Home-based nutritional supplementation significantly improved both PCS (MD = 13.54, 95 % CI: 0.73, 26.34, I2 = 80 %, p = 0.040) and MCS scores (MD = 8.69, 95 % CI: 2.98, 14.41, I2 = 0 %, p = 0.003, Fig. 8B and C, respectively) compared with the controls.
3.8. Effects of home-based combined physical exercise and nutritional interventions
As only one of the included RCTs used a combined physical exercise and nutritional intervention to treat sarcopenia (Li et al., 2021), the results are described narratively. This RCT found that a 12-week intervention of combined physical exercise and nutritional supplementation was effective in improving the SMI (p < 0.001, 95 % CI: 0.214, 0.581) and handgrip strength (p < 0.001, 95 % CI: 3.441, 8.907) and reducing fat mass (p < 0.001, 95 % CI: −4.717, −1.790) compared with the control group. When compared with the exercise-alone intervention, the combined intervention only demonstrated a significant reduction in fat mass (p =0.005, 95 % CI: −4.810, −0.878). However, none of these outcomes were significant in the combined intervention when compared with the nutrition-alone intervention.
3.9. Subgroup analyses
Compared with passive controls (usual care), home-based exercise interventions significantly improved knee extension strength (MD = 0.56 kg, 95 % CI: 0.09, 1.03, I2 = 0 %, p = 0.020, Appendix Fig. 1B) and reduced the time to complete the TUGT (MD = −1.41 s, 95 % CI: −2.28, −0.54, I2 = 0 %, p = 0.001, Appendix Fig. 1D) and led to a marginally significant increase in gait speed (MD = 0.12 m/s, 95 % CI: −0.00, 0.24, I2 = 53 %, p = 0.060, Appendix Fig. 2C), but did not affect the SMI, lean mass, body fat, BMI, handgrip strength, gait speed, or QoL (Appendix Figs. 1 and 2). Compared with active controls (group-based exercise), home-based exercise interventions significantly decreased gait speed (MD = −0.16 m/s, 95 % CI: −0.24, −0.07, I2 = 0 %, p = 0.0005, Appendix Fig. 3C) but caused no significant changes in lean mass, handgrip strength, and the time to complete the chair stand test (Appendix Fig. 3).
Compared with passive controls, home-based nutritional interventions significantly increased the ASM (MD = 0.47 kg, 95 % CI: 0.02, 0.91, I2 = 0 %, p = 0.040), but did not significantly affect the SMI, lean mass, body fat, BMI, handgrip strength, and gait speed (Appendix Fig. 4C). Compared with active controls, none of the outcomes (SMI, BMI, body fat, lean mass, handgrip strength, and gait speed) showed significant differences after home-based nutritional interventions (Appendix Fig. 5).
3.10. Adherence and compliance
In studies of home-based exercise interventions, the drop-out rates varied from 0 % to 40 % in the intervention groups and from 0 % to 44 % in the control groups. Only one RCT reported the reasons for drop-outs, which were mainly attendance failure in the intervention group and follow-up failure in the control group (Sen et al., 2021). Only two of the five RCTs on home-based exercise interventions reported an adherence rate, which was calculated as the number of sessions attended by each participant divided by the number of sessions they were expected to attend. This rate was 87.5 % in one study (Tsekoura et al., 2018), and >89 % for three different exercise movements and 71.7 % for walking in the other study (Maruya et al., 2016).
In studies of home-based nutritional interventions, the drop-out rates varied from 0 % to 40 % in the intervention groups and from 0 % to 51 % in the control groups. Only two RCTs provided the reasons for drop-outs, which were mainly health problems in the intervention group (Alemán-Mateo et al., 2012) and denture issues in the control group (Lin et al., 2021). Compliances were reported in three RCTs: 58 % (Björkman et al., 2020), 97 % (Lin et al., 2021), and 86 % (Cramer et al., 2016). One RCT only reported that the compliance was high, but did not provide specific data (Nasimi et al., 2021).
4. Discussion
In this SR, we summarized the components of non-pharmacological home-based interventions for community-dwelling older people with sarcopenia that can be implemented both during and after the COVID-19 pandemic and evaluated the effectiveness of these interventions on various health-related outcomes in this population. Home-based exercise (RE and walking) and home-based nutritional supplementation (protein and vitamin D supplements) were the home-based interventions mainly implemented in this population. The overall effects of home-based exercise interventions on muscle mass (the SMI, lean mass, body fat mass, and BMI), muscle strength (handgrip strength and knee extension strength), physical function (gait speed, time to complete the TUGT, and time to complete the chair stand test), and QoL were inconclusive. However, compared with passive controls, these interventions were effective in improving knee extension strength and reducing the time to complete the TUGT in older people with sarcopenia. Home-based nutritional interventions had overall beneficial effects on the ASM, gait speed, and QoL. Their beneficial effects on the ASM were consistent even when compared with passive controls. The results of this SR and meta-analysis indicate that home-based exercise interventions have the potential to improve muscle strength and physical function, while home-based nutritional supplements are effective in improving muscle mass, muscle strength, physical function, and QoL in community-dwelling older people with sarcopenia. The effects of home-based combined physical exercise and nutritional interventions still need further research as we found only one RCT on such a combined intervention.
4.1. Effects of home-based exercise interventions
The findings of this SR and meta-analysis showed that home-based exercise interventions were effective in improving muscle strength and physical function compared with the usual care. The beneficial effects of exercise, especially RE, on sarcopenia are well known. Our SR further confirmed the efficacy of exercise in alleviating sarcopenia when conducted at home, indicating that home-based exercise has the potential to circumvent the adverse effects of inactivity during isolation or quarantine on muscle mass and muscle function. As we mentioned earlier, home-based exercise interventions have advantages over center-based interventions, such as being more convenient, preferred by this population, and more practical, especially during the COVID-19 pandemic. Furthermore, almost all of the participants in the studies included in our SR had multiple comorbidities or reduced physical function. These groups are likely to be unwilling to go out and to prefer home-based exercise to center-based exercise. In addition, compared with center-based interventions, home-based exercise interventions can be performed in more flexible settings and are easier to incorporate into daily life, as people can perform exercises at any time and in a familiar environment (Dorresteijn et al., 2012).
4.2. Potential reasons for the small effect sizes of home-based exercise interventions
Although our SR found that home-based exercise interventions were effective in improving the muscle strength and physical function of older people with sarcopenia compared with passive controls, the effect sizes were smaller than those reported in an earlier SR of the effects of exercise interventions irrespective of the implemented settings (Lu et al., 2021). The reason for this difference might be the low intensity of exercises and poor intervention fidelity in the studies included in our SR. Moderate-intensity exercise, either from the beginning of or adopted progressively during the intervention, is strongly recommended in the International Exercise Recommendations in Older Adults (ICFSR) guidelines (Izquierdo et al., 2021) and previous reviews (Chen et al., 2021; Hurst et al., 2022). However, the moderate intensity requirement was only met in two of the five RCTs of home-based exercise interventions included in our SR (Chang et al., 2021; Tsekoura et al., 2018). As there is a dose–response relationship between exercise intensity and health outcomes, low-intensity exercise may not be able to relieve the symptoms of sarcopenia. Regarding intervention fidelity, although digital technologies that enable mutual communication (i.e., video conference platform and social media) have been widely used in health interventions during and after the COVID-19 pandemic (Kor et al., 2022), the majority of the included RCTs only used self-learning materials (i.e., booklets and CDs) to deliver the exercise interventions without conducting fidelity checks, such as checking whether the participants achieved the target intensity or performed the exercise program strictly following the intervention protocol (Chang et al., 2021; Maruya et al., 2016). This one-way delivery modality has a high risk of leading to inadequate progress among the exercise program participants, which further undermines the beneficial effect of home-based exercise on sarcopenia. In addition, one-way delivery of interventions and lack of intervention-fidelity checking may be why the drop-out rate in home-based exercise interventions was as high as 40 %. Although compliance is a major influencing factor for the effects of home-based exercise interventions and a key part of intervention fidelity, only two RCTs reported the compliance rates for RE (~80 %) and walking (71 %) (Maruya et al., 2016; Tsekoura et al., 2018), and the rates were much higher than those reported in another SR of home-based exercise interventions for older people (58 % for RE and 63 % for walking) (Mañas et al., 2021). However, due to the limited data, the high compliance with home-based exercise interventions found in our SR may not represent the compliance in the whole population of community-dwelling older people with sarcopenia. Thus, the compliance of older people with sarcopenia with home-based exercise and its facilitators and barriers need to be further explored.
4.3. Effects of home-based nutritional interventions
The results of this SR showed that home-based nutritional interventions were effective in improving the ASM, gait speed, and QoL, and the improvement in the ASM was consistent when compared with passive controls. However, no significant differences were observed in the SMI, BMI, fat mass, lean mass, handgrip strength, and gait speed post-intervention compared with active controls. These results suggest that active controls are potentially as beneficial as home-based nutritional interventions for sarcopenia. It is well known that nutritional interventions, especially protein supplements, are essential to increase muscle mass by stimulating muscle protein synthesis (Landers-Ramos and Dondero, 2019). Our SR further demonstrated that nutritional supplements or foods, when consumed at home with a regular diet, were also able to improve the ASM, gait speed, and QoL of older people with sarcopenia. It is especially important for older people with sarcopenia to consume nutritional supplements or foods at their homes (exactly as in our SR) as food diversity and nutrition intake have been greatly undermined during and after the COVID-19 pandemic (Kirwan et al., 2020). Inadequate nutrition intake leads to significant weight loss, further accelerating muscle loss (Leidy et al., 2007). However, contrary to our results, nutritional supplements in two studies showed no significant effect on gait speed (Gkekas et al., 2021; Wu et al., 2021) and ASM (Gkekas et al., 2021). This difference may be attributable to the use of different types of nutritional supplements.
Most of the studies on nutritional interventions for older people with sarcopenia have not described the intervention settings (Malafarina et al., 2013; Pinheiro et al., 2020). This might be because unlike exercise interventions, the intervention settings (e.g., centers, homes, and communities) do not influence the effects of nutritional interventions on sarcopenia. However, as we mentioned before, it is much more convenient and practical for this population to receive nutritional supplements or foods at home as they are often unwilling to go out due to the risk of getting infected, the serious symptoms and sequelae of COVID-19, and low mobility (Farrance et al., 2016; Kirwan et al., 2020). Moreover, home-based nutritional interventions are much easier to integrate into daily life than center- or laboratory-based nutritional interventions, which might further improve intervention compliance. Furthermore, as sarcopenia is caused by multiple factors, a balanced diet is crucial to treating it (Van Elswyk et al., 2022). Daily foods containing multiple nutrients could be more effective in treating sarcopenia than nutritional supplements that contain only one or a few certain nutrients. Apart from the beneficial effects, we should also pay attention to the potential adverse effects of home-based nutritional interventions. We found that daily foods have much less adverse effects than nutritional supplements. Early satiety (25 %) (Alemán-Mateo et al., 2012) was the only complaint reported by a small number of participants when using daily foods for the nutritional intervention, while adverse effects of nutritional supplements were much more common, such as gastrointestinal complaints [56 % (Björkman et al., 2020) and 28.5 % (Cramer et al., 2016)] and difficult defecation (10 %) (Bo et al., 2019).
Among the included secondary outcomes, QoL was the only indicator that contained a psychological domain in our SR and meta-analysis. Notably, our SR showed that home-based exercise had no significant effect on QoL, while home-based nutritional interventions improved both the physical and psychological domains of QoL. This difference could be because older people with sarcopenia are likely to have concerns about physical exercise even before participating in it and negative feelings when performing it (e.g., frustration, anxiety, distress, and depression) due to low physical function and exercise ability (Herrema et al., 2018). These concerns and negative feelings further damage their psychological health rather than improving it. In contrast, nutritional interventions do not induce such negative feelings. This indicates that paying attention to the psychology-enhancing component is crucial, especially in home-based exercise interventions, as it can reduce negative feelings and enhance positive emotional responses to exercise, thereby improving the experience of engaging in home-based exercise and the psychological well-being of older people with sarcopenia.
4.4. Clinical relevance
The COVID-19 pandemic has a great impact on people's lifestyle and the way of conducting research. It is imperative to find practical and acceptable ways to treat community-dwelling older people with sarcopenia during and after the COVID-19 pandemic. Notably, older people with sarcopenia prefer staying at home and doing exercise in a familiar environment. Our SR and meta-analysis demonstrated that home-based exercise and nutritional interventions were effective in improving several sarcopenia- and other health-related indicators in this population, providing health professionals with a pragmatic and promising approach to treat sarcopenia in this population.
4.5. Strengths and limitations
Our SR and meta-analysis has several strengths. First, to the best of our knowledge, this is the first SR and meta-analysis to explore the effects of non-pharmacological home-based interventions on various health-related outcomes in community-dwelling older people diagnosed with sarcopenia based on established criteria. This is important because if the settings are not defined (e.g., nursing homes, communities, and hospitals) and established sarcopenia diagnostic criteria are not followed, the characteristics of recruited participants may vary considerably (e.g., physical function and muscle mass), which may produce inconsistency in the effects of interventions on sarcopenia. Second, the methodology of this SR was guaranteed as we strictly followed the PRISMA guidelines and registered our protocol in Prospero (CRD42022297446). Third, the risk of missing relevant papers in our SR and meta-analysis was very low because of the comprehensive search and screening strategy that we adopted, through which we identified papers with subgroup analyses of the targeted study population.
Despite these advantages, this SR and meta-analysis has some limitations. First, the sample size was relatively small. Although 11 papers including 1136 participants were included, the numbers of studies and participants for some outcomes were relatively small (i.e., QoL). In addition, due to insufficient data, we could not perform meta-analyses of home-based combined exercise and nutritional interventions. Second, more than half of the included RCTs had moderate or high risks of bias. This may have decreased the overall quality of our SR and meta-analysis. Third, no eligible studies on home-based mind–body exercise interventions were found for inclusion in our SR, although there is increasing evidence of a correlation between sarcopenia and psychological health. This indicates that further studies on home-based mind–body exercise interventions and the outcome of psychological health are warranted. Fourth, the effectiveness of home-based interventions in participants who have recovered from COVID-19 remains unclear as our targeted population included community-dwelling older people with sarcopenia regardless of whether they had prior COVID-19.
5. Conclusion
This comprehensive SR and meta-analysis of RCTs showed that non-pharmacological home-based interventions to alleviate sarcopenia in community-dwelling older people mainly include home-based exercise (RE and walking) and nutritional interventions (protein and vitamin D supplementation), and that both could be implemented by health professionals and feasibly performed by older people with sarcopenia during and after the COVID-19 pandemic. Home-based exercise interventions are effective in circumventing inactivity and improving muscle strength and physical function in this population compared with passive controls (e.g., usual care, waitlist, and placebo), although attention should be paid to ensure intervention fidelity, especially in terms of following the recommended exercise intensity. Home-based nutritional interventions are effective in increasing muscle mass, physical function, and QoL. Compared with nutritional supplements, daily foods have fewer adverse effects and are easier to integrate into daily life. However, high-quality RCTs, especially those including psychological health-enhancing interventional elements and evaluating psychological health-related outcomes, are urgently needed.
Registration and protocol
The protocol of this SR and meta-analysis has been registered in the PROSPERO database (CRD42022297446).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgments
We would like to sincerely thank Ms. Lydia Ngai, a Librarian of The Hong Kong Polytechnic University, who gave us some valuable suggestions on our search strategies.
Section Editor: Christiaan Leeuwenburgh
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exger.2023.112128.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Alemán-Mateo H., Macías L., Esparza-Romero J., Astiazaran-García H., Blancas A.L. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: evidence from a randomized clinical trial using a protein-rich food. Clin. Interv. Aging. 2012;7:225–234. doi: 10.2147/cia.S32356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar A., Brach M., Trabelsi K., Chtourou H., Boukhris O., Masmoudi L., Bouaziz B., Bentlage E., How D., Ahmed M., Müller P., Müller N., Aloui A., Hammouda O., Paineiras-Domingos L.L., Braakman-Jansen A., Wrede C., Bastoni S., Pernambuco C.S., Hoekelmann A. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients. 2020;12(6):1583–1596. doi: 10.3390/nu12061583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L., Sharp G.A., Norton R.J., Dalal H., Dean S.G., Jolly K., Cowie A., Zawada A., Taylor R.S. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst. Rev. 2017;6 doi: 10.1002/14651858.CD007130.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth N.L., Chad K.E., Harrison E.L., Reeder B.A., Marshall S.C. Home versus center based physical activity programs in older adults. Cochrane Database Syst. Rev. 2005;2005(1) doi: 10.1002/14651858.CD004017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W., Sun Y., Zhang T., Zou L., Wu X., Wang D., Chen Z. Exercise programs for muscle mass, muscle strength and physical performance in older adults with sarcopenia: a systematic review and meta-analysis. Aging Dis. 2020;11(4):863–873. doi: 10.14336/ad.2019.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudart C., Zaaria M., Pasleau F., Reginster J.Y., Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman M.P., Suominen M.H., Kautiainen H., Jyväkorpi S.K., Finne-Soveri H.U., Strandberg T.E., Pitkälä K.H., Tilvis R.S. Effect of protein supplementation on physical performance in older people with sarcopenia–a randomized controlled trial [Article] J. Am. Med. Dir. Assoc. 2020;21(2):226–232. doi: 10.1016/j.jamda.2019.09.006. e221. [DOI] [PubMed] [Google Scholar]
- Bo Y., Liu C., Ji Z., Yang R., An Q., Zhang X., You J., Duan D., Sun Y., Zhu Y., Cui H., Lu Q. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: a double-blind randomized controlled trial [Article] Clin. Nutr. 2019;38(1):159–164. doi: 10.1016/j.clnu.2017.12.020. [DOI] [PubMed] [Google Scholar]
- Bruyère O., Beaudart C., Ethgen O., Reginster J.Y., Locquet M. The health economics burden of sarcopenia: a systematic review. Maturitas. 2019;119:61–69. doi: 10.1016/j.maturitas.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Cao L., Morley J.E. Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD-10-CM) code. J. Am. Med. Dir. Assoc. 2016;17(8):675–677. doi: 10.1016/j.jamda.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Chaabene H., Prieske O., Herz M., Moran J., Höhne J., Kliegl R., Ramirez-Campillo R., Behm D.G., Hortobágyi T., Granacher U. Home-based exercise programmes improve physical fitness of healthy older adults: a PRISMA-compliant systematic review and meta-analysis with relevance for COVID-19. Ageing Res. Rev. 2021;67 doi: 10.1016/j.arr.2021.101265. [DOI] [PubMed] [Google Scholar]
- Chang K.V., Hsu T.H., Wu W.T., Huang K.C., Han D.S. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing. 2017;46(5):738–746. doi: 10.1093/ageing/afx094. [DOI] [PubMed] [Google Scholar]
- Chang K.V., Wu W.T., Huang K.C., Han D.S. Effectiveness of early versus delayed exercise and nutritional intervention on segmental body composition of sarcopenic elders - a randomized controlled trial. Clin. Nutr. 2021;40(3):1052–1059. doi: 10.1016/j.clnu.2020.06.037. [DOI] [PubMed] [Google Scholar]
- Chen L.-K., Woo J., Assantachai P., Auyeung T.-W., Chou M.-Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., Kojima T., Kuzuya M., Lee J.S.W., Lee S.Y., Lee W.-J., Lee Y., Liang C.-K., Lim J.-Y., Lim W.S., Arai H. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020;21(3):300–307. doi: 10.1016/j.jamda.2019.12.012. e2. [DOI] [PubMed] [Google Scholar]
- Chen N., He X., Feng Y., Ainsworth B.E., Liu Y. Effects of resistance training in healthy older people with sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Eur. Rev. Aging Phys. Act. 2021;18(1):23. doi: 10.1186/s11556-021-00277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A.P., Barber S.E., Young J.B., Forster A., Iliffe S.J. Do home-based exercise interventions improve outcomes for frail older people? Findings from a systematic review. Rev. Clin. Gerontol. 2012;22(1):68–78. doi: 10.1017/s0959259811000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Collaboration Review Manager (RevMan). [5.3.5] 2019. https://revman.cochrane.org/
- Cramer J.T., Cruz-Jentoft A.J., Landi F., Hickson M., Zamboni M., Pereira S.L., Hustead D.S., Mustad V.A. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial [Article] J. Am. Med. Dir. Assoc. 2016;17(11):1044–1055. doi: 10.1016/j.jamda.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.-P., Rolland Y., Schneider S.M., Topinková E., Vandewoude M., Zamboni M. Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., Schneider S.M., Sieber C.C., Topinkova E., Vandewoude M., Visser M., Zamboni M., Writing Group for the European Working Group on Sarcopenia in Older P., the Extended Group for E. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet (London, England) 2019;393(10191):2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- Dorresteijn T.A., Rixt Zijlstra G.A., Van Eijs Y.J., Vlaeyen J.W., Kempen G.I. Older people's preferences regarding programme formats for managing concerns about falls. Age Ageing. 2012;41(4):474–481. doi: 10.1093/ageing/afs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essery R., Geraghty A.W., Kirby S., Yardley L. Predictors of adherence to home-based physical therapies: a systematic review. Disabil. Rehabil. 2017;39(6):519–534. doi: 10.3109/09638288.2016.1153160. [DOI] [PubMed] [Google Scholar]
- Farrance C., Tsofliou F., Clark C. Adherence to community based group exercise interventions for older people: a mixed-methods systematic review. Prev. Med. 2016;87:155–166. doi: 10.1016/j.ypmed.2016.02.037. [DOI] [PubMed] [Google Scholar]
- Gkekas N.K., Anagnostis P., Paraschou V., Stamiris D., Dellis S., Kenanidis E., Potoupnis M., Tsiridis E., Goulis D.G. The effect of vitamin D plus protein supplementation on sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Maturitas. 2021;145:56–63. doi: 10.1016/j.maturitas.2021.01.002. [DOI] [PubMed] [Google Scholar]
- Hale-Gallardo J., Kreider C.M., Castañeda G., LeBeau K., Varma D.S., Knecht C., Cowper Ripley D., Jia H. Meeting the needs of rural veterans: a qualitative evaluation of whole health coaches' expanded services and support during COVID-19. Int. J. Environ. Res. Public Health. 2022;19(20) doi: 10.3390/ijerph192013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrema A.L., Westerman M.J., van Dongen E.J.I., Kudla U., Veltkamp M. Combined protein-rich diet with resistance exercise intervention to counteract sarcopenia: a qualitative study on drivers and barriers of compliance. J. Aging Phys. Act. 2018;26(1):106–113. doi: 10.1123/japa.2017-0126. [DOI] [PubMed] [Google Scholar]
- Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M., Welch V. Cochrane handbook for systematic reviews of interventions version 6.2. 2021. https://training.cochrane.org/handbook/current
- Hoffmann T.C., Glasziou P.P., Boutron I., Milne R., Perera R., Moher D., Altman D.G., Barbour V., Macdonald H., Johnston M., Lamb S.E., Lamb S.E., Dixon-Woods M., McCulloch P., Wyatt J.C., Chan A.W., Michie S. [Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide] Gesundheitswesen. 2016;78(3):175–188. doi: 10.1055/s-0041-111066. [DOI] [PubMed] [Google Scholar]
- Hurst C., Robinson S.M., Witham M.D., Dodds R.M., Granic A., Buckland C., De Biase S., Finnegan S., Rochester L., Skelton D.A. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing. 2022;51(2) doi: 10.1093/ageing/afac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation WHO: At least 17 million people in the WHO European Region experienced long COVID in the first two years of the pandemic; millions may have to live with it for years to come. 2022. https://www.healthdata.org/news-release/who-least-17-million-people-who-european-region-experienced-long-covid-first-two-years Retrieved October 8, 2022 from.
- Izquierdo M., Merchant R., Morley J., Anker S., Aprahamian I., Arai H., Aubertin-Leheudre M., Bernabei R., Cadore E., Cesari M. International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J. Nutr. Health Aging. 2021;25(7):824–853. doi: 10.1007/s12603-021-1665-8. [DOI] [PubMed] [Google Scholar]
- Kirwan R., McCullough D., Butler T., Perez de Heredia F., Davies I.G., Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. Geroscience. 2020;42(6):1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kor P.P.K., Li M.L., Kwok D.K.S., Leung A.Y.M., Lai D.L.L., Liu J.Y.W. Evaluating the effectiveness of a 6-week hybrid mindfulness-based intervention in reducing the stress among caregivers of patients with dementia during COVID-19 pandemic: protocol of a randomized controlled trial. BMC Psychology. 2022;10(1):1–13. doi: 10.1186/s40359-022-00876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider C.M., Hale-Gallardo J., Kramer J.C., Mburu S., Slamka M.R., Findley K.E., Myers K.J., Romero S. Providers' shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.831762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.Y., Kwon K.S. Pharmacological interventions for treatment of sarcopenia: current status of drug development for sarcopenia. Ann. Geriatr. Med. Res. 2019;23(3):98–104. doi: 10.4235/agmr.19.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers-Ramos R.Q., Dondero K.R. Exercise and protein supplementation for prevention and treatment of sarcopenia. Curr. Geriatr. Rep. 2019;8(3):202–209. doi: 10.1007/s13670-019-00293-7. [DOI] [Google Scholar]
- Leidy H.J., Carnell N.S., Mattes R.D., Campbell W.W. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15(2):421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- Li Z., Cui M., Yu K., Zhang X., Li C., Nie X., Wang F. Effects of nutrition supplementation and physical exercise on muscle mass, muscle strength and fat mass among sarcopenic elderly: a randomized controlled trial. Appl. Physiol. Nutr. Metab. 2021;46(5):494–500. doi: 10.1139/apnm-2020-0643. [DOI] [PubMed] [Google Scholar]
- Lin C.C., Shih M.H., Chen C.D., Yeh S.L. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: an open-label, parallel-group study. Clin. Nutr. 2021;40(3):1323–1329. doi: 10.1016/j.clnu.2020.08.017. [DOI] [PubMed] [Google Scholar]
- Lin T.R., Huang X.Y., Hwu C.M. Exercise experiences of older adults with diabetes and sarcopenia: a phenomenological study. Clin. Nurs. Res. 2022;31(2):292–300. doi: 10.1177/10547738211039381. [DOI] [PubMed] [Google Scholar]
- Lu L., Mao L., Feng Y., Ainsworth B.E., Liu Y., Chen N. Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: a systematic review and meta-analysis. BMC Geriatr. 2021;21(1):708. doi: 10.1186/s12877-021-02642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafarina V., Uriz-Otano F., Iniesta R., Gil-Guerrero L. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J. Am. Med. Dir. Assoc. 2013;14(1):10–17. doi: 10.1016/j.jamda.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Mañas A., Gómez-Redondo P., Valenzuela P.L., Morales J.S., Lucía A., Ara I. Unsupervised home-based resistance training for community-dwelling older adults: a systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021;69 doi: 10.1016/j.arr.2021.101368. [DOI] [PubMed] [Google Scholar]
- Maruya K., Asakawa Y., Ishibashi H., Fujita H., Arai T., Yamaguchi H. Effect of a simple and adherent home exercise program on the physical function of community dwelling adults sixty years of age and older with pre-sarcopenia or sarcopenia. J. Phys. Ther. Sci. 2016;28(11):3183–3188. doi: 10.1589/jpts.28.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew A.J., Amog K., Phillips S., Parise G., McNicholas P.D., de Souza R.J., Thabane L., Raina P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56. doi: 10.1093/ageing/afy106. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Yoshida D., Honda T., Hata J., Shibata M., Hirakawa Y., Furuta Y., Kishimoto H., Ohara T., Kitazono T., Nakashima Y., Ninomiya T. Prevalence and mortality of sarcopenia in a community-dwelling older Japanese population: the Hisayama study. J. Epidemiol. 2021;31(5):320–327. doi: 10.2188/jea.JE20190289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasimi N., Sohrabi Z., Dabbaghmanesh M.H., Eskandari M.H., Bedeltavana A., Famouri M., Talezadeh P. A novel fortified dairy product and sarcopenia measures in sarcopenic older adults: a double-blind randomized controlled trial. J. Am. Med. Dir. Assoc. 2021;22(4):809–815. doi: 10.1016/j.jamda.2020.08.035. [DOI] [PubMed] [Google Scholar]
- Pandya S.P. Yoga education program for older women diagnosed with sarcopenia: a multicity 10-year follow-up experiment [Article] J. Women Aging. 2019;31(5):446–469. doi: 10.1080/08952841.2018.1510245. [DOI] [PubMed] [Google Scholar]
- Papadopoulou S.K., Tsintavis P., Potsaki P., Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J. Nutr. Health Aging. 2020;24(1):83–90. doi: 10.1007/s12603-019-1267-x. [DOI] [PubMed] [Google Scholar]
- Peter R.S., Nieters A., Kräusslich H.G., Brockmann S.O., Göpel S., Kindle G., Merle U., Steinacker J.M., Rothenbacher D., Kern W.V. Post-acute sequelae of covid-19 six to 12 months after infection: population based study. BMJ. 2022;379 doi: 10.1136/bmj-2022-071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro H.A., Cerceau V.R., Pereira L.C., Funghetto S.S., Menezes R.L.D. Nutritional intervention and functional exercises improve depression, loneliness and quality of life in elderly women with sarcopenia: a randomized clinical trial. Fisioterapia Mov. 2020;33 doi: 10.1590/1980-5918.033.ao32. [DOI] [Google Scholar]
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses The PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Schuch F.B., Bulzing R.A., Meyer J., López-Sánchez G.F., Grabovac I., Willeit P., Vancampfort D., Caperchione C.M., Sadarangani K.P., Werneck A.O., Ward P.B., Tully M., Smith L. Moderate to vigorous physical activity and sedentary behavior changes in self-isolating adults during the COVID-19 pandemic in Brazil: a cross-sectional survey exploring correlates. Sport Sci. Health. 2022;18(1):155–163. doi: 10.1007/s11332-021-00788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen E.I., Eyigor S., Dikici Yagli M., Ozcete Z.A., Aydin T., Kesiktas F.N., Aydin F.Y., Vural M., Sahin N., Karan A. Effect of home-based exercise program on physical function and balance in older adults with sarcopenia: a multicenter randomized controlled study. J. Aging Phys. Act. 2021;29(6):1010–1017. doi: 10.1123/japa.2020-0348. [DOI] [PubMed] [Google Scholar]
- Sepúlveda-Loyola W., Rodríguez-Sánchez I., Pérez-Rodríguez P., Ganz F., Torralba R., Oliveira D.V., Rodríguez-Mañas L. Impact of social isolation due to COVID-19 on health in older people: mental and physical effects and recommendations. J. Nutr. Health Aging. 2020;24(9):938–947. doi: 10.1007/s12603-020-1500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernán M.A., Hopewell S., Hróbjartsson A., Junqueira D.R., Jüni P., Kirkham J.J., Lasserson T., Li T., Higgins J.P.T. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.) 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- Thiebaud R.S., Funk M.D., Abe T. Home-based resistance training for older adults: a systematic review. Geriatr Gerontol Int. 2014;14(4):750–757. doi: 10.1111/ggi.12326. [DOI] [PubMed] [Google Scholar]
- Tsekoura M., Billis E., Tsepis E., Dimitriadis Z., Matzaroglou C., Tyllianakis M., Panagiotopoulos E., Gliatis J. The effects of group and home-based exercise programs in elderly with sarcopenia: a randomized controlled trial. J. Clin. Med. 2018;7(12) doi: 10.3390/jcm7120480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elswyk M.E., Teo L., Lau C.S., Shanahan C.J. Dietary patterns and the risk of sarcopenia: a systematic review and meta-analysis. Curr. Dev. Nutr. 2022;6(5) doi: 10.1093/cdn/nzac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Ageing and health. 2021. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health Retrieved 11 Octomber 2021 from.
- Wu P.Y., Huang K.S., Chen K.M., Chou C.P., Tu Y.K. Exercise, nutrition, and combined exercise and nutrition in older adults with sarcopenia: a systematic review and network meta-analysis. Maturitas. 2021;145:38–48. doi: 10.1016/j.maturitas.2020.12.009. [DOI] [PubMed] [Google Scholar]
- Yang M., Liu Y., Zuo Y., Tang H. Sarcopenia for predicting falls and hospitalization in community-dwelling older adults: EWGSOP versus EWGSOP2. Sci. Rep. 2019;9(1):17636. doi: 10.1038/s41598-019-53522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y., Wakabayashi H., Yamada M., Kim H., Harada A., Arai H. Interventions for treating sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J. Am. Med. Dir. Assoc. 2017;18(6) doi: 10.1016/j.jamda.2017.03.019. 553.e551-553.e516. [DOI] [PubMed] [Google Scholar]
- Zhu Y.Q., Peng N., Zhou M., Liu P.P., Qi X.L., Wang N., Wang G., Wu Z.P. Tai chi and whole-body vibrating therapy in sarcopenic men in advanced old age: a clinical randomized controlled trial. Eur. J. Ageing. 2019;16(3):273–282. doi: 10.1007/s10433-019-00498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.