Abstract
Despite our differences, there is much about the natural visual world that most observers perceive in common. Across adults, approximately 30% of the brain is activated in a consistent fashion while viewing naturalistic input. At what stage of development is this consistency of neural profile across individuals present? Here, we focused specifically on whether this mature profile is present in adolescence, a key developmental period that bridges childhood and adulthood, and in which new cognitive and social challenges are at play. We acquired fMRI data evoked by a movie shown twice to younger (9–14 years old) and older adolescents (15–19 years old) and to adults, and conducted three key analyses. First, we characterized the consistency of the neural response within individuals (across separate runs of the movie), then within individuals of the same age group, and, last, between age groups. The neural consistency within individuals was similar across age groups with reliable activation in largely overlapping but slightly different cortical regions. In contrast, somewhat differing regions exhibited higher within-age correlations in both groups of adolescents than in the adults. Last, across the whole cortex, we identified regions evincing different patterns of maturation across age. Together, these findings provide a fine-grained characterization of functional neural development in adolescence and uncover signatures of widespread change in cortical coherence that supports the emerging mature stereotypical responses to naturalistic stimuli. These results also offer a more nuanced account of development that obeys neither a rigid linear progression nor a large qualitative change over time.
INTRODUCTION
Despite vast individual differences, there is much about the visual world that humans perceive in common. For example, when viewing complex visual input such as a movie, similar neural responses are observed in approximately 30% of cortex of young adults, reflecting a stereotypical response to the input (Hasson, Malach, & Heeger, 2010; Hasson, Nir, Levy, Fuhrmann, & Malach, 2004). Importantly, this observed consistency across individuals is not a necessary condition of being a young adult, as individuals diagnosed with autism spectrum disorder do not exhibit this pattern of correlated responses among each other or with typically developing young adults (Hasson et al., 2009).
In this article, we examine the developmental sequence of these stereotyped neural responses by evaluating the patterns of whole-brain “neural coherence” in response to naturalistic, socially complex visual stimuli over the course of adolescence and early adulthood. As in much of the existing work investigating age-related differences in neural coherence (see below), we used fMRI to measure time-locked BOLD responses to the stimuli and adopted the intersubject correlation (inter-SC) approach (e.g., Nastase, Gazzola, Hasson, & Keysers, 2019; Hasson et al., 2004). Briefly, the inter-SC approach is useful for identifying brain regions that “process a stimulus in a consistent way across participants.” This analytic approach is data driven, does not require a model of the hemodynamic response function, and is relatively insensitive to idiosyncratic responses from individual participants (which are either induced by the stimulus or are spontaneous and unrelated to the stimulus; see Nastase et al., 2019). Therefore, regions in which the inter-SC is high (approaching 1) are interpreted to be involved in encoding and processing the stimulus in a stereotyped way across individuals. In contrast, regions with low inter-SC may reflect either idiosyncratic processing across individuals or little involvement in encoding/processing the stimulus. Therefore, age-related differences in the magnitude of inter-SC within regions in response to a complex stimulus can be the result of multiple underlying mechanisms including a change in the regions that participants use to encode/process stimuli with age; decreasing idiosyncratic processing across individuals within the set of regions that encode/process the stimuli with age; or a reduction in spontaneous, unrelated activity and noise among the regions that encode/process the stimulus. Any (or all) of these changes could result in the increase or decrease in the inter-SC of a region as a function of age.
Importantly, because the inter-SC approach effectively filters out subject-specific signals, a different approach is used to assess the relative contribution of subject-specific variables to changes in the development of consistent neural responses. To measure the contribution of idiosyncratic responses to age-related changes in neural coherence, we also computed “intrasubject correlations” (intra-SCs) for each participant (Golland et al., 2007). The intra-SC provides an estimate of the reliability of subject-specific responses over time. Regions with high intra-SC are reliably involved in encoding/processing a stimulus for individual participants. Critically, regions that are reliably involved in encoding information at the individual level may or may not be the same regions consistently encoding information across participants (as indicated by high inter-SC). It is essential to employ both approaches (inter-SC and intra-SC) to evaluate the full set of mechanisms that may be contributing to changes in neural coherence with age.
The Development of Neural Coherence
There are relatively few studies that have investigated age-related changes in neural coherence. The studies that do exist have largely used the inter-SC approach and specifically compare voxelwise time courses from individual young participants versus those from older (i.e., adult) participants. For example, in one study, children (aged 4–10 years) and young adults (aged 18–25 years) watched Sesame Street video clips during fMRI scanning (Cantlon & Li, 2013). The researchers computed inter-SCs within the child and adult groups separately (each child vs. all other children; each adult vs. all other adults) and between the child and adult groups (each child vs. all other adults). A contrast between the inter-SCs within age groups revealed that the children exhibited reduced neural coherence in many cortical regions compared to adults, including basic sensory, motor, and association cortices, indicating a difference in extent to which the two groups use these regions to encode/process the stimuli or evince heterogeneity between participants in their responses. At the same time, there were other regions where children showed enhanced neural coherence relative to adults (e.g., superior temporal cortex), indicating a more prominent role of these regions for encoding/processing information among the children relative to adults. These findings suggest that either the cortical regions that encode/process dynamic, complex visual stimuli change or the variability between responses within individuals are reduced as a function of age.
More recently, another study compared neural coherence in young children (aged 4 and 6 years) and adults while they viewed excerpts from the movie Toy Story (Moraczewski, Chen, & Redcay, 2018). The researchers used the inter-SC approach to estimate variation in within-age-group heterogeneity in neural coherence and between-age-group similarity to adult neural coherence (each child to adult group in each voxel). When they contrasted the within-age-group inter-SCs, they found stronger inter-SCs in the bilateral TPJ, middle and superior temporal, extrastriate, and superior parietal regions in the adults compared to both groups of children. Again, the adult–child difference could indicate that one of two mechanisms are at play; there is an age-related change in the regions implicated in encoding these complex visual stimuli, and/or there are decreasing heterogeneity/individual differences in the neural response profiles with age.
In a similar study, children (aged 3–12 years) and adults (aged 18–39 years) watched a short, animated film designed to elicit activation in the Theory of Mind (ToM) and pain networks (Richardson, 2019). In this study, the researchers largely focused on comparing age differences in the magnitude of inter-SCs within age groups between ROIs. For each age group, the inter-SC analyses were conducted by averaging the time courses of voxels within each ROI and then computing the correlations between ROI time courses for each participant, which generated correlations among ROIs within and between networks. They also computed these metrics between age groups (each child to an averaged adult time course) in each network. For both age groups, the ToM and pain networks exhibited higher within- than between-network inter-ROI correlations across participants, which increased as a function of age. In addition, the inter-ROI correlations across networks decreased as a function of age. Given the consistency of findings across both functional networks, the specificity of the mechanism driving the age-related changes in neural coherence among the ROIs is unclear because it could be related to an increasing role that these regions play in processing ToM and pain information and/or a reduction in the heterogeneity of the neural signals between individuals.
To our knowledge, only one study specifically investigated the relative magnitude of individual subject factors that contribute to age-related differences in neural coherence using the intra-SC approach. Richardson and Saxe (2020) conducted a secondary analysis of their original data (Richardson, Lisandrelli, Riobueno-Naylor, & Saxe, 2018) that included the analysis of a repeated presentation of the same movie in the scanner. Therefore, they were able to evaluate the reliability of neural coherence for individual participants among 3- to 4-year-old and 6- to 7-year-old children. The mean intra-SCs were quite low (ranging from r = .11–.25) and did not improve with age in this early to middle childhood age range. These results suggest that age-related changes in subject-level factors may play a large role in improving neural coherence as measured by inter-SC.
As evident, the existing literature is unclear about the process by which younger individuals, who appear to have less stereotypical neural responses, develop into adults who exhibit more coherent neural responses to the natural world. Here, we suggest that highly variable and unreliable neural responses among younger individuals contribute to age-related differences in neural coherence both within and between age groups.
Between-Subject Variability as a Mechanism for Age-Related Differences across Domains
There already exists evidence favoring the decreasing variability between individuals across multiple behavioral domains. For example, developmental changes in basic visuoperception could contribute to age-related differences in neural coherence, including changes in the perception of real-world objects (Freud, Plaut, & Behrmann, 2019; Freud & Behrmann, 2017) and the neural basis of size constancy of those objects (Nishimura, Scherf, Zachariou, Tarr, & Behrmann, 2015). In addition, working memory (Fry & Hale, 2000) and its neural basis (Scherf, Sweeney, & Luna, 2006) undergo age-related changes, which could influence the information processed as children and adolescents observe and interpret the world. These impressive age-related changes across multiple domains likely contribute to individual variability in the way information about the natural world is processed, which could result in relatively lower levels of neural coherence (i.e., inter-SC) among younger individuals. Another subject-level factor that could influence individual differences in neural coherence is the relative timing and tempo of puberty, which is related to structural (Herting & Sowell, 2017) and functional (Spielberg, Olino, Forbes, & Dahl, 2014; Forbes, Phillips, Silk, Ryan, & Dahl, 2011) brain development. Adolescence begins with the onset of pubertal development (Dahl, 2004), which triggers a cascade of neuroendocrine changes that continues for a period of 10 years (Susman et al., 2010), starting as early as 6 years old (Oerter, Uriarte, Rose, Barnes, & Cutler, 1990). As such, puberty might also influence developing neural coherence particularly in circuitry related to social information processing, and this may be especially evident during adolescence. Therefore, we propose that there may be widespread, whole-brain changes in the way neural activation develops into coherent responses as a function of age.
To evaluate this possibility, we too used the movie viewing approach that simulated the observation of naturalistic, complex social interactions and evaluated whole-brain changes in both inter-SC and intra-SC in younger and older adolescents and young adults. We evaluated three predictions of functional brain development. First, there is a long-standing prediction that the process of myelination follows a posterior-to-anterior gradient developmentally (Yakovlev & Lecours, 1967), and this might influence neural coherence to follow a similar posterior/anterior developmental gradient. A second prediction is that regions that function as major hubs (i.e., those that substantially impact the performance and efficiency of multiple regions) in adult neural networks, like the posterior cingulate cortex and the insula (see Menon, 2013), may become increasingly coherent in their functional activation as a function of age. Finally, we explore whether, within individual regions that evince age-related changes, the changes are linearly increasing with age, as might be predicted by a strict, chronological perspective of brain development.
Current Study
We evaluated the emergence of neural coherence across the full extent of adolescence as a developmental period. To do so, we investigated patterns of stereotypic neural responses in two groups of adolescents and compared them to those of young adults with the whole-brain neural responses derived from the BOLD signal while participants watch a socially complex movie. In this way, we aimed to approximate a more naturalistic viewing environment to the extent possible during fMRI scanning (see Cantlon, 2020).
The younger group of adolescents (aged 9–14 years) was likely to be actively undergoing pubertal development, based on age norms in the United States (Susman et al., 2010), as well as behavioral improvements in multiple cognitive and social domains relevant to interpreting the social interactions in the movie. In contrast, the older group of adolescents (aged 15–19 years) was likely to be approaching sexual maturity and adult levels of performance on many cognitive, social, and affective abilities relevant to understanding the movie. Participants were scanned using fMRI as they watched two iterations of the same movie clip of children and young adolescents engaging in complex peer interactions. Importantly, we compared patterns of within- and between-subject neural coherence to address three questions.
First, is there reliability in neural coherence within an individual (intra-SC) across repeated presentations of the movie, and does this change with age? Few studies assess test–retest reliability of the neural signal over development, and most existing studies assess reliability over very long intervals (e.g., months to years; Herting, Gautam, Chen, Mezher, & Vetter, 2018; Hasson et al., 2010).
Second, are there differences in neural coherence between individuals within each age group (inter-SC within group)? This allowed us to evaluate the variability within an age group and compare the within-group variability between groups. We predicted the least consistency or replicability of activation profile in the younger adolescents and also expected that their neural coherence might be evident in different regions than those documented for older adolescents and adults, given the possibility that they might employ different neural systems to interpret and process information (see Cantlon & Li, 2013).
Finally, how much does the between-age group profile of neural coherence vary (inter-SC across groups)? Specifically, we evaluated how replicable the coherence of the two adolescent groups was compared to each other and to the adult group. Note that, following recent guidelines (Elliott et al., 2020), we adopt the term “reliable” for the test–retest intra-SC approach and the term “replicable” or consistent for the inter-SC approach. The term “neural coherence” encompasses all of the above terms and is thus an umbrella term. The exploration of neural coherence across the whole brain provides a foundation for beginning to understand the kind of variability and the cortical locations where neural coherence emerges in the cortex from young adolescence to young adulthood.
METHODS
Participants
Participants included nine young adolescents (age range = 9–14 years; M = 11.0 years, SD = 1.7; seven male adolescents), nine older adolescents (age range = 15–19 years; M = 16.5 years, SD = 1.4; three male adolescents), and 10 adults (age range = 20–35 years; M = 25.3 years, SD = 4.4; five men). An additional three young adolescents, three older adolescents, and two adults were excluded from the analyses because of excessive head motion (>2.8 mm), technical problems during the scan (e.g., image distortion, no sound), or request to stop the scan before completion. All participants were typically developing, with no history of neurological or psychiatric disorders in themselves or in their first-degree relatives; had normal or corrected vision; and were right-handed, native English speakers. Before participating in the study, participants and/or their guardians provided written consent. Adolescents provided written assent. The protocol was approved by the University of Pittsburgh and Carnegie Mellon University Internal Review Boards, and participants were compensated for their participation.
Movie Task
The movie stimuli were created by extracting two clips from a G-rated movie, Escape to Witch Mountain (1975, Walt Disney Productions). We specifically chose a movie with a social plot and relatable characters not only to engage the participants but also to minimize group differences related to understanding the plot (see also Alexander et al., 2017). We selected an older movie (i.e., produced before any of the participants was born) that was likely to be novel to most participants to minimize a potential confound between age and familiarity that might impact patterns of neural activation.
Procedure
Immediately before the scanning session, all participants were trained in a mock scanner for approximately 20 min. Each participant practiced lying still while watching a movie inside the mock scanner with simulated scanner noises. Participants were instructed to use relaxation breathing and mental imagery (e.g., lying in own bed while watching a movie) and received feedback when they moved. This simulation procedure acclimates participants to the scanner environment, minimizes motion artifacts, and reduces anxiety in both children and adults (Scherf, Thomas, Doyle, & Behrmann, 2014). During this simulation session, participants watched the first clip with a duration of 10 min of the stimulus movie, Escape to Witch Mountain, to promote an understanding of the plot before observing the experimental clip. The second clip, the next 11.5 min of this movie, was selected as the stimulus for the experimental scanning paradigm.
Participants were scanned at the Brain Imaging Research Center in Pittsburgh on a Siemens 3-T Allegra Scanner, equipped with a quadrature birdcage head coil. During the scanning session, the movie stimuli were displayed using QuickTime on a rear-projection screen located inside the MR scanner. Participants wore MRI-compatible headphones and passively viewed stimuli in three functional runs, including two identical iterations (Run 1 and Run 2) of the movie task (see Figure 1) and a localizer scan that was not analyzed for this study. To ensure that the participants were watching the movie throughout the scan, we monitored their eye movements using an infrared video camera equipped with custom-built MRI telephoto lens (Model504LRO, Applied Sciences Laboratories). The total scanning time was approximately 45 min. After the scan, outside the magnet, participants answered questions about the characters and plot of the movie to verify that they understood the details of the narrative (e.g., What is the name of the young boy? Answer: Tony). All participants exhibited good comprehension of the characters and plot, and therefore, we included the data from all participants in the analysis.
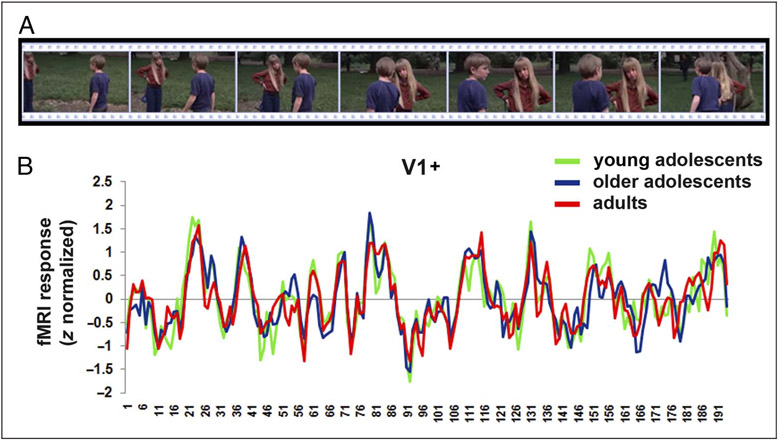
Figure 1.
(A) Excerpted sequence from the Escape to Witch Mountain movie, which all participants viewed (and heard) in the magnet. (B) The mean z-normalized fMRI response over the course of the movie duration extracted from area V1+ for all three groups of participants. The x-axis shows numbers representing TRs.
Functional EPI images were acquired in 35 AC–PC aligned slices that covered most of the brain (repetition time = 3000 msec, echo time = 35 msec, 64 × 64, 3-mm slice thickness, 3.2 × 3.2 mm in-plane resolution). High-resolution anatomical images were also acquired during the same scanning session using a 3-D magnetization prepared rapid gradient echo pulse sequence with 192 T1-weighted, straight sagittal slices (1-mm thickness).
MRI Processing and Data Analysis
Preprocessing
MRI processing was performed using BrainVoyager QX (Brain Innovation) together with in-house software written in MATLAB (The MathWorks). Preprocessing of the functional data included 3-D motion correction, filtering out low frequencies (e.g., slow drift) up to 10 cycles per experiment, linear trend removal, high-pass filtering (cutoff: 0.01 Hz), and spatial smoothing with a Gaussian filter (6-mm FWHM value). The first 25 and last 8 repetition times in each run were removed to eliminate preprocessing artifacts and to allow the hemodynamic responses to reach a steady state. The time-series data for each run of the task for each participant were spatially normalized into Talairach space, an approach validated in previous developmental studies (Burgund et al., 2002; and see Kamps, Hendrix, Brennan, & Dilks, 2020, demonstrating that registration between adults and children can be accomplished), and projected onto a reconstructed cortical surface from the high-resolution 3-D anatomical images. All analyses were computed in volume space (by voxel), although the figures display activation on the brain surface for illustrative purposes.
Only participants who exhibited motion of less than one voxel (3 mm) in all six directions, three translation parameters and three rotation parameters, for both runs of the movie were included in the fMRI analyses. In addition, we evaluated whether the mean motion in each of the six directions varied as a function of age group. To evaluate motion difference across the three groups, we computed mean relative framewise displacements (FDs) for each individual for each run of the movie task and compared the values in a repeated-measure analysis of variance. There was a significant effect of Run with higher FD for Run 2 (.021) than for Run 1 (.015), F(1, 2) = 10.6, p < .005, . There was also a main effect of Group, F(2, 26) = 3.9, p = .03, , but no interaction of Run × Group, F(2,26) = 1.2, p > .3, . An ANOVA with only two of the three groups, with p < .01 (for multiple test corrections), revealed no difference in mean FD across the two adolescent groups and no significant difference between the older adolescents and the adults but a significant difference between the younger adolescents and adults, F(1, 18) = 9.2, p = .008, . Given this last difference, the mean FD value for each participant (for Run 1) was included as a covariate in all analyses of group differences.
Sensory ROIs
To assess coherence in regions that reveal a high degree of neural coherence among adults and to enable us to benchmark our data against existing data (Hasson et al., 2004), we identified early auditory cortex (A1+) and early visual cortex (V1+). The early visual cortex (V1+) was defined anatomically along the lingual gyrus (Brodmann’s area 17/18). The early auditory cortex cluster (A1+) was defined as the set of voxels that correlated most highly with the stimulus audio envelope. To compute the correlation between the average BOLD signals and the audio envelope, we bandpass filtered the audio signal from the movie between 4 and 4000 Hz, extracted the envelope of the signal using a Hilbert transform, and then down-sampled the envelope to the sampling rate of the BOLD signal using an antialiasing low-pass finite impulse response filter.
Neural Coherence within Individuals across Movies (Test–Retest Reliability or Intra-SC)
Following our previous work (Hasson et al., 2009), intra-SC was computed in each voxel over the entire cortex for each participant as follows:
where r1(t) and r2(t) are the response time courses of a voxel in each of the presentations of the movie (change of BOLD signal with respect to voxel mean in a trial).
Neural Coherence within Each Age Group (Inter-SC within Group)
The inter-SCs within each age group were constructed on a voxelwise basis by comparing the time course of the BOLD signal for each voxel for a single individual with the average time course of the other participants in the same developmental group (see Hasson et al., 2004, 2009, 2010). This analysis was executed in two stages: first, using individual-participant level and then group-level analyses (for a review, see Nastase et al., 2019). First, inter-SCs were computed for each individual in each voxel using a leave-one-out approach. Briefly, the Pearson product–moment correlation was computed between each participant’s raw voxel time course and an averaged voxel time course, which was computed by averaging the voxel time courses of all the other individuals in the same age group, excluding the participant for whom the estimate was being computed. This generated a map of inter-SCs in each voxel for each participant separately in each age group.
As in our prior work, these inter-SC within-group maps were assessed for statistical significance using a phase-randomization procedure (see Lerner, Honey, Silbert, & Hasson, 2011). This is a nonparametric permutation approach. We applied a fast Fourier transformation to the time series, randomized the phase of each Fourier component, and inverted the Fourier transformation. This preserves the power spectrum in the data while making the expected correlation value between any pair of phase-randomized time courses to be 0. This phase randomization is performed at each iteration (~175,000) of the resampling procedure, before computing the inter-SC. After each iteration of the permutation test, the maximum correlation value across all voxels is aggregated into a null distribution of maximal correlation values (e.g., observed correlation values in the top 5% of the null distribution), which controls the family-wise error rate (FWER; for a review, see Nastase et al., 2019).
Group Differences in Correlation Maps (Intergroup Correlation)
Group differences were evaluated using multiple strategies. First, to compare the extent of coherence across groups, we computed the percentage of brain surface that exhibited a reliable response (i.e., number of voxels exhibiting test–retest reliable responses divided by the total number of voxels) for each individual. To evaluate effects of age, we submitted these scores to a one-way ANOVA with Group as the fixed factor. We also investigated the effects of age in a more continuous way by regressing age in years on the number of voxels exhibiting a test–retest reliable response.
Then, the inter-SC maps from all three groups were compared in a single voxelwise GLM with Age as a fixed factor and mean relative FD from Run 1 as a covariate. To assess the significance of a nonconstant model taking into account multiple comparisons, we performed multiple simulations of the same procedure with label permutations storing for each simulation critical p value across all voxels as noted above. More specifically, here, the age label for residuals between voxel correlations and GLM prediction for intact time courses were randomly permuted, and a new GLM estimation was obtained. p Values obtained from the permuted estimations from the whole brain were sorted, and the 5th percentile was recorded as an estimation of significance boundary. Using around 175,000 rounds of whole-brain permutations, the distribution of significance boundaries was obtained. The critical p value was selected as the 5th percentile of significance boundary distribution. By choosing the 5th percentile in null distribution for single randomized simulation, we are controlling per-family error rate (see Tukey, 1953) and, therefore, also FWER (see discussion over per-family error rate vs. FWER in Frane, 2015; Keselman, 2015).
Last, to illustrate the pattern of age-related change within each cluster, we computed the average inter-SC for each participant across the set of contiguous voxels from each cluster identified from the group comparison map and then, for descriptive purposes, plotted the values against age with the best fit function (linear, logarithmic, inverse, or quadratic).
RESULTS
The results are presented in three sections, corresponding to the three questions motivating this work. Figure 1A shows a sequence of images from the movie and the average time course of BOLD activation for each group in the primary visual area (V1+).
Are There Age-Related Changes in Intra-SC of Neural Coherence?
First, we assessed the degree of reliability in neural signals within each individual using intra-SC across the two presentations of the same movie. Figure 2 shows the voxels that exhibit reliable neural coherence within individuals among adults (Figure 2A), older adolescents (Figure 2B), and younger adolescents (Figure 2C). Visual inspection of the maps reveals that all three groups exhibited reliability within the sensory regions, A1+ and V1+. There was also reliability in all three groups in bilateral ventral temporal cortex, posterior and medial parietal cortex, full length of the middle temporal gyrus (MTG; from the TPJ terminating in close proximity to the temporal pole), and superior temporal gyrus (STG).
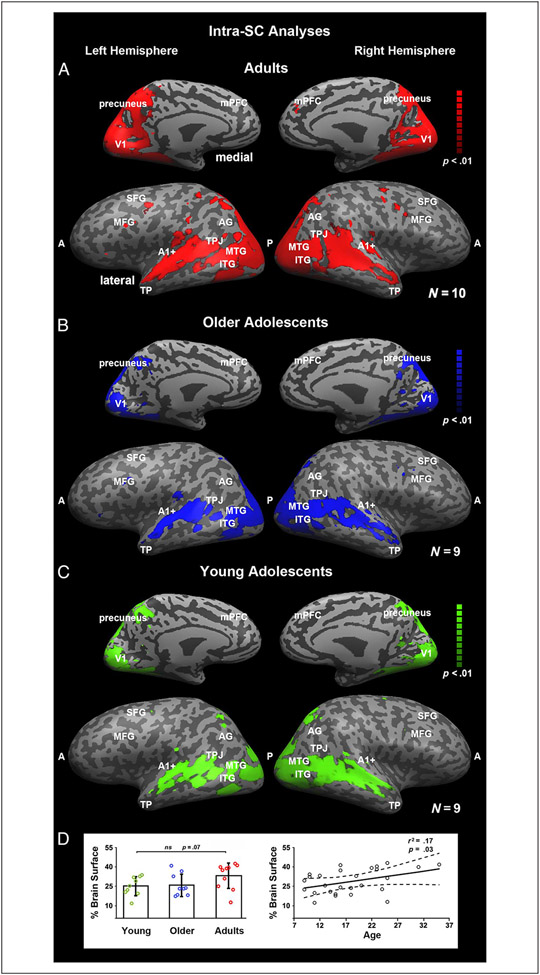
Figure 2.
Reliability of responses within participants. These maps illustrate the voxels that exhibit reliable responses across the two presentations of the movie stimulus within individuals as a function of group. The analyses were conducted using intra-SC on a voxelwise basis, and significance was assessed using multiple comparisons and a corrected phase randomization procedure (see Group Differences in Correlation Maps). “A” and “P” refer to the anterior and posterior portions of the brain, respectively, and many cortical landmarks are labeled for convenience of viewing. Adults (A), older adolescents (B), and younger adolescents (C) all exhibit reliability in responses bilaterally throughout the ventral visual pathway and full extent of the MTG and STG on the lateral surface and medially in the precuneus. (D) The mean percentage of brain surface exhibiting reliable responses (i.e., number of voxels exhibiting reliable responses divided by the total number of voxels) with 95% confidence interval for each group and proximity thresholding, and the regression result showing the age-related increase, with each individual as a function of age.
Consistent with previous findings in adults (Hasson et al., 2004), the adults (M = 32.1%, SD = 10.1) showed considerable overlap of signal across the two runs of the movie. This was also true for the older (M = 26.1%, SD = 8.6) and younger (M = 25.2%, SD = 7.3) adolescents (see Figure 2D for average brain surface per group). To examine whether the percentage of total brain surface exhibiting reliable coherence changes with age, we conducted a one-way ANOVA on the data from the three age groups. There was no significant main effect of Group, F(2, 26) = 2.3, p = .12 (Figure 2D, left). However, because age is a continuous variable, we then evaluated the change in the dependent measure over age using the entire distribution. There was a significant effect of Age, F(1, 26) = 5.3, p = .03, r2 = .17 (Figure 2D, right), reflecting an age-related increase in the amount of cortex exhibiting test–retest reliably in the coherence of neural responses. It should be noted, however, that this result is not robust statistically. If we remove the two points at the right end of the plot, the effect of Age is no longer significant, F(1, 24) = 1.5, p = .2, r2 = .06.
Are There Age-Related Changes in Neural Coherence across Individuals within Each Age Group?
We then evaluated the neural coherence of the patterns of activation across individuals within each age group elicited during the first viewing of the movie. In so doing, we determined how much variability (or consistency) exists among the individuals within each age group and whether that variability differs across the age groups. As the resulting voxelwise coherence pattern in Run 1 incorporated all the voxels obtained in the coherence pattern in Run 2, we elected to focus specifically on the Run 1 data. It is also possible that the change from Run 1 to Run 2 might differ across age, perhaps as a differential amount of adaptation or attention ensues, providing further support for the analysis of the Run 1 data exclusively.
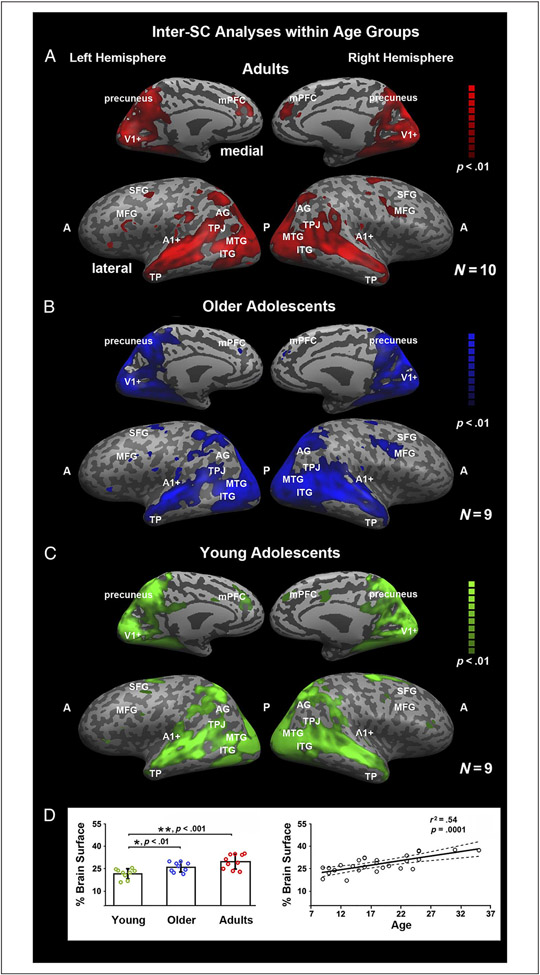
Figure 3 shows the voxelwise inter-SC maps of the whole cortex for adults (Figure 3A) and for older (Figure 3B) and younger (Figure 3C) adolescents. Among all three groups, as evident from visual inspection of the maps, there were consistent neural responses bilaterally in the visual and auditory cortices (see V1+ and A1+), inferior occipital gyrus, precuneus, inferior parietal lobule (IPL), TPJ, angular gyrus (AG), and supramarginal gyrus; throughout the length of the MTG and STG; and in the medial pFC (mPFC). As with the percentage of test–retest voxels, we computed the mean percentage of voxels that were consistently correlated between individuals within each age group (Figure 3D). We submitted these scores to a one-way ANOVA with Group as the fixed factor. There was a significant main effect of Group, F(2, 26) = 9.9, p < .001. Bonferroni-corrected post hoc tests (p < .01) revealed only one significant finding: The young adolescents (M = 21.4%, SD = 3.4) exhibited less consistency than the adults (M = 28.9%, SD = 4.8) who had a similar amount of voxel consistency as the older adolescents (M = 25.8%, SD = 3.3; see Figure 3D). We also investigated the relationship between age and percent consistency of cortex using a linear regression with age as the predictor. There was a significant effect of Age, F(1, 26) = 30.4, p < .0001, r2 = .54 (see Figure 3D). This indicates that consistency in neural coherence increases as a function of age from young adolescence to adulthood. To parallel the intra-SC analysis, we also removed the two points that fall to the right of the plot as they may be the source of the linear effect. The significant effect of Age is still present although slightly weaker in manifestation, F(1, 24) = 4.4, p < .05, r2 = .15.
Figure 3.
Coherence of neural responses within each age group. These maps illustrate the voxels that exhibit consistency in response profile across individuals within a group in response to viewing the first presentation of the movie stimulus. “A” and “P” refer to the anterior and posterior portions of the brain, respectively, and many cortical landmarks are labeled for convenience of viewing. The analyses were conducted using inter-SC on a voxelwise basis, and significance was assessed by using multiple comparisons and a corrected phase randomization procedure (see Neural Coherence within Each Age Group and Group Differences in Correlation Maps). Adults (A), older adolescents (B), and younger adolescents (C) all exhibit coherence in responses bilaterally throughout the ventral visual pathway, posterior parietal cortex, and full extent of the MTG and STG on the lateral surface as well as medially in the precuneus and posterior cingulate gyrus. The adults and young adolescents also exhibit coherence in a dorsal medial prefrontal region. (D) The mean percentage of brain surface exhibiting coherent responses (i.e., number of voxels showing coherent responses divided by the total number of voxels) with 95% confidence interval for each group and each individual as a function of age. Young adolescents exhibited less coherent response across the cortex than adults, and there was a linear trend across the whole age range in increasing the amount of cortical tissue that exhibited neural coherence.
Are There Age-Related Changes in Coherence of Neural Responses between Age Groups?
To explore differences in the consistency of neural responses between age groups, we conducted a voxelwise GLM with Age group as the fixed factor and FD as a covariate on the z-transformed inter-SC maps. Figure 4A illustrates the significant clusters of voxels exhibiting a main effect of age group (p < .05, permutation test, bounded by 5% false positive [see also Table 1]). We computed the mean inter-SC from each cluster for each participant, shown as a single circle in Figure 4B. Importantly, these data are not independent from the voxel selection process—we chose clusters based on age group effects and then examined the nature of the age effect. Therefore, we do not conduct statistical analyses of these data and only graph them to illustrate the nature or pattern of the relationship between age and change in inter-SCs and not to represent the magnitude of the effect size. To capture best fit function over age, we tested four commonly used curve fits, namely, linear, logarithmic, quadratic, and inverse, and then, where possible, reported the curve fit with the highest r2 and p value of the coefficient. Again, these fits should be treated with caution and considered descriptive, or qualitative, rather than providing a quantitative estimate of the age-related effect.
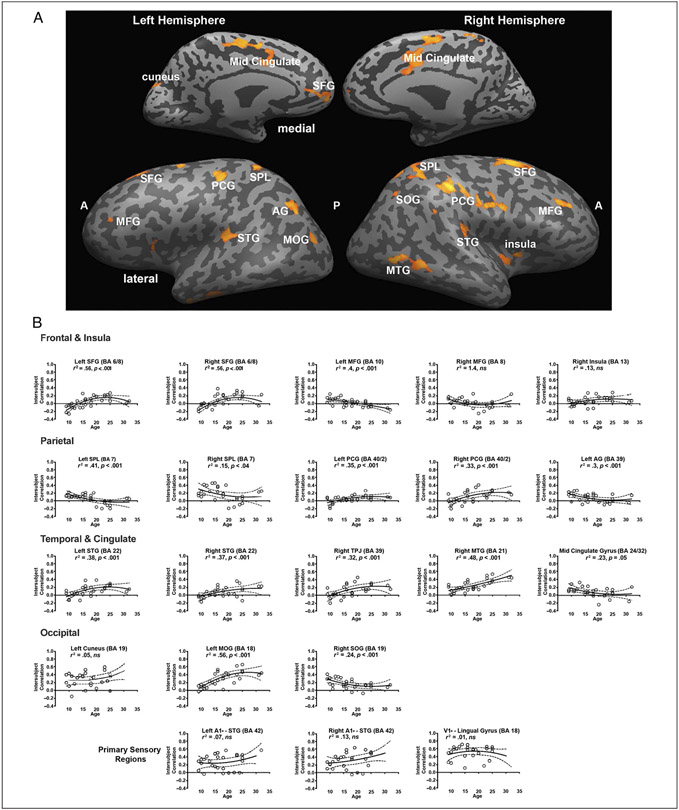
Figure 4.
Comparing neural coherence between groups. Group differences in neural coherence, or the consistency in response profile across individuals within a group in response to viewing the first presentation of the movie stimulus in A. “A” and “P” refer to the anterior and posterior portions of the brain, respectively, and many cortical landmarks are labeled for convenience of viewing. This analysis was conducted by submitting the z-transformed inter-SC maps to a GLM with Subjects as a random factor, Group as a fixed factor, and framewise displacement as a covariate. The map was corrected using a permutation-based method (see Group Differences in Correlation Maps). The regions identified as showing an effect of age are presented in B. To illustrate the direction of the age-related effect in each region, we averaged the inter-SCs across the voxels in each region for each participant and plotted the scores as a function of participant age with the best fit line. The primary sensory regions were defined separately. PCG = postcentral gyrus; MOG = middle occipital gyrus; SFG = superior frontal gyrus; SPL = superior parietal lobule; SOG = superior occipital gyrus.
Table 1.
Talairach Coordinates of the ROIs Exhibiting Main Effect of Age
| Talairach Coordinates |
Number of Voxels |
|||||
|---|---|---|---|---|---|---|
| ROI | Hem | BA | x | y | z | |
| Frontal lobe | ||||||
| Superior frontal gyrus | L | 6/8 | −15 | 16 | 50 | 1167 |
| Superior frontal gyrus | R | 6/8 | 21 | 8 | 52 | 1640 |
| MFG | L | 10 | −36 | 40 | 22 | 25 |
| MFG | R | 8 | 37 | 38 | 38 | 280 |
| Insular cortex | ||||||
| Insula | R | 13 | 35 | 14 | 8 | 106 |
| Cingulate cortex | ||||||
| Middle cingulate cortex | Mid | 24/32 | 1 | 5 | 41 | 1166 |
| Parietal lobe | ||||||
| AG | L | 39 | −40 | −63 | 35 | 445 |
| Postcentral gyrus | L | 40/2 | −41 | −28 | 46 | 349 |
| Postcentral gyrus | R | 40/2 | 44 | −29 | 35 | 1498 |
| Superior parietal lobule | L | 7 | −22 | −45 | 57 | 364 |
| Superior parietal lobule | R | 7 | 31 | −48 | 53 | 1063 |
| Temporal lobe | ||||||
| MTG | R | 21 | 57 | −55 | 0 | 1775 |
| TPJ | R | 39 | 45 | −61 | 34 | 1254 |
| STG | L | 22 | −46 | −35 | 16 | 484 |
| STG | R | 22 | 42 | −29 | 16 | 891 |
| Occipital lobe | ||||||
| Superior occipital gyrus | R | 19 | 32 | −58 | 38 | 47 |
| Middle occipital gyrus | L | 18 | −43 | −84 | 12 | 866 |
| Cuneus | L | 19 | −12 | −87 | 27 | 485 |
| Primary sensory regions | ||||||
| A1+ (superior temporal gyrus) | L | 42 | −54 | −17 | 8 | 756 |
| A1+ (superior temporal gyrus) | R | 42 | 54 | −15 | 6 | 326 |
| V1+ (lingual gyrus) | Mid | 18 | −1 | −79 | −9 | 1485 |
BA = Brodmann’s area; Hem = hemisphere; L = left; Mid = middle; R = right.
We also graphed the mean inter-SC for each participant from the sensory regions (i.e., A1+, V1+), and these are independent from the voxel selection process. We found no association between age and inter-SC in V1+, F(1, 26) = 0.10, p = .81. There was also no association with age and inter-SC in left A1+, F(1, 26) = 1.98, p = .17, but a marginal effect in right A1+, F(1, 26) = 4.52, p = .055. These results indicate that there is some degree of consistency between groups in the neural coherence of primary sensory regions, but there is possibly some small age-related change in consistency of neural coherence, as demonstrated in right A1+ .
Importantly, the regions (see Figure 4B) in which there were age-related changes in the consistency of neural coherence extended into all four lobes and also into the insular cortex (IC), indicating that they do not simply follow a posterior–anterior progression and are not clustered solely in the frontal cortex or anterior portions of the brain. Second, the patterns of age-related change in consistency differ in different regions (see value for each graph in Figure 4B). There was no association with age in some areas such as the right middle frontal gyrus (MFG), right insula, and left cuneus. In other regions, the best curve fit was positively linear, and this includes the right MTG and right postcentral gyrus. However, there were also regions in which there was a pattern of decreasing consistency with age such as the right and left superior parietal lobules, indicating that there is an age-related reduction in the extent to which alterations in neural coherence are observed in such regions. A few areas evinced a logarithmic curve fit including the left middle occipital gyrus, left STG, and right TPJ. Notably, there were also some regions that showed a quadratic shape function such as in the right STG. The fact that there are several different patterns of age-related effects is of particular interest, and we discuss the implications below.
DISCUSSION
This study was designed to investigate age-related changes in the profile of cortical coherence as individuals process socially complex, naturalistic visual input. We were specifically interested in understanding whether and how specific kinds of variability in neural coherence manifest as a function of age both within and between individuals (test–retest reliability, or intrasubject and intersubject consistency within and between groups). Furthermore, we specifically elected to put our focus on the period of adolescence when rapid age-related changes might be evident. In contrast with existing developmental work that has investigated neural coherence in younger children and that largely targeted the development of specific functional networks (Richardson et al., 2018; Cantlon & Li, 2013), we employed a more assumption-free and data-driven approach with the intention of characterizing potentially unique aspects of functional brain development in both younger and older adolescence.
As a developmental period, adolescence is accompanied by emerging behavioral stability in some domains and the formation of novel behaviors in other domains, and these changes occur in the context of substantial physical changes. All of the above changes make adolescence a particularly unique and interesting time to study how neural networks function and potentially reorganize to accommodate these changes within individuals and across age. Existing work using resting-state fMRI has helped to establish adolescence as an important developmental period for the functional organization of neural networks (Menon, 2013). Our goal was to evaluate age-related changes in the nature of neural coherence in different cortical regions as adolescents and adults process visual, social, affective, and cognitive information that approximates the world in a more natural way (see also Scherf, Behrmann, & Dahl, 2012). In so doing, we were also able to evaluate clear predictions from the literature describing models of functional brain development. The first prediction is that age-related improvements in neural coherence follow a posterior-to-anterior developmental gradient. The second prediction is that the posterior cingulate cortex and the insula may become increasingly coherent in their functional activation as a function of age because of their role as hubs in mature adult cortical function. The final prediction is that within-region developmental change would be characterized by a linear function.
To evaluate these predictions with respect to neural coherence and age-related changes, we scanned younger (aged 9–14 years) and older (aged 15–19 years) adolescents and young adults as they viewed two iterations of a movie clip illustrating a complex, social plot involving children and adolescents. We examined age-related changes in three types of neural coherence, where high coherence reflects increasing similarity in neural profile. First, neural coherence increased across age (with age used as a continuous variable) as reflected by the neural signal becoming more reliable within individuals across two viewings of the movie (intra-SC). Second, neural coherence increased with age as a function of increasing consistency in the neural signal between individuals of the same age group, all viewing the same stimulus (inter-SC within group). Third, the pattern of consistency in the neural signal changes as a function of age differed across cortical areas between three groups, with some regions evincing, for example, a positive linear curve fit whereas others revealed a quadratic fit. Each of these three analyses evaluated the contribution of specific signatures of consistency in the neural signal that could contribute to age-related changes in regional neural coherence across individuals as the adolescents and adults process and make sense of complex visual input.
Reliability and Replicability of Neural Coherence
There are limited data on the test–retest reliability of the neural signal in developmental populations, particularly on short time scales (e.g., less than several weeks or months). Here, we assessed the reliability of the neural signal across every voxel of the entire brain for the duration of the movie excerpt within each individual across the two presentations of the same movie. We predicted that, compared with the older individuals, younger individuals might still be processing the social nuances of the plot during the second viewing of the movie, which could reduce neural coherence across observations.
We found that, in each of the three age groups, approximately 30% of the cortex exhibited a reliable response across the two presentations of the movie, although the percentage increased statistically when age was used as a continuous variable. This finding converges with previous results using this same approach to test the reliability of neural coherence in young adults (Hasson et al., 2004). Importantly, the cortical regions that were reliably used to interpret the movies in each of the age groups were very similar and included the bilateral ventral temporal cortex, posterior parietal cortex, full length of the MTG, and STG (TPJ).
Individual Differences in Neural Coherence across Age—Changing Consistency
Next, we evaluated the neural coherence between individuals as they observed the first presentation of the movie. This was computed with the inter-SC to estimate the degree of individual differences in the neural network organization that each group uses to process the complex visual input. We predicted that adults would evince a similar degree of neural coherence in regions, including primary visual and auditory cortex, superior temporal cortex, and cingulate cortex, as has been reported in previous works using this approach (e.g., Hasson et al., 2004, 2010). We also predicted that younger adolescents would exhibit the largest individual differences, given the larger developmental transition that they are undergoing, and thus, should show the lowest intersubject coherence throughout most of the cortical regions.
Indeed, adults in this study demonstrated comparable neural coherence in sensory areas, like primary auditory and visual cortex, as in previous studies with average inter-SCs in A1+ and V1+. In addition, we observed extensive intersubject coherence among all three age groups in the same network of regions in which there was reliability in the neural coherence across iterations of the movie within individuals, including the bilateral ventral temporal cortex and along the lateral surface of the MTG and STG (see Figures 2 and 3). For each of the groups, there were coherent neural responses across individuals in approximately 27% of the cortex, which increased with age to around 35%. In the comparison of the inter-SC group maps, the adults showed more neural coherence in some frontal regions, insular regions, and posterior parietal regions, including TPJ and AG compared to younger adolescents, who exhibited more coherence in the middle cingulate than adults. Although the cortical areas that the adolescents and adults were using to process and interpret the complex visual and social information in the movie are largely overlapping, the important differences included regions that might be considered as a part of the mentalizing network but might also be considered as nodes in an attentional network or one engaged in ToM computations (i.e., mPFC, middle cingulate, TPJ, AG/posterior STG). These findings converge with those from the analyses of the reliability of the neural signal to suggest that this movie invokes multiple regions implicated in mentalizing in all three age groups and that there is differential weighting and/or recruitment of these specific regions that changes with age.
Finally, we compared the neural coherence between age groups in every voxel of the brain. This allowed us to generate a map with clusters of voxels that represented age-related changes in the magnitude of the inter-SC. Many clusters revealed a pattern of increasing neural coherence with age. These clusters were distributed throughout the brain and included many of the same regions that were identified in the previous neural coherence analyses. Again, these were regions that have been consistently implicated in mentalizing and ToM processing in both adults and children (for a review, see Mahy, Moses, & Pfeifer, 2014). There were also, however, regions showing a decrease in neural coherence with age and yet others that showed a more U-shaped function.
Age-Related Changes in Neural Coherence Supporting Complex Visual and Social Processing in Adolescence
Although we adopted a bottom–up approach in which we investigated age-related differences across the whole of cortex, nevertheless, across all three metrics of neural coherence (intraindividual, interindividual same age group, interindividual across age groups), our results revealed two central findings about the neural networks that adolescents and adults use to process complex visual and social information. Moreover, these two discoveries converge with the results of other studies. The first major result shows that the cortical networks invoked as adolescents and adults process complex visual and social input are largely similar and include higher-order object processing regions in the ventral visual pathway and regions implicated in processing social interactions that are distributed throughout the brain (see Redcay & Warnell, 2018). The second major result is that age-related differences in these networks are largely found in the extent to which individuals rely on particular regions within these networks. Specifically, younger adolescents rely more on the middle cingulate gyrus and less on the mPFC, precuneus, TPJ, insula, and frontal regions than do adults when processing and interpreting these complex visual scenes involving social interactions with peers. In summary, we have obtained evidence suggesting that the coherence profile of the brains of individuals in adolescence seems well suited to bridge between childhood to adulthood: In multiple cortical regions, the younger adolescents’ brain responses showed less consistency to each other than was true of the older adolescents who, in turn, showed less consistency in neural responses than adults (see Figure 3).
Given the series of findings we have reported, the implications for the proposed predictions become clear. First, it is not the case, in the range of ages we have tested, that there is a sequential progression in development from posterior to anterior. For example, as evident from Figure 3, the older adolescents have as much, if not more, coherence than the adults in the MFG, and in Figure 2, the younger adolescents show more coherence than the older adolescents and the adults in the IPL. It is also well established that higher-order association cortices mature after lower-order somatosensory and visual cortices (Gogtay et al., 2004) and that the structural covariance of networks (in which cortical thickness in one region influences the thickness of structurally and functionally connected regions) is coordinated or synchronized over the course of development (Alexander-Bloch, Raznahan, Bullmore, & Giedd, 2013). For example, individual sulcal patterns of ACC are fixed from childhood to adulthood, although quantitative anatomical ACC metrics may be changing dramatically (Cachia et al., 2016).
Second, there does not appear to be obvious large-scale reorganization or sculpting of regional hubs. Although adolescence is a time of large-scale reorganization in neural networks as a result of pubertal development and challenging social developmental tasks (Blakemore, Burnett, & Dahl, 2010), the results of the present investigation favor an interpretation that young adolescents use overlapping but slightly different networks when viewing and interpreting the movies. This is evident in a number of findings. Most of the cortex is not different (see all gray or noncolored regions in Figures 2 and 3) in inter-SC revealing that the networks are not that different. However, closer scrutiny reveals that the right IPL, for example, has a higher intra-SC in the younger adolescent group across the two viewings of the movie relative to other regions. In addition, this region shows decreasing inter-SCs with age during Run 1, and furthermore, the youngest group relies on this region more than the two older groups do to interpret visual stimuli. Together, these findings suggest that this region becomes less important with age. In contrast, the two older groups have more intra-SCs in the posterior left inferotemporal gyrus across the two viewings of the movie, and the inter-SCs in this region increase with age—indicating that this region becomes more integrated into the network with age. The findings are not easily accounted for by a framework in which heterogeneity of neural responses dominates the coherence among brains of adolescence and, subsequently, shares little commonality or substantial reorganization relative to the coherence profile among adult brains.
Last, change within a region is not necessarily linear. As shown in Figure 4, we see age-related changes that have different trajectories. Some of these changes are positively linear over age, and some are negatively linear, but there are as many of these as there are U-shaped functions with either upright or inverted “U.” Of course, the developmental profiles we uncover here only relate to the period of adolescence, the focus of this article. It would obviously be very interesting and important to adopt the same approach to examine the profiles and the three predictions we outline in younger children.
Together, these findings offer a more nuanced view of development and suggest that maturation is not an all-or-none phenomenon nor is it a lockstep sequential march across age. These results are consistent with findings from studies examining structural differences across age that have been explored by computing the similarity in the trajectory of cortical thickness within individuals and across age between any two cortical regions (Khundrakpam et al., 2019). Using this approach, the authors reported that changes in brain structure occurred in a coordinated fashion over time, that these changes were well aligned with functional connectivity (as in the default mode network), and that this individual-based structural covariance approach offered the possibility of tracking variability and may potentially provide a mechanistic explanation in cases with neural (and neurodevelopment) disorders.
We have examined whole-brain development in a bottom–up free manner and have been able to characterize changes in neural coherence at both the individual and group levels based on a somewhat naturalistic context in which the adolescents watch a movie in the scanner. With the advent of innovative acquisition and analytic approaches, whole-brain data can be relatively easily explored and analyses of individual and group similarity or variability can be better elucidated. Furthermore, additional data will license the use of regression analyses, which we only did sparingly given the relative paucity of individuals at each age and will permit closer statistical scrutiny of the within-region changes (which we refrained from doing given the nonindependence of definition and selection). Together, the findings reported here characterize the fine-grained developmental trajectories of regions of cortex engaged by naturalistic stimuli (as far as is possible in the bore of the magnet), perhaps the “messy information space” of development (Cantlon, 2020), and put a spotlight on the specific challenges of cortical development as the adolescent brain approximates the more mature, stable brain profile of adulthood.
Funding Information
This research was supported by a grant to Marlene Behrmann from the Foundation for the National Institutes of Health (https://dx.doi.org/10.13039/100000009), grant number: R01EY027018.
Diversity in Citation Practices
A retrospective analysis of the citations in every article published in this journal from 2010 to 2020 has revealed a persistent pattern of gender imbalance: Although the proportions of authorship teams (categorized by estimated gender identification of first author/last author) publishing in the Journal of Cognitive Neuroscience (JoCN) during this period were M(an)/M = .408, W(oman)/M = .335, M/W = .108, and W/W = .149, the comparable proportions for the articles that these authorship teams cited were M/M = .579, W/M = .243, M/W = .102, and W/W = .076 (Fulvio et al., JoCN, 33:1, pp. 3–7). Consequently, JoCN encourages all authors to consider gender balance explicitly when selecting which articles to cite and gives them the opportunity to report their article’s gender citation balance.
REFERENCES
- Alexander LM, Escalera J, Ai L, Andreotti C, Febre K, Mangone A, et al. (2017). An open resource for transdiagnostic research in pediatric mental health and learning disorders. Scientific Data, 4, 170181. 10.1038/sdata.2017.181, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Raznahan A, Bullmore E, & Giedd J (2013). The convergence of maturational change and structural covariance in human cortical networks. Journal of Neuroscience, 33, 2889–2899. 10.1523/JNEUROSCI.3554-12.2013, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, & Dahl RE (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31, 926–933. 10.1002/hbm.21052, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, et al. (2002). The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage, 17, 184–200. 10.1006/nimg.2002.1174, [DOI] [PubMed] [Google Scholar]
- Cachia A, Borst G, Tissier C, Fisher C, Plaze M, Gay O, et al. (2016). Longitudinal stability of the folding pattern of the anterior cingulate cortex during development. Developmental Cognitive Neuroscience, 19, 122–127. 10.1016/j.dcn.2016.02.011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF (2020). The balance of rigor and reality in developmental neuroscience. Neuroimage, 216, 116464. 10.1016/j.neuroimage.2019.116464, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, & Li R (2013). Neural activity during natural viewing of Sesame Street statistically predicts test scores in early childhood. PLoS Biology, 11, e1001462. 10.1371/journal.pbio.1001462, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE (2004). Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences, 1021, 1–22. 10.1196/annals.1308.001, [DOI] [PubMed] [Google Scholar]
- Elliott ML, Knodt AR, Ireland D, Morris ML, Poulton R, Ramrakha S, et al. (2020). What is the test–retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychological Science, 31, 792–806. 10.1177/0956797620916786, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, & Dahl RE (2011). Neural systems of threat processing in adolescents: Role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology, 36, 429–452. 10.1080/87565641.2010.550178, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frane AV (2015). Are per-family type I error rates relevant in social and behavioral science? Journal of Modern Applied Statistical Methods, 14, 12–23. 10.22237/jmasm/1430453040 [DOI] [Google Scholar]
- Freud E, & Behrmann M (2017). The life-span trajectory of visual perception of 3D objects. Scientific Reports, 7, 11034. 10.1038/s41598-017-11406-7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud E, Plaut DC, & Behrmann M (2019). Protracted developmental trajectory of shape processing along the two visual pathways. Journal of Cognitive Neuroscience, 31, 1589–1597. 10.1162/jocn_a_01434, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AF, & Hale S (2000). Relationships among processing speed, working memory, and fluid intelligence in children. Biological Psychology, 54, 1–34. 10.1016/S0301-0511(00)00051-X, [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, U.S.A, 101, 8174–8179. 10.1073/pnas.0402680101, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, et al. (2007). Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cerebral Cortex, 17, 766–777. 10.1093/cercor/bhk030, [DOI] [PubMed] [Google Scholar]
- Hasson U, Avidan G, Gelbard H, Vallines I, Harel M, Minshew N, et al. (2009). Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Research, 2, 220–231. 10.1002/aur.89, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Malach R, & Heeger DJ (2010). Reliability of cortical activity during natural stimulation. Trends in Cognitive Sciences, 14, 40–48. 10.1016/j.tics.2009.10.011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, & Malach R (2004). Intersubject synchronization of cortical activity during natural vision. Science, 303, 1634–1640. 10.1126/science.1089506, [DOI] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Chen Z, Mezher A, & Vetter NC (2018). Test–retest reliability of longitudinal task-based fMRI: Implications for developmental studies. Developmental Cognitive Neuroscience, 33, 17–26. 10.1016/j.dcn.2017.07.001, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, & Sowell ER (2017). Puberty and structural brain development in humans. Frontiers in Neuroendocrinology, 44, 122–137. 10.1016/j.yfrne.2016.12.003, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps FS, Hendrix CL, Brennan PA, & Dilks DD (2020). Connectivity at the origins of domain specificity in the cortical face and place networks. Proceedings of the National Academy of Sciences, U.S.A, 117, 6163–6169. 10.1073/pnas.1911359117, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keselman HJ (2015). Per family or familywise type I error control: “Eether, eyether, neether, nyther, Let’s call the whole thing off!”. Journal of Modern Applied Statistical Methods, 14, 24–37. 10.22237/jmasm/1430453100 [DOI] [Google Scholar]
- Khundrakpam BS, Lewis JD, Jeon S, Kostopoulos P, Itturia Medina Y, Chouinard-Decorte F, et al. (2019). Exploring individual brain variability during development based on patterns of maturational coupling of cortical thickness: A longitudinal MRI study. Cerebral Cortex, 29, 178–188. 10.1093/cercor/bhx317, [DOI] [PubMed] [Google Scholar]
- Lerner Y, Honey CJ, Silbert LJ, & Hasson U (2011). Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. Journal of Neuroscience, 31, 2906–2915. 10.1523/JNEUROSCI.3684-10.2011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy CE, Moses LJ, & Pfeifer JH (2014). How and where: Theory-of-mind in the brain. Developmental Cognitive Neuroscience, 9, 68–81. 10.1016/j.dcn.2014.01.002, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2013). Developmental pathways to functional brain networks: Emerging principles. Trends in Cognitive Sciences, 17, 627–640. 10.1016/j.tics.2013.09.015, [DOI] [PubMed] [Google Scholar]
- Moraczewski D, Chen G, & Redcay E (2018). Inter-subject synchrony as an index of functional specialization in early childhood. Scientific Reports, 8, 2252. 10.1038/s41598-018-20600-0, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastase SA, Gazzola V, Hasson U, & Keysers C (2019). Measuring shared responses across subjects using intersubject correlation. Social Cognitive and Affective Neuroscience, 14, 667–685. 10.1093/scan/nsz037, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Scherf KS, Zachariou V, Tarr MJ, & Behrmann M (2015). Size precedes view: Developmental emergence of invariant object representations in lateral occipital complex. Journal of Cognitive Neuroscience, 27, 474–491. 10.1162/jocn_a_00720, [DOI] [PubMed] [Google Scholar]
- Oerter KE, Uriarte MM, Rose SR, Barnes KM, & Cutler GB Jr., (1990). Gonadotropin secretory dynamics during puberty in normal girls and boys. Journal of Clinical Endocrinology and Metabolism, 71, 1251–1258. 10.1210/jcem-71-5-1251, [DOI] [PubMed] [Google Scholar]
- Redcay E, & Warnell KR (2018). A social-interactive neuroscience approach to understanding the developing brain. Advances in Child Development and Behavior, 54, 1–44. 10.1016/bs.acdb.2017.10.001, [DOI] [PubMed] [Google Scholar]
- Richardson H (2019). Development of brain networks for social functions: Confirmatory analyses in a large open source dataset. Developmental Cognitive Neuroscience, 37, 100598. 10.1016/j.dcn.2018.11.002, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H, Lisandrelli G, Riobueno-Naylor A, & Saxe R (2018). Development of the social brain from age three to twelve years. Nature Communications, 9, 1027. 10.1038/s41467-018-03399-2, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H, & Saxe R (2020). Development of predictive responses in theory of mind brain regions. Developmental Science, 23, e12863. 10.1111/desc.12863, [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, & Dahl RE (2012). Facing changes and changing faces in adolescence: A new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Developmental Cognitive Neuroscience, 2, 199–219. 10.1016/j.dcn.2011.07.016, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, & Luna B (2006). Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience, 18, 1045–1058. 10.1162/jocn.2006.18.7.1045, [DOI] [PubMed] [Google Scholar]
- Scherf KS, Thomas C, Doyle J, & Behrmann M (2014). Emerging structure–function relations in the developing face processing system. Cerebral Cortex, 24, 2964–2980. 10.1093/cercor/bht152, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Olino TM, Forbes EE, & Dahl RE (2014). Exciting fear in adolescence: Does pubertal development alter threat processing? Developmental Cognitive Neuroscience, 8, 86–95. 10.1016/j.dcn.2014.01.004, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Houts RM, Steinberg L, Belsky J, Cauffman E, Dehart G, et al. (2010). Longitudinal development of secondary sexual characteristics in girls and boys between ages 91/2 and 151/2 years. Archives of Pediatrics & Adolescent Medicine, 164, 166–173. 10.1001/archpediatrics.2009.261, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW (1953). The problem of multiple comparisons. In Braun H (Ed.), The collected works of John W. Tukey volume VIII, multiple comparisons: 1948–1983 (pp. 1–300). New York: Chapman & Hall. [Google Scholar]
- Yakovlev PI, & Lecours AR (1967). The myelogenetic cycles of regional maturation of the brain. In Minkowski A (Ed.), Regional development of the brain in early life (pp. 3–70). Oxford: Blackwell. [Google Scholar]