Summary
Background
This study aimed to: 1) assess the associations of biological age acceleration based on Klemera and Doubal's method (KDM-AA) with long-term risk of all-cause mortality; and 2) compare the association of KDM-AA with all-cause mortality among participants potentially at different stages of the cardiovascular disease (CVD) continuum.
Methods
The present study was based on a subpopulation of the China Kadoorie Biobank, with baseline survey during 2004–08. A total of 12,377 participants free of ischemic heart disease, stroke, or cancer at baseline were included, in which 8180 participants were identified to develop major coronary event (MCE), ischemic stroke (IS), intracerebral hemorrhage (ICH) or subarachnoid hemorrhage (SAH), and 4197 remained free of these cardiovascular diseases before 1 January 2014. These participants were followed up until 1 Jan 2018. KDM-AA was calculated by regressing biological age measurement, which was constructed based on baseline 16 physical and 9 biochemical markers using Klemera and Doubal's method, on chronological age. We estimated the associations of KDM-AA with the mortality risk using the hazard ratio (HR) and 95% confidence interval (CI) from Cox proportional hazard models. We assessed discrimination performance by Harrell's C-index and net reclassification index (NRI).
Findings
The participants who developed MCE (mean KDM-AA = 0.1 year, standard deviation [SD] = 1.6 years) or ICH/SAH (0.3 ± 1.5 years) during subsequent follow-up showed accelerated aging at baseline compared to those of IS (0.0 ± 1.2 years) and control (−0.3 ± 1.3 years) groups. The KDM-AA was positively associated with long-term risk of all-cause mortality (HR = 1.20; 95% CI: 1.17, 1.23), and the association was robust for participants potentially at different stages of the CVD continuum. Adding KDM-AA improved mortality prediction compared to the model only with sociodemographic and lifestyle factors in whole participants, with the Harrell's C-index increasing from 0.813 (0.807, 0.819) to 0.821 (0.815, 0.826) (NRI = 0.011; 95% CI: 0.003, 0.019).
Interpretation
In this middle-aged and elderly Chinese population, the KDM-AA is a promising measurement for biological age, and can capture the difference in cardiovascular health and predict the risk of all-cause mortality over a decade.
Funding
This work was supported by National Natural Science Foundation of China (82192904, 82192901, 82192900, 81941018). The CKB baseline survey and the first re-survey were supported by a grant from the Kadoorie Charitable Foundation Hong Kong. The long-term follow-up is supported by grants from the UK Wellcome Trust (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z), grants (2016YFC0900500) from the National Key R&D Program of China, National Natural Science Foundation of China (81390540, 91846303), and Chinese Ministry of Science and Technology (2011BAI09B01).
Keywords: Biological age, Blood biomarkers, Physical measurements, Mortality, Cardiovascular health
Research in context.
Evidence before this study
The aging pace in a population of the same chronological age varies between individuals. Biological age (BA), constructed by physical and biochemical markers, may help define whether aging is accelerated or delayed relative to chronological age. Also, this type of BA measurement tends to capture physiological alterations earlier than specific phenotypes and can be obtained at a lower cost than omics-based indicators. Klemera and Doubal's method (KDM), as one of the BA estimation algorithms, has shown superiority in predicting BA and mortality. Most studies on KDM-BA were conducted in western populations, and only a few in Asian populations recently.
Added value of this study
The present study was based on a subpopulation of the China Kadoorie Biobank cohort (CKB). A total of 12,377 participants free of ischemic heart disease, stroke, or cancer at baseline were included, in which some participants developed various subtypes of cardiovascular diseases (CVD) thereafter, and the others remained free of these CVDs for up to 10 years of follow-up. This study constructed BA based on baseline 16 physical and 9 biochemical markers using KDM and further calculated the biological age acceleration (KDM-AA).
The present study first suggests that the participants who subsequently developed major coronary event, ischemic stroke, or hemorrhagic stroke during follow-up showed varying degrees of accelerated aging at baseline. We exploited the advantages of CKB in outcome accuracy and examined the associations of the KDM-AA with long-term risk of all-cause mortality. Finally, we offered evidence that the association of KDM-AA with all-cause mortality was robust for participants potentially at different stages of the CVD continuum and those with different sociodemographic characteristics and lifestyles. The KDM-AA could improve the discrimination performance of prediction models.
Implications of all the available evidence
This study provides the comprehensive association analysis of KDM-AA with all-cause mortality by subgroups and also first uses general BA measurement for capturing the difference in cardiovascular health. The present study suggests that the KDM-AA may be a useful indicator for risk stratification, especially in a relatively healthy population.
Introduction
Aging is the ‘common soil' of age-related chronic diseases, the decline in activities of daily living, and death. Chronological age (CA) is a convenient measurement of the aging state of individuals. However, the aging pace in a population of the same CA varies between individuals, and variability in cognitive function1 and health status2 also increases with CA. Therefore, biological age (BA) was proposed to define whether aging was accelerated or delayed relative to chronological age.3
A variety of BA measurements have emerged recently, such as omics-based indicators (e.g., telomere length,4 Horvath's clock,5 and Hannum's clock6), clinical indicators (e.g., phenotypic age7 and BA measurement constructed under Klemera and Doubal's method [hereafter KDM-BA]),8 and phenotypic indicators (e.g., frailty phenotype,9 frailty index,10 and functional aging index11). There is yet no gold standard for measuring BA. By contrast, clinical indicators, usually constructed by physical and biochemical markers, tend to capture physiological alterations earlier than specific phenotypes and can be obtained at a lower cost than omics-based indicators.
Klemera and Doubal's method (KDM), as one of the BA estimation algorithms, has shown superiority in predicting BA and mortality.12,13 Although the number and types of markers included in KDM-BA construction differed slightly across studies, KDM-BA showed consistent associations with aging outcomes.8,12,14 Most studies on KDM-BA were conducted in western populations, and only a few in Asian populations recently.15, 16, 17, 18 A study in the Chinese population indicated that KDM-BA was predictive of all-cause mortality in two cohorts.15
The present study was based on a subpopulation of the China Kadoorie Biobank (CKB), a cohort of middle-aged and older adults with a mean follow-up period of approximately 10 years. We aimed to assess the associations of biological age acceleration (AA) based on KDM-BA (KDM-AA) at baseline with long-term risk of all-cause mortality. Biological AA was first seen in studies on DNA methylation age19 and is a deviation of BA from CA, reflecting the additional value of BA over CA. This study included over 10,000 participants free of ischemic heart disease (IHD), stroke, or cancer at baseline. Some participants had different subtypes of cardiovascular disease (CVD) diagnosed shortly after baseline and were more likely to have been in the stage of subclinical CVD at baseline, while others remained CVD-free after longer follow-up. This gives us an opportunity to compare the association of KDM-AA with all-cause mortality among participants potentially at different stages of the CVD continuum.
Methods
Study population
The baseline survey of the CKB cohort was launched in five urban and five rural areas during 2004–2008, enrolling a total of more than 0.5 million participants aged 30–79. The baseline design and the profile of CKB participants were described in detail previously.20,21 All participants provided written informed consent. The study protocol was approved by the Ethical Review Committee of the Chinese Center for Disease Control and Prevention (CDC, Beijing, China) and the Oxford Tropical Research Ethics Committee at the University of Oxford (Oxford, UK).
A subpopulation of CKB cohort was selected to measure a series of clinical biochemistry biomarkers. All participants who reported having doctor-diagnosed IHD, stroke, or cancer or self-reported statin users at baseline were first excluded from the selection process. The following participants with incident events documented during follow-up till the date of 1 January 2014 were identified: (1) major coronary event (MCE): fatal IHD (the 10th revision of the International Classification of Diseases, ICD-10: I20–I25) or nonfatal myocardial infarction (MI, I21–I23); (2) ischemic stroke (IS, I63 or I69.3); (3) intracerebral hemorrhage (ICH, I61 or I69.1) or subarachnoid hemorrhage (SAH, I60 or I69.0). Another group of participants was selected from those free of cardiovascular events till 1 January 2014, called the control group. Finally, 18,172 participants (the control group: n = 6341; MCE group: 1319; IS group: 5467; ICH/SAH group: 5045) were included for biochemistry tests.
The vital and disease status and hospitalization were updated for all participants as the follow-up continued. Due to updated outcome definition and case adjudication by expert physicians, the status of a few cases and controls aforementioned might be changed.22 However, the original grouping was retained in the present analysis.
Baseline data collection
At the baseline survey, trained staff interviewed participants to collect sociodemographic characteristics (e.g., age, sex, educational attainment), lifestyle factors, and personal medical and family histories. Information on tobacco smoking included smoking status (never, ever, or currently) and the main reason for smoking cessation in quitters. Questions about alcohol drinking included drinking frequency in the past 12 months (never, occasionally, monthly, weekly, or daily), the quantity of alcohol consumed in weekly drinkers, and past drinking status in non-weekly drinkers. We inquired about the frequency of dietary intake in the past 12 months (never/rarely, monthly, 1–3 days/week, 4–6 days/week, or daily) via a validated qualitative food frequency questionnaire, which covered 12 major food groups.23 The level of physical activity was assessed by multiplying the metabolic equivalent tasks (METs) values of activities by the typical duration of corresponding activities in hours per day and summing them together based on the 2011 update of compendium of physical activities.24
In the present study, we defined risky lifestyle factors (tobacco smoking, alcohol drinking, dietary habits, and physical activity) according to previous findings25, 26, 27 and adapted them to the present study. The risky dietary habits were defined according to the following criteria: eating vegetables less than daily, eating fruits less than daily, eating red meat daily or less than weekly, eating soybean <4 days per week, and eating fish less than weekly.25, 26, 27 One point was for one food item, and the total score was the risky dietary score, ranging from 0 to 5. The risky lifestyle factors included: currently smoking (including quitting due to illness); daily intake of pure alcohol ≥30 g for males, ≥15 g for females, or past drinking; the risky dietary score of 4–5 points; and the total physical activity level ranking in the lower age- (<50 years, 50–59 years, and ≥60 years) and sex-specific half. The risky lifestyle score was the number of the risky lifestyle factors, ranging from 0 (healthiest) to 4 (riskiest). Participants who scored 3 or 4 were categorized into the risky group.
Physical measurements included height, weight, waist circumference, hip circumference, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), and forced expiratory volume in 1 s (FEV1). Standing height and body weight was measured by a height meter and a TANITA TBF-300GS body fat meter, respectively. Waist circumference and hip circumference measurements were taken using a soft tape. Blood pressure and heart rate measurement were taken twice for each participant by a UA-779 digital sphygmomanometer. A third measurement was performed if the difference between the first two SBP measurements were greater than 10 mmHg, and the latter two readings were recorded. FEV1 was assessed by a spirometer, and two successful blows were recorded. We utilized mean values of heart rate, SBP, and DBP and the larger FEV1 in our construction of KDM-BA. All measurements were carried out under standard operating instructions. Previous literature can be referred to for details.28
Clinical biomarker measurement
For each participant, a 10 mL blood sample was collected at baseline without fasting requirement but with the time since last meal recorded. Random plasma glucose (RPG) was tested on-site using SureStep Plus meters (Lifescan, Johnson & Johnson). The participants with RPG of 7.8–11.0 mmol/L were invited to retest their fasting plasma glucose (FPG) the next morning. Clinical biochemistry tests were performed for 1 mL baseline plasma samples at the Wolfson Laboratory (Clinical Trial Service Unit & Epidemiological Studies Unit, UK). All biomarkers listed in Supplementary Table S1 were assayed using AU680 clinical chemical analyzer (Beckman Coulter Incorporation, UK), except that cystatin C was measured by BN Prospec nephelometer analyzer (Siemens, UK).29 All tests were performed using the manufacturer's standard reagents, calibrators, and settings.
A subset of participants was selected for high-throughput targeted NMR spectroscopy measurements at the Brainshake Laboratory (Kuopio, Finland).30 There was an overlapping of nine traits (albumin, creatinine, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, total triglyceride, apolipoprotein A1, apolipoprotein B, and RPG) between the clinical biochemistry tests and the NMR spectroscopy, with good agreement between measurements (r = 0.77–0.98).
Construction of biological age and age acceleration
A list of markers, including blood biomarkers and physical measurements, was considered in the construction of KDM-BA (Supplementary Table S2). For participants who reported taking antihypertensive medication (n = 2160), we added 15 and 10 mmHg to measured SBP and DBP, respectively.31 For participants with both random and fasting blood glucose levels measured, the fasting blood glucose value was used for the analysis (n = 657). According to distributions of markers, the log-transformation was used for biomarkers except for plasma albumin and all physical measurements.
According to the procedure proposed by Klemera,8 Levine,12 and a previous study,32,33 we constructed KDM-BA by sex. First, we calculated the Pearson's correlation coefficient of each marker with CA, and those with coefficients above a certain threshold (>0.10) were included in the construction of KDM-BA. The markers which showed a non-monotonic relationship with CA were excluded. Finally, sixteen markers remained in the calculation for both sexes. The remaining markers were then transformed into principal components (PCs) using principal component analysis based on the correlation matrix, which were further used to calculate the KDM-BA according to the Klemera and Doubal's method.8,12,32,33 KDM-AA was calculated by regressing KDM-BA on CA in the present study. The formula of calculation is in Supplementary methods.
Outcome ascertainment
During long-term follow-up, the vital status was ascertained through local Disease Surveillance Point (DSP) death registries using unique personal identification numbers, supplemented by annual checks with local residential records and active confirmation by contacting local communities or relatives.21 The causes of death, mainly derived from official death certificates, were supplemented by reviewing medical records and verbal autopsy based on symptoms and signs provided by informants (usually family members).34 The disease incidence was obtained by establishing linkage to the local disease registry system and national inpatient health insurance claim data in which >97% of participants were covered (these procedures also yielded a few additional deaths that had not been identified through death registries).34 All participants were followed until death or loss to follow-up. The data for the present analysis was censored on 1 Jan 2018.
Statistical analysis
Supplementary Fig. S1 illustrates the study design. Among 18,172 participants who had biochemical tests, we excluded (1) those with blood samples lipemic, icteric, haemolysed, or turbid (n = 3726); (2) those with values marked as under limit of detection (n = 1452); (3) those with missing values on any of the markers listed in Supplementary Table S2 (n = 167); (4) outliers, namely the participants with any marker lying outside of four standard deviations (SD, n = 450), leaving a total of 12,377 participants in the analysis.
We conducted the following analyses in whole participants and by four groups (control, MCE, IS, and ICH/SAH) separately, where appropriate. Baseline characteristics were compared among four groups using linear regression models for continuous variables and logistic regression models for categorical variables. The contrast was also made between included versus excluded participants. To assess the accuracy of KDM-BA in predicting CA of the aging model, we calculated the Pearson's correlation coefficient between KDM-BA and CA and root mean square error (RMSE) by regressing CA on KDM-BA.
We examined the association of KDM-AA with the risk of all-cause mortality using the hazard ratio (HR) and 95% confidence interval (CI) from Cox proportional hazard models. Cox models were stratified jointly by age groups in the 5-year interval, 10 study areas, and four groups (control, MCE, IS, and ICH or SAH), where appropriate, with CA as the underlying time scale. Models were adjusted for sex, fasting status (<8 or ≥8 h), and educational attainment (primary school and below, middle or high school, college or university). No major violations of the proportional hazards assumption for KDM-AA were identified. Our study further explored associations of KDM-AA with all-cause mortality stratified by sociodemographic (age groups, sex, urban or rural areas) and lifestyle factors (smoking, alcohol drinking, dietary habits, physical activity level, combined lifestyle factors) at baseline. The interactions between KDM-AA and the stratifying variables were tested using likelihood ratio tests by comparing models with and without product terms.
We also evaluated Cox models including CA only or KDM-AA additionally for predicting 10-year mortality risk by Harrell's C-index and net reclassification index (NRI) for the traditional 50% threshold.35,36 The basic models used time-on-study as the time scale and were stratified jointly by 10 study areas and four groups (control, MCE, IS, and ICH or SAH), adjusting for the same covariates as above, as well as urban or rural areas and lifestyle factors (smoking, alcohol drinking, dietary habits, physical activity level).
We performed sensitivity analysis by restricting the construction of KDM-BA by sex in the control group, as mentioned above. We then calculated PCs and KDM-BA for the case groups by sex using loadings of markers and weights of CA and PCs, which were derived from the control analysis. Considering the differences in standard deviation (SD) characteristics of KDM-AA between the primary and sensitivity analysis, we standardized KDM-AA by dividing SD to make the association estimates comparable. We also performed sensitivity analysis by excluding participants who reported taking antihypertensive medication.
We used TrueTrait function from WGCNA package in R (version 4.0.3) for the construction of KDM-BA and Stata (version 15.0) for other statistical analyses. Statistical significance was defined by the two-sided P-values <0.05.
This work was supported by National Natural Science Foundation of China (82192904, 82192901, 82192900, 81941018). The CKB baseline survey and the first re-survey were supported by a grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up is supported by grants from the UK Wellcome Trust (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z), grants (2016YFC0900500) from the National Key R&D Program of China, National Natural Science Foundation of China (81390540, 91846303), and Chinese Ministry of Science and Technology (2011BAI09B01).
Role of funding source
The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication.
Results
The four groups based on the disease status till the date of 1 January 2014 differed in their sociodemographic characteristic, lifestyle habits, and personal and family medical histories (Table 1). For example, participants in MCE and IS groups were younger than those in the control and ICH/SAH groups. IS and ICH/SAH groups had higher proportions of excessive alcohol drinking among men. All three case groups had a higher prevalence of hypertension and diabetes. Baseline characteristics between included versus excluded participants were similar in quantity except for the prevalence of diabetes (Supplementary Table S3). The mean values (SD) of blood biomarkers and physical measurements across the groups are shown in Supplementary Table S4.
Table 1.
Baseline characteristics of participants according to four separate groups (N = 12,377).
| All | Control | MCE | IS | ICH or SAH | P-value | |
|---|---|---|---|---|---|---|
| Number of participants | 12,377 | 4197 | 876 | 3837 | 3467 | |
| Sociodemographic characteristic | ||||||
| Age, year | 57.0 | 58.4 | 54.2 | 53.7 | 59.6 | <0.001 |
| Female, % | 6218 (50.2) | 2013 (48.0) | 377 (43.0) | 2137 (55.7) | 1691 (48.8) | <0.001 |
| Urban, % | 3690 (29.8) | 974 (23.2) | 277 (31.6) | 1681 (43.8) | 758 (21.9) | <0.001 |
| Middle school or above, % | 38.3 | 39.1 | 35.7 | 38.6 | 37.6 | 0.051 |
| Lifestyle | ||||||
| Current smokinga, % | ||||||
| Male | 69.6 | 67.1 | 77.8 | 72.1 | 67.7 | <0.001 |
| Female | 4.0 | 3.6 | 6.8 | 4.7 | 3.8 | 0.048 |
| Excessive alcohol drinkingb, % | ||||||
| Male | 23.7 | 19.9 | 22.1 | 27.2 | 25.7 | <0.001 |
| Female | 1.8 | 1.6 | 0.8 | 1.7 | 2.5 | 0.078 |
| Dietary habits, % | ||||||
| Eating fresh vegetables: not daily | 7.1 | 6.7 | 7.0 | 7.6 | 7.2 | 0.492 |
| Eating fresh fruits: not daily | 87.1 | 86.1 | 89.8 | 86.5 | 88.6 | <0.001 |
| Eating red meat: daily or less than weekly | 49.7 | 48.1 | 51.4 | 50.4 | 50.2 | 0.069 |
| Eating fish: less than weekly | 67.0 | 66.5 | 71.1 | 65.8 | 68.3 | <0.001 |
| Eating soybean: <4 days/week | 90.8 | 90.7 | 91.5 | 90.3 | 91.4 | 0.420 |
| Total physical activity, MET-h/d | 18.8 | 19.3 | 18.9 | 18.7 | 18.2 | 0.002 |
| Fasting time, h | 4.7 | 4.6 | 4.7 | 4.8 | 4.7 | 0.181 |
| Prevalent hypertension, % | 55.0 | 40.0 | 57.3 | 58.2 | 69.3 | <0.001 |
| Prevalent diabetes, % | 6.7 | 3.7 | 11.1 | 9.3 | 6.2 | <0.001 |
| Medication of, % | ||||||
| Antihypertensives | 17.5 | 10.5 | 19.2 | 20.1 | 22.5 | <0.001 |
| Antidiabetics | 2.4 | 1.5 | 4.6 | 3.3 | 1.8 | <0.001 |
| Family history of, % | ||||||
| Heart disease | 3.5 | 3.1 | 5.3 | 3.6 | 3.1 | 0.006 |
| Stroke | 21.6 | 19.2 | 21.5 | 22.7 | 23.2 | <0.001 |
| Cancer | 16.3 | 17.9 | 16.7 | 16.6 | 13.8 | <0.001 |
| Self-rated good health, % | 41.4 | 45.6 | 38.6 | 38.8 | 40.0 | <0.001 |
MET, metabolic equivalent of task; MCE: major coronary event; IS: ischemic stroke; ICH: intracranial hemorrhage; SAH: subarachnoid hemorrhage.
Sex and urban regions are presented as n (%). Other values are means or percentages with adjustment for age, sex, and study regions, where appropriate.
Former smoker who had stopped smoking for illness was categorized into the current smoker.
Daily intake of pure alcohol: ≥30 g for males, ≥15 g for females; former drinker was categorized into excessive alcohol drinker.
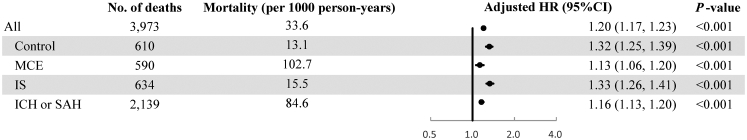
MCE group had the highest mortality rate (102.7 per 1000 person-years), followed by ICH/SAH group (84.6 per 1000 person-years), IS group (15.5 per 1000 person-years), and the control group (13.1 per 1000 person-years) (Fig. 1). As shown in Table 2, the median (interquartile range, IQR) time to the first diagnosis of the corresponding diseases was 4.4 (3.9) years for the MCE group, 4.6 (3.8) years for IS group, and 4.1 (3.6) years for ICH/SAH group. The median (IQR) time to death for the above three case groups is 5.8 (7.4) years, 11.0 (2.1) years, and 7.2 (7.1) years, respectively.
Fig. 1.
Associations of per 1-year increment of KDM age acceleration with all-cause mortality in all participants and four separate groups. KDM: Klemera and Doubal's method; MCE: major coronary event; IS: ischemic stroke; ICH: intracranial hemorrhage; SAH: subarachnoid hemorrhage; HR: hazard ratio. Cox models adjusted for sex, fasting status, and educational attainment, stratified jointly by age groups in the 5-year interval, study areas, and four groups.
Table 2.
Summary of CVD events during follow-up according to four separate groups.
| Control | MCE | IS | ICH or SAH | |
|---|---|---|---|---|
| Number of events | ||||
| CVD cases | 451 | 807 | 3562 | 3342 |
| MCE cases | 86 | 803 | 143 | 97 |
| IS cases | 321 | 25 | 3547 | 601 |
| ICH or SAH cases | 74 | 2 | 213 | 3316 |
| Incidence, per 1000 person-years | ||||
| CVD cases | 9.8 | 201.7 | 194.8 | 227.3 |
| MCE cases | 1.9 | 200.2 | 3.5 | 3.8 |
| IS cases | 7.0 | 4.4 | 193.5 | 25.9 |
| ICH or SAH cases | 1.6 | 0.3 | 5.3 | 224.0 |
| Median (IQR) time to events, year | ||||
| Deaths/loss to follow-up/1 Jan 2018 | 11.6 (1.9) | 5.8 (7.4) | 11.0 (2.1) | 7.2 (7.1) |
| CVD cases | 11.5 (2.1) | 4.4 (3.9) | 4.6 (3.8) | 4.1 (3.6) |
| MCE | 11.6 (1.9) | 4.4 (3.9) | 11.0 (2.1) | 7.2 (7.1) |
| IS cases | 11.5 (2.0) | 5.8 (7.3) | 4.6 (3.8) | 6.2 (6.7) |
| ICH or SAH cases | 11.6 (1.9) | 5.8 (7.4) | 11.0 (2.1) | 4.1 (3.6) |
IQR: interquartile range; CVD: cardiovascular disease; MCE: major coronary event; IS: ischemic stroke; ICH: intracranial hemorrhage; SAH: subarachnoid hemorrhage.
CVD cases correspond to any first occurrence of MCE, IS (ICD10: I63, I69.3), ICH (I61, I69.1), or SAH (I60, I69.0) till the censoring date of 1 Jan 2018. MCE refers to nonfatal myocardial infarction (I21-23) or fatal IHD (I20-25) here.
The distribution of KDM-AA showed statistically significant differences across groups (P < 0.001) (Table 3). The control group exhibited decelerated aging as a whole (KDM-AA [SD] = -0.3 [1.3] year), while the MCE (0.1 [1.6] year) and ICH/SAH (0.3 [1.5] year) groups displayed accelerated aging. The correlations between KDM-BA and CA were extremely high in the whole population and across groups (r = 0.98–0.99). The error of KDM-BA in predicting CA lies in the range from 1.24 years to 1.52 years. The control-derived KDM-AA demonstrated similar distribution but smaller difference and SD among groups (Supplementary Table S5).
Table 3.
Summary of CA and KDM-BA-related measurements for participants according to four separate groups.
| All | Control | MCE | IS | ICH or SAH | P-value | |
|---|---|---|---|---|---|---|
| CA, year | 57.0 (10.5) | 58.4 (11.0) | 54.2 (8.6) | 53.7 (9.4) | 59.6 (10.4) | <0.001 |
| KDM-BA, year | 57.0 (10.6) | 58.2 (11.1) | 54.3 (8.8) | 53.6 (9.5) | 59.9 (10.5) | <0.001 |
| KDM-AA, year | 0.0 (1.4) | −0.3 (1.3) | 0.1 (1.6) | 0.0 (1.2) | 0.3 (1.5) | <0.001 |
| RMSE, year | 1.39 | 1.33 | 1.52 | 1.24 | 1.50 | – |
| Pearson's correlation coefficient between KDM-BA and CA | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 | – |
KDM: Klemera and Doubal's method; CA: chronological age; BA: biological age; AA: age acceleration; RMSE: root mean square error; MCE: major coronary event; IS: ischemic stroke; ICH: intracranial hemorrhage; SAH: subarachnoid hemorrhage.
CA, KDM-BA, and KDM-AA measurements are presented as means (SD).
In the control group, accelerated aging was associated with an increased risk of mortality, with the adjusted HR (95% CI) for per 1-year increment of KDM-AA equal to 1.32 (1.25, 1.39) (Fig. 1). The HRs (95% CIs) for MCE, IS, and ICH/SAH groups were 1.13 (1.06, 1.20), 1.33 (1.26, 1.41), and 1.16 (1.13, 1.20), respectively. When the four groups merged into one, the positive association of KDM-AA with mortality remained robust (HR = 1.20; 95% CI: 1.17, 1.23). The associations of KDM-AA with mortality were also observed in all stratification analyses by sociodemographic and lifestyle factors at baseline. The strength of the association of KDM-AA with the risk of mortality was stronger in the participants aged <60, female, non-smokers, or non-excessive alcohol drinkers than in their counterparts (Supplementary Table S6).
Compared with models with CA included, the Harrell's C-indexes of models with KDM-AA included increased across all groups (increments = 0.008–0.020), and point estimates of NRIs varied from 0.011 to 0.073. The Harrell's C-indexes for models including only CA and both CA and KDM-AA in whole participants were 0.813 (95% CI: 0.807, 0.819) and 0.821 (0.815, 0.826), respectively (Table 4).
Table 4.
Harrell's C-index and NRI for CA and KDM-AA models in all participants and four separate groups.
| Harrell's C-index (95% CI) |
NRI (95% CI) | ||
|---|---|---|---|
| Basic model + CA | Basic model + CA + KDM-AA | ||
| All | 0.813 (0.807, 0.819) | 0.821 (0.815, 0.826) | 0.011 (0.003, 0.019) |
| Control | 0.775 (0.758, 0.793) | 0.794 (0.777, 0.811) | 0.073 (0.048, 0.099) |
| MCE | 0.641 (0.619, 0.664) | 0.652 (0.629, 0.674) | 0.019 (−0.020, 0.058) |
| IS | 0.745 (0.726, 0.765) | 0.765 (0.747, 0.784) | 0.036 (0.008, 0.065) |
| ICH or SAH | 0.665 (0.653, 0.677) | 0.676 (0.664, 0.687) | 0.021 (0.002, 0.040) |
NRI: net reclassification index; CA: chronological age; KDM: Klemera and Doubal's method; CI: confidence interval; AA: age acceleration; MCE: major coronary event; IS: ischemic stroke; ICH: intracranial hemorrhage; SAH: subarachnoid hemorrhage.
Basic Cox models were adjusted for sex, fasting status, educational attainment, urban or rural areas, and lifestyle factors (smoking, alcohol drinking, dietary habits, physical activity level), stratified jointly by study areas and four groups.
In the sensitivity analysis of restricting the construction of KDM-BA in the control group, the association estimates between KDM-AA and all-cause mortality in all participants and four separate groups were comparable to the HRs in the primary analysis and showed similar relative differences between groups (Supplementary Table S7). When the analysis was restricted to participants without taking antihypertensive medication, the results were consistent with the primary analysis, with most association estimates even slightly higher (Supplementary Table S8).
Discussion
This study constructed BA using KDM in a Chinese adult population, showing that the KDM-BA fit CA well. The participants who developed MCE or ICH/SAH during subsequent follow-up showed accelerated aging at baseline. The KDM-AA was positively associated with long-term risk of all-cause mortality and could improve the discrimination performance of models. The association of KDM-AA with all-cause mortality was robust for participants potentially at different stages of the CVD continuum and those with different sociodemographic characteristics and lifestyles.
In the present study, the KDM-BA was highly correlated with CA, with a small prediction error, partly because CA was included as one of the composite markers in the calculation. However, the associations of KDM-AA with death risk remained in our population of a wide age range after ruling out the influence of CA. Also, the discrimination performance with the additional inclusion of KDM-AA in models was better than that including CA. In previous studies of western populations, the association estimates of per 1-year increment of KDM-AA with all-cause mortality were around 1.10.12,32,37,38 A prospective study of 8119 Chinese participants aged 20–79 years between 2009 and 2015 waves utilized 12 biomarkers covering immune, cardiometabolic, liver, and renal function to calculate KDM-BA, demonstrating that per 1-year increment of KDM-AA was associated with a 14% (HR = 1.14; 95% CI: 1.08, 1.19) increased risk of all-cause mortality.15 A longitudinal study of 951 Taiwanese aged ≥54 years during 2000–2006 found KDM-BA improved discrimination of mortality over a basic model including CA and sex (base-model Harrell's C-index = 0.725; KDM-BA-model Harrell's C-index = 0.755).16
Previous studies have shown discrepancies in KDM-BA among participants at different stages of the disease course. A U.S. population study showed a difference in the KDM-BA among participants with diabetes, prediabetes, and age- and sex-matched controls.39 The study found that participants without diabetes were 2.69 years younger than those with prediabetes and 5.73 years younger than those with diabetes in KDM-BA.39 A study based on two Chinese adult cohorts found that participants with more chronic diseases over six years had higher baseline KDM-AA.15 In the present study, the constructed KDM-AA included biochemical markers covering liver and renal function, lipids, inflammation, and metabolism, and physical measurements related to cardiovascular, respiratory, and metabolic status. We found that even in the absence of IHD, stroke, or cancer at baseline, KDM-AA also somewhat distinguished individuals who might have been in the subclinical stage of CVD. Participants who subsequently developed the ICH/SAH or MCE showed an acceleration of aging, and they also had the earliest median time to subsequent onset of diseases compared to other groups. These findings support that KDM-AA as a composite measurement can capture multisystem aging, including vascular senescence.40 However, the association estimates between KDM-AA and death risk were slightly weaker in the ICH/SAH and MCE groups than in control and IS groups. It might be attributed to the already high death risk in these two groups, characterized by high case fatality and the earlier time to death, leaving relatively low additional predictive value for KDM-AA.
In our study, the association strength of KDM-AA with death risk was stronger in relatively healthy individuals, namely young adults, females, non-smokers, or non-excessive alcohol drinkers. These results were consistent with the above finding of stronger association in the control group, possibly with a similar explanation. A study based on the Dunedin Study birth cohort of young adults followed up over 12 years, from ages 26 to 38, showed variation in the pace of aging.41 Other studies, either constructing phenotypic age42 or KDM-AA,15 consistently found that the BA measurements could stratify the risk of death in participants with normal BMI and no defined diseases, with the association stronger in the young age group.42
This study has some advantages. The large sample size and long follow-up ensured robust results and an opportunity to observe the long-term effect of KDM-AA. The study population was characterized by a wide age range and various sociodemographic and lifestyle characteristics, making the conclusion more generalizable. Also, we could further analyze whether the performance of KDM-AA was consistent in the populations with diverse characteristics. The study included participants potentially at different stages of the CVD continuum, providing an opportunity to explore the differences in the performance of KDM-AA in different subpopulations.
Some limitations should be considered. First, the study participants were from a subpopulation of large prospective cohort study, and there existed an overrepresentation of people with poor cardiovascular health. However, all measurements were taken at baseline when all included participants were free of CVDs or cancer. All CVD cases were newly diagnosed during the follow-up. We restricted the construction of KDM-BA in the control group and then derived KDM-BA for the case groups, and there was no significant change in the results. Second, the composition of KDM-BA was limited to the available clinical markers and may miss some markers more relevant to aging, such as blood cell count. Although the types and number of clinical markers included in this study differed from previous studies, the associations of KDM-AA with mortality were consistent. This also suggests that the construction of KDM-BA doesn't need the same clinical markers as long as the markers cover a wide range of biological systems.
In conclusion, the KDM-AA, based on physical and biochemical markers, was a promising BA measurement in the middle-aged and elderly Chinese. The KDM-AA captured the difference in cardiovascular health and predicted the risk of all-cause mortality over a decade. It may be a useful indicator for risk stratification, especially in a relatively healthy population. The idea of constructing KDM-AA could be learned to maximize the use of data platforms for routine clinical practice to achieve effective and efficient risk stratification and early public health intervention.
Contributors
JL conceptualised and designed the paper. LL, ZC, and JC, as members of the CKB steering committee, designed and supervised the conduct of the whole study, obtained funding, and together with CY, YG, PP, LY, IM, RW, YC, HD, YL, SB, and RS acquired the data. LC, YZ, CY, YP, JL accessed and verified the data. LC and YZ analyzed the data and LC drafted the manuscript. JL contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content. All authors contributed to and approved the final manuscript, and were responsible for the decision to submit the manuscript. JL is the study guarantor.
Data sharing statement
The access policy and procedures for the dataset of this study are available at www.ckbiobank.org.
Declaration of interests
The authors declare that there is no conflict of interest.
Acknowledgments
The most important acknowledgment is to the participants in the study and the members of the survey teams in each of the 10 regional centres, as well as to the project development and management teams based at Beijing, Oxford and the 10 regional centres. This work was supported by National Natural Science Foundation of China (82192904, 82192901, 82192900, 81941018). The CKB baseline survey and the first re-survey were supported by a grant from the Kadoorie Charitable Foundation Hong Kong. The long-term follow-up is supported by grants from the UK Wellcome Trust (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z), grants (2016YFC0900500) from the National Key R&D Program of China, National Natural Science Foundation of China (81390540, 91846303), and Chinese Ministry of Science and Technology (2011BAI09B01).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104458.
Appendix A. Supplementary data
References
- 1.Bethlehem R.A.I., Paquola C., Seidlitz J., et al. Dispersion of functional gradients across the adult lifespan. Neuroimage. 2020;222 doi: 10.1016/j.neuroimage.2020.117299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris J.R., Pedersen N.L., McClearn G.E., Plomin R., Nesselroade J.R. Age differences in genetic and environmental influences for health from the Swedish Adoption/Twin Study of Aging. J Gerontol. 1992;47(3):P213–P220. doi: 10.1093/geronj/47.3.p213. [DOI] [PubMed] [Google Scholar]
- 3.Khan S.S., Singer B.D., Vaughan D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell. 2017;16(4):624–633. doi: 10.1111/acel.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 5.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannum G., Guinney J., Zhao L., et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine M.E., Lu A.T., Quach A., et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10(4):573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klemera P., Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Develop. 2006;127(3):240–248. doi: 10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol Series A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Mitnitski A.B., Mogilner A.J., Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkel D., Sternäng O., Jylhävä J., Bai G., Pedersen N.L. Functional aging index complements frailty in prediction of entry into care and mortality. J Gerontol Series A Biol Sci Med Sci. 2019;74(12):1980–1986. doi: 10.1093/gerona/glz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine M.E. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol Series A Biol Sci Med Sci. 2013;68(6):667–674. doi: 10.1093/gerona/gls233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jee H., Park J. Selection of an optimal set of biomarkers and comparative analyses of biological age estimation models in Korean females. Arch Gerontol Geriatr. 2017;70:84–91. doi: 10.1016/j.archger.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Chan M.S., Arnold M., Offer A., et al. A biomarker-based biological age in UK Biobank: composition and prediction of mortality and hospital admissions. J Gerontol Series A Biol Sci Med Sci. 2021;76(7):1295–1302. doi: 10.1093/gerona/glab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z. Development and validation of 2 composite aging measures using routine clinical biomarkers in the Chinese population: analyses from 2 prospective cohort studies. J Gerontol Series A Biol Sci Med Sci. 2021;76(9):1627–1632. doi: 10.1093/gerona/glaa238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaydosh L., Belsky D.W., Glei D.A., Goldman N. Testing proposed quantifications of biological aging in Taiwanese older adults. J Gerontol Series A Biol Sci Med Sci. 2020;75(9):1680–1685. doi: 10.1093/gerona/glz223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong X., Lu Y., Gao Q., et al. Estimating biological age in the Singapore longitudinal aging study. J Gerontol Series A Biol Sci Med Sci. 2020;75(10):1913–1920. doi: 10.1093/gerona/glz146. [DOI] [PubMed] [Google Scholar]
- 18.Jin S., Li C., Cao X., Chen C., Ye Z., Liu Z. Association of lifestyle with mortality and the mediating role of aging among older adults in China. Arch Gerontol Geriatr. 2022;98 doi: 10.1016/j.archger.2021.104559. [DOI] [PubMed] [Google Scholar]
- 19.Dhingra R., Nwanaji-Enwerem J.C., Samet M., Ward-Caviness C.K. DNA methylation age-environmental influences, health impacts, and its role in environmental epidemiology. Curr Environ Health Rep. 2018;5(3):317–327. doi: 10.1007/s40572-018-0203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z., Lee L., Chen J., et al. Cohort profile: the Kadoorie study of chronic disease in China (KSCDC) Int J Epidemiol. 2005;34(6):1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z., Chen J., Collins R., et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40(6):1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turnbull I., Clarke R., Wright N., et al. Diagnostic accuracy of major stroke types in Chinese adults: a clinical adjudication study involving 40,000 stroke cases. Lancet Reg Health West Pac. 2022;21 doi: 10.1016/j.lanwpc.2022.100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin C., Guo Y., Pei P., et al. The relative validity and reproducibility of food frequency questionnaires in the China Kadoorie Biobank study. Nutrients. 2022;14(4) doi: 10.3390/nu14040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ainsworth B.E., Haskell W.L., Herrmann S.D., et al. Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 25.Zhu N., Yu C., Guo Y., et al. Adherence to a healthy lifestyle and all-cause and cause-specific mortality in Chinese adults: a 10-year prospective study of 0.5 million people. Int J Behav Nutr Phys Activ. 2019;16(1):98. doi: 10.1186/s12966-019-0860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv J., Yu C., Guo Y., et al. Adherence to healthy lifestyle and cardiovascular diseases in the Chinese population. J Am Coll Cardiol. 2017;69(9):1116–1125. doi: 10.1016/j.jacc.2016.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv J., Yu C., Guo Y., et al. Adherence to a healthy lifestyle and the risk of type 2 diabetes in Chinese adults. Int J Epidemiol. 2017;46(5):1410–1420. doi: 10.1093/ije/dyx074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Z., Li J., Si J., et al. A large-scale genome-wide association analysis of lung function in the Chinese population identifies novel loci and highlights shared genetic etiology with obesity. Eur Respir J. 2021;58(4) doi: 10.1183/13993003.00199-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karim M.A., Kartsonaki C., Bennett D.A., et al. Systemic inflammation is associated with incident stroke and heart disease in East Asians. Sci Rep. 2020;10(1):5605. doi: 10.1038/s41598-020-62391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes M.V., Millwood I.Y., Kartsonaki C., et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71(6):620–632. doi: 10.1016/j.jacc.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobin M.D., Sheehan N.A., Scurrah K.J., Burton P.R. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 32.Li X., Ploner A., Wang Y., et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife. 2020;9 doi: 10.7554/eLife.51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Hägg S. Physiological age. Bio-protocol. 2020 bio-protocol.org/prep634 Preprint Date Available from: [Google Scholar]
- 34.Chen Y., Wright N., Guo Y., et al. Mortality and recurrent vascular events after first incident stroke: a 9-year community-based study of 0·5 million Chinese adults. Lancet Glob Health. 2020;8(4):e580–e590. doi: 10.1016/S2214-109X(20)30069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrell F.E., Jr., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Pencina M.J., D'Agostino R.B., Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine M.E., Crimmins E.M. Evidence of accelerated aging among African Americans and its implications for mortality. Soc Sci Med. 2014;118:27–32. doi: 10.1016/j.socscimed.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murabito J.M., Zhao Q., Larson M.G., et al. Measures of biologic age in a community sample predict mortality and age-related disease: the framingham offspring study. J Gerontol Series A Biol Sci Med Sci. 2018;73(6):757–762. doi: 10.1093/gerona/glx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahour N., Cortez B., Pan H., Shah H., Doria A., Aguayo-Mazzucato C. Diabetes mellitus correlates with increased biological age as indicated by clinical biomarkers. Geroscience. 2022;44(1):415–427. doi: 10.1007/s11357-021-00469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamczyk M.R., Nevado R.M., Barettino A., Fuster V., Andrés V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):919–930. doi: 10.1016/j.jacc.2019.11.062. [DOI] [PubMed] [Google Scholar]
- 41.Belsky D.W., Caspi A., Houts R., et al. Quantification of biological aging in young adults. Proc Natl Acad Sci U S A. 2015;112(30):E4104–E4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z., Kuo P.L., Horvath S., Crimmins E., Ferrucci L., Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. 2018;15(12) doi: 10.1371/journal.pmed.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.