Graphical abstract
Keywords: Sunlight, Pittsburgh Sleep Quality Index, Calciferol, Circadian rhythm
Abstract
Objectives
This study aimed to evaluate the association of vitamin D with sleep quality during the COVID-19 pandemic and the influence of daily sunlight on this association.
Methods
This cross-sectional, population-based study among adults stratified by multistage probability cluster sampling was conducted from October to December 2020 in the Iron Quadrangle region of Brazil. The outcome was sleep quality, evaluated by the Pittsburgh Sleep Quality Index. Vitamin D (25-hydroxyvitamin D) concentrations were determined by indirect electrochemiluminescence and a deficiency was classified as 25(OH)D < 20 ng/mL. To assess sunlight, the average daily sunlight exposure was calculated and was classified as insufficient when less than 30 min/d. Multivariate logistic analysis was used to estimate the association between vitamin D and sleep quality. A directed acyclic graph was used to select minimal and sufficient sets of adjustment variables for confounding from the backdoor criterion.
Results
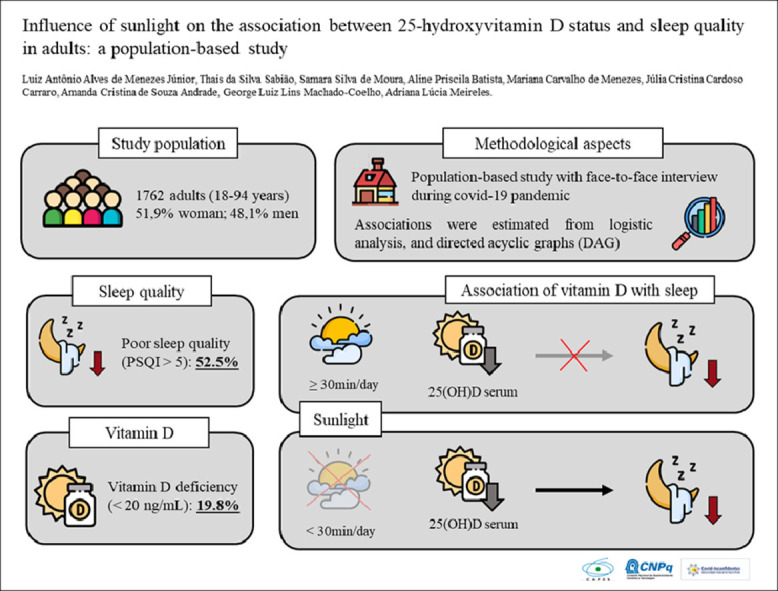
In a total of 1709 individuals evaluated, the prevalence of vitamin D deficiency was 19.8% (95% CI, 15.5–24.9%), and the prevalence of poor sleep quality was 52.5% (95% CI, 48.6–56.4%). In multivariate analysis, vitamin D was not associated with poor sleep quality in individuals with sufficient sunlight. Moreover, in individuals with insufficient sunlight, vitamin D deficiency was associated with poor sleep quality (odds ratio [OR], 2.02; 95% CI, 1.10–3.71). Furthermore, each 1-ng/mL increase in vitamin D levels reduced the chance of poor sleep quality by 4.2% (OR, 0.96; 95% CI, 0.92–0.99).
Conclusions
Vitamin D deficiency was associated with poor sleep quality in individuals with insufficient exposure to sunlight.
Introduction
Poor sleep quality is a severe public health problem in most countries, affecting 10-30% of the general population [1] and more than 50% of the elderly [2]. Epidemiological studies related to sleep have gained scientific notoriety since its effect on health and disease has been reported. About one-third of the general population has symptoms of insomnia [3], and poor sleep quality is associated with adverse health effects, including cerebrovascular diseases, cardiovascular diseases, hypertension, obesity, and diabetes [4]. Furthermore, sleep-related problems have been propagated on a large scale in modern society. The substantial increase in exposure to electronic devices has worsened sleep quality, making it a widespread public health implication and concern [4].
A recent factor that extensively affected the quality of sleep in the population was the COVID-19 pandemic [5], which greatly affected the population's daily life physically, economically, socially, and mentally. The confinement to home had mainly emotional effects, accompanied by fears of an imminent infection with a potentially fatal disease, concerns about one's economic well-being, experiencing unemployment, the insecurity of a lack of supplies, and the exacerbation of uncomfortable information by the media [6,7]. In this chaotic scenario, there has been a worsening in the occurrence of posttraumatic stress symptom predictors such as depression, anxiety, panic syndrome, negative mood impairments, cognition, and acute stress disorder [7]. These episodes of physiological hyperexcitation in the neural pathways experienced by the population during confinement promoted a deterioration in mental health and have been reported in several studies [6], [7], [8]. The great effects of the pandemic on mental health contribute to compromised sleep quality [6,8,9]. Furthermore, measures to reduce the movement of people in public and private collective spaces can decrease the intensity of zeitgebers (environmental cues), both photic (light) and non-photic (physical activity, social interactions, working hours, studying, and eating), all of which help regulate the circadian cycle [10]. Non-photic social zeitgebers, such as work and school during the day, contribute to preventing delays in sleep–wake phase rhythms. However, some people were confined because of the pandemic-related lockdown of COVID-19, disrupting their sleep–wake rhythms owing to the loss of zeitgebers, both photic and non-photic [11].
Even in the context of flexible social distancing and the use of personal protective equipment (such as masks), individuals were feeling insecure and avoided leaving their homes, when possible, which may have reduced their daily sunlight exposure [12]. Exposure to natural light, especially in the morning, is one of the main factors regulating the circadian cycle and inducing more profound and restorative sleep [13]. In addition, cutaneous production of vitamin D from exposure to sunlight is the main source of vitamin D, supplying about 90% of the daily demand [14].
Vitamin D has many characteristics, mainly regulating osteomineral physiology, especially calcium metabolism [15]. However, current evidence shows that the pleiotropic effects of vitamin D and its metabolites extend beyond bone-mineral metabolism and parathyroid gland activity, with effects linked to other potential areas, mainly sleep-related [16,17]. The causal mechanisms of the association between vitamin D and sleep quality are still unclear. Still, hypotheses for this association may be explained by the intracellular distribution of vitamin D receptors in areas of the brain that regulate the sleep–wake cycle or by proinflammatory mediators [18,19]. Experimental studies have shown that sleep-regulatory substances are inversely related to vitamin D levels [20]. In addition, vitamin D is also involved in the production of melatonin, an essential hormone in the regulation of circadian rhythms and sleep [19]. However, most studies that proposed such an investigation were conducted with specific populations, such as school-aged children [21], older individuals [22], and some occupational groups [23,24], with few studies evaluating the association of poor sleep quality in the general population, mainly during the COVID-19 pandemic. Furthermore, the influence of important determinants of the sleep–wake cycle, such as sunlight, the main synchronizer of the circadian cycle, remains to be elucidated [25].
Therefore, this study aimed to evaluate the association of vitamin D deficiency with sleep quality and the influence of daily sunlight on this association.
Methods
Study design
This is a cross-sectional, population-based cross-sectional survey by stratified, multistage probability cluster sampling, conducted between October and December 2020 in two medium-sized cities (Ouro Preto and Mariana) in the south-central region of Minas Gerais, known as Iron Quadrangle, one of the largest iron ore producing areas in Brazil [21].
The conglomerate carried out the sample design in three stages: census sector, household, and resident. This design was based on large national household surveys such as the National Household Sample Survey [26], Family Budget Survey (POF) [27], and Saúde em Beagá survey [28], and more recently, the EPICOVID19 study [29]. Therefore, in the study design, the census sectors were considered as primary sampling units, selected with probability proportional to the number of households, using as a measure of size the number of households, obtained from the synopsis of the 2010 census of population [30]. Before choosing the primary units, prior stratification was performed, considering the average income according to data from the 2010 demographic census of the Brazilian Institute of Geography and Statistics (IBGE), to avoid the risk of drawing a sample from non-representative sectors. Therefore, the representativeness of the different socioeconomic strata (<1 minimum wage, 1 to 3 minimum wages, and ≥4 minimum wages) was guaranteed in the final sample.
The secondary sampling units were the households, selected systematically using the updated listing of existing household units in the primary sampling units (selected census sectors). The household units are formed by private households with residents. After the selection of the census sectors, the household selection interval (k) was calculated for the systematic sampling according to the formula k = Ni / (xi/ni), where Ni is the total number of households in the census sector, xi is the sample size, and ni is the number of households to be selected in the census sector. In this way, a proportional number of homes per sector was obtained, covering the entire geographical area. The first household of the census sector was selected according to IBGE indications, and subsequently, the systematic sampling of the next household was done according to the household selection interval (k).
The tertiary sampling units were the individuals, selected from a simple random sampling. In the selected household, a list of all adult residents was made, and a simple random drawing of one resident to participate in the research was carried out. Based on the sample calculation, the minimum sample size was 1464 individuals. During the data collection process, we evaluated 1762 individuals; 25-hydroxyvitamin D was not analyzed in 53 owing to insufficient blood samples. Therefore, for this study, 1709 individuals were included, representing adult residents in the urban areas of the two cities. Face-to-face interviews were conducted in the residents’ homes; interviewers used an electronic form and adopted national protocols to contain the spread of the coronavirus, such as the use of personal protective equipment. The questionnaire was subdivided according to sociodemographic and economic aspects, living habits, general health conditions, and quality of sleep. All procedures were performed according to the Brazilian guidelines and the Declaration of Helsinki standards for research involving human beings and were approved by the research ethics committee (Ethics Submission Certificate no. 32815620.0.1001.5149). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Outcome variable: Sleep quality
Sleep quality was evaluated by the Pittsburgh Sleep Quality Index (PSQI) [31]. The Brazilian version of the PSQI had an overall reliability coefficient (Cronbach's α) of 0.82, indicating a high degree of internal consistency [32]. This instrument is composed of 19 questions categorized into seven components, each with a possible score of 0 to 3: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleep medication use, and daytime dysfunction. The sum of the scores produces an overall score, ranging from 0 to 21, where higher scores indicate poorer sleep quality [31]. In this study, sleep quality was classified as good when the PSQI score was less than 5 and as poor when the PSQI was 5 or greater [31,32].
Exposure variable: Vitamin D
Blood collection was performed by a trained professional by puncture in the region of the cubital fossa, with the participant seated and the arm supported on a table. A 7.5-mL S-monovette serum gel tube (SARSTEDT) was used for the collection. Subsequently, the samples were verified, stored in a cool box, and transported to the Laboratory of Epidemiology of the School of Medicine at the Federal University of Ouro Preto for processing and storage. In the laboratory, the serum tubes were centrifuged at 2500 rpm for 15 min; aliquots were prepared and stored in a freezer at –80°C for posterior analysis.
Vitamin D was determined by indirect electrochemiluminescence with competition principle in the Access 2 Immunoassay System (Beckman Coulter, USA) with a Roche Diagnostics commercial kit (Roche, Switzerland). For intralaboratory analysis, the coefficient of variation of the method ranges from 6.1% to 7.5%, and the correlation coefficient with high performance liquid chromatography tandem mass spectrometry (LC-MS/M) was 0.92 (data provided by the manufacturer). Furthermore, in previous studies, this method was performed against LC-S/MS [33], and LC-MS/MS in turn was standardized against the National Institute of Standards and Technology (NIST) standard [34]. To describe vitamin D levels, the cutoff points of 10, 20, and 30 ng/mL were used. Additionally, vitamin D concentrations were classified according to the Institute of Medicine as deficient when 25(OH)D was <20 ng/mL and sufficient when 25(OH)D was ≥20 ng/mL [35].
Stratification variable: Sunlight
Exposure to sunlight daily was assessed quantitatively from the following questions: “From Monday to Sunday, how many times a week and for how long are you exposed to the sun?” Subsequently, the average daily sunlight exposure was calculated from the following formula: [(weekly frequency of sunlight [0 to 7 days]) × (daily time of sunlight [min])] / 7. The value obtained was classified according to the National Heart, Lung, and Blood Institute (NHLBI), which suggests that daylight is critical for regulating daily sleep patterns and that individuals should be exposed to natural sunlight for at least 30 min each day. Therefore, we classified exposure as “insufficient” when less than 30 min/d of sunlight and “sufficient” when 30 min/d or more [36].
Covariates
The questionnaire included variables for possible confounding controls in the association between vitamin D and sleep quality analysis. The sociodemographic and economic variables evaluated were sex (female or male), age group (18-34 y, 35-59 y, or ≥60 y), marital status (single or married), living status (living alone or not living alone), current family income (≤2 minimum wages, >2 to ≤4 minimum wages, or >4 minimum wages), educational level (<8 y, 9-11 y, or ≥12 y of study), employment status during the pandemic (employed or not employed), and work-from-home schedule (percentage of active workers who were working at home). Self-reported race or skin color was evaluated using the categories proposed by the IBGE [37]. The participants were categorized as White, Black, Brown, and other races or skin colors (Indigenous or Asian).
Health conditions evaluated were self-reported chronic diseases (hypertension, diabetes, asthma, lung disease, chronic kidney disease, cancer, and heart or thyroid disease), which were dichotomized into morbidity (at least one disease) and without morbidity (no disease), and self-reported chronic physical pain (physical pain present for 3 mo or more). Furthermore, the following lifestyle variables were assessed: current smoking (yes or no), current alcohol drinking (yes or no), and physical activity per week (at least 150–300 min of moderate-intensity aerobic physical activity, at least 75–150 min of vigorous-intensity aerobic physical activity, or inactivity, when the recommendations were not reached) [38]. Nutritional status was evaluated by body mass index, calculated from self-reported height (m) and weight (kg) and classified as underweight (<18.5 kg/m2 if aged <60 y and < 23.0 kg/m2 if aged ≥60 y), eutrophic (18.5–24.9 kg/m2 if aged <60 years and 23.0–28.0 kg/m2 if aged ≥60 years), or overweight (≥25.0 kg/m2 if aged <60 y and ≥28.0 kg/m2 if aged ≥60 y), according to the World Health Organization (WHO) and Pan American Health Organization (PAHO) for adults and elderly individuals, respectively [39,40]. The anxiety and depression symptoms were evaluated by the seven-item Generalized Anxiety Disorder scale and Patient Health Questionnaire–9 scale, respectively. For both scales, scores equal to or above 10 were considered to determine the presence of anxiety and depression symptoms, respectively [41,42]. Moreover, we evaluated whether individuals used any vitamin D or mineral-fortified dietary supplements by self-report using the following questions: “In the past three months, have you used a vitamin-based dietary supplement, such as vitamin D or cholecalciferol or cod oil supplementation?” and “In the past three months, have you used any mineral supplements, such as multivitamins or minerals?” Responses were classified as yes or no.
Statistical analysis
The sample weight was calculated to adjust the natural weights of the design and/or to correct problems caused by the absence or refusal to answer, assigning different weights to the sample elements, corresponding to the inverse of the product from the probabilities included in the various selection stages [43]. When calculating the sample weights, we considered the probabilities of inclusion of the sample elements in the three stages: 1) probability of the census sector being randomized, 2) probability of the household being randomized, and 3) probability of the individual older than 18 y being randomized. The adjustment was applied to compensate for the non-response loss of interviews and calibration for the sample weight for the population totals by sex and age group to be consistent with the 2019 population projections.
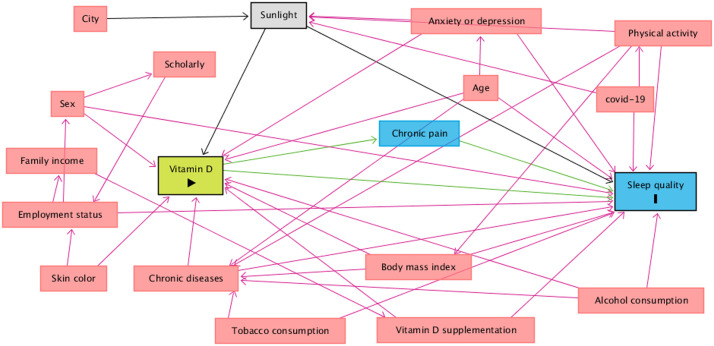
Furthermore, a theoretical causality model based on a directed acyclic graph (DAG) was developed according to the exposure variable (vitamin D), outcome (sleep quality), stratification (sunlight), and covariates, using the online software DAGitty, version 3.2. Causal connections (represented by arrows) were established between variables (Fig. 1 ). Each variable in the DAG is represented by a rectangle, and the colors have different meanings: green is the exposure variable, and blue circled by black is the response variable. Variables considered as potential confounders were included: in blue are the antecedents of the outcome variable, and those in red are the antecedents of the outcome and exposure variables. To avoid unnecessary adjustment, spurious associations, and estimation errors, the backdoor criterion was used to select a minimum set of confounding variables to fit the analyses [44]. The model was adjusted by the following minimum and sufficient set of variables: age (continuous variable), sex (male or female), skin color (White; Black; Brown or others race/skin colors—Indigenous and Asian), education (0– 8 y, 9–11 y, or ≥12 y), employment status (not workers or active workers), alcohol consumption (no or yes), body mass index (continuous variable [kg/m2]), presence of chronic diseases (no or yes), mental health (presence of anxiety or depression symptoms), physical activity (physically active or physically inactive), exposure to sunlight (continuous variable, min/d), and vitamin D supplementation (no or yes). The variance inflation factor assessed collinearity between covariates with the “subsetByVIF” package, considering a maximum cutoff point of 10 (VIF <10) [45,46]. Furthermore, the Hosmer–Lemeshow test and Akaike information criterion were used to assess the goodness-of-fit of the models.
Fig. 1.
Influence of sunlight on the association between 25-hydroxyvitamin D status and sleep quality in adults.
Unadjusted and adjusted logistic regressions were performed for the variables indicated by the DAG. Categorical variables were described as relative frequencies and 95% confidence intervals (CIs), and continuous variables were described as means and 95% CIs. All statistical analyses were performed considering the study design and sampling weighting factors using the “svy” package of Stata software, version 15.0. The significance level was set at 0.05.
Results
Characteristics of study participants
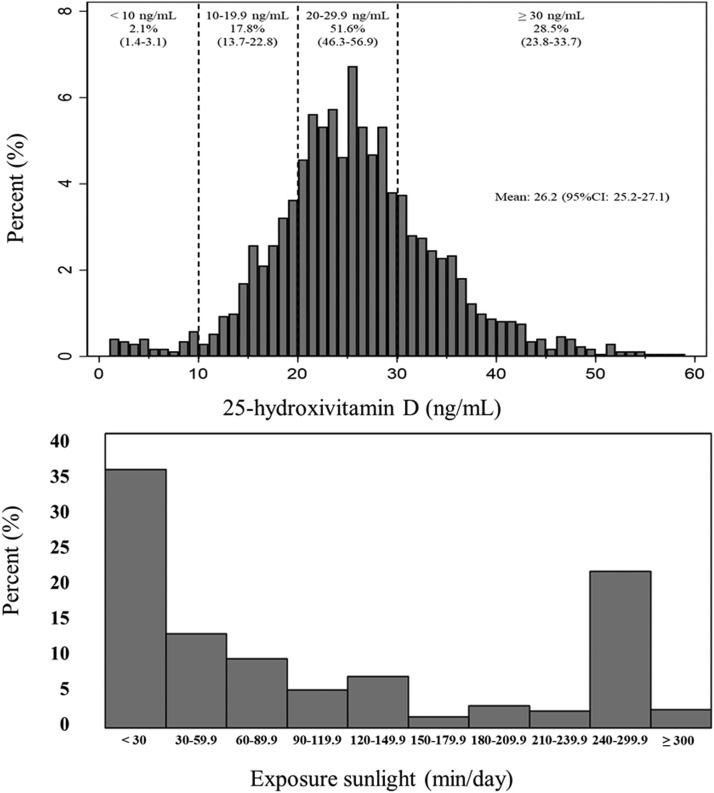
Among the 1709 individuals evaluated, the mean concentration of vitamin D was 26.2 ng/mL (95% CI, 25.2–27.1 ng/mL), with minimum and maximum values of 1.0 and 58.1 ng/mL, respectively. The prevalence of individuals with vitamin D values <10 ng/mL was 2.1% (95% CI, 1.4–3.1%), from 10 to 19.9 ng/mL was 17.8% (95% CI, 13.7–22.8%), from 20 to 29.9 ng/mL was 51.6% (95% CI, 46.3–56.9%) and ≥30 ng/mL was 28.5% (95% CI, 23.8–33.7%) (Fig. 2). Considering the classification of <20 ng/mL, 19.8% (95% CI, 15.5–24.9%) of the individuals had vitamin D deficiency. Poor sleep quality was observed in 52.5% of the sample (95% CI, 48.6–56.4%). Vitamin D deficiency was related to age (P < 0.001), education (P = 0.003), working status (P = 0.006), chronic disease (P = 0.004), alcohol consumption (P = 0.012), nutritional status (P = 0.018), physical activity (P = 0.002), anxiety symptoms (P = 0.005), depression symptoms (P = 0.024), vitamin D supplementation (P < 0.001), and exposure to sunlight (P = 0.045) (Table 1 ).
Fig. 2.
Distribution of 25-hydroxyvitamin D levels (ng/mL) and exposure to sunlight (min/day) in adults during the covid-19 pandemic.
Table 1.
Sociodemographic and health conditions in adults during the COVID-19 pandemic
| Characteristics | Total, % (95% CI) | Vitamin D |
P value* | |
|---|---|---|---|---|
| Sufficiency, % (95% CI) | Deficiency, % (95% CI) | |||
| Total | 80.2 (75.1–84.5) | 19.8 (15.5–24.9) | ||
| Sociodemographic | ||||
| Sex | ||||
| Male | 48.1 (41.0–55.2) | 47.9 (39.3–56.6) | 47.3 (42.3–62.8) | 0.933 |
| Female | 51.9 (44.7–59.0) | 52.1 (43.4–60.7) | 52.7 (42.3–62.8) | |
| Age, y | ||||
| Mean (95%CI) | 43.8 (42.5–45.2) | 40.2 (38.8–41.6) | 52.4 (49.6–55.2) | < 0.001 |
| 18-34 | 35.6 (31.1–40.3) | 35.0 (30.0–40.2) | 22.3 (14.2–33.3) | 0.001 |
| 35-59 | 45.6 (41.1–50.2) | 46.5 (41.4–51.7) | 35.6 (28.1–44.0) | |
| ≥60 | 18.8 (15.5–22.7) | 18.5 (14.8–23.0) | 42.1 (35.0–49.4) | |
| Skin color † | ||||
| White | 25.6 (20.8–31.2) | 23.9 (18.7–30.0) | 33.6 (22.2–47.3) | 0.163 |
| Black | 20.8 (16.0–26.4) | 20.2 (14.7–27.1) | 20.6 (15.1–27.5) | |
| Brown | 47.9 (41.5–54.4) | 49.5 (41.7–57.4) | 43.2 (32.6–54.5) | |
| Others | 5.7 (4.1–7.8) | 6.4 (4.4–9.1) | 2.6 (1.4–4.4) | |
| Marital status ‡ | ||||
| Married | 53.2 (47.2–59.2) | 53.0 (45.8–60.0) | 56.5 (44.4–67.9) | 0.604 |
| Not married | 46.8 (40.8–52.8) | 47.0 (40.0–54.2) | 43.5 (32.1–55.6) | |
| Education, y | ||||
| 0-8 | 31.2 (26.7–36.0) | 29.7 (24.8–35.2) | 33.6 (26.0–42.1) | 0.003 |
| 9-11 | 39.7 (35.6–43.9) | 40.3 (36.2–44.4) | 37.6 (26.6–50.0) | |
| ≥12 | 29.1 (23.8–35.1) | 30.0 (24.1–36.7) | 28.9 (20.5–38.9) | |
| Family income § | ||||
| ≤2 MW | 41.1 (35.6–46.8) | 45.6 (40.1–51.1) | 43.0 (32.9–53.6) | 0.867 |
| >2 to ≤4 MW | 32.0 (26.9–37.5) | 28.0 (22.2–34.7) | 34.9 (26.7–44.1) | |
| >4 MW | 26.9 (22.0–32.5) | 26.4 (20.9–32.8) | 22.1 (16.4–29.2) | |
| Work status ║ | ||||
| Not working | 47.5 (42.7–52.3) | 45.6 (42.7–53.4) | 57.0 (49.2–64.5) | 0.006 |
| Actively working | 52.5 (47.7–57.3) | 54.4 (45.6–62.9) | 43.0 (35.5–50.8) | |
| Working from home ¶ | ||||
| No | 79.7 (75.0–83.7) | 79.0 (74.0–83.3) | 80.6 (72.0–87.0) | 0.681 |
| Yes | 20.3 (16.2–25.0) | 21.0 (16.7–26.0) | 19.4 (13.0–27.9) | |
| City | ||||
| Mariana | 42.4 (30.2–55.7) | 39.1 (26.4–54.3) | 45.0 (32.4–58.3) | 0.202 |
| Ouro Preto | 57.6 (44.3–69.8) | 60.9 (45.7–73.6) | 55.0 (41.7–67.6) | |
| Health conditions | ||||
| Sleep quality # | ||||
| Good | 52.5 (48.6–56.4) | 47.6 (43.5–51.6) | 43.6 (31.6–56.5) | 0.563 |
| Poor | 47.5 (43.6–51.4) | 52.4 (48.4–56.5) | 56.4 (43.5–68.4) | |
| Chronic diseases ⁎⁎ | ||||
| No | 60.3 (54.4–65.8) | 51.4 (43.7–59.1) | 33.2 (25.2–42.3) | 0.004 |
| Yes | 39.7 (34.2–45.5) | 48.6 (40.9–56.3) | 66.8 (57.7–74.8) | |
| Chronic pain | ||||
| No | 65.7 (61.4–69.7) | 65.4 (60.3–70.2) | 63.8 (54.7–71.9) | 0.731 |
| Yes | 34.3 (30.3–38.6) | 34.6 (29.8–39.7) | 36.2 (28.1–45.3) | |
| Smoking | ||||
| No | 83.0 (78.6–86.7) | 84.3 (79.7–88.9) | 76.7 (68.6–86.0) | 0.227 |
| Yes | 17.0 (13.3–21.4) | 15.6 (11.1–20.3) | 23.3 (14.0–31.4) | |
| Alcohol consumption | ||||
| No | 41.8 (36.0–47.9) | 39.2 (29.5–43.9) | 51.8 (42.6–60.9) | 0.012 |
| Yes | 58.2 (52.1–64.0) | 60.8 (56.1–70.5) | 48.2 (39.1–57.4) | |
| Nutritional status †† | ||||
| BMI, kg/m2 | 26.7 (26.2–27.1) | 26.2 (25.7–26.7) | 27.6 (26.6–28.5) | 0.018 |
| Eutrophic | 41.0 (34.7–47.5) | 43.0 (34.1–52.5) | 39.9 (30.4–50.3) | 0.023 |
| Underweight | 2.9 (2.0–4.1) | 1.7 (1.1–2.6) | 4.2 (2.5–6.8) | |
| Overweight | 36.9 (29.5–44.9) | 40.2 (29.7–51.7) | 28.5 (22.4–35.4) | |
| Obesity | 19.2 (15.9–23.0) | 15.1 (11.6–19.5) | 27.4 (20.1–36.1) | |
| Physical activity ‡‡ | ||||
| Physically active | 30.8 (26.2–35.8) | 31.9 (25.8–39.0) | 23.5 (19.3–37.1) | 0.002 |
| Physically inactive | 69.2 (64.2–73.7) | 68.1 (61.0–74.2) | 76.5 (62.9–80.6) | |
| Mental health §§ | ||||
| Anxiety symptoms | 23.4 (19.5–27.7) | 18.9 (14.0–25.0) | 31.6 (25.3–38.6) | 0.005 |
| Depression symptoms | 15.8 (12.7–19.4) | 12.4 (9.0–16.9) | 22.0 (15.7–29.9) | 0.024 |
| Vitamin D supplementation | ||||
| No | 93.8 (91.8–95.3) | 92.5 (90.0–94.4) | 97.8 (95.9–98.8) | < 0.001 |
| Yes | 6.2 (4.7–8.2) | 7.5 (5.6–10.0) | 2.2 (1.2–4.1) | |
| Multivitamins or minerals supplementation | ||||
| No | 92.7 (90.4–94.6) | 92.0 (88.9–94.3) | 94.1 (89.9–96.6) | 0.355 |
| Yes | 7.3 (5.4–9.6) | 8.0 (5.7–11.1) | 5.9 (3.4–10.0) | |
| Exposure to sunlight ║║ | ||||
| Sufficient | 64.9 (59.0–70.4) | 66.5 (59.1–71.8) | 61.6 (50.7–71.2) | 0.045 |
| Insufficient | 35.1 (29.6–41.0) | 33.5 (28.2–40.9) | 38.6 (28.8–49.3) | |
BMI, body mass index; MW, minimum wage
Vitamin D concentrations were classified according to the Institute of Medicine as deficient when 25(OH)D <20 ng/mL and sufficient when 25(OH)D ≥20 ng/mL
Pearson χ2 test.
Other race or skin color included Indigenous and Asian.
Not married included widowed, divorced, or single.
Minimum wage value: BRL 1,045.00 ≈ USD 194.25 (1 USD = 5.3797 BRL).
Not working included unemployed, pensioner, or retiree.
Active workers who were working at home.
Poor sleep quality was determined by Pittsburgh Sleep Quality Index ≥ 5.
Chronic diseases were assessed by self-report and included hypertension, diabetes, asthma, lung disease, chronic kidney disease, cancer, and heart or thyroid disease.
Underweight (BMI <18.5 kg/m2 if aged <60 y or BMI <22.0 kg/m2 if aged >60 y), eutrophic (BMI 18.5–24.9 kg/m2 if aged <60 y or BMI 22.0–27.9 kg/m2 if aged >60 y), overweight (BMI 25.0–29.9 kg/m2 if aged <60 y or BMI 28.0–29.9 kg/m2 if aged >60 y), or obese (BMI >30.0 kg/m2).
Physically inactive (<150 min/wk of moderate physical activity or <75 min/wk of vigorous activity).
The seven-item Generalized Anxiety Disorder and Patient Health Questionnaire–9 scales were used to determining the presence of anxiety and depression symptoms, respectively.
Insufficient exposure to sunlight (<30 min/d) and sufficient (≥30 min/d).
Vitamin D and sleep quality
The mean total PSQI score of all participants was 6.33 (95% CI, 6.03–6.62). Individuals in the vitamin D sufficiency group showed a lower mean PSQI score (6.19; 95% CI, 5.90–6.47) when compared with individuals with vitamin D deficiency (7.02; 95% CI, 6.18–7.86) (P = 0.039). Regarding the PSQI components, individuals with vitamin D deficiency had poor subjective sleep quality (P = 0.004) and shorter sleep duration (P = 0.022). No significant differences in mean scores were found for the other categories of sleep latency, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction (Table 2 ).
Table 2.
Differences in overall PSQI score and scores for each item according to vitamin D level
| Variable | Mean (95% CI) | Vitamin D |
||
|---|---|---|---|---|
| Sufficiency Mean (95% CI) | Deficiency Mean (95% CI) | P value * | ||
| PSQI total score | 6.33 (6.03–6.62) | 6.19 (5.90–6.47) | 7.02 (6.18–7.86) | 0.039 |
| Subjective sleep quality | 1.04 (0.97–1.10) | 1.00 (0.93–1.07) | 1.20 (1.08–1.32) | 0.004 |
| Sleep latency | 1.48 (1.39–1.57) | 1.50 (1.39–1.61) | 1.43 (1.27–1.59) | 0.458 |
| Sleep duration | 0.80 (0.70–0.89) | 0.76 (0.67–0.85) | 1.00 (0.69–1.32) | 0.022 |
| Sleep efficiency | 0.72 (0.64–0.80) | 0.71 (0.62–0.79) | 0.79 (0.55–1.03) | 0.517 |
| Sleep disturbances | 1.35 (1.30–1.41) | 1.33 (1.27–1.39) | 1.45 (1.31–1.59) | 0.105 |
| Use of sleep medication | 0.31 (0.24–0.38) | 0.27 (0.20–0.35) | 0.42 (0.27–0.57) | 0.086 |
| Daytime dysfunction | 0.62 (0.56–0.69) | 0.61 (0.54–0.69) | 0.72 (0.55–0.89) | 0.177 |
PSQI, Pittsburgh Sleep Quality Index.
Vitamin D concentrations were classified according to the Institute of Medicine as deficient when 25(OH)D < 20 ng/mL and sufficient when 25(OH)D ≥ 20 ng/mL.
Comparisons made using the Student t test.
Evaluating the association of vitamin D with sleep quality, we found no association between vitamin D and sleep quality. When evaluating the model adjusted for confounding variables and stratified by sunlight exposure, we found that in individuals with sufficient exposure to sunlight, vitamin D was not associated with poor sleep quality. However, in individuals with insufficient sunlight exposure, vitamin D deficiency was associated with poor sleep (odds ratio [OR], 2.02; 95% CI, 1.10–3.71). Furthermore, each 1-ng/mL increase in vitamin D levels reduced the chance of having poor sleep quality by 4.2% (OR, 0.96; 95% CI, 0.92–0.99) (Table 3 ).
Table 3.
Association between vitamin D and sleep quality during the COVID-19 pandemic
| Total |
Exposure to sunlight † |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted * |
Sufficient (64.9%) |
Insufficient (35.1%) |
||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Vitamin D | |||||||||
| Sufficiency | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| Deficiency | 1.17 | 0.68–2.02 | 1.08 | 0.63–1.82 | 0.85 | 0.43–1.72 | 2.02 | 1.10–3.71 | |
| AIC | 2362.622 | 2280.924 | 1261.312 | 949.934 | |||||
| Continuous value | |||||||||
| ng/mL | 0.99 | 0.98–1.01 | 1.00 | 0.98–1.03 | 1.02 | 0.99–1.06 | 0.96 | 0.92–0.99 | |
| AIC | 2359.420 | 2190.415 | 1257.890 | 950.658 | |||||
AIC, Akaike information criterion.
The outcome variable evaluated was poor sleep quality (Pittsburgh Sleep Quality Index ≥5); vitamin D concentrations were classified according to the Institute of Medicine as deficient when 25(OH)D < 20 ng/mL and sufficient when 25(OH)D ≥ 20 ng/mL
Directed acyclic graph (DAG) was used to support the theoretical model for the adjusted analysis. Adjusted analysis was by the following minimum and sufficient set of variables: age (continuous variable), sex (male or female), skin color (white, black, brown, or other), education (0 to 8 y, 9 to 11 y, or >12 y), employment status (not working or actively working), alcohol consumption (no or yes), body mass index (continuous variable; kg/m2), presence of chronic diseases (no or yes), mental health (presence of anxiety or depression symptoms), physical activity (physically active or physically inactive), exposure to sunlight (continuous variable, min/d), and vitamin D supplementation (no or yes).
Analysis stratified by solar exposure and the minimum and sufficient set of variables is presented.
Discussion
To our knowledge, this is the first population-based study to examine the association between vitamin D and sleep quality in adults stratified by sunlight exposure. Our findings showed that vitamin D deficiency was only associated with poor sleep quality in individuals with insufficient sunlight exposure.
Impaired sleep quality and disturbances are relevant to health because alterations in sleep physiology are risk factors for obesity, hypertension, metabolic syndrome, metabolic changes, immune function, mental health, poor quality of life, and all-cause mortality [47]. Furthermore, poor sleep can severely affect social and work-related daytime performance and increases the risk of occupational and automobile accidents [48]. Therefore, the evaluation of sleep quality and factors that can alter it, such as vitamin D deficiency and sunlight exposure, is of great relevance for epidemiological and clinical research studies to quickly and accurately assess sleep disorders and impairments.
Two main axes, the homeostatic control of sleep and the circadian cycle system, regulate sleep. The circadian cycle system is controlled by an internal biological clock connected to a master clock called the circadian pacemaker, capable of controlling several physiological processes; the sleep–wake cycle is one of the most important [49]. This cycle is regulated by internal and external synchronizers, also called cues or zeitgebers. The main external synchronizer of circadian rhythms is light; when captured by photoreceptor cells in the retina, specifically melanopsin, it transmits luminous information via the optic nerve to the brain's main biological clock, the suprachiasmatic nucleus. From there, the impulses target various brain regions, including the pineal gland, which respond to light-induced stimuli to adjust circadian rhythms to the environmental light, regulating the rhythms of sleep and wakefulness using the hormones melatonin and cortisol [36,50]. In this way, our circadian rhythm aligns the sleep-wake cycle with day and night to create a stable cycle of restorative rest [51,52]. The way light alters the circadian rhythm depends on the intensity of the light and the exposure time. A higher luminance, measured in lux, increases the delay in the circadian rhythm. During the day, outdoor light intensities can reach illuminances of up to 100 000 lux in direct sunlight and 25 000 lux in full daylight. Light intensities in indoor rooms are considerably lower, and standard office lighting is only approximately 100 to 500 lux [53]. For this reason, the daytime sunlight source has a profound influence on the circadian rhythm and is indispensable for a good night's sleep [25]. Therefore, given that sunlight drives both vitamin D synthesis [14] and circadian rhythms [51], vitamin D may be involved in the transduction of light signals that regulate circadian rhythms [54].
Few population-based studies have evaluated the association between vitamin D levels and sleep. However, studies with these methods show similar results to our findings [22,55]. Dogan-Sander et al. [55] evaluated a subset of participants (n = 1045) from the LIFE-Adult-Study, a population-based study of 10 000 participants (18 to 79 y old), recruited between August 2011 and November 2014 in Leipzig, Germany. In this study, sleep was assessed objectively by actigraphic assessments and also subjectively by questionnaires of sleep quality (PSQI) and daytime sleepiness (the Epworth Sleepiness Scale). Multivariate models, adjusted for various confounding factors such as the influence of season, revealed significant associations of serum 25(OH)D concentration with the duration of nighttime sleep and the time of midsleep, assessed objectively. However, serum 25(OH)D concentration showed no significant association with subjective sleep quality and daytime sleepiness [55].
Recent studies of objective measurement [56,57], self-report [22,58,59], and systematic and meta-analytic analysis [16,20,60,61] of sleep have shown that lower vitamin D levels are associated with short sleep duration in adults, elderly individuals, and school-aged children [19,21,23,24,[62], [63], [64], [65], [66]]. A key point of discussion is that we found in the present study that vitamin D was associated with sleep quality only in individuals with low sunlight exposure. These results are corroborated by Choi et al., who found an association of lower 25(OH)D values with changes in sleep duration only in individuals with low sunlight exposure [67]. The same was observed in previous studies of mine workers in the same region as this study; when sleep was assessed by the gold standard method, polysomnography, workers with vitamin D deficiency were more likely to have a higher chance of obstructive sleep apnea, lower sleep efficiency–increased microarousal index, and lower arterial oxygen saturation [23,24]. However, in alternating shift workers, vitamin D was associated with sleep impairments independently of sunlight exposure. One hypothesis is that this population is characterized as a low-sunlight-exposure group, owing to occupational characteristics. Therefore, these workers had routines similar to confinement during the COVID-19 pandemic, because they were off-road truck drivers and spent most days in machinery with inaccessibility to sunlight, supporting our findings that vitamin D is associated with sleep quality only in individuals who are not adequately exposed to the sun. This may be related in part to the effect of natural light on the synchronization of the circadian cycle, and consequently, sleep. Thus, vitamin D deficiency in sun-exposed individuals does not directly interfere with the sleep–wake cycle. However, in individuals who do not have adequate sunlight habits, vitamin D deficiency becomes a relevant factor for poor sleep quality.
The association of vitamin D levels with sleep disorders is recent in the literature, and the main predictors of this relationship are not yet known. Experimental studies show that the sleep regulatory substances—tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and interleukin 1 (IL-1)—show inverse relationships with 25(OH)D [18,68]. A possible justification would be that individuals with higher degrees of systemic inflammation (such as obese individuals) probably behave in a way that promotes low vitamin D synthesis (avoiding sunlight, for example). However, Kuo et al. found that 25(OH)D inhibited tumor necrosis factor α production in macrophages after lipopolysaccharide stimulation, suggesting that these were causal, not spurious, relationships [69]. Furthermore, vitamin D can negatively regulate cyclooxygenase-2, an enzyme that controls the rate of production of prostaglandin D2, an important regulator of the sleep–wake cycle, implying that vitamin D deficiency may result in an increase in circulating prostaglandin D2, contributing to the occurrence of sleep disturbances [70].
Furthermore, in our study, we found that the group of individuals deficient in vitamin D had significantly more anxiety and depression. These results are corroborated by other studies, in which vitamin D supplementation ≥1,600 IU/day reduced anxiety symptoms [71] and ≥2,000 IU/day appeared to reduce depressive symptoms [72]. There are hypotheses for this association that can be highlighted. Vitamin D's active form can affect metabolism of serotonin, increasing serotonin synthesis by inducing the expression of the tryptophan hydroxylase 2 (TPH2) gene. Low serotonin levels are also related to depression and anxiety [73], which is one of the most important factors associated with acute and chronic sleep deprivation [74]. Indirectly, the regulation of serotonin synthesis may increase the synthesis of melatonin, the main sleep-inducing hormone [75,76]. Vitamin D may reduce the increase in neuronal calcium levels, which are known to alter brain function. In this hypothesis, it is suggested that vitamin D plays a role in maintaining the expression of calcium bombs and buffers that reduce their neuronal levels, which may explain how it acts to reduce the onset of depression [77], [78], [79]. Furthermore, vitamin D also regulates the release of nerve growth factor, a molecule essential for neuron survival of hippocampal and cortical neurons, which are essential for mental health and sleep–wake cycle mechanisms [80].
Moreover, some current evidence shows that vitamin D may be involved in the regulation of the circadian clock, because vitamin D receptors have been identified in brain regions that regulate sleep, including the hypothalamus, prefrontal cortex, central gray matter of the midbrain, substantia nigra, and raphe nuclei [81,82]. High concentrations of vitamin D receptors have been found in structures of the brainstem and the suprachiasmatic nucleus, the “master clock” of the circadian system, known as pacemaker cells, which play an important role in the first stage of sleep and its maintenance [83]. Furthermore, a study by Gutierrez-Monreal et al. demonstrated that the active form of vitamin D, 1,25(OH)2D3, was able to synchronize the expression of two important genes of the circadian clock in stem cells, BMAL1 and PER2 [84]. These authors further suggested that because the circadian rhythm influences many physiological processes, this may be the key to a better understanding of the mechanisms by which 1,25(OH)2D3 mediates its many cellular functions [84].
It should be noted that the control of most of the determinants of sleep quality involves the implementation of measures that are difficult to manage because they include the complexity of socioeconomic and psychosocial factors. However, the results of our study indicate that public policies aimed at improving sleep quality should also be considered in the context of strategies that involve behavioral changes. Therefore, we recommend building actions that target direct exposure to sunlight, except for those individuals with extraordinary sensitivity, as an effective measure for improving sleep quality and maintaining adequate serum levels of vitamin D. In addition, although sun exposure is the main contributor to the maintenance of serum vitamin D, it is noted, as observed in this study, that a high prevalence of vitamin D deficiency is a reality even in tropical countries. Therefore, it is necessary to implement policies and recommendations to achieve adequate intake and status of vitamin D, such as expanding the incentive for supplementation beyond risk groups such as babies, elderly individuals, and pregnant individuals, as well as increasing food fortification.
Our study has some strengths. To our knowledge, this is the first population-based study to evaluate the association of vitamin D with sleep quality in adults during the COVID-19 pandemic. In addition, the sample design brings robustness to the study: 1) a representative random sample of the resident population from different socioeconomic strata, 2) assessment by household survey, and 3) a face-to-face study during the COVID-19 pandemic. Furthermore, the DAG was used to guide analysis plans and decisions about possible confounders.
However, although our findings provide relevant insights, this study has limitations in some areas that deserve to be mentioned. First, the information obtained was self-reported, so the individuals’ perception may overestimate and/or underestimate findings compared with objective measures. However, the evaluation of sleep quality needs to be done subjectively, since it considers factors intrinsic to the individuals’ perception of their sleep. The social construct of race and skin color based on the IBGE classification when compared with scales for assessing skin color, such as Fitzgerald's, may reduce the flexibility of the respondents’ choices regarding self-report. However, the IBGE classification is widely used in national studies [85], [86], [87], [88], and in a study conducted by Travassos et al., it was demonstrated that the self-assessment of the IBGE, as adopted in our study, represents the same construct of race and skin color of the assessment scales [89]. A further limitation of the study is the subjective assessment of sun exposure, since this may imply recall bias. However, it should be noted that self-reported measures are widely used in epidemiological studies [67,90,91], and some studies have shown a good correlation between objective and subjective measures of sun exposure assessment [92,93]. Although we adjusted the association models for potential confounders, our study cannot ignore residual confounding. Because of the design of the study, it is impossible to construct internally homogeneous strata about the real confounding variable. The controlled variable is an imperfect or incomplete substitute for the real confounding factor (such as psychological distress, adjusted for anxiety and depression symptoms) or because other confounding effects in the hypotheses are ignored or not measured [94]. It is possible that people with psychological distress exhibit abstinence behavior, performing few activities in the sun, and thus find themselves grouped in the low-sun-exposure group, when at the same time they also have particularly low sleep quality owing to their psychological condition. However, it is important to note that the hypotheses have been carefully defined according to the current scientific literature and articulated in counterfactual terms to construct theories that can support the presumptions of the analyses. In this regard, the incorporation of directed graphical models is of great importance and provides robustness to the study [95].
Conclusion
Vitamin D was not associated with poor sleep quality in individuals with sufficient sunlight exposure. However, in individuals with low sunlight, we found that low serum vitamin D levels were associated with poor sleep quality. In modern society, owing to social, occupational, and cultural issues, opportunities for exposure to sunlight are inevitably low. Therefore, maintaining an adequate serum vitamin D level can be important for healthy sleep quality.
Acknowledgments
The authors acknowledge the support of the Federal University of Ouro Preto and the Research and Education Group in Nutrition and Collective Health for their support and incentive, the Clinical Analysis Pilot Laboratory of the Pharmacy School of the Federal University of Ouro Preto for the biochemical analyses, and the support of the Municipal Health Secretariats of the municipalities.
Footnotes
This study was supported by the Brazilian Council for Scientific and Technological Development, Coordination for the Improvement of Higher Education Personnel–Brazil (CAPES) [0676/2020; nº 88881.504995/2020-01], the Foundation for Research Support of the State of Minas Gerais (FAPEMIG) (nº001/2021; APQ-02445–21), and finance code 001 for a Ph.D. student scholarship.
References
- 1.Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Fam Med Primary Care. 2016;5:780–784. doi: 10.4103/2249-4863.201153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018:141017–141024. doi: 10.5664/jcsm.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohayon MM. Epidemiological overview of sleep disorders in the general population. Sleep Med Res. 2011;2:1–9. [Google Scholar]
- 4.Chattu V, Manzar M.D, Kumary S, Burman D, Spence D, Pandi-Perumal S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel) 2018;7:1. doi: 10.3390/healthcare7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mello MT, de Silva A, Guerreiro RDC, Da-Silva FR, Esteves AMU, Poyares D, et al. Sleep and COVID-19: considerations about immunity, pathophysiology, and treatment. Sleep science. (Sao Paulo. Brazil) 2020;13(3):199–209. doi: 10.5935/1984-0063.20200062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta K, Tripathi M. Sleep and COVID-19. Neurology India. 2021;69:26–31. doi: 10.4103/0028-3886.310073. [DOI] [PubMed] [Google Scholar]
- 7.Liu N, Zhang F, Wei C, Jia Y, Shang Z, Sun L, et al. Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: gender differences matter. Psychiatr Res. 2020;287 doi: 10.1016/j.psychres.2020.112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi R, Socci V, Talevi D, Mensi S, Niolu C, Pacitti F, et al. COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. Front Psychiatr. 2020;11:790. doi: 10.3389/fpsyt.2020.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Di Y, Ye J, Wei W. Study on the public psychological states and its related factors during the outbreak of coronavirus disease 2019 in some regions of China. Psychol Health Med. 2021;26:13–22. doi: 10.1080/13548506.2020.1746817. [DOI] [PubMed] [Google Scholar]
- 10.Guichard K, Geoffroy PA, Taillard J, Micoulaud-Franchi JA, Royant-Parola S, Poirot I, et al. Stratégies de gestion de l'impact du confinement sur le sommeil : une synthèse d'experts. Médecine Du Sommeil. 2020;17:108–112. [Google Scholar]
- 11.Otsuki R, Matsui K, Yoshiike T, Nagao K, Utsumi T, Tsuru A, et al. Decrease in social zeitgebers is associated with worsened delayed sleep-wake phase disorder: findings during the pandemic in Japan. Front Psychiatr. 2022;13 doi: 10.3389/fpsyt.2022.898600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talic S, Shah S, Wild H, Gasevic D, Maharaj A, Ademi Z, et al. Effectiveness of public health measures in reducing the incidence of COVID-19, SARS-CoV-2 transmission, and COVID-19 mortality: systematic review and meta-analysis. BMJ. 2021;17 doi: 10.1136/bmj-2021-068302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang AM, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, et al. Human responses to bright light of different durations. J Physiol. 2012;590:3103–3112. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 16.Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The association between vitamin D deficiency and sleep disorders: a systematic review and meta-analysis. Nutrients. 2018;10:1395. doi: 10.3390/nu10101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai YH, Fang TC. The pleiotropic effect of vitamin D. ISRN Nephrol. 2013:1–6. doi: 10.5402/2013/898125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellia A, Garcovich C, D'Adamo M, Lombardo M, Tesauro M, Donadel G, et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med. 2013;8:33–40. doi: 10.1007/s11739-011-0559-x. [DOI] [PubMed] [Google Scholar]
- 19.Romano F, Muscogiuri G, di Benedetto E, Zhukouskaya VV, Barrea L, Savastano S, et al. Vitamin D and sleep regulation: is there a role for vitamin D? Curr Pharm Des. 2021;26:2492–2496. doi: 10.2174/1381612826666200310145935. [DOI] [PubMed] [Google Scholar]
- 20.Abboud M. Vitamin D supplementation and sleep: a systematic review and meta-analysis of intervention studies. Nutrients. 2022;14:1076. doi: 10.3390/nu14051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong QH, Li SX, Li H, Chen Q, Li XY, Xu GZ. 25-Hydroxyvitamin D status and its association with sleep duration in Chinese schoolchildren. Nutrients. 2018;10:1013. doi: 10.3390/nu10081013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, Chang JH, Kim DY, Kang JW. Association between self-reported sleep duration and serum vitamin D level in elderly Korean adults. J Am Geriatr Soc. 2014;62:2327–2332. doi: 10.1111/jgs.13148. [DOI] [PubMed] [Google Scholar]
- 23.de Menezes Júnior LAA, Fajardo VC, de Freitas SN, Machado-Coelho GLL, de Oliveira FLP, do Nascimento Neto RM, Meireles AL. Rotating shift workers with vitamin D deficiency have a higher risk of obstructive sleep apnea. Sleep Breath. 2023 doi: 10.1007/s11325-022-02603-4. [e-pub ahead of print] accessed February 23. [DOI] [PubMed] [Google Scholar]
- 24.Menezes-Júnior LAA, de Fajardo VC, Freitas SN, de Pimenta FAP, Oliveira FLP, de Machado-Coelho GLL, et al. Hypovitaminosis D is associated with sleep disorders in workers on alternating shifts with cardiovascular risk factors. MedRxiv 2021. 05.04.21256625.
- 25.Blume C, Garbazza C, Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie (Berl) 2019;23:147–156. doi: 10.1007/s11818-019-00215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.do Nascimiento Silva PL, Pessoa DGC, Lila MF. Análise estatística de dados da PNAD: incorporando a estrutura do plano amostral—statistical analysis of data from PNAD: incorporating the sample design. Ciência & Saúde Coletiva. 2002;7:659–670. [Google Scholar]
- 27.Brazilian Institute of Geography and Statistics Pesquisa de orçamentos familiares 2017-2018 : análise do consumo alimentar pessoal no Brasil. IBGE, Coordenação de Trabalho e Rendimento. 2020:120. [Google Scholar]
- 28.Meireles AL, Xavie CC, Proietti FA, Caiaffa WT. Influence of individual and socio-environmental factors on self-rated health in adolescents. Rev Bras Epidemiol. 2015;18:538–551. doi: 10.1590/1980-5497201500030002. [DOI] [PubMed] [Google Scholar]
- 29.Hallal PC, Hartwig FP, Horta BL, Silveira MF, Struchiner CJ, Vidaletti LP, et al. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8:e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brazilian Institute of Geography and Statistics. Atlas do censo demográfico 2010. 2013. Available at: https://biblioteca.ibge.gov.br/index.php/biblioteca-catalogo?view=detalhes&id=264529. Accessed February 23, 2023.
- 31.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, da Silva Miozzo IC, de Barba MEF, Menna Barreto SS. Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011;12:70–75. doi: 10.1016/j.sleep.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Vogeser M, Kyriatsoulis A, Huber E, Kobold U. Candidate reference method for the quantification of circulating 25-hydroxyvitamin D3 by liquid chromatography—tandem mass spectrometry. Clin Chem. 2004;50:1415–1417. doi: 10.1373/clinchem.2004.031831. [DOI] [PubMed] [Google Scholar]
- 34.Vogeser M, Kyriatsoulis A, Huber E, Kobold U. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88 doi: 10.1093/ajcn/88.2.511S. 511S-2S. [DOI] [PubMed] [Google Scholar]
- 35.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Heart Lung And Blood Institute. In brief: your guide to healthy sleep. Available at: https://www.nhlbi.nih.gov/files/docs/public/sleep/healthysleepfs.pdf. Accessed February 23, 2023.
- 37.Kabad JF, Bastos JL, Santos RV. Raça, cor e etnia em estudos epidemiológicos sobre populações brasileiras: revisão sistemática na base PubMed. Physis: Revista de Saúde Coletiva 2012;22:895–918.
- 38.World Health Organization. WHO guidelines on physical activity, sedentary behaviour, and sleep for children under 5 years of age. Available at: https://apps.who.int/iris/bitstream/handle/10665/325147/WHO-NMH-PND-2019.4-eng.pdf?sequence=1&isAllowed=y%0Ahttp://www.who.int/iris/handle/10665/311664%0Ahttps://apps.who.int/iris/handle/10665/325147. Accessed February 23, 2023. [PubMed]
- 39.World Health Organization. Physical status: the use and interpretation of anthropometry. Technical Report Series No. 854. Available at: https://apps.who.int/iris/handle/10665/37003. Accessed February 23, 2023. [PubMed]
- 40.Pan American Health Organization (PAHO). XXXVI Reunión del Comité Asesor de Investigaciones en Salud - Encuesta Multicêntrica - Salud Beinestar y Envejecimeiento (SABE) en América Latina y el Caribe. Preliminary report, 2001. Available at: https://iris.paho.org/handle/10665.2/6210. Accessed February 23, 2023.
- 41.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 42.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szwarcwald CL, Damacena GN. Amostras complexas em inquéritos populacionais: planejamento e implicações na análise estatística dos dados. Revista Brasileira de Epidemiologia. 2008;11:38–45. [Google Scholar]
- 44.Cortes TR, Faerstein E, Struchiner CJ. Use of causal diagrams in epidemiology: application to a situation with confounding. Cade Saude Publica. 2016;32 doi: 10.1590/0102-311X00103115. [DOI] [PubMed] [Google Scholar]
- 45.Hair JF. Multivariate data analysis: an overview. In: International encyclopedia of statistical science. Springer Berlin Heidelberg.
- 46.Plummer W, Dupont WD, Plummer W, Dupont WD. SUBSETBYVIF: Stata module to select a subset of covariates constrained by VIF. Econ Papers. 2019. Available at: https://EconPapers.repec.org/RePEc:boc:bocode:s458635. Accessed February 23, 2023.
- 47.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Léger D, Bayon V, Ohayon MM, Philip P, Ement P, Metlaine A, et al. Insomnia and accidents: cross-sectional study on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23:143–152. doi: 10.1111/jsr.12104. [DOI] [PubMed] [Google Scholar]
- 49.National Institute of Neurological and Sleep Disorders. Brain basics: understanding sleep. Available at: https://www.ninds.nih.gov/health-information/patient-caregiver-education/brain-basics-understanding-sleep. Accessed February 23, 2023.
- 50.Sleep Healthy. Harvard Medical School; 2023. External factors that influence sleep.http://healthysleep.med.harvard.edu/healthy/science/how/external-factors Available at: Accessed February 23. [Google Scholar]
- 51.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Ann Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 52.Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health. 2001;25:85–93. [PMC free article] [PubMed] [Google Scholar]
- 53.Spitschan M, Aguirre GK, Brainard DH, Sweeney AM. Variation of outdoor illumination as a function of solar elevation and light pollution. Sci Rep. 2015;6:26756. doi: 10.1038/srep26756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucock M, Jones P, Martin C, Beckett E, Yates Z, Furst J, Veysey M. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310–322. doi: 10.1177/2156587215580491. [DOI] [PubMed] [Google Scholar]
- 55.Dogan-Sander E, Willenberg A, Batmaz İ, Enzenbach C, Wirkner K, Kohls E, et al. Association of serum 25-hydroxyvitamin D concentrations with sleep phenotypes in a German community sample. PLoS ONE. 2019;14:1–17. doi: 10.1371/journal.pone.0219318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massa J, Stone KL, Wei EK, Harrison SL, Barrett-Connor E, Lane NE, et al. Vitamin D and actigraphic sleep outcomes in older community-dwelling men: the MrOS Sleep Study. Sleep. 2015;38:251–257. doi: 10.5665/sleep.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piovezan RD, Hirotsu C, Feres MC, Cintra FD, Andersen ML, Tufik S, et al. Obstructive sleep apnea and objective short sleep duration are independently associated with the risk of serum vitamin D deficiency. PLoS ONE. 2017;12:1–11. doi: 10.1371/journal.pone.0180901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung YS, Chae CH, Kim YO, Son JS, Kim CW, Park HO, et al. The relationship between serum vitamin D levels and sleep quality in fixed day indoor field workers in the electronics manufacturing industry in Korea. Ann Occup Environ Med. 2017;29:25. doi: 10.1186/s40557-017-0187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beydoun MA, Gamaldo AA, Canas JA, Beydoun HA, Shah MT, McNeely JM, et al. Serum nutritional biomarkers and their associations with sleep among US adults in recent national surveys. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0103490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, He J, Yun J. The association between serum vitamin D and obstructive sleep apnea: an updated meta-analysis. Resp Res. 2020;21:1–12. doi: 10.1186/s12931-020-01554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mirzaei-Azandaryani Z, Abdolalipour S, Mirghafourvand M. The effect of vitamin D on sleep quality: a systematic review and meta-analysis. Nutr Health. 2022;28:515–526. doi: 10.1177/02601060221082367. [DOI] [PubMed] [Google Scholar]
- 62.Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. 2012;79:132–135. doi: 10.1016/j.mehy.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 63.Lee HJ, Choi H, Yoon IY. Impacts of serum vitamin D levels on sleep and daytime sleepiness according to working conditions. J Clin Sleep Med. 2020;16:1045–1054. doi: 10.5664/jcsm.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCarty DE, Chesson AL, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. 2014;18:311–319. doi: 10.1016/j.smrv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Mosavat M, Smyth A, Arabiat D, Whitehead L. Vitamin D and sleep duration: is there a bidirectional relationship? Horm Mol Biol Clin Investig. 2020:41. doi: 10.1515/hmbci-2020-0025. [DOI] [PubMed] [Google Scholar]
- 66.Neighbors CLP, Noller MW, Song SA, Zaghi S, Neighbors J, Feldman D, et al. Vitamin D and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med. 2018;43:100–108. doi: 10.1016/j.sleep.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Choi JH, Lee B, Lee JY, Kim CHH, Park B, Kim DY, et al. Relationship between sleep duration, sun exposure, and serum 25-hydroxyvitamin D status: a cross-sectional study. Sci Rep. 2020;10:4168. doi: 10.1038/s41598-020-61061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khoo AL, Chai LYA, Koenen HJPM, Sweep FCGJ, Joosten I, Netea MG, et al. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin Exp Immunol. 2011;164(1):72–79. doi: 10.1111/j.1365-2249.2010.04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuo YT, Kuo CH, Lam KP, Chu YT, Wang WL, Huang CH, et al. Effects of vitamin D3 on expression of tumor necrosis factor-α and chemokines by monocytes. J Food Sci. 2010;75:H200–H204. doi: 10.1111/j.1750-3841.2010.01704.x. [DOI] [PubMed] [Google Scholar]
- 70.Swami S, Krishnan AV, Feldman D. Vitamin D metabolism and action in the prostate: implications for health and disease. Molecular and Cellular Endocrinology. 2011;347:61–69. doi: 10.1016/j.mce.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu C, Zhang Y, Wang T, Lin Y, Yu J, Xia Q, et al. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020;10:e01760. doi: 10.1002/brb3.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mikola T, Marx W, Lane MM, Hockey M, Loughman A, Rajapolvi S, et al. The effect of vitamin D supplementation on depressive symptoms in adults: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2022;11:1–18. doi: 10.1080/10408398.2022.2096560. [DOI] [PubMed] [Google Scholar]
- 73.Correia AS, Vale N. Tryptophan metabolism in depression: a narrative review with a focus on serotonin and kynurenine pathways. Int J Mol Sci. 2022;23:8493. doi: 10.3390/ijms23158493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox RC, Olatunji BO. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord. 2016;37:104–129. doi: 10.1016/j.janxdis.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Portas CM, Bjorvatn B, Ursin R. Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog Neurobiol. 2000;60:13–35. doi: 10.1016/s0301-0082(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 76.Ceolin G, Mano GPR, Hames NS, Antunes LDC, Brietzke E, Riege DK, et al. Vitamin D, depressive symptoms, and COVID-19 pandemic. Front Neurosci. 2021;15 doi: 10.3389/fnins.2021.670879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anjum I, Jaffery SS, Fayyaz M, Samoo Z, Anjum S. The role of vitamin D in brain health: a mini literature review. Cureus. 2018;10:e2960. doi: 10.7759/cureus.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69:80–92. doi: 10.1124/pr.116.013227. [DOI] [PubMed] [Google Scholar]
- 79.Harrison PJ, Hall N, Mould A, Al-Juffali N, Tunbridge EM. Cellular calcium in bipolar disorder: systematic review and meta-analysis. Mol Psychiatr. 2019;26:4106–4116. doi: 10.1038/s41380-019-0622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gezen-Ak D, Dursun E, Yilmazer S. The effect of vitamin D treatment on nerve growth factor release from hippocampal neurons. Nöro Psikiyatri Arşivi. 2014;51:157. doi: 10.4274/npa.y7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stumpf WE, Bidmon HJ, Li L, Pilgrim C, Bartke A, Mayerhofer A, et al. Nuclear receptor sites for vitamin D-soltriol in midbrain and hindbrain of Siberian hamster assessed by autoradiography. Histochemistry. 1992;98:155–164. doi: 10.1007/BF00315874. [DOI] [PubMed] [Google Scholar]
- 82.Stumpf WE, O'Brien LP. 1,25(OH)2 vitamin D3 sites of action in the brain. Histochemistry. 1987;87:393–406. doi: 10.1007/BF00496810. [DOI] [PubMed] [Google Scholar]
- 83.Musiol IM, Stumpf WE, Bidmon HJ, Heiss C, Mayerhofer A, Bartke A. Vitamin D nuclear binding to neurons of the septal, substriatal and amygdaloid area in the siberian hamster brain. Neuroscience. 1992;48:841–848. doi: 10.1016/0306-4522(92)90272-4. [DOI] [PubMed] [Google Scholar]
- 84.Gutierrez-Monreal MA, Cuevas-Diaz Duran R, Moreno-Cuevas JE, Scott SP. A role for 1α,25-dihydroxyvitamin D3 in the expression of circadian genes. J Biol Rhythms. 2014;29:384–388. doi: 10.1177/0748730414549239. [DOI] [PubMed] [Google Scholar]
- 85.dos Santos MPA, Nery JS, Goes EF, da Silva A, dos Santos ABS, Batista LE, et al. População negra e COVID-19: reflexões sobre racismo e saúde. Estudos Avançados. 2020;34:225–244. [Google Scholar]
- 86.da Silveira Moreira R. Epidemiologia e a categoria das raças: reflexões onto-epistemológicas. Cadernos de Saúde Pública. 2021;37 doi: 10.1590/0102-311X00133721. [DOI] [PubMed] [Google Scholar]
- 87.Barbosa REC, Fonseca GC, de Azevedo DSDS, Simões MRL, Duarte ACM, de Alcântara MA. Prevalência e fatores associados à autoavaliação negativa de saúde entre trabalhadores da rede municipal de saúde de Diamantina, Minas Gerais. Epidemiologia e Serviços de Saúde. 2020;29 doi: 10.5123/S1679-49742020000200013. [DOI] [PubMed] [Google Scholar]
- 88.Fabíola M, Araújo S, Araújo De Souza T, de Almeida A, Iii M, Carla De Souza, J, et al. Fatores associados aos problemas de sono e ao uso de medicação para dormir em brasileiros.
- 89.Travassos C, Laguardia J, Marques PM, Mota JC, Szwarcwald CL. Comparison between two race/skin color classifications in relation to health-related outcomes in Brazil. Int J Equity Health. 2011;10:1–8. doi: 10.1186/1475-9276-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu CL, Li Y, Freedman DM, Fears TR, Kwok R, Chodick G. Alexander, et al . Assessment of lifetime cumulative sun exposure using a self-administered questionnaire: reliability of two approaches. Cancer Epidemiol Biomarkers Prev. 2009;18:464–471. doi: 10.1158/1055-9965.EPI-08-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cui Y, Gong Q, Huang C, Guo F, Li W, Wang Y, et al. The relationship between sunlight exposure duration and depressive symptoms: a cross-sectional study on elderly Chinese women. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0254856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Køster B, Søndergaard J, Nielsen JB, Allen M, Olsen A, Bentzen J. The validated sun exposure questionnaire: association of objective and subjective measures of sun exposure in a Danish population-based sample. Br J Dermatol. 2017;176:446–456. doi: 10.1111/bjd.14861. [DOI] [PubMed] [Google Scholar]
- 93.Eshtiaghi P, Khosravi-Hafshejani T, Sara G, Lui H, Kalia S. Assessment of sun-safety education behavior via spectrophotometric evaluation: a preliminary study. Photodermatol Photoimmunol Photomed. 2022;38:451–458. doi: 10.1111/phpp.12768. [DOI] [PubMed] [Google Scholar]
- 94.Reichenheim ME, Moraes CL. Alguns Pilares Para a Apreciação Da Validade de Estudos Epidemiológicos. Rev Bras Epidemiol. 1998;1(2):131–148. doi: 10.1590/S1415-790X1998000200004. [DOI] [Google Scholar]
- 95.Pearl J. Causality: models, reasoning and inference.